Roasting Extraction of Lithium from Fly Ash: A Study of Influential Parameters and Mechanisms
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Pretreatment Procedures
2.2.1. Ultrasonic Treatment
2.2.2. Roasting Activation
2.3. Leaching Procedures
2.4. Analytical Methods
3. Results and Discussion
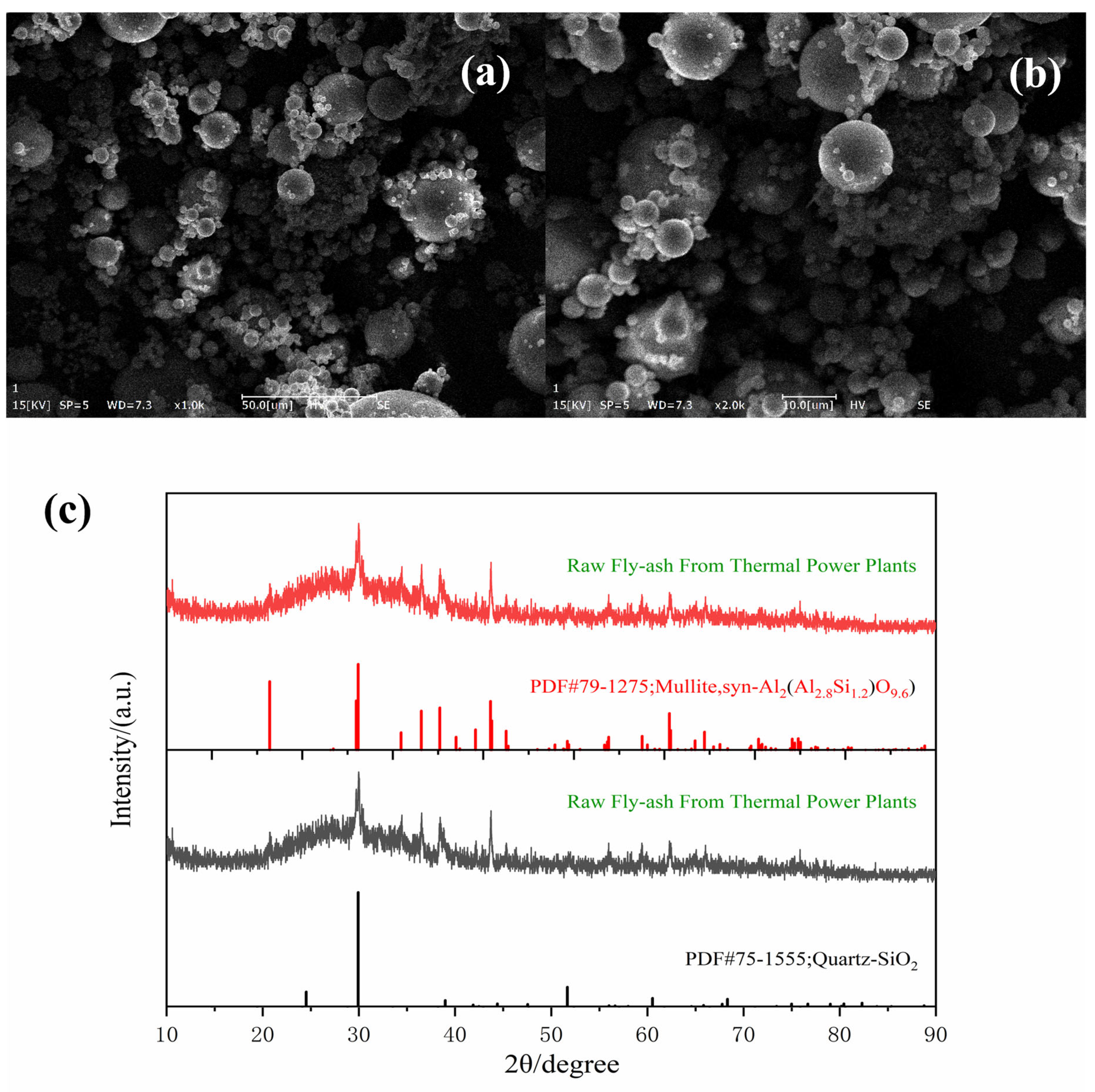
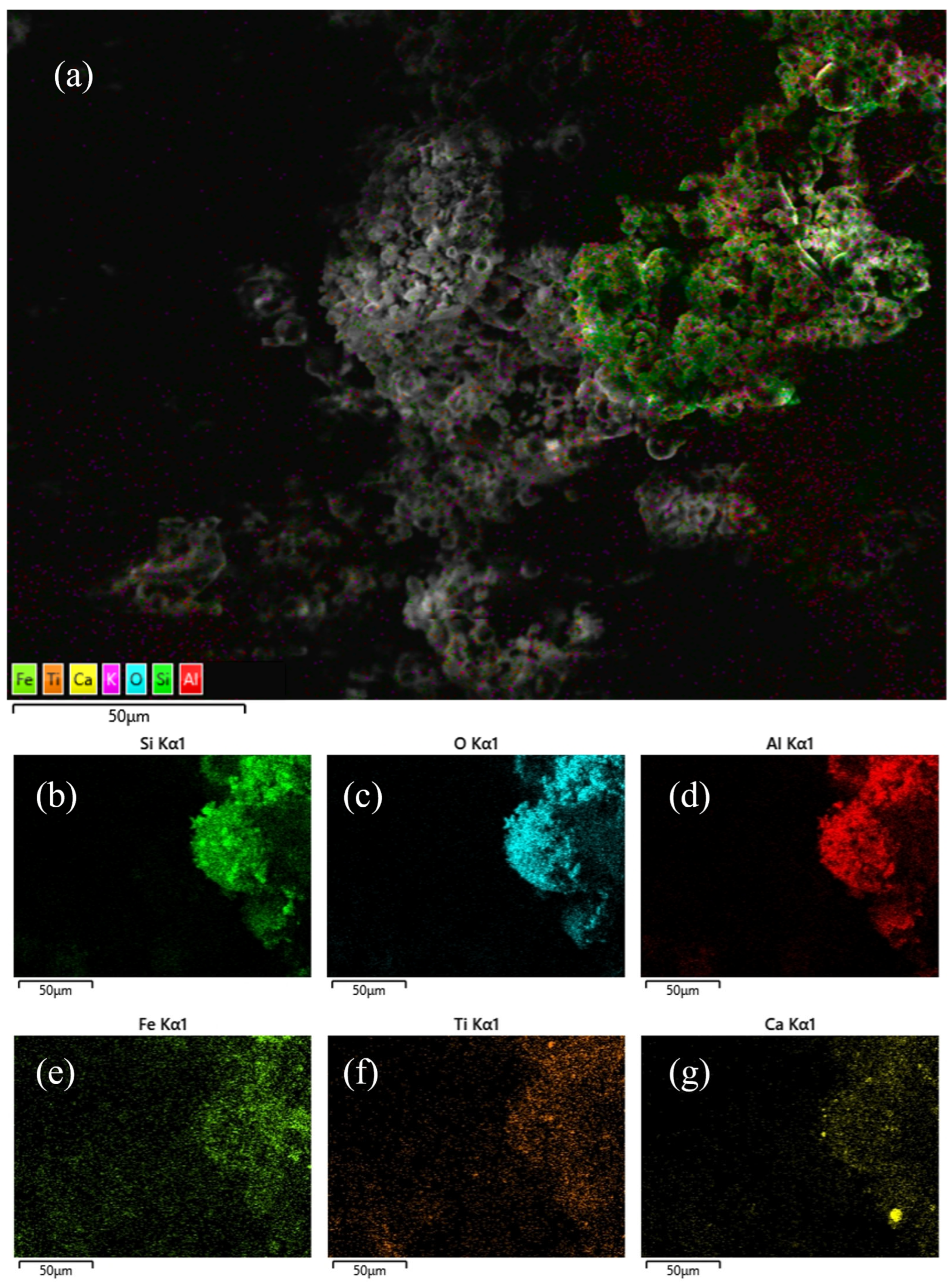
3.1. Characterization of Original Fly Ash
3.2. Roasting Effects
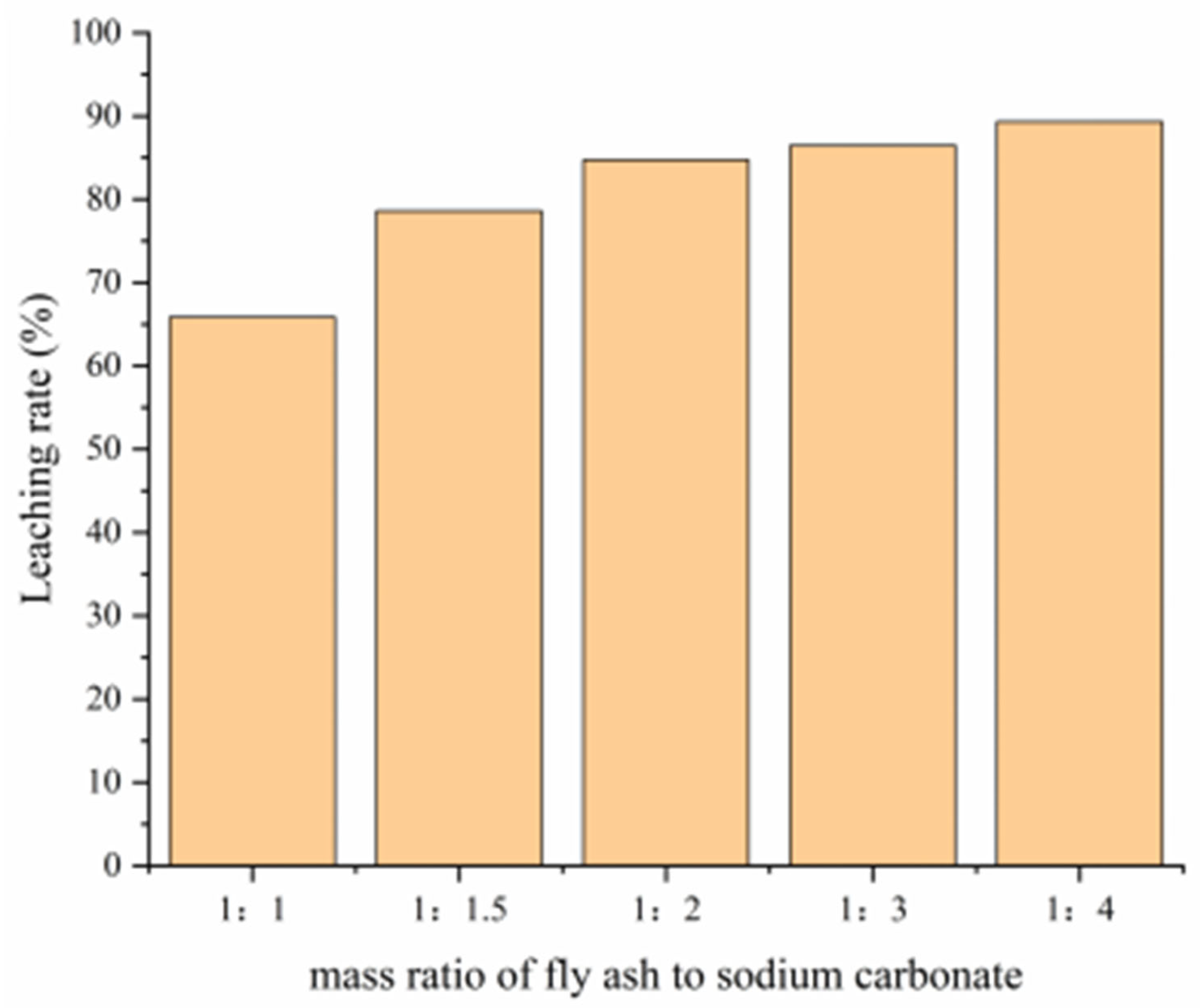
3.2.1. Sodium Carbonate Addition Ratio
3.2.2. Roasting Time
3.2.3. Roasting Analysis
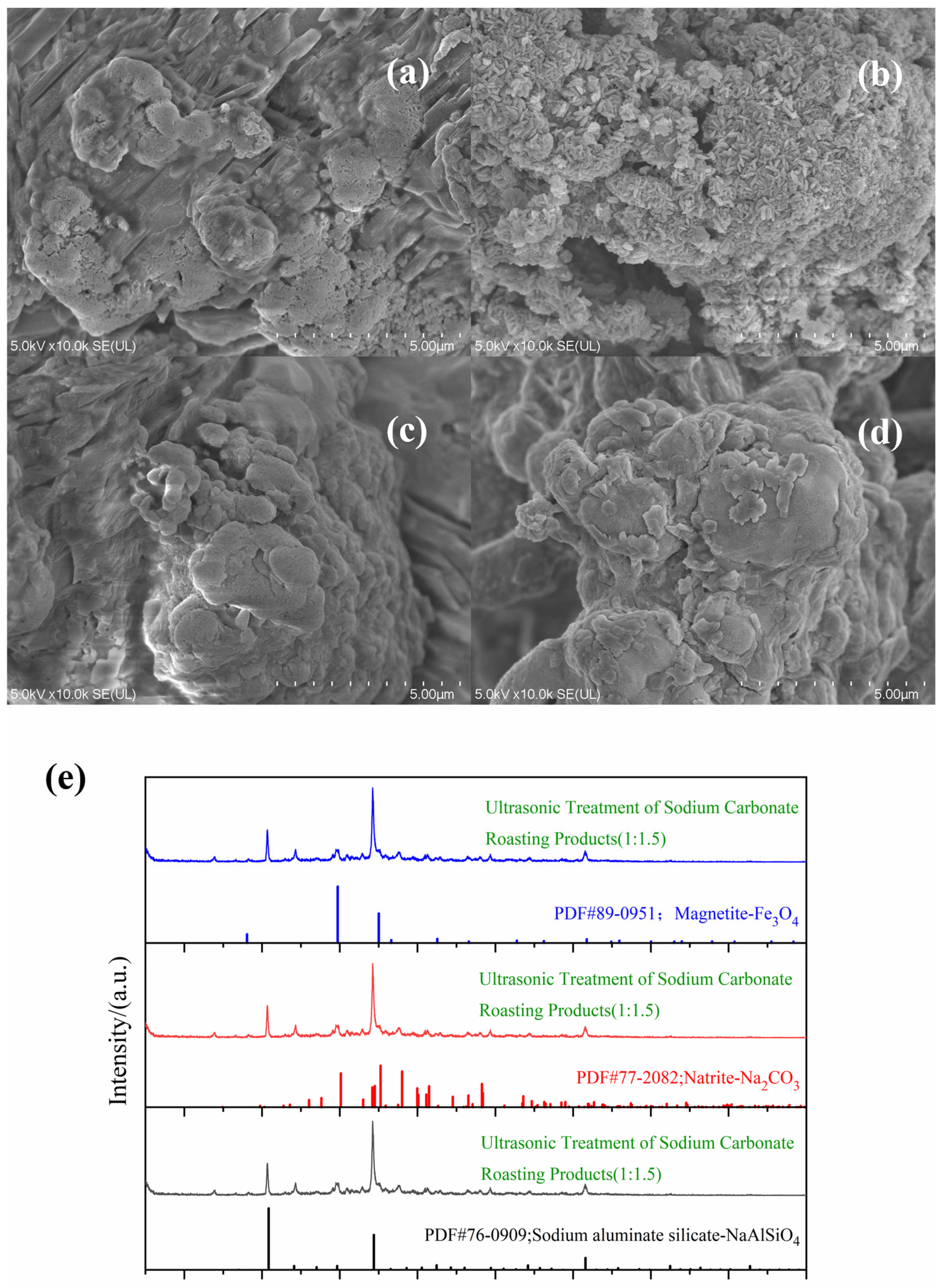
3.3. Ultrasonic Pretreatment Effects
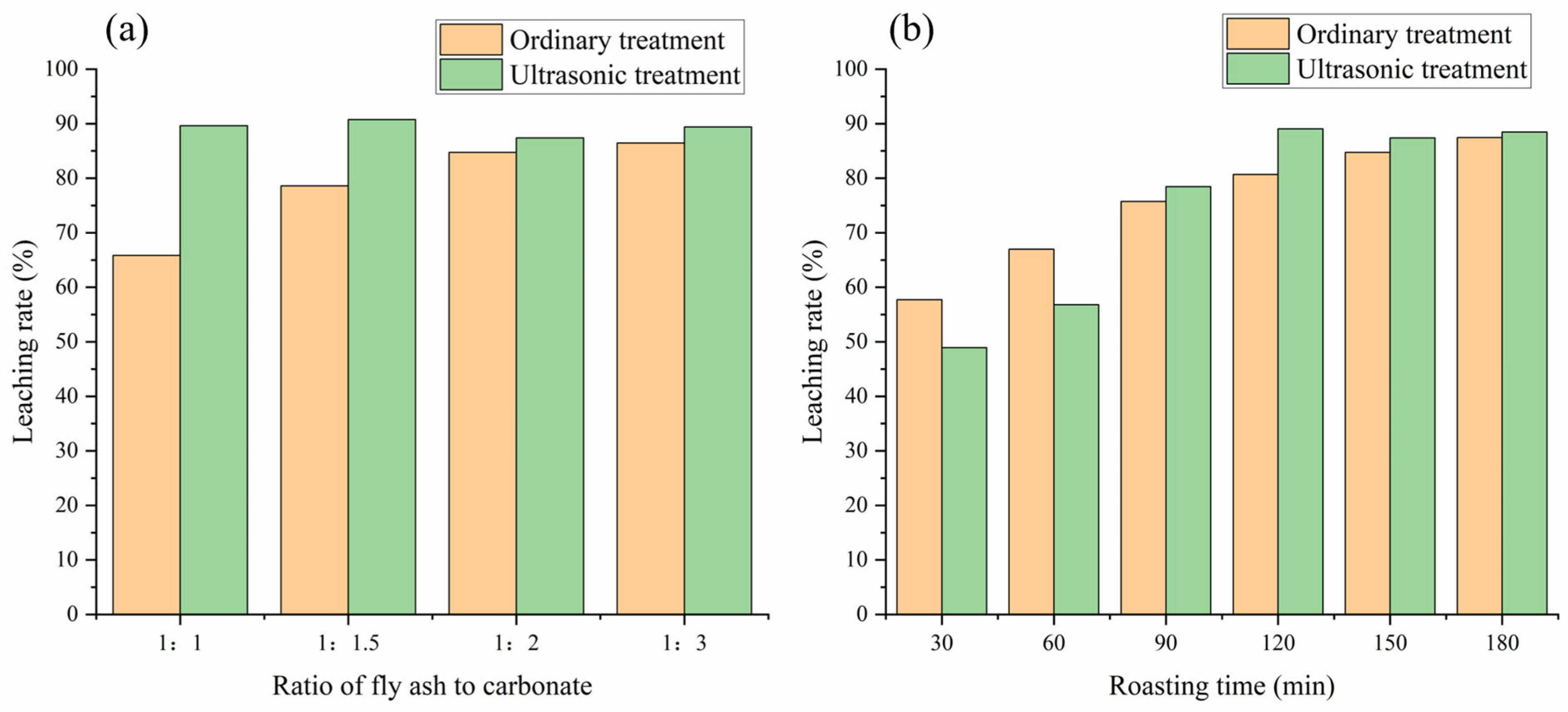
3.3.1. Comparison Analysis
3.3.2. Ultrasonic Treatment Analysis
3.4. Acid Leaching Effects
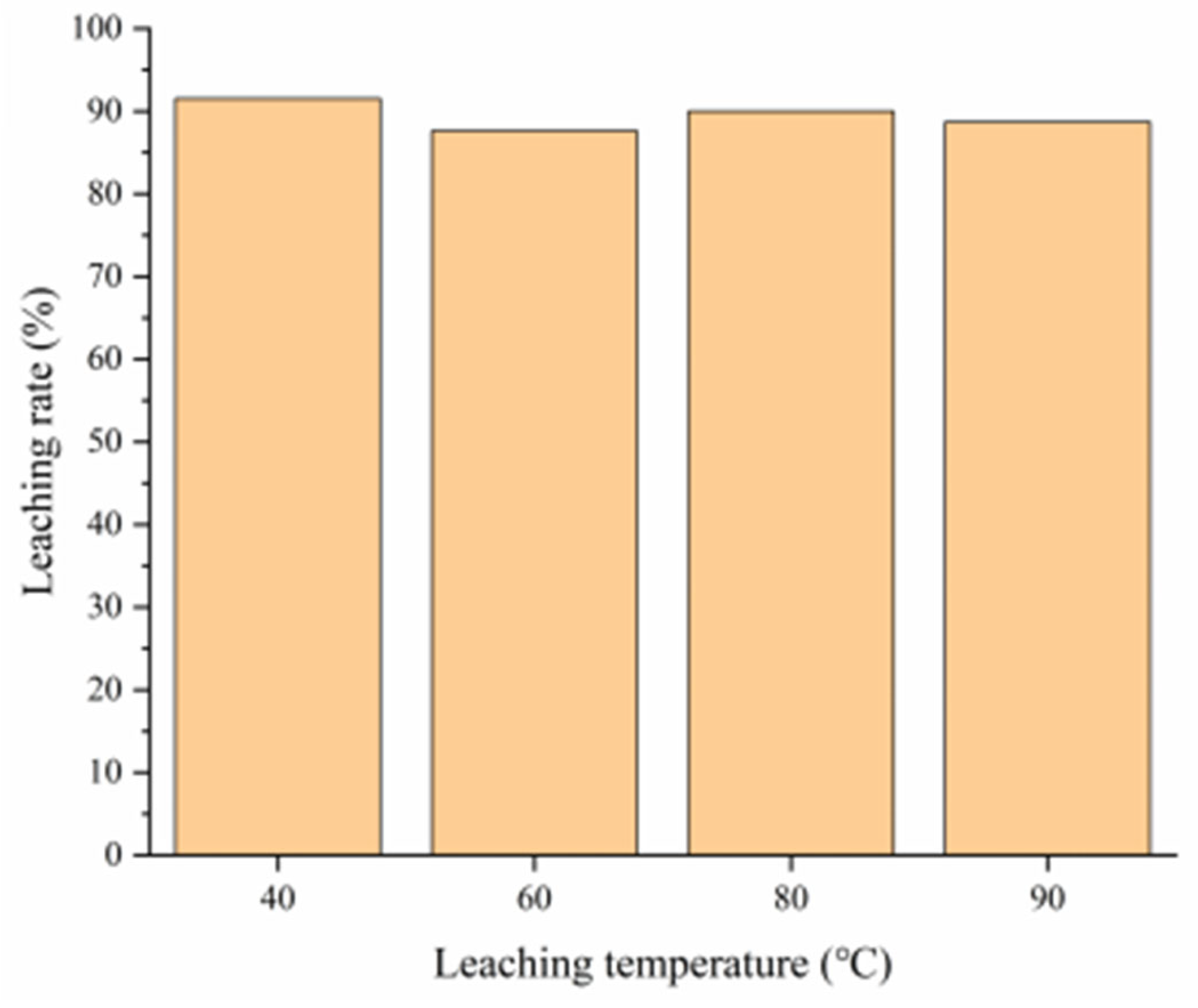
3.4.1. Effect of Leaching Temperature
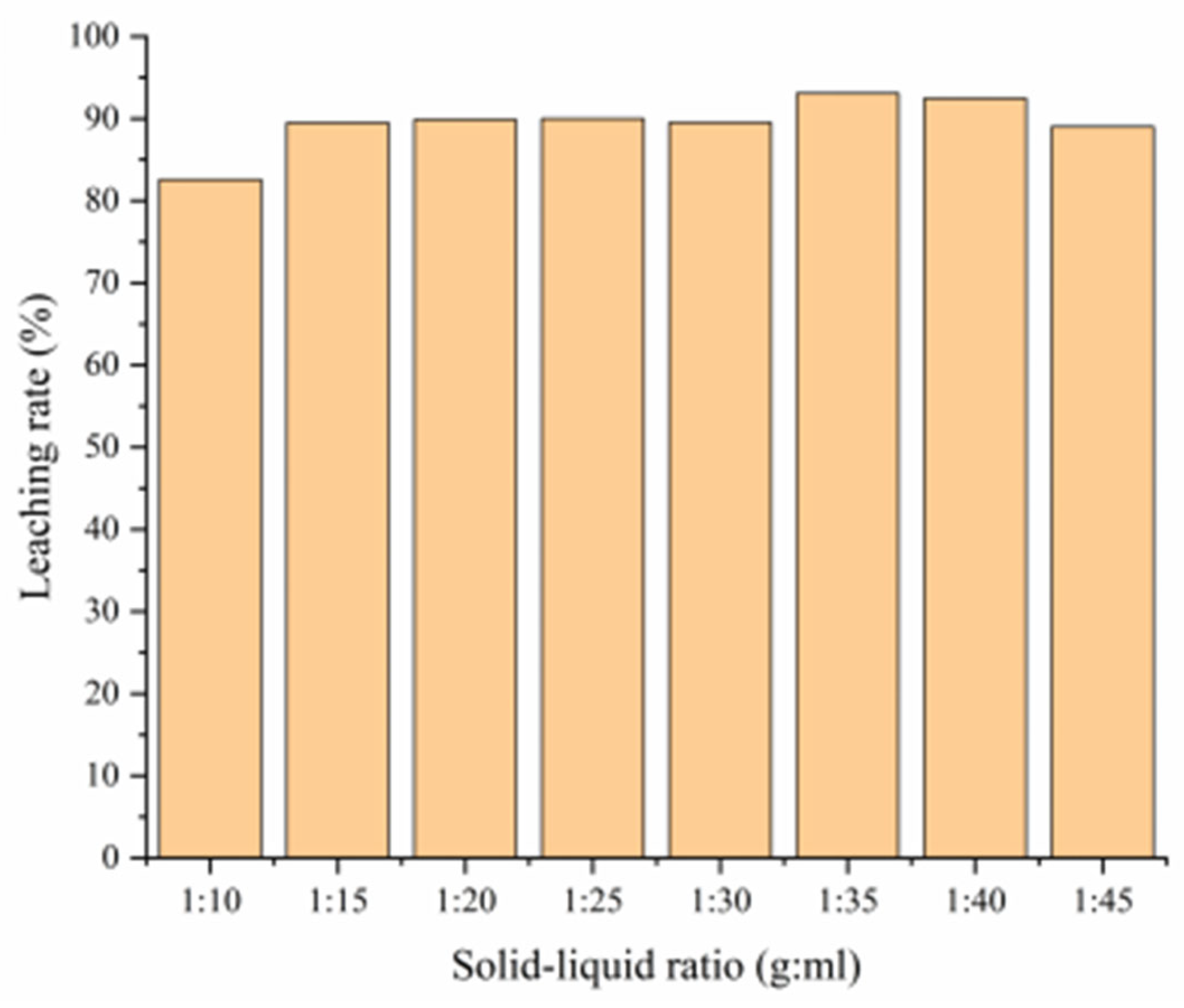
3.4.2. Effect of Solid–Liquid Ratio
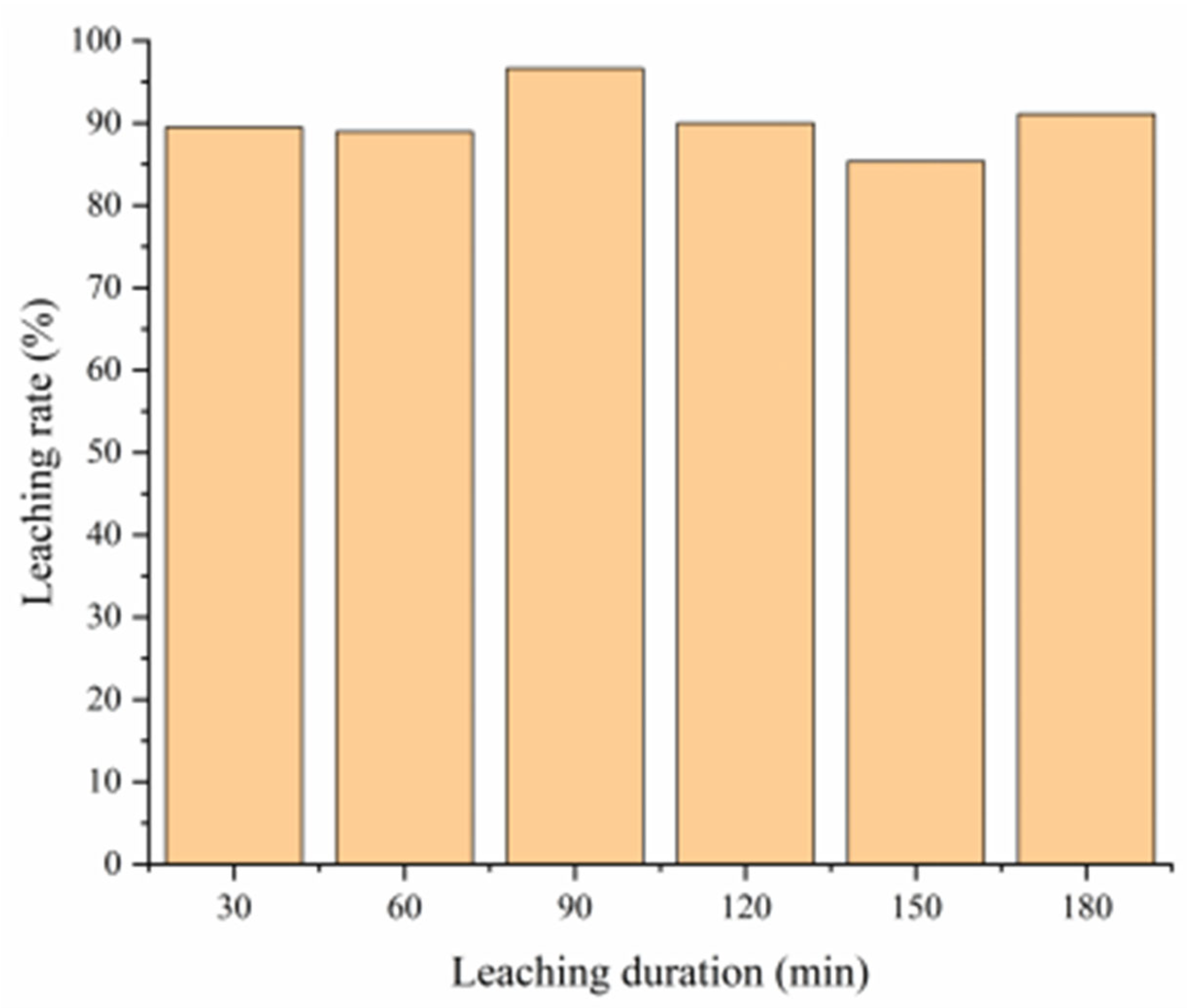
3.4.3. Effect of Leaching Time
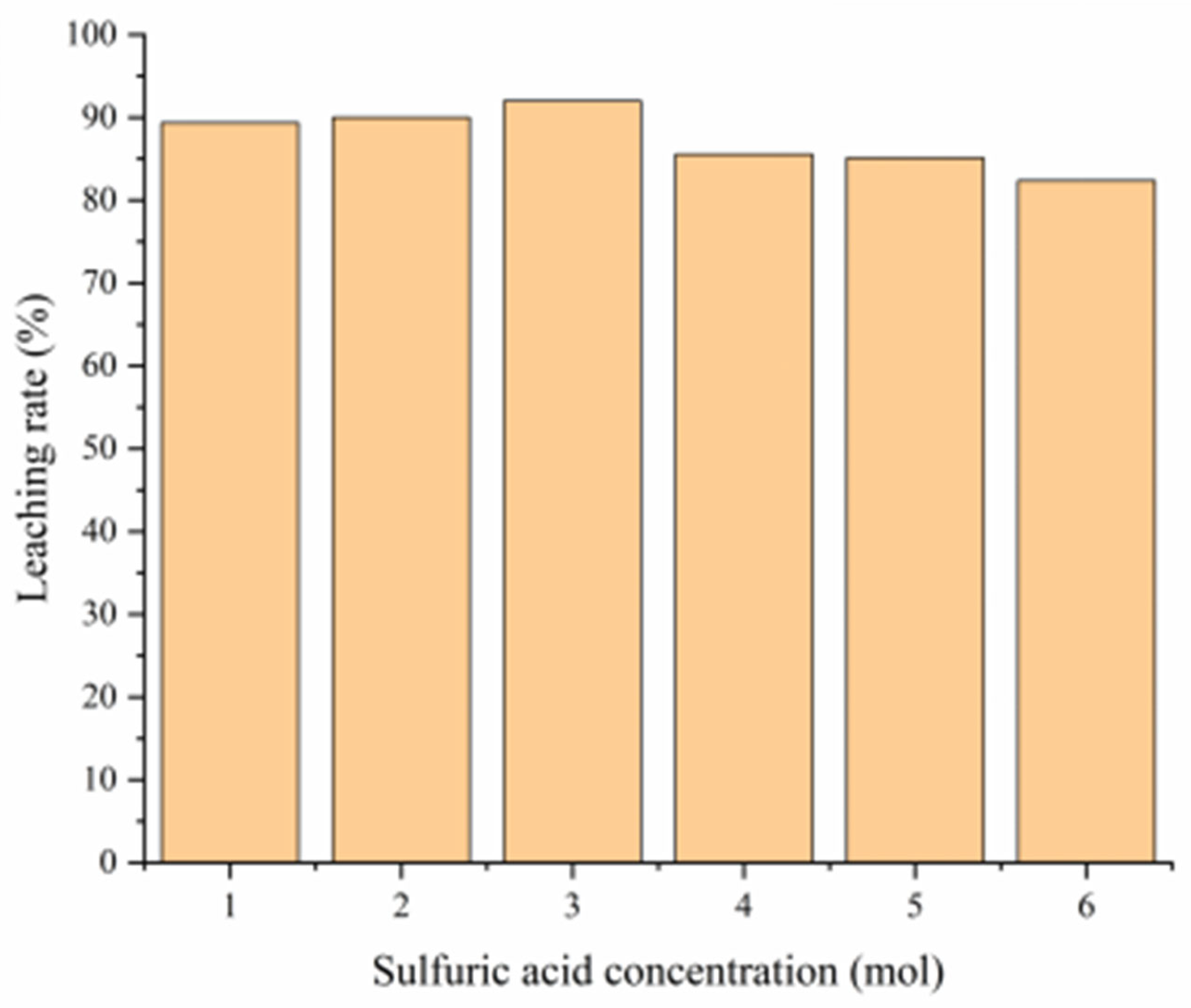
3.4.4. Influence of Leaching Agent Concentration
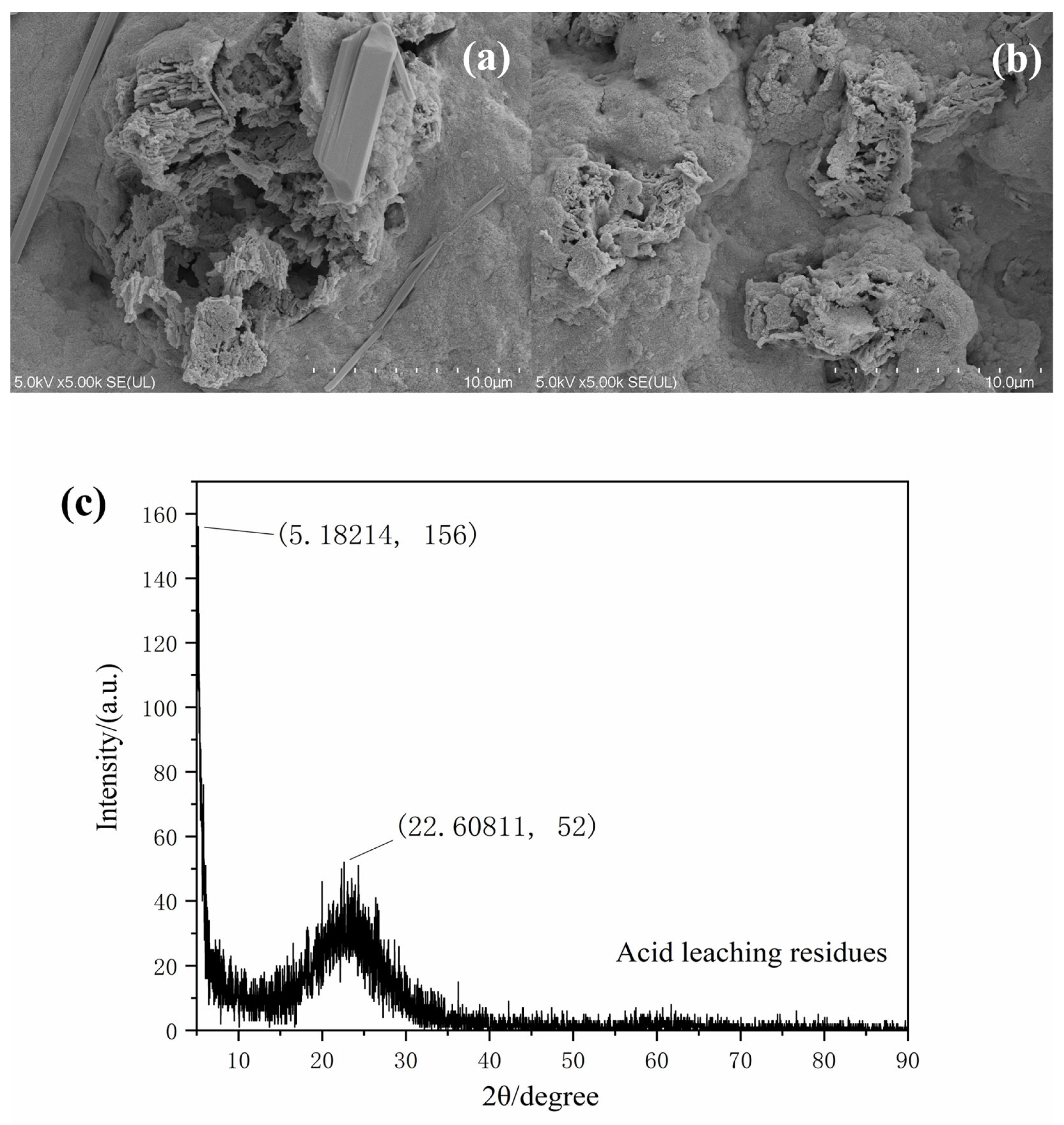
3.4.5. Acid Leaching Process Characterization Analysis
3.5. Orthogonal Experiment Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Wang, Q. Global lithium resource exploration and development and supply and demand situation analysis. Chin. Min. Ind. 2016, 25, 11–15+24. [Google Scholar]
- Zhiqin, X.; Wenbin, Z.; Bihai, Z.; Liangshu, S.; Guangwei, L.; Xudong, C.; Yulong, Q. Research on the Strategy and Continental Dynamics of new energy lithium ore—Commemorating the 100th anniversary of the School of Earth Science and Engineering of Nanjing University. Acta Geol. Sin. 2021, 95, 2937–2954. [Google Scholar] [CrossRef]
- Shanjun, P.; Tailiang, F.; Moran, L.; Renliang, L. Research progress on extraction of lithium from fly ash. Contemp. Chem. Res. 2024, 19, 34–37. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research–global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Cheng, J.; Zuo, Z.; Zhan, C.; Guo, H. Study on dynamic evolution and early warning of comprehensive risk of lithium resources in China. J. Nat. Resour. 2024, 39, 528–546. [Google Scholar]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Hu, P.; Hou, X.; Zhang, J.; Li, S.; Wu, H.; Damo, A.J.; Li, H.; Wu, Q.; Xi, X. Distribution and occurrence of lithium in high-alumina-coal fly ash. Int. J. Coal Geol. 2018, 189, 27–34. [Google Scholar] [CrossRef]
- Nizar, I.K.; Al Bakri, A.M.M.; Rafiza, A.; Kamarudin, H.; Alida, A.; Zarina, Y. Study on physical and chemical properties of fly ash from different area in Malaysia. Key Eng. Mater. 2014, 594, 985–989. [Google Scholar]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Zheng, D. Forecast of China’s energy consumption and coal demand in 2025. J. Coal Sci. 2019, 44, 1949–1960. [Google Scholar] [CrossRef]
- Bai, X.; Ding, H.; Lian, J.; Ma, D.; Yang, X.; Sun, N.; Xue, W.; Chang, Y. Coal production in China: Past, present, and future projections. In Coal Geology of China; Routledge: London, UK, 2020; pp. 5–17. [Google Scholar]
- Cui, X. Demand Forecast and Supply and Demand Analysis of Lithium Ore Resources in China. Master’s Thesis, China University of Geosciences, Wuhan, China, 2017. [Google Scholar]
- Nandihalli, N.; Chouhan, R.K.; Kuchi, R.; Hlova, I.Z. Aspects of spodumene lithium extraction techniques. Sustainability 2024, 16, 8513. [Google Scholar] [CrossRef]
- Liu, D.; Sun, S.; Yu, J. Research and development of lithium extraction technology from salt lake brine. J. Chem. Eng. 2018, 69, 141–155. [Google Scholar]
- Xu, Z.; Liang, J.; Li, H.; Guo, J. Research status and prospect of lithium extraction from lithium containing resources. Complex Util. Valuab. Miner. 2021, 5, 32–37. [Google Scholar]
- Sun, B.; Wang, G.; Zeng, F.; Liu, C.; Xie, X. Discovery of spodumene in lithium rich coal and its geological significance. In Proceedings of the First National mineral Exploration Conference, Hefei, China, 11–13 October 2021; p. 4. [Google Scholar]
- Li, H.; Zhang, J.; Wang, C.; Li, S.; Cao, S.; Hu, P.; Zhu, Q. Construction and research progress of clean recycling technology of high alumina coal ash associated resources. Clean Coal Technol. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Hu, P. Study on the Occurrence State and Leaching Rule of Lithium in High Alumina Coal Ash during Pre-Desiliconization. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2018. [Google Scholar]
- Yang, J. Study on Comprehensive Extraction Technology of Aluminum and Lithium from Coal Ash in Pingshuo. Master’s Thesis, Hebei University of Technology, Tianjin, China, 2013. [Google Scholar]
- Zang, J. Experimental Study on Lithium Leaching from Fly Ash of a Power Plant in Guizhou. Master’s Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Wang, Q. Study on Kinetics and Separation Mechanism of Lithium and Gallium by Microwave Enhanced Leaching from High Alumina Ash. Master’s Thesis, Hebei University of Technology, Tianjin, China, 2024. [Google Scholar]
- Bo, P.; Li, S.; Wu, S.; Lu, Q.; Qin, Z. Experimental study on leaching of valuable lithium from fly ash in Pingshuo Mining area. Chem. Res. Appl. 2019, 31, 1351–1356. [Google Scholar]
- Xu, H.; Liu, C.; Mi, X.; Wang, Z.; Han, J.; Zeng, G.; Liu, P.; Guan, Q.; Ji, H.; Huang, S.J.S.; et al. Extraction of lithium from coal fly ash by low-temperature ammonium fluoride activation-assisted leaching. Sep. Purif. Technol. 2021, 279, 119757. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, C.; Xu, S.; Shi, J.; Hu, Y.; Liu, G. High-efficiency extraction of aluminum and lithium from coal fly ash using a novel sodium pyrosulfate mechanochemical activation-Sodium persulfate pressure leaching technology. J. Clean. Prod. 2023, 423, 138841. [Google Scholar] [CrossRef]
- Rui, H. Study on technology and mechanism of extracting lithium from hydrochloric acid leaching solution of high alumina ash by solvent extraction. Master’s Thesis, Xidian University, Xi’an, China, 2020. [Google Scholar]
- Xu, Z.; Wang, X.; Sun, S. Performance of a synthetic resin for lithium adsorption in waste liquid of extracting aluminum from fly-ash. Chin. J. Chem. Eng. 2022, 44, 115–123. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Xu, M.; Yao, B.; Zhang, C. Preparation of Manganese Dioxide Lithium Ion Sieve and Its Application in Lithium Extraction from Coal Fly Ash. Appl. Sci. 2024, 14, 1463. [Google Scholar] [CrossRef]


| Elements | Compounds | ||||
|---|---|---|---|---|---|
| Element | Conc | Unit | Element | Conc | Unit |
| Si | 38.758 | wt% | SiO2 | 45.239 | wt% |
| Al | 25.744 | wt% | Al2O3 | 30.777 | wt% |
| Fe | 18.364 | wt% | Fe2O3 | 11.23 | wt% |
| Ca | 5.204 | wt% | CaO | 3.56 | wt% |
| K | 4.58 | wt% | TiO2 | 3.524 | wt% |
| S | 3.802 | wt% | K2O | 2.253 | wt% |
| Elements | Compounds | ||||
|---|---|---|---|---|---|
| Element | Conc | Unit | Element | Conc | Unit |
| Si | 70.429 | wt% | SiO2 | 74.604 | wt% |
| S | 25.513 | wt% | SO32− | 23.353 | wt% |
| Cl | 0.808 | wt% | Al2O3 | 0.506 | wt% |
| Ca | 0.77 | wt% | TiO2 | 0.373 | wt% |
| Ti | 0.749 | wt% | CaO | 0.357 | wt% |
| Fe | 0.611 | wt% | Fe2O3 | 0.256 | wt% |
| Al | 0.381 | wt% | Cl− | 0.256 | wt% |
| Levels | Factors | |||
|---|---|---|---|---|
| A/g:g | B/min | C/mol·L−1 | D/g:mL | |
| 1 | 1:1 | 60 | 2 | 1:30 |
| 2 | 1:1.5 | 90 | 3 | 1:35 |
| 3 | 1:2 | 120 | 4 | 1:40 |
| Levels | Factors | Lithium Leaching Rate/% | ||||
|---|---|---|---|---|---|---|
| Numbers | A | B | C | D | ||
| 1 | 1 | 1 | 1 | 1 | 98.67 | |
| 2 | 1 | 2 | 3 | 2 | 88.36 | |
| 3 | 1 | 3 | 2 | 3 | 93.78 | |
| 4 | 2 | 1 | 3 | 3 | 88.24 | |
| 5 | 2 | 2 | 2 | 1 | 96.12 | |
| 6 | 2 | 3 | 1 | 2 | 98.68 | |
| 7 | 3 | 1 | 2 | 2 | 91.14 | |
| 8 | 3 | 2 | 1 | 3 | 91.11 | |
| 9 | 3 | 3 | 3 | 1 | 98.04 | |
| K1 | 280.81 | 278.05 | 288.47 | 292.83 | T = 844.15 | |
| K2 | 283.04 | 275.59 | 281.04 | 278.19 | ||
| K3 | 280.30 | 290.51 | 274.64 | 273.13 | ||
| k1 | 93.60 | 92.68 | 96.16 | 97.61 | Y = 93.79 | |
| k2 | 94.35 | 91.86 | 93.68 | 92.73 | ||
| k3 | 93.43 | 96.84 | 91.55 | 91.04 | ||
| R | 0.91 | 4.97 | 4.61 | 6.57 | ||
| Impact Order | D > B > C > A | |||||
| Optimal Level | D1B3C1A2 | |||||
| Optimum Condition | 1:1.5; 120 min; 2 mol/L; 1 g:30 mL | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, J.; Lv, L.; Mu, X.; Ren, Y.; Zhu, G. Roasting Extraction of Lithium from Fly Ash: A Study of Influential Parameters and Mechanisms. Appl. Sci. 2025, 15, 1280. https://doi.org/10.3390/app15031280
Li F, Liu J, Lv L, Mu X, Ren Y, Zhu G. Roasting Extraction of Lithium from Fly Ash: A Study of Influential Parameters and Mechanisms. Applied Sciences. 2025; 15(3):1280. https://doi.org/10.3390/app15031280
Chicago/Turabian StyleLi, Fayue, Jingfeng Liu, Longjiao Lv, Xiwei Mu, Yuting Ren, and Guocheng Zhu. 2025. "Roasting Extraction of Lithium from Fly Ash: A Study of Influential Parameters and Mechanisms" Applied Sciences 15, no. 3: 1280. https://doi.org/10.3390/app15031280
APA StyleLi, F., Liu, J., Lv, L., Mu, X., Ren, Y., & Zhu, G. (2025). Roasting Extraction of Lithium from Fly Ash: A Study of Influential Parameters and Mechanisms. Applied Sciences, 15(3), 1280. https://doi.org/10.3390/app15031280






