Featured Application
Barley grains are highly valuable in human nutrition because they contain dietary fiber, polyphenols, and carotenoids, which provide various health benefits and contribute to the prevention of non-communicable diseases. By examining how cultivation practices and varieties affect these beneficial compounds, we can develop barley with an improved nutritional profile while minimizing the risks of mycotoxins, thereby safeguarding consumer health. This research supports advancements in crop management, food quality, and safety, benefiting both farmer profitability and public health in Poland. The insights gained from this study can inform the development and selection of barley varieties that offer higher yields, enhanced nutritional value, and reduced susceptibility to mycotoxin contamination, all tailored to Polish agroclimatic conditions. The findings also endorse the use of certain cereal varieties and optimized nitrogen fertilization to lower chemical inputs, decrease environmental impact, and maintain high productivity, which aligns with sustainable farming goals.
Abstract
Barley has consistently been ranked among the four most grown cereals in the world. Integrated agronomic approaches, combining a selection of optimal genotypes and growing conditions, may help to provide high yields of quality and safe barley grains. This study aimed to assess the yield, polyphenol and carotenoid content, and mycotoxin presence in grains of four winter barley varieties—Hobbit, Zoom, Galation, and Sandra—grown under different nitrogen (N) fertilization levels. High-performance liquid chromatography (HPLC) was used to analyze bioactive compounds, while liquid chromatography tandem mass spectrometry (LC-MS/MS) was applied to determine mycotoxin occurrence. Results showed that Hobbit and Zoom had higher yields, with Hobbit benefiting from higher N fertilization levels. While no significant differences in phenolic acids (sum) were observed among the barley varieties tested (av. 80.50 ± 6.78 mg/100 g), higher N levels raised flavonoid content (46.78 ± 4.35 vs. 38.82 ± 3.54 mg/100 g). Zoom was characterized by the highest total polyphenol levels (130.45 ± 12.50 mg/100 g). Among the 14 mycotoxins tested, only two were frequently found in the grain samples (DON and 15-Ac-DON), with Sandra being the least and Galation the most susceptible. The N fertilization doses did not significantly impact mycotoxin levels in grains. The insights gained from this study can inform the development and selection of barley varieties and growing conditions that offer optimized yields, enhanced nutritional value, and reduced susceptibility to mycotoxin contamination, tailored to the producers’ and consumers’ expectations and to sustainable farming goals.
Keywords:
cereals; Hordeum vulgare; food safety; phenolic compounds; phenolic acids; flavonoids; lutein; β-carotene; deoxynivalenol; HPLC; LC-MS/MS 1. Introduction
In global agriculture, barley (Hordeum vulgare) holds a significant position. For years, it has consistently been ranked as the fourth most grown cereal species in the world after wheat, rice, and maize [1]. In the period 2015–2021, 60.7% of the world’s production of this cereal came from Europe, making it the leading continent in terms of production volume [2].
Barley is used as malt, food, and livestock feed [3]. It contains high levels of dietary fiber, particularly soluble fractions, such as β-glucans and arabinoxylans, and many important vitamins, such as vitamin E and B-group vitamins [4,5]. It is also a good source of phosphorus and manganese [6], as well as phenolic compounds, carotenoids, and other antioxidants that contribute to its health-promoting properties [7,8].
Due to the soluble dietary fiber and bioactive compounds in barley composition, its regular consumption has the potential to contribute to the reduction of serum low-density lipoprotein cholesterol levels, to modulate gut microbiota, and to decrease postprandial blood glucose levels, thus contributing to the promotion of cardiovascular health, the prevention of diabetes, and the support of the digestive system [9,10].
Plant secondary metabolites, including phenolic compounds, play significant roles in plant reproduction, growth, and inner protection against pests and pathogens [11]. The main groups of phenolics present in cereal grains, flavonoids and phenolic acids, are known to be free radical scavengers [12,13]. Therefore, their consumption has been suggested to be linked to numerous health benefits, including the prevention of such non-communicable diseases as cancers and cardiovascular problems [14,15,16].
Carotenoids are another important group of plant phytochemicals which confer nutritional quality to cereal grains and cereal-based products [17]. They play a crucial role in photosynthesis and photoprotection in plants. For humans, carotenoids are precursors of vitamin A, which is necessary for maintaining health [18]. Neither humans nor animals are able to synthesize carotenoid compounds, so their supply from diet is necessary to reap health benefits. They are antioxidants suggested to enhance immune system functions and to play a role in the prevention of retinal degradation, liver cancer, and sunburn [19]. One of the main carotenoid compounds of barley is lutein, which is accumulated in a yellow spot of the retina in the eye and protects it from damage by absorbing UV radiation [20].
Microscopic filamentous fungi of the genera Fusarium, Penicillium, and Aspergillus produce mycotoxins—secondary metabolites with strong toxicity potential [21]. Infection with these fungi can lead to a reduction in the quantity, quality, and safety of grains [22]. The most common mycotoxins include deoxynivalenol (DON); fumonisins; trichothecenes, especially T-2 toxin and HT-2 toxin; zearalenone (ZEN); ochratoxins, especially ochratoxin A (OTA); and aflatoxins [21,23]. The most prevalent mycotoxins in barley are deoxynivalenol (DON) and its derivatives—3-acetyldeoxynivalenol (3-AcDON) and 15-acetyldeoxynivalenol (15Ac-DON)—which are often associated with Fusarium infections. The HT-2 and T-2 toxins are also common in barley, with the former occurring more often [24]. These trichothecenes are known for their acute toxicity, posing a high risk to health at low doses. Ochratoxin A (OTA) can also be found in barley samples, although it is less common [25]. It is produced by the genus Aspergillus. OTA is known for its toxic effects on the kidneys, liver, nervous system, and immune system. Moreover, its embryotoxic and teratogenic effects have been proven in many species. Ochratoxin A was classified as a possible carcinogen in humans by the International Agency for Research on Cancer in 1993 [26]. Aflatoxins are also mutagenic and carcinogenic [27]. Poor hygienic conditions during transportation and storage, wetness, heavy rains, and high temperatures promote the production of these toxins.
There are two groups of fungi that produce mycotoxins in food: those that invade before harvest, called field fungi, and those that invade after harvest, called storage fungi [21]. At the very beginning of the production chain, the use of resistant varieties can be helpful in limiting the growth of mold and mycotoxin contamination of food raw materials and products [28]. Scientific literature points to the impact of nitrogen fertilization on the risk of mycotoxin contamination of cereal grains [29,30,31].
Excessive nitrogen use in plant cultivation is a very important topic, as it has a negative impact on the ecosystem, significantly increases the environmental footprint of agricultural production, and can also affect the quality and safety of crops. Therefore, this study aimed to examine the yield as well as selected quality and safety features (polyphenol and carotenoid content and mycotoxin presence in grain) of four winter barley varieties from a controlled field experiment in which different doses of nitrogen fertilizers were used in cultivation. Barley produced in Poland is widely used for animal feed, malt, and human consumption. Hence, the presented study on the content of bioactive compounds and mycotoxin contamination of the grain of this cereal may be especially of interest to producers, enabling them to select varieties characterized by good yields using effective doses of nitrogen and by high quality and safety values, which are important from the point of view of consumers expecting high-quality and safe food. In addition, the obtained results may constitute a premise for further research to confirm and popularize effective barley cultivation methods and varieties.
2. Materials and Methods
2.1. Field Experiment
Barley grains came from the field experiment conducted at the Agricultural Experimental Station in Osiny Farm (57°47′ N, 22°05′ E), belonging to the Institute of Soil Science and Plant Cultivation—State Research Institute in Puławy, Poland. The experiments were conducted on Albic Luvisols (developed in loamy sand on loam). The experiment was set up using the randomized subblock design, with three field replications. The experimental factors included 4 varieties of winter barley and nitrogen fertilization rates of 60, 90, 120, and 150 kg N ha−1. Among the varieties used, there was one traditional barley pollination variety (Sandra), and there were 3 new hybrid varieties (Zoom, Hobbit, and Galation), which are usually characterized by higher yielding potential than traditional varieties. The forecrop was winter rape. Barley was sown at the optimal time and with the optimal sowing rate. The crop protection strategy applied followed the recommendations set by the Institute of Plant Protection—National Research Institute. The cultivation was carried out under the weather conditions shown in Table 1. The meteorological data were collected from the Automatic Agrometeorological Station located in the proximity of the experimental station in Osiny (φ = 51°47′ N, λ = 22°05′ E).

Table 1.
Weather conditions, including monthly average air temperature (°C), total monthly rainfall (mm), and long-term averages (1981–2010), in the season of barley cultivation, at the Osiny experimental station.
2.2. Chemicals
Acetone (HPLC grade) from Sigma-Aldrich (Poznań, Poland); acetonitrile (HPLC grade) from Chempur (Piekary Śląskie, Poland); carotenoids (lutein and β-carotene) and phenolics standards (HPLC grade 99.00–99.99% pure) from Fluka and Sigma-Aldrich (Poznań. Poland); ethyl acetate from Sigma Aldrich (Poznań, Poland); magnesium carbonate (ultrapure) from Chempur (Piekary Śląskie, Poland); methanol (HPLC grade) from Chempur (Piekary Śląskie, Poland); ortho-phosphoric acid from Chempur (Piekary Śląskie, Poland); acetonitrile for LC-MS, ammonium formate and formic acid for mass spectrometry from Sigma-Aldrich (Steinheim, Germany), methanol hypergrade for LC-MS from Merck (Darmstadt, Germany); certified liquid standards of mycotoxins from Romer Labs Diagnostic (Tulin, Austria); water, ultra-pure grade (18.2 MΩ cm), from a Milli-Q Academic A10 Water Purification System (Millipore Ltd., Bedford, MA, USA).
2.3. Dry Matter Content
The dry matter content in the grains was analyzed by a weight method, following the Standard PN-R-04013:1988 [32], as previously presented by Kazimierczak et al. [33].
2.4. HPLC Analysis of Phenolic Acids and Flavonoids
The concentration of phenolic compounds (phenolic acids and flavonoids) was determined by the high-performance liquid chromatography (HPLC) method, as previously described by Kazimierczak et al. [33], using Shimadzu equipment (USA Manufacturing Inc., Canby, OR, USA). The 100 mg samples of grain material were mixed with 5 mL of 80% methanol, vortexed (using a Micro-Shaker 326M, Premeo, Marki, Poland) and incubated for 10 min at 30 °C and 5.5 kHz in an ultrasonic bath, then samples were centrifuged (10 min at 6000× rpm at 5 °C). Following a second centrifugation (5 min, 12,000× rpm at 0 °C) of the acquired supernatants, 900 μL of the clear supernatants were transferred to HPLC vials for analysis. The HPLC-set included the following: two LC-20AD pumps, a CBM-20A controller, a SIL-20AC column oven, and a UV/Vis SPD-20 AV spectrometer, manufactured by USA Manufacturing Inc. Using the Synergy Fusion-RP, Å-80 Phenomenex column (250 × 4.60 mm), the phenolic compounds were separated at a flow rate of 1 mL min−1. For phenolic acids, the wavelength was 250 nm, while for flavonoids, it was 370 nm. The identification of individual compounds was based on Fluka and Sigma-Aldrich (Warsaw, Poland) standards with HPLC purities of 99.00–99.99%. The HPLC chromatograms presenting time of retention and peaks of the phenolic acids and flavonoids identified in barley grain samples are presented in Figures S1 and S2 (Supplementary Materials).
2.5. HPLC Analysis of Carotenoids
The identification and quantification of carotenoids were carried out through the HPLC method with the use of the HPLC equipment described above. A weighed amount (400 mg) of powdered grain sample was extracted with cold acetone, and then magnesium carbonate was added. The samples were incubated in a cold ultrasonic bath (5.5 kHz, 0 °C, 15 min). After extraction, the samples were centrifuged (5500× rpm, 2 °C, 10 min). Then, 1 mL of centrifuged extract was used for the next step of analysis. A total of 200 µL of supernatant was injected into a Max RP Å-80 column (250 × 4.6 mm, Phenomenex, Warsaw, Poland). For analytical purposes, two mobile phases were used. The first mobile phase (A) contained 90% acetonitrile and 10% methanol. The second phase (B) contained 68% methanol and 32% ethyl acetate. A flow of 1 mL min−1 was used with the following time program: 1.00–14.99 min, Phase A 100%; 15.00–22.99 min, Phase A 40% and Phase B 60%; and 24.00–28.00 min, Phase A 100%. The wavelengths used for detection were 450 nm for carotenes and 445 nm for xanthophylls. The carotenoids were identified based on Fluka and Sigma-Aldrich external standards (lutein, β-carotene). The HPLC chromatograms presenting time of retention and peaks of the carotenoids identified in barley grain samples are presented in Figures S3 and S4 (Supplementary Materials).
2.6. LC-MS/MS Analysis of Mycotoxins
The study comprises analysis of 14 mycotoxins, including 3-acetyldeoxynivalenol (3-AcDON), 15-acetyldeoxynivalenol (15-AcDON), aflatoxin B1 (AFLB1), aflatoxin B2 (AFLB2), aflatoxin G1 (AFLG1), aflatoxin G2 (AFLG2), deoxynivalenol, fumonisin B1 (FB1), fumonisin B2 (FB2), ochratoxin A (OTA), nivalenol (NIV), T-2 toxin (T-2), HT-2 toxin (HT-2), and zearalenone (ZEA). The mycotoxins were extracted from barley grain samples according to the method described in an earlier publication by the authors [33] based on the method developed by Spanjer et al. [34]. The method included sample extraction with water (30 min) and acetonitrile (2 h), centrifugation of the sample, and 20-fold dilution of the extract, before instrumental analysis, with LC-phase solution [33].
Instrumental analysis was performed using an LC-MS/MS system consisting of an Eksigent Expert UltraLC 100-XL chromatograph (Eksigent Technologies, Dublin, CA, USA, equipped with a Kinetex C18 100Å, 100 mm × 2.1 mm, 2.6 µm core-shell column (Phenomenex, Torrance, CA, USA) kept at 40 °C, coupled with QTRAP 6500 tandem mass spectrometry (AB Sciex Instruments, Foster City, CA, USA). Data acquisition was conducted using Analyst Software version 1.6.2 (AB Sciex) in multiple reaction monitoring (MRM) mode for 16.0 min and data processing using MultiQuant Software version 3.0.2 (AB Sciex). The LC-MS/MS method used was optimized as described in Reference [33]. The chromatographic parameters were unchanged. Two mobile phases, solvent A, consisting of 0.1% formic acid and 5 mM ammonium formate in water, and solvent B, consisting of 0.1% formic acid and 5 mM ammonium formate in methanol, were used. The gradient elution, at a flow rate of 0.5 mL min−1, was started at 2% B and held for 1.0 min, then increased to 98% B in 9 min and held for 3.0 min; then, within 0.1 min, the mobile phase composition returned to the initial state, and the column re-equilibration phase was held for 2.9 min. The mass spectrometer, as in previous studies, was used in positive and negative electrospray ionization mode and operated at a capillary voltage of 4500 V or −4500 V, a desolvation temperature of 500 °C, an entrance potential (EP) of 10 V or −10 V, with nitrogen as the curtain gas (CUR), nebulizer (GS1), and auxiliary gas (GS2), at pressures of 30, 60, and 50 psi, respectively, and with nitrogen as the collision gas. For each mycotoxin, the parent ion and two daughter ions were selected with optimized parameters for ion transitions, including collision energy, declustering, and cell exit potential, as well as the determined retention time (RT). All the parameters were presented in the Supplementary Materials of the authors’ above-mentioned paper [33]. LC-MS/MS chromatograms of representative barley samples contaminated by mycotoxins are shown in Figure S6 in the Supplementary Materials.
For the study, a stock mixture of certified solvent standards (Romer Labs Diagnostic, Tulin, Austria) in acetonitrile was prepared with the following concentrations of individual mycotoxins: AFLB2 and AFLG2 (0.025 μg mL−1); AFLB1 and AFLG1 (0.1 μg mL−1); OTA (0.5 μg mL−1); FB1, FB2, T-2, and HT-2 (5.0 μg mL−1); and ZEA, DON, 3-AcDON, 15-AcDON, and NIV (20 μg mL−1). The stock mixture was diluted by 50–5000-fold with acetonitrile to get working solutions. In addition, the isotopically labelled standard solution of 13C17–aflatoxin B1 (13C17-AB1) in acetonitrile at a concentration of 0.01 μg mL−1 was prepared, and its 50-fold dilution was used as an internal standard (ILS). The stock standard mixture and working solutions were kept at −20 °C.
Calibration mixtures were prepared from the working mixtures by pipetting 100 µL of a given concentration of the working standard mixture, 50 µL of internal standard (ILS), and 850 µL of LC mobile phase (95% A and 5% B) into the chromatographic vials. Concentrations of individual mycotoxins in the calibration mixtures were in different ranges, for AFLB1 and AFLG1 from 0.002–1 ng mL−1; for AFLB2 and AFLG2 from 0.0005–0.25 ng mL−1; for OTA from 0.01–5 ng mL−1; for FB1, FB2, HT-2, T-2, and ZEA from 0.1–50 ng mL−1; and for DON, 3-AcDON, 15-AcDON, and NIV from 0.4–200 ng mL−1. Calibration concentration in solvent corresponded to a 20 times higher value of mycotoxin content in a sample, expressed in µg kg−1. Internal standard, 13C17-AB1 (ILS), was added prior to the injection into the LC-MS/MS and was used to control the accuracy of calibration standard and sample extract injections. For quantitation, 1/x weighted, multi-point calibration curves were used. All calibration curves, developed using MultiQuant software, which were used to determine mycotoxin content, are presented in Figure S5 in the Supplementary Materials.
The method was validated statistically and by recovery studies for linearity, precision, repeatability, and reproducibility. Validation experiments were performed by spiking the matrix control sample with the stock standard mixture and by using several European Reference Materials (ERM-BE375, ERM–BE376, ERM-BC717), produced and certified under responsibility of the Institute for Reference Materials and Measurements (IRMM) of the European Commission’s Joint Research Centre (Sigma Aldrich, Steinheim, Germany). The results showed that recoveries were in the range of 70–120% and precision (RSD) ≤ 20%. The method’s suitability was confirmed in proficiency tests organized by TestQual Proficiency Testing Schemes (Murcia, Spain) and in the Check Sample Survey (CSS) organized by Romer Labs.
The limit of quantification (LOQ) determination for mycotoxins was performed using a calibration approach recommended by “Guidelines for the estimation of LOD and LOQ for the measurement of contaminants in feed and food” [35]. The LOQ was estimated as equivalent to 3.3 × LOD, based on a linear multipoint calibration curve including the lowest calibration level (LCL), characterized by R2 ≥ 0.998. For simplicity, however, the LOQ for all mycotoxins was assumed to be equivalent to the lowest calibration level (LCL) except for HT-2 and T-2, for which it was established as ½ LCL.
Consequently, the LOQ was set at 0.01 μg kg−1 for AFLB2 and AFLG2, 0.04 μg kg−1 for AFLB1 and AFLG1, 0.2 μg kg−1 for OTA, 20 μg kg−1 for FB1, 2 μg kg−1 for FB2 and ZEA, 1 μg kg−1 for HT-2 and T-2, 8 μg kg−1 for DON, 3-AcDON, 15-AcDON, and NIV.
2.7. Statistical Analyses
The effects of barley variety and nitrogen dose (N-dose) on the grain compositional parameters were assed using two-way analysis of variance (ANOVA), followed by the post hoc Tukey’s test, and differences were considered significant at p < 0.05. Principal component analysis (PCA) was used to investigate interrelations between the yield, identified bioactive compounds content, and the occurrence of mycotoxins in all barley varieties tested. Additionally, Spearman’s Correlation analysis was performed to assess possible linear associations between the yield, concentrations of polyphenols, carotenoids, and the mycotoxin occurrence in grain samples. The Statgraphics 18 Software (Statgraphics Technologies, Inc., The Plains, VA, USA) was used for ANOVA analysis. The R statistical environment (R Foundation for Statistical Computing, Vienna, Austria) was used to plot data with post hoc test results, for PCA picturing, and for the calculation and visualization of correlation analysis results [36].
3. Results and Discussion
3.1. Grain Yield
Significant differences in grain yield were found depending on the barley variety. At the same time, no such effect was observed depending on the dose of nitrogen applied to the crop in the case of three of the four varieties tested. Only in the case of the Hobbit variety, it was shown that higher doses of nitrogen applied to the crop stimulated an increase in yield. Similar grain yields for this variety were obtained by applying 120 and 150 kg of nitrogen per hectare. A comparison of mean yields for individual varieties showed significantly higher yields for Hobbit and Zoom than for the Galation and Sandra varieties (Table 2).

Table 2.
Total grain yields (tonnes per hectare) of four barley varieties in relation to different nitrogen fertilization levels (N-dose) in the controlled field trial in Poland.
Zoom, Hobbit, and Galation are hybrid varieties that have higher yielding potential than conventional genotypes (population varieties) such as Sandra. This is because hybrids obtained by crossing different genetic lines, thanks to heterosis, are characterized by greater vitality and greater development than conventional genotypes [37,38,39]. The results obtained in our study correspond to the results of other authors [38,40,41]. Tuppad et al. showed that increased nitrogen levels considerably influenced the yields, which were highest when 120 kg N ha−1 was applied [41].
3.2. Polyphenols and Carotenoids Content
Barley grains are rich in secondary metabolites such as polyphenols, represented mainly by phenolic acids and flavonoids. These bioactive compounds, known for their health-promoting antioxidant properties, make barley a valuable resource for developing food products and animal feeds [42].
Ferulic and p-coumaric acid are the main phenolic acids in barley grains [43], known for their antioxidant properties [44]. Other phenolic acids, reported in the scientific literature to represent smaller amounts in the grains, include caffeic and sinapic acids, belonging together with ferulic and p-coumaric acids to the group of hydroxycinnamic acids, and also vanilic and protocatechuic acids, belonging to the group of hydroxybenzoic acids [45]. The highest concentration of phenolic acids is found in bran and whole grains [46].
Flavonoids detected in barley grains include, among others, quercetin and kaempferol [47]. Secondary metabolite synthesis in plants is influenced by many factors such as the plant genotype, the environment, agronomic practices, and stress conditions [41] e.g., higher flavonoid content occurs in the purple grain varieties, which have the Ant2 gene [48]. Since these compounds are known to play a role in plant defense against stress [49], abiotic stress factors were previously shown to enhance phenolic acids and flavonoid content in cereal grains. Another abiotic stress factor, nitrogen deficiency, was also suggested to trigger the accumulation of phenolic compounds in grains [50]. Scientific literature suggests that nitrogen limitation may promote secondary metabolite synthesis by redirecting plant metabolism towards carbon-based assimilates [41,49]. On the other hand, some researchers indicate the opposite, that nitrogen fertilization can increase the total phenolic content, with this effect being dependent on the dose of nitrogen [51,52,53]. The content of these compounds is also affected by harvest time, plant maturity, part of the grain, and post-harvest storage conditions [54].
In our study in the case of phenolic acids, no significant differences were observed in the content of the sum of these compounds between barley grains grown at different nitrogen rates, nor was there any variation between varieties in this respect (Table 3). The impact of genetic background and N fertilization levels could have been overweighted by some other subtle abiotic and biotic factors not specifically controlled in the experiment [41].

Table 3.
Phenolic acid concentrations (mg/100 g) in the grain of four barley varieties grown in different conditions of nitrogen fertilization (N-dose) in the controlled field trial in Poland.
However, as shown in Table 3, some significant differences were found regarding the content of individual identified phenolic acids, both depending on nitrogen dose and barley variety. This concerned phenolic acids occurring in smaller quantities in barley grains, such as gallic, caffeic, t-cinnamic, p-coumaric, and sinapic acids, but no differences were found in the content of the dominant ferulic acid (Table 3). The interaction means showing the character of interactions concerning the effect of nitrogen fertilization rate and barley variety on phenolic acid content are presented in Figure 1.
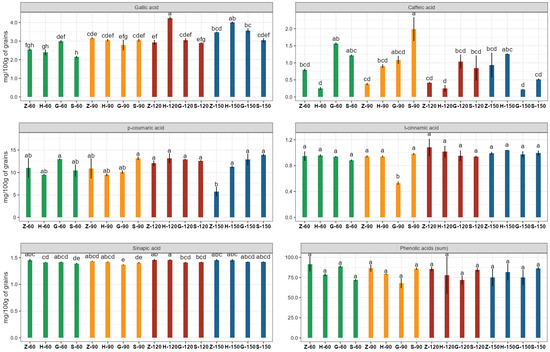
Figure 1.
Phenolic acid concentrations in grains of four different barley varieties grown with four different N fertilization doses. Data are presented as means ± standard errors (SE). Mean values followed by different letters (a–h) are significantly different at p < 0.05. Capital letters and numbers below bars indicate the name of the cultivar and the dose of fertilizer: Z—Zoom; H—Hobbit; G—Galation; S—Sandra; 60—60 kg N/ha; 90—90 kg N/ha; 120—120 kg N/ha; 150—150 kg N/ha.
The Sandra and Galation varieties were characterized by the highest caffeic and p-coumaric acid concentrations among the varieties tested. Sandra grain also had the highest t-cinnamic acid contents. The Hobbit variety stood out in terms of a higher content of gallic and sinapic acid. Similarly, the Zoom variety was among the richest in sinapic acid (Table 3). As mentioned, the ferulic acid was identified to be a dominant phenolic acid in the tested barley grains. On average, it accounted for approximately 78% of the sum of all identified phenolic acids. This result is closely in line with previous studies showing ferulic and p-coumaric acids as the major low-molecular-weight phenolic acids in the grain of barley [33,55,56].
Flavonoids are the second group of polyphenols present at high levels in barley grains. They play an important role in cereal plants, contributing to various physiological functions and helping with adaptation to environmental challenges [57,58]. They take part in, among others, the processes of seed maturation and dormancy, which are essential for the proper development and survival of cereal crops [57] and act as UV-scavengers, protecting plants from the adverse effects of ultraviolet radiation [59]. They are involved in both biotic and abiotic stress responses [57,58]. In our study, the applied doses of nitrogen in barley cultivation significantly affected the concentration of flavonoids in grains. It turned out that significantly more flavonoids were identified in grain grown with higher nitrogen rates (120 and 150 kg N ha−1). This result is consistent with the observations of other researchers, who claimed that the dose of 120 kg N ha−1 generally improves plant growth and nutrient content [51,52]. Significant differences were also observed among the studied barley varieties. The Hobbit variety was richest in flavonoids (Table 4).

Table 4.
Flavonoid concentrations (mg/100 g) in the grain of four barley varieties grown in different conditions of nitrogen fertilization (N-dose) in the controlled field trial in Poland.
Among the flavonoids detected in our study, apigenin and kaempferol were found in the highest concentrations in barley grain, and their content depended on both the variety and the fertilization. Similar apigenin contents were found in grain grown with doses of 90, 120, and 150 kg N ha−1, and they were significantly higher than at the dose of 60 kg N ha−1. In the case of kaempferol, higher contents were identified in grain grown with doses of 60, 120, and 150 kg N ha−1 in comparison to 90 kg N ha−1. Among the varieties tested, Sandra and Galation contained the least apigenin and kaempferol, respectively. The interactions’ means concerning the effect of nitrogen rates and barley variety on flavonoid content are presented in Figure 2. According to the literature, the dominant flavonoids in barley vary depending on the grain color and type, which includes varieties such as purple, black, blue, yellow, and white barley [60].
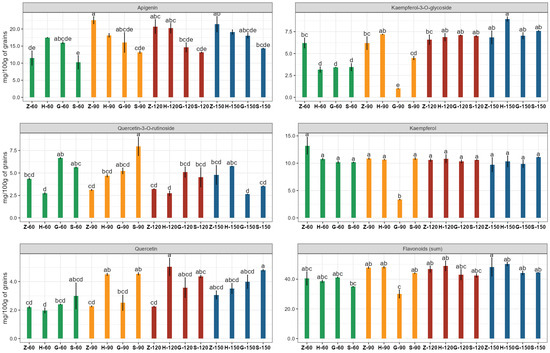
Figure 2.
Flavonoid concentrations in grains of four different barley varieties grown with four different N fertilization doses. Data are presented as means ± standard errors (SE). Mean values followed by different letters (a–e) are significantly different at p < 0.05. Capital letters and numbers below bars indicate the name of the cultivar and the dose of fertilizer. Z—Zoom; H—Hobbit; G—Galation; S—Sandra; 60—60 kg N/ha; 90—90 kg N/ha; 120—120 kg N/ha; 150—150 kg N/ha.
A comparison of the content of the sum of identified polyphenols in grains cultivated with the different nitrogen doses showed no statistical differences. When comparing the studied barley varieties, Zoom stood out in terms of the concentration of these compounds (Table 5). The Zoom variety also showed a tendency towards a higher lutein content in the grain compared to the other varieties, while barley grain grown with different N-fertilizer doses did not differ in terms of the content of the identified carotenoids, namely lutein and β-carotene (Table 5). As reported by Movludi et al. [61], higher nitrogen rates were shown to reduce carotenoid levels in barley under stress conditions (drought). On the other hand, it is possible that moderate nitrogen doses may be beneficial for maintaining or slightly increasing the concentration of these compounds [51].

Table 5.
Total polyphenols, lutein, and β-carotene content in four barley varieties grown in different conditions of nitrogen fertilization (N-dose) in the controlled field trial in Poland.
3.3. Mycotoxins Occurrence
Amongst the analyzed mycotoxins, deoxynivalenol (DON) and 15-acetyldeoxynivalenol (15-AcDON) were the most frequently detected in the barley grains tested (Table 6). Of the 14 mycotoxins analyzed, only four were detected in the tested samples. Aflatoxins B1, B2, G1, and G2 were not detected in any of the samples, and neither were OTA, 3-acetyldeoxynivalenol (3-AcDON), and nivalenol (NIV). Fumonisin B2 was detected in only one case, similarly to T-2 and HT-2 toxins. Among the barley varieties, Sandra was found to be the least susceptible to mycotoxin accumulation, and only at the highest nitrogen fertilization was it found to contain small amounts of DON. In contrast, the Hobbit, Zoom, and Galation varieties, irrespective of nitrogen fertilization conditions, contained mycotoxin DON, while in the case of the Hobbit and Galation varieties at fertilization levels of 90, 120, and 150 kg nitrogen per hectare, 15-AcDON mycotoxin was present in the grain (Table 6). However, DON concentrations were far lower than the maximum permissible levels defined for this mycotoxin in the current EU regulation (which is 1000 μg kg−1 for unprocessed cereals) [62] which decreased from 1250 μg kg−1 set in the previous EU regulation [63]. The detected concentrations of T-2 and HT-2 toxins were also far below the maximum limit, set as 150 μg kg−1 for the sum of these two toxins in Commission Regulation (EU) No. 2024/1038 [64]. Regarding fumonisins, the current EU regulation No. 2023/915 does not set the maximum limits for barley [65].

Table 6.
Mycotoxins occurrence (μg/kg) in grains of four barley varieties grown in different conditions of nitrogen fertilization (N-dose) in the controlled field trial in Poland 1.
Deoxynivalenol (DON) and 15-acetyldeoxynivalenol (15-AcDON) are produced by Fusarium graminearum and Fusarium culmorum during cereal production. Both species are considered significant contributors to Fusarium head blight, a common fungal disease that often affects barley and other small-grain cereals [66]. These toxins are primarily produced during and after the flowering stage (anthesis) to early grain-filling stages of cereals such as wheat, barley, and maize. Infection risk is highest when warm, wet, and humid conditions occur during flowering, as the fungi infect open flowers and begin colonizing developing kernels. The fungi can continue to produce DON and 15-AcDON as the grain matures, especially if moist conditions persist up to harvest [67,68]. In our study, during the experiment in May, when barley was earing, high rainfall was recorded, almost twice as high as the long-term average (Table 1). In this case, this could have been the reason for the presence of these mycotoxins in the grain.
DON and 15-AcDON are commonly found in food and feed samples due to a combination of biological and stability factors. There is a notable prevalence of 15-AcDON-producing Fusarium strains, with certain species, such as F. graminearum, primarily biosynthesizing 15-AcDON as an intermediate metabolite during the production of DON [69,70]. This results in a higher natural occurrence of 15-AcDON compared to 3-AcDON in contaminated cereal grains [70]. Additionally, 15-AcDON often coexists with DON in grains, further increasing its detection frequency [71]. Stability during storage and processing also plays a crucial role; studies indicate that both DON and 15-AcDON demonstrate greater stability during grain storage than 3-AcDON, enhancing their durability and likelihood of detection [71].
Some authors point to the impact of nitrogen fertilization on the risk of mycotoxin contamination of cereal grains. In some cases, a higher risk of mycotoxin contamination has been associated with higher nitrogen fertilization rates during cultivation [29]. However, another study found that nitrogen fertilization reduced the Fusarium mycotoxin content in barley grains, suggesting that well-supplied plants may be more resistant [30]. The studies investigating the impact of agricultural factors and practices on mycotoxin contamination indicate that high organic matter content in the soil and diverse crop rotations are linked to a lower risk of Fusarium mycotoxin contamination, while high doses of mineral nitrogen fertilizers, overuse of certain fungicides and herbicides, and lack of crop rotation may increase the risk of contamination [31]. After harvest, fungal development and mycotoxin production can be prevented in grain storage under controlled humidity and temperature conditions. Reducing the storage period to less than six months can also be beneficial. Keeping the temperature at 10–15 °C and relative humidity at 65% can be effective [72].
Pathogenic fungi pose a risk and can make infected grain unsuitable for human consumption or animal feed production. These toxic secondary metabolites can cause various adverse effects in animals and humans. Mycotoxins are a serious food and feed safety threat concerning cereal-based foods and feeds. Particularly, infants, small children, farm animals, and pets are at greater risk of mycotoxicosis. The molds producing mycotoxins can cause acute toxicity as well as initiate/contribute to the development of a number of non-communicable diseases, including immunity suppression, growth impairment, neural defects, and cancers [73]. For this reason, it is essential to research varieties and growing conditions of cereals that indicate a lower risk of mycotoxin contamination.
3.4. Relations Between Experimental Factors and Parameters Tested
To gain a deeper understanding of the similarities and differences among the examined barley grain samples regarding their polyphenolic and carotenoid content as well as mycotoxin contamination, a principal component analysis (PCA) was conducted (Figure 3a,b, respectively). The PCA of bioactive compounds in barley samples of different varieties (Zoom, Galation, Hobbit, and Sandra) cultivated with different N fertilization levels (60, 90, 120, and 150 kg N ha−1) revealed that the first main component explains 39.56% of the total variance, while the second component explains 17.22%.
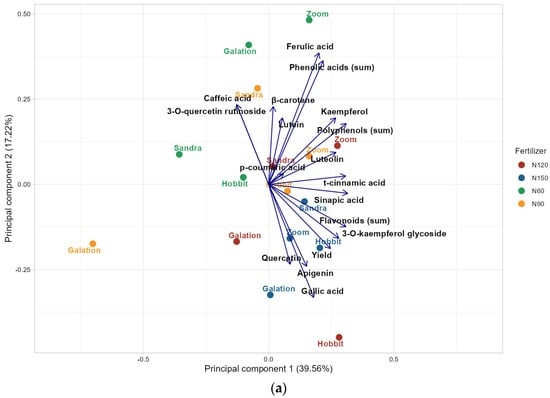
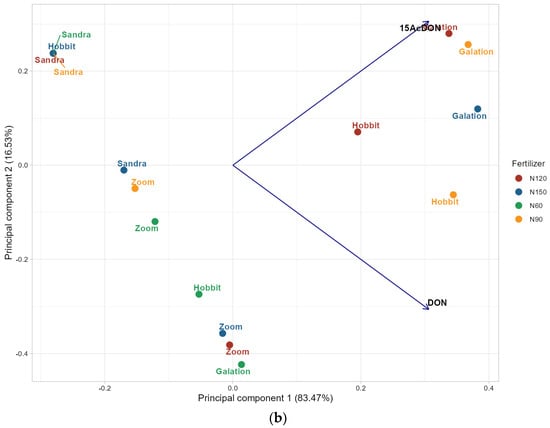
Figure 3.
The plot of principal component analysis (PCA) showing relationships between the chemical composition of grains of the studied barley varieties grown with different doses of N fertilizer: (a) carotenoids, phenolic acids, and flavonoids; (b) mycotoxins. 60 kg N ha−1 (N60), 90 kg N ha−1 (N90), 120 kg N ha−1 (N120), 150 kg N ha−1 (N150), 15-acetyldeoxynivalenol (15-AcDON), and deoxynivalenol (DON).
The PCA score plot shows that samples coming from the field plots fertilized with 60 kg and 150 kg N ha−1 tend to cluster in opposite quadrants, indicating distinct profiles of bioactive compounds at these fertilization levels. No clear grouping of results based on barley variety was observed, suggesting that N fertilization level has a more pronounced effect on the studied bioactive compounds than genotype. Lower nitrogen application (60 kg N ha−1) was associated with higher contents of phenolic acids, particularly ferulic and caffeic acid, as well as flavonoids such as quercetin-3-O-rutinoside and kaempferol, in grains. Conversely, the highest fertilization level (150 kg N ha−1) was related to increased levels of gallic acid, quercetin, apigenin, and the kaempferol-3-O-glycoside. Additionally, the highest fertilization dose was linked to increased grain yields, indicating a potential trade-off between bioactive compound profiles and productivity (Figure 3a). The demonstrated relationships are also confirmed by other authors studying cereals [74,75].
The PCA of 15-AcDON and DON mycotoxins’ contents in barley samples revealed that the first principal component explains 83.47% of the total variance, while the second component explains 16.53%. The biplot clearly distinguishes the barley varieties Hobbit and Galation from samples subjected to nitrogen fertilization at 90 and 120 kg N ha−1, with these groups associated with higher concentrations of 15-AcDON and DON mycotoxins. Specifically, the Hobbit and Galation samples from the highest and intermediate nitrogen treatments tend to exhibit elevated mycotoxin levels, indicating a correlation between increased nitrogen fertilization and mycotoxin contamination. This is confirmed by other studies, which have shown that increasing the nitrogen dose accelerates the development of Fusarium (fungi producing DON and 15-AcDON) and increases the production of mycotoxins, especially at high humidity during flowering [30]. Conversely, the varieties Sandra and Zoom cluster closely together in the PCA space, suggesting similar mycotoxin occurrence profiles across different fertilization levels. Notably, the Sandra samples, despite varying nitrogen applications, remain the most similar within their group (Figure 3b). These findings, similar to another study [76], underscore the influence of both barley variety and nitrogen fertilization on mycotoxin contamination, with certain varieties and treatment levels associated with higher toxin accumulation.
The Spearman correlation analysis revealed several significant relationships between phytochemical contents, crop yield, and fertilization levels in barley cultivation (Figure 4).
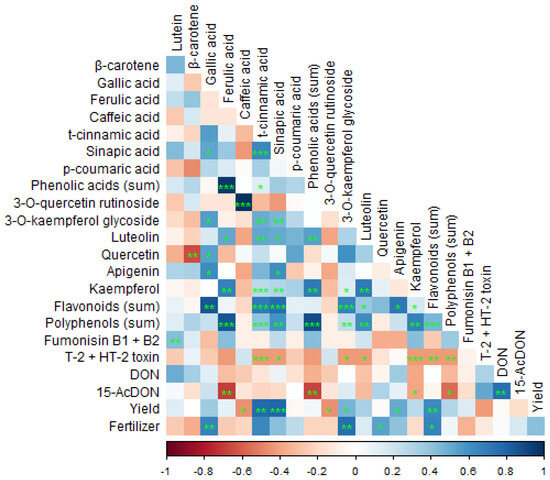
Figure 4.
Spearman’s correlation between the concentrations of bioactive compounds (phenolic acids, flavonoids and carotenoids), mycotoxins identified in barley samples, fertilization doses, and yield. Color (red/blue) and color intensity indicate the direction and the strength of the association. Green signs reflect statistical significance of the correlation at the level of p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***); 15-acetyldeoxynivalenol (15-AcDON), deoxynivalenol (DON).
Notably, barley grain yield was strongly and positively correlated with the levels of p-coumaric acid and sinapic acid, suggesting that higher concentrations of these phenolic acids may be associated with increased biomass production. Additionally, a moderate yet positive correlation was observed between yield and the total flavonoid content, particularly with apigenin, indicating that flavonoid accumulation could also be linked to plant productivity. Conversely, a negative correlation was identified between barley yield and the levels of caffeic acid and quercetin-3-O-rutinoside, implying that elevated concentrations of these compounds might be associated with reduced yields or stress responses. Fertilization levels showed positive correlations with gallic acid, the amount of kaempferol-3-O-glycoside, quercetin, and the sum of flavonoids tested, suggesting that increased nitrogen input may promote the biosynthesis of these secondary metabolites. However, this result is not typical based on past experiments.
The mycotoxin 15-AcDON demonstrated a strong and negative correlation with ferulic acid and kaempferol, suggesting that higher concentrations of 15-AcDON are associated with lower levels of these phenolic compounds. Additionally, the sum of mycotoxins T-2 and HT-2 exhibited negative correlations with various polyphenols, including p-coumaric acid, sinapic acid, kaempferol, quercetin-3-O-rutinoside, and kaempferol-3-O-glycoside, indicating an inverse relationship between these toxins and specific antioxidant constituents. Conversely, fumonisins B1 + B2 showed a positive correlation solely with lutein, implying a potential association between these mycotoxins and carotenoid content. Further analysis highlighted that flavonoid levels correlated with lutein. Our findings, as well as others, suggest complex interactions between mycotoxin contamination and the phytochemical profile of barley, which may be influenced by cultivation practices and environmental factors [77,78,79].
4. Conclusions
In conclusion, it is important to highlight the complex interactions between genotype, nitrogen fertilization, phytochemical composition, and mycotoxin contamination in barley, emphasizing the need for integrated breeding and crop management strategies to optimize yield, grain quality, and safety. Our research showed that genetic factors are the primary determinants of grain yield, with hybrid varieties (mainly Hobbit and Zoom) yielding better than the conventional one (Sandra). Nitrogen fertilization significantly impacted certain flavonoid contents, particularly in hybrids, but had little effect on overall yield. Mycotoxin accumulation (mainly DON and 15-Ac-DON) in grains varied by barley variety, with Sandra showing the least susceptibility, while Hobbit, Zoom, and Galation contained mycotoxins regardless of nitrogen levels. Higher contents of antioxidant compounds in barley may correlate with reduced mycotoxin levels, although this relationship is complex.
Optimal nitrogen management is essential to increase yields and phenolic concentrations while minimizing the risk of mycotoxin contamination of the grain. Therefore, breeding should aim to combine high yields with resistance to mycotoxins and favorable phytochemical profiles to enhance both productivity and grain quality and safety.
The current study, although showing some important limitations, provides a significant insight into future research focused on determining the impact of different rates of nitrogen fertilizers and barley varieties, not only on yield, but also on important quality and safety characteristics of barley grain. The research was conducted in specific regions of Poland, which may not represent barley growing conditions in other countries or climates. Therefore, the results may not be universally applicable. Moreover, only four winter barley varieties were examined (Hobbit, Zoom, Galation, and Sandra). The findings may not reflect the performance of other barley varieties that could exhibit different responses to nitrogen fertilization and mycotoxin contamination. Additionally, this study investigated only a limited range of nitrogen fertilization levels. Future research could explore a broader range and more specific N application rates to determine the optimal conditions for maximizing yield and grain quality and minimizing mycotoxin contamination risk. Results may vary across different years due to variations in weather patterns, pest pressures, and soil health, which can affect plant growth and mycotoxin levels. By addressing these limitations in future research, the understanding of how cultivation practices and environmental variables influence the quality and safety of barley could be further enhanced.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126904/s1. Figures S1 and S2: Chromatograms showing retention times for the polyphenols identified in barley grains; Figures S3 and S4: Chromatogram showing retention times for the carotenoids identified in barley grains; Figure S5: Standard calibration curves for fourteen analyzed mycotoxins; Figure S6: LC-MS/MS example chromatograms of mycotoxin solutions versus representative barley samples contaminated by mycotoxins.
Author Contributions
Conceptualization, R.K., D.Ś.-T., D.L., and B.G.; data curation, R.K., D.Ś.-T., K.K., E.H., and A.N.; formal analysis, K.K., M.Ż.-K., E.H., A.N., and A.H.-K.; funding acquisition, R.K., D.L., and B.G.; investigation, R.K., D.Ś.-T., K.K., J.W., D.L., A.N., and A.H.-K.; methodology, R.K., E.H., D.L., A.N., A.H.-K., R.T.-S., and B.G.; resources, E.H., D.L., and B.G.; supervision, R.K. and D.Ś.-T.; visualization, R.K., D.Ś.-T., K.K., M.Ż.-K., and R.T.-S.; writing—original draft, R.K., D.Ś.-T., K.K., J.W., A.N., and A.H.-K.; writing—review and editing, R.K., D.Ś.-T., K.K., J.W., M.Ż.-K., E.H., D.L., A.N., A.H.-K., R.T.-S., and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Higher Education with funds of the Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS) for scientific research.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We gratefully acknowledge the technical support of Tomasz Strok in the preparation of barley grain samples.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| 13C17-AB1 | 13C17–aflatoxin B1 |
| 15Ac-DON | 15-acetyldeoxynivalenol |
| 3-AcDON | 3-acetyldeoxynivalenol |
| AFLB1 | Aflatoxin B1 |
| AFLB2 | Aflatoxin B2 |
| AFLG1 | Aflatoxin G1 |
| AFLG2 | Aflatoxin G2 |
| ANOVA | Analysis of variance |
| CSS | Check sample survey |
| CUR | Curtain gas |
| DON | Deoxynivalenol |
| FB1 | Fumonisin B1 |
| FB2 | Fumonisin B2 |
| GAE | Gallic acid equivalent |
| HPLC | High-performance liquid chromatography |
| HT-2 | HT-2 toxin |
| ILS | Internal standard |
| IRMM | Institute for Reference Materials and Measurements |
| LCL | Lowest calibration level |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| LDL | Low-density lipoprotein |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MRM | Multiple reaction monitoring |
| N | Nitrogen |
| NIV | Nivalenol |
| OTA | Ochratoxin A |
| PCA | Principal component analysis |
| RT | Retention time |
| T-2 | T-2 toxin |
| ZEN | Zearalenone |
References
- Rodríguez, A.; van Grinsven, H.J.M.; van Loon, M.P.; Doelman, J.C.; Beusen, A.H.W.; Lassaletta, L. Costs and Benefits of Synthetic Nitrogen for Global Cereal Production in 2015 and in 2050 under Contrasting Scenarios. Sci. Total Environ. 2024, 912, 169357. [Google Scholar] [CrossRef] [PubMed]
- Soare, E.; Bold, N.; Stoicea, P.; David, L.; Dobre, C.A.; Firățoiu, A.R. Survey on the Worldwide Barley Production and Trade. Bulg. J. Agric. Sci. 2023, 29, 119–124. [Google Scholar]
- Meints, B.; Vallejos, C.; Hayes, P. Multi-Use Naked Barley: A New Frontier. J. Cereal Sci. 2021, 102, 103370. [Google Scholar] [CrossRef]
- Yuan, C.; Hu, R.; He, L.; Hu, J.; Liu, H. Extraction and Prebiotic Potential of β-Glucan from Highland Barley and Its Application in Probiotic Microcapsules. Food Hydrocoll. 2023, 139, 108520. [Google Scholar] [CrossRef]
- Chen, L.; Cui, C.; Wang, Z.; Che, F.; Chen, Z.; Feng, S. Structural Characterization and Antioxidant Activity of β-Glucans from Highland Barley Obtained with Ultrasonic–Microwave-Assisted Extraction. Molecules 2024, 29, 684. [Google Scholar] [CrossRef]
- Panizo-Casado, M.; Déniz-Expósito, P.; Rodríguez-Galdón, B.; Afonso-Morales, D.; Ríos-Mesa, D.; Díaz-Romero, C.; Rodríguez-Rodríguez, E.M. The Chemical Composition of Barley Grain (Hordeum vulgare L.) Landraces from the Canary Islands. J. Food Sci. 2020, 85, 1725–1734. [Google Scholar] [CrossRef]
- Kaur, A.; Purewal, S.S.; Phimolsiripol, Y.; Punia Bangar, S. Unraveling the Hidden Potential of Barley (Hordeum vulgare): An Important Review. Plants 2024, 13, 2421. [Google Scholar] [CrossRef]
- Hussain, A.; Larsson, H.; Johansson, E. Carotenoid Extraction from Locally and Organically Produced Cereals Using Saponification Method. Processes 2021, 9, 783. [Google Scholar] [CrossRef]
- Pandey, S.; Kunwar, N. Impact of Barley Product on Human Health and Inhibiting Factors for Consuming the Barley Product. Asian J. Agric. Ext. Econ. Sociol. 2023, 41, 146–152. [Google Scholar] [CrossRef]
- Matsuoka, T.; Hosomi, K.; Park, J.; Goto, Y.; Nishimura, M.; Maruyama, S.; Murakami, H.; Konishi, K.; Miyachi, M.; Kawashima, H.; et al. Relationships between Barley Consumption and Gut Microbiome Characteristics in a Healthy Japanese Population: A Cross-Sectional Study. BMC Nutr. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Dixon, R.A.; Dickinson, A.J. A Century of Studying Plant Secondary Metabolism—From “What?” To “Where, How, and Why?”. Plant Physiol. 2024, 195, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Hajji, T.; Mansouri, S.; Vecino-Bello, X.; Cruz-Freire, J.M.; Rezgui, S.; Ferchichi, A. Identification and Characterization of Phenolic Compounds Extracted from Barley Husks by LC-MS and Antioxidant Activity in Vitro. J. Cereal Sci. 2018, 81, 83–90. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review. Nutrients 2024, 16, 10. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Amarowicz, R. Natural Phenolic Compounds Protect LDL against Oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 677–679. [Google Scholar] [CrossRef]
- Trono, D. Carotenoids in Cereal Food Crops: Composition and Retention throughout Grain Storage and Food Processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef]
- Niaz, M.; Zhang, B.; Zhang, Y.; Yan, X.; Yuan, M.; Cheng, Y.Z.; Lv, G.; Fadlalla, T.; Zhao, L.; Sun, C.; et al. Genetic and Molecular Basis of Carotenoid Metabolism in Cereals. Theor. Appl. Genet. 2023, 136, 1–14. [Google Scholar] [CrossRef]
- Paznocht, L.; Kotíková, Z.; Šulc, M.; Lachman, J.; Orsák, M.; Eliášová, M.; Martinek, P. Free and Esterified Carotenoids in Pigmented Wheat, Tritordeum and Barley Grains. Food Chem. 2018, 240, 670–678. [Google Scholar] [CrossRef]
- Kumar, P.; Banik, S.P.; Ohia, S.E.; Moriyama, H.; Chakraborty, S.; Wang, C.K.; Song, Y.S.; Goel, A.; Bagchi, M.; Bagchi, D. Current Insights on the Photoprotective Mechanism of the Macular Carotenoids, Lutein and Zeaxanthin: Safety, Efficacy and Bio-Delivery. J. Am. Nutr. Assoc. 2024, 43, 505–518. [Google Scholar] [CrossRef]
- Tola, M.; Kebede, B. Occurrence, Importance and Control of Mycotoxins: A Review. Cogent. Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, Z.; Hou, J.; Yao, Y. Contamination and Control of Mycotoxins in Grain and Oil Crops. Microorganisms 2024, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, Toxicity, Production and Detection of Fusarium Mycotoxin: A Review. FPPN 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Shi, H.; Schwab, W.; Yu, P. Natural Occurrence and Co-Contamination of Twelve Mycotoxins in Industry-Submitted Cool-Season Cereal Grains Grown under a Low Heat Unit Climate Condition. Toxins 2019, 11, 160. [Google Scholar] [CrossRef]
- Sinphithakkul, P.; Poapolathep, A.; Klangkaew, N.; Imsilp, K.; Logrieco, A.F.; Zhang, Z.; Poapolathep, S. Occurrence of Multiple Mycotoxins in Various Types of Rice and Barley Samples in Thailand. J. Food Prot. 2019, 82, 1007–1015. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Zadravec, M.; Markov, K.; Lešić, T.; Frece, J.; Petrović, D.; Pleadin, J. Biocontrol Methods in Avoidance and Downsizing of Mycotoxin Contamination of Food Crops. Processes 2022, 10, 655. [Google Scholar] [CrossRef]
- Krnjaja, V.; Mandić, V.; Lević, J.; Stanković, S.; Petrović, T.; Vasić, T.; Obradović, A. Influence of N-Fertilization on Fusarium Head Blight and Mycotoxin Levels in Winter Wheat. Crop Prot. 2015, 67, 251–256. [Google Scholar] [CrossRef]
- Hofer, K.; Barmeier, G.; Schmidhalter, U.; Habler, K.; Rychlik, M.; Hückelhoven, R.; Hess, M. Effect of Nitrogen Fertilization on Fusarium Head Blight in Spring Barley. Crop Prot. 2016, 88, 18–27. [Google Scholar] [CrossRef]
- Bernhoft, A.; Wang, J.; Leifert, C. Effect of Organic and Conventional Cereal Production Methods on Fusarium Head Blight and Mycotoxin Contamination Levels. Agronomy 2022, 12, 797. [Google Scholar] [CrossRef]
- Polish Norm PN-EN 12145 2001; Fruits and Vegetable Juices-Determination of Dry Matter-Gravimetric Method. The Polish Committee for Standarization: Warsaw, Poland, 2001.
- Kazimierczak, R.; Średnicka-Tober, D.; Leszczyńska, D.; Nowacka, A.; Hallmann, E.; Barański, M.; Kopczyńska, K.; Gnusowski, B. Evaluation of Phenolic Compounds and Carotenoids Content and Mycotoxins Occurrence in Grains of Seventeen Barley and Eight Oat Cultivars Grown under Organic Management. Appl. Sci. 2020, 10, 6369. [Google Scholar] [CrossRef]
- Spanjer, M.; Rensen, P.; Scholten, J. LC-MS/MS Multi-Method for Mycotoxins after Single Extraction, with Validation Data for Peanut, Pistachio, Wheat, Maize, Cornflakes, Raisins and Figs. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; UR 28099; Publications Office of the European Union: Luxembourg, 2016; pp. 1–58. ISBN 9789279617683. [Google Scholar]
- A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024.
- Mühleisen, J.; Piepho, H.P.; Maurer, H.P.; Longin, C.F.H.; Reif, J.C. Yield Stability of Hybrids versus Lines in Wheat, Barley, and Triticale. Theor. Appl. Genet. 2014, 127, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Scapino, M.; Meloni, R.; Blandino, M. A Comparison of the Agronomic Management of a Winter Barley Hybrid and a Conventional Genotype: Effect of the Seeding Rate, Soil Tillage and Nitrogen Fertilization. Front. Agron. 2025, 7, 1546989. [Google Scholar] [CrossRef]
- Maresma, Á.; Martínez-Casasnovas, J.A.; Santiveri, F.; Lloveras, J. Nitrogen Management in Double-Annual Cropping System (Barley-Maize) under Irrigated Mediterranean Environments. Eur. J. Agron. 2019, 103, 98–107. [Google Scholar] [CrossRef]
- Preiti, G.; Calvi, A.; Romeo, M.; Badagliacca, G.; Bacchi, M. Seeding Density and Nitrogen Fertilization Effects on Agronomic Responses of Some Hybrid Barley Lines in a Mediterranean Environment. Agronomy 2021, 11, 1942. [Google Scholar] [CrossRef]
- Tuppad, P.; Kishore, A.; Kharad, S.S.; Sharma, J.D. Effect of Nitrogen Levels on the Growth and Yield of Barley (Hordeum vulgare L.) Varieties. Ecol. Environ. Conserv. 2023, 29, 156–160. [Google Scholar] [CrossRef]
- Dang, B.; Zhang, W.G.; Zhang, J.; Yang, X.J.; Xu, H. De Evaluation of Nutritional Components, Phenolic Composition, and Antioxidant Capacity of Highland Barley with Different Grain Colors on the Qinghai Tibet Plateau. Foods 2022, 11, 2025. [Google Scholar] [CrossRef]
- Han, Z.; Gao, H.; Ye, L.; Adil, M.F.; Ahsan, M.; Zhang, G. Identification of QTLs Associated with P-Coumaric Acid and Ferulic Acid in Barley. Euphytica 2019, 215, 198. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Choo, T.M.; Dhillon, S.; Rabalski, I. Free and Bound Phenolic Acids and Total Phenolics in Black, Blue, and Yellow Barley and Their Contribution to Free Radical Scavenging Capacity. Cereal Chem. 2012, 89, 198–204. [Google Scholar] [CrossRef]
- Gałązka, A.; Gawryjołek, K.; Żuchowski, J. Evaluation of the Content of Phenolic Acids and Their Antioxidant Activity in Winter Cereal Seeds. J. Elem. 2017, 22, 593–605. [Google Scholar] [CrossRef]
- Gani, A.; SM, W.; FA, M. Whole-Grain Cereal Bioactive Compounds and Their Health Benefits: A Review. J. Food Process. Technol. 2012, 3, 146–156. [Google Scholar] [CrossRef]
- Yang, T.; Duan, C.; Zeng, Y.; Du, J.; Yang, S.; Pu, X.; Yang, S. HPLC Analysis of Flavonoids Compounds of Purple, Normal Barley Grain. Adv. Mat. Res. 2013, 634–638, 1486–1490. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Mock, H.-P.; Kukoeva, T.V.; Bö Rner, A.; Khlestkina, E.K. Regulation of the Flavonoid Biosynthesis Pathway Genes in Purple and Black Grains of Hordeum Vulgare. PLoS ONE 2016, 11, e0163782. [Google Scholar] [CrossRef]
- Han, Z.; Ahsan, M.; Adil, M.F.; Chen, X.; Nazir, M.M.; Shamsi, I.H.; Zeng, F.; Zhang, G. Identification of the Gene Network Modules Highly Associated with the Synthesis of Phenolics Compounds in Barley by Transcriptome and Metabolome Analysis. Food Chem. 2020, 323, 126862. [Google Scholar] [CrossRef]
- Mencin, M.; Abramovič, H.; Jamnik, P.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Abiotic Stress Combinations Improve the Phenolics Profiles and Activities of Extractable and Bound Antioxidants from Germinated Spelt (Triticum spelta L.) Seeds. Food Chem. 2021, 344, 128704. [Google Scholar] [CrossRef]
- Nowak, R.; Szczepanek, M.; Kobus-Cisowska, J.; Stuper-Szablewska, K.; Graczyk, R.; Błaszczyk, K. Relationships Between Photosynthetic Efficiency and Grain Antioxidant Content of Barley Genotypes Under Increasing Nitrogen Rates. Agriculture 2024, 14, 1913. [Google Scholar] [CrossRef]
- Surányi, S.; Izsáki, Z. Plant Analysis Application for Environmentally Friendly Fertilization of Winter Barley (Hordeum vulgare L.). Appl. Ecol. Environ. Res. 2018, 14, 5213–5226. [Google Scholar] [CrossRef]
- Wang, S.; Peng, J.; Dong, W.; Wei, Z.; uz Zafar, S.; Jin, T.; Liu, E. Optimizing Irrigation and Nitrogen Fertilizer Regimes to Increase the Yield and Nitrogen Utilization of Tibetan Barley in Tibet. Agronomy 2024, 14, 1775. [Google Scholar] [CrossRef]
- Liu, R.H. Whole Grain Phytochemicals and Health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive Phytochemicals in Barley. J. Food Drug. Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of Phenolic Content in Different Barley Varieties and Corresponding Malts by Liquid Chromatography-Diode Array Detection-Electrospray Ionization Tandem Mass Spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Shirley, B.W. Flavonoids in Seeds and Grains: Physiological Function, Agronomic Importance and the Genetics of Biosynthesis. Seed. Sci. Res. 1998, 8, 415–422. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Pu, Z.; Wang, J.; Zheng, Y.; Li, Y.; Wei, Y. Regulation, Evolution, and Functionality of Flavonoids in Cereal Crops. Biotechnol Lett. 2013, 35, 1765–1780. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Jin, H.M.; Dang, B.; Zhang, W.G.; Zheng, W.C.; Yang, X.J. Polyphenol and Anthocyanin Composition and Activity of Highland Barley with Different Colors. Molecules 2022, 27, 3411. [Google Scholar] [CrossRef]
- Movludi, A.; Ebadi, A.; Jahanbakhsh, S.; Davari, M.; Parmoon, G. The Effect of Water Deficit and Nitrogen on the Antioxidant Enzymes’ Activity and Quantum Yield of Barley (Hordeum vulgare L.). Not. Bot. Horti Agrobot. Cluj Napoca 2014, 42, 398–404. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EU) 2024/1022 of 8 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food. Off. J. Eur. Union 2024, 1–4. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EU) 2024/1038 of 9 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of T-2 and HT-2 Toxins in Food. Off. J. Eur. Union 2024, 1–5. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EU) 2023/915 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 103–157. [Google Scholar]
- Mesterhazy, A. Updating the Breeding Philosophy of Wheat to Fusarium Head Blight (FHB): Resistance Components, QTL Identification, and Phenotyping—A Review. Plants 2020, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Borowik, P.; Dyshko, V.; Tkaczyk, M.; Okorski, A.; Polak-Śliwińska, M.; Tarakowski, R.; Stocki, M.; Stocka, N.; Oszako, T. Analysis of Wheat Grain Infection by Fusarium Mycotoxin-Producing Fungi Using an Electronic Nose, GC-MS, and QPCR. Sensors 2024, 24, 326. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Khaneghah, A.; Kamani, M.H.; Fakhri, Y.; Coppa, C.F.S.C.; de Oliveira, C.A.F.; Sant’Ana, A.S. Changes in Masked Forms of Deoxynivalenol and Their Co-Occurrence with Culmorin in Cereal-Based Products: A Systematic Review and Meta-Analysis. Food Chem. 2019, 294, 587–596. [Google Scholar] [CrossRef]
- Gonçalves, C.; Stroka, J. Cross-Reactivity Features of Deoxynivalenol (DON)-Targeted Immunoaffinity Columns Aiming to Achieve Simultaneous Analysis of DON and Major Conjugates in Cereal Samples. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1053–1062. [Google Scholar] [CrossRef]
- Alizadeh, A.; Braber, S.; Akbari, P.; Kraneveld, A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol and Its Modified Forms: Are There Major Differences? Toxins 2016, 8, 334. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Zhang, Y.; Lu, M.; Sun, L.; Li, W.; Hu, X.; Wang, B. Retention of Deoxynivalenol and Its Derivatives during Storage of Wheat Grain and Flour. Food Control 2016, 65, 177–181. [Google Scholar] [CrossRef]
- Ham, H.; Baek, J.; Lee, M.; Lee, T.; Hong, S.K.; Lee, S. Change of Fungi and Mycotoxin in Hulled Barley under Different Conditions and Period. Korean J. Food Preserv 2017, 24, 857–864. [Google Scholar] [CrossRef]
- Yu, J.; Pedroso, I.R. Mycotoxins in Cereal-Based Products and Their Impacts on the Health of Humans, Livestock Animals and Pets. Toxins 2023, 15, 480. [Google Scholar] [CrossRef]
- Stumpf, B.; Yan, F.; Honermeier, B. Influence of Nitrogen Fertilization on Yield and Phenolic Compounds in Wheat Grains (Triticum Aestivum L. Ssp. Aestivum). J. Plant Nutr. Soil Sci. 2019, 182, 111–118. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, S.; Feng, D.; Duan, N.; Rong, L.; Wu, Z.; Shen, Y. Effect of Different Doses of Nitrogen Fertilization on Bioactive Compounds and Antioxidant Activity of Brown Rice. Front. Nutr. 2023, 10, 1071874. [Google Scholar] [CrossRef] [PubMed]
- Krnjaja, V.; Mandić, V.; Petrović, T.; Stanković, S.; Lučev, M.; Obradović, A.; Mićić, N. Fusarium Spp. Infection, Mycotoxin Contamination, and Some Agronomic Traits in Winter Barley as Affected by N Fertilization under Serbia Conditions. Chil. J. Agric. Res. 2024, 84, 632–643. [Google Scholar] [CrossRef]
- Chhaya, R.S.; O’Brien, J.; Cummins, E. Feed to Fork Risk Assessment of Mycotoxins under Climate Change Influences—Recent Developments. Trends Food Sci. Technol. 2022, 126, 126–141. [Google Scholar] [CrossRef]
- Beccari, G.; Tini, F.; Jørgensen, H.J.L. Editorial: Current Advances in the Metabolism of Mycotoxins in Plants. Front. Plant Sci. 2023, 14, 1343855. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, S.; Du, Z.; An, Y.; Zhang, C.; Yao, Y. Contamination Status and Control of Mycotoxins in Grain and Oil Crops. J. AGRO-Environ. Sci. 2022, 41, 2680–2687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).