Evidence of the Formation of Crystalline Aluminosilicate Phases in Glass-Ceramics by Calcination of Alkali-Brick Aggregates, Enabling Cs+, Rb+, Co2+, and Sr2+ Encapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Brick Materials
2.2. Chemicals
2.3. ICP-OES and ICP-MS Analyses
2.4. X-Ray Diffraction (XRD)
2.5. Electron Microscopy
2.6. Nitrogen Adsorption–Desorption Isotherms
2.7. Thermal (TGA and DSC) Analyses
2.8. Corrosion-Resistance Experiments
3. Results
3.1. XRD Analysis
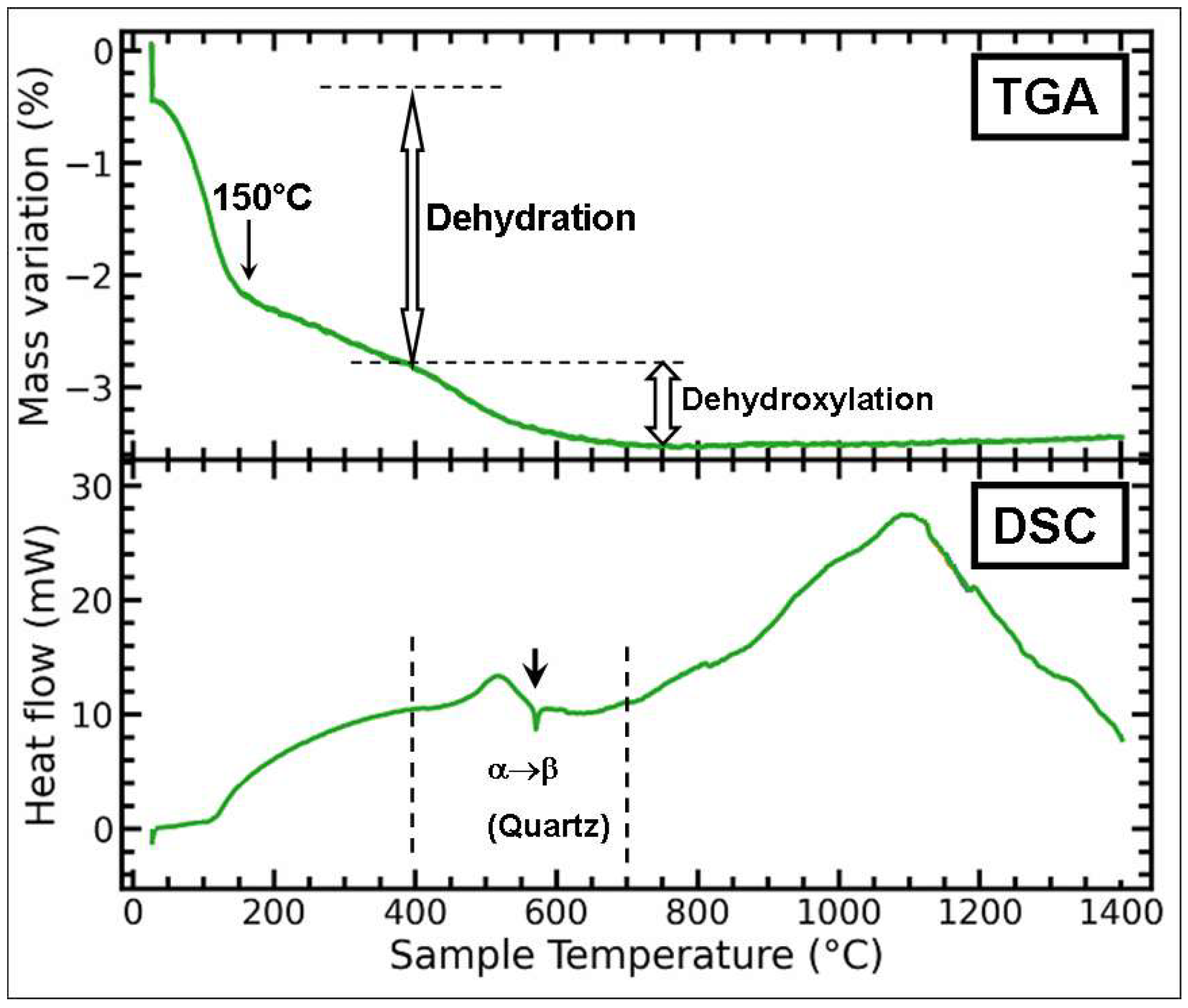
3.2. Thermo-Gravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
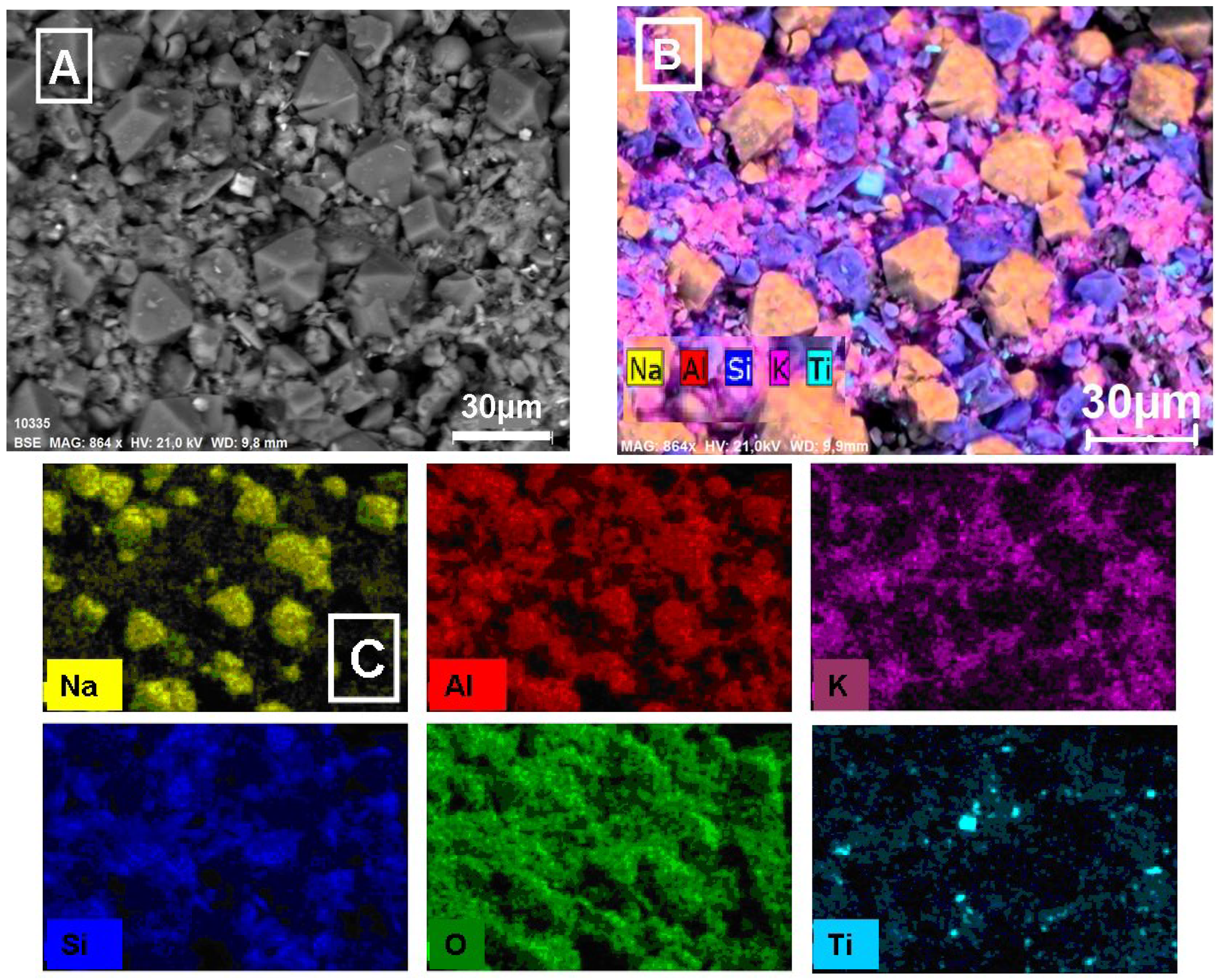
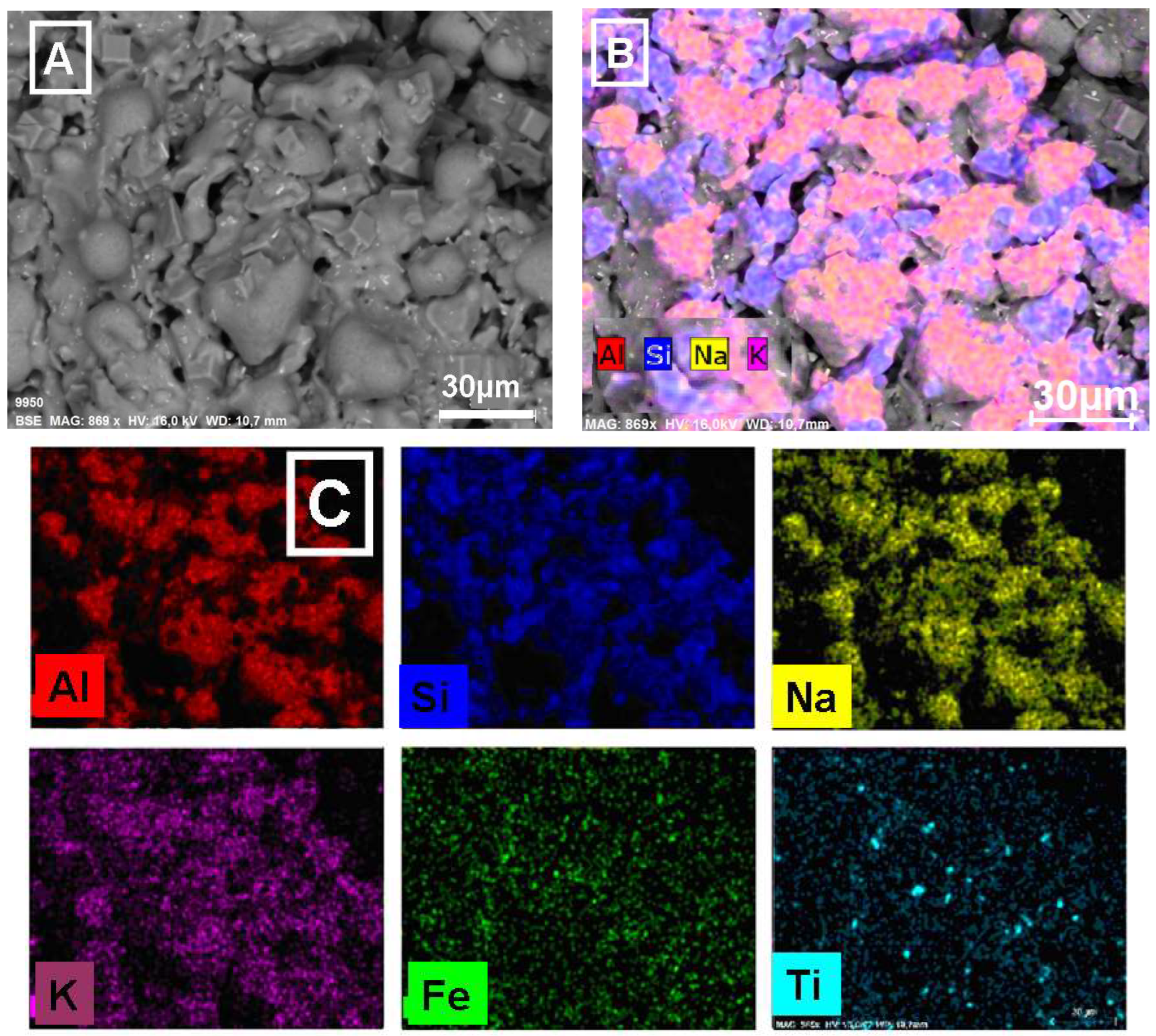
3.3. ESEM/EDS Studies
3.3.1. Microscopic Analysis of Raw Brick
3.3.2. Microscopic Analysis of Alkali-Activated Brick
3.3.3. Microscopic Analysis of Calcined Alkali-Brick
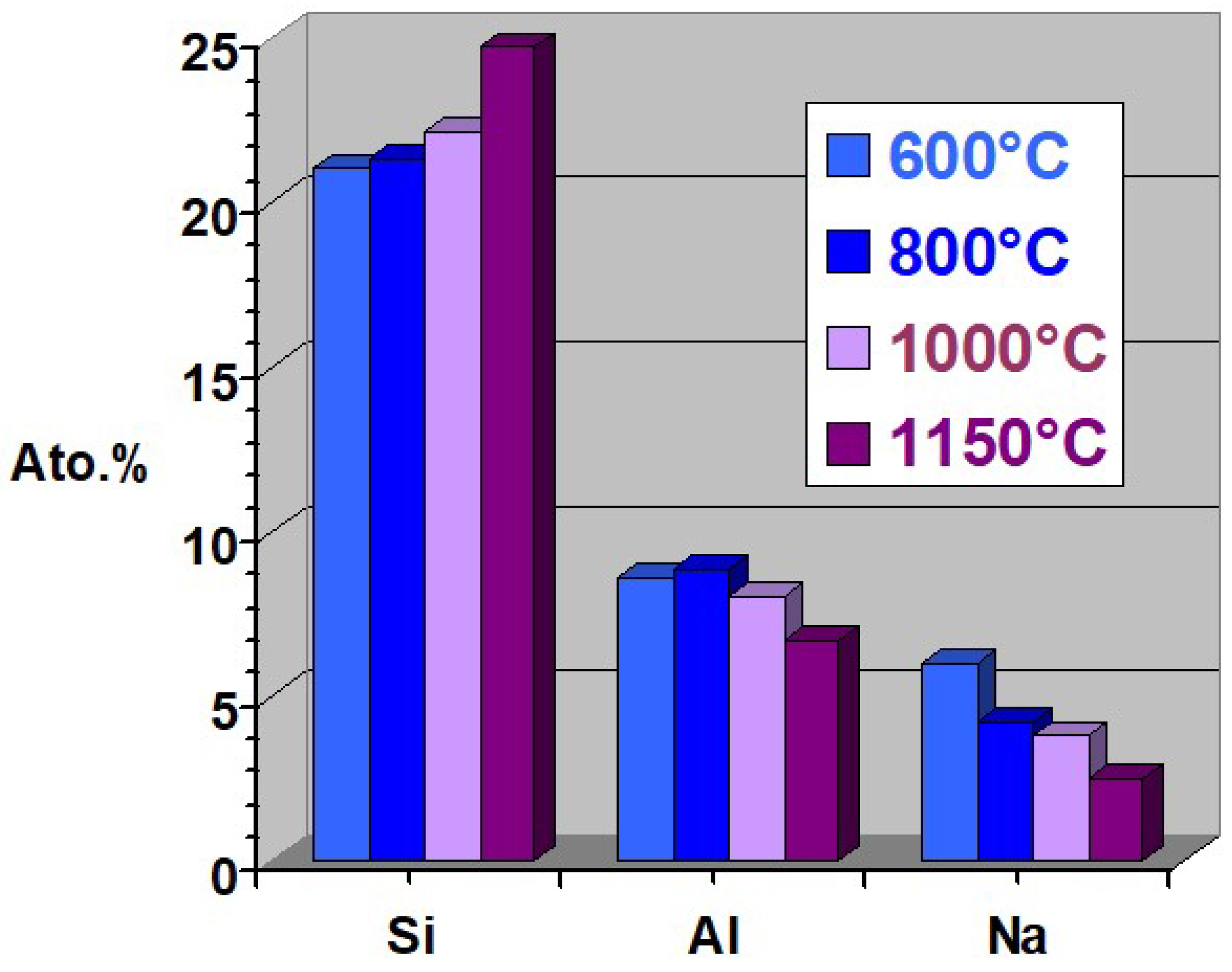
3.4. Implication of Fluxing Agent(s) in Thermal Process
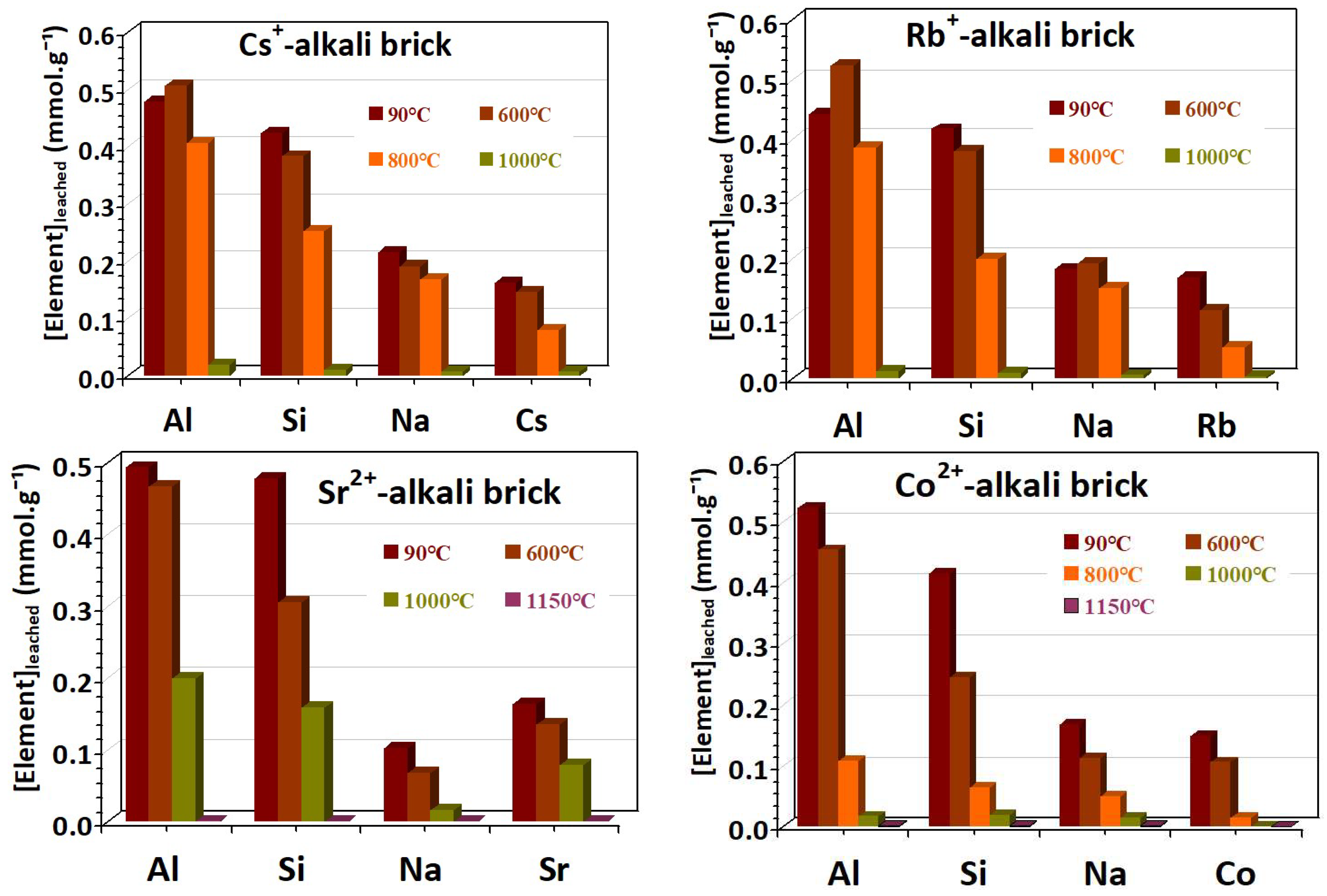
3.5. Acid Corrosion Resistance of Brick Materials
4. Conclusions
5. Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kochkin, B.; Malkovsky, V.; Yudintsev, S.; Petrov, V.; Ojovan, M. Problems and perspectives of borehole disposal of radioactive waste. Prog. Nucl. Energy 2021, 139, 103867. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Liu, J.; Ma, H.; Wang, C.; Zhang, H.; Wang, J. Progress on rock mechanics research of Beishan granite for geological disposal of high-level radioactive waste in China. Rock Mech. Bull. 2023, 2, 100046. [Google Scholar] [CrossRef]
- Zou, L.; Cvetkovic, V. Disposal of high-level radioactive waste in crystalline rock: On coupled processes and site development. Rock Mech. Bull. 2023, 2, 100061. [Google Scholar] [CrossRef]
- Chen, T.Y.; Yen, S.H.; Ferng, Y.M. Development of thermal-hydraulic coupling model for deep-geological disposal of high-level radioactive wastes. Nucl. Eng. Des. 2024, 424, 113227. [Google Scholar] [CrossRef]
- Neeway, J.J.; Emerson, H.P.; Asmussen, R.M.; Fujii Yamagata, A.L.; Meyer, P.D. Review of intermediate-scale field tests in support of disposal of waste forms. Chemosphere 2024, 347, 140625. [Google Scholar] [CrossRef]
- Jacques, D.; Phung, Q.T.; Perko, J.; Seetharam, S.C.; Maes, N.; Liu, S.; Yu, L.; Rogiers, B.; Laloy, E. Towards a scientific-based assessment of long-term durability and performance of cementitious materials for radioactive waste conditioning and disposal. J. Nucl. Mater. 2021, 557, 153201. [Google Scholar] [CrossRef]
- Shih, P.Y. Properties and FTIR spectra of lead phosphate glasses for nuclear waste immobilization. Mater. Chem. Phys. 2003, 80, 299–304. [Google Scholar] [CrossRef]
- Caurant, D.; Majerus, O.; Loiseau, P.; Bardez, I.; Baffier, N.; Dussossoy, J.L. Crystallization of neodymium-rich phases in silicate glasses developed for nuclear waste immobilization. J. Nucl. Mater. 2006, 354, 143–162. [Google Scholar] [CrossRef]
- Donald, I.W.; Metcalfe, B.L.; Fong, S.K.; Gerrard, L.A.; Strachan, D.M.; Scheele, R.D. A glass-encapsulated calcium phosphate wasteform for the immobilization of actinide-, fluoride-, and chloride-containing radioactive wastes from the pyrochemical reprocessing of plutonium metal. J. Nucl. Mater. 2007, 361, 78–93. [Google Scholar] [CrossRef]
- Tan, S.; Ojovan, M.I.; Hyatt, N.C.; Hand, R.J. MoO3 incorporation in magnesium aluminosilicate glasses. J. Nucl. Mater. 2015, 458, 335–342. [Google Scholar] [CrossRef]
- Yang, Y.; Ning, X.; Luo, S.; Dong, F.; Li, L. Electron irradiation effects of SrZrO3 ceramic for radioactive strontium immobilization. Procedia Environ. Sci. 2016, 31, 330–334. [Google Scholar] [CrossRef]
- Wei, Y.; Luo, P.; Wang, J.; Wen, J.; Zhan, L.; Zhang, X.; Yang, S.; Wang, J. Microwave-sintering preparation, phase evolution and chemical stability of Na1-2xSrxZr2(PO4)3 ceramics for immobilizing simulated radionuclides. J. Nucl. Mater. 2020, 540, 152366. [Google Scholar] [CrossRef]
- Tao, Y.; Zhenyu, L.; Chunrong, R.; Yuanyuan, W.; Zhichao, H.; Xin, H.; Jie, W.; Mengliang, L.; Qiubai, D.; Khan, K.; et al. Study on solidification properties of chemically bonded phosphate ceramics for cesium radionuclides. Ceram. Int. 2020, 46, 14964–14971. [Google Scholar] [CrossRef]
- Yıldırım, A.C.; Toda, K.; Saito, T. Determination of the sorption mechanisms of sodium-alkalinized metakaolin-based geopolymers. Appl. Clay Sci. 2024, 251, 107303. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Zhang, W.; Ye, J.; Wang, R. Solidification performance and mechanism of typical radioactive nuclear waste by geopolymers and geopolymer ceramics: A review. Prog. Nucl. Energy 2024, 169, 105106. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Wang, J.; Liu, K.; Wen, J.; Xu, J.; Jiang, P. Temperature dependences of phase composition, densification, and thermal/chemical stability of Sr0.5Zr2(PO4)3-Nd0.5Sm0.5PO4 composite ceramics for nuclear waste form. J. Eur. Ceram. Soc. 2024, 44, 116806. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Zhang, Z.; Ke, Z.; Shan, C.; Cao, X.; Ma, L.; Peng, S. A novel method to convert Cs-polluted soil into pollucite-base glass-ceramics for Cs immobilization. Chem. Eng. J. 2020, 385, 123844. [Google Scholar] [CrossRef]
- Li, B.; Chen, C.; Zhang, Y.; Yuan, L.; Deng, H.; Qian, W. Preparation of glass-ceramics from chromite-containing tailings solidified with Red Mud. Surf. Interfaces 2021, 25, 101210. [Google Scholar] [CrossRef]
- Tribet, M.; Jégou, C.; Miro, S.; Delrieu, J.; Doreau, F.; Peuget, S. Trivalent actinides and lanthanides incorporation and partitioning in UMo glass-ceramics. J. Nucl. Mater. 2023, 585, 154634. [Google Scholar] [CrossRef]
- Pu, B.; Wang, F.; Xu, Y.; Gu, Y.; Liu, L.; Liao, Q. Effect of Nd2O3 on the phase composition, structure and aqueous durability of SiO2-B2O3-CaO-Na2O-TiO2 glass-ceramics. J. Non-Cryst. Solids 2024, 628, 122841. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Z.; Zeng, S.; Chen, C.; Feng, W.; Zhou, X. The mechanical properties and chemical durability of granite wastes based glass-ceramics for immobilization of high-level nuclear wastes. Ceram. Int. 2024, 50, 24907–24912. [Google Scholar] [CrossRef]
- Nevolina, L.A.; Shtenberg, M.V.; Gladkochub, E.A.; Koroleva, O.N. Structural features and crystallization of Na2O-Cs2O-B2O3-SiO2 glasses for radioactive waste immobilization. Materialia 2024, 36, 102134. [Google Scholar] [CrossRef]
- Kim, K.W.; Shon, W.J.; Oh, M.K.; Yang, D.; Foster, R.I.; Lee, K.Y. Evaluation of dynamic behavior of coagulation-flocculation using hydrous ferric oxide for removal of radioactive nuclides in wastewater. Nucl. Eng. Technol. 2019, 51, 738–745. [Google Scholar] [CrossRef]
- Hossain, F. Natural and anthropogenic radionuclides in water and wastewater: Sources, treatments and recoveries. J. Environ. Radioact. 2020, 225, 106423. [Google Scholar] [CrossRef]
- Kutsevol, N.; Kuziv, Y.; Cabrera, T.; Husson, S.M.; DeVol, T.A.; Bliznyuk, V. Biodegradable star-like polymer flocculants for rapid, efficient purification of water contaminated with industrial radionuclides. Sep. Purif. Technol. 2021, 273, 118630. [Google Scholar] [CrossRef]
- Ye, D.; Gao, Q.; Li, T.; Wu, X.; Wu, Y. Photo-Fenton catalytic anti-fouling membranes for efficient elimination of radionuclides and organic contaminants. Desalination 2023, 553, 116461. [Google Scholar] [CrossRef]
- Adli Nor Azman, M.; Goh, P.S.; Ismail, A.F.; Jamaluddin, K.; Wong, K.Y.; Syazwan Sahril, A. Forward osmosis as a contemporary treatment solution for mitigating radionuclide pollution in water bodies. J. Environ. Chem. Eng. 2024, 12, 112542. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Tam, L.J.; Goh, P.S.; Azman, N.; Ismail, A.F.; Jamaluddin, K.; Arthanareeswaran, G. Treatment of radionuclide-containing wastewater using thin film composite reverse osmosis membrane with spray coating-assembled titania nanosheets. J. Environ. Chem. Eng. 2023, 11, 110540. [Google Scholar] [CrossRef]
- Sordyl, J.; Chamberlain, C.E.; Sweet, T.F.M.; Burns, P.C.; Cronberger, K.; Manecki, M. Immobilization of uranium from aqueous solutions by room-temperature precipitation of pyromorphite [Pb5(PO4)3Cl]. Polyhedron 2024, 252, 116891. [Google Scholar] [CrossRef]
- Han, W.; Zhang, Y.; Liu, R.; Sun, Y.; Li, M. Removal of Re3+, Cs+, Sr2+, Ba2+ from molten salt electrolyte by precipitation and solidification of glass-ceramics. J. Non-Cryst. Solids 2023, 606, 122208. [Google Scholar] [CrossRef]
- Palamarchuk, M.; Egorin, A.; Tokar, E.; Tutov, M.; Marinin, D.; Avramenko, V. Decontamination of spent ion-exchangers contaminated with cesium radionuclides using resorcinol-formaldehydes resins. J. Hazard. Mater. 2017, 321, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Canner, A.J.; Pepper, S.E.; Hayer, M.; Ogden, M.D. Removal of radionuclides from a HCl steel decontamination stream using chelating ion exchange resins—Initial studies. Prog. Nucl. Energy 2018, 104, 271–279. [Google Scholar] [CrossRef]
- Li, B.; Chen, M. Estimation of radionuclides migration mechanism in thermal decomposition of spent ion exchange resin in nuclear power plant. J. Anal. Appl. Pyrolysis 2023, 173, 106089. [Google Scholar] [CrossRef]
- Aamir Hafeez, M.; Singh, B.K.; Yang, S.H.; Kim, J.; Kim, B.; Shin, Y.; Um, W. Recent advances in Fenton-like treatment of radioactive ion exchange resins. Chem. Eng. J. Adv. 2023, 14, 100461. [Google Scholar] [CrossRef]
- Matskevich, A.I.; Markin, N.S.; Palamarchuk, M.S.; Tokar, E.A.; Egorin, A.M. Decontamination of spent ion-exchange resins from the nuclear fuel cycle using chemical decontamination and direct current. J. Clean. Prod. 2024, 449, 141658. [Google Scholar] [CrossRef]
- Sang, H.; Mao, C.; Ming, F.; Xu, L.; Wei, Y.; Wu, Y. Selective separation and immobilization process of 137Cs from high-level liquid waste based on silicon-based heteropoly salt and natural minerals. Chem. Eng. J. 2022, 449, 137842. [Google Scholar] [CrossRef]
- Jain, S.; Banthia, N.; Troczynski, T. Conditioning of simulated cesium radionuclides in NaOH-activated fly ash-based geopolymers. J. Clean. Prod. 2022, 380, 134984. [Google Scholar] [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption behaviour of pollutants: Heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): A review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef]
- Niu, X.; Elakneswaran, Y.; Islam, C.R.; Provis, J.L.; Sato, T. Adsorption behaviour of simulant radionuclide cations and anions in metakaolin-based geopolymer. J. Hazard. Mater. 2022, 429, 128373. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Sarwar, M.T.; Yang, H. Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. Appl. Clay Sci. 2023, 232, 106796. [Google Scholar] [CrossRef]
- Akiyamaa, D.; Duhamel, C.; Kirishima, A. Immobilization of radioactive waste by an aluminum silicate matrix formed from fly ash or bentonite. J. Nucl. Mater. 2023, 574, 154151. [Google Scholar] [CrossRef]
- Erdogmus, E.; Sutcu, M.; Hossain, S.; Bayram, M.; Sarı, A.; Gencel, O.; Ozbakkaloglu, T. Effect of molding pressure and firing temperature on the properties of ceramics from natural zeolite. Constr. Build. Mater. 2023, 402, 132960. [Google Scholar] [CrossRef]
- Dehou, S.C.; Mabingui, J.; Lesven, L.; Wartel, M.; Boughriet, A. Improvement of Fe (II)-adsorption capacity of FeOOH-coated brick in solutions, and kinetics aspects. J. Water Resour. Prot. 2012, 4, 464–473. [Google Scholar] [CrossRef][Green Version]
- Allahdin, O.; Dehou, S.C.; Wartel, M.; Recourt, P.; Trentesaux, M.; Mabingui, J.; Boughriet, A. Performance of FeOOH-brick based composite for Fe(II) removal from water in fixed bed column and mechanistic aspects. Chem. Eng. Res. Des. 2013, 91, 2732–2742. [Google Scholar] [CrossRef]
- Allahdin, O.; Wartel, M.; Recourt, P.; Revel, B.; Ouddane, B.; Billon, G.; Mabingui, J.; Boughriet, A. Adsorption capacity of iron oxyhydroxide-coated brick for cationic metals and nature of ion surface interactions. Appl. Clay Sci. 2014, 90, 141–149. [Google Scholar] [CrossRef]
- Allahdin, O.; Wartel, M.; Mabingui, J.; Boughriet, A. Kinetics of divalent Metals (Cd2 +, Cu2+, Pb2+, Zn2+) adsorption onto a modified brick. Am. Chem. Sci. J. 2014, 4, 687–705. [Google Scholar] [CrossRef]
- Allahdin, O.; Wartel, M.; Mabingui, J.; Boughriet, A. Implication of electrostatic forces on the adsorption capacity of a modified brick for the removal of divalent cations from water. Am. J. Anal. Chem. 2015, 6, 11–25. [Google Scholar] [CrossRef]
- Allahdin, O. Élimination (par Adsorption sur la Brique Activée) de Polluants Métalliques dans les eaux de la République Centrafricaine et les pays en voie de Développement: Aspects Texturaux, Physicochimiques, (Electro)-Cinétiques et Thermodynamiques. Ph.D. Thesis, University of Lille, Villeneuve d’Ascq, France, 2014; p. 177. Available online: https://ori.univ-lille1.fr/thematic-search.html?menuKey=these&id=allahdin (accessed on 24 November 2014).
- Ben Sghaier, R.; Net, S.; Allahdin, O.; Bessadok, S.; Sahyoun, W.; Ouddane, B.; Ben Hassan-Chehimi, D. Removal of bisphenol A and 4-nonylphenol from water by using a modifed brick–ferrihydrite coated. Chem. Pap. 2023, 77, 3937–3946. [Google Scholar] [CrossRef]
- Ben Sghaier, R.; Allahdin, O.; Net, S.; Bessadok, S.; Shayoun, W.; Ouddane, B.; Latrous, L. Application of modified bricks ferrihydrite-coated for the elimination of hormones from contaminated water: Case of 17α-ethynylestradiol, testosterone and estrone. Chem. Afr. 2024, 7, 2737–2747. [Google Scholar] [CrossRef]
- Boughriet, A.; Allahdin, O.; Poumaye, N.; Tricot, G.; Revel, B.; Lesven, L.; Wartel, M. Micro-Analytical Study of a Zeolites/GeoPolymers/Quartz Composite, Dielectric Behaviour and Contribution to Brønsted Sites Affinity. Ceramics 2022, 5, 908–927. [Google Scholar] [CrossRef]
- Boughriet, A.; Allahdin, O.; Poumaye, N.; Doyemet, G.; Tricot, G.; Revel, B.; Ouddane, B.; Wartel, M. Alkali-Activated Brick Aggregates as Industrial Valorized Wastes: Synthesis and Properties. Ceramics 2023, 6, 1765–1787. [Google Scholar] [CrossRef]
- Poumaye, N.; Allahdin, O.; Wartel, M.; Boughriet, A. Insights into characterization and adsorptive behaviour of zeolitized brick in water toward cadmium (A very toxic heavy metal to humans). Int. J. Pharm. Pharm. Res. 2018, 13, 1–29. [Google Scholar] [CrossRef]
- Poumaye, N.M. Transformation Chimique et Structurale d’un Constituant de Brique en Zéolite: Application à l’Elimination des Contaminants Métalliques dans le Traitement des eaux. Ph.D. Thesis, University of Lille, Villeneuve d’Ascq, France, 2020; p. 177. Available online: https://www.theses.fr/253318424 (accessed on 12 July 2020).
- Poumaye, N.; Allahdin, O.; Lesven, L.; Wartel, M.; Boughriet, A. Adsorption of iron (II) on sodic Zeolites—Bearing brick (in batch): Insights into interfacial chemical processes and thermodynamic equilibria. Int. J. Sci. Res. Methodol. 2019, 11, 88–119. Available online: https://www.researchgate.net/publication/331249158 (accessed on 12 November 2019).
- Allahdin, O.; Poumaye, N.; Wartel, M.; Boughriet, A. Correlation analysis between cationic metal characteristics and ion-exchange performance of brick-derived zeolites: A comprehensive mechanistic explanation. Mater. Chem. Phys. 2022, 276, 125353. [Google Scholar] [CrossRef]
- Boughriet, A.; Doyemet, G.; Poumaye, N.; Allahdin, O.; Wartel, M. Insight into adsorption kinetics of Cs+, Rb+, Co2+, and Sr2+ on a zeolites-based composite: Comprehensive diffusional explanation and modelling. Appl. Sci. 2024, 14, 3511. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Li, H.; Li, J.; Hu, D.; Xiang, Y.; Han, L.; Peng, X. Immobilization of strontium and cesium by aluminosilicate ceramics derived from metakaolin geopolymer-zeolite A composite via 1100 °C heating treatment. Ceram. Int. 2022, 48, 15236–15242. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.; Elsevier: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Ban, T.; Okada, K. Analysis of local cation arrangement in mullite using 29Si magic-angle spinning nuclear magnetic resonance spectra. J. Am. Ceram. Soc. 1993, 76, 2491–2496. [Google Scholar] [CrossRef]
- Carroll, D.L.; Kemp, T.F.; Bastow, T.J.; Smith, M.E. Solid-state NMR characterisation of the thermal transformation of a Hungarian white illite. Solid State Nucl. Magn. Reson. 2005, 28, 31–43. [Google Scholar] [CrossRef]
- Sharma, P.; Song, J.S.; Han, M.H.; Cho, C.H. GIS-NaP1 zeolite microspheres as potential water adsorption material: Influence of initial silica concentration on adsorptive and physical/topological properties. Sci. Rep. 2016, 6, 22734. [Google Scholar] [CrossRef]
- Fu, L.; Jiang, L.; Liao, K.; An, J.; Huang, W.; Sun, X.; Li, T.; He, Y. Adsorption behavior of welan gum on quartz sand in reservoir. J. Pet. Sci. Eng. 2021, 205, 108850. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Liu, H.; Wang, R.; Qin, Z.; Zhu, M. Surface modifications of short quartz fibers and their influence on the physicochemical properties and in vitro cell viability of dental composites. Dent. Mater. 2024, 40, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, D.; Guo, X.; Navrotsky, A. Energetics and structural evolution of Na–Ca exchanged zeolite A during heating. Phys. Chem. Chem. Phys. 2015, 17, 9241–9247. [Google Scholar] [CrossRef]
- Norton, G.A. The determination of quartz using differential scanning calorimetry. Thermochim. Acta 1994, 231, 295–304. [Google Scholar] [CrossRef]
- Alahdin, O.; Wartel, M.; Tricot, G.; Revel, B.; Boughriet, A. Hydroxylation and dealumination of ametakaolinite-rich brick under acid conditions, and their influences on metal adsorption: One- and two-dimensional (1H,27Al,23Na,29Si) MAS NMR, and FTIRstudies. Microporous Mesoporous Mater. 2016, 226, 360–368. [Google Scholar] [CrossRef]
- Sathupunya, M.; Glari, E.; Wongkasemjit, S. ANA and GIS zeolite synthesis directly from alumatrane and silatrane by sol-gel process and microwave technique. J. Eur. Ceram. Soc. 2002, 22, 2305–2314. [Google Scholar] [CrossRef]
- Zubowa, H.L.; Kosslick, H.; Müller, D.; Richter, M.; Wilde, L.; Fricke, R. Crystallization of phase-pure zeolite NaP from MCM-22-type gel compositions under microwave radiation. Microporous Mesoporous Mater. 2008, 109, 542–548. [Google Scholar] [CrossRef]
- Huo, Z.; Xu, X.; Lü, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L. Synthesis of zeolite NaP with controllable morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Behin, J.; Kazemian, H.; Rohani, S. Sonochemical synthesis of zeolite NaP from clinoptilolite. Ultrason. Sonochem. 2016, 28, 400–408. [Google Scholar] [CrossRef]
- Seliem, M.K.; Kormarneni, S. Equilibrium and kinetic studies for dissociation of iron from aqueous solution by synthetic Na-A zeolites: Statistical modelling and optimization. Microporous Mesoporous Mater. 2016, 228, 266–274. [Google Scholar] [CrossRef]
- Tontisirin, S. Synthesis and characterization of co-crystalline zeolite composite of LSX/A. Microporous Mesoporous Mater. 2017, 239, 123–129. [Google Scholar] [CrossRef]
- Illite Information. Available online: https://webmineral.com/data/illite/shtml (accessed on 13 November 2023).
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 3rd ed.; The Mineralogical Society: London, UK, 2013; p. 498. [Google Scholar] [CrossRef]
- Kosanovicc, C.; Subotic, B. Preparation of mullite micro-vessels by a combined treatment of zeolite A. Microporous Mesoporous Mater. 2003, 66, 311–319. [Google Scholar] [CrossRef]
- Schneider, H.; Fischer, R.X.; Schreuer, J. Mullite: Crystal structure and related properties. J. Am. Ceram. Soc. 2015, 98, 2948–2967. [Google Scholar] [CrossRef]
- Ghorbel, A.; Fourati, M.; Bouaziz, J. Microstructural evolution and phase transformation of different sintered kaolins powder compacts. Mater. Chem. Phys. 2008, 112, 876–885. [Google Scholar] [CrossRef]
- De Aza, A.H.; Turrillas, X.; Rodriguez, M.A.; Duran, T.; Pen, P. Time-resolved powder neutron diffraction study of the phase transformation sequence of kaolinite to mullite. J. Eur. Ceram. Soc. 2014, 34, 1409–1421. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; You, Y.; Cheng, W.; Dong, M.; Zhu, Z.; Liu, J.; Xie, W.; Wang, L.; Zhang, X.; et al. Finite element simulations of thermal stress distribution in thermal barrier coatings with different mullite whisker arrangements. Ceram. Int. 2024, 50, 43397–43413. [Google Scholar] [CrossRef]
- Chen, P.; Gu, X.; Zhou, X.; He, Z.; Zhang, Q.; Chen, J.; Yu, J.; Song, H.; Xuan, W.; Ren, Z. Green mullite whiskers prepared from zeolite waste at low-temperature. Ceram. Int. 2024, 50, 47051–47066. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Gao, Q.; Li, S.; Chen, R.; Wen, H.; Li, C. Fly ash coated with alumina sol for improving strength and thermal insulation of mullite porous ceramics. Constr. Build. Mater. 2024, 416, 135013. [Google Scholar] [CrossRef]
- Shi, Z. Green manufacturing of silicate materials using desert sand as a raw-material resource (Review). Constr. Build. Mater. 2022, 338, 127539. [Google Scholar] [CrossRef]
- Wang, W.; Song, J.; Shi, Z.; Sun, W.; Xue, L. Crystallization behavior and properties of quartz glass-ceramics synthesized from desert sand. Ceram. Int. 2024, 50, 370–383. [Google Scholar] [CrossRef]
- Torres, P.; Manjate, R.S.; Quaresma, S.; Fernandes, H.R.; Ferreira, J.M.F. Development of ceramic floor tile compositions based on quartzite and granite sludges. J. Eur. Ceram. Soc. 2007, 27, 4649–4655. [Google Scholar] [CrossRef]
- Phase Equilibria and Phase Diagrams—Open Petrology. Available online: https://opengeology.org/petrology/ (accessed on 8 November 2024).


| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Raw brick | 72.74 ± 1.56 | 8.72 ± 1.32 | 2.82 ± 0.03 | <0.010 | 0.130 ± 0.033 | 0.138 ± 0.008 | 1.031 ± 0.021 | 1.554 ± 0.039 |
| Activated-brick | 69.87 ± 1.43 | 8.79 ± 0.21 | 2.82 ± 0.16 | 0.043 ± 0.025 | 0.138 ± 0.030 | 1.929 ± 0.057 | 1.071 ± 0.012 | 1.007 ± 0.591 |
| Heating Temperature | 90 °C | 600 °C | 800 °C | 900 °C | 1000 °C |
|---|---|---|---|---|---|
| BET surface area (SBET) in m2/g | 19.319 | 14.421 | 4.254 | 0.801 | 0.002(6) |
| Single point adsorption total pore volume of pores (∅ < 313.549 nm at P/Po ≤ 0.9942) in cm3/g | 0.0397 | 0.0353 | 0.0200 | 0.0031 | 0.0017 (at P/Po ≤ 0.2716) |
| BJH Adsorption cumulative volume of pores (1.700 nm ≤ ∅ ≤ 300.000 nm) in cm3/g | 0.0363 | 0.0323 | 0.0205 | 0.0028 | - |
| Adsorption average pore width (4 V/A by BET) in nm | 8.22 | 9.78 | 18.80 | 15.53 | - |
| BJH Adsorption average pore diameter (4 V/A) in nm | 12.71 | 13.45 | 17.30 | 27.52 | - |
| BJH Desorption average pore diameter (4 V/A) in nm | 12.46 | 13.23 | 16.16 | 28.58 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boughriet, A.; Doyemet, G.; Poumaye, N.; Alaimo, V.; Ventalon, S.; Bout-Roumazeilles, V.; Wartel, M. Evidence of the Formation of Crystalline Aluminosilicate Phases in Glass-Ceramics by Calcination of Alkali-Brick Aggregates, Enabling Cs+, Rb+, Co2+, and Sr2+ Encapsulation. Appl. Sci. 2025, 15, 1379. https://doi.org/10.3390/app15031379
Boughriet A, Doyemet G, Poumaye N, Alaimo V, Ventalon S, Bout-Roumazeilles V, Wartel M. Evidence of the Formation of Crystalline Aluminosilicate Phases in Glass-Ceramics by Calcination of Alkali-Brick Aggregates, Enabling Cs+, Rb+, Co2+, and Sr2+ Encapsulation. Applied Sciences. 2025; 15(3):1379. https://doi.org/10.3390/app15031379
Chicago/Turabian StyleBoughriet, Abdel, Gildas Doyemet, Nicole Poumaye, Véronique Alaimo, Sandra Ventalon, Viviane Bout-Roumazeilles, and Michel Wartel. 2025. "Evidence of the Formation of Crystalline Aluminosilicate Phases in Glass-Ceramics by Calcination of Alkali-Brick Aggregates, Enabling Cs+, Rb+, Co2+, and Sr2+ Encapsulation" Applied Sciences 15, no. 3: 1379. https://doi.org/10.3390/app15031379
APA StyleBoughriet, A., Doyemet, G., Poumaye, N., Alaimo, V., Ventalon, S., Bout-Roumazeilles, V., & Wartel, M. (2025). Evidence of the Formation of Crystalline Aluminosilicate Phases in Glass-Ceramics by Calcination of Alkali-Brick Aggregates, Enabling Cs+, Rb+, Co2+, and Sr2+ Encapsulation. Applied Sciences, 15(3), 1379. https://doi.org/10.3390/app15031379





