Abstract
The objective of this study was to investigate the distribution of pyrolysis products and the chemical reaction kinetics of a novel composite, GO@CL-20. The GO@CL-20 composite powder was synthesized using a solvent–non-solvent method. The thermal decomposition process of GO@CL-20 was analyzed through thermogravimetric differential scanning calorimetry (TG-DSC). The results indicate that the incorporation of graphene oxide (GO) reduces the activation energy of the sample, thereby catalyzing the thermal decomposition process of the complex. Subsequently, single pulse shock tube experiments were conducted to assess ignition delay time distribution, from which corresponding data on pyrolysis product distribution for GO@CL-20 were obtained. The findings regarding ignition delay times demonstrate that adding GO decreases the energy within the complex system and mitigates its reactivity, consequently prolonging ignition delay times. An important carbon and nitrogen molecule, C2N2, was identified in the pyrolysis product distribution; notably, its yield increased progressively with higher concentrations of GO. Finally, mass transfer characteristics and sensitivity analyses for GO@CL-20 samples were performed using CHEMKIN software to preliminarily determine pyrolysis reaction pathways. The results reveal that incorporating GO can significantly alter the thermal decomposition behavior of the entire system; moreover, C2N2 exhibits a high cleavage rate along this reaction pathway—findings that align well with experimental observations. This study aims to enhance understanding of CL-20 and GO reaction kinetics—materials with extensive applications in military operations as well as aviation and aerospace—and provides valuable insights for propellant development.
1. Introduction
The role of propellants in rockets and missiles is of great consequence. The assessment of propellant performance is contingent upon several key factors, including high energy density, insensitivity, a brief ignition delay time, and a rapid response rate [1,2,3,4,5]. At the present time, a considerable number of researchers are engaged in efforts to enhance combustion technology in order to meet the demands of military and civilian applications [6,7,8]. It is well established that the thermal decomposition properties of propellants exert a direct influence on the combustion characteristics of propellants. The use of combustion catalysts to modulate the thermal decomposition properties of propellants represents an effective and straightforward approach to achieving this goal. Consequently, the investigation of the physicochemical effects of new efficient combustion catalysts in the thermal decomposition process of propellants has emerged as a prominent area of research in recent years.
Since the discovery of fire and the subsequent invention of explosives, carbon has constituted an indispensable element in these materials. Among the plethora of carbon materials, graphene has attracted considerable attention due to its exceptional physical properties, which have the potential to be harnessed in a multitude of fields [9,10,11,12]. Graphene oxide (GO) is a derivative of graphene that offers a number of advantages, including a low density, low cost, good electrical and thermal conductivity, and favorable mechanical properties. These attributes render a promising functional additive for high-energy materials, either alone or in combination with other functional catalysts, with the objective of enhancing the comprehensive performance of high-energy materials. Consequently, graphene oxide (GO) is a particularly advantageous material in the field of high-energy materials in comparison to other materials. The carbon skeleton of graphene oxide contains a substantial number of oxygen-containing functional groups, which exhibit strong hydrophilicity and surface activity. Moreover, graphene oxide exerts a more pronounced effect on the thermal decomposition of 2,4,6,8,10,12-hexanonitro-2,4,6,8,10,12-hexaazadiourea (CL-20), cyclic tetramethylene-trinitramine (HMX), or ammonium perchlorate (AP) than the purely energy-containing components [9,13,14].
GO is currently employed as a functional additive for propellants, explosives, and launching agents in the field of energetic materials, as shown in Figure 1. In recent years, a substantial body of research has been conducted by both domestic and foreign researchers. In a recent study, Yu et al. investigated a novel formulation of CL-20-based explosives containing a reduced graphene oxide modification, resulting in the development of a low-sensitivity energy-containing material [15,16]. Memon et al. [17] prepared AP composites with a GO mass fraction of 2% using the recrystallisation and fast stirring collision method. The lower exothermic peak temperatures of GO/AP composites prepared by the rapid collision method in comparison with those of pristine AP, and the consistent exothermic peak temperatures of AP, can be primarily attributed to the effects of rapid stirring collision and the numerous functional groups on the GO surface. The restriction of AP crystal growth results in a reduction of particle size and an encapsulation effect, which increases the specific surface area of AP and improves the heat transfer efficiency, thereby enhancing its thermal decomposition performance. The presented evidence substantiates the assertion that GO exerts a catalytic effect on the thermal decomposition performance of AP. Yu Jiaying et al. [18] prepared CL-20/GO composites with a GO mass fraction of 2% by the ultrasonic composite method. The resulting morphology was that of folded circular particles, with the loading of CL-20 forming a continuous structure between the GO sheets. The peak exothermic and weight loss temperatures of the composites were reduced due to the lower decomposition and exothermic temperatures of GO, whereas the maximum exothermic rate of the composites was reduced, which was mainly due to the lower exothermicity of GO itself as well as the intertwining of CL-20 between the GO sheets.CL-20 is a high-energy-density compound with excellent detonation properties. In practical applications, CL-20 is typically employed in conjunction with other additives to augment its combustion performance. GO plays a pivotal role in the combustion of propellants. On the one hand, GO can be used to load metals and metal oxides. GO can promote the pyrolysis of oxidants and allow the full reaction of NO2 released from them. It would be beneficial to investigate the kinetics of the combustion reaction between GO and CL-20 [19,20,21,22].
Figure 1.
Molecular structure diagram of graphene oxide.
In this study, a composite of graphene oxide (GO) with CL-20 was initially prepared in sufficient quantity by the solvent–non-solvent method. Subsequently, the preliminary thermal decomposition properties of GO@CL-20 were investigated by thermogravimetric differential scanning calorimetry (TG-DSC). Moreover, the pyrolysis product distribution of the complex was subjected to experimental investigation. The ignition delay time and pyrolysis properties of the complex at high temperatures were investigated in detail using a single pulse excitation tube experimental system combined with gas chromatography. The pyrolysis products were subjected to qualitative and quantitative analysis. The distribution of the concentration of the products and residual reactants with pyrolysis temperature was investigated. In light of the aforementioned findings, the high-temperature pyrolysis characteristics and sensitivity coefficients of GO@CL-20 were subjected to detailed analysis using the chemical kinetics software Chemkin 2020. Subsequently, the decomposition reaction paths of the complex were plotted based on the aforementioned results. The findings of this research provide a reliable foundation for further optimization of the combustion mechanism of CL-20.
2. Materials and Methods
2.1. Reagents and Materials
The GO@CL-20 used in this article was prepared by the solvent–non-solvent method. 490 mg of CL-20 was ultrasonically dispersed in 30 mL of deionized water, and after uniform dispersion, it was stirred at room temperature for 4 h. Then, 20 mL of a 0.5 g/L graphene oxide dispersion was added dropwise to the above mixture, and stirred at room temperature for another 10 h. The resulting mixture was filtered, and the filter cake was washed three times with ultrapure water. After vacuum drying at 60 °C for 24 h, the CL-20/GO composite material was prepared. In this study, three different compositions of composites were prepared, with the mass ratios of graphene oxide (GO) being 1%, 1.5%, and 2% for comparative analysis. Pure CL-20 was used as the control group and these four groups of samples were used together for experimental comparison. The specific components are as shown in Table 1.

Table 1.
Experimental GO@CL-20 sample (mass percentage (w%)).
Graphene oxide was purchased from Huayuan Chemical Co., Ltd., Shanghai Huayi Group in Shanghai, China. CL-20 was provided by the Xi’an Modern Chemical Research Institute in Xi’an, China.
2.2. Experiment Methods
2.2.1. Thermogravimetric Differential Scanning Calorimetry (TG-DSC)
In order to study the properties of the preliminary thermal decomposition of the GO@CL-20 complex, the thermal decomposition process of GO@CL-20 with a mass of approximately 0.3 mg was tested using a thermogravimetric differential scanning calorimeter (TG-DSC), model STA449F3, manufactured by NETZSCH in Bavaria, Germany, in the temperature range of 40–400 °C. The temperature was increased at a rate of 5.0, 10.0, 15.0, and 20.0 K per minute, with argon serving as the protective gas and a gas flow rate of 70 mL per minute.
2.2.2. High-Temperature Cracking Experiment
The shock tube was constructed from 304 stainless steel, featuring a driver section measuring 3.05 m in length and a driven section measuring 1.5 m in length, both with an internal diameter of 44 mm. The critical component, the dump chute, is attached to the low-pressure section of the shock tube via a baffle valve, also with an internal diameter of 44 mm. This valve serves to prevent the formation of multiple reflected shock waves within the shock tube during experiments, ensuring that the shock wave propagates as a single pulse. As illustrated in Figure 2, five pressure sensors were installed on the side wall of the low-pressure section near the end cap to monitor the pressure rise at various time points. By measuring the distance between these sensors, the velocity of the incident shock wave was determined. The analysis and calculation of the shock wave’s speed were performed using the widely recognized Gaseq 0.7 software, developed by Professor Morley of Columbia University [23,24,25,26]. This software includes a module for reflected shock, which calculates the state of the low-pressure region after the shock wave reflects from the shock tube, providing data on the temperature and pressure of the low-pressure region post-reflection.
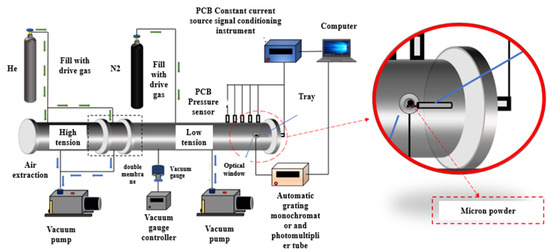
Figure 2.
Schematic diagram of single-pulse shock tube structure.
Prior to conducting any experiments, 100 mg of energetic material powder was weighed using an electronic analytical balance and evenly spread onto a thin blade. Subsequently, the blade was mounted on the adjusted support rod and positioned within the low-pressure chamber of the shock tube. The high-pressure chamber and low-pressure chamber were evacuated using a mechanical pump and a turbomolecular pump, respectively. Once the low-pressure chamber has been evacuated to an ultimate vacuum of 3 × 10−2 Pa by the turbomolecular pump, the diluted gas is filled to the predetermined pressure. To prevent the energetic material powder from rising prematurely from the blade before breaking the film, it is necessary to control the rate of vacuuming while vacuuming the low-pressure chamber. The experiment demonstrated that if the pressure in the low-pressure chamber decreased at a rate of 1 kPa/s, the energetic material powder would remain on the blade after vacuuming [27,28,29].
The outer wall of the shock tube is wrapped with heating tape and insulation material. When N2 is used as a diluting gas, it is sufficient to maintain the entire experimental system at room temperature (25 °C). Once the low-pressure chamber is filled with gas, it is then filled into the high-pressure chamber and the middle section. The film-breaking experiment is then conducted once the pressure reaches the specified level. At the moment of film breaking, the high-pressure gas exerts a force on the low-pressure gas, resulting in the generation of a shock wave. As the shock wave passes through the blade, it raises and disperses the energetic material powder on the blade. The tail cap reflects the shock wave, which then passes through the energetic material powder, heating it to the expected temperature.
The specific gas chromatography model used in this paper is Agilent GC7820A in Shanghai, China. The Agilent GC7820A gas chromatograph is straightforward to operate and offers dependable analytical outcomes, rendering an optimal choice for routine analysis. The system is equipped with EZChrom Elite 3.2.0 software, which enables the user to modify the operational parameters of the gas chromatograph and display the status of the chromatograph [30,31]. The chromatograph is equipped with a hydrogen flame ionization detector (FID) and a thermal conductivity detector (TCD).
2.3. Simulation Methods
Researchers have commenced studying the pyrolysis process of fuels through numerical simulation, employing established chemical reaction mechanisms and the thermodynamic and transport properties of gas mixtures. Numerical simulation methods hold significant importance for modeling the chemical kinetics of combustion. By comparing computational outcomes with experimental findings, one can assess the precision of the established model and conduct various detailed kinetic analyses, reaction pathway analyses, and sensitivity analyses for fundamental reactions. This facilitates the refinement and simplification of chemical kinetic models.
The software utilized in this study, CHEMKIN 2020, consists of two components: a gas-phase reaction kinetics solver and a surface chemical kinetics solver. It encompasses a subroutine library, a property library, a preprocessor, and an application reactor, enabling a more comprehensive investigation of chemical reaction kinetics. CHEMKIN 2020 operates by reading the reaction rates of the elementary reactions from the mechanism and accessing the thermodynamic data files of relevant substances via the FORTRAN 95 interpreter, upon which calculations are performed based on the input initial parameters. Upon completion of the calculations, the corresponding results are exported according to the user’s requirements.
3. Results and Discussion
3.1. Thermal Behaviors of Composites
3.1.1. Differential Scanning Calorimetry (DSC)
In this study, thermogravimetric differential scanning calorimetry (TG-DSC) was utilized to investigate the preliminary thermal decomposition properties of GO@CL-20 complexes. The thermal decomposition process was analyzed for samples weighing approximately 0.3 mg over a temperature range of 40–400 °C. The heating rates were set at 5.0, 10.0, 15.0, and 20.0 K/min, with argon serving as the protective gas at a flow rate of 70 mL/min. The experimental results are presented in Figure 3, which illustrates the thermal behavior of the complexes when heated from 40 °C to 400 °C at different rates. Figure 4 displays the linear fit curves derived from the preliminary thermal decomposition reactions, calculated using the Kissinger and Ozawa methods and described by Equations (1) and (2). Relevant parameters are summarized in Table 2.
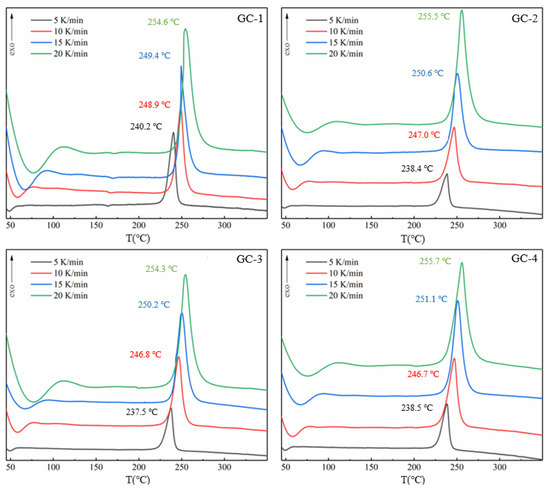
Figure 3.
DSC curves of GC-1, GC-2, GC-3, and GC-4 at different heating rates.
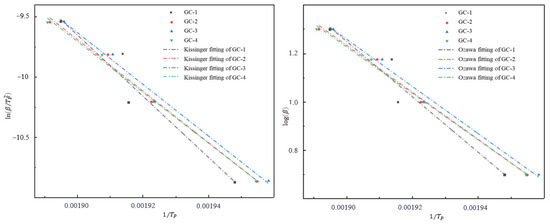
Figure 4.
Linear fitting curves of GC-1, GC-2, GC-3, and GC-4 based on Kissinger and Ozawa methods.

Table 2.
Non-isothermal kinetic parameters of GC-1, GC-2, GC-3, and GC-4.
The DSC curves in Figure 5 depict the thermal behavior of CL-20 and its mixtures with additives at heating rates of 5, 10, 15, and 20 °C/min and a temperature range of 40–400 °C. Initial analyses of GC-1 samples without graphene oxide revealed a one-step low-temperature decomposition phase. The initial decomposition temperatures of GC-1 were 220.5 °C, 221.9 °C, 225.4 °C, and 223.6 °C as the heating rate increased. The corresponding peak decomposition temperatures were 240.2 °C, 248.9 °C, 249.4 °C, and 254.6 °C. These findings generally align with the previously reported decomposition temperatures of CL-20, with the differences falling within acceptable limits.
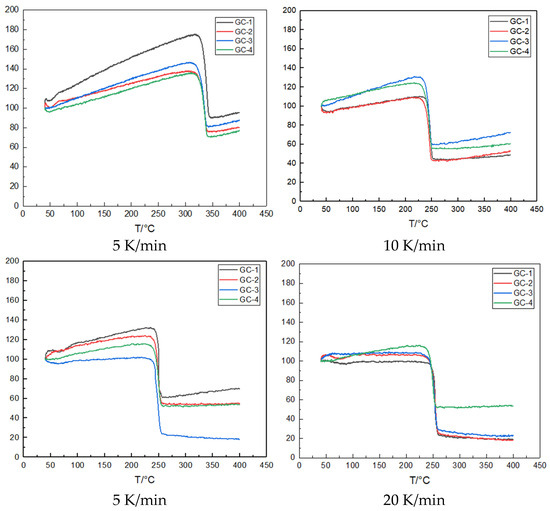
Figure 5.
TG curves of GC-1, GC-2, GC-3, and GC-4 at different heating rates.
The non-isothermal kinetic behavior of the mixture was investigated using DSC. As illustrated in Figure 3, as the heating rate increases the exothermic peak of CL-20 shifts to higher temperatures. There is no significant difference in the heat flow curves of the three types of graphene-CL-20 mixtures; all exhibit a single exothermic peak, albeit with varying peak temperatures. At heating rates of 5 and 10 °C/min, the peak temperature of the DSC curves for CL-20 samples containing graphene oxide is lower than that of pure CL-20 samples. The peak temperatures for different samples at various heating rates are summarized in Table 2.
As illustrated in the Figure 4, an increase in the heating rate results in a shift of the exothermic peak of CL-20 to higher temperatures. The heat flow curves of the three types of graphene mixed with CL-20 exhibit no discernible difference, displaying a single exothermic peak with varying peak temperatures. The peak temperature of the DSC curves of CL-20 samples containing graphene oxide is observed to be lower than that of pure CL-20 samples at heating rates of 5 and 10 °C min−1. The peak temperatures of the various samples under different heating rates are presented in tabular form.
Kissinger equation:
Among them, β represents the heating rate, with the unit of °C·min−1; Tp is the peak temperature, with the unit of °C; EK is the activation energy calculated by the Kissinger method, with the unit of kJ/mol; R is the Boltzmann gas constant, with a value of 8.314 and the unit of J/(mol·K); A refers to the leading factor.
Ozawa equation:
Among them, T is the characteristic temperature, with the unit of °C; EO is the activation energy calculated by the Ozawa method, with the unit of kJ/mol; α is the weight loss percentage; and G(α) is the integral form of the phase transformation mechanism function.
Given that this study focuses on the initial stage of the overall thermal decomposition of the sample and primarily investigates the effects of temperature and heating rate on the reaction, it is reasonable to assume that the α values at the peak temperatures (Tp) of each thermal spectrum under different heating rates (β) are approximately equal. Consequently, G(α) can be treated as a constant [32].
After disregarding the influence of the conversion rate on the Ozawa equation, the Kissinger and Ozawa equations were used to fit the peak temperature at different heating rates, and the thermal decomposition activation energies were obtained from the fitted equations, which are listed in the Table 2. The activation energies obtained by the two methods are very close, which indicates that the results are highly reliable. The average thermal decomposition activation energy of CL-20 is 209.98 kJ·mol−1, and the average activation energy of GC-2, GC-3, and GC-4 is lower than that of pure CL-20. The thermal decomposition activation energy of GC-4 is the lowest, being 34.60 kJ·mol−1 lower than that of GC-1. It can be observed that within a specific range, as the amount of GO content increases, the thermal decomposition activation energy of GO@CL-20 reaches its maximum. The results indicated that the introduction of graphene oxide would catalyze the thermal decomposition of CL-20.
3.1.2. Thermogravimetry (TG)
The TG results for the three samples, subjected to various heating rates, are presented. The observed behavior of the mass loss curves corroborates the one-step decomposition noted above in the DSC experiments. The mean mass loss observed across all samples during the heating process was approximately 88%.
As shown in Figure 5, thermogravimetric analysis results show that the TG curves of the four samples show one-step weight loss. Once decomposition begins, the samples will rapidly lose weight in a short time and the curves show a steep state in the longitudinal direction, resulting in a violent decomposition process. In addition, taking pure CL-20 sample GC-1 as an example, the initial weight loss temperature is as follows: at 220.5 °C, 221.9 °C, 225.4 °C, and 223.6 °C, the initial weight loss temperature corresponds to the initial decomposition temperature of the DSC curve, maintaining a high degree of consistency.
3.1.3. Ignition Delay Time (IDT) Experiment
To further investigate the thermodynamic properties of GO@CL-20 complexes, the ignition delay time of GO@CL-20 was measured using a single-pulse excitation tube. In this experiment, the pressure was maintained at 10.0 bar and the temperature range was set from 2000 to 2600 K. A 100 mg sample of the powder was placed on the carrier platform within the tube. High-temperature and high-pressure shocks were generated by rupturing a diaphragm and the resulting data were collected via sensors to determine the ignition delay time for the relevant tests. The results are shown in Figure 6.
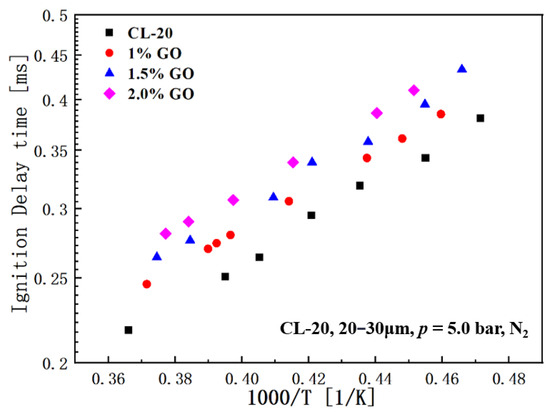
Figure 6.
Distribution of IDT with temperature for each sample.
As illustrated in the experimental results graph, the ignition delay time progressively increases with the rising concentration of graphene oxide (GO) in the sample. A detailed examination of the data points indicates that an increase of merely 0.5% in GO content leads to an approximate 20% extension in ignition delay time. This phenomenon can be attributed to the role of GO within the material’s overall composition. The incorporation of GO effectively lowers the energy threshold necessary for the composite system to achieve ignition. Consequently, the GO@CL-20 composite system exhibits reduced sensitivity and enhanced resistance to ignition, thereby significantly prolonging the ignition delay time. This effect is especially pronounced in propellant combustion scenarios. In practical applications, such as rocket propulsion systems, the presence of GO can enhance safety and control by delaying the onset of combustion. This characteristic is crucial for ensuring propellant reliability and performance, as it facilitates better management of the combustion process, mitigates the risk of premature ignition, and improves overall system stability.
3.2. Distribution of Cracking Products
The high-temperature cracking behavior of CL-20@GO was investigated using a single-pulse shock tube at a pressure of 10.0 bar and a temperature range of 1500–2200 K. The cracking products of CL-20@GO were identified using a flame ionization detector (FID). The cracking products were subjected to qualitative and quantitative analysis using a flame ionization detector (FID), resulting in the identification of six distinct cracking products. The pyrolysis process yielded three principal products. The identified products were allene, ethene, and methane. The concentrations of these products were found to be correlated with the pyrolysis temperature. As illustrated in Figure 7, the concentration of these products exhibits a tendency to increase and subsequently decline as the pyrolysis temperature rises. When the temperature is raised to a certain point, these hydrocarbon molecules break down into smaller, lower molecular weight gaseous molecules, such as CO2 and NO2. Furthermore, three additional products were identified: propene, 2-butene, and 1,3-butadiene. The presence of the 2-butene molecule was contingent upon the addition of a GO molecule. It is reasonable to posit that this molecule was produced by the decomposition of the GO.
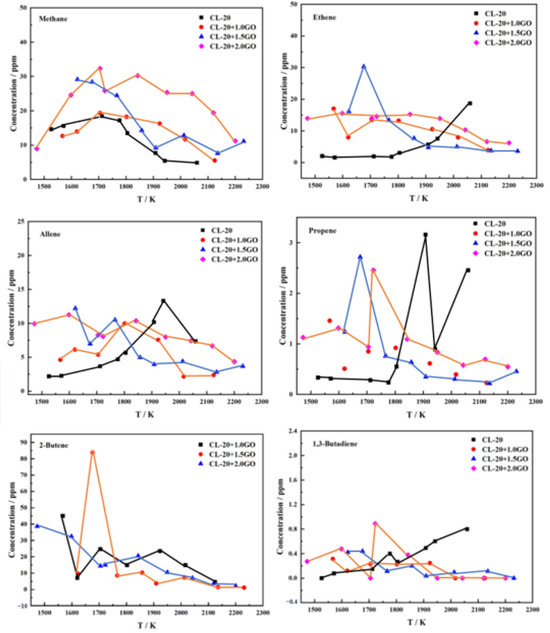
Figure 7.
Distribution of decomposition products as a function of temperature.
Figure 7 illustrates the abundance of hydrocarbon products, including allene, ethene, and methane, among the pyrolysis products. The content of pyrolysis products attains its maximum value at 1750 K. The pyrolysis products of CL-20@GO are the most sensitive to temperature. It can be concluded that at 1750 K, CL-20@GO is within the temperature range at which it is most susceptible to cracking. A considerable quantity of pyrolysis products can be obtained, as well as a certain amount of propylene, 1,3-butadiene, and other pyrolysis products that are difficult to observe at other temperatures.
The concentrations of the pyrolysis products, including allene, ethylene, and methane, exhibit a comparable pattern of change to that of the pyrolysis temperature, with concentrations increasing gradually as the temperature rises. Subsequently, the concentration of the products declines as the pyrolysis temperature rises. The maximum concentration of methane is observed at approximately 1700 K, with a GC-4 sample decomposing at this temperature to produce approximately 32 ppm methane. The temperature at which the highest concentration of ethylene is observed is approximately 1650 K, with 100 mg of GC-3 yielding approximately 30 ppm of the compound. In contrast, the temperature at which the highest concentration of allene is observed is higher, at approximately 1850 K, with 100 mg of GC-4 yielding up to 12 ppm of the compound. Furthermore, the temperature dependence of the concentrations of propene, 2-butene, and 1,3-butadiene exhibits a comparable pattern, with peaks occurring to some extent.
The high temperature cracking products follow a similar trend with temperature, reaching a certain peak in yield. In the distribution plot of 2-butene, sample GC-3 has a high cascade extra around 1700 K. The reason is presumed to be that the residual gas in the pipeline is not cleaned up. In terms of product content, methane, ethene, and allene are still the three main products with decreasing content in that order.
Concurrently, the gas chromatography analysis revealed that the yields of a minor carbon-nitrogen molecule, C2N2, were sufficiently elevated. Figure 8 illustrates the peak area of the resulting C2N2 plotted against the four samples, which is directly proportional to the direct yield of the decomposition product. It can thus be concluded that the yield of C2N2 increases gradually with the addition of GO. Furthermore, a considerable quantity of C2N2 molecules constituted a significant component of the decomposition products of GO@CL-20. This also provides some data support for subsequent analysis and speculation on the decomposition pathway.
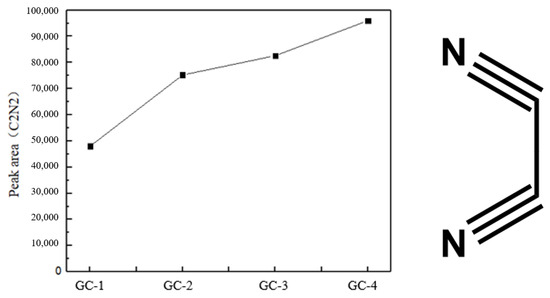
Figure 8.
Peak area of pyrolysis product C2N2 and the molecular structure of C2N2.
3.3. Sensitivity Analysis in Reaction
Upon completion of the high-temperature pyrolysis experiments in a single-pulse shock tube, we conducted an in-depth analysis of the pyrolysis process of the GO@CL-20 complex and its constituent molecules using CHEMKIN 2020 software and the NUIGMech 1.1 chemical kinetics mechanism. This approach enabled us to accurately simulate and analyze the concentration distribution of pyrolysis products as a function of temperature. The simulation provided valuable insights into how varying temperatures influence the concentrations of pyrolysis products during fuel pyrolysis, which is essential for understanding and optimizing propellant performance across different applications. Additionally, we performed numerical sensitivity analyses to examine the unique characteristics of the fuel pyrolysis process. By evaluating available chemical reaction mechanisms and the thermodynamic properties of gas mixtures, our objective was to identify the critical factors that impact the efficiency of fuel pyrolysis. Figure 9 and Figure 10 present the results obtained from simulation.
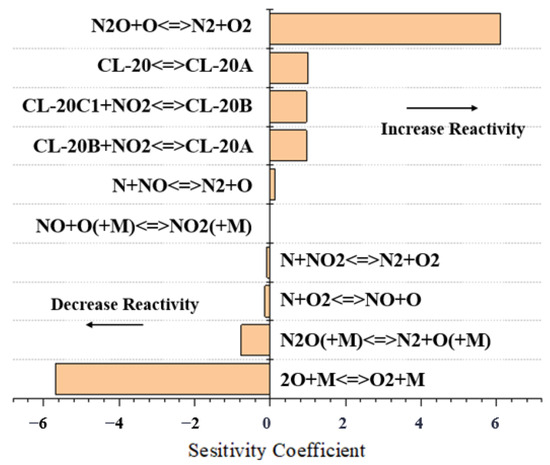
Figure 9.
Sensitivity analysis results of CL-20 for 2000 K at 10 bar.
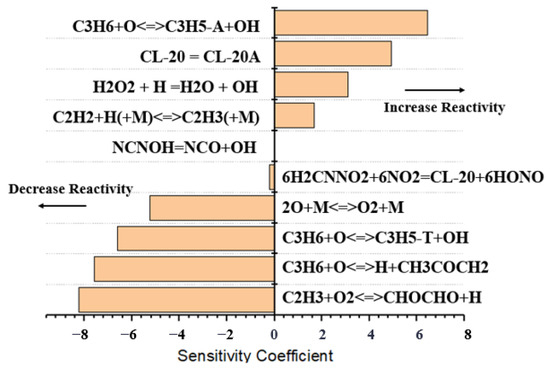
Figure 10.
Sensitivity analysis results of CL-20@GO for 2000 K at 10 bar.
The sensitivity coefficients on the horizontal axis of the figure represent the sensitivity of each radical reaction to the propellant pyrolysis conversion rate. In the sensitivity analysis of pure CL-20 reactions, the sensitivity coefficients of small-molecule nitrogen-containing reactions are higher, which plays an almost decisive role on the H-related reactions in the whole CL-20 decomposition reaction pathway, while for the decomposition reaction of CL-20@GO (GC-4, for example), in positively correlated reactions, the sensitivity of the C3H6 and O reaction (C3H6 + O <=> C3H5-A + OH) and the sensitivity coefficient of the C3H6-O reaction (C3H6 + O <=> C3H5-A + OH) in the positive correlation reaction is significantly higher than that of the other cleavage channels under the same conditions, reaching 6.46 at 3000 K. On the contrary, the sensitivity coefficient of the C2H3-O2 reaction in the negative correlation reaction is −8.24, which indicates that the GO has a very important impact on the high-temperature pyrolysis process of CL-20 to some extent. It is then involved in the subsequent reactions between the small molecules. Small molecules such as C2N2, the main product of CL-20, also showed high cracking rates in shock tube experiments.
3.4. Reaction Path Analysis
Given the significant presence and pivotal role of CL-20 and GO molecules in this type of propellant, their reaction paths and fluxes within the CL-20@GO composite at 2000 K and 10 bar nitrogen were modeled using the detailed kinetic mechanism implemented via CHEMKIN software. Specifically, the cracking reaction curves for both CL-20 and GO were plotted, along with the detailed kinetic mechanisms based on the preliminary construction of NEPE. The simulations captured the reaction paths and fluxes of CL-20 and GO within the composite under the specified conditions. Additionally, the respective cracking reactions, total reaction paths, and fluxes of CL-20 and GO were visualized. The visualization results are shown in Figure 11 and Figure 12.
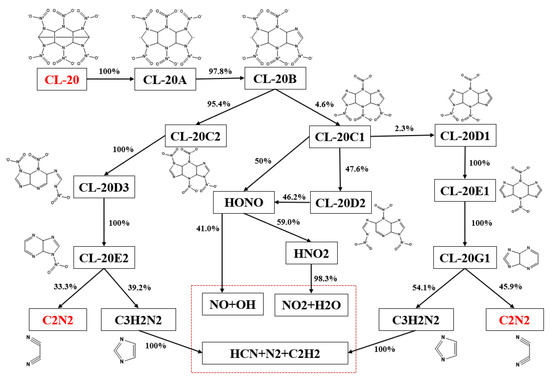
Figure 11.
Reaction paths and fluxes of CL-20.
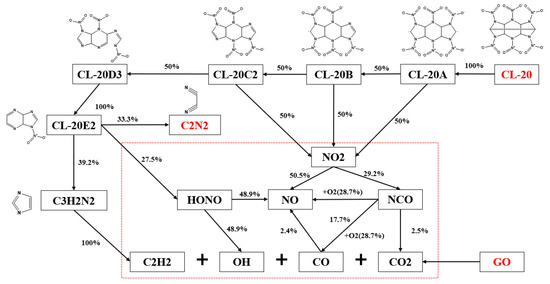
Figure 12.
Reaction paths and fluxes of GO@CL-20.
Due to the high-temperature environment, the tendency of CL-20 molecular decomposition is more favorable towards ring-opening reactions. Therefore, as shown in Figure 11, the initial decomposition of CL-20 primarily involves ring-opening to form CL-20A, which also has the potential to break the N-NO2 bond and directly generate H2CNNO2 through self-opening. Subsequently, a series of successive N-NO2 bond cleavage and ring-opening reactions occur. The resulting small molecule products are mainly NO2, C2N2, CO, H2, HCN, and N2, which are consistent with the high-temperature pyrolysis documented in the literature. On the other hand, the production of C2N2 aligns with the significant amounts observed in the experiments, further supporting the proposed initial decomposition pathway of this species.
In the initial stage of GO decomposition, a large number of hydrocarbon products are produced. From the basic mechanism, it decomposes in one step into functional products such as HCOOH and OH and hydrocarbon products such as C2H2 and CO2 and their derivatives. These small hydrocarbon products (e.g., C2H3 and C3H6) can contribute to the initial decomposition of CL-20 accordingly. These findings are consistent with the existing literature. The reaction channels are in complete agreement with the descriptions in the relevant literature. The presence of these small hydrocarbon molecules and C2N2 further corroborates the accuracy and reliability of the high-temperature pyrolysis pathways for CL-20 and GO@CL-20. This provides a solid foundation for future research into the combustion reaction kinetics of GO and CL-20.
4. Conclusions
The TG-DSC and single-pulse shock tube experiments conducted in this study have yielded the distribution of core pyrolysis products for the GO@CL-20 complex. Additionally, CHEMKIN simulations were employed to obtain sensitivity analyses, reaction pathways, and flux distributions. These results provide a solid foundation for subsequent quantum chemical calculations and serve as valuable references for model construction. The following conclusions can be drawn from the obtained data:
- (1)
- The thermal decomposition process of GO@CL-20 was investigated using thermogravimetric differential scanning calorimetry (TG-DSC). The results demonstrated that the addition of graphene oxide (GO) lowered the activation energy of the sample, thereby accelerating the thermal decomposition of the composite. Subsequently, single-pulse shock tube experiments were conducted to evaluate the ignition delay time distribution, providing detailed data on the pyrolysis product distribution of GO@CL-20. Analysis of the ignition delay time revealed that the incorporation of GO reduced the internal energy of the composite system, leading to decreased reactivity and extended ignition delay times. Notably, an important carbon-nitrogen molecule, C2N2, was identified in the pyrolysis products, with its yield increasing progressively as the concentration of GO increased.
- (2)
- Mass transfer characteristics and sensitivity analysis of GO@CL-20 samples were performed using CHEMKIN software to preliminarily determine the pyrolysis reaction pathways. The results demonstrated that the introduction of graphene oxide (GO) significantly altered the thermal decomposition behavior of the entire system. Additionally, C2N2 exhibited a notably high decomposition rate along this reaction pathway, findings which are in excellent agreement with experimental observations. Meanwhile, H2CNNO2 played a crucial bridging role between the two macromolecules.
- (3)
- The fitting results obtained using the Kissinger and Ozawa equations show close agreement. Specifically, the average activation energy for the thermal decomposition of CL-20 is 209.98 kJ/mol. In comparison, the average activation energies of GC-2, GC-3, and GC-4 are lower than that of pure CL-20. Notably, the activation energy for the thermal decomposition of GC-4 is reduced by 34.60 kJ/mol. These findings indicate that the introduction of graphene oxide exerts a catalytic effect on the thermal decomposition of CL-20.
Author Contributions
Conceptualization, X.F. and X.A.; methodology, X.A.; software, X.B.; validation, R.H. and Z.G.; data curation, X.A.; writing—original draft preparation, X.A.; writing—review and editing, X.F. and Z.G.; visualization, X.A.; funding acquisition, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 06101213).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Specially acknowledge to the Xi’an Institute of Modern Chemistry and Northwestern Polytechnical University for the experimental materials and equipment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nielsen, A.T.; Chafin, A.P.; Christian, S.L.; Moore, D.W.; Nadler, M.P.; Nissan, R.A. Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 1998, 54, 11793–11812. [Google Scholar] [CrossRef]
- Bennion, J.C.; Chowdhury, N.; Kampf, J.W.; Matzger, A.J. Hydrogen Peroxide Solvates of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12- hexaazaisowurtzitane. Angew. Chem. 2016, 55, 13118–13121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Deng, M.; Qi, X.; Nie, F.; Zhang, Q. A promising high-energy-density material. Nat. Commun. 2017, 8, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Bazaki, H.; Kawabe, S.; Miya, H.; Kodama, T. Synthesis and Sensitivity of Hexanitrohexaaza-isowurtzitane (HNIW). Propellants Explos. Pyrotech. 1998, 23, 333–336. [Google Scholar] [CrossRef]
- Vandewiele, M.N.; Magoon, R.G.; Geem, V.M.K. Kinetic Modeling of Jet Propellant-10 Pyrolysis. Energy Fuels 2014, 29, 1. [Google Scholar] [CrossRef]
- Vandewiele, M.N.; Magoon, R.G.; Geem, V.M.K. Experimental and Modeling Study on the Thermal Decomposition of Jet Propellant-10. Energy Fuels 2014, 28, 8. [Google Scholar] [CrossRef]
- Liang, J.; Cao, S.; Li, F. A comparative single-pulse shock tube experiment and kinetic modeling study on pyrolysis of cyclohexane, methylcyclohexane and ethylcyclohexane. Def. Technol. 2023, 20, 137–148. [Google Scholar] [CrossRef]
- Niklas, Z.; Christer, F.; Jen, K. Small Skeletal Kinetic Reaction Mechanism for Ethylene-Air Combustion. Energy Fuels 2017, 31, 12. [Google Scholar]
- Kee, R.J.; Rupley, F.M.; Miller, J.A. CHEMKIN-III: A FORTRAN chemical kinetics. package for the analysis of gas-phase chemical and plasma kinetics. Report No. SAND-96-8216; Sandia National Lab: Livermore, CA, USA, 1996; pp. 10–40. [Google Scholar] [CrossRef]
- Zhou, C.W.; Li, Y.; Burke, U. An experimental and chemical kinetic modeling study of 1, 3-butadiene combustion: Ignition delay time and laminar flame speed measurements. Combust. Flame 2018, 197, 423–438. [Google Scholar] [CrossRef]
- Yionon, J.; Yost, R.A.; Bulusu, S. The thermal decomposition of explosives by pyrolysis gas chromatography–mass spectrometry. J. Chromatogr. A 1994, 688, 231–242. [Google Scholar] [CrossRef]
- Huwei, L.; Ruonong, F. Investigation of thermal decomposition of HMX and RDX by pyrolysis gas chromatography. Thermochim. Acta 1989, 138, 167–171. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.W.; Somers, K.P. The oxidation of 2-butene: A high pressure ignition delay, kinetic modeling study and reactivity comparison with isobutene and 1-butene. Proc. Combust. Inst. 2017, 36, 403–411. [Google Scholar] [CrossRef]
- Gao, H.; Li, S.; Yao, Z. Experimental Study on Thermal and Catalytic Decomposition of a Dual-Mode Ionic Liquid Propellant. E3S Web Conf. 2021, 257, 01041. [Google Scholar] [CrossRef]
- Yu, J.Y.; Wang, J.H.; Liu, Y.C. Preparation and Thermal Properties of CL-20/GO Nano-composite Energetic Materials. Sci. Technol. Eng. 2017, 17, 93–96. [Google Scholar]
- Yu, J.Y. Preparation and Properties of CL-20/Open-Multiwalled Carbon Nanotubes Composite Energetic Materials. Chin. J. Explos. Propellants 2017, 40, 72–76. [Google Scholar] [CrossRef]
- Memon, N.K.; McBain, A.W.; Son, S.F. Graphene oxide/ammonium perchlorate composite material for use in solid propellants. J. Propuls. Power 2016, 32, 682–686. [Google Scholar] [CrossRef]
- Zhang, G. Theoretical study of the gas-phase pyrolysis initiation reaction of hexanitrohexaaza-isowoodzane. Energy-Contain. Mater. 2000, 8, 149–154. [Google Scholar]
- Yalamanchi, K.K.; Li, Y.; Wang, T. Large-scale thermochemistry calculations for combustion models. Appl. Energy Combust. Sci. 2022, 12, 100084. [Google Scholar]
- Wang, E. NEPE propellant combustion mechanism study. J. Fire 2000, 4, 24–26. [Google Scholar]
- Gough, R.V.; Widegren, J.A.; Bruno, T.J. Thermal Decomposition Kinetics of the Thermally Stable Jet Fuels JP-7, JP-TS and JP-900. Energy Fuels 2014, 28, 5. [Google Scholar] [CrossRef]
- Wu, F.; Wang, S.Y.; Pang, A.M. Survey of NEPE propellant combustion performance. Flight Missile 2003, 7, 51–55. [Google Scholar]
- Weinrottera, M.; Kopeceka, H.; Wintnera, E. Application of laser Ignition to hydrogen-air mixtures at high pressures. Int. J. Hydrogen Energy 2005, 30, 319–326. [Google Scholar] [CrossRef]
- Phuoc, T.X. Laser-induced spark ignition fundamental and applications. Opt. Lasers Eng. 2006, 44, 351–397. [Google Scholar] [CrossRef]
- Ulas, A.; Kuo, K.K. Laser-induced ignition of solid propellants for gas generators. Fuel 2008, 88, 639–646. [Google Scholar] [CrossRef]
- Zhuang, X. Shock tube experiment and dynamic simulation of JP-10 cracking. Chin. J. Mech. Mech. 2019, 51, 85–93. [Google Scholar]
- Liang, J.H. Study on ignition characteristics of RP-3 aviation kerosene. Chin. J. Power 2014, 46, 352–360. [Google Scholar]
- Han, X.; Miroslaw, L.; Xu, R. A high pressure shock tube study of pyrolysis of real jet fuel Jet A. Proc. Combust. Inst. 2018, 37, 189–196. [Google Scholar] [CrossRef]
- He, R.N. Pyrolysis Characteristics Study of Allene and Propyne Based on Single Pulse Shock Tube. Master’ Thesis, North University of China, Taiyuan, China, 2022. [Google Scholar] [CrossRef]
- Tang, W.; Brezinsky, K. Chemical kinetic simulations behind reflected shock waves. Int. J. Chem. Kinet. 2010, 38, 75–97. [Google Scholar] [CrossRef]
- Panigrahy, S.; Liang, J.; Nagaraja, S.S. A comprehensive experimental and improved kinetic modeling study on the pyrolysis and oxidation of propyne. Proc. Combust. Inst. 2020, 38, 479–488. [Google Scholar] [CrossRef]
- Koga, N. Ozawa’s kinetic method for analyzing thermoanalytical curves: History and theoretical fundamentals. J. Therm. Anal. Calorim. 2013, 113, 1527–1541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).