Differences in EEG Functional Connectivity in the Dorsal and Ventral Attentional and Salience Networks Across Multiple Subtypes of Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Depression Scales
2.3. EEG Data Processing
2.4. Procedure
3. Results
3.1. Age, Sex, and SDS Scores
3.2. MDD Subtypes
3.2.1. Theta Band
3.2.2. Alpha Band
3.2.3. Beta Band
3.2.4. Gamma Band
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, D.; Ruan, W.; Peng, Y.; Lu, Z.; Wang, D. Associations of depression, sleep disorder with total and cause-specific mortality: A prospective cohort study. J. Affect. Disord. 2022, 298, 134–141. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington DC, USA, 2022. [Google Scholar]
- LeMoult, J. From stress to depression: Bringing together cognitive and biological science. Curr. Dir. Psychol. Sci. 2020, 29, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Ferster, C.B. A Functional Analysis of Depression. Am. Psychol. 1973, 28, 857–870. [Google Scholar] [CrossRef]
- Dougher, M.J.; Hackbert, L. A Behavior-Analytic Account of Depression and a Case Report Using Acceptance-Based Procedures. Behav. Anal. 1994, 17, 321–334. [Google Scholar] [CrossRef]
- Bolling, M.; Kohlenberg, R.; Parker, C. Behavior Analysis and Depression. In Clinical Behavior Analysis; Dougher, M.J., Ed.; Context Press: Reno, NV, USA, 1999; pp. 127–153. [Google Scholar]
- Kanter, J.; Cautilli, J.; Busch, A.; Baruch, D. Towards a comprehensive functional analysis of depressive behavior: Five environmental factors and a possible sixth and seventh factor. Int. J. Behav. Consult. Ther. 2011, 7, 5–14. [Google Scholar] [CrossRef][Green Version]
- Santos, M.M.; Nagy, G.A.; Kanter, J.W.; López, S.R. Applying a Process-Oriented Model of Cultural Competence to Behavioral Activation for Depression. Cogn. Behav. Pract. 2021, 28, 127–146. [Google Scholar] [CrossRef]
- Darwin, C. Recollections of the development of my mind and character. In Autobiographies; Penguin: London, UK, 2002. [Google Scholar]
- Sturmey, P. Functional Analysis in Clinical Psychology; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Stein, A.T.; Carl, E.; Cuijpers, P.; Karyotaki, E.; Smits, J.A. Looking beyond depression: A meta-analysis of the effect of behavioral activation on depression, anxiety, and activation. Psychol. Med. 2021, 51, 1491–1504. [Google Scholar] [CrossRef]
- Ostergaard, S.; Jensen, S.; Bech, P. The heterogeneity of the depressive syndrome: When numbers get serious. Acta Psychiatr. Scand. 2011, 124, 495–496. [Google Scholar] [CrossRef]
- Baumeister, H.; Parker, G. Meta-review of depressive subtyping models. J. Affect. Disord. 2012, 139, 126–140. [Google Scholar]
- Sharpley, C.F.; Bitsika, V. Differences in neurobiological pathways of four “clinical content” subtypes of depression. Behav. Brain Res. 2013, 256, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, C.F.; Bitsika, V. Validity, reliability and prevalence of four ‘Clinical Content’ subtypes of depression. Behav. Brain Res. 2014, 259, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Beijers, L.; Wardenaar, K.; van Loo, H.; Schoevers, R. Data-driven biological subtypes of depression: Systematic review of biological approaches to depression subtyping. Mol. Psychiatry 2019, 24, 888–900. [Google Scholar] [CrossRef]
- Buch, A.; Liston, C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology 2021, 46, 156–175. [Google Scholar] [CrossRef]
- Pandya, M.; Altinay, M.; Malone, D.A.; Anand, A. Where in the brain is depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef]
- Raz, A.; Buhle, J. Typologies of attentional networks. Nat. Rev. Neurosci. 2006, 7, 367–379. [Google Scholar] [CrossRef]
- Huang, H.; Chen, C.; Rong, B.; Wan, Q.; Chen, J.; Liu, Z.; Zhou, Y.; Wang, G.; Wang, H. Resting-state functional connectivity of salience network in schizophrenia and depression. Sci. Rep. 2022, 12, 11204. [Google Scholar] [CrossRef]
- Han, S.; Cui, Q.; Wang, X.; Li, L.; Li, D.; He, Z.; Guo, X.; Fan, Y.; Guo, J.; Sheng, W.; et al. Resting state functional network switching rate is differently altered in bipolar disorder and major depressive disorder. Hum. Brain Mapp. 2020, 41, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage 2014, 99, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S.; Wisco, B.E.; Lyubomirsky, S. Rethinking rumination. Perspect. Psychol. Sci. 2008, 3, 400–424. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Sharpley, C.F.; Agnew, L.L. The New England Mental Health Study, 1st ed.; University of New England: Amidale, NSW, Australia, 2016. [Google Scholar]
- Zung, W. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- Zung, W. From art to science: The diagnosis and treatment of depression. Arch. Gen. Psychiatry 1973, 29, 328–337. [Google Scholar] [CrossRef]
- DeJonge, J.; Baneke, J. The Zung Self-rating Depression Scale: A replication study on reliability, validity and prediction. Psychol. Rep. 1989, 64, 833–834. [Google Scholar] [CrossRef]
- Gabrys, J.; Peters, K. Reliability, discriminant and predictive validity of the Zung Self-Rating Depression Scale. Psychol. Rep. 1985, 57, 1091–1096. [Google Scholar] [CrossRef]
- Schaefer, A.; Brown, J.; Watson, C.; Plenel, D.; DeMotts, J.; Howard, M.; Norman, P.; Balleweg, B.J.; Douglas, A. Comparison of the validities of the Beck, Zung and MMPI depression scales. J. Consult. Clin. Psychol. 1985, 53, 415–418. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Marco-Pallarés, J.; Grau, C.; Ruffini, G. Combined ICA-LORETA analysis of mismatch negativity. Neuroimage 2005, 25, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): A review. Methods Find. Exp. Clin. Pharmacol. 2002, 24 (Suppl. C), 91–95. [Google Scholar] [PubMed]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: Exact, zero error localization. arXiv 2007, arXiv:07103341. [Google Scholar]
- Fox, M.D.; Corbetta, M.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. USA 2006, 103, 10046–10051. [Google Scholar] [CrossRef]
- Raichle, M.E. The restless brain. Brain Connect. 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Japee, S.; Holiday, K.; Satyshur, M.D.; Mukai, I.; Ungerleider, L.G. A role of right middle frontal gyrus in reorienting of attention: A case study. Front. Syst. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Suo, X.; Ding, H.; Li, X.; Zhang, Y.; Liang, M.; Zhang, Y.; Yu, C.; Qin, W. Anatomical and functional coupling between the dorsal and ventral attention networks. Neuroimage 2021, 232, 117868. [Google Scholar] [CrossRef]
- Astafiev, S.V.; Shulman, G.L.; Corbetta, M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. Eur. J. Neurosci. 2006, 23, 591–596. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Margulies, D.S.; Kelly, A.C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 2007, 37, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Rothbart, M.K.; Sheese, B.E.; Tang, Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn. Affect. Behav. Neurosci. 2007, 7, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, D.Y.; Camarillo-Rodriguez, L.; Serruya, M.D.; Herweg, N.A.; Waldman, Z.J.; Wanda, P.A.; Sharan, A.D.; Weiss, S.A.; Sperling, M.R. Contribution of left supramarginal and angular gyri to episodic memory encoding: An intracranial EEG study. Neuroimage 2021, 225, 117514. [Google Scholar] [CrossRef]
- Kambeitz-Ilankovic, L.; Koutsouleris, N.; Upthegrove, R. The potential of precision psychiatry: What is in reach? Br. J. Psychiatry 2022, 220, 175–178. [Google Scholar] [CrossRef]
- Tozzi, L.; Zhang, X.; Pines, A.; Olmsted, A.M.; Zhai, E.S.; Anene, E.T.; Chesnut, M.; Holt-Gosselin, B.; Chang, S.; Stetz, P.C.; et al. Personalized brain circuit scores identify clinically distinct biotypes in depression and anxiety. Nat. Med. 2024, 30, 2076–2087. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.; Williams, J. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]

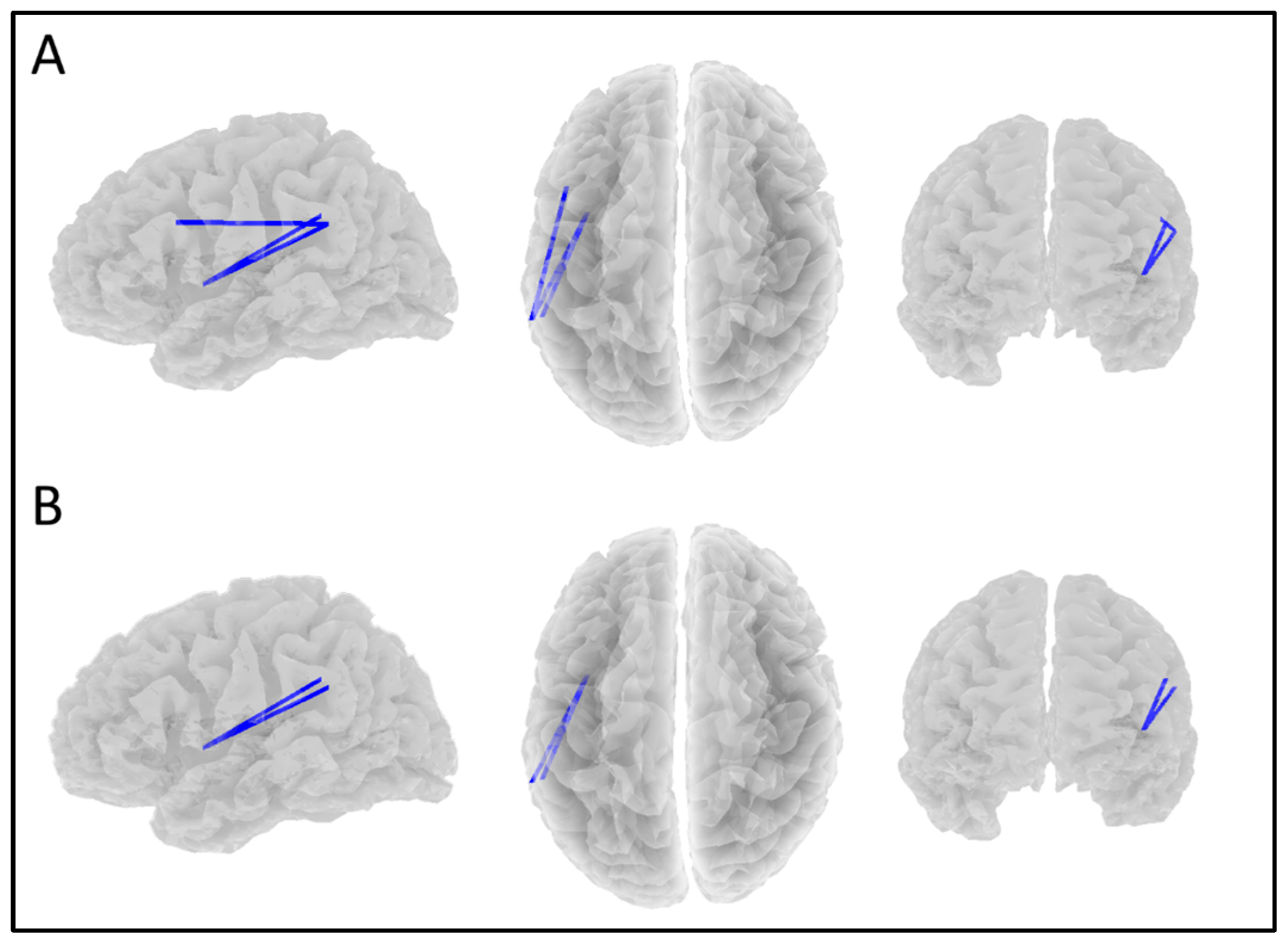
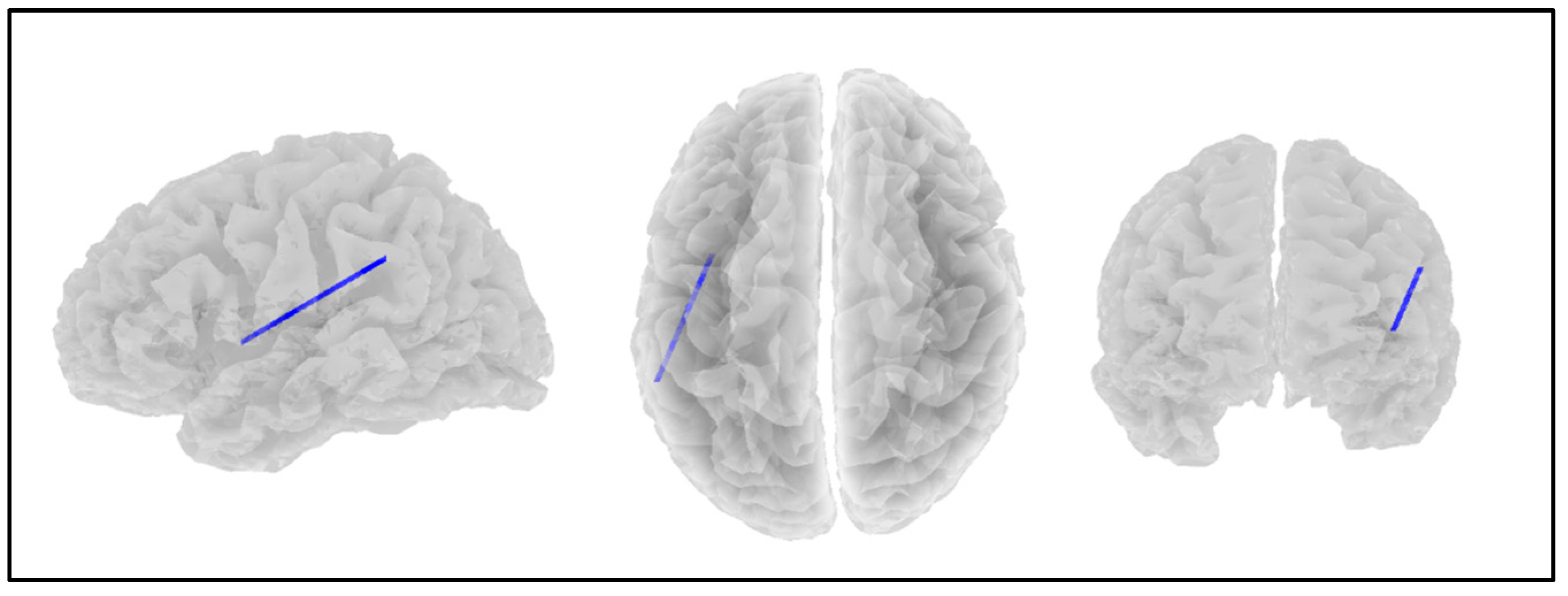
| Network | Location | MNI (X, Y, Z) |
|---|---|---|
| Dorsal Attention Network | left frontal eye field | −25, 12, 55 |
| right frontal eye field | 28, −10, 53 | |
| left posterior intraparietal sulcus | −22, −68, 46 | |
| right posterior intraparietal sulcus | 20, −67, 51 | |
| Ventral Attention Network | left middle frontal gyrus | −47, 14, 32 |
| right middle frontal gyrus | 47, 14, 32 | |
| left supramarginal gyrus | −57, −43, 34 | |
| right supramarginal gyrus | 57, −43, 34 | |
| Salience Network | dorsal anterior cingulate | 0, −21, 36 |
| left anterior prefrontal cortex | −35, 45, 30 | |
| right anterior prefrontal cortex | 32, 45, 30 | |
| left insula | −41, 3, 6 | |
| right insula | 41, 3, 6 | |
| left lateral parietal lobule | −62, −45, 30 | |
| right lateral parietal lobule | 62, −45, 30 |
| ROI | Network | r | p |
|---|---|---|---|
| left frontal eye field | DAN | 0.311 | 0.082 |
| right middle frontal gyrus | VAN | ||
| right middle frontal gyrus | VAN | 0.342 | 0.037 |
| dorsal anterior cingulate | SN | ||
| dorsal anterior cingulate | SN | 0.323 | 0.064 |
| left insula | SN | ||
| left insula | SN | 0.325 | 0.060 |
| left frontal eye field | DAN | ||
| left posterior IPS | DAN | 0.323 | 0.063 |
| left middle frontal gyrus | VAN | ||
| left middle frontal gyrus | VAN | 0.326 | 0.059 |
| right posterior IPS | DAN |
| ROI | Network | r | p |
|---|---|---|---|
| right insula | SN | 0.309 | 0.049 |
| left posterior IPS | DAN | ||
| left posterior IPS | DAN | 0.358 | 0.009 |
| left middle frontal gyrus | VAN | ||
| left middle frontal gyrus | VAN | 0.330 | 0.022 |
| right posterior IPS | DAN | ||
| right posterior IPS | DAN | 0.310 | 0.047 |
| right insula | SN | ||
| right frontal eye field | DAN | 0.289 | 0.089 |
| left posterior IPS | DAN |
| ROI | Network | r | p |
|---|---|---|---|
| right insula | SN | 0.294 | 0.096 |
| left posterior IPS | DAN | ||
| left posterior IPS | DAN | 0.300 | 0.082 |
| left middle frontal gyrus | VAN |
| ROI | Network | r | p |
|---|---|---|---|
| right supramarginal gyrus | VAN | −0.273 | 0.046 |
| left insula | SN | ||
| left insula | SN | −0.279 | 0.037 |
| left lateral parietal lobule | SN | ||
| left lateral parietal lobule | SN | −0.253 | 0.092 |
| right middle frontal gyrus | VAN |
| Anhedonic | SDS | ||||
|---|---|---|---|---|---|
| ROI | Network | r | p | r | p |
| right supramarginal gyrus | VAN | −0.266 | 0.040 | −0.239 | 0.097 |
| left insula | SN | ||||
| left insula | SN | −0.271 | 0.032 | −0.239 | 0.093 |
| left lateral parietal lobule | SN | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, I.D.; Sharpley, C.F.; Bitsika, V.; Vessey, K.A.; Williams, R.J.; Jesulola, E.; Agnew, L.L. Differences in EEG Functional Connectivity in the Dorsal and Ventral Attentional and Salience Networks Across Multiple Subtypes of Depression. Appl. Sci. 2025, 15, 1459. https://doi.org/10.3390/app15031459
Evans ID, Sharpley CF, Bitsika V, Vessey KA, Williams RJ, Jesulola E, Agnew LL. Differences in EEG Functional Connectivity in the Dorsal and Ventral Attentional and Salience Networks Across Multiple Subtypes of Depression. Applied Sciences. 2025; 15(3):1459. https://doi.org/10.3390/app15031459
Chicago/Turabian StyleEvans, Ian D., Christopher F. Sharpley, Vicki Bitsika, Kirstan A. Vessey, Rebecca J. Williams, Emmanuel Jesulola, and Linda L. Agnew. 2025. "Differences in EEG Functional Connectivity in the Dorsal and Ventral Attentional and Salience Networks Across Multiple Subtypes of Depression" Applied Sciences 15, no. 3: 1459. https://doi.org/10.3390/app15031459
APA StyleEvans, I. D., Sharpley, C. F., Bitsika, V., Vessey, K. A., Williams, R. J., Jesulola, E., & Agnew, L. L. (2025). Differences in EEG Functional Connectivity in the Dorsal and Ventral Attentional and Salience Networks Across Multiple Subtypes of Depression. Applied Sciences, 15(3), 1459. https://doi.org/10.3390/app15031459






