Mapping Frozen Fish Quality via Machine Learning for Predictive Spoilage Kinetics Under Subzero Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Dataset for Computational Analyses
2.2. Raw Material and Sample Preparation
2.3. Physicochemical Analyses
2.4. Biochemical Analyses
2.4.1. Determination of Total Volatile Basic-Nitrogen (TVB-N)
2.4.2. Determination of Trimethylamine-Nitrogen (TMA-N)
2.4.3. Determination of Thiobarbituric Acid (TBA)
2.4.4. Determination of Free Fatty Acids (FFA)
2.5. Statistical Analyses
2.6. Machine Learning (ML) Modeling
2.6.1. Dataset Preparation
2.6.2. Implemented Algorithms
2.6.3. Hyperparameter Optimization
2.6.4. Model Training and Validation
3. Results
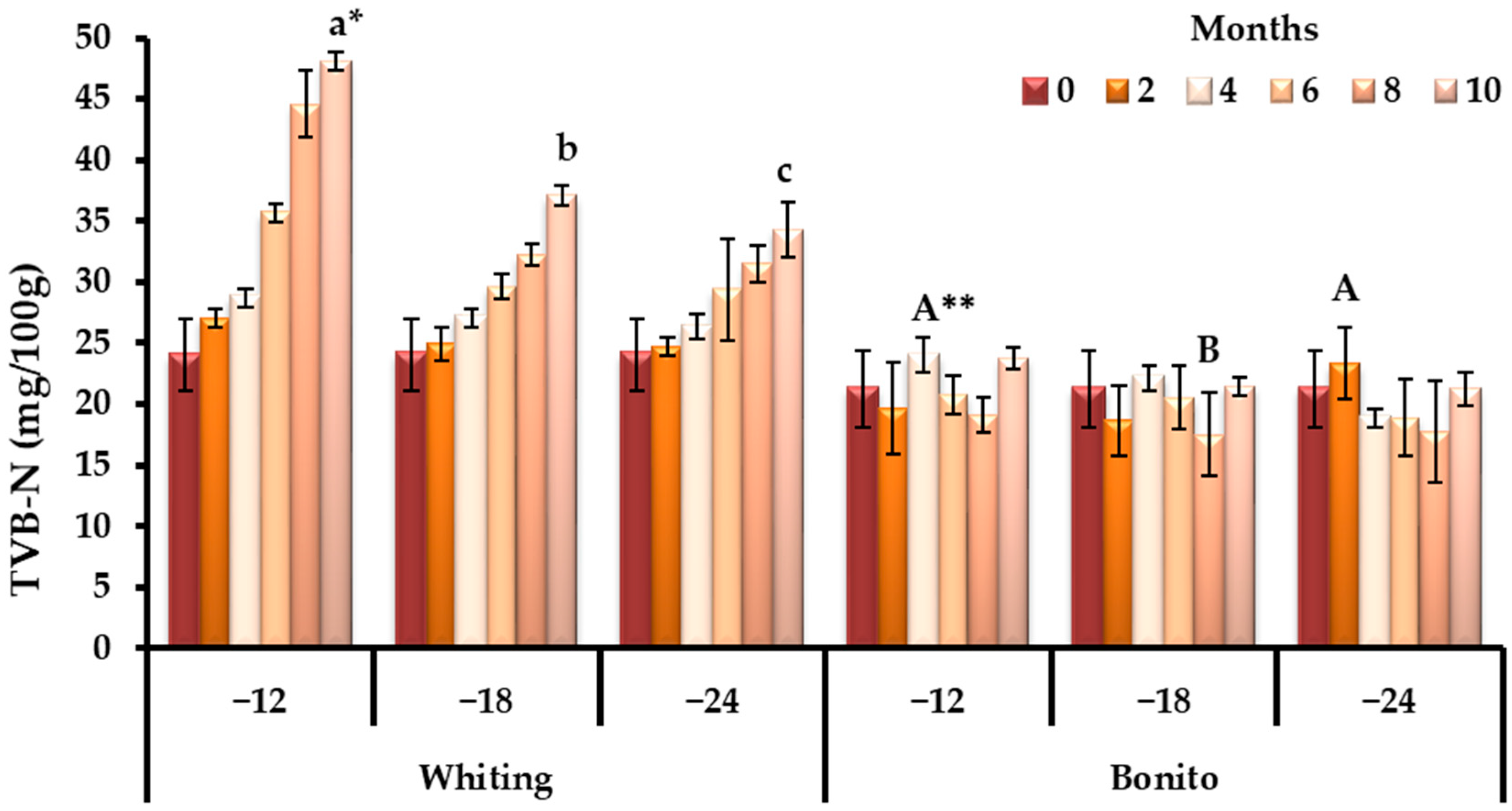
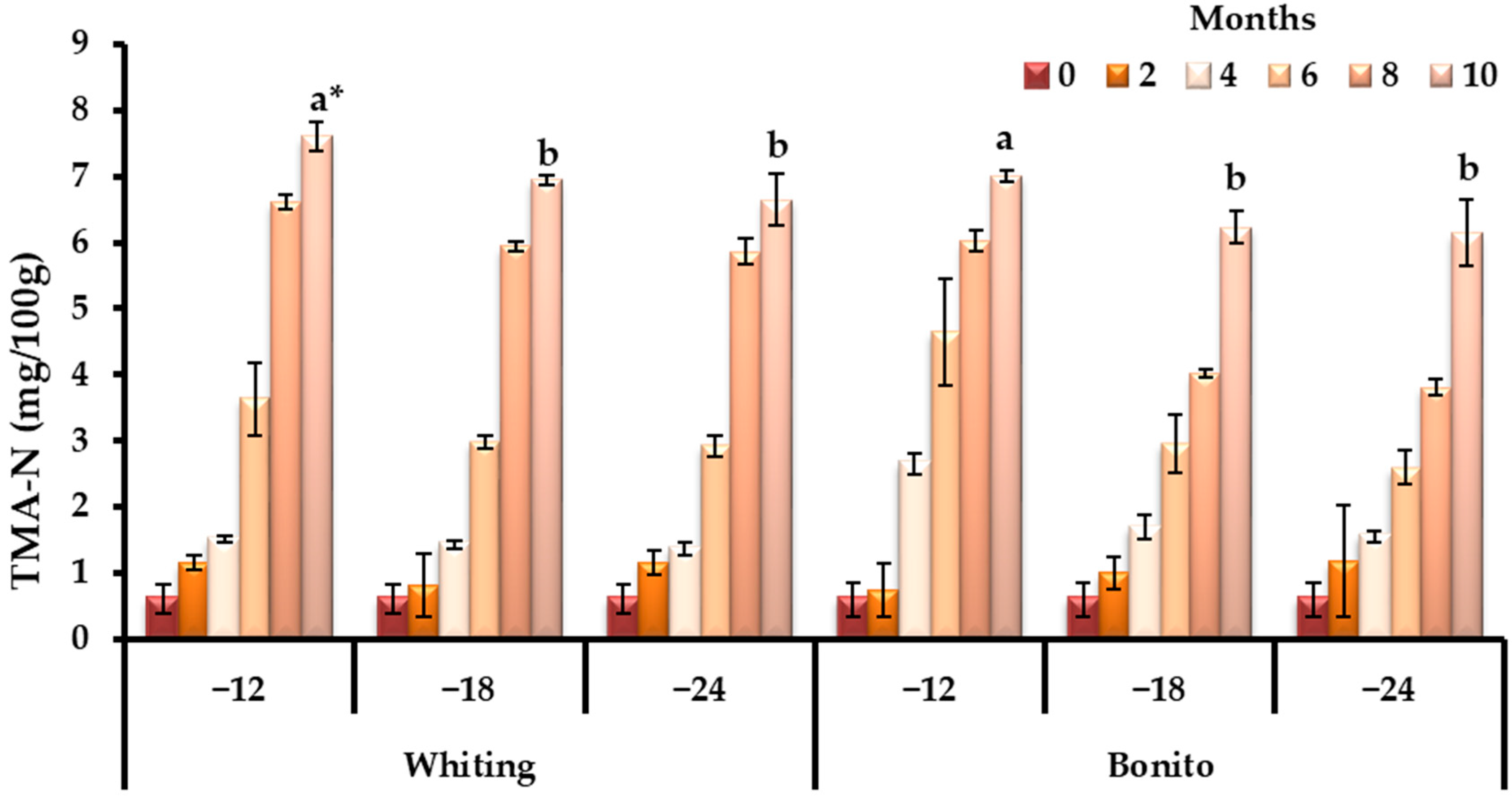
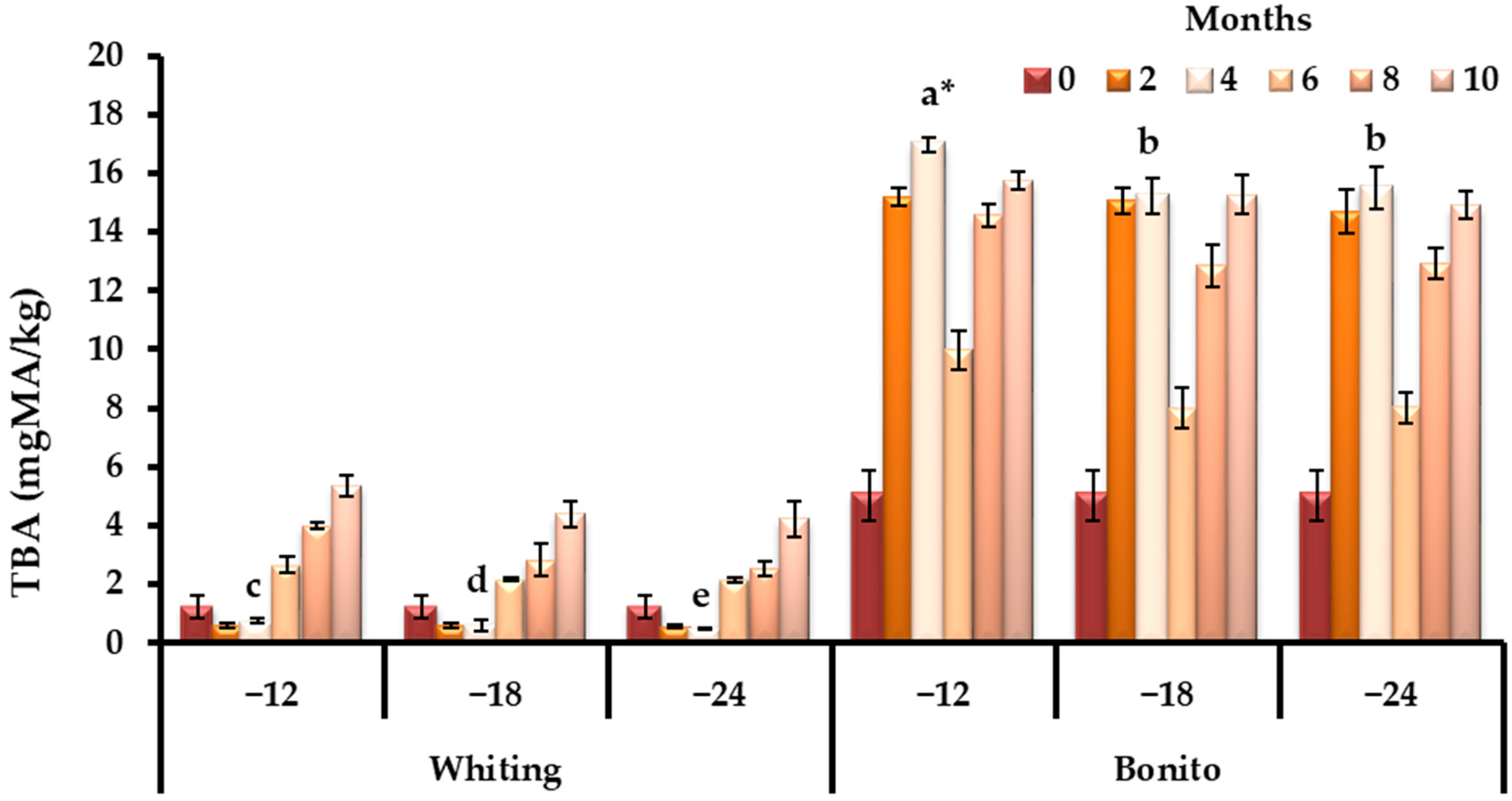
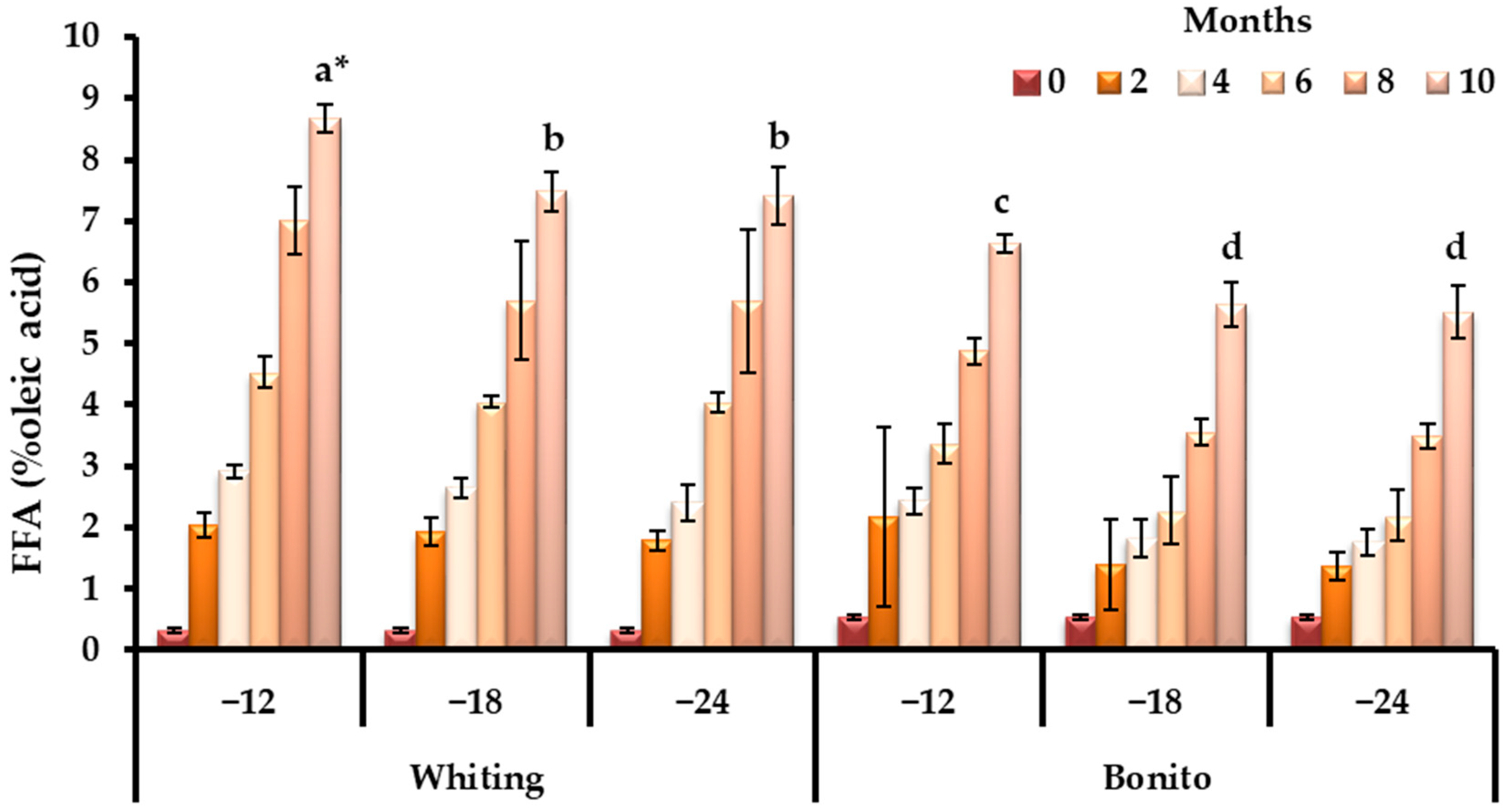
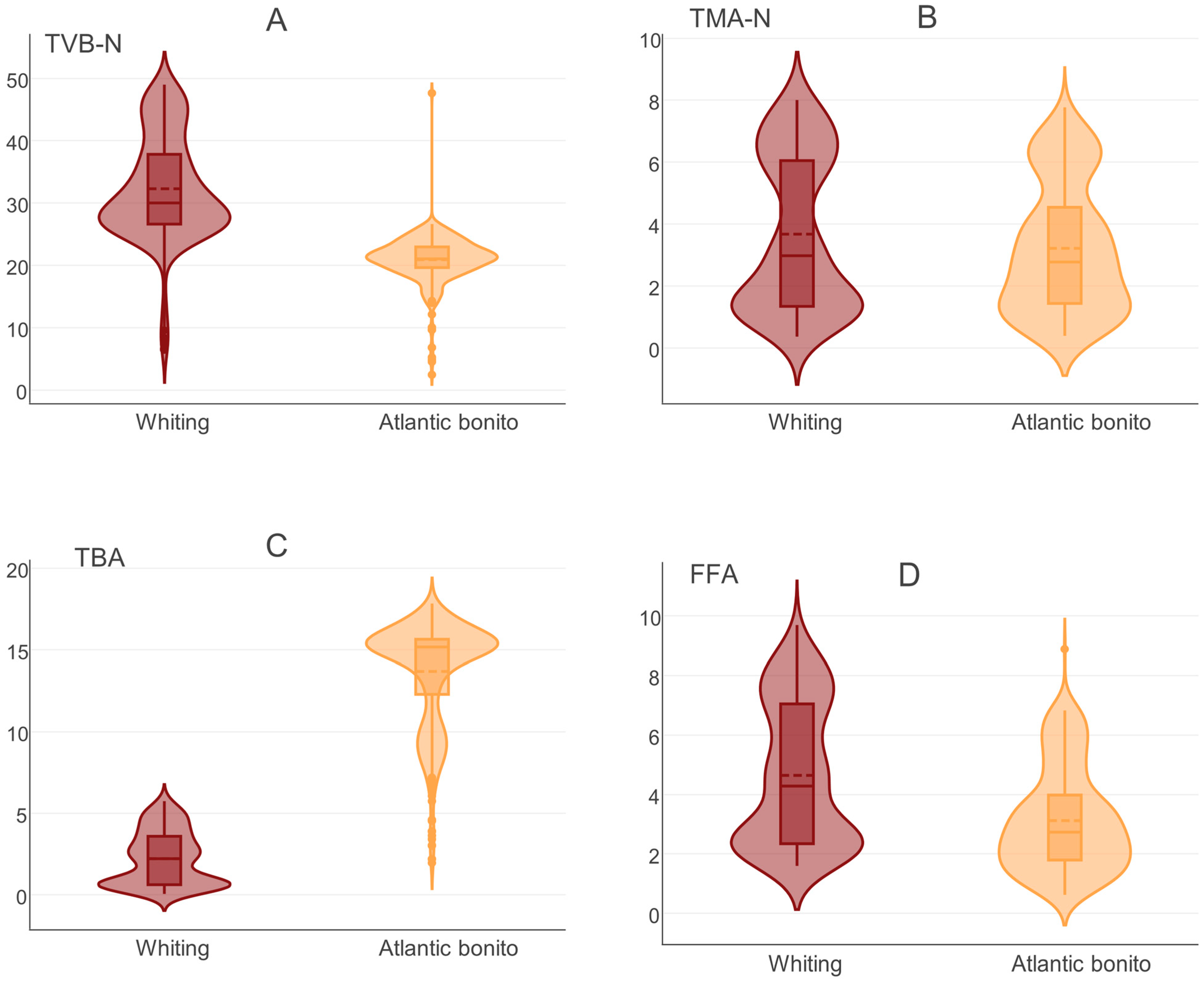
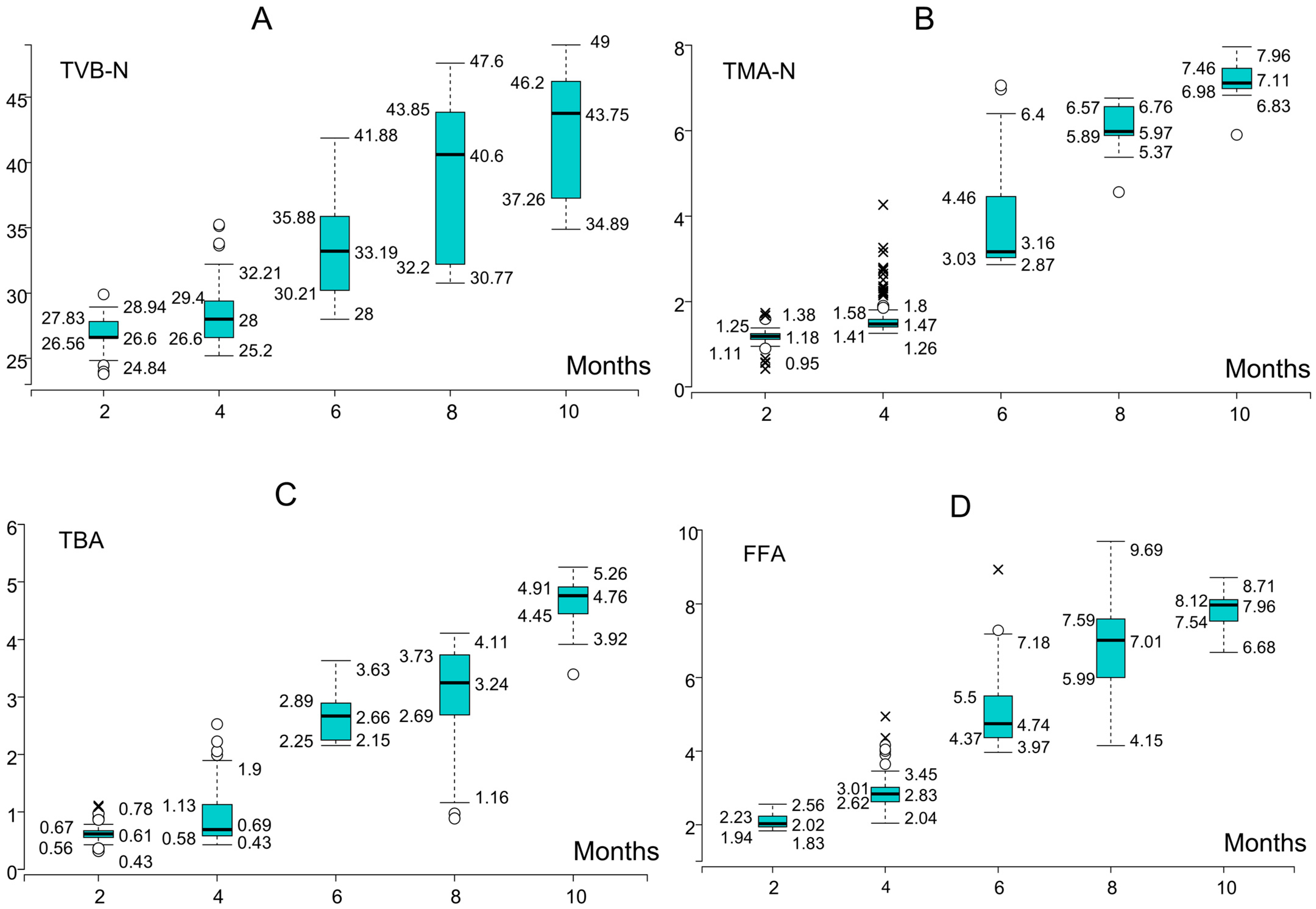
3.1. Changes in Biochemical Quality Indicators During Storage
3.2. Descriptive Statistics
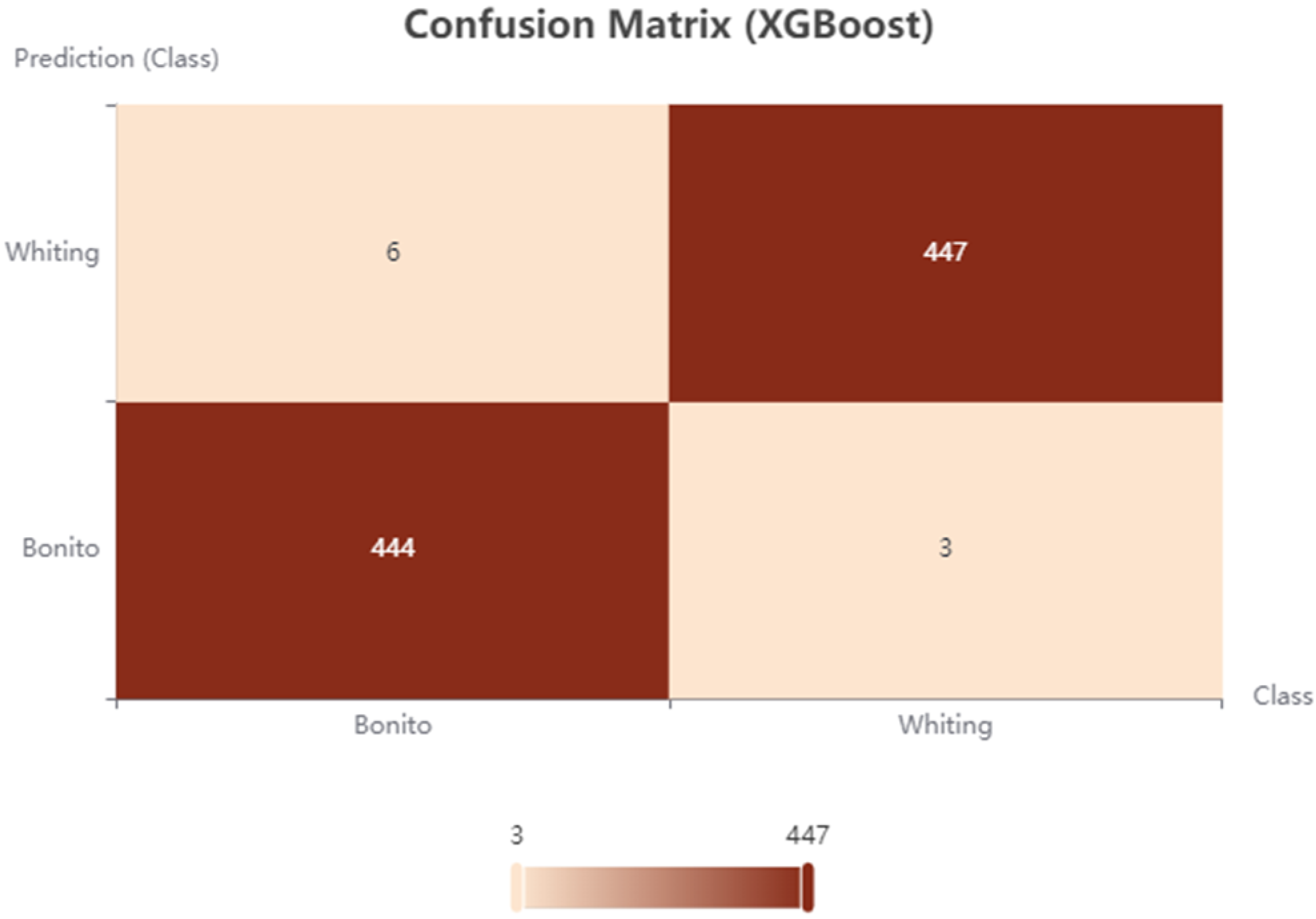
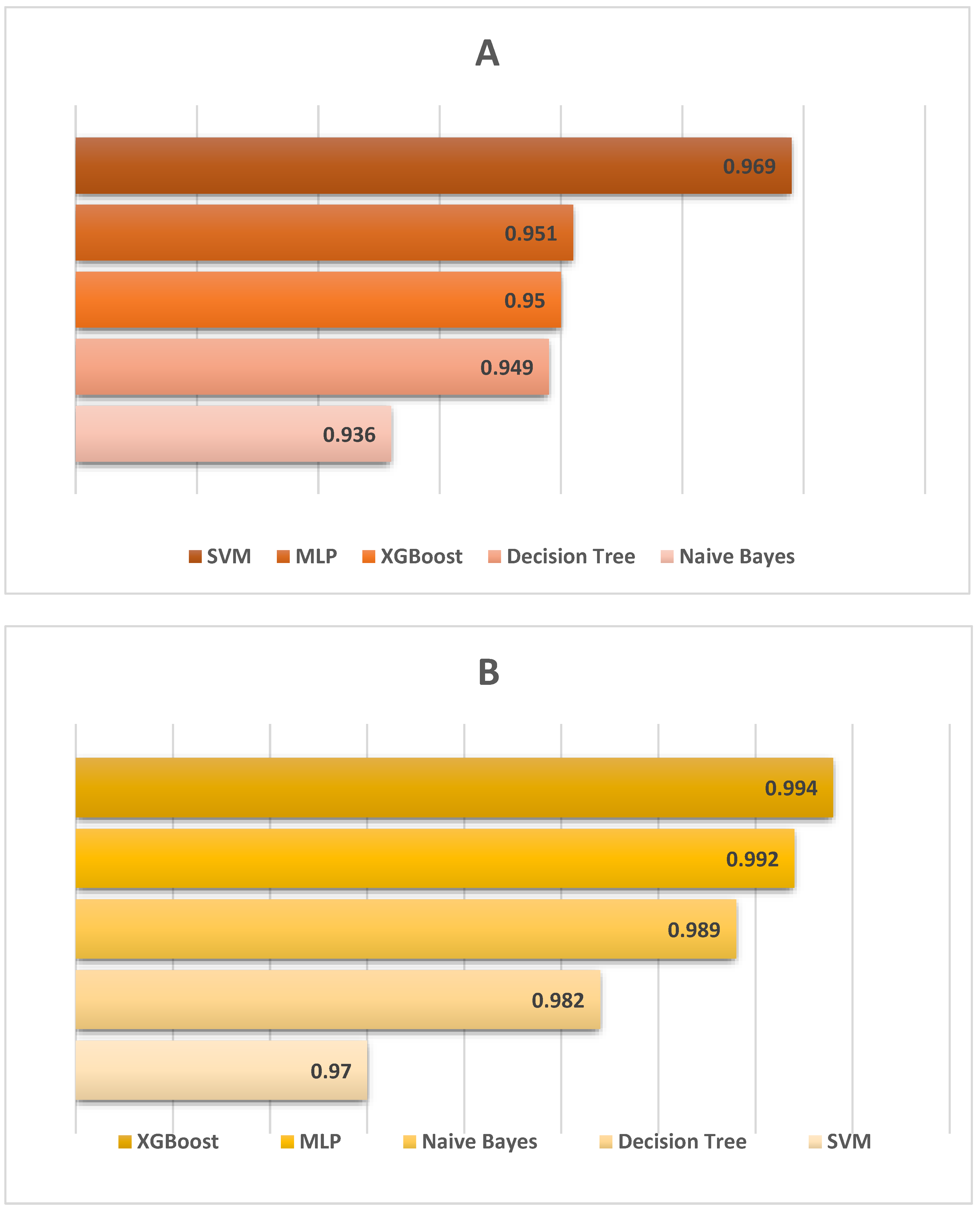
3.3. Machine Learning (ML) Classification Performance
3.4. Machine Learning (ML) Regression Performance
3.5. Model Performance Visualization and Interpretability
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TVB-N | Total Volatile Basic Nitrogen |
| TMA-N | Trimethylamine Nitrogen |
| TBA | Thiobarbituric Acid |
| FFA | Free Fatty Acids |
| AOAC | Association of Official Analytical Chemists |
| TCA | Trichloracetic Acid |
| H2SO4 | Sulfuric Acid |
| MA | Malondialdehyde |
| NaOH | Sodium Hydroxide |
| N × 6.25 | Nitrogen-to-Protein Conversion Factor (for crude protein) |
| HCl | Hydrochloric Acid |
| UV/VIS | Ultraviolet–Visible (spectrophotometer) |
| PUFA | Polyunsaturated Fatty Acids |
| RF | Random Forest |
| NIR | Near-Infrared Spectroscopy |
| MRI | Magnetic Resonance Imaging |
| UV | Ultraviolet |
| TBARS | Thiobarbituric Acid Reactive Substances |
| WHC | Water Holding Capacity |
| TVC | Total Viable Counts |
| EC | Electrical Conductivity |
| K-value | ATP Degradation Index |
| BP NN | Backpropagation Neural Network |
| LSTM | Long Short-Term Memory network |
| RBFNN | Radial Basis Function Neural Network |
| SVR | Support Vector Regression |
| CNN | Convolutional Neural Network |
| MPLS | Modified Partial Least Squares |
| SVM | Support Vector Machine |
| NB | Naïve Bayes |
| DT | Decision Tree |
| MLP | Multilayer Perceptron |
| XGBoost | Extreme Gradient Boosting |
| SRT | Simple Regression Tree |
| PR | Polynomial Regression |
| RFR | Random Forest Regression |
| SMOTE | Synthetic Minority Oversampling Technique |
| RBF | Radial Basis Function (kernel) |
| ETSFormer | Transformer Architecture Based on Exponential Smoothing |
| MAE | Mean Absolute Error |
| RMSE | Root Mean Square Error |
| MAPE | Mean Absolute Percentage Error |
| R2 | Coefficient of Determination |
| F1 | F1-score (harmonic mean of precision and recall) |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| CV | Cross-Validation |
| κ (Kappa) | Cohen’s Kappa Coefficient |
| ACC | Accuracy |
| P (PPV) | Precision/Positive Predictive Value |
| R (Recall) | Recall/Sensitivity/True Positive Rate |
| AUC-ROC | Area Under the Receiver Operating Characteristic Curve |
| TP | True Positive |
| TN | True Negative |
| FP | False Positive |
| FN | False Negative |
References
- Ramadhan, A.H.; Yu, D.; Hlaing, K.S.S.; Jiang, Q.; Xu, Y.; Xia, W. Effects of packaging and storage time on lipid and protein oxidation and modifications in texture characteristics of refrigerated grass carp (Ctenopharyngodon idellus) fish muscles. Int. J. Food Sci. Technol. 2025, 60, vvaf082. [Google Scholar] [CrossRef]
- Wei, H.; Tian, Y.; Yamashita, T.; Ishimura, G.; Sasaki, K.; Niu, Y.; Yuan, C. Effects of thawing methods on the biochemical properties and microstructure of pre-rigor frozen scallop striated adductor muscle. Food Chem. 2020, 319, 126559. [Google Scholar] [CrossRef]
- Çaklı, Ş. Su Ürünleri Işleme Teknolojisi; Ege Üniversitesi Yayınları, Su Ürünleri Fakültesi: Bornova, Türkiye, 2008; Yayın No: 77; p. 513. (In Turkish) [Google Scholar]
- Nakazawa, N.; Okazaki, E. Recent research on factors influencing the quality of frozen seafood. Fish. Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef]
- Tian, J.; Walayat, N.; Ding, Y.; Liu, J. The role of trifunctional cryoprotectants in the frozen storage of aquatic foods: Recent developments and future recommendations. Compr. Rev. Food Sci. Food Saf. 2022, 21, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Varlık, C. Balık ve kanatlı etlerinin soğutulması, dondurulması ve depolanması. In Proceedings of the Soğuk Tekniği Uygulamaları Semineri, April 1987; Tübitak Marmara Araştırma Enstitüsü: Gebze, Türkiye, 1987; pp. 1–11. (In Turkish). [Google Scholar]
- Sikorski, Z.E.; Pan, B.S. Preservation of seafood quality. In Seafoods: Chemistry, Processing, Technology and Quality; Shahidi, F., Botta, J.R., Eds.; Chapman & Hall: Boca Raton, FL, USA, 1994; pp. 335–360. [Google Scholar]
- Liciardello, J.J. The Seafood Industry; Van Nostrand Reinhold: New York, NY, USA, 1990; p. 435. [Google Scholar]
- Göğüş, A.K.; Kolsarıcı, N. Su Ürünleri Teknolojisi; Ankara Üniversitesi Ziraat, Fakültesi Yayınları: Ankara, Türkiye, 1992; p. 358. (In Turkish) [Google Scholar]
- Uzunkuşak, A. Gıda maddelerinin dondurulması prensipleri ve donmuş gıda endüstrisinin tekamülü. Et Endüstrisi Derg. 1971, 5, 29–36. (In Turkish) [Google Scholar]
- Gülyavuz, H.; Ünlüsayın, M. Su Ürünleri Işleme Teknolojisi; Süleyman Demirel Üniversitesi, Eğirdir Su Ürünleri Fakültesi: Isparta, Türkiye, 1999. (In Turkish) [Google Scholar]
- French, J.S.; Kramer, D.E.; Kennish, J.M. Protein hydrolysis in coho and sockeye salmon during partially frozen storage. J. Food Sci. 1988, 53, 1014–1017. [Google Scholar] [CrossRef]
- Ludorff, W.; Meyer, V. Fische und Fischerzeugnisse; AVI Publishing Company: New York, NY, USA, 1973. [Google Scholar]
- Khayat, A.; Schwall, D. Lipid oxidation in seafood. Food Technol. 1983, 37, 130–140. [Google Scholar]
- Shamberger, R.J.; Shamberger, B.A.; Willis, C.E. Antioxidants and cancer. IV. Initiating activity of malonaldehyde as a carcinogen. J. Natl. Cancer Inst. 1974, 53, 177–183. [Google Scholar]
- Hultin, H.O. Oxidation of lipids in seafoods. In Seafoods: Chemistry, Processing Technology and Quality; Shahidi, F., Botta, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 49–74. [Google Scholar]
- Yang, Z.; Yan, J.; Xie, J. Effect of vacuum and modified atmosphere packaging on moisture state, quality, and microbial communities of grouper (Epinephelus coioides) fillets during cold storage. Food Res. Int. 2023, 173, 113340. [Google Scholar] [CrossRef]
- Sikorski, Z.E. Seafood: Resources, Nutritional Composition, and Preservation; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Verma, J.; Srikar, L.N. Protein and lipid changes in pink perch (Nemipterus japonicus) mince during frozen storage. J. Food Sci. Technol. 1994, 31, 238–240. [Google Scholar]
- Simeonidou, S.; Govaris, A.; Vareltzis, K. Effect of frozen storage on the quality of whole fish and fillets of horse mackerel (Trachurus trachurus) and Mediterranean hake (Merluccius mediterraneus). Z. Lebensm. Unters. ForschA 1997, 204, 405–410. [Google Scholar] [CrossRef]
- Polymenidinis, A.; Kotter, H.C.L.; Terplan, G. Kühlen und Gefrieren von Fleisch. Fleischwirtschaft 1978, 5, 702–711. (In German) [Google Scholar]
- Bland, J.M.; Yagiz, Y.; Balaban, M.O.; Marshall, M.R.; Otwell, W.S. Comparison of sensory and instrumental methods for the analysis of texture of cooked individually quick frozen and fresh-frozen catfish fillets. Food Sci. Nutr. 2018, 6, 1692–1705. [Google Scholar] [CrossRef]
- Zhuang, S.; Guo, H.; Wang, Y.; Zhou, Y.; Liu, J.; Liu, Z.; Luo, Y. Spoilage-related microbiota in fish and crustaceans during storage: Research progress and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 252–288. [Google Scholar] [CrossRef]
- Sikorski, Z.E.; Kolakowska, A. Freezing of marine food. In Seafood: Resources, Nutritional Composition and Preservation; Sikorski, Z.E., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 111–124. [Google Scholar]
- Sikorski, Z.E.; Kolakowska, A. Changes in proteins in frozen stored fish. In Seafood Proteins; Sikorski, Z.E., Ed.; Chapman & Hall: Boca Raton, FL, USA, 1994; pp. 234–254. [Google Scholar]
- Connel, J.J. Changes in the eating quality of frozen stored cod and associated chemical and physical changes. In Freezing and Irradiation of Fish; Fishing News (Books); FAO: Rome, Italy, 1969; pp. 323–336. [Google Scholar]
- Ke, P.J.; Ackman, R.G.; Linke, B.A.; Nash, D.M. Differential lipid oxidation in various parts of frozen mackerel. J. Food Technol. 1977, 12, 37–47. [Google Scholar] [CrossRef]
- Tolstorebrov, I.; Eikevik, T.M.; Bantle, M. Effect of low and ultra-low temperature applications during freezing and frozen storage on quality parameters for fish. Int. J. Refrig. 2016, 63, 37–47. [Google Scholar] [CrossRef]
- Alsailawi, H.; Mudhafar, M.; Abdulrasool, M. Effect of frozen storage on the quality of frozen foods—A review. J. Chem. 2020, 14, 86–96. [Google Scholar] [CrossRef]
- Kundakçı, A. Haskefal ve sazan balıklarının dondurularak saklanması sırasında lipidlerdeki değişmeler. Doctoral Dissertation, Ege Üniversitesi, İzmir, Türkiye, 1979. (In Turkish). [Google Scholar]
- Ciarlo, A.; Boeri, R.; Giannini, D. Storage life of frozen blocks of Patagonian hake (Merluccius hubbsi) filleted and minced. J. Food Sci. 1985, 50, 723–726. [Google Scholar] [CrossRef]
- Licciardello, J.J.; Elinor, M.R. Frozen storage characteristics of cownose ray (Rhinoptera bonasus). J. Food Qual. 1988, 11, 71–76. [Google Scholar] [CrossRef]
- Fazal, A.; Srikar, L.N. Changes in flesh lipids of seer fish during frozen storage. J. Food Sci. Technol. 1987, 24, 321–324. [Google Scholar]
- Varlık, C.; Yolcular, H. Dondurulmuş lüfer ve hamsinin depolanması. Gıda Sanayi 1987, 2, 39–42. (In Turkish) [Google Scholar]
- Vareltzis, K.; Zetou, F.; Tsiaras, I. Textural deterioration of chub mackerel (Scomber japonicus collias) and smooth hound (Mustellus mustellus L.) in frozen storage in relation to chemical parameters. Lebensm.-Wiss. Technol. 1988, 21, 206–211. [Google Scholar]
- Srikar, L.N.; Seshadari, H.; Fazal, A. Changes in lipids and proteins of marine catfish (Tachysurus dussumieri) during frozen storage. Int. J. Food Sci. Technol. 1989, 24, 653–658. [Google Scholar] [CrossRef]
- Liu, D.; Wu, T.; Wang, C.; Yu, Y.; Li, H. Effects of ultrasound treatment on muscle structure, volatile compounds, and small molecule metabolites of salted Culter alburnus fish. Ultrason. Sonochem. 2023, 97, 106440. [Google Scholar] [CrossRef] [PubMed]
- Konig, A.J.; Mol, T.H. Quantitative quality tests for frozen fish: Soluble protein and free fatty acid content as quality criteria for hake (Merluccius capensis) stored at –18 °C. J. Sci. Food Agric. 1991, 54, 449–458. [Google Scholar] [CrossRef]
- Boran, M. Farklı işlem uygulanarak dondurulan hamsilerde muhafaza süresince oluşan kalite değişiklikleri üzerine bir araştırma. Master’s Thesis, Karadeniz Teknik Üniversitesi, Trabzon, Türkiye, 1991. (In Turkish). [Google Scholar]
- Polvi, S.M.; Ackman, R.G.; Lall, S.P.; Saunders, R.L. Stability of lipids and omega–3 fatty acids during frozen storage of Atlantic salmon. J. Food Process. Preserv. 1991, 15, 167–181. [Google Scholar] [CrossRef]
- Çaklı, Ş.; Tokur, B.; Çelik, U.; Taşkaya, L. No-frost koşullarda depolanan sardalya balıklarının (Sardina pilchardus) fiziksel, kimyasal ve duyusal değerlendirilmesi. In Proceedings of the Akdeniz Balıkçılık Kongresi Bildirileri, İzmir, Türkiye, 9–11 April 1997; pp. 733–740. (In Turkish). [Google Scholar]
- Simunovic, S.; Jankovic, S.; Baltic, T.; Nikolic, D.; Djinovic-Stojanovic, J.; Lukic, M.; Parunovic, N. Histamine in canned and smoked fishery products sold in Serbia. Meat Technol. 2019, 60, 8–16. [Google Scholar] [CrossRef]
- Careche, M.; Li-Chan, E.C.Y. Structural changes in cod myosin after modification with formaldehyde or frozen storage. J. Food Sci. 1997, 62, 717–723. [Google Scholar] [CrossRef]
- Álvarez, C.; Aubourg, S.P.; Pérez-Martín, R.I.; Gallardo, J.M.; Sotelo, C.G. Consequences of frozen storage for nutritional value of hake. Food Sci. Technol. Int. 1999, 5, 493–499. [Google Scholar] [CrossRef]
- Dönmez, M.; Tatar, O. Fileto ve bütün olarak dondurulmuş gökkuşağı alabalığının (Oncorhynchus mykiss W.) muhafazası süresince yağ asitleri bileşimlerindeki değişmelerin araştırılması. Ege J. Fish. Aquat. Sci. 2001, 18, 1–8. (In Turkish) [Google Scholar]
- Baygar, T.; Özden, Ö.; Üçok, D. Dondurma ve çözündürme işleminin balık kalitesi üzerine etkisi. Turk. J. Vet. Anim. Sci. 2004, 28, 173. (In Turkish) [Google Scholar]
- Aubourg, S.P.; Piñeiro, C.; González, M.J. Quality loss related to rancidity development during frozen storage of horse mackerel (Trachurus trachurus). J. Am. Oil Chem. Soc. 2004, 81, 671–678. [Google Scholar] [CrossRef]
- Puchała, R.; Białowąs, H.; Pilarczyk, M. Influence of cold and frozen storage on carp (Cyprinus carpio) flesh quality. Pol. J. Food Nutr. Sci. 2005, 55, 103–106. [Google Scholar]
- Geirsdottir, M.; Hlynsdottir, H.; Thorkelsson, G.; Sigurgisladottir, S. Solubility and viscosity of herring (Clupea harengus) proteins as affected by freezing and frozen storage. J. Food Chem. Toxicol. 2007, 72, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Baron, C.P.; Kjærsgaard, I.V.H.; Jessen, F.; Jacobsen, C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J. Agric. Food Chem. 2007, 55, 8118–8125. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.A.F.; Sant’Ana, H.M.P.; Campos, F.M.; Costa, N.M.B.; Silva, M.T.C.; Salaro, A.L.; Franceschini, C.C. Fatty acid composition of three freshwater fishes under different storage and cooking processes. Food Chem. 2007, 103, 1080–1090. [Google Scholar] [CrossRef]
- Duarte, A.M.; Silva, F.; Pinto, F.R.; Barroso, S.; Gil, M.M. Quality assessment of chilled and frozen fish—Mini review. Foods 2020, 9, 1739. [Google Scholar] [CrossRef]
- Walayat, N.; Tang, W.; Wang, X.; Yi, M.; Guo, L.; Ding, Y.; Liu, J.; Ahmad, I.; Ranjha, M.M.A.N. Quality evaluation of frozen and chilled fish: A review. eFood 2023, 4, e67. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, X.; Chen, L.; Liu, M.; Zheng, M.; Liu, J.; Liu, H. Changes in quality characteristics based on protein oxidation and microbial action of ultra-high pressure-treated grass carp (Ctenopharyngodon idella) fillets during magnetic field storage. Food Chem. 2024, 434, 137464. [Google Scholar] [CrossRef]
- Tokur, B.; Kandemir, S. Dondurulmuş balıklarda farklı çözündürme şekillerinin protein kalitesine olan etkileri. J. Fish. Sci. 2008, 2, 100–106. (In Turkish) [Google Scholar] [CrossRef]
- Keyvan, A.; Moini, S.; Ghaemi, N.; Haghdoost, A.A.; Jalili, S.; Pourkabir, M. Effect of frozen storage time on the lipid deterioration and protein denaturation during Caspian Sea white fish (Rutilus frisii kutum). J. Fish. Aquat. Sci. 2008, 3, 404–409. [Google Scholar] [CrossRef]
- Beyter, N. Farklı ticari yemlerle beslemenin gökkuşağı alabalıklarının (Oncorhynchus mykiss) büyüme performansına, balık eti bileşimine ve yağ asitleri profiline etkisi. Master’s Thesis, Ankara Üniversitesi, Ankara, Türkiye, 2008. (In Turkish). [Google Scholar]
- Widjaja, W.P.; Abdulamir, A.S.; Abu Bakar, F.; Saari, N.B.; Ishak, Z.B.; Hamid, A.A. Lipid quality deterioration of Bagridae catfish (Mystus nemurus) during storage. Res. J. Biol. Sci. 2009, 4, 525–530. [Google Scholar]
- Wang, P.A.; Martinez, I.; Olsen, R.L. Myosin heavy chain degradation during post mortem storage of Atlantic cod (Gadus morhua L.). Food Chem. 2009, 115, 1228–1233. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Stoforos, N.G.; Taoukis, P.S. Quality and Shelf-Life Modeling of Frozen Fish at Constant and Variable Temperature Conditions. Foods 2020, 9, 1893. [Google Scholar] [CrossRef]
- Kong, C.; Wang, H.; Li, D.; Zhang, Y.; Pan, J.; Zhu, B.; Luo, Y. Quality changes and predictive models of radial basis function neural networks for brined common carp (Cyprinus carpio) fillets during frozen storage. Food Chem. 2016, 201, 327–333. [Google Scholar] [CrossRef]
- Kong, C.; Duan, C.; Zhang, Y.; Shi, C.; Luo, Y. Changes in lipids and proteins of common carp (Cyprinus carpio) fillets under frozen storage and establishment of a radial basis function neural network (RBFNN). Foods 2023, 12, 2741. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Wang, H.; Hong, H.; Yu, X.; Luo, Y. Establishment of the Arrhenius model and the radial basis function neural network (RBFNN) model to predict quality of thawed shrimp (Solenocera melantho) stored at different temperatures. Food Chem. 2016, 202, 327–333. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Wang, H.; Hong, H.; Yu, X.; Luo, Y. Comparison between the Arrhenius model and the radial basis function neural network (RBFNN) model for predicting quality changes of frozen shrimp (Solenocera melantho). Int. J. Food Prop. 2017, 20, 2711–2723. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, J.; Li, X.; Wang, J.; Yi, S.; Zhu, W.; Xu, Y.; Li, J. Prediction of the freshness of horse mackerel (Trachurus japonicus) using e-nose, e-tongue, and colorimeter based on biochemical indexes analyzed during frozen storage of whole fish. Food Chem. 2023, 402, 134325. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Li, Y.; Zeng, F.; Qiu, X.; Li, G.; Li, C. Non-destructive prediction of total volatile base nitrogen using color-texture features of UV-induced fluorescence image for freeze–thaw treated frozen-whole-round tilapia. J. Sci. Food Agric. 2024, 104, 2574–2586. [Google Scholar] [CrossRef]
- Park, S.-K.; Cho, J.-S.; Won, D.-H.; Kim, S.S.; Lim, J.-H.; Choi, J.H.; Yun, D.-Y.; Park, K.-J.; Lee, G. Quality differences in frozen mackerel according to thawing method: Potential classification via hyperspectral imaging. Foods 2024, 13, 4005. [Google Scholar] [CrossRef] [PubMed]
- Anderssen, K.E.; Syed, S.; Stormo, S.K. Quantification and mapping of tissue damage from freezing in cod by magnetic resonance imaging. Food Control. 2021, 123, 107734. [Google Scholar] [CrossRef]
- Currò, S.; Savini, F.; Fasolato, L.; Indio, V.; Tomasello, F.; Rampazzo, G.; Zironi, E.; Pagliuca, G.; Gazzotti, T.; Prandini, L.; et al. Application of near-infrared spectroscopy as an at-line method for the evaluation of histamine in tuna fish (Thunnus albacares). Food Control. 2025, 167, 110778. [Google Scholar] [CrossRef]
- Gonçalves, D.B.; Santos, C.S.P.; Pinho, T.; Queirós, R.; Vaz, P.D.; Bloore, M.; Satta, P.; Kovács, Z.; Casal, S.; Hoffmann, I. Near infrared reflectance spectroscopy coupled to chemometrics as a cost-effective, rapid, and non-destructive tool for fish fraud control: Monitoring source, condition, and nutritional value of five common whitefish species. J. AOAC Int. 2021, 104, 53–60. [Google Scholar] [CrossRef]
- Tan, M.; Wang, J.; Li, P.; Xie, J. Storage time prediction of glazed frozen squids during frozen storage at different temperatures based on neural network. Int. J. Food Prop. 2020, 23, 1663–1677. [Google Scholar] [CrossRef]
- Lan, W.; Yang, X.; Gong, T.; Xie, J. Predicting the shelf life of Trachinotus ovatus during frozen storage using a back propagation (BP) neural network model. Aquac. Fish. 2022, 8, 62–70. [Google Scholar] [CrossRef]
- Chen, S.; Tao, F.; Pan, C.; Hu, X.; Ma, H.; Li, C.; Zhao, Y.; Wang, Y. Modeling quality changes in Pacific white shrimp (Litopenaeus vannamei) during storage: Comparison of the Arrhenius model and Random Forest model. J. Food Process. Preserv. 2020, 45, e14999. [Google Scholar] [CrossRef]
- Hu, K.; Hu, T.; Yan, W.; Dong, W.; Zuo, M.; Zhang, Q. Quality grading and prediction of frozen Zhoushan hairtails in China based on ETSFormer. Sustainability 2023, 15, 15566. [Google Scholar] [CrossRef]
- Guo, M.; Lin, H.; Feng, H.; Cao, L.; Sui, J.; Wang, X.; Wang, K. Deep learning algorithm-assisted non-destructive detection of TBARS values of salmon flesh using multi-modal molecular spectra fusion. Food Chem. 2025, 492, 145649. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Gu, Y.; Fu, Y.; Zhang, X.; Hu, J. Atlantic salmon adulteration authentication by machine learning using bioimpedance non-destructive flexible sensing. Microchem. J. 2023, 196, 109638. [Google Scholar] [CrossRef]
- Sakai, A.; Yagi, M.; Yasutomi, S.; Mizuno, K.; Suzuki, K.; Goto, K. Machine learning approach for frozen tuna freshness inspection using low-frequency A-mode ultrasound. IEEE Access 2023, 11, 107379–107393. [Google Scholar] [CrossRef]
- Castro-Silupu, W.; Saavedra-García, M.; Avila-George, H.; De la Torre-Gomora, M.; Bruno-Tech, A. Probabilistic or Convolutional-LSTM neuronal networks: A comparative study of discrimination capacity on frozen–thawed fish fillets. In Proceedings of the 11th International Conference on Software Process Improvement (CIMPS), Acapulco, Guerrero, Mexico, 19–21 October 2022; pp. 112–118. [Google Scholar] [CrossRef]
- Giannakourou, M.; Taoukis, P. Changes during Food Freezing and Frozen Storage. Foods 2021, 10, 2525. [Google Scholar] [CrossRef]
- Feng, H.; Wang, W.; Chen, B.; Zhang, X. Evaluation on frozen shellfish quality by blockchain-based multi-sensors monitoring and SVM algorithm during cold storage. IEEE Access 2020, 8, 297772. [Google Scholar] [CrossRef]
- Feng, H.; Fan, J.; Ji, Y.; Glamuzina, B.; Ma, R. Reliable quality traceability for tilapia cold chain using blockchain and machine learning techniques. J. Food Process Eng. 2024, 47, e70016. [Google Scholar] [CrossRef]
- Fan, L.; Xian, C.; Tang, S.; Ding, W.; Wang, X. Effect of frozen storage temperature on lipid stability of hepatopancreas of Eriocheir sinensis. LWT 2022, 154, 112513. [Google Scholar] [CrossRef]
- Fan, L.; Xiao, T.; Xian, C.; Ding, W.; Wang, X. Effect of short-term frozen storage on taste of gonads of female Eriocheir sinensis and the classification of taste quality combined with sensory evaluation and fuzzy logic model. Food Chem. 2022, 378, 132105. [Google Scholar] [CrossRef]
- Kono, S.; Nabetani, H.; Sagara, Y. Evaluation methodology based on ice crystal morphology during freezing and storage of frozen foods and its evolution to actual operation. J. Jpn. Soc. Food Sci. 2018, 65, 290–308. [Google Scholar] [CrossRef]
- Li, L.; Cao, R.; Zhao, L.; Liu, N.; Sun, H.; Zhang, Z.; Sun, Y. Near-infrared spectroscopy combined with explainable machine learning for storage time prediction of frozen Antarctic krill. Foods 2025, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 1995. [Google Scholar]
- Varlık, C.; Uğur, M.; Gökoğlu, N.; Gün, H. Su Ürünlerinde Kalite Kontrol Ilke ve Yöntemleri; Gıda Teknolojisi Derneği: İstanbul, Türkiye, 1993. (In Turkish) [Google Scholar]
- Pearson, D.; Cox, H. Determination of Total Volatile Bases in Flesh Foods; The Chemical Analyses Inc.: Miami, FL, USA, 1962. [Google Scholar]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A distillation method for quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Saddle River, NJ, USA, 1999. [Google Scholar]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W. H. Freeman: New York, NY, USA, 1995. [Google Scholar]
- Tsakanikas, P.; Karnavas, A.; Panagou, E.Z.; Nychas, G.-J.E. A machine learning workflow for raw food spectroscopic classification in a future industry. Sci. Rep. 2020, 10, 11212. [Google Scholar] [CrossRef]
- Wang, X.; Bouzembrak, Y.; Oude Lansink, A.G.J.M.; van der Fels-Klerx, H.J. Application of machine learning to the monitoring and prediction of food safety: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 416–434. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, X.; Zhang, Y.; Jiang, H. Machine learning approaches for predicting food quality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 6012–6036. [Google Scholar] [CrossRef]
- Rish, I. An empirical study of the naïve Bayes classifier. In Proceedings of the IJCAI 2001 Workshop on Empirical Methods in Artificial Intelligence, Seattle, WA, USA, 4–6 August 2001; pp. 41–46. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman and Hall/CRC: Boca Raton, FL, USA, 1984. [Google Scholar]
- Hornik, K.; Stinchcombe, M.; White, H. Multilayer feedforward networks are universal approximators. Neural Netw. 1989, 2, 359–366. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Seber, G.A.F. Nonlinear regression models. In The Linear Model and Hypothesis: A General Unifying Theory; Springer: Berlin/Heidelberg, Germany, 2015; pp. 117–128. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the 14th International Joint Conference on Artificial Intelligence, Montreal, QC, Canada, 20–25 August 1995; Morgan Kaufmann: San Francisco, CA, USA, 1995; pp. 1137–1145. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef]
- Powers, D.M.W. Evaluation: From precision, recall and F-measure to ROC, informedness, markedness and correlation. J. Mach. Learn. Technol. 2011, 2, 37–63. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Schormüller, J. Handbuch der Lebensmittelchemie, Band III/2, Tierische Lebensmittel: Eier, Fleisch, Buttermilch; Springer: Berlin/Heidelberg, Germany, 1968. (In German) [Google Scholar]
- Aydın, I.; Gökoglu, N. Effects of temperature and time of freezing on lipid oxidation in anchovy (Engraulis encrasicholus) during frozen storage. Eur. J. Lipid Sci. Technol. 2014, 116, 996–1001. [Google Scholar] [CrossRef]
- Chu, Y.; Ding, Z.; Wang, J.; Xie, J.; Ding, Y. Factors affecting the quality of frozen large yellow croaker (Pseudosciaena crocea) in cold chain logistics: Retention time and temperature fluctuation. Food Chem. X 2023, 18, 100742. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Li, M.; Shi, C.; Zhang, J.; Yang, X. Determination of salmon freshness by computer vision based on eye color. Food Packag. Shelf Life 2022, 34, 100984. [Google Scholar] [CrossRef]
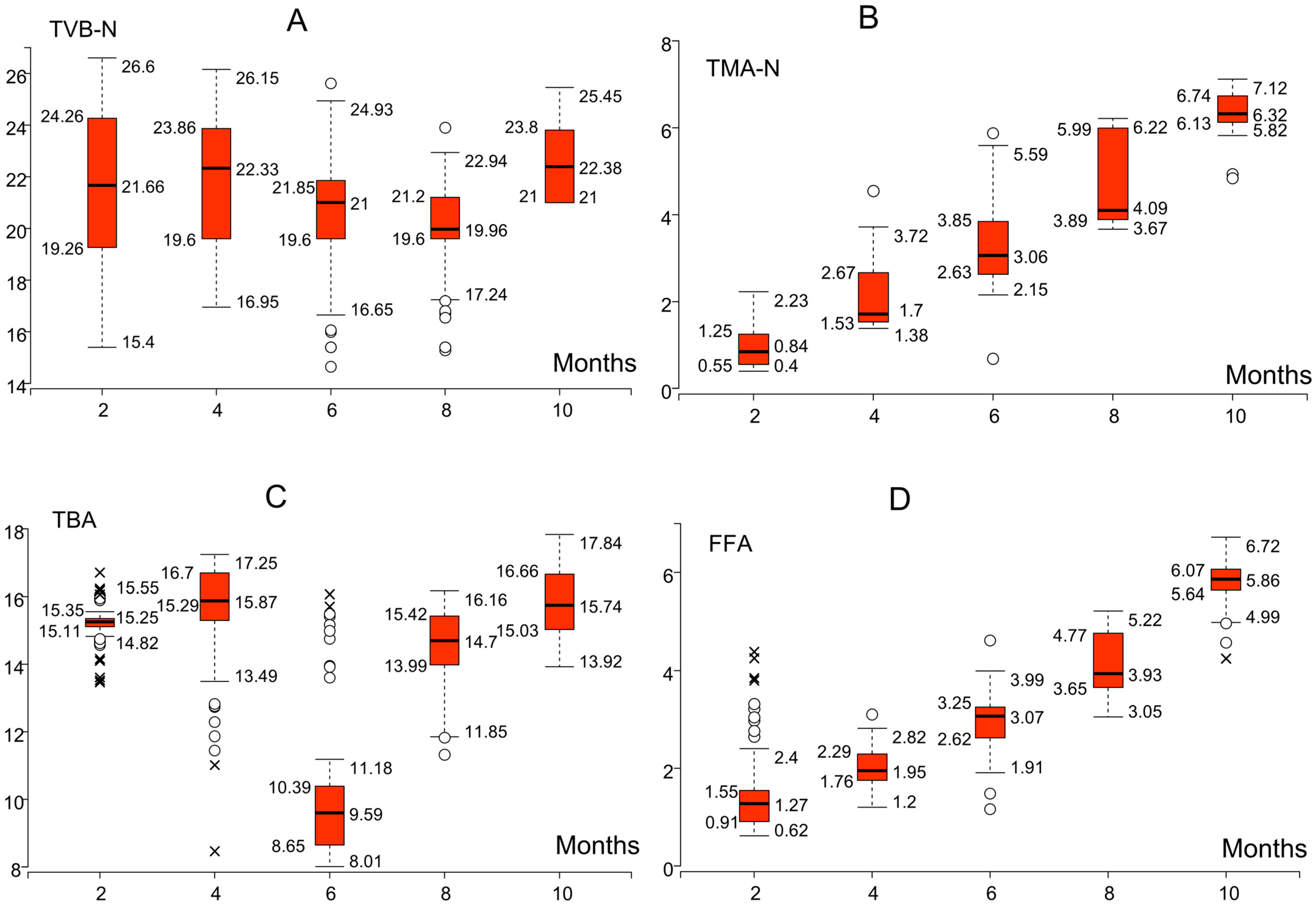
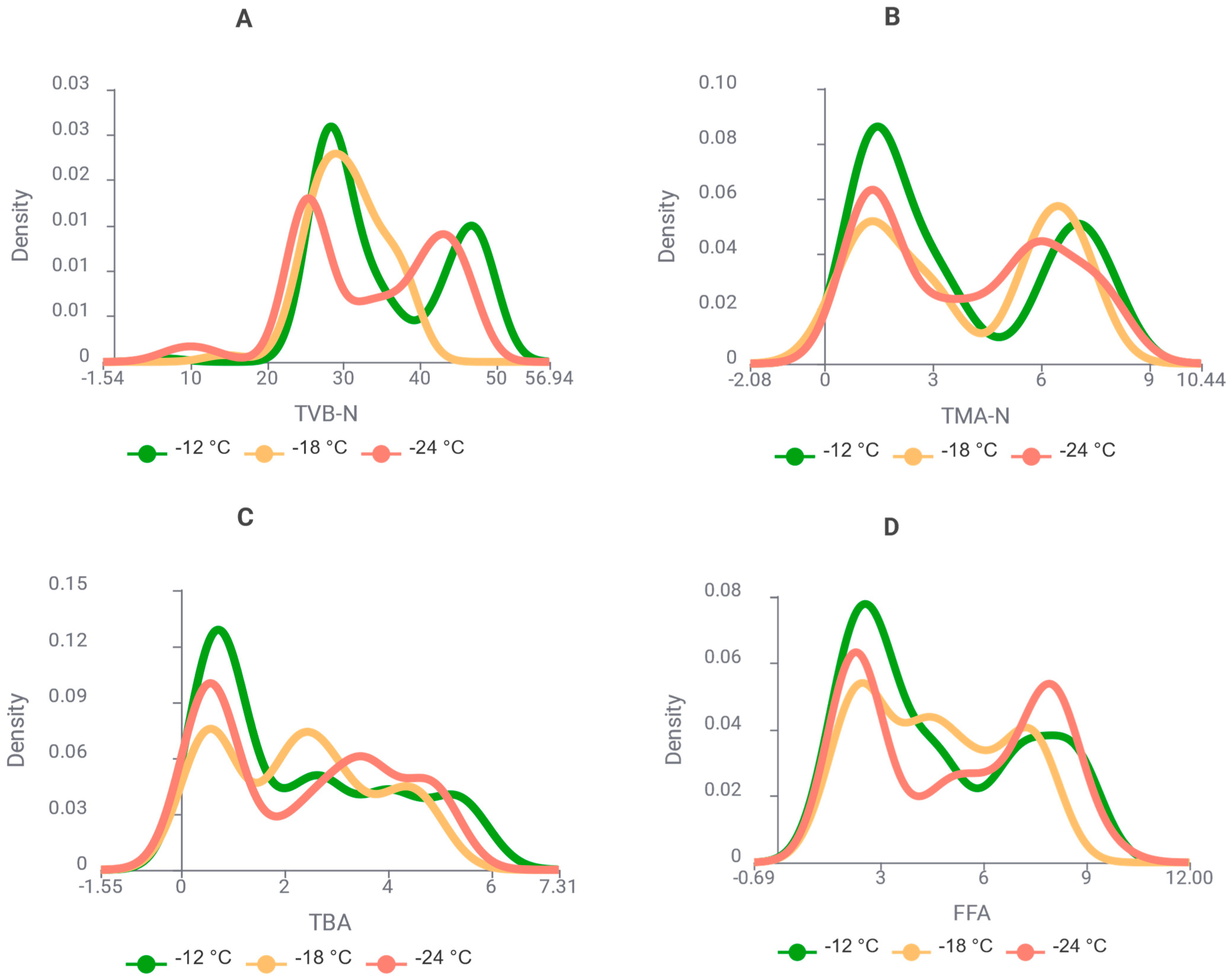
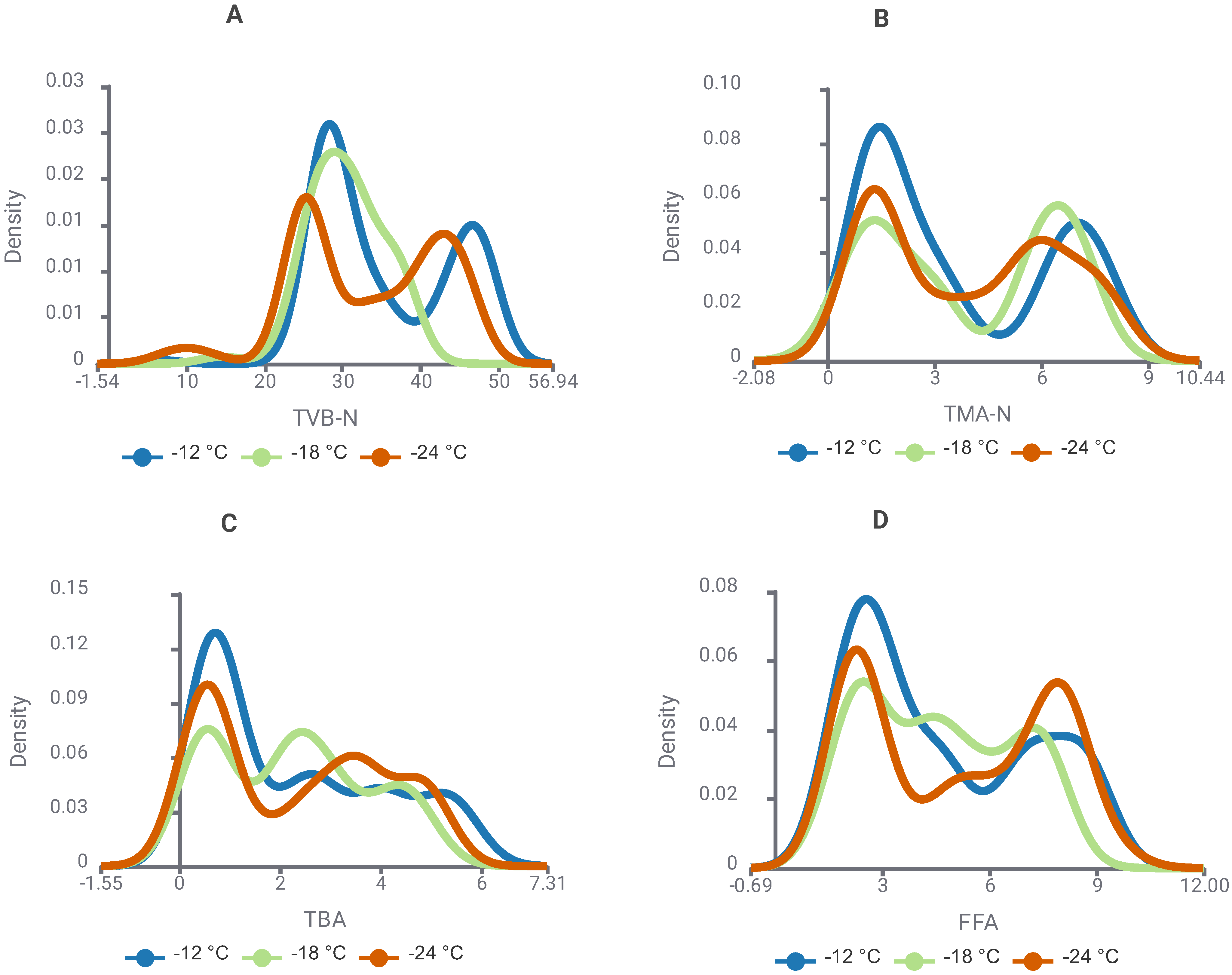
| Algorithm * | Key Hyperparameters |
|---|---|
| NB | Default prob: 0.0001; Min std dev: 0.0001; Threshold std dev: 0.0 |
| SVM (RBF) | Kernel: RBF; Sigma (γ): 2.7 |
| MLP | Hidden layers: 1; Neurons/layer: 10; Max iterations: 100 |
| DT | Quality measure: Gini; Min records/node: 2; Reduced error pruning; Avg split point |
| RFR | Number of trees (n_estimators): 100; Maximum depth: None; Minimum samples per split: 2; Minimum samples per leaf: 1; Criterion: Gini; Bootstrap: True; Random state: 42 |
| XGBoost | Objective: Binary logistic; Features: TVB-N, TMA-N, TBA, FFA, Temp, Month; Boosting rounds: 100; Base score: 0.5; Threads: 6 |
| Species | Dry Matter | Crude Ash | Crude Lipid | Crude Protein | pH |
|---|---|---|---|---|---|
| Whiting | 18.10 ± 1.27 | 2.91 ± 0.78 | 0.77 ± 0.32 | 15.67 ± 1.47 | 7.17 ± 0.05 |
| Atlantic bonito | 38.67 ± 4.37 | 4.01 ± 0.66 | 12.59 ± 2.53 | 19.89 ± 0.25 | 5.99 ± 0.06 |
| Indicator * | Min | Max | Mean | SD | Variance | Skewness | Kurtosis | Overall Sum | Median |
|---|---|---|---|---|---|---|---|---|---|
| TVB-N | 2.4 | 49 | 26.65157 | 8.621121 | 74.32372 | 0.735935 | 0.427197 | 23,986.41 | 24.93658 |
| TMA-N | 0.3604 | 8.0012 | 3.449748 | 2.292323 | 5.254747 | 0.447713 | −1.25768 | 3104.773 | 2.879266 |
| TBA | 0.0544 | 17.8376 | 7.904814 | 6.275193 | 39.37805 | 0.194933 | −1.68023 | 7114.332 | 5.278907 |
| FFA | 0.622 | 9.6937 | 3.886142 | 2.200128 | 4.840563 | 0.690118 | −0.6648 | 3497.528 | 3.099634 |
| Algorithm * | Accuracy (%) | Error (%) | Cohen’s Kappa |
|---|---|---|---|
| NB | 98.111 | 1.889 | 0.962 |
| SVM | 96.889 | 3.111 | 0.938 |
| MLP | 94.667 | 5.333 | 0.893 |
| DT | 98.444 | 1.556 | 0.969 |
| XGBoost | 98.889 | 1.111 | 0.978 |
| Model * | Class (Biochemical Profile—Species) | R | P | Specificity | F-Measure |
|---|---|---|---|---|---|
| MLP | TVB-N/TMA-N/TBA/FFA profile—M. merlangus | 0.907 | 0.996 | 0.995 | 0.949 |
| TVB-N/TMA-N/TBA/FFA profile—S. sarda | 0.995 | 0.898 | 0.907 | 0.944 | |
| SVM | TVB-N/TMA-N/TBA/FFA profile—M. merlangus | 0.991 | 0.947 | 0.949 | 0.968 |
| TVB-N/TMA-N/TBA/FFA profile—S. sarda | 0.949 | 0.991 | 0.991 | 0.970 | |
| NB | TVB-N/TMA-N/TBA/FFA profile—M. merlangus | 0.964 | 1.000 | 1.000 | 0.981 |
| TVB-N/TMA-N/TBA/FFA profile—S. sarda | 1.000 | 0.962 | 0.964 | 0.981 | |
| DT | TVB-N/TMA-N/TBA/FFA profile—M. merlangus | 0.987 | 0.982 | 0.982 | 0.984 |
| TVB-N/TMA-N/TBA/FFA profile—S. sarda | 0.982 | 0.987 | 0.987 | 0.984 | |
| XGBoost | TVB-N/TMA-N/TBA/FFA profile—M. merlangus | 0.982 | 0.996 | 0.995 | 0.989 |
| TVB-N/TMA-N/TBA/FFA profile—S. sarda | 0.995 | 0.982 | 0.982 | 0.989 |
| Algorithm * | R2 | RMSE | MAE | MAPE |
|---|---|---|---|---|
| SRT | 0.960 | 0.662 | 0.208 | 0.047 |
| PR | 0.907 | 1.008 | 0.791 | 0.172 |
| RFR | 0.986 | 0.392 | 0.791 | 0.048 |
| Study | Species | ML Method | Prediction Accuracy | Best Indicators |
|---|---|---|---|---|
| [72] | Trachinotus ovatus | BPNN | R2 = 0.8642–0.9904; error ≤ 10% | TVB-N, water retention |
| [71] | Glazed squid | LSTM, BPNN | MAPE 5.01% | WHC, texture, sulfhydryl, weight loss |
| [111] | Large yellow croaker | LSTM | MAPE 7.78%; RMSE 7.94 | Centrifugal loss, TVB-N, K-value, whiteness |
| [112] | Salmon filets | RBFNN, Support Vector Regression (SVR) | RBFNN < 5%; SVR < 10% | TBA, TVB-N, Total Viable Counts (TVC), K-value, sensory |
| [73] | Pacific white shrimp | RF | R2 close to 1; RMSE < 0.1 | Sensory, pH, texture, TBA, TVC |
| [63] | Thawed shrimp | RBFNN | Error ± 2% | TVB-N, aerobic counts, K-value, hypoxanthine, pH, Electrical Conductivity (EC), sensory |
| Study | Species | Quality Parameter | Method | Performance |
|---|---|---|---|---|
| [69] | Tuna | Histamine | Modified partial least squares (MPLS), SVM | R2 = 0.88–0.97 |
| [66] | Tilapia | TVB-N | CNN | Accuracy > 97%; RMSE = 0.0115 |
| [63] | Shrimp | TVB-N, aerobic counts, K-value, pH | RBFNN | Error ± 2% |
| [61] | Carp | Quality indices | RBFNN | Error < 5% |
| [62] | Carp | Lipid/protein indices | RBFNN | Error < 10% |
| [73] | Shrimp | Sensory, pH, TBA, TVC | RF | R2 ≈ 1; RMSE < 0.1 |
| [74] | Hairtail | TBARS, carbonyl, sulfhydryl | Transformer (ETSFormer) | F1 = 92–98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meriç Turgut, İ.; Gerdan Koc, D. Mapping Frozen Fish Quality via Machine Learning for Predictive Spoilage Kinetics Under Subzero Conditions. Appl. Sci. 2025, 15, 12611. https://doi.org/10.3390/app152312611
Meriç Turgut İ, Gerdan Koc D. Mapping Frozen Fish Quality via Machine Learning for Predictive Spoilage Kinetics Under Subzero Conditions. Applied Sciences. 2025; 15(23):12611. https://doi.org/10.3390/app152312611
Chicago/Turabian StyleMeriç Turgut, İlknur, and Dilara Gerdan Koc. 2025. "Mapping Frozen Fish Quality via Machine Learning for Predictive Spoilage Kinetics Under Subzero Conditions" Applied Sciences 15, no. 23: 12611. https://doi.org/10.3390/app152312611
APA StyleMeriç Turgut, İ., & Gerdan Koc, D. (2025). Mapping Frozen Fish Quality via Machine Learning for Predictive Spoilage Kinetics Under Subzero Conditions. Applied Sciences, 15(23), 12611. https://doi.org/10.3390/app152312611







