Cortical Tuber Types in Tuberous Sclerosis Complex: Need for New MRI-Based Classification System Incorporating Changes in Susceptibility Weighted Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Imaging Selection Criteria
2.2. MRI Evaluation and Classification Model
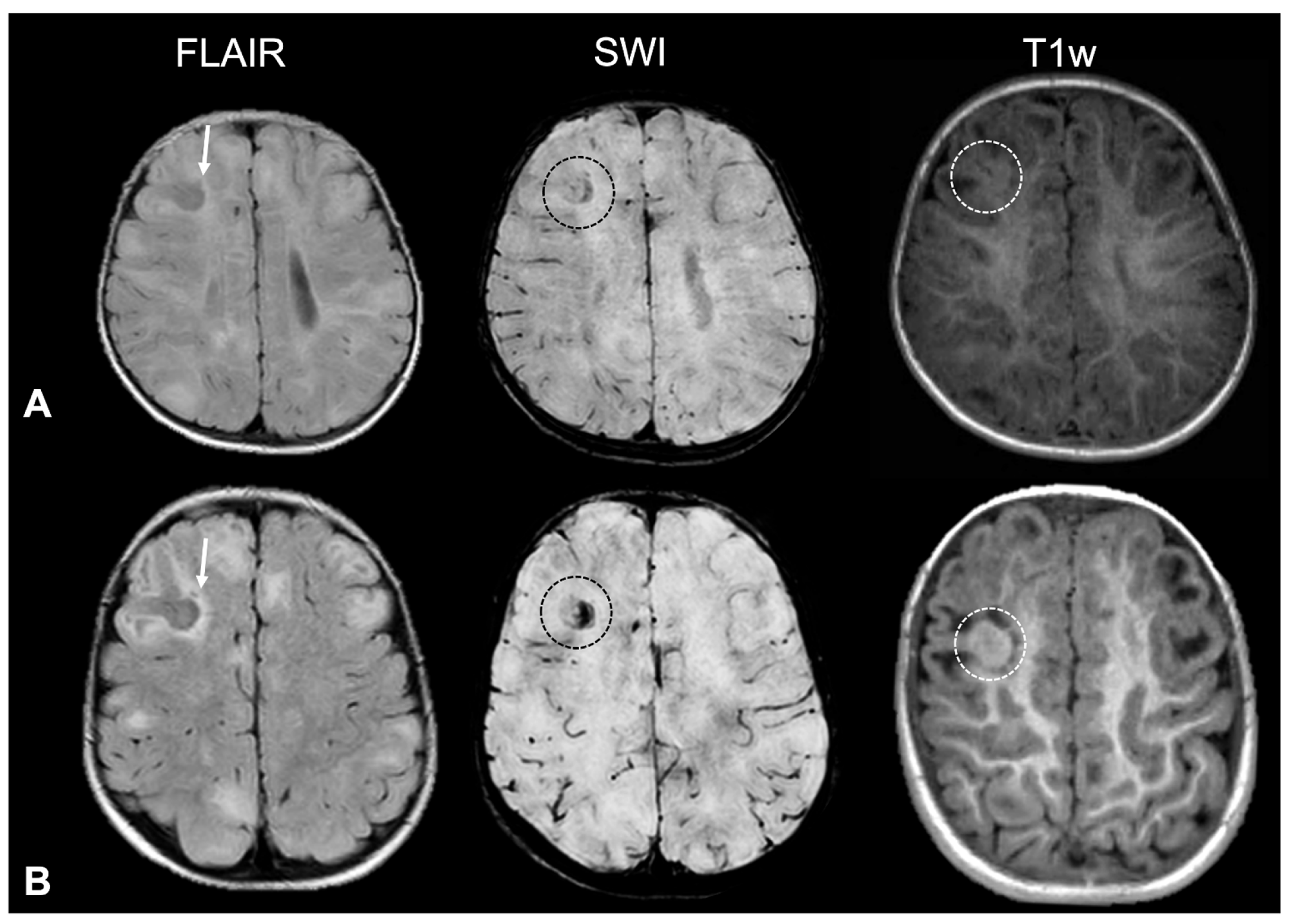
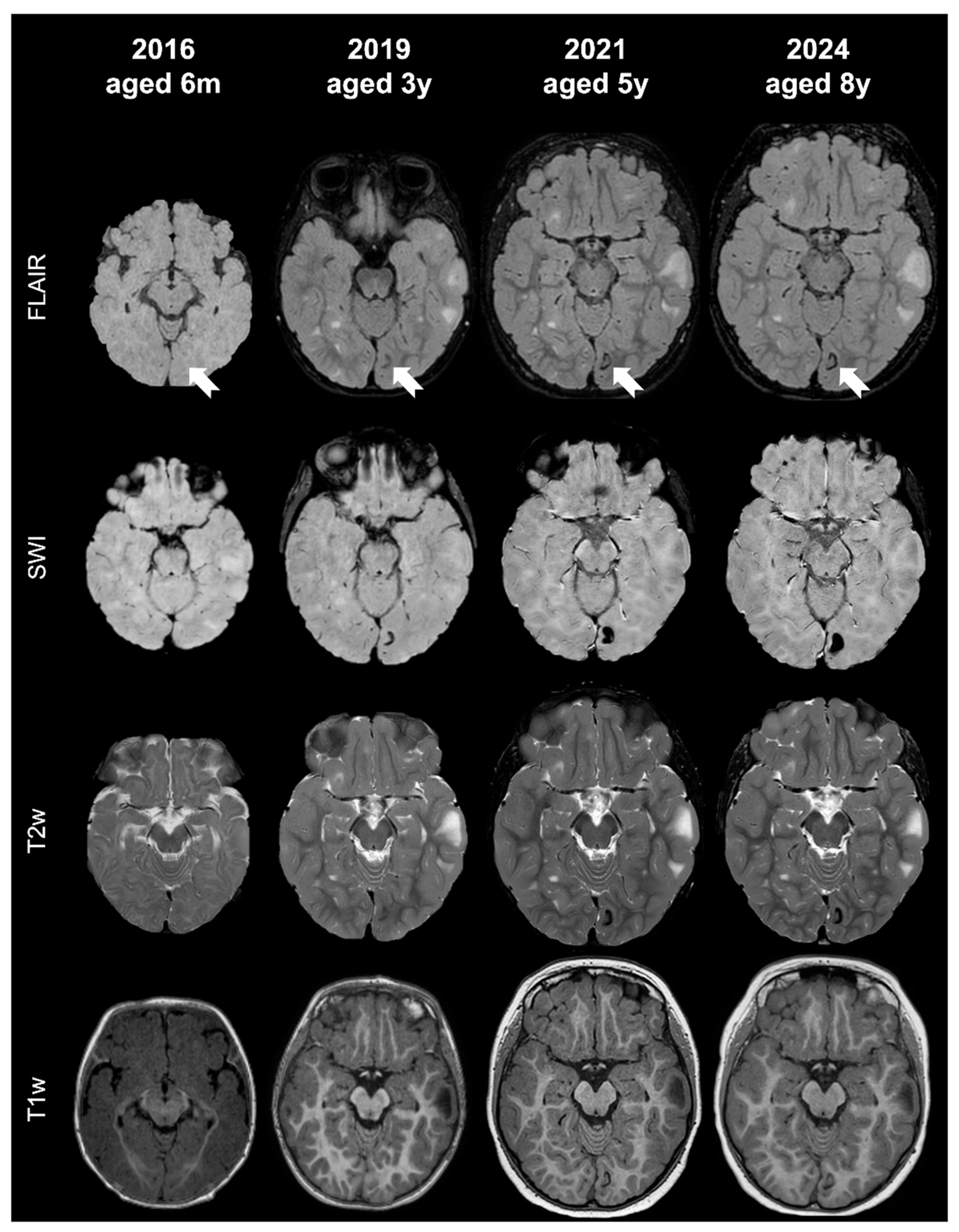
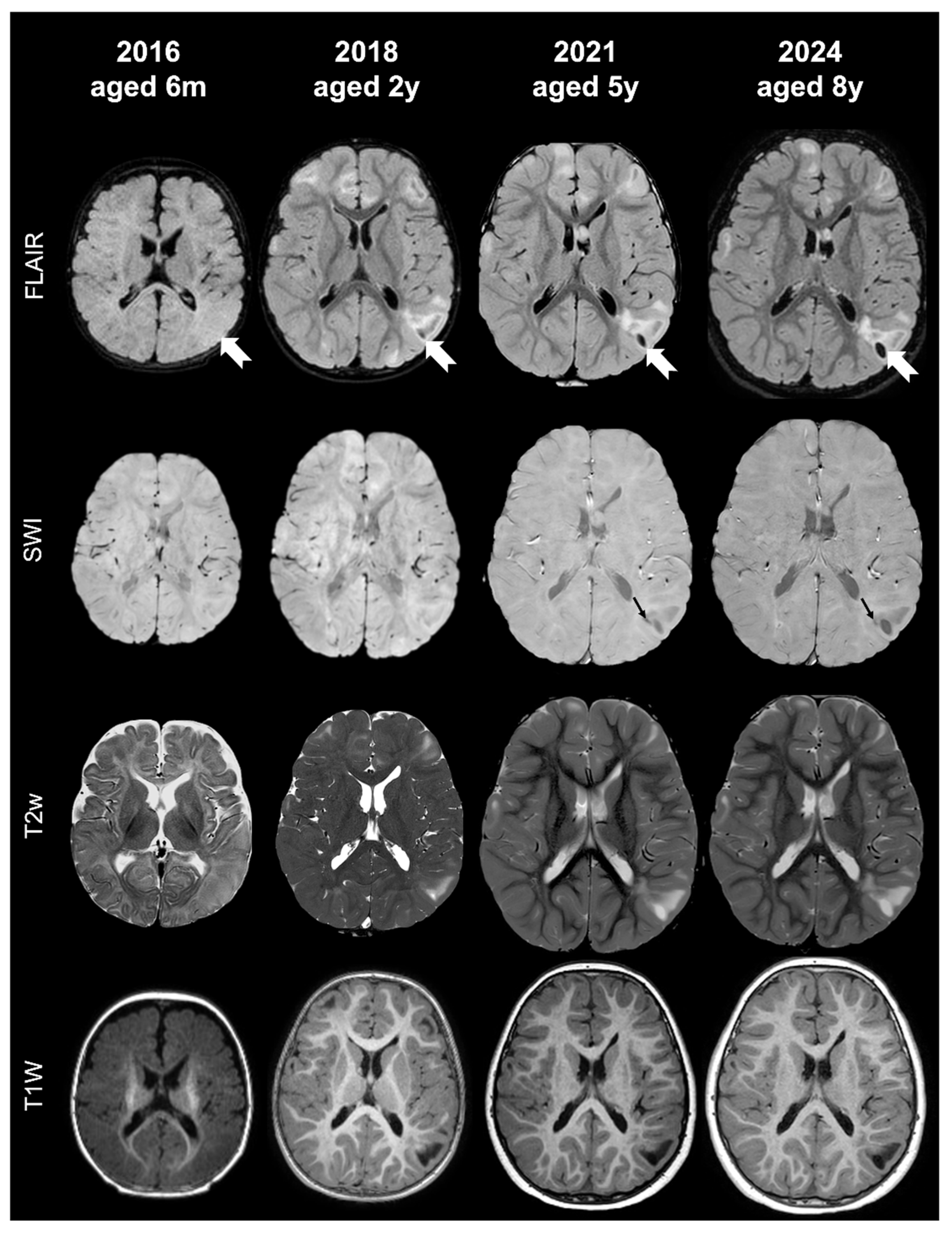
- Tuber A (corresponding to former tuber A in the classification by Gallagher et al., 2010 [27]): isointense on volumetric T1-weighted images and subtly hyperintense on T2-weighted images, with no mass effect, no distortion of the gyral folding pattern, and no calcifications on SWI.
- Tuber B (corresponding to former tuber B in the classification by Gallagher et al., 2010 [27]): hypointense on volumetric T1-weighted images and homogeneously hyperintense on T2-weighted images, with no well-defined borders, minimal mass effect, slight disruption of the gyral pattern, and no calcifications on SWI.
- Tuber C: hypointense on volumetric T1-weighted images and homogeneously hyperintense on T2-weighted images with inner calcifications on SWI, further divided into the following:
- Tuber D: hypointense on volumetric T1-weighted images and homogeneously hyperintense on T2-weighted images with a central cystic area of vacuolization, regardless of the presence of associate calcification(s).
2.3. Statistical Analysis
3. Results
4. Discussion
- Tuber A: isointense on volumetric T1-weighted images and subtly hyperintense on T2-weighted images, with no mass effect, no distortion of the gyral folding pattern, and no calcifications on SWI
- Tuber B: hypointense on volumetric T1-weighted images and homogeneously hyperintense on T2-weighted images, with no well-defined borders, minimal mass effect, slight disruption of the gyral pattern, and no calcifications on SWI
- Tuber C: hypointense on volumetric T1-weighted images and homogeneously hyperintense on T2-weighted images with inner calcifications on SWI, further divided into the following:
- Tuber C1: with subtle, non-confluent, pinpoint-like calcifications on SWI (micro-calcified)
- Tuber C2: with large, confluent, linear or curvilinear calcifications on SWI (macro-calcified)
- Tuber D: hypointense on volumetric T1-weighted images and homogeneously hyperintense on T2-weighted images, with a central cystic area of vacuolization, regardless of associated calcification(s).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| CT | cortical tuber |
| ICC | intraclass correlation coefficient |
| MRgLITT | magnetic resonance-guided laser interstitial thermal therapy |
| MRI | magnetic resonance imaging |
| mTOR | mechanistic target of rapamycin complex |
| NMI | no mutation identified |
| RML | radial migration line |
| SEGA | sub-ependymal giant cell astrocytoma |
| SEN | sub-ependymal nodule |
| SWI | susceptibility-weighted imaging |
| TSC | tuberous sclerosis complex |
| WML | white matter lesion |
References
- Crino, P.B. mTOR Signaling in Epilepsy: Insights from Malformations of Cortical Development. Cold Spring Harb. Perspect. Med. 2015, 5, a022442. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Nastro, A.; Cicala, D.; De Liso, M.; Covelli, E.M.; Cinalli, G. Neuroimaging in tuberous sclerosis complex. Child’s Nerv. Syst. 2020, 36, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- Umeoka, S.; Koyama, T.; Miki, Y.; Akai, M.; Tsutsui, K.; Togashi, K. Pictorial Review of Tuberous Sclerosis in Various Organs. RadioGraphics 2008, 28, e32. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Aronow, M.E.; Bebin, E.M.; Bissler, J.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Fuchs, Z.; Gosnell, E.S.; Gupta, N.; et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef]
- Moavero, R.; Kotulska, K.; Lagae, L.; Benvenuto, A.; Gialloreti, L.E.; Weschke, B.; Riney, K.; Feucht, M.; Krsek, P.; Nabbout, R.; et al. Is autism driven by epilepsy in infants with Tuberous Sclerosis Complex? Ann. Clin. Transl. Neurol. 2020, 7, 1371–1381. [Google Scholar] [CrossRef]
- Curatolo, P.; Moavero, R.; de Vries, P.J. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015, 14, 733–745. [Google Scholar] [CrossRef]
- Cusmai, R.; Moavero, R.; Bombardieri, R.; Vigevano, F.; Curatolo, P. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy Behav. 2011, 22, 735–739. [Google Scholar] [CrossRef]
- Curatolo, P.; Napolioni, V.; Moavero, R. Autism Spectrum Disorders in Tuberous Sclerosis: Pathogenetic Pathways and Implications for Treatment. J. Child. Neurol. 2010, 25, 873–880. [Google Scholar] [CrossRef]
- Pereira, C.C.d.S.; Dantas, F.D.G.; Baratela, W.A.d.R.; da Costa, F.A.; Lucato, L.T.; Kok, F. Tuberous sclerosis complex: Clinical, genetic and 7T-MRI neuroimaging findings. Brain Dev. 2025, 47, 104386. [Google Scholar] [CrossRef]
- Tsai, V.; Parker, W.E.; Orlova, K.A.; Baybis, M.; Chi, A.W.; Berg, B.D.; Birnbaum, J.F.; Estevez, J.; Okochi, K.; Sarnat, H.B.; et al. Fetal Brain mTOR Signaling Activation in Tuberous Sclerosis Complex. Cereb. Cortex 2014, 24, 315–327. [Google Scholar] [CrossRef]
- Dabora, S.L.; Jozwiak, S.; Franz, D.N.; Roberts, P.S.; Nieto, A.; Chung, J.; Choy, Y.-S.; Reeve, M.P.; Thiele, E.; Egelhoff, J.C.; et al. Mutational Analysis in a Cohort of 224 Tuberous Sclerosis Patients Indicates Increased Severity of TSC2, Compared with TSC1, Disease in Multiple Organs. Am. J. Hum. Genet. 2001, 68, 64–80. [Google Scholar] [CrossRef]
- Nijman, M.; Yang, E.; Jaimes, C.; Prohl, A.K.; Sahin, M.; Krueger, D.A.; Wu, J.Y.; Northrup, H.; Stone, S.S.; Madsen, J.R.; et al. Limited utility of structural MRI to identify the epileptogenic zone in young children with tuberous sclerosis. J. Neuroimaging 2022, 32, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Jeong, A.; Nakagawa, J.A.; Wong, M. Predictors of Drug-Resistant Epilepsy in Tuberous Sclerosis Complex. J. Child. Neurol. 2017, 32, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, M.; Jurkiewicz, E.; Domańska-Pakieła, D.; Syczewska, M.; Łojszczyk, B.; Chmielewski, D.; Kotulska, K.; Kuczyński, D.; Kmieć, T.; Dunin-Wąsowicz, D.; et al. Cerebral tuber count and its impact on mental outcome of patients with tuberous sclerosis complex. Epilepsia 2011, 52, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Chu-Shore, C.J.; Major, P.; Camposano, S.; Muzykewicz, D.; Thiele, E.A. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 2010, 51, 1236–1241. [Google Scholar] [CrossRef]
- Moavero, R.; Napolitano, A.; Cusmai, R.; Vigevano, F.; Figà-Talamanca, L.; Calbi, G.; Curatolo, P.; Bernardi, B. White matter disruption is associated with persistent seizures in tuberous sclerosis complex. Epilepsy Behav. 2016, 60, 63–67. [Google Scholar] [CrossRef]
- Sahin, M.; Henske, E.P.; Manning, B.D.; Ess, K.C.; Bissler, J.J.; Klann, E.; Kwiatkowski, D.J.; Roberds, S.L.; Silva, A.J.; Hillaire-Clarke, C.S.; et al. Advances and Future Directions for Tuberous Sclerosis Complex Research: Recommendations from the 2015 Strategic Planning Conference. Pediatr. Neurol. 2016, 60, 1–12. [Google Scholar] [CrossRef]
- Jesmanas, S.; Norvainytė, K.; Gleiznienė, R.; Šimoliūnienė, R.; Endzinienė, M. Different MRI-defined tuber types in tuberous sclerosis complex: Quantitative evaluation and association with disease manifestations. Brain Dev. 2018, 40, 196–204. [Google Scholar] [CrossRef]
- Zhang, M.-N.; Zou, L.-P.; Wang, Y.-Y.; Pang, L.-Y.; Ma, S.-F.; Huang, L.-L.; Gao, Y.; Lu, Q.; Franz, D.N. Calcification in cerebral parenchyma affects pharmacoresistant epilepsy in tuberous sclerosis. Seizure 2018, 60, 86–90. [Google Scholar] [CrossRef]
- Hulshof, H.M.; Benova, B.; Krsek, P.; Kyncl, M.; Lequin, M.H.; Belohlavkova, A.; Jezdik, P.; Braun, K.P.J.; Jansen, F.E. The epileptogenic zone in children with tuberous sclerosis complex is characterized by prominent features of focal cortical dysplasia. Epilepsia Open 2021, 6, 663–671. [Google Scholar] [CrossRef]
- Holmes, G.L.; Stafstrom, C.E. Tuberous Sclerosis Complex and Epilepsy: Recent Developments and Future Challenges. Epilepsia 2007, 48, 617–630. [Google Scholar] [CrossRef]
- Gallagher, A.; Kovach, A.; Stemmer-Rachamimov, A.; Rosenberg, A.E.; Eskandar, E.; Thiele, E.A. Metaplastic bone in a cortical tuber of a young patient with tuberous sclerosis complex. Neurology 2011, 76, 1602–1604. [Google Scholar] [CrossRef]
- Gallagher, A.; Madan, N.; Stemmer-Rachamimov, A.; Thiele, E.A. Progressive calcified tuber in a young male with tuberous sclerosis complex. Dev. Med. Child. Neurol. 2010, 52, 1062–1065. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Z.; Wei, X. Investigation of quantitative susceptibility mapping (QSM) in diagnosis of tuberous sclerosis complex (TSC) and assessment of associated brain injuries at 1.5 Tesla. J. Clin. Transl. Res. 2020, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhuang, H.; Guo, Y.; Sharma, R.D.; Zhang, Q.; Li, J.; Lu, S.; Xu, L.; Chan, Q.; Yoneda, T.; et al. The appearance of magnetic susceptibility objects in SWI phase depends on object size: Comparison with QSM and CT. Clin. Imaging 2022, 82, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Tonduti, D.; Pichiecchio, A.; Uggetti, C.; Bova, S.M.; Orcesi, S.; Parazzini, C.; Chiapparini, L. How to look for intracranial calcification in children with neurological disorders: CT, MRI, or both of them? Neurol. Sci. 2022, 43, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.; Grant, E.P.; Madan, N.; Jarrett, D.Y.; Lyczkowski, D.A.; Thiele, E.A. MRI findings reveal three different types of tubers in patients with tuberous sclerosis complex. J. Neurol. 2010, 257, 1373–1381. [Google Scholar] [CrossRef]
- Northrup, H.; Krueger, D.A.; Roberds, S.; Smith, K.; Sampson, J.; Korf, B.; Kwiatkowski, D.J.; Mowat, D.; Nellist, M.; Povey, S.; et al. Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013, 49, 243–254. [Google Scholar] [CrossRef]
- Russo, C.; Coluccino, S.; De Leva, M.F.; Graziano, S.; Russo, C.; Mazio, F.; De Liso, M.; Cicala, D.; Nastro, A.; Palladino, F.; et al. Cortical Tubers’ Transformation in Pediatric Patients Diagnosed with Tuberous Sclerosis Complex: A Retrospective Longitudinal MRI Analysis. J. Clin. Med. 2025, 14, 7665. [Google Scholar] [CrossRef]
- Mühlebner, A.; Van Scheppingen, J.; Hulshof, H.M.; Scholl, T.; Iyer, A.M.; Anink, J.J.; Ouweland, A.M.W.V.D.; Nellist, M.D.; Jansen, F.E.; Spliet, W.G.M.; et al. Novel Histopathological Patterns in Cortical Tubers of Epilepsy Surgery Patients with Tuberous Sclerosis Complex. PLoS ONE 2016, 11, e0157396. [Google Scholar] [CrossRef]
- Ruppe, V.; Dilsiz, P.; Reiss, C.S.; Carlson, C.; Devinsky, O.; Zagzag, D.; Weiner, H.L.; Talos, D.M. Developmental brain abnormalities in tuberous sclerosis complex: A comparative tissue analysis of cortical tubers and perituberal cortex. Epilepsia 2014, 55, 539–550. [Google Scholar] [CrossRef]
- Crino, P.B. Molecular Pathogenesis of Tuber Formation in Tuberous Sclerosis Complex. J. Child. Neurol. 2004, 19, 716–725. [Google Scholar] [CrossRef]
| N | % | MEAN | SD | ||
|---|---|---|---|---|---|
| Age (years) | First MRI | - | - | 4.9 | 4.8 |
| Last MRI | - | - | 8.9 | 5.3 | |
| Gender | Overall | 57 | 100% | - | - |
| Male | 24 | 42% | - | - | |
| Female | 33 | 58% | - | - | |
| Mutation | TSC1 | 12 | 21% | - | - |
| TSC2 | 25 | 44% | - | - | |
| NMI | 20 | 35% | - | - | |
| Time between first and last MRI (years) | - | - | 4.0 | 2.7 | |
| Number of MRI examinations between first and last MRI | - | - | 2.6 | 3.5 | |
| Lesion Type | Timepoint | N (%) | Proportion of Total CTs (%) | Mean Number ± SD |
|---|---|---|---|---|
| Calcified CTs (Type C, total) | At diagnosis | 36/57 (63%) | 19% | 3.1 ± 4.9 |
| Last follow-up | 44/57 (77%) | 24% | 4.4 ± 6.3 | |
| Type C1 (micro-calcified) | At diagnosis | — | 92% of Type C | 2.8 ± 4.8 |
| Last follow-up | — | 87% of Type C | 3.9 ± 5.8 | |
| Type C2 (macro-calcified) | At diagnosis | — | 8% of Type C | 0.2 ± 0.8 |
| Last follow-up | — | 13% of Type C | 0.6 ± 1.5 | |
| Cystic CTs (Type D) | At diagnosis | 2/57 (3%) | 0.3% | 0.1 ± 0.3 |
| Last follow-up | 4/57 (7%) | 0.5% | 0.1 ± 0.4 |
| Tuber Type | Signal on T1w Sequences | Signal on T2w Sequences | Calcification(s) on SWI | Cystic Component(s) |
|---|---|---|---|---|
| A | isointense | hyperintense | absent | absent |
| B | hypointense | hyperintense | absent | absent |
| C1 | hypo/hyperintense | hyperintense | micro-calcified | absent |
| C2 | hypo/hyperintense | hyperintense | macro-calcified | absent |
| D | hypointense | hyperintense | absent/present | present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, C.; Coluccino, S.; De Leva, M.F.; Graziano, S.; Cristofano, A.; Russo, C.; Cicala, D.; Cinalli, G.; Varone, A.; Covelli, E.M. Cortical Tuber Types in Tuberous Sclerosis Complex: Need for New MRI-Based Classification System Incorporating Changes in Susceptibility Weighted Imaging. Appl. Sci. 2025, 15, 12486. https://doi.org/10.3390/app152312486
Russo C, Coluccino S, De Leva MF, Graziano S, Cristofano A, Russo C, Cicala D, Cinalli G, Varone A, Covelli EM. Cortical Tuber Types in Tuberous Sclerosis Complex: Need for New MRI-Based Classification System Incorporating Changes in Susceptibility Weighted Imaging. Applied Sciences. 2025; 15(23):12486. https://doi.org/10.3390/app152312486
Chicago/Turabian StyleRusso, Camilla, Simone Coluccino, Maria Fulvia De Leva, Stefania Graziano, Adriana Cristofano, Carmela Russo, Domenico Cicala, Giuseppe Cinalli, Antonio Varone, and Eugenio Maria Covelli. 2025. "Cortical Tuber Types in Tuberous Sclerosis Complex: Need for New MRI-Based Classification System Incorporating Changes in Susceptibility Weighted Imaging" Applied Sciences 15, no. 23: 12486. https://doi.org/10.3390/app152312486
APA StyleRusso, C., Coluccino, S., De Leva, M. F., Graziano, S., Cristofano, A., Russo, C., Cicala, D., Cinalli, G., Varone, A., & Covelli, E. M. (2025). Cortical Tuber Types in Tuberous Sclerosis Complex: Need for New MRI-Based Classification System Incorporating Changes in Susceptibility Weighted Imaging. Applied Sciences, 15(23), 12486. https://doi.org/10.3390/app152312486







