Aspect-Related Mechanical Properties of the Cortical Bone in the Third Metacarpal Bone of Mares
Abstract
1. Introduction
2. Materials and Methods
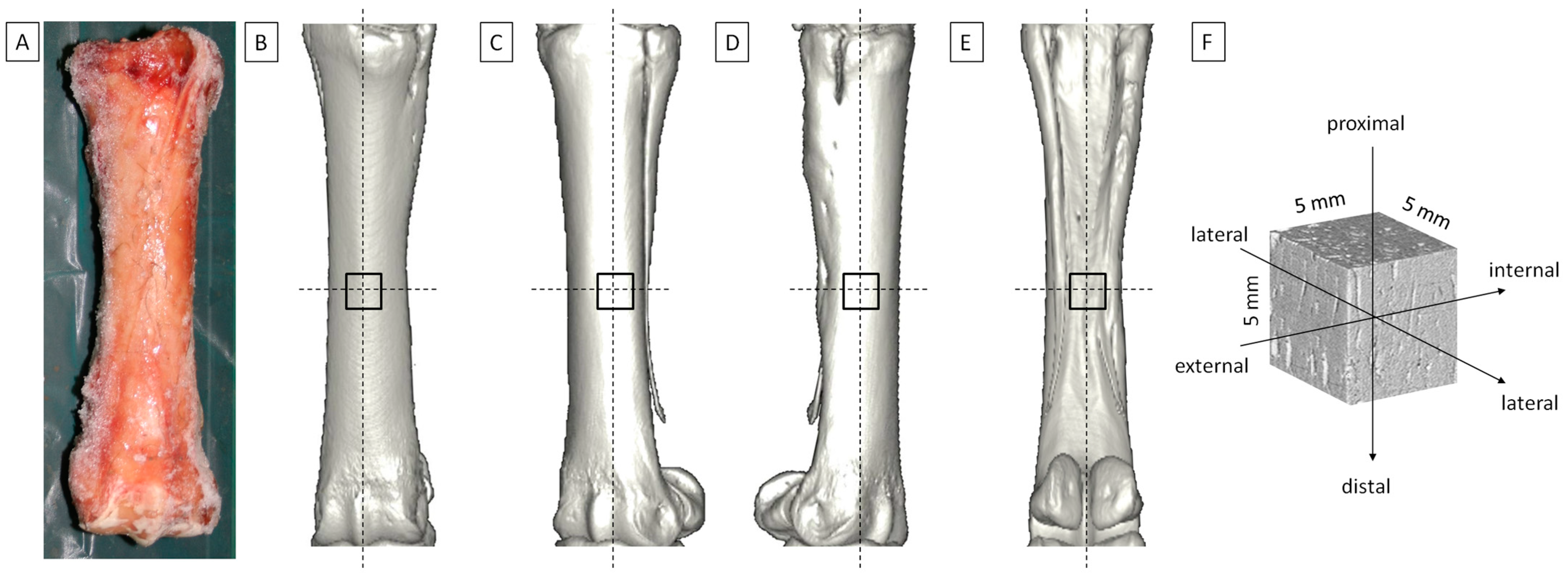
2.1. Biological Samples
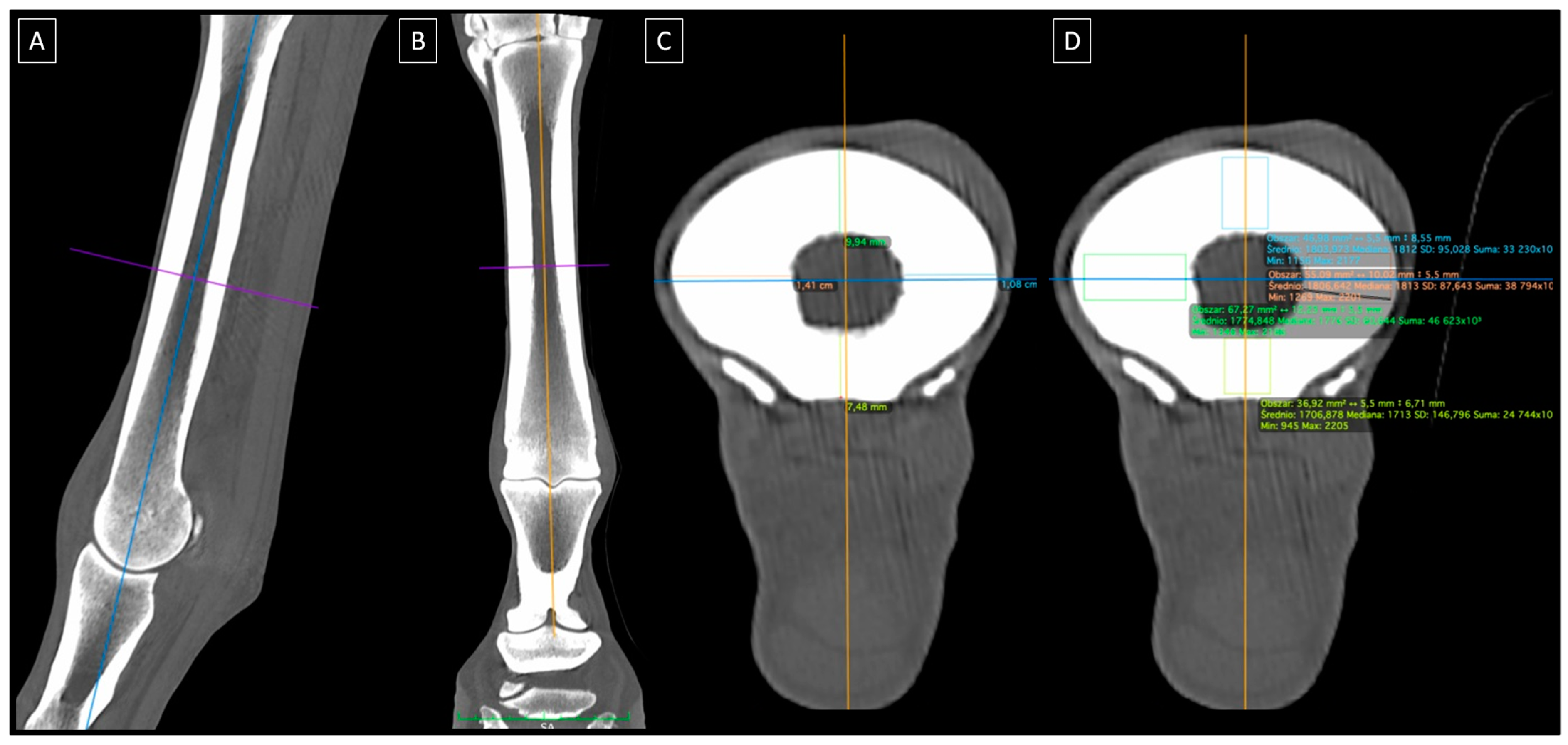
2.2. CT Imaging
2.3. Mechanical Tests
2.3.1. 3-Point Bending Test
2.3.2. Uniaxial Compression Test
2.4. Statistical Analysis
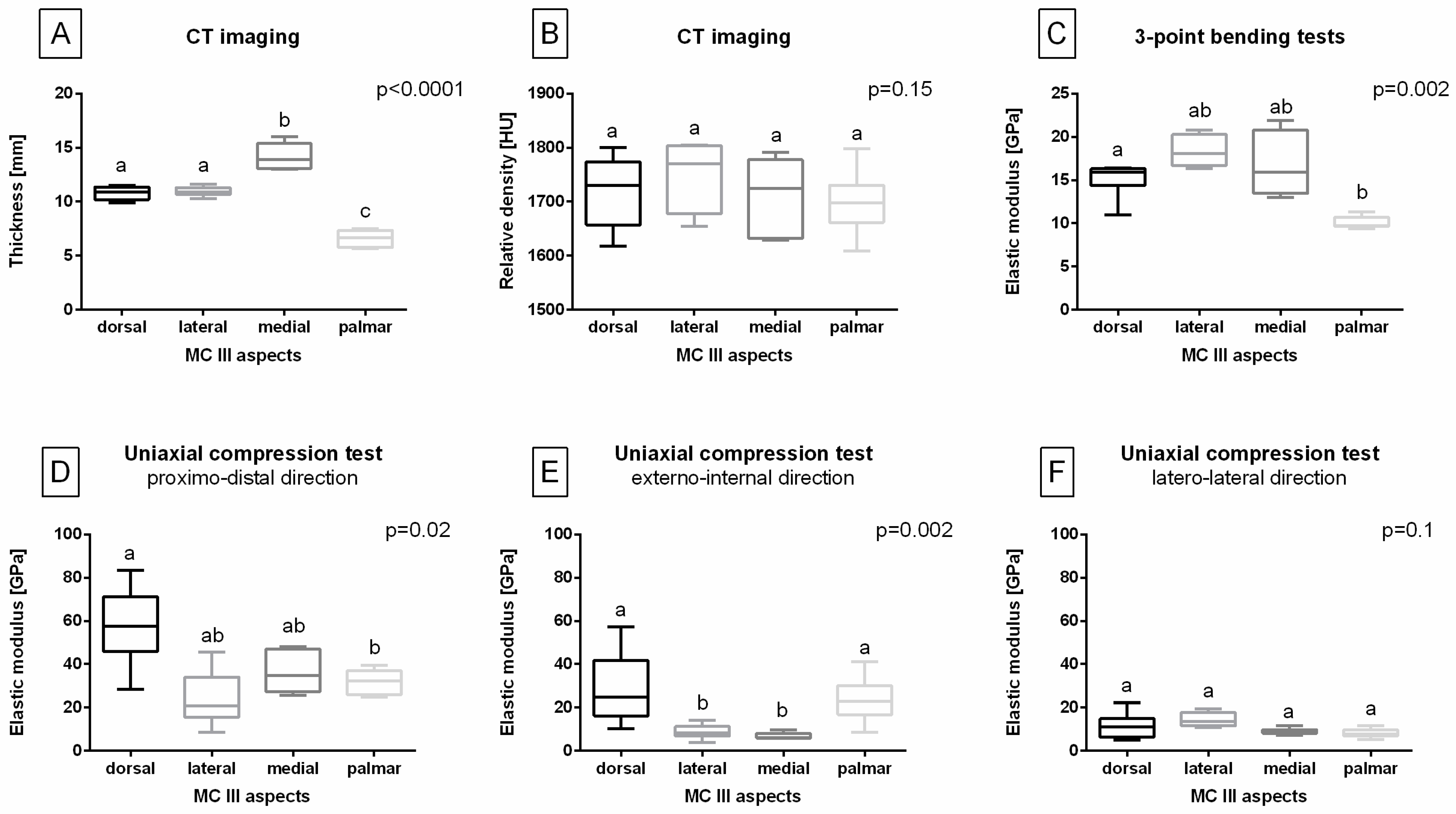
3. Results
3.1. Aspect-Related Properties of the MC III
3.2. Load Direction-Related Properties of the MC III
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bischofberger, A.S.; Fürst, A.; Auer, J.; Lischer, C. Surgical management of complete diaphyseal third metacarpal and metatarsal bone fractures: Clinical outcome in 10 mature horses and 11 foals. Equine Vet. J. 2009, 41, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Bogers, S.H.; Rogers, C.W.; Bolwell, C.; Roe, W.; Gee, E.; McIlwraith, C.W. Quantitative comparison of bone mineral density characteristics of the distal epiphysis of third metacarpal bones from Thoroughbred racehorses with or without condylar fracture. Am. J. Vet. Res. 2016, 77, 32–38. [Google Scholar] [CrossRef]
- Moulin, N.; François, I.; Coté, N.; Alford, C.; Cleary, O.; Desjardins, M.R. Surgical repair of propagating condylar fractures of the third metacarpal/metatarsal bones with cortical screws placed in lag fashion in 26 racehorses (2007–2015). Equine Vet. J. 2018, 50, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Lischer, C.; Klaus, C. Diaphyseal fractures of the Third Metacarpal and Third Metatarsal Bones. In Fractures in the Horse; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Steel, C.; Ahern, B.; Zedler, S.; Vallance, S.; Galuppo, L.; Richardson, J.; Whitton, C.; Young, A. Comparison of Radiography and Computed Tomography for Evaluation of Third Carpal Bone Fractures in Horses. Animals 2023, 13, 1459. [Google Scholar] [CrossRef]
- Morgan, R.; Dyson, S. Incomplete longitudinal fractures and fatigue injury of the proximopalmar medial aspect of the third metacarpal bone in 55 horses. Equine Vet. J. 2012, 44, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Reardon, R.J.; Boden, L.; Stirk, A.J.; Parkin, T.D.H. Accuracy of distal limb fracture diagnosis at British racecourses 1999–2005. Vet. Rec. 2014, 174, 477. [Google Scholar] [CrossRef]
- Wright, I.M.; Nixon, A.J. Fractures of the condyles of the third metacarpal and metatarsal bones. In Equine Fracture Repair; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Misheff, M.M.; Alexander, G.R.; Hirst, G.R. Management of fractures in endurance horses. Equine Vet. Educ. 2010, 22, 623–630. [Google Scholar] [CrossRef]
- Young, N.; Corletto, F.; Wright, I. Predicting return to racing after repair of fractures of the metacpal/metatarsal condyles in Thoroughbred racehorses. Vet. Surg. 2022, 51, 753–762. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.R.; Watkins, J.P.; Glickman, N.W.; Hawkins, J.F.; Glickman, L.T. Complete fractures of the third metacarpal or metatarsal bone in horses: 25 cases (1980–1996). J. Am. Vet. Med. Assoc. 1998, 213, 847–850. [Google Scholar] [CrossRef]
- Turek, B.; Potyński, A.; Wajler, C.; Szara, T.; Czopowicz, M.; Drewnowska, O. Biomechanical study in vitro on the use of self-designed external fixator in diaphyseal III metacarpal fractures in horses. Pol. J. Vet. Sci. 2015, 18, 323–332. [Google Scholar] [CrossRef]
- Donati, B.; Fürst, A.E.; Hässig, M.; Jackson, M.A. Epidemiology of fractures: The role of kick injuries in equine fractures. Equine Vet. J. 2018, 50, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Sarrafian, T.L.; Case, J.T.; Kinde, H.; Daft, B.M.; Read, D.H.; Moore, J.D.; Stover, S.M. Fatal musculo-skeletal injuries of Quarter Horse racehorses: 314 cases (1990–2007). J. Am. Vet. Med. Assoc. 2012, 241, 935–942. [Google Scholar] [CrossRef]
- Springer, S.; Jenner, F.; Tichy, A.; Grimm, H. Austrian veterinarians’ attitudes to euthanasia in equine practice. Animals 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.M.; Whitton, R.C.; Kawcak, C.E.; Stover, S.M.; Pandy, M.G. Evaluation of a subject-specific finite-element model of the equine metacarpophalangeal joint under physiological load. J. Biomech. 2014, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shaktivesh, S.; Malekipour, F.; Whitton, R.C.; Hitchens, P.L.; Lee, P.V. Fatigue behavior of subchondral bone under simulated physiological loads of equine athletic training. J. Mech. Behav. Biomed. Mater. 2020, 110, 103920. [Google Scholar] [CrossRef] [PubMed]
- McCarty, C.A.; Thomason, J.J.; Gordon, K.D.; Burkhart, T.A.; Milner, J.S.; Holdsworth, D.W. Finite-element analysis of bone stresses on primary impact in a large-animal model: The distal end of the equine third metacarpal. PLoS ONE 2016, 11, e0159541. [Google Scholar] [CrossRef][Green Version]
- Słowiński, J.; Roszak, M.; Krawiec, K.; Henklewski, R.; Jamroziak, K. Numerical Analysis of Stabilization of a Horse’s Third Metacarpal Bone Fracture for Prediction of the Possibility of Bone Union. Appl. Sci. 2024, 14, 7976. [Google Scholar] [CrossRef]
- Lescun, T.B.; McClure, S.R.; Ward, M.P.; Downs, C.; Wilson, D.A.; Adams, S.B.; Hawkins, J.F.; Reinertson, E.L. Evaluation of transfixation casting for treatment of third metacarpal, third metatarsal, and phalangeal fractures in horses: 37 cases (1994–2004). J. Am. Vet. Med. Assoc. 2007, 230, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Brianza, S.; Brighenti, V.; Lansdowne, J.L.; Schwieger, K.; Bouré, L. Finite element analysis of a novel pin-sleeve system for external fixation of distal limb fractures in horses. Vet. J. 2011, 190, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Turek, B.; Potyński, A.; Drewnowska, O. Own-design external fixator for the treatment of diaphyseal fractures of the third metacarpal bone in horses. Med. Weter. 2016, 72, 197–202. [Google Scholar]
- Lescun, T.B.; Adams, S.B.; Main, R.P.; Nauman, E.A.; Breur, G.J. Finite Element Analysis of Six Transcortical Pin Parameters and Their Effect on Bone–Pin Interface Stresses in the Equine Third Meta-carpal Bone. Vet. Comp. Orthop. Traumatol. 2020, 33, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Boros, K.; Dyson, S. Magnetic resonance imaging, computed tomographic and radiographic findings in the metacarpophalangeal joints of 40 non-lame Thoroughbred Yearlings. Animals 2023, 13, 3466. [Google Scholar] [CrossRef]
- Nagy, A.; Dyson, S. Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 31 Warmblood Showjumpers in Full Work and Competing Regularly. Animals 2024, 14, 1417. [Google Scholar] [CrossRef]
- Skedros, J.G.; Dayton, M.R.; Sybrowsky, C.L.; Bloebaum, R.D.; Bachus, K.N. The influence of collagen fiber orientation and other histocompositional characteristics on the mechanical properties of equine cortical bone. J. Exp. Biol. 2006, 209, 3025–3042. [Google Scholar] [CrossRef] [PubMed]
- Novitskaya, E.; Chen, P.-Y.; Lee, S.; Castro-Ceseña, A.; Hirata, G.; Lubarda, V.A.; McKittrick, J. Anisotropy in the compressive mechanical properties of bovine cortical bone and the mineral and protein constituents. Mater. Sci. Eng. 2011, 7, 3170–3177. [Google Scholar] [CrossRef]
- Glade, M.J.; Luba, N.K.; Schryver, H.F. Effects of age and diet on the development of mechanical strength by the third metacarpal and metatarsal bones of young horses. J. Anim. Sci. 1986, 63, 1432–1444. [Google Scholar] [CrossRef]
- Jackson, B.F.; Lonnell, C.; Verheyen, K.; Wood, J.L.N.; Pfeiffer, D.U.; Price, J.S. Gender differences in bone turnover in 2-year-old horses. Equine Vet. J. 2014, 46, 303–310. [Google Scholar]
- Marsiglia, M.F.; Yamada, A.L.M.; Agreste, F.R.; de Sá, L.R.M.; Nieman, R.T.; da Silva, L.C.L.C. Morphological analysis of third metacarpus cartilage and subchondral bone in Thoroughbred racehorses: An ex vivo study. Anat. Rec. 2022, 305, 3385–3397. [Google Scholar] [CrossRef]
- de Oliveira Pereira, L.; de Souza, A.F.; Yamada, A.L.M.; de Andrade Salgado, D.R.; De Zoppa, A.L.D.V. Radiographic Texture of the Trabecular Bone in the Proximal Phalanx of Horses. Int. J. Equine Sci. 2024, 3, 107–114. [Google Scholar]
- Rho, J.Y.; Currey, J.D.; Zioupos, P.; Pharr, G.M. The anisotropic Young’s modulus of equine secondary osteones and interstitial bone determined by nanoindentation. J. Exp. Biol. 2001, 204, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.T.; Burstein, A.H. The elastic and ultimate properties of compact bone tissue. J. Biomech. 1975, 8, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, W.; Grynpas, M.D. Anisotropy of the Young’s modulus of bone. Nature 1977, 270, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Les, C.M.; Keyak, J.H.; Stover, S.M.; Taylor, K.T.; Kaneps, A.J. Estimation of material properties in the equine metacarpus with use of quantitative computed tomography. J. Orthop. Res. 1994, 12, 822–833. [Google Scholar] [CrossRef]
- Rubio-Martínez, L.M.; Cruz, A.M.; Gordon, K.; Hurtig, M.B. Mechanical properties of subchondral bone in the distal aspect of third metacarpal bones from Thoroughbred racehorses. Am. J. Vet. Res. 2008, 69, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Leahy, P.D.; Smith, B.S.; Easton, K.L.; Kawcak, C.E.; Eickhoff, J.C.; Shetye, S.S.; Puttlitz, C.M. Correlation of mechanical properties within the equine third metacarpal with trabecular bending and multi-density micro-computed tomography data. Bone 2010, 46, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.E.; Entwistle, R.C.; Arens, A.M.; Garcia, T.C.; Christiansen, B.A.; Fyhrie, D.P.; Stover, S.M. Mechanical and morphological properties of trabecular bone samples obtained from third metacarpal bones of cadavers of horses with a bone fragility syndrome and horses unaffected by that syndrome. Am. J. Vet. Res. 2012, 73, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Hounsfield, G.N. Nobel Award address. Computed medical imaging. Med. Phys. 1980, 7, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.S.; Mao, Z.; Spengler, D.M. Young’s modulus, bending strength, and tissue physical properties of human compact bone. J. Orthop. Res. 1990, 8, 592–603. [Google Scholar] [CrossRef]
- Odgaard, A.; Linde, F. The underestimation of Young’s modulus in compressive testing of cancellous bone specimens. J. Biomech. 1991, 24, 691–698. [Google Scholar] [CrossRef]
- Biewener, A.A. Allometry of quadrupedal locomotion: The scaling of duty factor, bone curvature, and limb orientation to body size. J. Exp. Biol. 1983, 105, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Shahkhosravi, N.A.; Bellenzani, M.C.; Davies, H.M.; Komeili, A. The influence of equine limb conformation on the biomechanical responses of the hoof: An in vivo and finite element study. J. Biomech. 2021, 128, 110715. [Google Scholar] [CrossRef]
- Barnes, G.; Pinder, D. In-vivo tendon tension and bone strain measurement and correlation. J. Biomech. 1974, 7, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.S.; McLeod, K.J.; Rubin, C.T. Technical note: Characterizing bone’ strain distributions in vivo using three triple rosette strain gages. J. Biomech. 1992, 25, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.; Mills, E.; Turner, A.; Simonen, F. In vivo and analytical studies of forces and moments in equine long bone. J. Biomech. 1977, 10, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Mills, E.; Gabel, A. In vivo measurement of bone strain in the horse. Am. J. Vet. Res. 1975, 36, 1573–1579. [Google Scholar]
- Sauer, F.J.; Hellige, M.; Beineke, A.; Geburek, F. Osteoarthritis of the coxofemoral joint in 24 horses: Evaluation of radiography, ultrasonography, intra-articular anesthesia, treatment, and outcome. Equine Vet. J. 2024, 57, 101–104. [Google Scholar] [CrossRef]
- Wang, X.; Thomas, C.D.L.; Clement, J.G.; Das, R.; Davies, H.; Fernandez, J.W. A mechanostatistical approach to cortical bone remodelling: An equine model. Biomech. Model. Mechanobiol. 2016, 15, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Les, C.M.; Stover, S.M.; Keyak, J.H.; Taylor, K.T.; Willits, N.H. The distribution of material properties in the equine third metacarpal bone serves to enhance sagittal bending. J. Biomech. 1997, 30, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, S.; Giorgi, M.; Dall’Ara, E. Validation of finite element models of the mouse tibia using digital volume correlation. J. Mech. Behav. Biomed. Mater. 2018, 86, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.J.; Burd, M.A.; Craig, J.J.; Craig, M. Radiographic calibration for analysis of bone mineral density of the equine third metacarpal bone. J. Equine Vet. Sci. 2013, 33, 1131–1135. [Google Scholar] [CrossRef]
- Turek, B.; Borowska, M.; Jankowski, K.; Skierbiszewska, K.; Pawlikowski, M.; Jasiński, T.; Domino, M. A Preliminary Protocol of Radiographic Image Processing for Quantifying the Severity of Equine Osteoarthritis in the Field: A Model of Bone Spavin. Appl. Sci. 2024, 14, 5498. [Google Scholar] [CrossRef]
- McClure, S.R.; Glickman, L.T.; Glickman, N.W.; Weaver, C.M. Evaluation of dual-energy x-ray absorptiometry for in situ measurement of bone mineral density of equine metacarpi. Am. J. Vet. Res. 2001, 62, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Skierbiszewska, K.; Szałaj, U.; Turek, B.; Sych, O.; Jasiński, T.; Łojkowski, W.; Domino, M. Radiological properties of nano-hydroxyapatite compared to natural equine hydroxyapatite quantified using dual-energy CT and high-field MR. Nanomedicine 2024, 61, 102765. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to calculate sample size in animal studies. J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turek, B.; Mikułowski, G.; Szara, T.; Dołasiński, M.; Jasiński, T.; Domino, M. Aspect-Related Mechanical Properties of the Cortical Bone in the Third Metacarpal Bone of Mares. Appl. Sci. 2025, 15, 1593. https://doi.org/10.3390/app15031593
Turek B, Mikułowski G, Szara T, Dołasiński M, Jasiński T, Domino M. Aspect-Related Mechanical Properties of the Cortical Bone in the Third Metacarpal Bone of Mares. Applied Sciences. 2025; 15(3):1593. https://doi.org/10.3390/app15031593
Chicago/Turabian StyleTurek, Bernard, Grzegorz Mikułowski, Tomasz Szara, Michał Dołasiński, Tomasz Jasiński, and Małgorzata Domino. 2025. "Aspect-Related Mechanical Properties of the Cortical Bone in the Third Metacarpal Bone of Mares" Applied Sciences 15, no. 3: 1593. https://doi.org/10.3390/app15031593
APA StyleTurek, B., Mikułowski, G., Szara, T., Dołasiński, M., Jasiński, T., & Domino, M. (2025). Aspect-Related Mechanical Properties of the Cortical Bone in the Third Metacarpal Bone of Mares. Applied Sciences, 15(3), 1593. https://doi.org/10.3390/app15031593





