Abstract
Hydrogel is one of the most prominent biomaterials in therapeutic and biomedical engineering, benefiting from its biocompatibility, chemical/physical tunability, and wide versatility to various fabrication techniques. One remarkable advance in the latest hydrogel research is the micro/nanofabrication technologies, which utilize unique mechanical and chemical properties of hydrogel, various chemical reaction mechanisms, and multidisciplinary approaches to realize innovative systems at these size scales. This review reports a comprehensive overview on the latest advances in fabrication of hydrogel-based micro- and nano-systems with an emphasis on their biomedical and therapeutic applications. Challenges and prospects are discussed from the material, fabrication, and system design perspectives to develop effective, personalized, and versatile hydrogel-based therapies.
1. Introduction
Hydrogel-based systems have emerged noticeably over the past decades, largely driven by their ability to mimic biological environments, tunable properties, and biocompatibility [1,2,3,4]. Traditional drug delivery methods often require frequent administrations or high dosages, which can lead to systemic toxicity and inefficient therapeutic outcomes. Hydrogels have been highlighted as a promising material to enable controlled and localized drug release, significantly improving therapeutic efficacy while minimizing side effects. Their tunable physical and chemical properties make them highly adaptable to various clinical needs, supporting applications including sustained and stimuli-responsive drug release [5,6,7,8,9].
One of the most compelling attributes of hydrogels is the similarity to biological tissues and their microenvironments, which allows them to integrate seamlessly into physiological settings. This makes hydrogels particularly effective for applications in oncology, immunotherapy, precision medicines, and regenerative medicines. By incorporating responsive functionality or tailoring permeability, hydrogels can precisely regulate mass transfer of drugs, degradation kinetics, or targetability [9,10]. Additionally, their ability to stabilize delicate and fragile biomolecules, including proteins and nucleic acids, underscores their potential toward advanced therapeutic technologies [11,12,13,14].
Despite their promising potential, hydrogel-based drug delivery systems still face significant challenges, including their mechanical stability, limited drug loading efficiency, undesirable bioactivity, and more importantly, translational barriers from laboratory research to clinical applications [4,15,16]. Moreover, the integration of hydrogel systems into multidisciplinary fields such as nanotechnology and molecular biology requires a robust understanding of the interplay between material science and biological systems.
While hydrogels in bulk form have been extensively studied and reviewed [5,6,10,16,17,18], their particulate forms, microgels and nanogels, remain underexplored. These types of hydrogels are designed as discrete particles at micro- and nanoscale dimensions and offer unique advantages including high surface area-to-volume ratio, enhanced permeability, and versatility in targeting specific cellular pathways. Furthermore, controlled synthesis methods for these systems are not comprehensively reviewed, leaving a gap between the growing interests from the diverse fields of research and the practical implications.
This review addresses two objectives: to provide fundamentals of hydrogel as bioengineering materials and their engineering strategies, and to discuss effective and controllable synthesis strategies for microgels and nanogels. It aims to bridge the theoretical principles and practical translation into experimental processes, with emphasis on the complexity and controllability of fabrication strategies. This review illustrates the material properties of selected synthetic and natural hydrogels, and the progress through conventional bulk synthesis methods. Next, the detailed guidelines on fabricating microgels and nanogels, including the implantation of functional properties, are discussed. This review concludes with an exploration of the recent applications of particulate-formed hydrogels and potential directions. The goal of this review is to deliver a foundational explanation for new researchers, offering practical insights for synthesis and functionalization, and fostering innovative research in the expanding field of hydrogel particle-based drug delivery systems.
2. Hydrogel Materials
The biocompatible nature of various hydrogels is mainly decided by the characteristics of the materials that comprise the hydrogels. In addition, unique functionalities of hydrogels including stimuli-responsive behaviors, self-healable gels, dynamic mechanical strength, and high-water retention ratio could be rendered based on the types of hydrogel materials [15,16,19,20]. Hydrogel materials can be categorized into synthetic hydrogels, natural hydrogels, and hybrid hydrogels that combine two or more hydrogel materials (Figure 1). One common discrepancy between synthetic and natural hydrogels is the strategy of gel formation. Synthetic hydrogel is often produced from precursors, or monomers, via bond formation to yield vastly crosslinked polymeric networks, whereas natural hydrogels can be easily produced from natural polysaccharides or polypeptides through non-covalent interactions induced by external triggers such as thermal cycle.
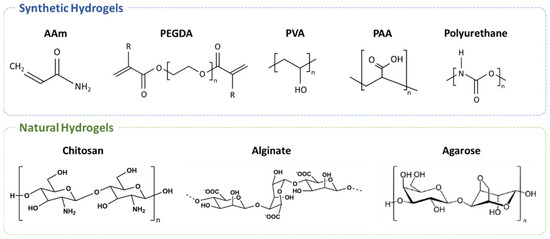
Figure 1.
Chemical structures of the selected hydrogel and precursors categorized into synthetic and natural materials. These structures mainly illustrate the backbone and intrinsically present functional groups of each polymeric hydrogel material, where functionalities can serve as crosslinking points to form complex networks of these polymeric materials.
2.1. Synthetic Hydrogels
2.1.1. Acrylamide and Its Derivatives
Acrylamide (AAm) and its derivatives are the most widely studied hydrogel precursors that can consecutively form hydrogels via chemical and physical mechanisms, including free radical polymerization as a commonly adapted chemical synthesis technique (Table 1). A precursor is a building block of a synthetic hydrogel and generally exhibits chemical functionalities which can be utilized to crosslink with another precursor in the presence of crosslinkers such as N,N’-methylenebisacrylamide [15,19].
AAm hydrogels exhibit high water absorptivity and sensitivity to their surrounding environments, such as pH, temperature, and chemical composition, which can trigger a physical or structural shift in gels, and they can stably retain a large amount of water within their network to undergo volumetric swelling without rupturing structural integrity. Common derivatives of AAm include polyacrylamide (PAAm), poly(N-isopropylacrylamide) (PNIPAm), poly(N-isopropylmethacrylate) (PNIPMAm), and poly(N,N-diethylacrylamide) (PDEAm). Among those, PNIPAm, PNIPMAm, and PDEAm are famously known as thermo-responsive polymers [15,19,20].
One noticeable difference among the thermo-responsive AAm hydrogels is the classification of amine functional groups [21]. PDEAm has a tertiary amine group that can be protonated through the physiological or acidic condition, rendering the PDEAm hydrogels positively charged, and more broadly, sensitive. PNIPAm and PNIPMAm both have secondary amine groups and are generally known as neutral. Both hydrogels lack charged groups and could minimize the Coulombic interaction with cell membranes, thereby exhibiting relatively better biocompatibility and lower cytotoxicity [19,22].
The thermo-responsiveness of PDEAm, PNIPAm, and PNIPMAm are well represented by their lower critical solution temperature (LCST), a specific temperature at which the hydrogel transitions from a hydrophilic state to a hydrophobic state, often being described as a swollen state and collapsed state, respectively. This phenomenon causes the hydrogel dehydrates to be above their LCST, allowing the initially loaded solution within the hydrogel matrix to be “squeezed out”. PNIPAm hydrogel has a lower critical solution temperature (LCST) of approximately 32 °C, making it particularly attractive to clinical applications, whereas PNIPMAm hydrogels exhibit a slightly higher LCST of around 40 °C due to the steric hindrance caused by the additional methyl group on its precursor as well as the significantly higher dehydration than PNIPAm above the LCST. PDEAm has been increasingly utilized in biomedical studies, owing to its LSCT similar to that of PNIPAm and its tertiary amide structure that serves as a hydrogen bonding acceptor [15,23,24]. However, the thermo-responsive characteristics of AAm hydrogels could suffer from thermal and mechanical hysteresis, ultimately limiting their applicability in repetitive uses. This side effect tends to be more pronounced in alkyl acrylamide hydrogels due to their high thermal sensitivity.
2.1.2. Polyethylene Glycol and Its Derivatives
Poly(ethylene glycol) diacrylate (PEGDA) hydrogel is represented by its promising potential in biomedical and clinical applications, including biocompatibility, flexibility, high water permeability, strong tissue adhesion, and intrinsically low cytotoxicity [25,26,27]. Owing to these advantages, PEGDA hydrogels have been increasingly adapted in tissue engineering, drug delivery, and bio-adhesive applications. PEGDA precursors are commercially available at various molecular weights from two hundred and fifty to over a few thousand g/mol, allowing us to combine short- and long-chain precursors to tune crosslinking density, elasticity, mechanical strength, and cell viability [28,29].
The molecular weight of the PEGDA precursor plays a significant role in determining its overall characteristics, for example, higher-molecular-weight PEGDA tends to form more stretchable hydrogels with higher tensile strength owing to longer chains between two acrylate end groups, but it has been reported that enhancing the mechanical strength of PEGDA hydrogels could result in decreased cell viability [30]. However, the cytotoxicity of PEGDA hydrogels is also affected by total precursor concentration and precursor composition, making its overall dependency highly non-linear and difficult to estimate. The reasoning for this complex trend is based on two separate hypotheses. One suggests that higher crosslinking density, caused by lower-molecular-weight PEGDAs, renders the pores of a hydrogel network smaller, limiting the mass transfer rate of nutrients and growth factors as well as suppressing cell motility, hence providing opposing effects to cell proliferation and viability [25,31,32]. Another hypothesis is that the precursor with longer chains allows higher chain mobility and increased gel elasticity, providing a suitable environment for the seeded cells, whereas the precursor with shorter chains could adversely affect cells more severely than that with longer chains. For residual precursors, smaller molecules exhibit a faster diffusion rate than larger molecules within a gel matrix, while its acrylate end groups remain active to affect nearby cells [30,33].
In the aspects of hydrophilicity and swelling, PEG itself exhibits high water solubility, but its oligomeric or polymeric chain length directly affects the hydrophilicity and water absorption capacity of the resulting hydrogels. PEGDA hydrogels made from lower-molecular-weight PEGDA, and hence having a higher crosslinking density, are less hydrophilic and more mechanically robust than the hydrogels synthesized from higher-molecular-weight PEGDA. This phenomenon restricts water uptake and swelling and directly impacts the rate of biodegradation which makes it suitable for clinical long-term implants and stress-bearing scaffolds.
Conventionally, PEGDA hydrogel is formed via UV-induced photopolymerization by taking advantage of its relatively high solubility to commercially available photoinitiators (Table 1). When combined with an adequate photoinitiator, such as Irgacure 2959 or 2-hydroxy-2-methylpropiophenone (HMP), and a light source, the precursors could readily form a crosslinking network without any need for heat or electrical bias, which allows light-controlled synthesis as well as tunable crosslinking density and mechanical stability [3,31,34].
Poly(ethylene glycol) dimethacrylate (PEGDMA) is another derivative of PEG-based hydrogel that has minimal toxicity and is modifiable to be bio-inert [35]. It also exhibits similar reaction characteristics to PEGDA, which allows facile control over its crosslinking density, water retention, and mechanical stability. In the aspect of biomedical applications, this controllability enables PEGDA hydrogels to sustain or to tailor the release dynamics of bioactive molecules or to resemble the physiochemical properties of an extracellular matrix to aid the interactions at the vascular interfaces or bond scaffolds [31,34]. Bio-adhesive properties become particularly useful for wound closure of sensitive tissues, where the benefits of low swelling ratio and facile applications are emphasized, and the risk of pressure-induced hemostasis is critical. However, PEG-based hydrogels share critical challenges such as cytotoxic effects caused over prolonged exposure by a residual photoinitiator or unreacted precursors and deviation in swelling behavior [35,36,37].
2.1.3. Polyvinyl Alcohol
Poly(vinyl alcohol) (PVA) is a water-soluble, bio-degradable synthetic polymer that has a chemical formula of [CH2CH(OH)]n and is commercially available in a wide range of molecular weights from a few thousands to over a hundred thousand; in addition, it has been widely adapted in biomedical applications since the 1970s for cartilage repair and wound dressing [38,39,40]. PVA is conventionally synthesized from vinyl acetate via free radical polymerization followed by hydrolysis of an acetate moiety into a hydroxyl group [27,41]. Hydroxyl group side groups in PVA have major impacts on rheological properties and mechanical strength of the gels, and it also possesses the capability of forming hydrogen bonding with neighboring molecules [38,42,43].
PVA hydrogels are formed via various mechanisms including hydrolysis, alcoholysis, or physically induced chain aggregation like the freeze–thaw method. PVA polymers exhibit excellent biocompatibility, especially against blood and tissue cells and high thermal stability. However, low bioactivity and weak mechanical stability limit the applications of PVA hydrogels in biomedical fields, especially for regenerative therapies [44,45,46,47].
2.1.4. Poly(acrylic Acid)
Poly(acrylic acid) (PAA) is a versatile ionizable polymer, primarily classified as an anionic polymer due to its abundance of carboxylic acid (-COOH) groups [48,49]. PAA hydrogels exhibit efficient and tunable responsiveness to pH changes, and this pH-responsive nature makes them highly attractive in drug delivery systems for oral administration and tumor-targeted drugs, where the system undergoes pH shifts and subsequently releases drugs at a specific site [48,50,51]. In addition, physical and chemical properties of PAA resemble the extracellular matrix, and it has been increasingly utilized in tissue engineering, biomedical sensing, and diagnostics [15].
The pKa value of PAA is approximately 5 and tunable within the range of 4 to 7 by altering its molecular weight, polydispersity, and chemical functionalities, which renders PAA polymers ionizable and responsive in different pH environments [48,51,52]. Carboxylic groups remain protonated in acidic conditions, more specifically at below the pKa value, rendering the PAA hydrogel hydrophobic and low in water retention. The swelling behavior of PAA hydrogels is determined by the ionic strength and pH of the surrounding medium. At above the pKa, deprotonation occurs that induces electrostatic repulsion between neighboring polymers, leading to the swelling of the PAA hydrogels. Reversible swelling–deswelling behavior under pH changes also makes PAA a promising candidate for soft actuator and artificial muscles.
The common synthesis strategies of PAA hydrogels are free radical polymerization and photopolymerization, specifically inducing polymerization reactions at the carbon–carbon double bond present in its monomer structure [49,53,54,55]. Besides free radical initiators, photoinitiators are also highly compatible with PAA hydrogel, enabling rapid and spatially controlled gelation under UV or visible light. Also, hydrogen bonding between carboxyl groups in PAA can form reversible networks, which is crucial for self-healing or dynamic responsiveness. Combined with their excellent swelling characteristics, PAA hydrogels present promising potentials especially toward advanced drug delivery; however, they often suffer from brittleness and limited mechanical strength, which are significantly pronounced in nanoscale applications such as hydrogel nanoparticles [8,56,57,58]. The brittle nature of PAA hydrogels is primarily due to the dense crosslinking of polymer chains, which is directly related to their limited biodegradability as well. Enhancing degradability through biodegradable crosslinkers or copolymerization with natural polymers like chitosan or alginate has been highlighted as an alternative strategy [53,59]; however, this poses practical challenges like maintaining uniformity and reproducibility in large-scale synthesis.
2.1.5. Polyurethane-Based Hydrogels
Polyurethane(PU)-based hydrogels are represented by their exceptional elasticity and mechanical durability [60,61]. Polyurethanes are copolymers consisting of alternating soft and hard chain segments, such as polyols and isocyanates. Soft segments increase flexibility and elasticity of the resulting gels, whereas the hard segments contribute to mechanical strength and structural integrity [54,60,61,62]. This unique characteristic enables PU-based hydrogels to be tailored to exhibit high tensile strength and excellent water retention at the same time.
The hydrophilicity of PU hydrogels can be determined by the abundance of hydrophilic functional groups, commonly known as functionalities capable of forming hydrogen bonds such as hydroxyl, carboxyl, and amino groups, in its polymeric backbone. High hydrophilicity promotes facile swelling in aqueous solutions just like other hydrogels, but PU hydrogel offers an efficient strategy to finely tune its property though adjusting the ratio of hard-to-soft segments, molecular weight, and crosslinking density [5,61,62,63,64,65].
The main synthesis strategies of PU hydrogels are chemical crosslinking and physical crosslinking. Chemical crosslinking utilizes the spontaneous reaction between diisocynates and polyols, which results in the formation of a covalently linked network of urethane bonds [61]. Physical crosslinking mechanisms rely on intermolecular interactions, such as van der Waals force or hydrogen bonding, to create a network of reversible and dynamic bonds. Advanced polymerization techniques such as photopolymerization or solvent-free copolymerization have been adapted into the synthesis of PU hydrogels as well.
PU hydrogels are well known as a core element of wound healing patches and soft tissue applications, owing to their biocompatibility, flexibility, and ability to absorb wound exudates while maintaining the physical environment to stimulate therapeutic efficacy or native healing process (Table 1) [40,66,67]. PU hydrogels are also widely utilized in drug delivery systems by taking advantage of their controllable permeability and degradability, which makes PU a suitable material for sustained drug release, especially at the proximity of the circulatory system or even within the blood vessels. A unique aspect of PU hydrogels is their self-healing properties, which are imperative for the material specialized for durable and responsive devices. Despite the advantages, limited biodegradability and potential cytotoxicity caused by residual isocyanate moieties are major drawbacks for the implantable or injectable form of PU-based hydrogels [40,46,61,62,63].

Table 1.
Key features and biomedical applications of common synthetic hydrogels.
Table 1.
Key features and biomedical applications of common synthetic hydrogels.
| Hydrogel Materials | Crosslinking Methods | Functional Features | Applications | Ref. |
|---|---|---|---|---|
| Acrylamide (AAM) and derivatives |
|
|
| [15,19,24] |
| Poly(ethylene glycol) diacrylate (PEGDA) |
|
|
| [28,36,37] |
| Poly(vinyl alcohol) (PVA) |
|
|
| [42,43,47,59] |
| Poly(acrylic acid) (PAA) |
|
|
| [15,49,52,55] |
| Polyurethane (PU) |
|
|
| [60,61,62,63,67] |
2.2. Natural Hydrogels
2.2.1. Chitosan
Chitosan-based hydrogels are among the most extensively studied natural hydrogel material, benefiting from their excellent biocompatibility, degradability, and unique physicochemical characteristics (Table 2) [17]. Chitosan is naturally derived from chitin, a polysaccharide abundantly found in the exoskeletons of shellfish like shrimps or crabs, or the cell walls of fungi. Its chemical structure includes repeating units of glucosamine and N-acetylglucosamine, which provide chemical moieties for additional modifications and crosslinking. Three major functional groups are a primary amino group at C2, and a primary and secondary hydroxyl group at C6 and C3, respectively [17,68,69].
The pH-dependent behavior of chitosan is governed by the amino groups which become protonated in acidic pH, enhancing solubility that subsequently allows controlled gelation. Additionally, chitosan exhibits strong antimicrobial and antifungal activities. These unique phenomena make chitosan hydrogel highly suitable for controlled drug delivery, wound healing and infection control, and tissue regeneration [53,59,69,70]. Currently, chitosan hydrogels are found in emergency kits for uncontrolled bleeding, owing to their abilities to agglutinate red blood cells, activate platelets, and maintain a moist environment in the vicinity of the hydrogel. These hemostatic properties make chitosan hydrogel ideal for wound care, where timely gelation, stable adhesion, and strong antimicrobial activity are all critically important to the patient’s survival [40,66].
The chemical structure of chitosan molecules contains carboxyl and amino groups, which enable facile chemical modifications to implant functionalities via the click chemistry or EDC/NHS method [71,72]. The water solubility of chitosan molecules varies by the degree of deacetylation (DD), where an acetyl group contributes to inducing the crystallization of chitosan molecules and dramatically decreases their solubility to water and other common solvents. The DD of chitosan is also hypothesized to be a major factor determining the antimicrobial activity of chitosan hydrogels, as the lower density of the acetyl group allows a higher level of positive net charge from the protonation of amine groups, subsequently strengthening its antibacterial activity [73,74,75]. Chitosan is partially water-soluble when its DD level at above 70% and becomes highly soluble at a DD above 95%. The DD level also influences the antimicrobial activity of chitosan hydrogels, as a higher degree increases the net positive charge, thereby enhancing the antibacterial effects.
However, conventional techniques to chemically tune chitosan molecules are often complex and time-consuming. Moreover, intravenous applications of chitosan have been reported to induce and accelerate immune responses, emphasizing the importance of key considerations in developing new drug delivery systems [76].
The chitosan hydrogels could be formed via both physical and chemical methods. Physical crosslinking relies on ionic interactions and hydrogen bonding between functional groups, or hydrophobic interactions at a larger scale, and their non-covalent association enables a reversible and self-healing constructed gel network. On the other hand, chemical crosslinking results in the formation of stable, covalently crosslinked gel networks, by utilizing additional components like glutaraldehyde, carbodiimide, or genipin [17,59,68,77].
Applications of chitosan-based hydrogels, as highlighted above, span a wide range of both academic and industrial fields. Besides the hemostatic performance, chitosan hydrogels are employed in tissue engineering as scaffolds, benefiting from the similarity of their physical and chemical properties to those of the extracellular matrix, which enables cell proliferation and differentiation. Chitosan has been increasingly utilized in drug delivery systems for oral, ocular, transdermal, and injectable formulations, where their biodegradability and mucoadhesive properties promote drug retention and absorption over extensive periods [71,72,75,77,78].
The main challenges of chitosan-based hydrogel are the limited aqueous solubility and low mechanical strength. Chitosan quickly loses solubility as the pH rises from acidic conditions and becomes practically insoluble at neutral and basic pH environments, which restricts their utilities in physiological applications [68,77]. An alternative approach is to decrease the average molecular weight of chitosan, which is generally over 100 kDa when obtained from chitin, via hydrolysis or oxidative reactions; however, this pathway still poses critical challenges like limited controllability over the final molecular weight and scalability [78,79,80,81].
2.2.2. Agarose
Agarose is a natural polysaccharide derived from marine algae and is composed of alternating D-galactose and 3,6-anhydro-L-galactopyranose units. It has an uncharged molecular structure, which renders agarose to exhibit similar properties to the extracellular matrix, making it an excellent material for biomedical applications [82,83]. Agarose is particularly valued for its thermo-reversible behavior, which allows it to transition between sol and gel states.
The synthesis of agarose hydrogels typically involves a heat-assisted sol–gel transition. At ambient conditions, agarose exists in an entangled state, tightly held together via hydrogen bonds, which appears as a solid powder and is hard to dissolve in aqueous solutions. When dispersed in a solution and heated to 80–90 °C, these molecular entanglements uncoil, resulting in a fully dissolved sol. Upon cooling, the agarose molecules re-associate to form helices, which bundle into a macroscale network stabilized by hydrogen bonding. This network creates the gel, and since hydrogen bonding is a non-covalent intermolecular interaction, this structure is thermo-reversible and can be returned to the sol state when reheated above its melting temperature [83,84,85,86]. Also, the mechanical stiffness or elasticity of the resulting agarose gel can be controlled by adjusting thermal profiles [82].
Agarose hydrogels are widely used in cell cultures and in vivo implantations largely owing to their excellent biocompatibility and ease of handling. Agarose also exhibits similar characteristics to the extracellular matrix, which supports stable cell adhesion and proliferation, while allowing facile control over gel stiffness, making it one of the most practically suitable soft materials for tissue engineering [83,87,88,89]. Additionally, agarose hydrogels are commonly utilized in electrophoresis and biomolecular separation for proteins and nucleic acids.
Major challenges of agarose hydrogels in biomedical applications are insufficient mechanical stability, intrinsic hydrophobicity, and ironically, its thermo-reversible nature. Agarose is relatively hydrophobic compared to other polysaccharides, which makes chemical modification necessary to promote directed intermolecular interactions [84,88,89,90,91]. Although the reversible phase transition between the sol state and gel state is straightforward, the mobility of the agarose molecules and association dynamics between bundled agarose chains, or between water molecules and agarose chains, directly affects this phase transition. The accumulative and combined effects of those lead to thermal hysteresis of native agarose gel, which is often observed as shifts in gelation temperature over repetition [92].
2.2.3. Alginate
Alginate is a naturally occurring anionic polysaccharide commonly extracted from seaweed, composed of alternating blocks of mannuronic acid and guluronic acid groups, which are called the M unit and G unit, respectively [93]. The ratio of M and G units and the sequence significantly influence the overall characteristics of alginate hydrogels. Alginate hydrogels are commonly highlighted for their biocompatibility, biodegradability, and minimal toxicity and have been widely used in pharmaceutical and biomedical fields (Table 2) [93,94,95].
Generally, alginate hydrogels are synthesized via ionic crosslinking. In the presence of divalent cations such as calcium (Ca2+) or magnesium (Mg2+), the G blocks of adjacent alginate chains bind together to form stable crosslinking points, yielding a three-dimensional network of alginate molecules [93,96]. This gelation process can occur under physiological conditions, which makes it highly compatible with the applications that require the preservation of bioactivity or encapsulation of cells or proteins. Alginate can be covalently crosslinked using a similar group of crosslinking agents like glutaraldehyde, genipin, or uronic acid [97].
The versatility of alginate hydrogels is emphasized most with its wide range of tunability, including mechanical strength, degradation mechanism, bioactivity, and biochemical modification with peptides or growth factors. Alginate hydrogels are widely utilized in drug delivery systems, owing to their capacity to encapsulate therapeutic agents within their matrix and release them in a controlled manner [23,94,95,98,99]. Additionally, the gentle gelation process offers great potential toward the injectable formulation of alginate, which allows the implantation of functional hydrogel at a desired site in the body with minimal invasive procedures [100,101,102].
Interestingly, this gentle gelation process makes alginate the most suitable for 3D bioprinting technologies, since it can stay as a relatively low-viscosity solution and quickly turn into a soft gel when a divalent cation is introduced, enabling the precise fabrication of alginate-based scaffolds with complex architectures for tissue and organ regeneration [103,104,105]. However, one significant drawback of alginate hydrogel is the limited control over the rate of biodegradation since environmental heterogeneity and a diverse involvement of divalent cations, such as calcium and magnesium, facilitate various biological processes within the human body [88,103,104,106]. This unpredictability becomes more critical for the application of alginate hydrogels in the circulatory systems.
2.2.4. Matrigel
Matrigel is a natural hydrogel derived from the extracellular matrix of Engelbreth–Holm–Swarm (EHS) mouse sarcoma cells. It is rich in structural proteins such as laminin, collagen IV, entactin, and growth factors as well as cytokines, enabling the most native ECM environment [107]. Benefiting from its unique composition, Matrigel serves as a robust scaffold for cell adhesion, differentiation, and proliferation in in vitro cell culture models [13,108,109].
Matrigel intrinsically exhibits thermosensitive properties and undergoes phase transition from a liquid state to a gel state over about 10 °C temperature increase. Once it becomes a gel, it provides a three-dimensional environment conducive to the growth and maintenance of various cell types, including stem cells and cancer cells. It is very easy to handle and can be directly applied to in vivo applications as well [91,110].
Applications of Matrigel are extensive in the biomedical field and are most widely used in cancer and neural cell research for studying cell differentiation and growth, metastasis of tumor cells, etc. (Table 2). Matrigel also largely contributed to the development of organoid and 3D cell culture systems, making it indispensable for modeling organ development and disease progression [111,112]. Recently, Matrigel has also been widely utilized in vascular cells and angiogenesis studies [13,112,113,114]. While Matrigel has been transformative in biomedical research, its predefined composition and possible heterogeneity inherited from the fact that it originated from a biological source remains a major challenge.

Table 2.
Key features and biomedical applications of common natural hydrogels.
Table 2.
Key features and biomedical applications of common natural hydrogels.
| Hydrogels | Source | Crosslinking Methods | Functional Features | Applications | Ref. |
|---|---|---|---|---|---|
| Chitosan | Exoskeleton of shellfish, fungi cells |
|
|
| [17,59,68,74] |
| Agarose | Marine algae |
|
|
| [82,86,87,89] |
| Alginate | Seaweed |
|
|
| [26,93,94,99] |
| Matrigel | Derived from mouse sarcoma |
|
|
| [107,109,111,113] |
2.3. Hybrid Hydrogels
Hybridization is an advanced strategy to amplify and to combine desirable properties by creating a unique matrix of two or more hybrid materials. This approach could efficiently overcome the limitations of individual materials, such as the insufficient mechanical strength of natural hydrogels and the lack of bioactivity of synthetic hydrogels, as well as offer enhanced functionalities for complex biomedical applications.
Hybrid hydrogel is an extremely diverse category and could not be simply classified without overlooking the critical perspective of each case; however, there are resemblances in their experimental approaches which include the interpenetrating polymer network (IPN), blending, copolymerization, and layer-by-layer assembly (Figure 2).
IPNs generally involve two or more polymeric materials that are physically entangled without covalent bonds between each other [115]. This approach is known to be highly efficient for enhancing mechanical strength, swelling behavior and stability, and stimuli responsiveness. Compared to blending, IPN is often described as an interwoven network of two or more polymers that retains individual properties and functions synergistically [41,116,117].
Blending is a more straightforward strategy that combines natural hydrogel materials like chitosan or alginate with synthetic hydrogels such as polyacrylamides or polyurethanes [18,59,118]. Blending is often used to describe a homogeneous mixture of two or more hydrogel materials that does not necessarily involve chain entanglement, with a primary aim to balance the advantages of each component such as mechanical stability or bioactivity [118,119,120].
Copolymerization is a type of polymerization reaction that involves two or more types of monomers forming a chain composed of more than one monomer. It is often applied to synthetic hydrogels, where different types of monomers are sequentially fed during the controlled radical polymerization process like atom transfer radical polymerization (ATRP) or reversible addition fragmentation chain transfer (RAFT) [121,122]. Notable differences in copolymerization from other approaches are the precise control over the resulting polymer in the molecular scale, including composition, molecular weight, statistical uniformity, and sequence of monomers, which are especially crucial in nanoscale hydrogel composites [123,124,125,126,127].
Layer-by-layer assembly (LbL) is a repetitive process that yields a sequentially multilayered composite of varying materials [128,129,130]. The most common example is an alternating deposition of oppositely charged polymeric materials, and each layer is stably incorporated with previously applied layers via electrostatic attraction. Another approach has made noticeable advancements by adapting additive manufacturing technologies. Hydrogels are increasingly adapted into 3D printings, owing to their unique characteristics, including dynamic sol–gel transition, tunable viscosity, and selective gelation [130]. One major advantage of the LbL assembly is the precise spatial control over the hydrogel by incorporating a certain group of materials within the deposition sequence, allowing us to design a multilayer hybrid for a specific application such as drug release or tissue scaffolding [119,120,131].
Nanocomposite formation incorporates various nanomaterials such as graphene oxide, silica nanoparticles, or other inorganic nanocrystals with hydrogel material to improve mechanical properties and thermal stability, and to implant unique functionalities [56,132]. Nanomaterials can be either dispersed into precursors of hydrogel or directly grafted onto polymeric chains [54,118,133,134,135,136].
Though the aims of hybridization vary from case to case, major considerations must be made in designing hybrid hydrogels. First, the biocompatibility and safety of compositions need to be evaluated, especially for their cytotoxicity, immunogenicity, and long-term safety in physiological settings. Second, the compatibility of each component must be accounted for. Physically incomparable mixtures often result in macro-phase separation, resulting in a significant loss in functionalities. Chemical compatibility refers to near-inert reactivity between major components, except designated reactions or intermolecular interactions. Also, the scalability of the hybridization mechanism and synthesis process must not be overlooked, especially for the strategies that involve chemical reactions [123,137,138]. Reaction dynamics and selectivity of reaction pathways could significantly differ from the lab-scale synthesis as the overall system increases, ultimately posing a serious risk of altering the chemical and physical properties of the resulting hybrid gels [124].
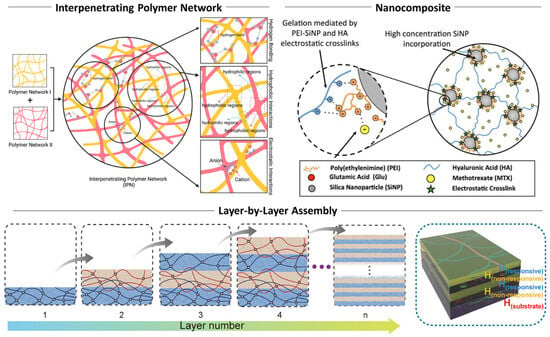
Figure 2.
Hybridization strategies of multi-component hydrogel systems. Representative approaches include IPN, incorporation of secondary functional nanomaterials, and multilayered 2D or pseudo-2D hydrogel systems. Reproduced with permission from [117]. Licensed under CC BY 4.0, adapted from [131,136].
3. Fabrication of Hydrogels
To maximize the utilities of hydrogel materials that we discussed in the earlier sections, it is important to highlight the series of synthesis technologies as well. The versatility and functionality of hydrogels largely depend on their preparation methods, which have evolved from traditional bulk synthesis to precise and controllable synthesis methods.
Hydrogel synthesis techniques can be categorized differently from the physical and chemical perspectives. Physically, the hydrogel system can be considered a continuum where the scale of system itself is substantially larger than the scale of molecular interactions and dynamics [20,58]. This type of system is often called “bulk”. On the other hand, in miniaturized systems at the scales of cells or microorganisms, the overall characteristics of the material, governing interaction, and stability of the hydrogel itself can appear very different. The chemical perspective focuses on the fundamentals of the network formation between hydrogels or precursor materials, and the strategy to engineer these networks, ultimately enabling us to design and realize advanced hydrogel systems [16,90,139] (Figure 3).
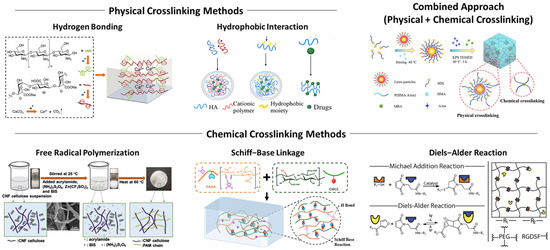
Figure 3.
Conventional approaches for the fabrication of hydrogel systems at bulk scale, highlighted by physical, chemical, and combined crosslinking methods. Reproduced with permission from [135,140,141]. Licensed under CC BY 4.0, adapted from [142,143].
3.1. Bulk Hydrogel vs. Hydrogel Particles
Hydrogels are conventionally synthesized via the formation of a continuous network of polymers through physical or chemical crosslinking in a single step. This process can create large-scale structures like scaffolds, membranes, or injectable hydrogels. Bulk hydrogels tend to exhibit uniform properties across the structure, because the functionalities of bulk hydrogel mostly originated from its building block. For example, the powerful antioxidant activity of chitosan hydrogel is based on the strong tendency of protonation of amino groups present in the chitosan molecule [11,73,78].
Hydrogel particles conventionally refer to a discrete phase of hydrogel at micro- and nanoscales within a liquid medium, also known as a colloidal system [115,144,145,146]. Particle synthesis using hydrogel material focuses on creating micro- or nanoscale hydrogels, whose surface-to-volume ratio becomes high enough to exhibit surface-oriented functionalities. Also, smaller-scale hydrogels exhibit much faster diffusional mass transfer and become more eligible for intravenous injections. Combined with exceptional biocompatibility and biodegradability and a large surface area for functionalization, hydrogel particle systems construct a highly convincing new approach for advanced drug delivery systems [115,147,148,149,150].
3.2. Physcial Crosslinking
Physical crosslinking has been the most facile approach to form hydrogels of various materials, which relies on non-covalent interactions to create three-dimensional polymeric networks. Distinguished from chemical methods, the physical approach omits the need for crosslinking agents, which poses a potential risk of cytotoxicity, and is thereby generally considered to be more biocompatible and safer to use.
3.2.1. Hydrogen Bonding
Hydrogen bonding is a special type of intermolecular interaction that occurs between molecules with highly polarized bonds, including hydroxyl or amine groups [69,151]. Hydrogen bonds are formed between a hydrogen atom bound to an electronegative atom and another electronegative atom, like nitrogen, oxygen, or fluorine. These bonds are reversible and weaker than covalent bonds, allowing the gel constructed via hydrogen bonding to be highly responsive to environmental conditions like pH and temperature [19,76]. For example, lowering the pH of the aqueous solution protonates chemical moieties, promoting hydrogen bonding. PVA and gelatin-based hydrogel systems are common examples, but the hydrogel solely relies on hydrogen bonding and typically exhibits weaker mechanical stability [42,146,152].
3.2.2. Hydrophobic Interaction
Hydrophobic interactions occur when hydrophobic segments of amphiphilic polymers aggregate in aqueous environments to minimize exposure to water. These interactions are driven primarily by entropy, as the system reduces the ordered structure of water molecules around hydrophobic regions, increasing overall entropy. This process is significantly prominent for amphiphilic polymers such as Pluronic® and results in the formation of micelles, where the hydrophobic cores act as crosslinking points within the hydrogel network [69,138,143]. Hydrophobic interactions can be finely tuned by altering the number and chemical structure of hydrophobic groups, allowing precise control over the swelling dynamics and hysteresis, water retention, and drug loading efficiency [84,153]. The reversible nature of hydrophobic interactions also enhances the injectability of gels. Furthermore, dense hydrophobic structures facilitate the rapid reformation of crosslinking points upon mechanical disruption, making these hydrogels ideal for applications requiring self-healing and reusability [19,154,155].
3.2.3. Ionic Crosslinking
Ionic crosslinking follows the most straightforward mechanism in physical crosslinking, relying on electrostatic interactions between oppositely charged molecules [93,110]. It is typically achieved by introducing oppositely charged multivalent ions or particles into a polyelectrolyte solution, creating a localized electrostatic assembly between multiple polymeric chains and a divalent cation which serves as a crosslinking point within the hydrogel network. For instance, alginate hydrogels are conventionally formed when divalent cations, such as Ca²⁺, Ba²⁺, or Mg²⁺, interact with the guluronate blocks of alginate chains [93,96,97]. These electrostatic interactions result in the formation of polyelectrolyte complexes, creating a stable, reversible hydrogel network.
Ionic crosslinking offers the advantage of mild reaction conditions, making it highly suitable for encapsulating sensitive biological materials such as cells or proteins. However, the strength and stability of the hydrogel depend on factors like ion concentration, type of crosslinking ion, and environmental ionic strength.
3.3. Chemical Crosslinking
Generally, chemical approaches necessitate the use of additional components including crosslinkers or photoinitiators. However, this strategy yields a highly stable crosslinking point, which renders the overall hydrogel robust and stable at varying conditions. However, intrinsic toxicity of photoinitiators or limited biodegradation kinetics caused by dense crosslinking points must be carefully addressed prior to administration. Here, we highlight a series of synthesis methods selected based on the applicability and ease of controllability.
3.3.1. Condensation Reaction
Condensation reactions, such as EDC/NHS-mediated coupling, leverage functional groups like carboxyl (-COOH) and amine (-NH2) groups on the polymer chains [156,157]. In typical condensation reactions, the reaction involves the removal of water molecules as the bond is formed between two functional groups. EDC/NHS coupling is widely utilized to form an amide bond between carboxyl and amine groups through an intermediate step involving carbodiimide activation. While the EDC/NHS still forms a byproduct like urea, it resembles more of a catalyzed reaction than a conventional condensation reaction [157].
Generally, these reactions are known to be highly specific and efficient, as they form covalent bonds that contribute to robust mechanical properties of hydrogels. The spontaneity of this reaction simplifies the fabrication process, progressing without continuous external input other than thermal energy [158]. However, it poses challenges in controlling reaction kinetics and ensuring network uniformity [159]. To form a crosslinked network instead of linear polymerization, the presence of multifunctional groups capable of bonding with multiple polymer chains is essential for creating a stable, three-dimensional hydrogel matrix [143,160]. Additionally, the nature of byproducts generated during condensation reactions, such as water, alcohol, or ammonia, can significantly influence the process. For example, volatile byproducts like alcohol or ammonia require careful removal prior to biomedical uses to maintain biocompatibility and structural integrity [161,162].
3.3.2. Free Radical Polymerization
Free radical polymerization is one of the most widely used methods for synthetic hydrogels. Radicals play a central role in this technique by initiating the polymerization reaction between initially stable monomers upon activation [125]. The mechanism of this reaction consists of three steps: initiation, propagation, and termination. Radicals can be generated from chemical or thermal initiators, which produce reactive radical species when certain criteria are met (initiation) [124,125,163]. Radicals, with their unpaired electrons, are highly reactive and extremely unstable chemical species. In this process, the radical from the initiator attacks a monomer, which makes the monomer radical and causes it to then immediately react with another monomer, as if a monomer is added to a growing polymer chain (propagation). When a monomer has more than two reactive sites or a crosslinker is used, this process creates a three-dimensional polymer network. Since the hydrogel synthesis via radical polymerization commonly occurs within a liquid phase, two polymeric chains with radical ends could collide with each other and immediately react to form a covalent bond between two ends, or one sufficiently long polymeric chain with two or more radical sites could undergo the same process, resulting in the elimination of radicals (termination) [164,165]. The chance of termination naturally increases as the monomer concentration decreases, or the size of the polymeric chain in the propagation stage increases as the reaction progresses.
Synthetic hydrogels such as polyacrylamide, polyacrylic acid, and polyvinyl alcohol are commonly fabricated using this method, and this technique is widely compatible with other monomers with a carbon–carbon double bond or a vinyl group [18,110,166,167]. While it provides cost-effective scalability and a broad range of monomer compatibility, controlling the uniformity of the network and minimizing free radical side reactions remain challenging.
A significant concern in free radical polymerization, especially in biomedical applications, is the toxicity of chemical initiators or their residual byproducts [106,167,168]. Benefiting from the high permeability and liquid contents of hydrogels, residues could be further removed via simply extensive rinsing or dialysis. Recently, more “bio-tolerable” initiators like lithium phenyl-2,4,6-trimetylbenzoylphosphinate (LAP) have been increasingly used in various hydrogel or biopolymer systems as a more fundamental solution [169,170,171].
3.3.3. Photopolymerization
The photopolymerization of hydrogel is fundamentally similar to the free radical polymerization, except the external trigger that activates radical generation is ultraviolet or visible light [3,172]. With recent advances in digital light processing (DLP) or laser-based lithography techniques like stereolithography, this approach allows spatial and temporal control over gelation and is suitable for creating patterned hydrogel structures at the microscale [104,168,173]. The same approach is found in stereolithography, a type of additive manufacturing technique that forms solid microstructures within a pool of liquid resin, where the optical resolution determines the minimum size of realizable structures [4,106,130].
The major difference between photopolymerization and other radical polymerization is the mechanism of radical initiation. A photoinitiator is an essential reagent in photopolymerization which undergoes photolysis under the irradiation of an appropriate wavelength and produces radical species. Photoinitiators include benzoin and benzophenone compounds and their derivatives, or commercially available types including Irgacure 2959, Irgacure 1173, or riboflavin, can efficiently absorb a certain wavelength within the range of UV to visible light, depending on the types and number of functional groups [169,170,174]. Compared with conventional thermally initiated radical polymerization, a light-controlled mechanism offers powerful advantages for hydrogel synthesis by allowing the excellent spatial selectivity of radical generation and absence of heat throughout the entire process. Irgacure 2959, for example, is commonly used for hydrogel synthesis because of its relatively low cytotoxicity and acceptable tolerability [29,105,171]. These benefits enable facile fabrications of biologically complex systems like cell-laden hydrogel platforms or growth factor-loaded scaffolds.
However, hydrogel synthesis via photopolymerization often suffers from two major drawbacks: cytotoxicity of residual photoinitiators and limited range of water-soluble photoinitiators [174,175]. First, the removal of a residual photoinitiator becomes more challenging for hydrogels of higher solid composition and bulkier structure. Since their passive diffusion rate drastically decreases as crosslinking progresses, much extensive rinsing or dialysis is required to drive out the residues from the interior of the hydrogel to a sufficient level; on the other hand, the same residues could cause long-term cytotoxic effects in in vivo settings. Second, the most commercially available photoinitiators used in hydrogel synthesis exhibit extremely poor solubility to water of approximately 10 mg/L or less. This inevitably necessitates extensive UV exposure to compensate for limited photoinitiator concentration, which renders the reaction environment incompatible with cells or other UV-sensitive reagents [104,167,173,176].
3.3.4. Schiff Base Linkage
Schiff base reactions occur between the primary amine group and carbonyl group, resulting in the formation of imine bonds, carbon–nitrogen double bonds. It follows the basic reaction mechanism of a nucleophile like an amine group and an electrophile like an aldehyde group, and the resulting bond is at a dynamic equilibrium but more commonly considered to be a pseudo-covalent bond. Also, in tissue environments, aldehyde groups contribute to form strong adherence to cells, which is an essential feature for injectable or implantable hydrogel systems [110].
Schiff base linkage is reversible and pH sensitive, which makes it suitable for designing dynamic hydrogel behaviors like self-healing [177,178]. The formation of linkage is spontaneous, and when a physical discontinuity occurs within the hydrogel, the broken Shiff base linkage reforms, thereby regaining its structural integrity. The spontaneity of this reaction can be maintained under mild conditions without any additional reagents, offering promising potential regarding biomedical applications [178,179].
3.3.5. Diels–Alder Reaction
The most widely utilized click chemistry in hydrogel synthesis is the Diels–Alder reaction. The concept of click chemistry is largely based on its practical advantages including versatility, simplicity, and efficiency [180]. The Diels–Alder reaction is a highly selective and efficient cycloaddition reaction between a diene compound and a dienophile like maleimide, and it exhibits rapid reaction kinetics in aqueous solution [141].
The Diels–Alder reaction does not mandate the use of any initiators or coupling agent nor produce byproducts yet could form a covalent bond at generally high yield and maintain its reactivity and specificity at varying conditions [141,152]. One unique feature is that the stereochemistry of functional groups on participating molecules determines the stereochemistry of the resulting structure, enabling three-dimensional control over molecular structures [181]. This method of crosslinking is highly versatile as far as the portion of participating molecules suffice the criteria of the diene–dienophile pair; thus, it is widely compatible for both natural and synthetic hydrogels.
4. Paradigm Shift: Bulk Hydrogels to Hydrogel Micro/Nanoparticles
Hydrogels, widely utilized in bioengineering applications like scaffolds and coatings, have recently seen transformative advances through the miniaturizations of a bulk system into the micro- and nanoscale, known as microgels and nanogels. This physical transition offers new unique properties like dynamic responsiveness along with the benefits of the reduced size scale, opening new potential regarding hydrogel-based biomedical technologies.
Miniaturizing bulk hydrogels into nanogels introduces a series of distinctive attributes including increased surface area, rapid responsive kinetics, enhanced drug delivery, and mechanical flexibility [84,182,183]. The surface-to-volume ratio drastically increases as the size of the system reduces to nanoscale, which facilitates an extensive surface for additional functionalization. This increased ratio can directly affect the overall characteristics of the hydrogel because the surface-driven properties become more pronounced than the volume-driven properties as the surface area increases. Additionally, transport phenomena of heat and mass generally occur at faster rates in a smaller-scale system. For example, a shorter diffusion length enables faster swelling and deswelling, or drug release, when it is triggered via pH shift or temperature shift. Thereby, miniaturization enables rapid and reversible responses to stimuli such as pH, temperature, or light, allowing more precisely controllable hydrogel systems [5,8,10,184].
For drug delivery systems, nanoparticles exhibit a unique passive tumor-targeting phenomenon called the enhanced permeability and retention (EPR) effect [10,102,184,185]. This phenomenon is caused by the formation of blood vessels with high vascular permeability in the tumor microenvironment. Abnormal angiogenesis is not directly related to the onset of a tumor, but it is critical for the fate of the tumor since it requires higher vessel density as its size exceeds a few millimeters. The EPR effect describes how nanoparticles below 200 nm in size show selective accumulation within and near the tumor sites, while the same particles still cannot efficiently pass through the endothelial barriers of normal vessels [184,186,187,188,189]. This property offers a distinct advantage over bulk hydrogels, which often lack directive tumor targeting and exhibit slower drug release kinetics due to their larger size and slower responsiveness [190,191].
Thin-film hydrogels, a unique class of hydrogel systems, offer a distinctive approach bridging the gap between bulk scale hydrogels and micro/nanogels. Thin-film hydrogels are typically micrometers thin and present a highly adaptable platform that can serve as a coating or membrane [191]. Macroscopic film systems can contribute to the overall mechanical stability or provide a protective interface, while nanoscale thin films can leverage selective permeability and rapid stimuli responsiveness, making them suitable for biosensors or drug delivery patches. However, thin-film hydrogels remain fundamentally different from microgels and nanogels despite their versatility. Thin films lack the discrete and particulate nature of micro/nanogels, limiting their application to superficial interfaces, such as skin or dressing, or hard interfaces.
However, miniaturized hydrogel systems like microgels and nanogels also exhibit inherent disadvantages compared to their bulk counterparts. One major limitation is reduced mechanical stability, which restricts their use in load-bearing applications or high-shear environments [15,136]. Also, the fabrication of microgel and nanogel often involves complex and lengthy processes, requiring additional equipment or precise process control. The uncontrolled synthesis of these systems may lead to reduced retention at the target site or premature clearance by the immune system.
Another significant challenge arises from the biocompatibility and cytotoxic effect associated with functionalized hydrogels [69,171]. While hydrogel materials are generally considered to be non-toxic and chemically inert, the use of crosslinking agents, surfactants, or other additives during the synthesis process contributes to the cytotoxicity of the resulting systems. Traces of unreacted monomers or degradation byproducts also adversely interact with surrounding biological tissues, potentially inducing inflammation or immune responses. These issues require further investigation regarding the choice of reagents and purification techniques to ensure clinical safety.
Furthermore, the interactions of particulate forms of hydrogel with biological systems necessitates thorough examination beyond material compatibility [185]. In particular, the behavior of nanoparticles in physiological environments, including protein adsorption, clearance, and biodistribution, could be largely governed by their physical sizes and surface functionalities and plays a crucial role in determining therapeutic efficacy [134,192]. While the EPR effect offers tumor-specific targeting, challenges remain in achieving uniform distribution across the tumor cells and avoiding off-target accumulation. Lastly, the long-term stability of nanoparticles within a biological system and clearance pathways must be carefully optimized to improve therapeutic outcomes.
The promising potential of hydrogels lies in their unique properties as versatile biomedical materials, further enhanced by systematic miniaturization into microgels and nanogels. While the advantages, such as enhanced targeting, faster response kinetics, and improved functionalization, significantly expand their applications, the limitations including mechanical fragility and production complexity underscore the need for creative solutions as well. Here, we highlight two key aspects of hydrogel micro- and nanoparticles toward an advanced drug delivery system: how to design and implement stimuli-responsive properties and how to synthesize them in a controllable manner.
4.1. Designing Stimuli-Responsive Properties
Stimuli-responsive hydrogels are engineered to undergo physical and chemical transformation when specific environmental changes occur, including pH, temperature, and light. These phenomena allow dynamic control over the properties of hydrogels, which is an essential feature in precision medicine, tissue engineering, sensing, and diagnostics. Most of all, the ability to reversibly respond to external stimuli is imperative for highly controllable drug delivery systems, especially when it is combined with the EPR effect.
4.1.1. pH Sensitivity
The basis of the pH-sensitive behavior of hydrogels is the ionizable functional groups present in their molecular structure, where the dissociation or protonation of these chemical moieties modulates the swelling behavior, mechanical strength, and permeability of a hydrogel. Anionic hydrogels contain acidic functionalities such as a carboxyl group, which could deprotonate at higher pH levels [76]. Deprotonation of an acidic group renders its surface potential negative, increasing electrostatic repulsion and swelling. This behavior is suitable for the controlled drug release in basic environments such as the intestines, where the pH value begins at 6 and reaches approximately 7.4. PAA hydrogels are widely adapted in the DDS specialized in oral administration, since the micro/nanogels could encapsulate drug molecules at their interior to physically separate them from the surrounding environment [53,150,193]. This allows us to avoid undesirable interactions between normal cells and the drugs in the acidic gastric environment and to release them in the basic intestinal tract.
Cationic hydrogels contain basic functionalities, like amine groups of chitosan, and these groups can protonate in acidic conditions, at below their dissociation constant (pKa), inducing extensive swelling of the gels [68,194]. This behavior is advantageous in tumor-targeted therapy, since the tumor microenvironment exhibits a slightly acidic pH ranging from 5.6 to 6.8 due to the high level of glycolysis and hypoxia [13,110,195].
4.1.2. Temperature Sensitivity
Temperature-responsive hydrogels could undergo phase transition induced by temperature changes across certain thresholds, more commonly referred to as the lower critical solution temperature (LCST) and upper critical solution temperature (UCST). In the simplest form, hydrogels can be described as a polymeric network comprising the skeletal structure of a gel and an aqueous phase filling this structure driven by the hydrophilicity of the gel material [21,64,193]. Each critical temperature describes a thermodynamic point where the phase behavior transitions between hydrated (soluble) and collapsed (insoluble) states. LCST refers to the temperature below which a polymeric phase remains soluble with a solvent, hence hydrated, and above which it becomes insoluble, and the gel becomes collapsed [63,64,196].
Above the LCST, the hydrogel is dominated by hydrophobic interactions within the polymeric network, causing the hydrogel to expel water molecules and a steep reduction in its volume [193]. This volumetric contraction is a primary mechanism for the selective onset of drug release, and it is particularly useful when the LCST is within the range of body temperatures such as PNIPAM. The concept of UCST is the opposite of LCST, and the polymeric phase stays insoluble with a solvent and becomes soluble below and above this threshold, respectively. At high temperatures, thermal energy becomes sufficient to disrupt hydrogen bonding or ionic coupling, and this mechanism is often dominated by types of polymer–polymer interactions including hydrogen bonding and ionic interactions over polymer–solvent interactions [197,198].
The thermo-responsive behavior of hydrogels is largely determined by the chemical properties and compositions of hydrogel material, but it is also affected by other parameters such as crosslinking density, polymer architecture, or types of incorporated additives [197,198,199]. Conventionally, LCST-type hydrogels are more common in both synthetic and natural materials. PNIPAM, its derivatives, and poly(vinyl methyl ether) exhibit an LCST of body temperature, and various copolymers including Pluronic® series exhibit a wider range of LCST. For natural hydrogels, polysaccharide-based types are known as LCST type; for example, methylcellulose and hydroxypropyl cellulose exhibit an LCST of approximately 60 °C and 40 °C, respectively [200,201]. UCST characteristics can be found in copolymer-based or ionically crosslinked systems, to achieve strong polymer–polymer interactions such as poly(acrylamide-co-acrylonitrile) or chitosan/alginate blend. In addition, gelatin is the most common UCST-type hydrogel material that exhibits an LCST of 40 °C [146,202,203].
4.1.3. Light Sensitivity
The photo-responsive property is represented by the benefits of light as a triggering signal, for their minimally destructive and highly penetrative nature to biological settings. Light-sensitive hydrogels utilize photosensitive agents or certain chemical structures which could absorb specific wavelengths of light and undergo structural or chemical changes. This mechanism is highly compatible with thermo-sensitive hydrogels and allows highly precise spatiotemporal control of external stimulation, making them suitable for in vivo targeted therapy and diagnostics [70,84,204].
Photosensitivity can be categorized into photothermal and photochemical effects. Photothermal effects are based on the conversion of photo energy into heat, which rapidly dissipates into its vicinity and raises the local temperature. Combined with a thermo-responsive hydrogel, phase transition can be induced by the absorption of light instead of external heat, avoiding undesirable thermal damage, especially when the target location is far from externally accessible points like skin. Also, the effective penetration of light into tissue is deeper for near-infrared (NIR) or longer wavelengths of light than visible or ultraviolet, which offers a facile onset mechanism for non-invasive anticancer treatment [205,206]. Gold nanoparticles and carbon nanomaterials are the most utilized photosensitive agents for this purpose. Gold nanoparticles exhibit a unique phenomenon called the localized surface plasmon resonance (LSPR), which makes them effectively absorb a certain wavelength of light depending on its size [205]. The LSPR of gold nanoparticles can be tuned to absorb NIR light by synthesizing into a rod-shape and can induce synergistic effects for plasmonic photothermal therapy (PPTT) [70,123,207]. Basically, PPTT follows a stepwise mechanism starting from the selective accumulation of gold nanorods at the target followed by irradiation of an NIR laser, inducing highly effective absorption of photo energy and localized heat, which results in a sufficiently high temperature to induce cell damage and death [208,209].
Photochemical effects utilize certain chemical bonds or functional groups that are directly affected by the absorption of certain light. Azobenzene or spiropyran groups can undergo reversible conformational changes, called photoisomerization [210,211]. Azobenzene can be “photo-switched” between cis- and trans-form by absorptions of UV and visible light, respectively, whereas spiropyran can form its open-ring isomer called merocyanine under UV and visible light. Both changes induce the changes in porosity, elasticity, and volume of the hydrogels [212,213]. Chemical functional groups like coumarin could form photo-cleavable bonds upon UV irradiation, making it suitable for photo-degradable hydrogel systems and on-demand drug release. Coumarin-functionalized polymers could be crosslinked via dimerization of two coumarin groups under UV light of around 360 nm and can be disconnected under deeper UV light of around 250 nm [214,215]. This approach provides the most direct manipulation of a hydrogel network structure, but the destructive effects and insufficient tissue penetration of UV light must be carefully considered.
4.2. Hydrogel Microparticle Synthesis
The synthesis of hydrogel microparticles can be classified into three major approaches: microfluidic emulsification, spraying and drying, and sonication-based methods.
4.2.1. Microfluidic Emulsification
Microfluidics is a class of fluid dynamics with an emphasis on microscale systems and has increasingly adapted into diverse fields, benefiting from the advances in microfabrication technologies. A microfluidic system allows precise control of one or more types of fluid flow and their fluid dynamic behaviors including mixing, phase separation, and flow profiles [216,217,218]. When two immiscible fluids are injected into a microfluidic system in the same direction, two fluids form an interphase along their primary flow direction. Depending on their flow rate difference, interfacial tension, or shear stress, one fluid could become disconnected (dispersed phase), which naturally takes a spherical form, while the other fluid surrounds it (continuous phase), forming a continuous flow of uniformly sized droplets [219]. The size of the droplets can be controlled by adjusting the same parameters, allowing the precision synthesis of droplets measuring from sub-micron to several millimeters.
Microfluidic techniques leverage the precise control of droplet formation within the microchannel to create hydrogel microparticles (Figure 4). One significant advantage of microfluidic synthesis is the ability to produce monodisperse particles with highly controlled size and morphology, which is essential for precise dosing and uniform cellular responses. This precision also allows the fabrication of advanced microarchitectures including core–shell structures, Janus particles, and multi-compartmental designs [217,218,219,220].
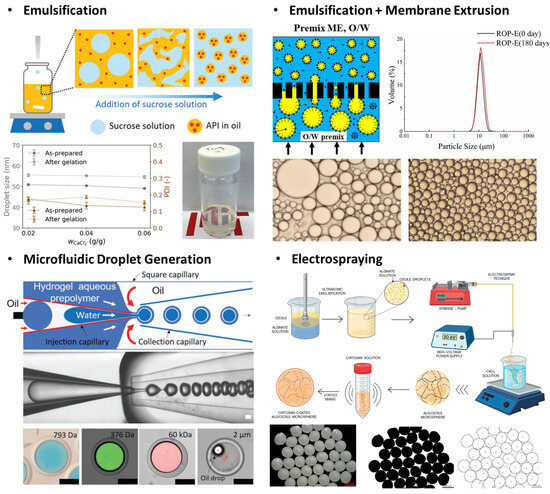
Figure 4.
Synthesis technologies of hydrogel micro- and nanoparticles (microgels and nanogels). Emulsification involves immiscible fluids, forming a discrete volume of hydrogel precursors that define the physical size of the microgel. The achievable size of emulsion varies by the types of emulsification technique, size of the system, etc. Electrospraying generally exhibits large reductions from the aerosol size to the resulting solid particles. Reproduced with permission from [221,222,223]. Licensed under CC BY 4.0, adapted from [224,225].
Emulsion, commonly referred to as droplets, is a minute volume of a fluid dispersed in another immiscible fluid phase, which is directly applicable by making a discrete phase for hydrogel materials and required additives to induce crosslinking. In this approach, a hydrogel microparticle is formed via a stepwise process, droplet formation, and hydrogel crosslinking, which can be modified to introduce drug molecules, buffer exchange, or purification by modifying microfluidic designs [182,226]. For example, a microfluidic orifice is an effective design to formulate localized high-interfacial tension sites, pinching an initially continuous hydrogel solution to form droplets. Also, a complex microfluidic design can be realized via photolithography to combine a curved microchannel with a droplet generator [218]. In this design, a centripetal force exerted on a denser phase induces radially outward flow perpendicular to the primary flow direction, enabling the high throughput of hydrogel microparticle synthesis within a compact form factor device [219,227].
One careful consideration that needs to be made for the microfluidic approach is the high operational pressure [182,188]. For the simplest straight cylindrical microchannel, the flow dynamic behavior in this model is well described by the Hagen–Poiseuille equation.
where ΔP is the pressure drop, μ is the viscosity of the fluid, Q is the volumetric flow rate, and Lc and rc are the length and radius of the microchannel, respectively. As described in the equation above, narrowing down a microchannel incurs a steep increase in the pressure required to maintain the flow rate. A high-pressure operation poses practical challenges, including limited throughput and scalability. However, the smallest size of droplet achievable in a microfluidic design is largely affected by the overall size scale of the design; therefore, miniaturization is essential to push the minimum size of a hydrogel particle [58,219].
4.2.2. Spray Drying
Spraying and drying methods rely on the atomization of a liquid-phase hydrogel precursor into fine droplets, or aerosols, followed by the removal of the solvent phase resulting in solidified microparticles. These techniques are highly practical from the perspectives of cost and scalability, and can adequately incorporate any heat-sensitive compounds. Atomization is achieved through mechanical forces or electronic forces, such as air pressure or Coulombic attraction, respectively [32]. The solidification principle also varies by the type of atomization, where mechanical spraying usually accompanied with heated air drying and electrospraying utilizes electrostatic repulsion to achieve stability and to remove solvent contents. This approach can be configured almost “chemistry-independent” compared to other microparticle synthesis methods, but it is also applicable to restrict the secondary chemical process within the isolated aerosols by loading precursors that can be thermally crosslinked or by using additional crosslinkers in the initial solution [228].
Electrospraying is highly compatible with charge-bearing polymeric materials, and the process requires a strong potential bias to form an electric field between the spraying nozzle and collecting platform. This method is also advantageous for encapsulating bioactive agents, by avoiding the thermal stress associated with a conventional drying process [229,230,231]. Though this process is already widely adapted in various particle syntheses, maintaining the uniformity and controllability of aerosols or droplets down to the nanoscale remains challenging due to the relatively high viscosity of polymer-laden solutions [99].
4.2.3. Sonication
Sonication methods leverage sound waves to agitate particulates or to induce cavitation, the formation and collapse of microbubbles in a liquid medium, by utilizing the wave frequencies ranging from 15 kHz to 400 kHz [232,233]. This process generates high shear spots, where a liquid phase is temporarily disrupted into dispersions (bubbles) or a binary mixture of immiscible fluids into relatively stable emulsions. Emulsions produced from sonication require secondary stabilization such as chemical crosslinking or gelation, and the size of emulsion is controlled by the frequency, power input, and time of sonication, with a general trend of a smaller emulsion size being produced as more powerful and longer sonication is applied [232,234].
Natural polymers like polysaccharides or synthetic polymers could simply be applied to this approach by utilizing certain functional groups and also undergo physical fragmentation during this high-energy process, which could be useful to form smaller-sized particles. For example, thiol groups could help to simplify the stabilization step, by directly forming disulfide bonds which serve as crosslinking points, as demonstrated by Park et al. In this approach, a thiolated heparin and PEG matrix formed a complex of a polymer-rich phase, followed by sonication-aided disulfide linkage formation to stabilize the crosslinked gel matrix [235]. Seshadri et al. reported that high methoxyl pectic (HMP) is directly applicable to the sonication method for controllable synthesis. HMP with a high degree of esterification exhibits increased hydrophobic interactions and hydrogen bonding and reduced ionic interactions, and in this case, hydrogen bonding serves as a crosslinking point. Hence, high-intensity ultrasonic waves are used to form droplets of HMP, while dimethyl sulfoxide is used to enhance polymer chain mobility as well as to reduce solution viscosity, and extensive sonication time helped to facilitate the size controllable synthesis. During this process, high shear and localized heating also caused the breakdown of high-molecular-weight polymer chains, allowing HMP microgels of 10–50 μm in diameter to be formed while maintaining a high encapsulation efficiency of 85% [236].
Ultrasonication is a facile method to produce micro- and nano-emulsions with minimal use of a surfactant via cavitation, but it continuously disrupts the existing emulsions and generates new ones, and prolonged treatment could cause degradation of the polymer under high shear environments. Nanoscale particles can still be formed by repetitive ultrasonication, but this poses a risk of thermal damage to bioactive materials or drugs as high-power sonication could rapidly increase the solution temperature in a few minutes even under cooling [53,234].
4.3. Hydrogel Nanoparticle Synthesis
Nanoscale hydrogel particles, commonly referred to as nanogels, have emerged as a transformative advance toward biomedical, pharmaceutical, and environmental engineering. As described in the previous section, nanoparticles, as a physical format, offer great advantages toward therapeutic agents specialized for the human circulatory system, with features such as controlled drug release and precise targeting. However, the synthesis strategies of nanosized hydrogels are limited to a few principles including polymerization and membrane-mediated methods, which also limits the range of applicable biomaterials and the tunability of physical and chemical characteristics (Figure 4).
4.3.1. Microfluidics-Assisted Polymerization
The most conventional bottom-up approach to produce nanogels is the polymerization process, which can be coupled with a droplet-based system to constrain the reaction to a finite volume and to allow more uniform and rapid thermodynamic control. The microfluidic system offers an efficient strategy to realize this reaction environment, by formulating extremely small and uniformly sized droplets surrounded by a chemically inert fluid [216,217]. In this microfluidic system, immiscible fluids flow through microchannels, where droplet generation is governed by hydrodynamic focusing and droplet breakup at flow junctions, and the produced droplets serve as a microreactor for nanogel formation, stabilized by subsequent crosslinking or polymerization processes such as ATRP or RAFT [58,122,237]. The microscopic dimension of the microchannel facilitates precise control over droplet size and polymerization reaction kinetics.
Controlled polymerization is a fundamental process that enables the synthesis of nanogels, and it provides a controllability over molecular weight distribution, functional groups, and stimuli-responsive behavior [124,165]. Approaches to forming nanogels via polymerization can be categorized into dispersion polymerization and precipitation polymerization. Dispersion polymerization occurs in a homogeneous solvent system where a monomer (precursor) and initiator are soluble at the beginning of the reaction. As polymerization progresses, the growing polymer chain becomes less soluble and begins to phase separate, which can be illustrated as the appearance of a small stabilized solid-like phase rich in polymer chains. With the aid of surfactants or ligands, this process results in the dispersion of spherical particles. The size of the resulting particle can be controlled by the number of monomers and reaction temperature and could reach sub-microns in diameter [124,164]. Precipitation polymerization also begins with a similar system, but generally in the absence of a stabilizer to induce precipitation. Once the polymeric solid matter precipitates, polymerization continues on the surface and leads to particle growth [122,238]. Comparing two approaches, dispersion polymerization generally produces larger-sized polymeric particles but with better size uniformity and scalability. The stability of the solid-rich phase also benefits the stable encapsulation of drug particles. Precipitation polymerization requires a simpler system and could synthesize smaller particles at the scale of tens of nanometers, but size controllability is largely affected by the nucleation mechanism, which is more difficult to control.
Controlled polymerization methods such as ATPR and RAFT are another imperative factor in nanogel synthesis, which offer strategies to control the kinetics of nucleation and polymerization [164]. ATRP employs a transition metal catalyst to manipulate polymer growth, ensuring a controlled polymerization rate and narrow molecular weight distribution. RAFT polymerization leverages chain transfer agents to regulate how monomers are incorporated into growing polymeric chains, enabling the synthesis of nanoscale hydrogel particles as well as copolymerization with other precursors [122,123,124].
4.3.2. Microfluidic Mixing
Microfluidic mixing is another microfluidic approach that induces precipitation and self-assembly of nanoparticles. The fundamental principle of this method is based on a continuous mixing between two or more miscible phases that occurs within the microfluidic channel, whereas emulsification requires a combination of immiscible fluids. In a reduced size scale, mixing in a straight flow path can be confined to the diffusion at the interfaces of miscible fluids with minimal contribution of convective flow [239,240,241]. This process requires a continuous flow of fluids, and the degree of mixing of two fluids becomes independent of time, but it depends more on the distance from the junction where two fluids conjoin, which is called “controlled diffusive mixing” [182]. The degree of mixing can be controlled by the operational parameters including flow rates of each fluid, as well as design parameters such as microchannel dimensions, angle of the junction, etc. The precipitation of the solute can be controlled in this environment [242,243]. If a certain compound was dissolved in one solvent but is insoluble to another one, the continuous mixing between two solvents comprises a dynamic environment for the solute where the solvent composition constantly changes as bulk flow progresses. Thereby, the solute experiences a gradual decrease in solubility as mixing occurs, inducing precipitations of nanoparticles. Benefiting from the controllable manner of the diffusion process, the resulting particle size can be controlled by manipulating the same parameters.
Controlled self-assembly of the block copolymer or amphiphilic molecules within the microfluidic mixer is another spontaneous process to synthesize nanoparticles such as micelles or nanogels driven by non-covalent interactions. Self-assembly is a unique thermodynamic phenomenon where small building blocks spontaneously construct ordered, higher-scale supramolecular structures, largely driven by enthalpic gain [182,187,243]. A common example of self-assembly is the formation of bubbles in the presence of surfactants, or vesicle formation from lipid molecules. The underlying principle is similar to the precipitation of nanoparticles, except the dynamic change in solvent composition within a mixer allows us to control the self-assembly process to a certain extent.
To summarize the two approaches, nano-precipitation produces polymeric nanoparticles and is suitable for encapsulating small molecules that exhibit similar properties or high affinity to the polymer material. On the other hand, controlled self-assembly can form nanogels, micelles, or vesicles through non-covalent interactions, and often utilizes amphiphilic or block copolymers as basic building blocks. Both techniques require precise manipulation of fluid dynamic conditions to acquire the controllability of synthesis, which can be achieved by taking advantage of microfluidic systems.
4.3.3. Emulsification and Membrane Extrusion
Membrane-mediated emulsification is a highly effective approach for producing nanogels and particularly suitable for large-scale synthesis. In this technique, a preformed dispersed phase (emulsion) is forced through a microporous membrane under high pressure, where each emulsion experiences a high level of shear stress and deformation, resulting in overall size reduction. The size of nanodroplets generated in this method is dictated by the pore size of the membrane, applied inlet pressure, and initial size of emulsions [57,186,244].
Subsequent stabilization is imperative because emulsions become increasingly unstable as they become smaller due to the increased surface area and mechanical disruption accompanied with membrane extrusion. Nano-emulsion destabilization can occur through three mechanisms: coalescence, flocculation, and Ostwald ripening [245]. Coalescence occurs when emulsions merge to form larger droplets, which is a spontaneous phenomenon to reduce the total interfacial area. It is easily driven by the insufficient stabilization of the nano-emulsion interface, eventually leading to macro-phase separation. Flocculation describes the aggregation of emulsions without merging, as if they are stuck together. The fate of flocculation is governed by inter-droplet forces; however, it is generally seen as a “delayed” mechanism leading to macro-phase separation. Ostwald ripening involves the diffusion of a smaller emulsion into larger ones as if they are absorbed. This phenomenon is driven by differences in Laplace pressure, where a smaller radius of curvature exhibits a larger value, making it very challenging to maintain smaller groups of emulsions. Over time, this process causes the average size of emulsion to increase and the size distribution to broaden, destabilizing nano-emulsions.
Despite the fundamental challenges, the membrane extrusion coupled with emulsification offers tight control over particle size with minimal energy compared to conventional nano-emulsion synthesis methods. This technique has been widely adapted for encapsulating bioactive reagents, including protein, nucleic acids, and small drug molecules.
4.3.4. Template-Assisted Synthesis
Template-assisted synthesis is still at its early stage, but it has been increasingly gaining interest for its potential benefits as a straightforward, controllable strategy of nanogels compatible with various conventional hydrogel chemistries [246,247]. The most basic form of this approach is the previously discussed nano-emulsion method. Nanoscale emulsion could encapsulate the required precursors and crosslinkers for hydrogel formation, which serves basically as a nanoscale batch reactor (Figure 5). One major drawback is that the reliable formation of emulsion is often limited to hundreds of nanometers, and further miniaturization is accompanied by a substantial increase in the heterogeneity of emulsions.
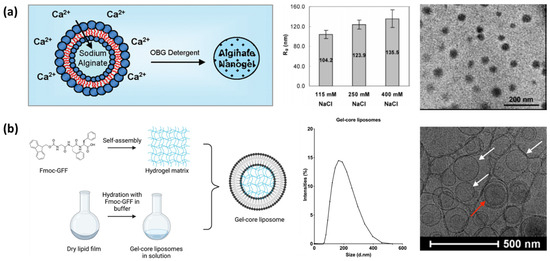
Figure 5.
Template-assisted synthesis of nanogels. (a) First demonstration of alginate nanogel prepared by using liposome as a temporary template. (b) Similar approach demonstrated using glycine diphenylalanine (GFF) as a precursor and liposome as a template. Both approaches showed successful synthesis of nanogels at the 100–200 nm scale but lacked controllability and uniformity as confirmed in the size distributions and transmittance electron microscopy. White arrows and a red arrow indicate unilamellar and multilamellar vesicles, respectively. Reproduced with permission from [246]. Licensed under CC BY 4.0, adapted from [247].
However, the concept of a nanoscale template that is compatible with conventional hydrogel synthesis methods offers an attractive strategy to realize sub-100 nm hydrogel nanoparticles. The template-assisted approach leverages pre-formed templates to dictate the geometry, size, and the characteristics of nanogels, where the templates such as nanoparticle, nanofibers, or nano-emulsions provide the physical framework for chemical synthesis (Figure 5a). The precursor of hydrogel is crosslinked within or around the template, which can be subsequently removed, leaving behind structured nanogels such as porous or hollow interiors. Most commonly used sacrificial colloidal templates are polymer nanoparticles made of polystyrene, poly(methyl methacrylate), or melamine, and inorganic nanoparticles like silica. However, these templates are hard solid particles, and the gelation chemistry is limited to the proximity of their surface and thus is not suitable to produce nanogels. Also, the selective removal of a polymeric template is generally achieved by adding or changing to a good solvent, which naturally undergoes swelling from solvent uptake before dissolution, leading to mechanical ruptures of the synthesized gel architecture [248,249].
Recently, another study utilized a liposome as a nanoscale aqueous reactor for photopolymerization of precursors (Figure 5b) [247]. The microfluidic methods could produce liposomes from below 30 nm to above 100 nm with exceptional stability [187], which provide an aqueous interior and selectively removable exterior with a small amount of surfactant. Both internal and external phases of a liposome are aqueous due to its vesicular nature, which makes it highly compatible with hydrogel and bioactive materials. Although it requires improvements and optimization in various aspects, this strategy shows great promise for the realization of nanogels that can incorporate large biomolecules or biological agents such as proteins, nucleic acids, or even cells.
5. Therapeutic and Biomedical Applications of Micro- and Nano-Hydrogels
Hydrogel micro- and nanoparticles have emerged as transformative tools in various therapeutic applications. Their unique properties, including high water content, dynamic mechanical properties, and biocompatibility, enable the targeted delivery of therapeutic agents with enhanced efficacy and reduced systematic toxicity, as well as efficient development of new therapeutics.
5.1. Anticancer Treatment
Hydrogel-mediated anticancer therapies can be broadly classified into chemotherapy and immunotherapy. Chemotherapy remains a major cancer treatment method, by accompanying cytotoxic drugs to affect rapidly dividing tumor cells. Hydrogel-based drug delivery systems are expected to provide versatile platforms for improving the delivery and stability of chemotherapeutic agents while addressing several limitations of conventional therapies.
The basis of the chemotherapeutic approach of hydrogels is the encapsulation of drugs within the hydrogel matrix to enhance their solubility and bioavailability and to prevent degradation. The conventional chemotherapeutic agents such as doxorubicin (DOX) or paclitaxel are classified as small molecules that function directly with nucleic acids or proteins within tumor cells, leading to apoptosis (Figure 6a) [250]. The effectiveness of these drugs is significantly limited when delivered intravenously, due to several pharmacokinetic and pharmacodynamic challenges. First, the non-specific distribution of drugs caused by poor tumor targeting often results in cytotoxic effects on healthy tissues and organs, also known as common side effects of chemotherapy, like damage to bone marrow, the gastrointestinal tract, and hair follicles. Second, molecular drugs generally exhibit a high clearance rate, either via renal excretion, hepatic metabolism, or bindings to serum proteins. Also, some of these agents are extremely hydrophobic and require solubilizing agents like ethoxylated castor oil (Cremophor® EL) or DMSO, which poses additional toxicity [100,251,252].
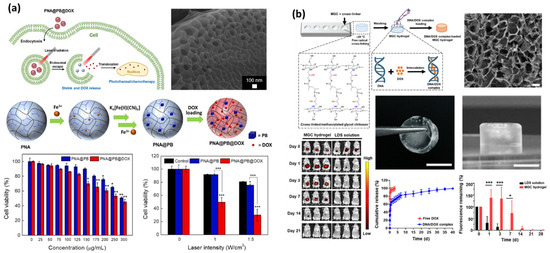
Figure 6.
Applications of micro- and nanogels in anticancer treatment. (a) Nanoscale hydrogels served as an effective drug carrier that can selectively accumulate within the tumor. (b) More conventional approach taken using the macroscale hydrogel incorporated with a chemotherapeutic drug, demonstrating sustained release of the drug. (* p < 0.05, ** p < 0.01, *** p < 0.001). Reproduced with permission from [207]. Licensed under CC BY 4.0, adapted from [11].
Incorporation of chemotherapeutic drugs into nanoscale hydrogels offers promising potential to overcome these challenges and to enhance the therapeutic efficacy. Their high-water content and tunable properties allow sustained and controlled drug release, mitigating the systemic toxicity. Also, nanogels could passively target tumor sites via the EPR effect, enabling the selective accumulation of drugs in tumor tissues. Encapsulation provides a physical boundary between the drugs and plasma, and it allows us to protect drugs from rapid clearance [8,186].
Immunotherapy is another major classification of anticancer therapy, which harnesses the patient’s immune system to recognize and eliminate cancer cells [253]. Unlike chemotherapy, which directly interacts with rapidly dividing cells, immunotherapy, as famously represented by the chimeric antigen receptor T cell (CAR T-cell) therapies, activates immune responses to achieve a more targeted and longer-lasting effect. To introduce major strategies, the checkpoint inhibitor utilizes the fact that the cancer cell exploits immune checkpoint pathways (PD-1/PD-L1, CTLA-4) to avoid T-cell activation and immune responses, and inhibits these pathways to suppress this mechanism. The cytokine-based approach utilizes signaling proteins, like interleukins or interferons, to boost immune response to cancer [11,186,254]. In adoptive cell therapy, such as CAR T therapy, the immune cells collected from a patient are rendered to enhance their anticancer activity. This process usually follows the collection of T-cells, genetic modification, cell expansion, and re-injection into the patient.
The advantages of immunotherapy are prominent and highly expected to realize anticancer technologies with high-specificity, immunological memory, and minimal side effects. However, immune modulation requires careful considerations, including the risks of cytokine release syndrome (cytokine storm), graft vs. host diseases (GVHDs), neurologic immune-related adverse effects, collateral damage on off-target cells, and downregulation of tumor cells.
Seo et al. investigated a methacrylated glycol chitosan (MGC) hydrogel system for post-surgical cancer therapy, aiming to combine sustained drug release and immunotherapy (Figure 6b). Chitosan was selected for its biocompatibility and pH-responsive properties and served as a scaffold for immune cell activation as well as a drug carrier. The resulting hydrogel encapsulated DNA/doxorubicin complexes and demonstrated sustained release over 40 days, which showed suppressed tumor recurrence and metastasis and confirmed immunogenic cell death and a damage-associated molecular pattern, while achieving an 80% survival rate over 60 days in preclinical models. The MGC hydrogel also promoted cytotoxic T lymphocyte infiltration, directly affecting the tumor microenvironment (TME) [11].
Jo et al. developed a poly(N-isopropylacrylamide-co-acrylic acid)/Prussian blue (PNA@PB) microgel system for synergistic photothermal chemotherapy. This composite microgel leveraged NIR light to induce photothermal effects and enabled controlled drug release and tumor ablation. Prussian blue served as photothermal agent to raise the system temperature above the LCST of the copolymer upon irradiation of an 808 nm NIR laser, causing the volumetric contraction of the microgel and release of DOX. The resulting microgel showed 51% drug encapsulation efficiency and a controlled release of 36% under NIR. Effective tumor cell apoptosis was confirmed in vitro, while in vivo tests showed tumor growth suppression in a xenograft mouse model [207].
Yu et al. developed a photothermal-responsive, injective hydrogel by combining chitosan-capped gold nanoparticles and Olaparib-liposomes for enhanced antitumor therapy. Through an approach combining mild photothermal activity and inhibiting tumor DNA damage repair, this hydrogel demonstrated in vivo suppression of breast tumor growth, localized tumor ablation, and reactive oxygen species (ROS) generation [70].
Fang et al. demonstrated a zwitterionic hydrogel system designed for post-operative breast cancer treatment by utilizing a macrophage membrane and STING agonist. The zwitterionic component helped to reduce protein adsorption and immune recognition, while ensuring stability and reduced off-target effects of the hydrogel system. This hydrogel system incorporates macrophage membrane-coated nanoparticles and 2′,3′-cGAMP to achieve tumor-targeted drug delivery and TME remodeling. They reported the controlled release of DOX, triggered by the acidic TME, DNA damage-induced immunogenic cell death, and enhanced CD8+ T cell filtration as confirmed in in vivo studies [102].
While the applications described above are considered a direct anticancer therapy, hydrogel plays an imperative role in supportive anticancer technology, such as gel dosimetry [47,255]. Fricke gel dosimeters, hydrogel matrices composed of ferrous ions (Fe2+), experience radiation-induced oxidation to ferric ions (Fe3+) that allows the quantification of spatial dose distributions both in vivo and in vitro [47,256]. In these applications, hydrogels such as PVA or gelatin help to stabilize radicals and provide tissue-like properties, which are indispensable features for validating and optimizing radiation delivery in stereotactic radiotherapy and proton therapy. Gel dosimetry, as a supportive anticancer therapy, directly impacts the overall efficacy of radiotherapy, ultimately enhancing the survival rate of patients. Accurate dose measurement and 3D mapping ensure that high doses are delivered to the target sites while minimally damaging the surrounding healthy tissues [47,257].
While most research in gel dosimetry is conducted at bulk scales in the form of injectable or implantable hydrogels, miniaturizing these systems into a micro- and nanoscale format offers transformative potential as radiotherapy techniques become increasingly complex, as in intensity-modulated radiotherapy (IMRT). Microgel systems consisting of advanced gel formulations for lower ion diffusion, such as glutaraldehyde-crosslinked PVA hydrogel, open opportunities for high-precision dose measurement and real-time dose tracking, bridging the gap between theoretical dosimetry and clinical precision [47].
5.2. Advanced Drug Screening
Drug screening is a critical step in pharmaceutical development that identifies and evaluates potential therapeutic compounds [258]. Traditional drug screening models such as in vitro 2D cell culture or animal models often fail to mimic the complexity of human tissues and their microenvironment, which contributes to the extremely low success rate of drug development. Recent advances in the three-dimensional scaffolds specialized in mimicking human tissue environments, also known as organoids, have been highlighted for their ability to emulate physiological conditions of complex tissue or even organs [13,14,259,260]. Hydrogel is an essential element to realize complex environments of ECM or tumor sites, enabling high-throughput, multiplexed platforms for drug screening.
From the material perspective, PEG and polysaccharides have been widely adapted for this application. PEG-based hydrogels are particularly valued for the facile tunability of their properties and intrinsic biocompatibility, which enables controllability over the stiffness, porosity, or degradation kinetics of hydrogels. Polysaccharides can mimic the native ECM effectively while exhibiting essential features such as antimicrobial activity or pH sensitivity [176]. Although the application of microgels and nanogels in drug screening remains in its early stages, the focus on their unique advantages for drug delivery systems has been growing with the advances in organoid technologies. The synergistic combinations of hydrogels as drug carriers and diverse organoid models such as brains, intestines, and cancer pose an eminent potential to bridge the gap between in vitro and in vivo models, ultimately toward a significant advancement in preclinical drug development.
PEG-based hydrogel 3D matrices consisting of alkoxysilane-modified PEG reported by Enyedi et al. compared the impacts of 3D and 2D environments to drug resistance evaluation, where a 3D model supported A2058 melanoma spheroid formation and showed an increased survival rate and higher resistance to daunorubicin treatment (Figure 7a) [261]. Gebeyehu et al. utilized a polysaccharide-based bioink, VitroGel, to develop 3D tumor scaffolds for chemotherapeutic agent screening. The resulting system remained stable in physiological settings and successfully formed spheroids from non-small-cell lung cancer-derived xenograft cells. A drug sensitivity study revealed that the 3D-printed scaffolds showed significantly higher IC50 values for docetaxel, DOX, and erlotinib than 2D culture, highlighting the enhanced resistance associated with 3D TME (Figure 7b) [176]. Lan and Starly developed a 2D–3D hybrid co-culture platform based on alginate to encapsulate HepG2 liver cells and MCF-7 breast cancer cells. Ionically crosslinked hydrogel showed acceptable cell viability of >80% over 3 days without diffusion-limited nutrient deficiency. Overall, 3D models showed greater correlation with in vivo LD50 values and successfully demonstrated simultaneous hepatotoxicity and drug evaluations [98].
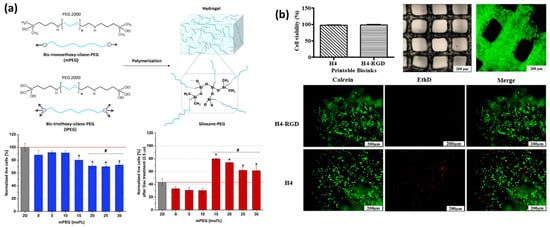
Figure 7.
Hydrogel-based drug screening platforms. The materialistic benefits of hydrogel are especially suitable for 3D cell culture systems, allowing it to mimic the in vivo setting closer than the conventional 2D culture systems. (a) PEG/polysaccharide-based system, and (b) chemically modified PEG-based 3D culture system. (*,# p < 0.05). Licensed under CC BY 4.0, adapted from [176,261].
5.3. Drug Delivery Across Blood–Brain Barrier
The blood–brain barrier (BBB) poses the most challenging obstacle in therapeutic technologies for the central nervous system (CNS) [262]. This highly selective barrier, composed of tightly connected brain endothelial cells supported by astrocytes and pericytes, is the most critical defense for maintaining neural homeostasis, but it also restricts the passage of nearly 98% of small molecular drugs and almost all large-molecule-based therapeutics. This impermeability limits administrable options for neurological disorders, glioblastoma, neurodegenerative diseases, and CNS infections [149,192,202].
Hydrogel materials are emerging as pivotal candidates for BBB-targeted DDS. Gelatin methacrylate, GelMA, is a photo-crosslinkable material derived from denatured collagen with excellent capacity to mimic the ECM [173]. Recent studies highlight the versatility of GelMA in applications requiring 3D scaffolds and drug encapsulation of both therapeutic agents and cells. PVA has also been increasingly utilized for the encapsulation of hydrophobic and hydrophilic drugs by leveraging the ease of chemical modification, which also enhances its compatibility with various biomolecules and targeting ligands [152].
Recently, Lopez et al. synthesized chitosan (CS)-based nanogels functionalized with a tricarbocyanine (CNN) fluorescent probe to investigate its BBB traverse efficiency. Nanogel was ionically crosslinked using tripolyphophate, and the resulting particle sizes were measured as 273 and 435 nm, for CS and CS-CNN nanogels, respectively. Successful cellular uptake and endo/lysosomal escape were confirmed in neuroblastoma cells, and selective accumulation in mice brain was achieved within 2 h [194]. An in vitro BBB model made from GelMA-based hydrogel was recently reported by Augustine et al. This system is composed of a GelMA–astrocyte layer over endothelial cells to form a BBB-like physiological environment and is used to investigate the migration of triple-negative breast cancer cells, where up to 100% reduction was achieved at 20 μM of cisplatin concentration (Figure 8) [202]. Another advanced 3D BBB model was developed using PEG functionalized with RGD and IKVAVA peptides, by constructing an astrocyte-embedded hydrogel matrix and endothelial cells seeded on its surface. Barrier integrity was assessed using transendothelial electrical resistance (TEER) to estimate vascular permeability, and it was found to be closest to the in vivo BBB environment when the combination of RGD and IKVAV modification was applied. These findings emphasize the potential synergistic impacts of micro/nanogels and hydrogel-based platforms toward overcoming BBB and the discovery of advanced CNS therapeutic technologies [263].
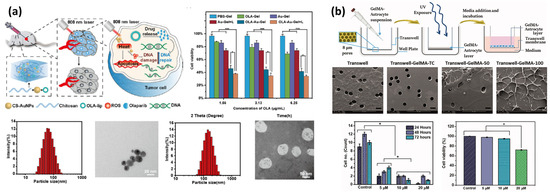
Figure 8.
Applications of nanogels and hydrogel-based platforms of the BBB models. (a) Chitosan nanoparticles serve as a carrier with photothermal and antitumor agents encapsulated in the interior. An NIR laser triggered photothermal effects to induce localized heating as well as burst release of drugs. (b) Three-dimensional construction of a hydrogel with seeded cells mimicking astrocytes in the brain-like environment, allowing a more structured approach to understand the BBB. Reproduced with permission [70]. (* p < 0.05, *** p < 0.001). Licensed under CC BY 4.0, adapted from [202].
6. Perspectives and Conclusions
The exploration of newly emerging hydrogel-based systems, microgels and nanogels, highlights an eminent advance in the fields of drug delivery and therapeutic technologies. These forms of hydrogels show unique advantages that extend beyond conventional benefits of hydrogels, especially the ability to control their properties down to cellular interactions and pathways. By focusing on the fundamental properties of hydrogels and engineering strategies, this review outlined the potential of synthetic and natural materials to overcome the critical challenges in therapeutic applications.
With an emphasis on the synthesis and functionalization of microgels and nanogels, we discussed detailed guidelines on fabricating these systems, not only in theoretical principles but also their practical methodologies. As the main objective of this review is to bridge the gap between diverse fields, this review offers actionable insights to researchers from varying backgrounds, including those who are new to hydrogel particle synthesis.
Despite the availability of various synthesis methods for microgels and nanogels, further advancements are imperative to realize the impactful technologies in next-generation therapeutic applications. Recent approaches have demonstrated promising potential in addressing the limitations of controllability and functionalization of hydrogel-based systems. These efforts also opened new opportunities for integration with state-of-the-art technologies, offering an effective strategy to create truly innovative hydrogel systems.
This review serves as a starting point for continued exploration with practical guidance for new researchers in the field. The need for ongoing innovation and collaboration across diverse fields is clear, and the promising potential of microgels and nanogels open new opportunities to leap forward toward the next generation of biomedical technologies.
Author Contributions
Conceptualization, J.Y.H. and S.S.; investigation and writing—draft preparation, Y.C., H.Y.K. and J.Y.H.; writing—review and editing, J.Y.H. and S.S.; supervision, J.Y.H. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00253941) and the Gachon University research fund of GCU-202110350001.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, H.; Tang, S.Y.; Yun, G.; Li, H.; Zhang, Y.; Qiao, R.; Li, W. Modular and Integrated Systems for Nanoparticle and Microparticle Synthesis—A Review. Biosensors 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Koh, W.G.; Lee, H.J. Enhanced Hepatotoxicity Assessment through Encapsulated HepG2 Spheroids in Gelatin Hydrogel Matrices: Bridging the Gap from 2D to 3D Culture. Eur. J. Pharm. Biopharm. 2024, 202, 114417. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, D.G.; Lee, S.; Park, J.; Jung, H.W. Crosslinking Dynamics and Gelation Characteristics of Photo- and Thermally Polymerized Poly(Ethylene Glycol) Hydrogels. Materials 2020, 13, 3277. [Google Scholar] [CrossRef]
- Nie, J.; Gao, Q.; Fu, J.; He, Y. Grafting of 3D Bioprinting to In Vitro Drug Screening: A Review. Adv. Healthc. Mater. 2020, 9, e1901773. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Tan, B.; Huang, L.; Wu, Y.; Liao, J. Advances and Trends of Hydrogel Therapy Platform in Localized Tumor Treatment: A Review. J. Biomed. Mater. Res. A 2021, 109, 404–425. [Google Scholar] [CrossRef]
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef]
- Ghosh Majumdar, A.; Pany, B.; Parua, S.S.; Mukherjee, D.; Panda, A.; Mohanty, M.; Das, B.; Si, S.; Mohanty, P.S. Stimuli-Responsive Nanogel/Microgel Hybrids as Targeted Drug Delivery Systems: A Comprehensive Review. Bionanoscience 2024, 14, 3496–3521. [Google Scholar] [CrossRef]
- de Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Jaymand, M. Hydrogel-Based Drug Delivery Systems for Synergistic Chemo/Hyperthermia Therapy of Cancer: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2024, 95, 105581. [Google Scholar] [CrossRef]
- Seo, H.S.; Han, J.H.; Lim, J.; Bae, G.H.; Byun, M.J.; Wang, C.P.J.; Han, J.; Park, J.; Park, H.H.; Shin, M.; et al. Enhanced Postsurgical Cancer Treatment Using Methacrylated Glycol Chitosan Hydrogel for Sustained DNA/Doxorubicin Delivery and Immunotherapy. Biomater. Res. 2024, 28, 0008. [Google Scholar] [CrossRef]
- Recktenwald, M.; Kaur, M.; Benmassaoud, M.M.; Copling, A.; Khanna, T.; Curry, M.; Cortes, D.; Fleischer, G.; Carabetta, V.J.; Vega, S.L. Antimicrobial Peptide Screening for Designing Custom Bactericidal Hydrogels. Pharmaceutics 2024, 16, 860. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Park, D.; Lee, J.; Jung, S.; Baek, K.; Sung, K.E.; Lee, J.; Jeon, N.L. Microfluidic High-Throughput 3D Cell Culture. Nat. Rev. Bioeng. 2024, 2, 453–469. [Google Scholar] [CrossRef]
- Ko, J.; Hyung, S.; Cheong, S.; Chung, Y.; Li Jeon, N. Revealing the Clinical Potential of High-Resolution Organoids. Adv. Drug Deliv. Rev. 2024, 207, 115202. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic Acid/Acrylamide Based Hydrogels and Its Properties—A Review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Doghish, A.S.; Zewail, M.B.; Abdelfatah, A.M.; Noshy, M.; Mohammed, O.A.; El-Dakroury, W.A. Smart/Stimuli-Responsive Chitosan/Gelatin and Other Polymeric Macromolecules Natural Hydrogels vs. Synthetic Hydrogels Systems for Brain Tissue Engineering: A State-of-the-Art Review. Int. J. Biol. Macromol. 2024, 260, 129323. [Google Scholar] [CrossRef]
- Hanyková, L.; Šťastná, J.; Krakovský, I. Responsive Acrylamide-Based Hydrogels: Advances in Interpenetrating Polymer Structures. Gels 2024, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Kolouchová, K.; Lobaz, V.; Beneš, H.; De La Rosa, V.R.; Babuka, D.; Švec, P.; Černoch, P.; Hrubý, M.; Hoogenboom, R.; Štěpánek, P.; et al. Thermoresponsive Properties of Polyacrylamides in Physiological Solutions. Polym. Chem. 2021, 12, 5077–5084. [Google Scholar] [CrossRef]
- Litowczenko, J.; Gapiński, J.; Markiewicz, R.; Woźniak, A.; Wychowaniec, J.K.; Peplińska, B.; Jurga, S.; Patkowski, A. Synthesis, Characterization and in Vitro Cytotoxicity Studies of Poly-N-Isopropyl Acrylamide Gel Nanoparticles and Films. Mater. Sci. Eng. C 2021, 118, 111507. [Google Scholar] [CrossRef]
- Işıklan, N.; Altınışık, Z. Development and Characterization of Dual Sensitive Poly(N,N-Diethyl Acrylamide) Grafted Alginate Microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 352–362. [Google Scholar] [CrossRef]
- Tie, B.S.H.; Halligan, E.; Zhuo, S.; Keane, G.; Geever, L. Synthesis of NVCL-NIPAM Hydrogels Using PEGDMA as a Chemical Crosslinker for Controlled Swelling Behaviours in Potential Shapeshifting Applications. Gels 2023, 9, 248. [Google Scholar] [CrossRef]
- Mohammad Mehdipour, N.; Kumar, H.; Kim, K.; Sundararaj, U.; Shor, R.J.; Natale, G. Manipulating Mechanical Properties of PEG-Based Hydrogel Nanocomposite: A Potential Versatile Bio-Adhesive for the Suture-Less Repair of Tissue. J. Mech. Behav. Biomed. Mater. 2024, 150, 106285. [Google Scholar] [CrossRef]
- Kavand, A.; Noverraz, F.; Gerber-Lemaire, S. Recent Advances in Alginate-Based Hydrogels for Cell Transplantation Applications. Pharmaceutics 2024, 16, 469. [Google Scholar] [CrossRef]
- Dolati, M.; Vandghanooni, S.; Veisi, K.; Jaymand, M. Coactive Chemoradiotherapy Using Polysaccharides- and Synthetic Polymers-Based Hydrogels for Cancer Treatment: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100463. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef]
- Sanchez Noriega, J.L.; Chartrand, N.A.; Valdoz, J.C.; Cribbs, C.G.; Jacobs, D.A.; Poulson, D.; Viglione, M.S.; Woolley, A.T.; Van Ry, P.M.; Christensen, K.A.; et al. Spatially and Optically Tailored 3D Printing for Highly Miniaturized and Integrated Microfluidics. Nat. Commun. 2021, 12, 5509. [Google Scholar] [CrossRef] [PubMed]
- Rekowska, N.; Arbeiter, D.; Seitz, H.; Mau, R.; Riess, A.; Eickner, T.; Grabow, N.; Teske, M. The Influence of PEGDA’s Molecular Weight on Its Mechanical Properties in the Context of Biomedical Applications. Curr. Dir. Biomed. Eng. 2022, 8, 181–184. [Google Scholar] [CrossRef]
- Yasmin, M.; Gupta, M.; Shukla, J.P. Molecular Interactions and Structural Effects on Mixing Pentanol in Polyethylene Glycol Diacrylate and Polyethylene Glycol Dimethacrylate. J. Mol. Liq. 2011, 164, 212–217. [Google Scholar] [CrossRef]
- Melnik, E.; Kurzhals, S.; Mutinati, G.C.; Beni, V.; Hainberger, R. Electrochemical Diffusion Study in Poly(Ethylene Glycol) Dimethacrylate-Based Hydrogels. Sensors 2024, 24, 3678. [Google Scholar] [CrossRef]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Kennedy, J.E.; Higginbotham, C.L. Mechanical Properties and Thermal Behaviour of PEGDMA Hydrogels for Potential Bone Regeneration Application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1219–1227. [Google Scholar] [CrossRef]
- Li, Y.; Dennis Tolley, H.; Lee, M.L. Monoliths from Poly(Ethylene Glycol) Diacrylate and Dimethacrylate for Capillary Hydrophobic Interaction Chromatography of Proteins. J. Chromatogr. A 2010, 1217, 4934–4945. [Google Scholar] [CrossRef]
- Burke, G.; Cao, Z.; Devine, D.M.; Major, I. Preparation of Biodegradable Polyethylene Glycol Dimethacrylate Hydrogels via Thiol-Ene Chemistry. Polymers 2019, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.H.; Recalde Phillips, S.Y.; Ruben, E.; Estes, M.; Rajavel, V.; Baig, T.; Paleti, C.; Landsgaard, K.; Lee, R.H.; Guda, T.; et al. Impact of PEG Sensitization on the Efficacy of PEG Hydrogel-Mediated Tissue Engineering. Nat. Commun. 2024, 15, 3283. [Google Scholar] [CrossRef] [PubMed]
- Johannsmeier, S.; Nguyen, M.T.T.; Hohndorf, R.; Dräger, G.; Heinemann, D.; Ripken, T.; Heisterkamp, A. PEGDMA Hydrogels for Cell Adhesion and Optical Waveguiding. ACS Appl. Bio Mater. 2020, 3, 7011–7020. [Google Scholar] [CrossRef]
- Luthfianti, H.R.; Waresindo, W.X.; Edikresnha, D.; Hapidin, D.A.; Noor, F.A.; Elfahmi, E.; Khairurrijal, K. Bioactive Compounds-Loaded Polyvinyl Alcohol Hydrogels: Advancements in Smart Delivery Media for Biomedical Applications. Mater. Res. Express 2024, 11, 062002. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hyu, H.S. Development and Evaluation of Polyvinyl Alcohol-Hydrogels as an Artificial Atrticular Cartilage for Orthopedic Implants. Materials 2010, 3, 2753–2771. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Romero García, A.; Bercea, M. Recent Advances in Poly(Vinyl Alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Upadhyay, N.K.; Surekha, P.; Roy, P.K. Synthesis, Characterization and Efficacy of Chemically Crosslinked PVA Hydrogels for Dermal Wound Healing in Experimental Animals. J. Appl. Polym. Sci. 2009, 111, 1400–1408. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, Z.; Zhang, Q.; Guo, Z.; Li, X.; Chen, W.; Wei, X.; Li, P. Tough Physically Crosslinked Poly(Vinyl Alcohol)-Based Hydrogels Loaded with Collagen Type I to Promote Bone Regeneration in Vitro and in Vivo. Int. J. Biol. Macromol. 2024, 261, 129847. [Google Scholar] [CrossRef]
- Bray, J.C.; Merrill, E.W. Poly(Vinyl Alcohol) Hydrogels for Synthetic Articular Cartilage Material. J. Biomed. Mater. Res. 1973, 7, 431–443. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Gan, L.; Zhang, L.; Yang, L.; Wu, P. Application of Biomedical Microspheres in Wound Healing. Int. J. Mol. Sci. 2023, 24, 7319. [Google Scholar] [CrossRef]
- Gallo, S.; Locarno, S.; Brambilla, E.; Lenardi, C.; Pignoli, E.; Veronese, I. Dosimetric Characterization of Double Network Fricke Hydrogel Based on PVA-GTA and Phenylalanine Peptide Derivative. J. Phys. D Appl. Phys. 2024, 7, 075303. [Google Scholar] [CrossRef]
- Elliott, J.E.; MacDonald, M.; Nie, J.; Bowman, C.N. Structure and Swelling of Poly(Acrylic Acid) Hydrogels: Effect of PH, Ionic Strength, and Dilution on the Crosslinked Polymer Structure. Polymers 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Shin, W.; Chung, K. Preparation and Characterization of Poly(Acrylic Acid)-Based Self-Healing Hydrogel for 3D Shape Fabrication via Extrusion-Based 3D Printing. Materials 2023, 16, 2085. [Google Scholar] [CrossRef] [PubMed]
- Nho, Y.C.; Park, J.S.; Lim, Y.M. Preparation of Poly(Acrylic Acid) Hydrogel by Radiation Crosslinking and Its Application for Mucoadhesives. Polymers 2014, 6, 890–898. [Google Scholar] [CrossRef]
- Fuhrer, L.M.; Sun, S.; Boyko, V.; Kellermeier, M.; Cölfen, H. Tuning the Properties of Hydrogels Made from Poly(Acrylic Acid) and Calcium Salts. Phys. Chem. Chem. Phys. 2020, 22, 18631–18638. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Mechanisms of Mucoadhesion of Poly(Acrylic Acid) Hydrogels. J. Am. Assoc. Pharm. Sci. 1987, 4, 457–464. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan Cross-Linked Poly(Acrylic Acid) Hydrogels: Drug Release Control and Mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Pourmadadi, M.; Farokh, A.; Rahmani, E.; Eshaghi, M.M.; Aslani, A.; Rahdar, A.; Ferreira, L.F.R. Polyacrylic Acid Mediated Targeted Drug Delivery Nano-Systems: A Review. J. Drug Deliv. Sci. Technol. 2023, 80, 104169. [Google Scholar] [CrossRef]
- Martínez, G.; Begines, B.; Pajuelo, E.; Vázquez, J.; Rodriguez-Albelo, L.M.; Cofini, D.; Torres, Y.; Alcudia, A. Versatile Biodegradable Poly(Acrylic Acid)-Based Hydrogels Infiltrated in Porous Titanium Implants to Improve the Biofunctional Performance. Biomacromolecules 2023, 24, 4743–4758. [Google Scholar] [CrossRef] [PubMed]
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Mastella, P.; Todaro, B.; Luin, S. Nanogels: Recent Advances in Synthesis and Biomedical Applications. Nanomaterials 2024, 14, 1300. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Wang, S.H.; Wu, F.G. Nanogels: Synthesis, Properties, and Recent Biomedical Applications. Prog. Mater. Sci. 2023, 139, 101167. [Google Scholar]
- Seifi, S.; Shamloo, A.; Barzoki, A.K.; Bakhtiari, M.A.; Zare, S.; Cheraghi, F.; Peyrovan, A. Engineering Biomimetic Scaffolds for Bone Regeneration: Chitosan/Alginate/Polyvinyl Alcohol-Based Double-Network Hydrogels with Carbon Nanomaterials. Carbohydr. Polym. 2024, 339, 122232. [Google Scholar] [CrossRef]
- Sonzogni, A.S.; Retamar, L.; Muhando, M.; Cabrera, G.; Gugliotta, L.M.; Minari, R.J.; Ronco, L.I. Polyurethane Based Thin Hydrogels for Sustained Protein Delivery. Polymers 2024, 294, 126704. [Google Scholar] [CrossRef]
- Kamaci, M. Polyurethane-Based Hydrogels for Controlled Drug Delivery Applications. Eur. Polym. J. 2020, 123, 109444. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough Hydrophilic Polyurethane-Based Hydrogels with Mechanical Properties Similar to Human Soft Tissues. J. Mater. Chem. B 2019, 7, 3512–3519. [Google Scholar] [CrossRef]
- Georgiana, M.; Kaya, A.; Popa, L.; Dinu-Pirvu, C.E.; Plugariu, I.-A.; Madalina Gradinaru, L.; Avadanei, M.; Rosca, I.; Nita, L.E.; Maxim, C.; et al. Thermosensitive Polyurethane-Based Hydrogels as Potential Vehicles for Meloxicam Delivery. Pharmaceuticals 2023, 16, 1510. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chen, C.W.; Hung, K.C.; Tsai, Y.C.; Li, S. Thermo-Responsive Polyurethane Hydrogels Based on Poly(ε-Caprolactone) Diol and Amphiphilic Polylactide-Poly(Ethylene Glycol) Block Copolymers. Polymers 2016, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Cascone, M.G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 2023, 8, 74. [Google Scholar] [CrossRef]
- Witthayaprapakorn, C.; Molloy, R.; Nalampang, K.; Tighe, B.J. Design and Preparation of a Bioresponsive Hyd rogel for Biomedical Application as a Wound Dressing. Adv. Mat. Res. 2008, 55–57, 681–684. [Google Scholar] [CrossRef]
- Wei, H.; Wang, X.; Liu, W.; Dong, S.; Dai, T.; Luo, F.; Li, Z.; Tan, H.; Li, J. Investigating the Structure and Properties of Polyurethane Hydrogels with Varying Soft and Hard Segments. J. Polym. Sci. 2024, 62, 3979–3991. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Fletes-Vargas, G.; Espinosa-Andrews, H.; Cervantes-Uc, J.M.; Limón-Rocha, I.; Luna-Bárcenas, G.; Vázquez-Lepe, M.; Morales-Hernández, N.; Jiménez-Ávalos, J.A.; Mejía-Torres, D.G.; Ramos-Martínez, P.; et al. Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers 2023, 15, 2203. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.; Meng, X.; Jiang, T.; Zhao, X. Chitosan Thermosensitive Hydrogel Based on DNA Damage Repair Inhibition and Mild Photothermal Therapy for Enhanced Antitumor Treatment. Biomacromolecules 2023, 24, 3755–3766. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in Vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Mushtaq, A.; Li, L.; A, A.; Grøndahl, L. Characterisation of Products from EDC-Mediated PEG Substitution of Chitosan Allows Optimisation of Reaction Conditions. Int. J. Biol. Macromol. 2022, 221, 204–211. [Google Scholar] [CrossRef]
- Lu, Y.; Lou, X.; Jiang, J.; Wang, J.; Peng, X.; Yao, H.; Wu, J. Antioxidative, Anti-Inflammatory, Antibacterial, Photo-Cross-Linkable Hydrogel of Gallic Acid-Chitosan Methacrylate: Synthesis, In Vitro, and In Vivo Assessments. Biomacromolecules 2024, 25, 4358–4373. [Google Scholar] [CrossRef]
- Mathaba, M.; Daramola, M.O. Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef]
- Amor, I.B.; Hemmami, H.; Laouini, S.E.; Abdelaziz, A.G.; Barhoum, A. Influence of Chitosan Source and Degree of Deacetylation on Antibacterial Activity and Adsorption of AZO Dye from Water. Biomass Convers. Biorefin. 2024, 14, 16245–16255. [Google Scholar] [CrossRef]
- Asadi, K.; Samiraninezhad, N.; Akbarizadeh, A.R.; Amini, A.; Gholami, A. Stimuli-Responsive Hydrogel Based on Natural Polymers for Breast Cancer. Front. Chem. 2024, 12, 1325204. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial Activity and Mechanism of Chitosan with Ultra High Molecular Weight. Carbohyd Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation, Properties, and Application of Low-Molecular-Weight Chitosan. Handb. Chitin Chitosan 2020, 453–471. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Waheed, A. A Review on Super-Wettable Porous Membranes and Materials Based on Bio-Polymeric Chitosan for Oil-Water Separation. Adv. Colloid. Interface Sci. 2022, 303, 102635. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Xu, X.W.; Chen, F.Q.; Weng, H.F.; Chen, J.; Ru, Y.; Xiao, Q.; Xiao, A.F. Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Mar. Drugs 2023, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Jarosz, A.; Kapusta, O.; Gugała-Fekner, D.; Barczak, M. Synthesis and Characterization of Agarose Hydrogels for Release of Diclofenac Sodium. Materials 2023, 16, 6042. [Google Scholar] [CrossRef]
- Tako, M.; Nakamura, S. Gelation Mechanism of Agarose. Carbohydr. Res. 1988, 180, 277–284. [Google Scholar] [CrossRef]
- Sacco, P.; Piazza, F.; Marsich, E.; Abrami, M.; Grassi, M.; Donati, I. Ionic Strength Impacts the Physical Properties of Agarose Hydrogels. Gels 2024, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Y.; Tian, Y.; Liu, Z.J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef]
- Grolman, J.M.; Singh, M.; Mooney, D.J.; Eriksson, E.; Nuutila, K. Antibiotic-Containing Agarose Hydrogel for Wound and Burn Care. J. Burn. Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of Synthesis of Hydrogels … A Review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K.; Kumar Shukla, V. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of Alginate-Based Hydrogels: Crosslinking Strategies and Biomedical Applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Arora, S. Recent Advancements in Alginate Hydrogels: A Comprehensive Review. Darpan Int. Res. Anal. 2024, 12, 120–130. [Google Scholar] [CrossRef]
- Donati, I.; Christensen, B.E. Alginate-Metal Cation Interactions: Macromolecular Approach. Carbohydr. Polym. 2023, 321, 121280. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Lan, S.F.; Starly, B. Alginate Based 3D Hydrogels as an in Vitro Co-Culture Model Platform for the Toxicity Screening of New Chemical Entities. Toxicol. Appl. Pharmacol. 2011, 256, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Xiao, M.; Ma, J.; Qu, Y.; Zou, L.; Zhang, J. Nano-in-Micro Alginate/Chitosan Hydrogel via Electrospray Technology for Orally Curcumin Delivery to Effectively Alleviate Ulcerative Colitis. Mater. Des. 2022, 221, 110894. [Google Scholar] [CrossRef]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels 2023, 9, 533. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, S.; Hu, Q.; Zhang, J.; King, J.A.; Wang, Y.; Wei, Z.; Lu, J.; He, Z.; Kong, X.; et al. Injectable Zwitterionic Physical Hydrogel with Enhanced Chemodynamic Therapy and Tumor Microenvironment Remodeling Properties for Synergistic Anticancer Therapy. ACS Nano 2023, 17, 24883–24900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. B Eng. 2022, 238. [Google Scholar] [CrossRef]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Sarker, M.D.; Sharma, N.K.; Barhoumi, Z.; Chen, X. Printability of 3D Printed Hydrogel Scaffolds: Influence of Hydrogel Composition and Printing Parameters. Appl. Sci. 2020, 10, 292. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From Discovery and ECM Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliv. Rev. 2014, 79, 3–18. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, L.; Li, Y.; Gu, X.; Liu, Z.; Han, X.; Li, W.; Ma, W. Activating the Healing Process: Three-Dimensional Culture of Stem Cells in Matrigel for Tissue Repair. BMC Biotechnol. 2024, 24, 36. [Google Scholar] [CrossRef]
- Lingard, E.; Dong, S.; Hoyle, A.; Appleton, E.; Hales, A.; Skaria, E.; Lawless, C.; Taylor-Hearn, I.; Saadati, S.; Chu, Q.; et al. Optimising a Self-Assembling Peptide Hydrogel as a Matrigel Alternative for 3-Dimensional Mammary Epithelial Cell Culture. Biomater. Adv. 2024, 160, 213847. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue Extracellular Matrix Hydrogels as Alternatives to Matrigel for Culturing Gastrointestinal Organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Song, J.; Lee, Y.; Choi, N.; Kim, H.N. Understanding Organotropism in Cancer Metastasis Using Microphysiological Systems. Lab. Chip 2024, 24, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Godbey, W.T.; McPherson, G.L.; John, V.T. Microgel, Nanogel and Hydrogel-Hydrogel Semi-IPN Composites for Biomedical Applications: Synthesis and Characterization. Colloid. Polym. Sci. 2006, 284, 1121–1129. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Cona, C.; Bailey, K.; Barker, E. Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels. Polymers 2024, 16, 2050. [Google Scholar] [CrossRef] [PubMed]
- Marzini Irranca, S.; García Schejtman, S.D.; Rosso, A.P.; Coronado, E.A.; Martinelli, M. Hybrid Nanogels by Direct Mixing of Chitosan, Tannic Acid and Magnetite Nanoparticles: Processes Involved in Their Formation and Potential Catalytic Properties. Soft Matter 2023, 19, 8378–8385. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid Hydrogels for Biomedical Applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Yin, R.; Tarnsangpradit, J.; Gul, A.; Jeong, J.; Hu, X.; Zhao, Y.; Wu, H.; Li, Q.; Fytas, G.; Karim, A.; et al. Organic Nanoparticles with Tunable Size and Rigidity by Hyperbranching and Cross-Linking Using Microemulsion ATRP. Proc. Natl. Acad. Sci. USA 2024, 121. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, A.; Sentoukas, T.; Selianitis, D.; Balafouti, A.; Pispas, S. Using RAFT Polymerization Methodologies to Create Branched and Nanogel-Type Copolymers. Materials 2024, 17, 1947. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Cong, Y.; Liu, L.; Li, L. Free Radical Polymerization of Gold Nanoclusters and Hydrogels for Cell Capture and Light-Controlled Release. ACS Appl. Mater. Interfaces 2021, 13, 19360–19368. [Google Scholar] [CrossRef] [PubMed]
- Pandey, T.; Sharma, A.; Sandhu, A.; Pandey, V. Controlled Polymerization in Microfluidics: Advancement in Nanogel Synthesis. SPE Polym. 2024, 5, 113–126. [Google Scholar] [CrossRef]
- Bassenheim, D.; Mitterbauer, M.; Liska, R.; Knaack, P. Novel Thermal Initiator Systems for Radical Induced Cationic Frontal Polymerization. Polym. Chem. 2024, 15, 2229–2234. [Google Scholar] [CrossRef]
- Tymetska, S.; Shymborska, Y.; Stetsyshyn, Y.; Budkowski, A.; Bernasik, A.; Awsiuk, K.; Donchak, V.; Raczkowska, J. Thermoresponsive Smart Copolymer Coatings Based on P(NIPAM-Co-HEMA) and P(OEGMA-Co-HEMA) Brushes for Regenerative Medicine. ACS Biomater. Sci. Eng. 2023, 9, 6256–6272. [Google Scholar] [CrossRef]
- Shymborska, Y.; Stetsyshyn, Y.; Awsiuk, K.; Raczkowska, J.; Bernasik, A.; Janiszewska, N.; Da̧bczyński, P.; Kostruba, A.; Budkowski, A. Temperature- and PH-Responsive Schizophrenic Copolymer Brush Coatings with Enhanced Temperature Response in Pure Water. ACS Appl. Mater. Interfaces 2023, 15, 8676–8690. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-Layer Assembled Polymeric Thin Films as Prospective Drug Delivery Carriers: Design and Applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef]
- Liu, Y.; Bethel, K.; Singh, M.; Zhang, J.; Ashkar, R.; Davis, E.M.; Johnson, B.N. Comparison of Bulk- vs Layer-by-Layer-Cured Stimuli-Responsive PNIPAM–Alginate Hydrogel Dynamic Viscoelastic Property Response via Embedded Sensors. ACS Appl. Polym. Mater. 2022, 4, 5596–5607. [Google Scholar] [CrossRef]
- Lee, U.N.; Day, J.H.; Haack, A.J.; Bretherton, R.C.; Lu, W.; Deforest, C.A.; Theberge, A.B.; Berthier, E. Layer-by-Layer Fabrication of 3D Hydrogel Structures Using Open Microfluidics. Lab. Chip 2020, 20, 525–536. [Google Scholar] [CrossRef]
- Xu, R.; Hua, M.; Wu, S.; Ma, S.; Zhang, Y.; Zhang, L.; Yu, B.; Cai, M.; He, X.; Zhou, F. Continuously Growing Multi-Layered Hydrogel Structures with Seamless Interlocked Interface. Matter 2022, 5, 634–653. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite Nanoparticles: Synthesis Methods—A Comparative Review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef]
- Wang, T.; Ding, J.; Chen, Z.; Zhang, Z.; Rong, Y.; Li, G.; He, C.; Chen, X. Injectable, Adhesive Albumin Nanoparticle-Incorporated Hydrogel for Sustained Localized Drug Delivery and Efficient Tumor Treatment. ACS Appl. Mater. Interfaces 2024, 16, 9868–9879. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, C.; Wu, Q.; Xie, W.; Kim, W.-Y.; Lee, S.-Y.; Gwon, J. A Stretchable Solid-State Zinc Ion Battery Based on a Cellulose Nanofiber–Polyacrylamide Hydrogel Electrolyte and a Mg0.23V2O5·1.0H2O Cathode. J. Mater. Chem. A Mater. 2020, 8, 18327–18337. [Google Scholar] [CrossRef]
- Newham, G.; Evans, S.D.; Ong, Z.Y. Mechanically Tuneable Physical Nanocomposite Hydrogels from Polyelectrolyte Complex Templated Silica Nanoparticles for Anionic Therapeutic Delivery. J. Colloid. Interface Sci. 2022, 617, 224–235. [Google Scholar] [CrossRef]
- Manno, M.; Emanuele, A.; Martorana, V.; Bulone, D.; San Biagio, P.L.; Palma-Vittorelli, M.B.; Palma, M.U. Multiple Interactions between Molecular and Supramolecular Ordering. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1999, 59, 2222–2230. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Xie, C.; Liu, G.; Wang, L.; Yang, Q.; Liao, F.; Yang, X.; Xiao, B.; Duan, L. Synthesis and Properties of Injectable Hydrogel for Tissue Filling. Pharmaceutics 2024, 16, 430. [Google Scholar] [CrossRef]
- Wei, L.; Wang, X.; Fu, J.; Yin, J.; Hu, J. A Physically Cross-Linked Double Network Polysaccharides/Ca2+ Hydrogel Scaffold for Skeletal Muscle Tissue Engineering. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131410. [Google Scholar] [CrossRef]
- Koehler, K.C.; Anseth, K.S.; Bowman, C.N. Diels-Alder Mediated Controlled Release from a Poly(Ethylene Glycol) Based Hydrogel. Biomacromolecules 2013, 14, 538–547. [Google Scholar] [CrossRef]
- Mohammed, M.; Devnarain, N.; Elhassan, E.; Govender, T. Exploring the Applications of Hyaluronic Acid-Based Nanoparticles for Diagnosis and Treatment of Bacterial Infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1799. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Ren, X.; Gao, G. The Role of Chemical and Physical Crosslinking in Different Deformation Stages of Hybrid Hydrogels. Eur. Polym. J. 2018, 100, 86–95. [Google Scholar] [CrossRef]
- Le Goff, G.C.; Srinivas, R.L.; Hill, W.A.; Doyle, P.S. Hydrogel Microparticles for Biosensing. Eur. Polym. J. 2015, 72, 386–412. [Google Scholar] [CrossRef]
- Zhai, M.; Wu, P.; Liao, Y.; Wu, L.; Zhao, Y. Polymer Microspheres and Their Application in Cancer Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 6556. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.T.; Wang, C.; Wang, D.A. Cell Delivery with Genipin Crosslinked Gelatin Microspheres in Hydrogel/Microcarrier Composite. Compos. Sci. Technol. 2010, 70, 1909–1914. [Google Scholar] [CrossRef]
- Azadi, S.; Osanloo, M.; Zarenezhad, E.; Farjam, M.; Jalali, A.; Ghanbariasad, A. Nano-Scaled Emulsion and Nanogel Containing Mentha Pulegium Essential Oil: Cytotoxicity on Human Melanoma Cells and Effects on Apoptosis Regulator Genes. BMC Complement. Med. Ther. 2023, 23, 6. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, F.; Cai, J. Advances in the Delivery of Bioactive Compounds Using Starch-Based Nanogels: Formation Mechanism, Contemporary Challenges, and Prospects. J. Agric. Food Res. 2024, 16, 101142. [Google Scholar] [CrossRef]
- Wang, J.; Viola, M.; Migliorini, C.; Paoletti, L.; Arpicco, S.; Di Meo, C.; Matricardi, P. Polysaccharide-Based Nanogels to Overcome Mucus, Skin, Cornea, and Blood-Brain Barriers: A Review. Pharmaceutics 2023, 15, 2508. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Singh, C.; Kaushik, A.; Saleem, S.; Kumar, A. Recent Advances in Stimuli-Responsive Tailored Nanogels for Cancer Therapy; from Bench to Personalized Treatment. J. Mater. Chem. B 2023, 12, 382–412. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and Crosslinking Strategies for Hyaluronic Acid-based Hydrogel Biomaterials. Smart Med. 2023, 2, e20230029. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Forouz, F.; Hipwood, L.; Clegg, J.; Jeffery, P.; Gough, M.; van Wyngaard, T.; Pyke, C.; Adams, M.N.; Bray, L.J.; et al. GelMA, Click-Chemistry Gelatin and Bioprinted Polyethylene Glycol-Based Hydrogels as 3D Ex Vivo Drug Testing Platforms for Patient-Derived Breast Cancer Organoids. Pharmaceutics 2023, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Polo Fonseca, L.; Trinca, R.B.; Felisberti, M.I. Amphiphilic Polyurethane Hydrogels as Smart Carriers for Acidic Hydrophobic Drugs. Int. J. Pharm. 2018, 546, 106–114. [Google Scholar] [CrossRef]
- Wang, R.; Liu, L.; He, X.; Xia, Z.; Zhao, Z.; Xi, Z.; Yu, J.; Wang, J. Dynamic Crosslinked Injectable Mussel-Inspired Hydrogels with Adhesive, Self-Healing, and Biodegradation Properties. Polymers 2023, 15, 1876. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Shepherd, D.V.; Shepherd, J.H.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M. The Process of EDC-NHS Cross-Linking of Reconstituted Collagen Fibres Increases Collagen Fibrillar Order and Alignment. APL Mater. 2015, 3, 14902. [Google Scholar] [CrossRef]
- Palazon, F.; Montenegro Benavides, C.; Léonard, D.; Souteyrand, É.; Chevolot, Y.; Cloarec, J.P. Carbodiimide/NHS Derivatization of COOH-Terminated SAMs: Activation or Byproduct Formation? Langmuir 2014, 30, 4545–4550. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Yokozawa, T. Converting Step-Growth to Chain-Growth Condensation Polymerization. Macromolecules 2007, 40, 4093–4101. [Google Scholar] [CrossRef]
- Hua, J.; Li, Z.; Xia, W.; Yang, N.; Gong, J.; Zhang, J.; Qiao, C. Preparation and Properties of EDC/NHS Mediated Crosslinking Poly (Gamma-Glutamic Acid)/Epsilon-Polylysine Hydrogels. Mater. Sci. Eng. C 2016, 61, 879–892. [Google Scholar] [CrossRef]
- He, W.; Chen, K.; Gao, W.; Duan, R.; Li, Z.; Li, B.; Xia, J.; Zhao, Y.; Liu, W.; Zhou, H.; et al. A Sequential Physical and Chemical Dual Crosslinked Multifunctional Hydrogel with Enhanced Mechanical and Osteogenic Effects for Vascularized Bone Tissue Regeneration. Mater. Des. 2024, 237, 112563. [Google Scholar] [CrossRef]
- Nishi, K.; Akizuki, S.; Toda, T.; Matsuyama, T.; Ida, J. Advanced Light-Tolerant Microalgae-Nitrifying Bacteria Consortia for Stable Ammonia Removal under Strong Light Irradiation Using Light-Shielding Hydrogel. Chemosphere 2022, 297, 134252. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Hepatic, Metabolic and Toxic Effects of Ethanol: 1991 Update. Alcohol. Clin. Exp. Res. 1991, 15, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Fielding, L.A. PH-Responsive Nanogels Generated by Polymerization-Induced Self-Assembly of a Succinate-Functional Monomer. Macromolecules 2024, 57, 3496–3501. [Google Scholar] [CrossRef]
- Grubbs, R.B.; Grubbs, R.H. 50th Anniversary Perspective: Living Polymerization—Emphasizing the Molecule in Macromolecules. Macromolecules 2017, 50, 6979–6997. [Google Scholar] [CrossRef]
- Ivchenko, P.V. Controlled Polymerization. Polymers 2023, 15, 1379. [Google Scholar] [CrossRef] [PubMed]
- Koide, H.; Okishima, A.; Hoshino, Y.; Kamon, Y.; Yoshimatsu, K.; Saito, K.; Yamauchi, I.; Ariizumi, S.; Zhou, Y.; Xiao, T.H.; et al. Synthetic Hydrogel Nanoparticles for Sepsis Therapy. Nat. Commun. 2021, 12, 5552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Yang, J.J.; Osada, Y.; Gong, J.P. Synthetic Hydrogels as Scaffolds for Manipulating Endothelium Cell Behaviors. Chin. J. Polym. Sci. (Engl. Ed.) 2011, 29, 23–41. [Google Scholar] [CrossRef]
- Wu, G.H.; Hsu, S.H. Review: Polymeric-Based 3D Printing for Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar] [CrossRef]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly Efficient Water-Soluble Visible Light Photoinitiators. J. Polym. Sci. Part. A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Ghazali, H.S.; Askari, E.; Seyfoori, A.; Naghib, S.M. A High-Absorbance Water-Soluble Photoinitiator Nanoparticle for Hydrogel 3D Printing: Synthesis, Characterization and in Vitro Cytotoxicity Study. Sci. Rep. 2023, 13, 8577. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Soleimani Rad, J. Chemical Gelling of Hydrogels-Based Biological Macromolecules for Tissue Engineering: Photo- and Enzymatic-Crosslinking Methods. Int. J. Biol. Macromol. 2019, 139, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Shan, J.; Gong, X.; Sun, Y.; Sang, L.; Ding, X.; Zhou, H.; Yu, M. GelMA Hydrogel: A Game-Changer in 3D Tumor Modeling. Mater. Today Chem. 2024, 38, 102111. [Google Scholar] [CrossRef]
- Tomal, W.; Petko, F.; Galek, M.; Świeży, A.; Tyszka-Czochara, M.; Środa, P.; Mokrzyński, K.; Ortyl, J. Water-Soluble Type I Radical Photoinitiators Dedicated to Obtaining Microfabricated Hydrogels. Chem. Mater. 2024, 36, 6421–6439. [Google Scholar] [CrossRef]
- Pyszka, I.; Jędrzejewska, B. Design of Dyes Based on the Quinoline or Quinoxaline Skeleton towards Visible Light Photoinitiators. Int. J. Mol. Sci. 2024, 25, 4289. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, A.; Surapaneni, S.K.; Huang, J.; Mondal, A.; Wang, V.Z.; Haruna, N.F.; Bagde, A.; Arthur, P.; Kutlehria, S.; Patel, N.; et al. Polysaccharide Hydrogel Based 3D Printed Tumor Models for Chemotherapeutic Drug Screening. Sci. Rep. 2021, 11, 372. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Hsu, S.-h. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Morozova, S.M. Recent Advances in Hydrogels via Diels–Alder Crosslinking: Design and Applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.G.; Hill, R.K. Stereochemistry of the Diels-Alder Reaction. Chem. Rev. 1961, 61, 537–562. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D.L. Microfluidic Synthesis of Lipid-Based Nanoparticles for Drug Delivery: Recent Advances and Opportunities. Lab. Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Oveysi, M.; Rezvani, A.; Karim Khani, M.M.; Bazargan, V.; Nejat, A.; Varshochian, R.; Marengo, M. Size Prediction of Drug-Loaded Polymeric (PLGA) Microparticles Prepared by Microfluidics. J. Drug Deliv. Sci. Technol. 2024, 98, 105776. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New Progress and Prospects: The Application of Nanogel in Drug Delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Kendre, P.N.; Satav, T.S. Current Trends and Concepts in the Design and Development of Nanogel Carrier Systems. Polym. Bull. 2019, 76, 1595–1617. [Google Scholar] [CrossRef]
- Han, J.Y.; La Fiandra, J.N.; DeVoe, D.L. Microfluidic Vortex Focusing for High Throughput Synthesis of Size-Tunable Liposomes. Nat. Commun. 2022, 13, 6997. [Google Scholar] [CrossRef]
- Han, J.Y.; Chen, Z.; Devoe, D.L. Scalable Liposome Synthesis by High Aspect Ratio Microfluidic Flow Focusing. Methods Mol. Biol. 2023, 2622, 87–93. [Google Scholar] [CrossRef]
- Sanati, M.; Amin Yavari, S. Liposome-Integrated Hydrogel Hybrids: Promising Platforms for Cancer Therapy and Tissue Regeneration. J. Control. Release 2024, 368, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Tran, V.T.; Mredha, M.T.I.; Na, J.Y.; Seon, J.-K.; Jeon, I. Thin-Film Hydrogels with Superior Stiffness, Strength, and Stretchability. Extrem. Mech. Lett. 2020, 37, 100720. [Google Scholar] [CrossRef]
- Cheng, S.; Lou, Z.; Zhang, L.; Guo, H.; Wang, Z.; Guo, C.; Fukuda, K.; Ma, S.; Wang, G.; Someya, T.; et al. Ultrathin Hydrogel Films toward Breathable Skin-Integrated Electronics. Adv. Mater. 2023, 35, 2206793. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Ma, C.; Tang, H.; Hao, H.; Li, M.; Luo, X.; Yang, M.; Gao, L.; Li, J. Advances in Hydrogels of Drug Delivery Systems for the Local Treatment of Brain Tumors. Gels 2024, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C.L. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef] [PubMed]
- Rivera López, E.; Samaniego López, C.; Spagnuolo, C.C.; Berardino, B.G.; Alaimo, A.; Pérez, O.E. Chitosan-Tricarbocyanine-Based Nanogels Were Able to Cross the Blood–Brain Barrier Showing Its Potential as a Targeted Site Delivery Agent. Pharmaceutics 2024, 16, 964. [Google Scholar] [CrossRef]
- Singh, A.; Poling, H.M.; Spence, J.R.; Wells, J.M.; Helmrath, M.A. Gastrointestinal Organoids: A next-Generation Tool for Modeling Human Development. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G375. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, C.; Xiao, C.; Ding, J.; Zhuang, X.; Huang, Y.; Chen, X. Decisive Role of Hydrophobic Side Groups of Polypeptides in Thermosensitive Gelation. Biomacromolecules 2012, 13, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Santhamoorthy, M.; Phan, T.T.V.; Kim, S.C. PNIPAm-Based PH and Thermoresponsive Copolymer Hydrogel for Hydrophobic and Hydrophilic Drug Delivery. Gels 2024, 10, 184. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical Properties of PNIPAM Based Hydrogels: A Review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable Hydrogels Delivering Therapeutic Agents for Disease Treatment and Tissue Engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef]
- Hrubý, M. Thermoresponsive Polymers as Promising New Materials for Local Radiotherapy. Appl. Radiat. Isot. 2005, 63, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Bubli, S.Y.; Smolag, M.; Blackwell, E.; Lin, Y.C.; Tsavalas, J.G.; Li, L. Inducing an LCST in Hydrophilic Polysaccharides via Engineered Macromolecular Hydrophobicity. Sci. Rep. 2023, 13, 14896. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Zahid, A.A.; Mraiche, F.; Alam, K.; Al Moustafa, A.E.; Hasan, A. Gelatin-Methacryloyl Hydrogel Based in Vitro Blood–Brain Barrier Model for Studying Breast Cancer-Associated Brain Metastasis. Pharm. Dev. Technol. 2021, 26, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Günter, E.A.; Melekhin, A.K.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. Preparation, Physicochemical Characterization and Swelling Properties of Composite Hydrogel Microparticles Based on Gelatin and Pectins with Different Structure. Int. J. Biol. Macromol. 2024, 258, 128935. [Google Scholar] [CrossRef]
- Rizwan, A.; Ali, I.; Jo, S.H.; Vu, T.T.; Gal, Y.S.; Kim, Y.H.; Park, S.H.; Lim, K.T. Facile Fabrication of NIR-Responsive Alginate/CMC Hydrogels Derived through IEDDA Click Chemistry for Photothermal–Photodynamic Anti-Tumor Therapy. Gels 2023, 9, 961. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.-M.; Kim, M.; Nam, J.-M.; Lee, J.-H. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.R.; Song, Y.J.; Lee, N.Y. Polydopamine-Mediated Gold Nanoparticle Coating Strategy and Its Application in Photothermal Polymerase Chain Reaction. Lab. Chip 2025. [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Lee, H.; Lee, S.; Lee, T.S. Improving Cancer Chemotherapy through Photothermally Triggered Drug Release from Poly(N-Isopropylacrylamide-Co-Acrylic Acid)/Prussian Blue Hydrogel. Mater. Des. 2023, 233, 112243. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, S.P.; Liao, A.H.; Yang, Y.C.; Lee, C.R.; Wu, C.H.; Wu, P.C.; Liu, T.M.; Wang, C.R.C.; Li, P.C. Synergistic Delivery of Gold Nanorods Using Multifunctional Microbubbles for Enhanced Plasmonic Photothermal Therapy. Sci. Rep. 2014, 4, 5685. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A.; Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed]
- Ruskowitz, E.R.; Deforest, C.A. Photoresponsive Biomaterials for Targeted Drug Delivery and 4D Cell Culture. Nat. Rev. Mater. 2018, 3, 17087. [Google Scholar] [CrossRef]
- Meeks, A.; Lerch, M.M.; Schroeder, T.B.H.; Shastri, A.; Aizenberg, J. Spiropyran Photoisomerization Dynamics in Multiresponsive Hydrogels. J. Am. Chem. Soc. 2022, 144, 219–227. [Google Scholar] [CrossRef]
- Bai, Z.; Guo, L.; Zhao, D.; Wang, Y. Photochromic Spiropyran-Based Dual-Emitting Luminescent Hybrid Films for Dynamic Information Anticounterfeiting. ACS Appl. Mater. Interfaces 2024, 16, 44018–44025. [Google Scholar] [CrossRef] [PubMed]
- Beninatto, R.; Barbera, C.; De Lucchi, O.; Borsato, G.; Serena, E.; Guarise, C.; Pavan, M.; Luni, C.; Martewicz, S.; Galesso, D.; et al. Photocrosslinked Hydrogels from Coumarin Derivatives of Hyaluronic Acid for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 96, 625–634. [Google Scholar] [CrossRef]
- Azagarsamy, M.A.; McKinnon, D.D.; Alge, D.L.; Anseth, K.S. Coumarin-Based Photodegradable Hydrogel: Design, Synthesis, Gelation, and Degradation Kinetics. ACS Macro Lett. 2014, 3, 515–519. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Do, H.D.K.; Nam, N.N.; Dan, T.T.; Trinh, K.T.L.; Lee, N.Y. Droplet-Based Microfluidics: Applications in Pharmaceuticals. Pharmaceuticals 2023, 16, 937. [Google Scholar] [CrossRef]
- Tutoni, G.G.; McDonald, S.M.; Zhong, R.; Lu, A.; Huang, T.J.; Becker, M.L. Microfluidic Assembly of Degradable, Stereocomplexed Hydrogel Microparticles. J. Am. Chem. Soc. 2024, 146, 14705–14714. [Google Scholar] [CrossRef]
- Duncanson, W.J.; Lin, T.; Abate, A.R.; Seiffert, S.; Shah, R.K.; Weitz, D.A. Microfluidic Synthesis of Advanced Microparticles for Encapsulation and Controlled Release. Lab. Chip 2012, 12, 2135–2145. [Google Scholar] [CrossRef]
- Long, F.; Guo, Y.; Zhang, Z.; Wang, J.; Ren, Y.; Cheng, Y.; Xu, G. Recent Progress of Droplet Microfluidic Emulsification Based Synthesis of Functional Microparticles. Glob. Chall. 2023, 7, 2300063. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jin, S.; Zhang, C.; Ren, J.; Jing, W.; Wei, X. Microfluidics-Assisted Synthesis of Hydrogel Microparticles with Acoustic-Magnetic Control. Chem. Eng. Sci. 2023, 281, 119082. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Kim, E.; Nam, C.; Choi, Y.; Lee, Y.-J.; Weitz, D.A.; Lee, H.; Choi, C.-H. Hydrogel Microcapsules with a Thin Oil Layer: Smart Triggered Release via Diverse Stimuli. Adv. Funct. Mater. 2021, 31, 2009553. [Google Scholar] [CrossRef]
- Yuan, M.; Na, X.; Liu, J.; Zhang, Y.; Wei, Y.; Ma, G. Preparation, Characterization and in Vivo Efficacy Evaluation of Ropivacaine O/W Emulsion by Premix Membrane Emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128316. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Preparation of Microparticles and Nanoparticles Using Membrane-Assisted Dispersion, Micromixing, and Evaporation Processes. Particuology 2024, 84, 30–44. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Russo, T.; Toto, E.; Santonicola, M.G. Fabrication of Alginate/Ozoile Gel Microspheres by Electrospray Process. Gels 2024, 10, 52. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, L.; Doyle, P.S. Nanoemulsion-Loaded Capsules for Controlled Delivery of Lipophilic Active Ingredients. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Y.; Zhou, Y.; Islam, K.; Liu, Y. Microfluidic Droplet-Assisted Fabrication of Vessel-Supported Tumors for Preclinical Drug Discovery. ACS Appl. Mater. Interfaces 2023, 15, 15152–15161. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.M.; Razzaghi, A.; Ramachandran, A.; Mikkonen, K.S. Emulsion Characterization via Microfluidic Devices: A Review on Interfacial Tension and Stability to Coalescence. Adv. Colloid. Interface Sci. 2022, 299, 102541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Z.; Cai, Y.; Li, H.Y. Chitosan-Based Spray-Dried Mucoadhesive Microspheres for Sustained Oromucosal Drug Delivery. Powder Technol. 2017, 312, 124–132. [Google Scholar] [CrossRef]
- Li, W.; Wei, H.; Liu, Y.; Li, S.; Wang, G.; Guo, T.; Han, H. An in Situ Reactive Spray-Drying Strategy for Facile Preparation of Starch-Chitosan Based Hydrogel Microspheres for Water Treatment Application. Chem. Eng. Process.—Process Intensif. 2021, 168, 108548. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Russo, T.; Vella, S.; Toto, E.; Santonicola, M.G. Electrospray Fabrication of PH-Responsive Microspheres for the Delivery of Ozoile. Macromol. Symp. 2024, 413, 2400031. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Placido, M.; Toto, E.; Santonicola, M.G. Dual-Responsive Alginate/PNIPAM Microspheres Fabricated by Microemulsion-Based Electrospray. Polymers 2024, 16, 2765. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Sato, A.C.K. Sonication Technique to Produce Emulsions: The Impact of Ultrasonic Power and Gelatin Concentration. Ultrason. Sonochem. 2019, 52, 286–293. [Google Scholar] [CrossRef]
- Widnersson, I.; Teo, B.M.; Ashokkumar, M.; Grieser, F. Sonochemical Synthesis and Characterisation of Thermoresponsive Microgel Particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 342–348. [Google Scholar] [CrossRef]
- Pérez-González, J.; Muñoz-Castro, Y.; Rodríguez-González, F.; Marín-Santibáñez, B.M.; Medina-Bañuelos, E.F. Influence of Sonication on the Molecular Characteristics of Carbopol® and Its Rheological Behavior in Microgels. Gels 2024, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Mok, H.; Park, T.G. Synthesis, Characterization, and Intracellular Delivery of Reducible Heparin Nanogels for Apoptotic Cell Death. Biomaterials 2008, 29, 3376–3383. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Weiss, J.; Hulbert, G.J.; Mount, J. Ultrasonic Processing Influences Rheological and Optical Properties of High-Methoxyl Pectin Dispersions. Food Hydrocoll. 2003, 17, 191–197. [Google Scholar] [CrossRef]
- Srivastava, S.; Saha, S.; Jakhmola, V. Nanogel: Types, Methods of Preparation, Limitation, Evaluation and Application—A Systematic Review. Int. J. Drug Deliv. Technol. 2023, 13, 1631–1639. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, R.; Gou, Q.; Lai, J.; Li, X. Precipitation Polymerization: A Powerful Tool for Preparation of Uniform Polymer Particles. Polymers 2022, 14, 1851. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A Review on Microfluidic-Assisted Nanoparticle Synthesis, and Their Applications Using Multiscale Simulation Methods. Discover Nano 2023, 18, 18. [Google Scholar] [CrossRef]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; Devoe, D.L.; Gaitan, M. Microfluidic Mixing and the Formation of Nanoscale Lipid Vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Uno, S.; Niwa, A.; Okada, Y.; Tokeshi, M. Microfluidic Technologies and Devices for Lipid Nanoparticle-Based RNA Delivery. J. Control Release 2022, 344, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. One-Step Production Using a Microfluidic Device of Highly Biocompatible Size-Controlled Noncationic Exosome-like Nanoparticles for RNA Delivery. ACS Appl. Bio Mater. 2021, 4, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Fujishima, Y.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. Understanding the Formation Mechanism of Lipid Nanoparticles in Microfluidic Devices with Chaotic Micromixers. PLoS ONE 2017, 12, e0187962. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Kupikowska-Stobba, B.; Kasprzak, M. Fabrication of Nanoparticles for Bone Regeneration: New Insight into Applications of Nanoemulsion Technology. J. Mater. Chem. B 2021, 9, 5221–5244. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Vreeland, W.N.; Lacerda, S.H.D.P.; Locascio, L.E.; Gaitan, M.; Raghavan, S.R. Liposome-Templated Supramolecular Assembly of Responsive Alginate Nanogels. Langmuir 2008, 24, 4092–4096. [Google Scholar] [CrossRef] [PubMed]
- Duché, G.; Heu, C.; Thordarson, P. Development and Characterization of Nanoscale Gel-Core Liposomes Using a Short Self-Assembled Peptide Hydrogel: Implications for Drug Delivery. ACS Appl. Nano Mater. 2023, 6, 14745–14755. [Google Scholar] [CrossRef]
- Hong, J.; Char, K.; Kim, B.-S. Hollow Capsules of Reduced Graphene Oxide Nanosheets Assembled on a Sacrificial Colloidal Particle. J. Phys. Chem. Lett. 2010, 1, 3442–3445. [Google Scholar] [CrossRef]
- Li, B.; Yang, X.; Xia, L.; Majeed, M.I.; Tan, B. Hollow Microporous Organic Capsules. Sci. Rep. 2013, 3, 2128. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Alimohammadvand, S.; Kaveh Zenjanab, M.; Mashinchian, M.; Shayegh, J.; Jahanban-Esfahlan, R. Recent Advances in Biomimetic Cell Membrane–Camouflaged Nanoparticles for Cancer Therapy. Biomed. Pharmacother. 2024, 177, 116951. [Google Scholar] [CrossRef] [PubMed]
- Farasati Far, B.; Isfahani, A.A.; Nasiriyan, E.; Pourmolaei, A.; Mahmoudvand, G.; Karimi Rouzbahani, A.; Namiq Amin, M.; Naimi-Jamal, M.R. An Updated Review on Advances in Hydrogel-Based Nanoparticles for Liver Cancer Treatment. Livers 2023, 3, 161–189. [Google Scholar] [CrossRef]
- Goldberg, M.S. Improving Cancer Immunotherapy through Nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- He, W.; Zhang, Y.; Qu, Y.; Liu, M.; Li, G.; Pan, L.; Xu, X.; Shi, G.; Hao, Q.; Liu, F.; et al. Research Progress on Hydrogel-Based Drug Therapy in Melanoma Immunotherapy. BMB Rep. 2024, 57, 71–78. [Google Scholar] [CrossRef]
- Strom, T.J.; Wilder, R.B.; Fernandez, D.C.; Mellon, E.A.; Saini, A.S.; Hunt, D.C.; Pow-Sang, J.M.; Spiess, P.E.; Sexton, W.J.; Poch, M.A.; et al. A Dosimetric Study of Polyethylene Glycol Hydrogel in 200 Prostate Cancer Patients Treated with High-Dose Rate Brachytherapy ± Intensity Modulated Radiation Therapy. Radiother. Oncol. 2014, 111, 126–131. [Google Scholar] [CrossRef]
- Lazzaroni, S.; Liosi, G.M.; D’Agostino, G.; Marconi, R.P.; Mariani, M.; Buttafava, A.; Dondi, D. The Role of Hydrogels in the Radical Production of the Fricke-Gel-Dosimeter. Radiat. Phys. Chem. 2018, 142, 137–140. [Google Scholar] [CrossRef]
- Locarno, S.; Arosio, P.; Curtoni, F.; Piazzoni, M.; Pignoli, E.; Gallo, S. Microscopic and Macroscopic Characterization of Hydrogels Based on Poly(Vinyl-Alcohol)–Glutaraldehyde Mixtures for Fricke Gel Dosimetry. Gels 2024, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumacheva, E. Hydrogel Microenvironments for Cancer Spheroid Growth and Drug Screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, N.; Liu, B.; Li, S.; Zhang, H. Current Advances of 3D-Bioprinted Microfluidic Models with Hydrogel Bioinks and Their Applications in Drug Screening. Int. J. Bioprint 2024, 10, 172–196. [Google Scholar] [CrossRef]
- Royse, M.K.; Fowler, M.; Mai, A.K.; He, Y.; Durante, M.R.; Buist, N.; Procopio, A.; Xu, J.; Veiseh, O. Development of a 3D Printed Perfusable in Vitro Blood-Brain Barrier Model for Use as a Scalable Screening Tool. Biomater. Sci. 2024, 12, 4363–4375. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, K.N.; Enyedi, G.; Lajkó, E. Three-Dimensional, PEG-Based Hydrogels Induce Spheroid Formation and Enhance Viability of A2058 Melanoma Cells. FEBS Open Bio 2023, 13, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Luo, Y.; Hu, X.; Chu, J.; Li, S.; Hao, M.; Zhuang, J.; Liu, Y.; Gao, J.; Yin, Y. Emerging Hydrogel Therapies for Translating Brain Disease: Materials, Mechanisms, and Recent Research. Mater. Today Adv. 2024, 22, 100490. [Google Scholar] [CrossRef]
- Ahmad, N.; Kiriako, G.; Saliba, J.; Abla, K.; El-Sabban, M.; Mhanna, R. Engineering a 3D Biomimetic Peptides Functionalized-Polyethylene Glycol Hydrogel Model Cocultured with Endothelial Cells and Astrocytes: Enhancing In Vitro Blood-Brain Barrier Biomimicry. Mol. Pharm. 2024, 21, 4664–4672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
