Effectiveness of Digital Health Tools for Asthma Self-Management: A Systematic Review and Meta-Analysis of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Sources and Search Strategy
2.4. Selection and Extraction of Information
- Study characteristics: Main author, publication year, country.
- Participant details: Sample size per group, age distribution, proportion of men.
- Intervention specifics: Type of technological resource, application context, duration.
- Outcomes: All reported clinical outcomes of interest. This extracted information was subsequently verified for accuracy and completeness by two other researchers (C.L.P. and F.E.C.M.).
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
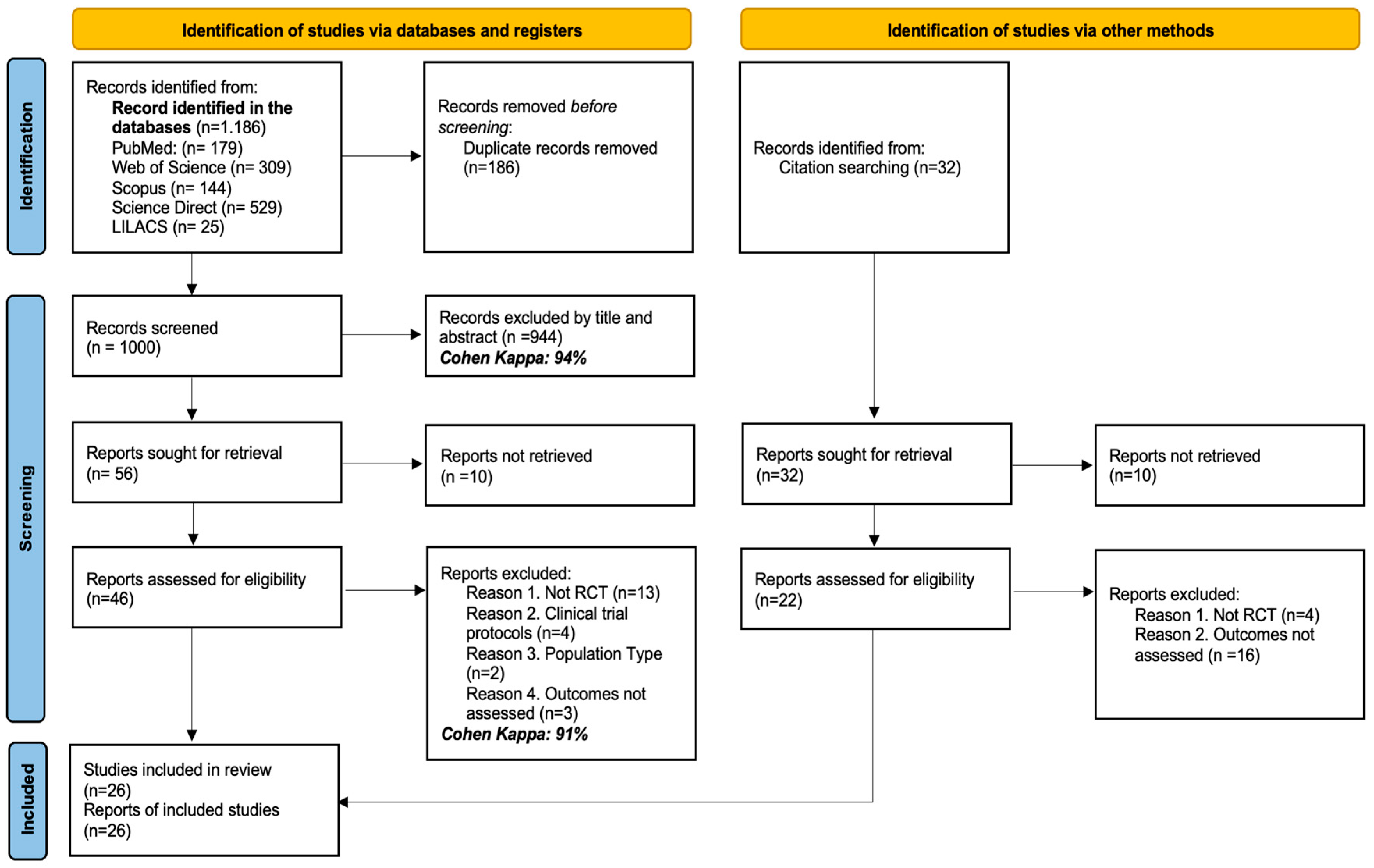
3.1. Studies Identified for the Review
3.2. Characteristics of the Studies Included in the Review
3.3. Characteristics of the Population and the Intervention Applied
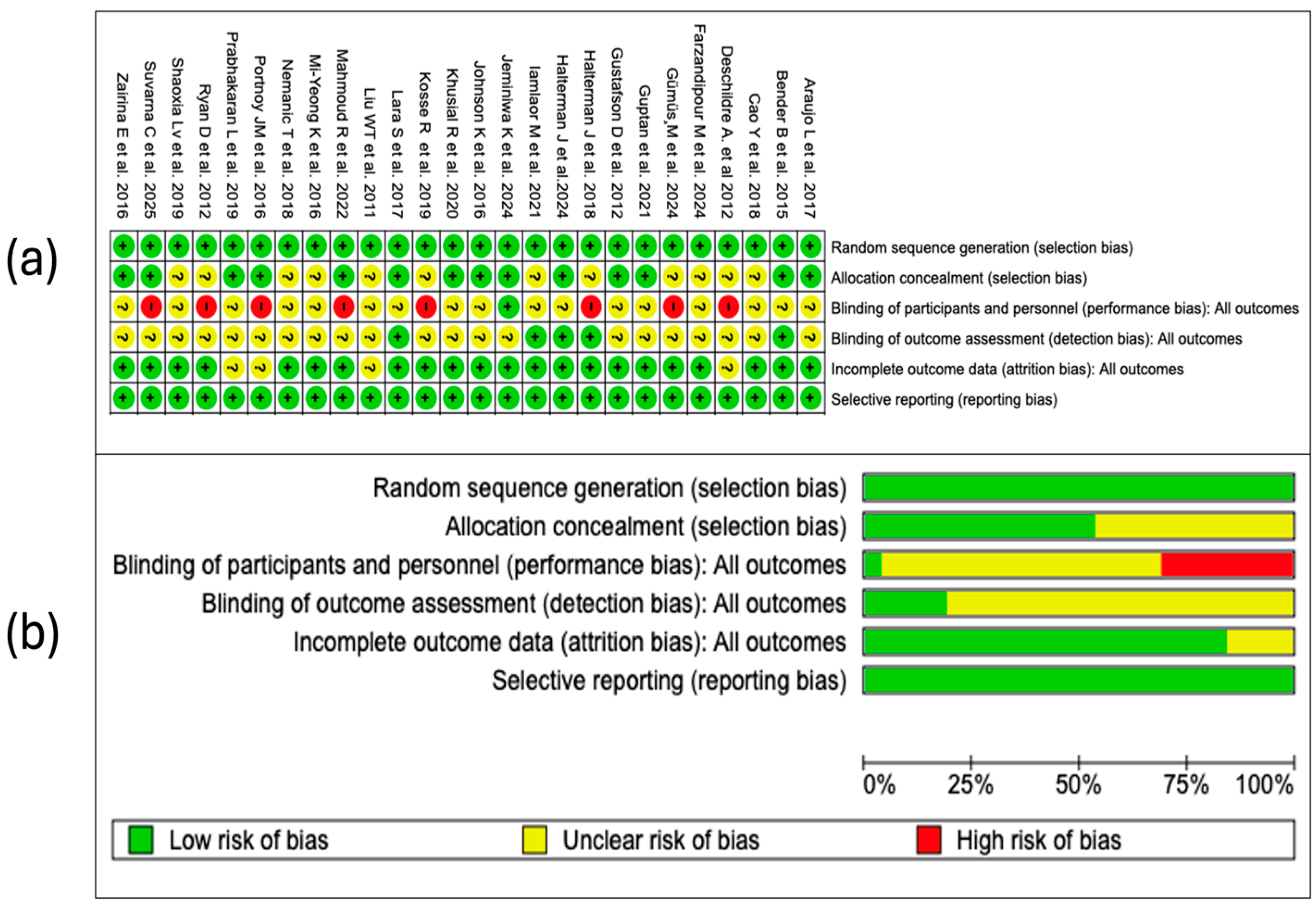
3.4. Findings from the Bias Risk Analysis
3.4.1. Random Sequence Generation
3.4.2. Allocation Concealment
3.4.3. Blinding of Participants and Personnel
3.4.4. Blinding in the Assessment of Outcomes
3.4.5. Incomplete Outcomes
3.4.6. Selective Reporting
3.4.7. Summary of Risk of Bias
3.5. Qualitative Synthesis of Scientific Evidence
3.5.1. Treatment Adherence
3.5.2. Disease Exacerbations
3.6. Meta-Analysis
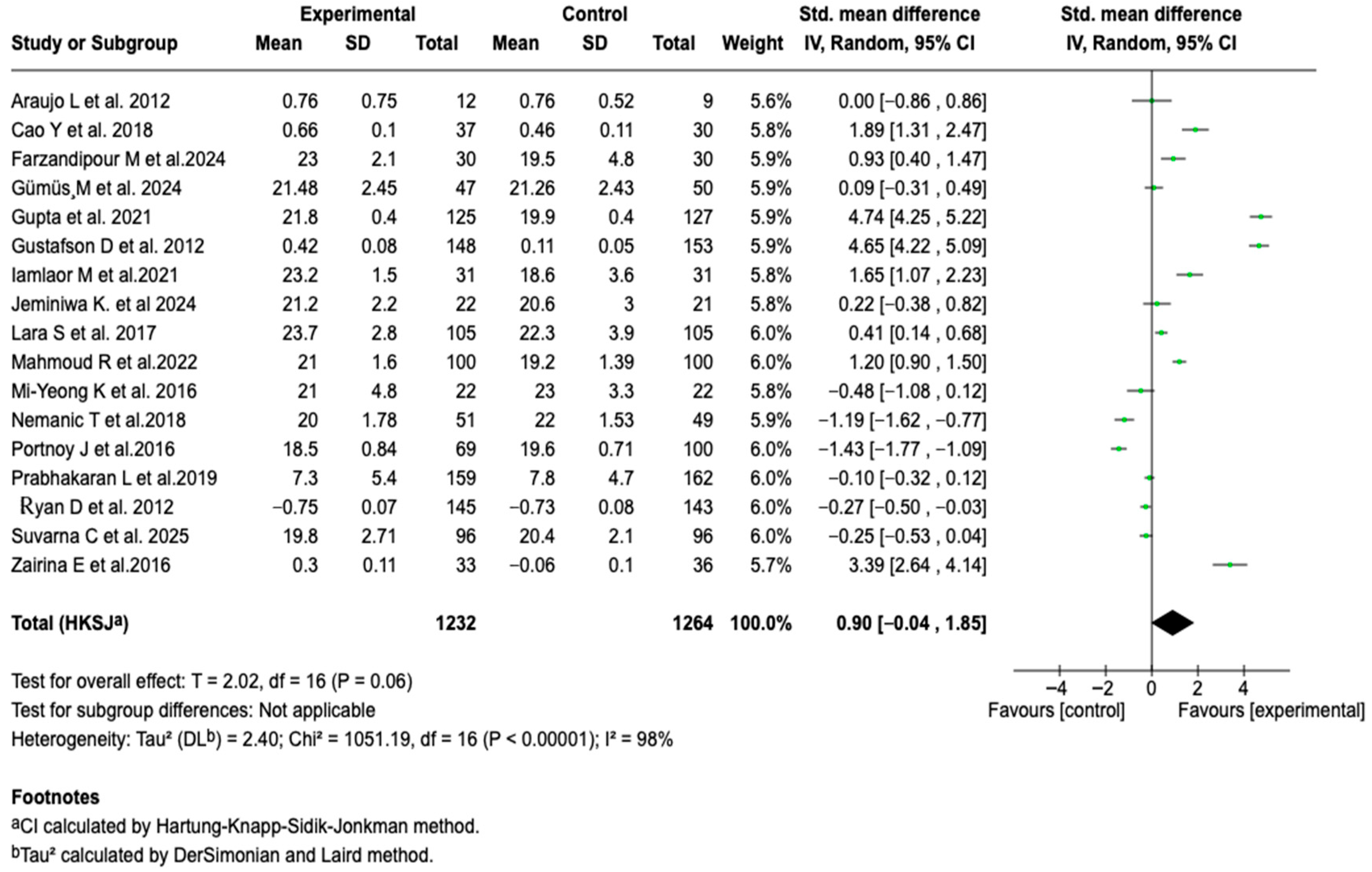
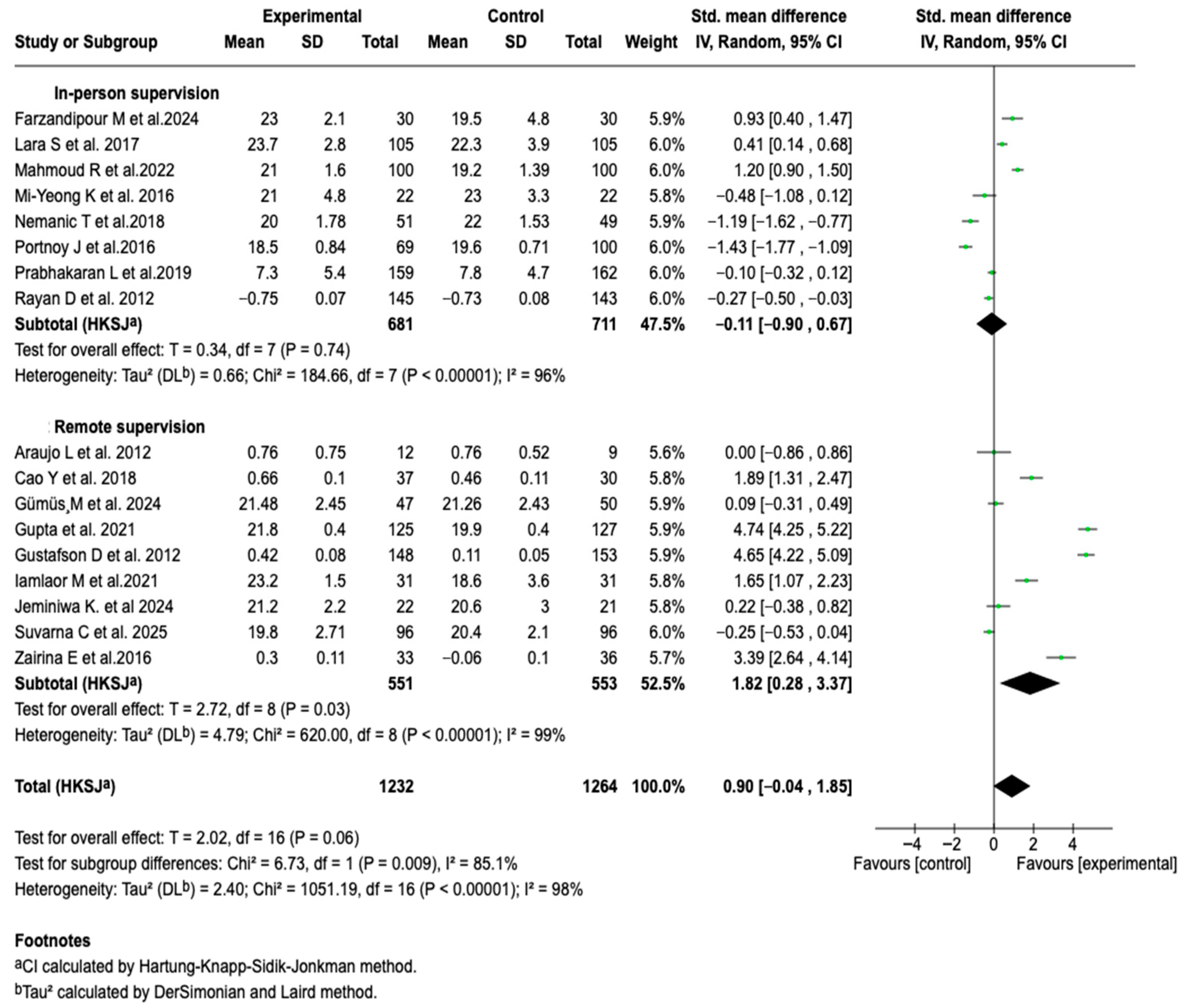
3.6.1. Asthma Control
3.6.2. Pulmonary Function
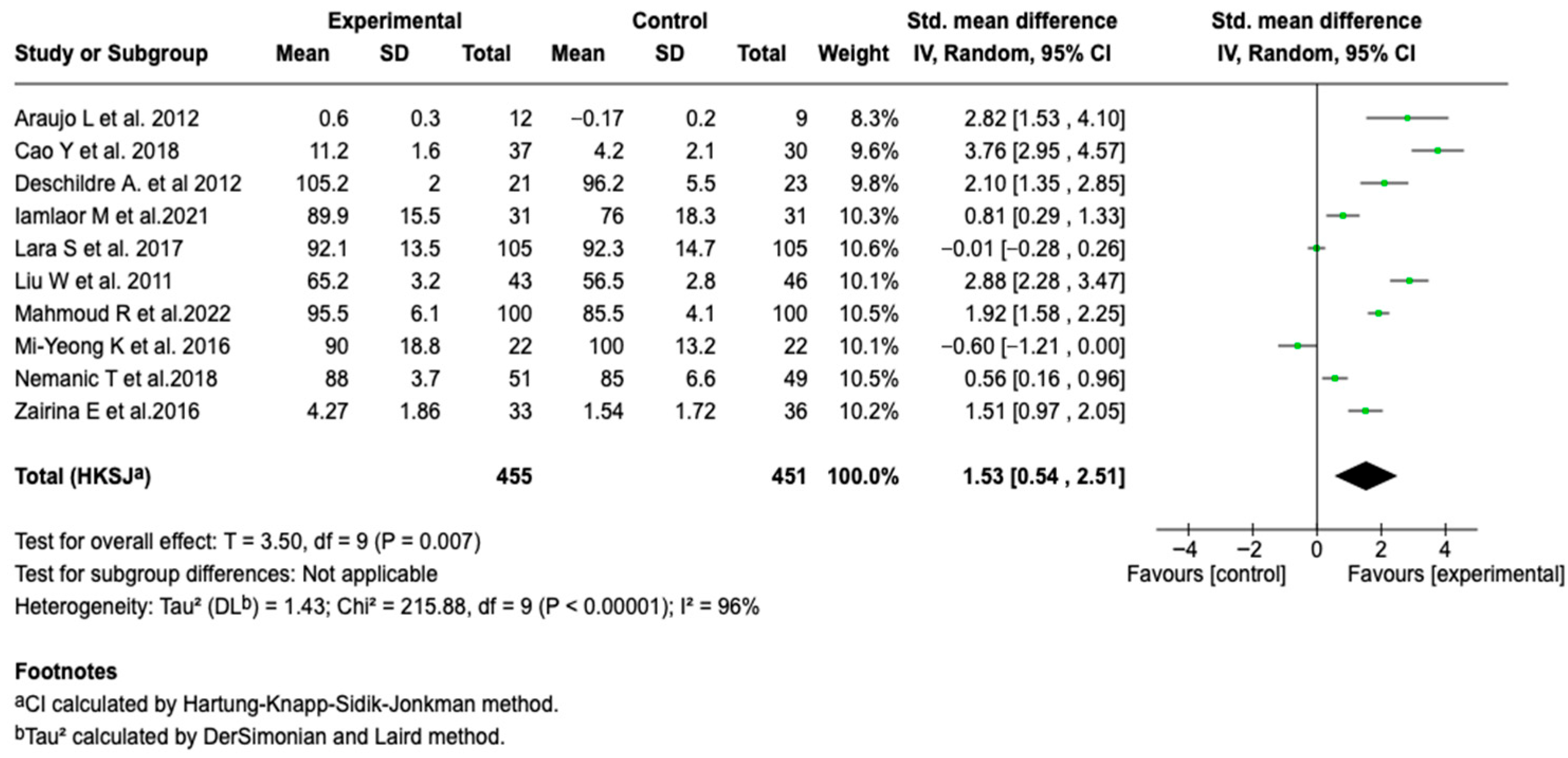
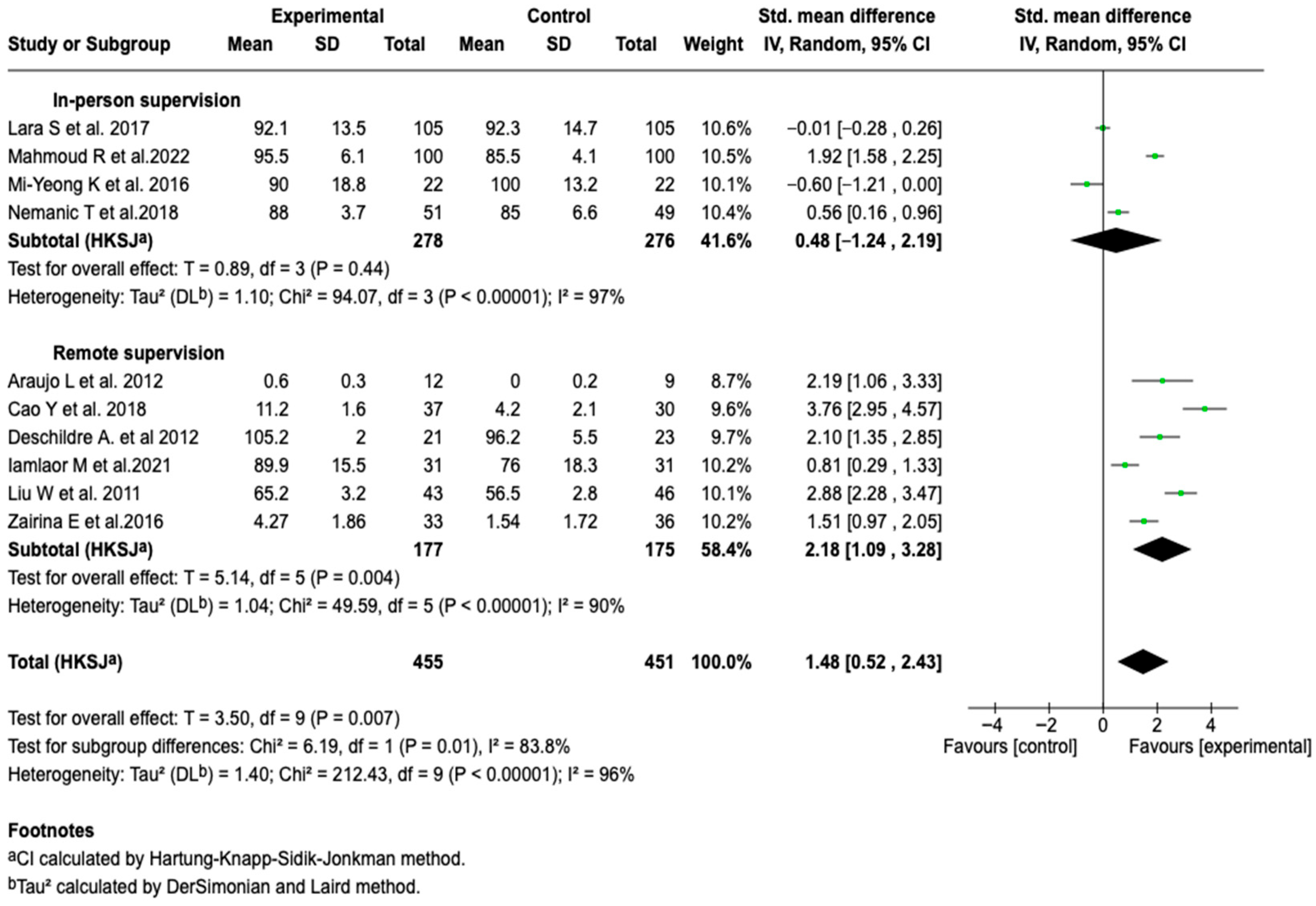
Forced Expiratory Volume in the First Second (FEV1)
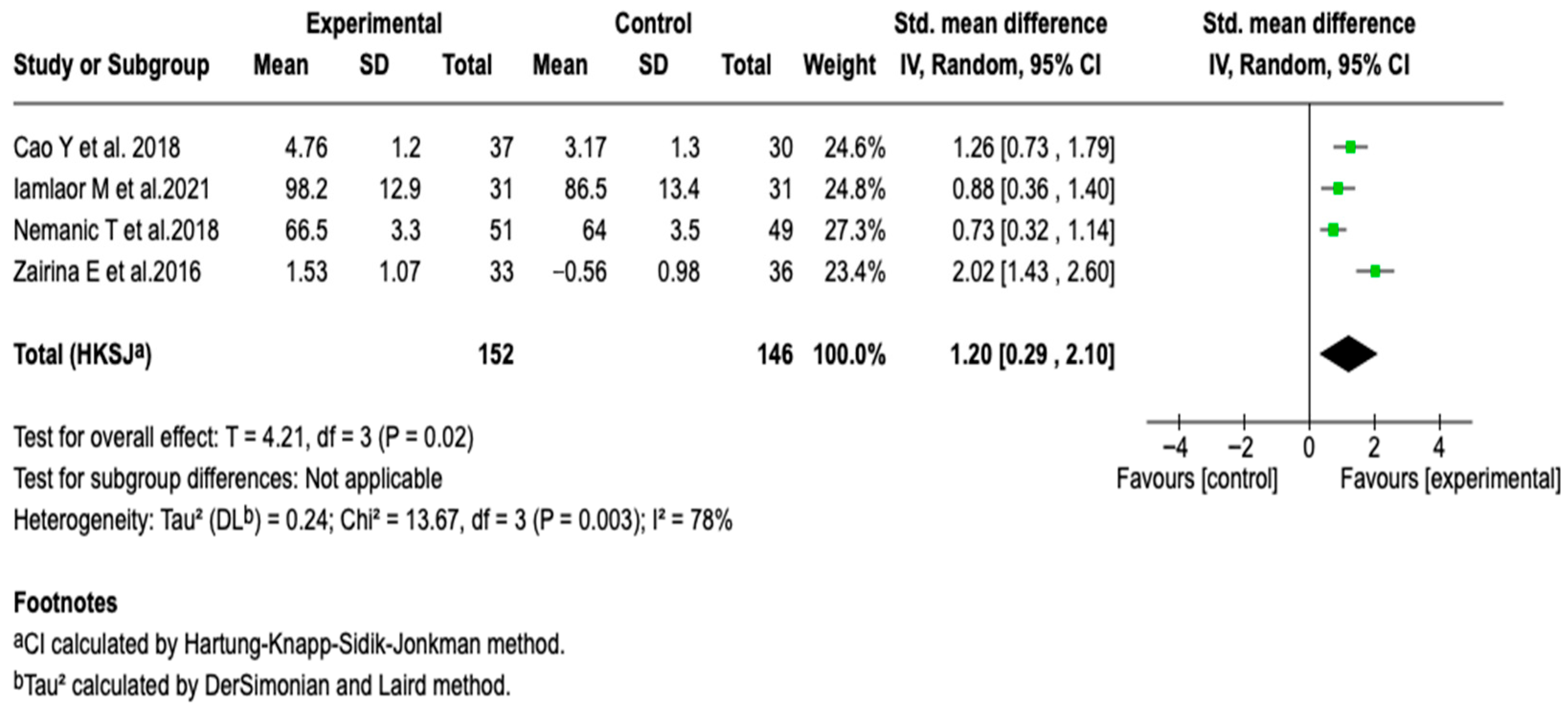
FEV1/FVC
Peak Expiratory Flow
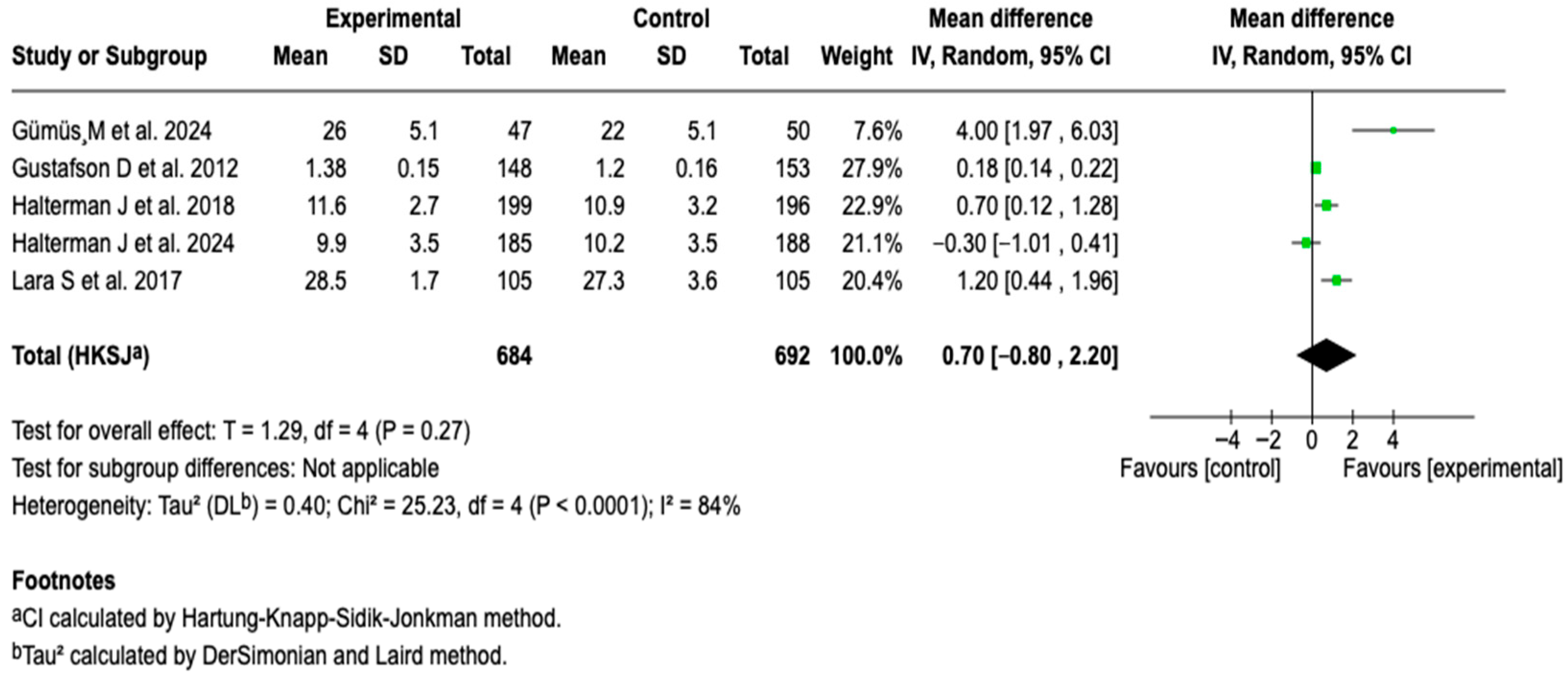
3.6.3. Symptom-Free Days
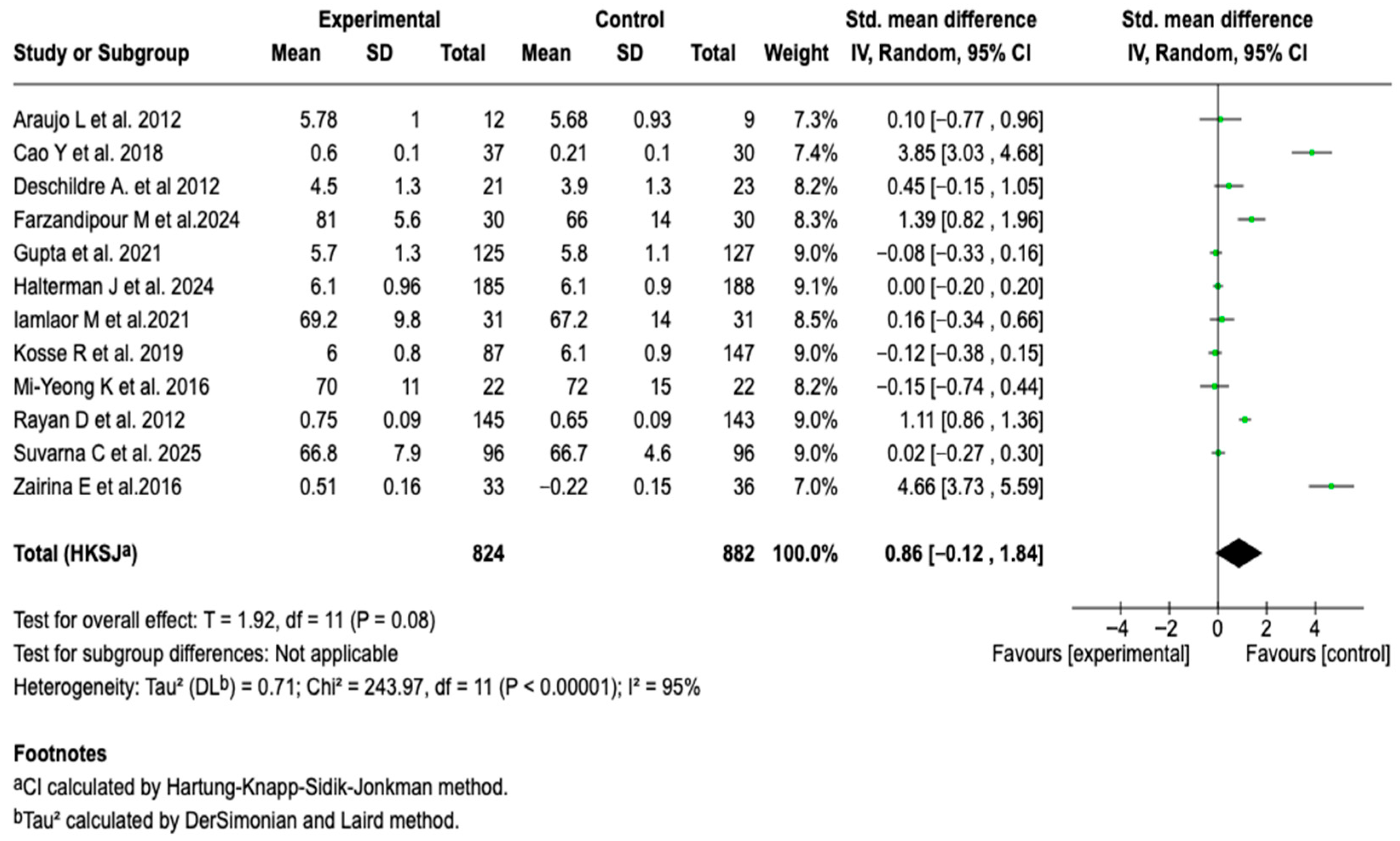
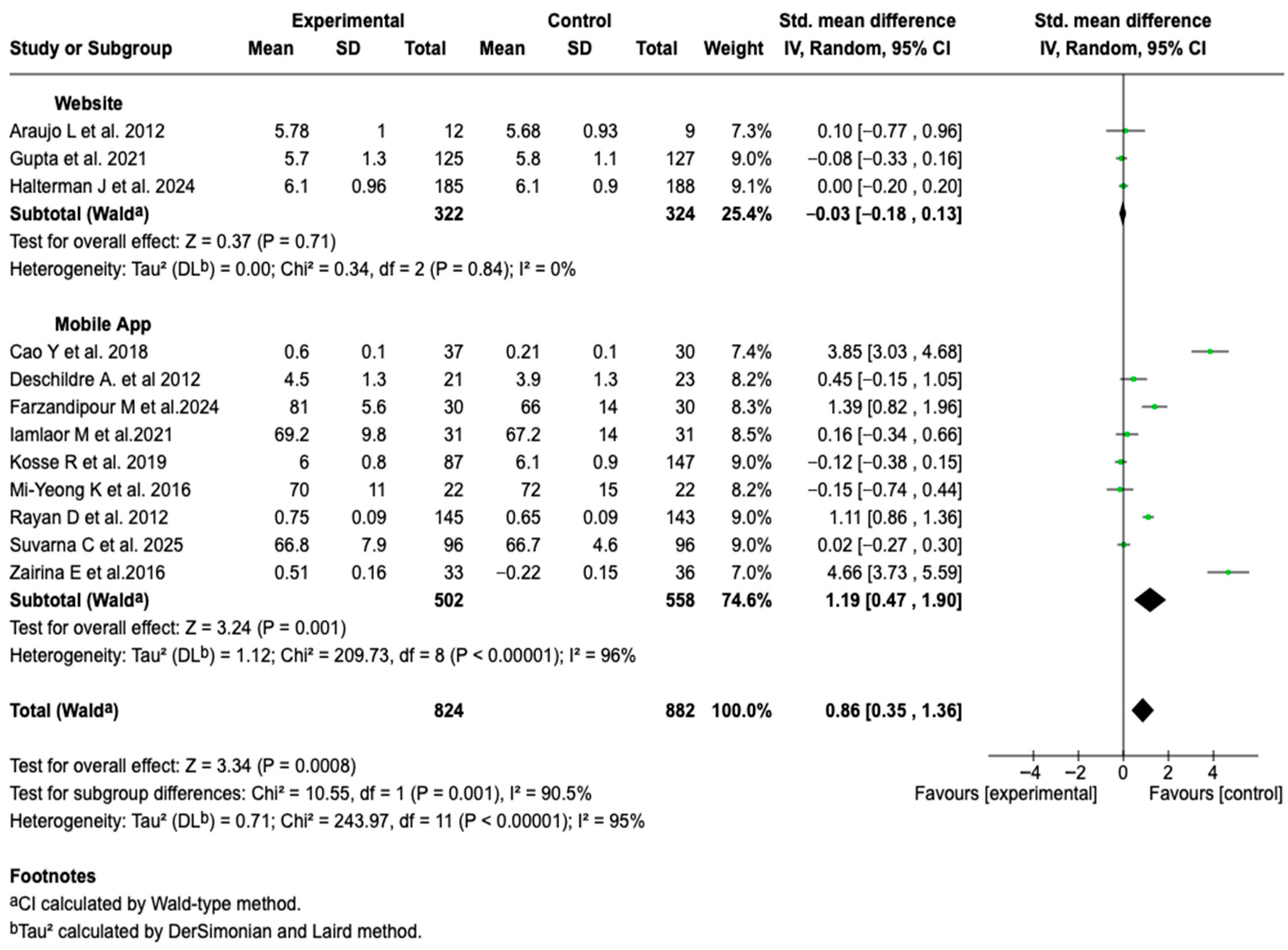
3.6.4. Health-Related Quality of Life
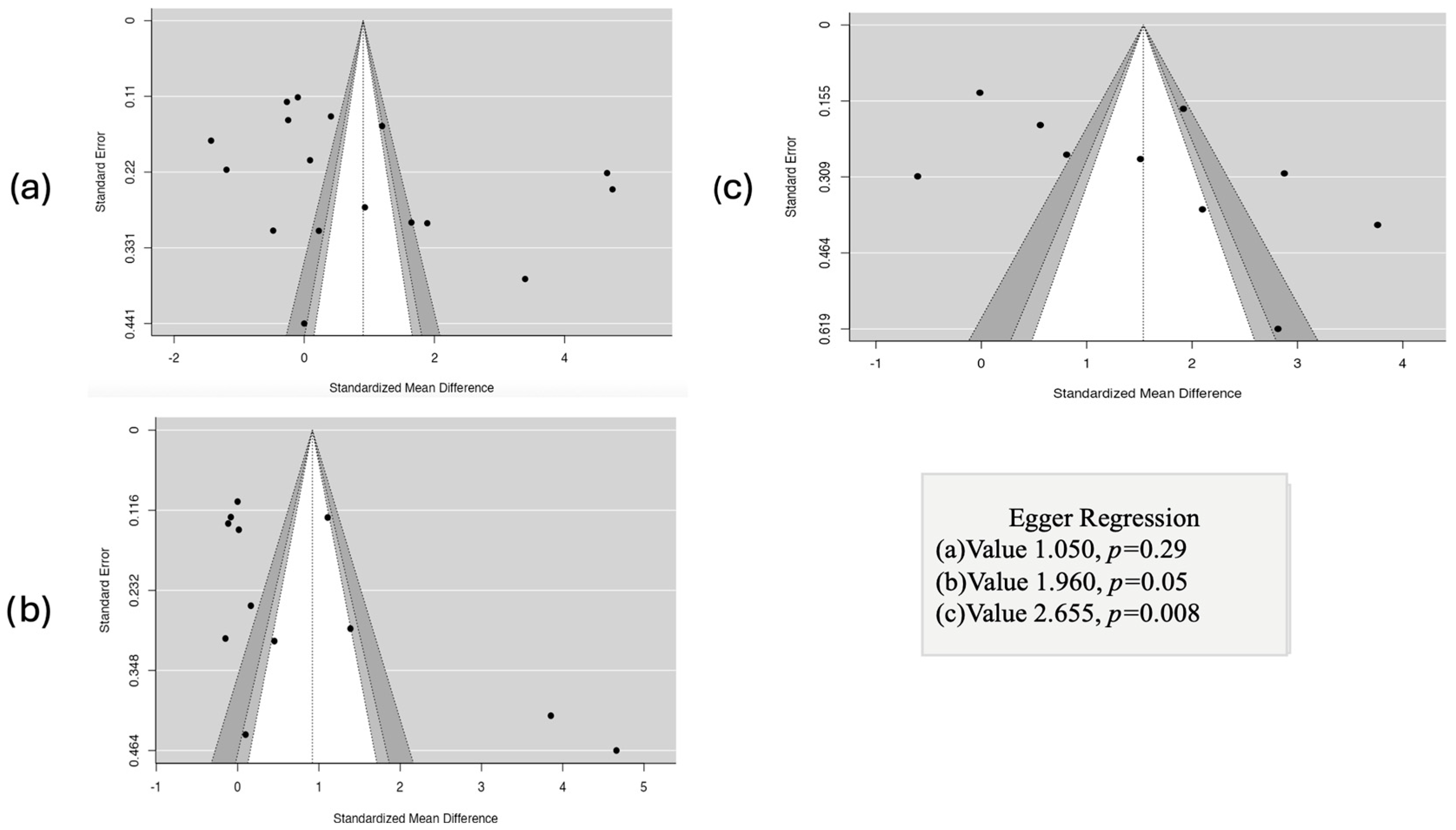
3.7. Results of Publication Bias Assessment
3.8. Results of the GRADE Certainty of Evidence Assessment
4. Discussion
4.1. Main Findings of the Review
4.2. Comparison with Pior Research
4.3. Limitations of the Included Studies
4.4. Limitations of the Review
4.5. Strengths of the Review
4.6. Clinical and Public Health Implications
4.7. Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenzel, S.E. Asthma Phenotypes: The Evolution from Clinical to Molecular Approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K.; et al. Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived with Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Melén, E.; Lehtimäki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef]
- Marcano Belisario, J.S.; Huckvale, K.; Greenfield, G.; Car, J.; Gunn, L.H. Smartphone and Tablet Self Management Apps for Asthma. Cochrane Database Syst. Rev. 2013, 2013, CD010013. [Google Scholar] [CrossRef]
- Morrison, D.; Wyke, S.; Agur, K.; Cameron, E.J.; Docking, R.I.; MacKenzie, A.M.; McConnachie, A.; Raghuvir, V.; Thomson, N.C.; Mair, F.S. Digital Asthma Self-Management Interventions: A Systematic Review. J. Med. Internet Res. 2014, 16, e51. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; FitzGerald, J.M.; Bateman, E.D.; Bacharier, L.B.; Becker, A.; Brusselle, G.; Buhl, R.; Cruz, A.A.; Fleming, L.; Inoue, H.; et al. GINA 2019: A Fundamental Change in Asthma Management: Treatment of Asthma with Short-Acting Bronchodilators Alone Is No Longer Recommended for Adults and Adolescents. Eur. Respir. J. 2019, 53, 1901046. [Google Scholar] [CrossRef] [PubMed]
- Gatheral, T.L.; Rushton, A.; Evans, D.J.; Mulvaney, C.A.; Halcovitch, N.R.; Whiteley, G.; Eccles, F.J.; Spencer, S. Personalised Asthma Action Plans for Adults with Asthma. Cochrane Database Syst. Rev. 2017, 2017, CD011859. [Google Scholar] [CrossRef] [PubMed]
- Holley, S.; Morris, R.; Knibb, R.; Latter, S.; Liossi, C.; Mitchell, F.; Roberts, G. Barriers and Facilitators to Asthma Self-management in Adolescents: A Systematic Review of Qualitative and Quantitative Studies. Pediatr. Pulmonol. 2017, 52, 430–442. [Google Scholar] [CrossRef]
- Miller, L.; Schüz, B.; Walters, J.; Walters, E.H. Mobile Technology Interventions for Asthma Self-Management: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2017, 5, e57. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, J.; Chiang, V.; Choi, T.; Wang, Y.; Sun, L.; Wu, Y. Effectiveness of mHealth Interventions for Asthma Self-Management: A Systematic Review and Meta-Analysis. Stud. Health Technol. Inf. 2018, 250, 144–145. [Google Scholar]
- Chongmelaxme, B.; Lee, S.; Dhippayom, T.; Saokaew, S.; Chaiyakunapruk, N.; Dilokthornsakul, P. The Effects of Telemedicine on Asthma Control and Patients’ Quality of Life in Adults: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2019, 7, 199–216.e11. [Google Scholar] [CrossRef] [PubMed]
- Exarchos, K.P.; Beltsiou, M.; Votti, C.-A.; Kostikas, K. Artificial Intelligence Techniques in Asthma: A Systematic Review and Critical Appraisal of the Existing Literature. Eur. Respir. J. 2020, 56, 2000521. [Google Scholar] [CrossRef]
- Chan, A.; De Simoni, A.; Wileman, V.; Holliday, L.; Newby, C.J.; Chisari, C.; Ali, S.; Zhu, N.; Padakanti, P.; Pinprachanan, V.; et al. Digital Interventions to Improve Adherence to Maintenance Medication in Asthma. Cochrane Database Syst. Rev. 2022, 2022, CD013030. [Google Scholar] [CrossRef]
- Liscano, Y.; Anillo Arrieta, L.A.; Montenegro, J.F.; Prieto-Alvarado, D.; Ordoñez, J. Early Warning of Infectious Disease Outbreaks Using Social Media and Digital Data: A Scoping Review. Int. J. Environ. Res. Public Health 2025, 22, 1104. [Google Scholar] [CrossRef]
- Liscano, Y.; Bernal, L.M.; Díaz Vallejo, J.A. Effectiveness of AI-Assisted Digital Therapies for Post-Stroke Aphasia Rehabilitation: A Systematic Review. Brain Sci. 2025, 15, 1007. [Google Scholar] [CrossRef]
- Kandola, A.; Edwards, K.; Straatman, J.; Dührkoop, B.; Hein, B.; Hayes, J. Digital Self-Management Platform for Adult Asthma: Randomized Attention-Placebo Controlled Trial. J. Med. Internet Res. 2024, 26, e50855. [Google Scholar] [CrossRef]
- Ljungberg, H.; Carleborg, A.; Gerber, H.; Öfverström, C.; Wolodarski, J.; Menshi, F.; Engdahl, M.; Eduards, M.; Nordlund, B. Clinical Effect on Uncontrolled Asthma Using a Novel Digital Automated Self-Management Solution: A Physician-Blinded Randomised Controlled Crossover Trial. Eur. Respir. J. 2019, 54, 1900983. [Google Scholar] [CrossRef]
- Anawade, P.A.; Sharma, D.; Gahane, S. A Comprehensive Review on Exploring the Impact of Telemedicine on Healthcare Accessibility. Cureus 2024, 16, e55996. [Google Scholar] [CrossRef] [PubMed]
- Herrero Martín, S.; Hueto Pérez De Heredia, J.; Cuesta Remón, A.; Gómez Fernández, M.; Antón, M.M.; Cabasés, J.; García Rey, R.; Cebollero Rivas, P. Is a Mobile Application Useful for Patients with Moderate-Severe Asthma? Arch. Bronconeumol. 2021, 57, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Aparicio, M.; Almonacid, C.; Calvín Lamas, M.; Delgado, J.; Gandolfo-Cano, M.; López-Carrasco, V.; Vega, J.M.; Díaz-Pérez, D.; Villamañán, E. Telemedicina y asma grave en nuestro entorno: Reflexiones sobre la experiencia de los profesionales y propuestas para hacerla realidad. Open Respir. Arch. 2023, 5, 100239. [Google Scholar] [CrossRef]
- Hui, C.Y.; Walton, R.; McKinstry, B.; Jackson, T.; Parker, R.; Pinnock, H. The Use of Mobile Applications to Support Self-Management for People with Asthma: A Systematic Review of Controlled Studies to Identify Features Associated with Clinical Effectiveness and Adherence. J. Am. Med. Inform. Assoc. 2017, 24, 619–632. [Google Scholar] [CrossRef] [PubMed]
- McLean, G.; Murray, E.; Band, R.; Moffat, K.R.; Hanlon, P.; Bruton, A.; Thomas, M.; Yardley, L.; Mair, F.S. Interactive Digital Interventions to Promote Self-Management in Adults with Asthma: Systematic Review and Meta-Analysis. BMC Pulm. Med. 2016, 16, 83. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Prieto-Alvarado, D.E.; Parada-Gereda, H.M.; Molano, D.; Martinez, Y.L.; Tafurt, G.P.R.; Masclans, J.-R. Risk Factors and Outcomes of Ventilator-Associated Pneumonia in Patients with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Crit. Care 2025, 85, 154922. [Google Scholar] [CrossRef]
- Cruz Mosquera, F.E.; Perlaza, C.L.; Naranjo Rojas, A.; Murillo Rios, S.; Carrero Gallego, A.; Fischersworring, S.I.; Rodríguez, J.S.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina 2025, 61, 489. [Google Scholar] [CrossRef] [PubMed]
- Cruz Mosquera, F.E.; Murillo, S.R.; Naranjo Rojas, A.; Perlaza, C.L.; Castro Osorio, D.; Liscano, Y. Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis. Medicina 2024, 60, 1725. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, F.E.C.; De La Rosa Caldas, M.; Naranjo Rojas, A.; Perlaza, C.L.; Liscano, Y. Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis. Children 2025, 12, 591. [Google Scholar] [CrossRef]
- Zairina, E.; Abramson, M.J.; McDonald, C.F.; Li, J.; Dharmasiri, T.; Stewart, K.; Walker, S.P.; Paul, E.; George, J. Telehealth to Improve Asthma Control in Pregnancy: A Randomized Controlled Trial. Respirology 2016, 21, 867–874. [Google Scholar] [CrossRef]
- Farzandipour, M.; Heidarzadeh Arani, M.; Sharif, R.; Nabovati, E.; Akbari, H.; Anvari, S. Improving Asthma Control and Quality of Life via a Smartphone Self-Management App: A Randomized Controlled Trial. Respir. Med. 2024, 223, 107539. [Google Scholar] [CrossRef]
- Suvarna, K.C.; Kumar, P.; Singh, K.; Kumar, J.; Goyal, J.P. Comparison of Telemedicine versus In-Person Visit for Control of Asthma in Children Aged 7–17 Years: A Randomized Controlled Trial. Indian J. Pediatr. 2025, 92, 467–473. [Google Scholar] [CrossRef]
- Jeminiwa, R.; Garza, K.B.; Chou, C.; Franco-Watkins, A.; Fox, B.I. Effects of Framed Mobile Messages on Beliefs, Intentions, Adherence, and Asthma Control: A Randomized Trial. Pharmacy 2024, 12, 10. [Google Scholar] [CrossRef]
- Halterman, J.S.; Fagnano, M.; Tremblay, P.; Butz, A.; Perry, T.T.; Wang, H. Effect of the Telemedicine Enhanced Asthma Management Through the Emergency Department (TEAM-ED) Program on Asthma Morbidity: A Randomized Controlled Trial. J. Pediatr. 2024, 266, 113867. [Google Scholar] [CrossRef]
- Gümüş, M.; Yardimci, F.; Duman Şenol, H.; Demir, E. Virtual Care for Paediatric Asthma: A Randomized Controlled Trial. Int. J. Nurs. Pract. 2024, 30, e13290. [Google Scholar] [CrossRef]
- Mahmoud, R.A.A.; Boshra, M.S.; Saeed, H.; Abdelrahim, M.E.A. The Impact of the Clip-Tone Training Device and Its Smartphone Application to Pressurized Metered-Dose Inhaler in Adult Asthmatics. J. Asthma 2023, 60, 227–234. [Google Scholar] [CrossRef]
- Gupta, R.S.; Fierstein, J.L.; Boon, K.L.; Kanaley, M.K.; Bozen, A.; Kan, K.; Vojta, D.; Warren, C.M. Sensor-Based Electronic Monitoring for Asthma: A Randomized Controlled Trial. Pediatrics 2021, 147, e20201330. [Google Scholar] [CrossRef]
- Iamlaor, U.; Taneepanichskul, S. Effectiveness of Asthma Self-Care Program Through Mobile Line Application (SALA) on Lung Function among Asthma Patients in Angthong Hospital: A Randomized Control Trial. J. Med. Assoc. Thai 2021, 104, 264–270. [Google Scholar] [CrossRef]
- Khusial, R.J.; Honkoop, P.J.; Usmani, O.; Soares, M.; Simpson, A.; Biddiscombe, M.; Meah, S.; Bonini, M.; Lalas, A.; Polychronidou, E.; et al. Effectiveness of myAirCoach: A mHealth Self-Management System in Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 1972–1979.e8. [Google Scholar] [CrossRef]
- Prabhakaran, L.; Chun Wei, Y. Effectiveness of the eCARE Programme: A Short Message Service for Asthma Monitoring. BMJ Health Care Inf. 2019, 26, e100007. [Google Scholar] [CrossRef]
- Shaoxia, L.; Ye, X.; Wang, Z.; Xia, W.; Qi, Y.; Wang, W.; Chen, Y.; Cai, X.; Qian, X. A Randomized Controlled Trial of a Mobile Application-assisted Nurse-led Model Used to Improve Treatment Outcomes in Children with Asthma. J. Adv. Nurs. 2019, 75, 3058–3067. [Google Scholar] [CrossRef]
- Kosse, R.C.; Bouvy, M.L.; De Vries, T.W.; Koster, E.S. Effect of a mHealth Intervention on Adherence in Adolescents with Asthma: A Randomized Controlled Trial. Respir. Med. 2019, 149, 45–51. [Google Scholar] [CrossRef]
- Nemanic, T.; Sarc, I.; Skrgat, S.; Flezar, M.; Cukjati, I.; Marc Malovrh, M. Telemonitoring in Asthma Control: A Randomized Controlled Trial. J. Asthma 2019, 56, 782–790. [Google Scholar] [CrossRef]
- Halterman, J.S.; Fagnano, M.; Tajon, R.S.; Tremblay, P.; Wang, H.; Butz, A.; Perry, T.T.; McConnochie, K.M. Effect of the School-Based Telemedicine Enhanced Asthma Management (SB-TEAM) Program on Asthma Morbidity: A Randomized Clinical Trial. JAMA Pediatr. 2018, 172, e174938. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, S.-H.; Zhu, D.; Xu, F.; Chen, Z.-H.; Shen, H.-H.; Li, W. WeChat Public Account Use Improves Clinical Control of Cough-Variant Asthma: A Randomized Controlled Trial. Med. Sci. Monit. 2018, 24, 1524–1532. [Google Scholar] [CrossRef]
- Lara, S.; Roukema, J.; Boehmer, A.L.M.; Brouwer, M.L.; Hugen, C.A.C.; Niers, L.E.M.; Sprij, A.J.; Rikkers-Mutsaerts, E.R.V.M.; Rottier, B.L.; Donders, A.R.T.; et al. A Virtual Asthma Clinic for Children: Fewer Routine Outpatient Visits, Same Asthma Control. Eur. Respir. J. 2017, 50, 1700471. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Waller, M.; De Lurgio, S.; Dinakar, C. Telemedicine Is as Effective as In-Person Visits for Patients with Asthma. Ann. Allergy Asthma Immunol. 2016, 117, 241–245. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Lee, S.-Y.; Jo, E.-J.; Lee, S.-E.; Kang, M.-G.; Song, W.-J.; Kim, S.-H.; Cho, S.-H.; Min, K.-U.; Ahn, K.-H.; et al. Feasibility of a Smartphone Application Based Action Plan and Monitoring in Asthma. Asia Pac. Allergy 2016, 6, 174–180. [Google Scholar] [CrossRef]
- Johnson, K.B.; Patterson, B.L.; Ho, Y.-X.; Chen, Q.; Nian, H.; Davison, C.L.; Slagle, J.; Mulvaney, S.A. The Feasibility of Text Reminders to Improve Medication Adherence in Adolescents with Asthma. J. Am. Med. Inform. Assoc. 2016, 23, 449–455. [Google Scholar] [CrossRef]
- Bender, B.G.; Cvietusa, P.J.; Goodrich, G.K.; Lowe, R.; Nuanes, H.A.; Rand, C.; Shetterly, S.; Tacinas, C.; Vollmer, W.M.; Wagner, N.; et al. Pragmatic Trial of Health Care Technologies to Improve Adherence to Pediatric Asthma Treatment: A Randomized Clinical Trial. JAMA Pediatr. 2015, 169, 317. [Google Scholar] [CrossRef]
- Ryan, D.; Price, D.; Musgrave, S.D.; Malhotra, S.; Lee, A.J.; Ayansina, D.; Sheikh, A.; Tarassenko, L.; Pagliari, C.; Pinnock, H. Clinical and Cost Effectiveness of Mobile Phone Supported Self Monitoring of Asthma: Multicentre Randomised Controlled Trial. BMJ 2012, 344, e1756. [Google Scholar] [CrossRef]
- Gustafson, D.; Wise, M.; Bhattacharya, A.; Pulvermacher, A.; Shanovich, K.; Phillips, B.; Lehman, E.; Chinchilli, V.; Hawkins, R.; Kim, J.-S. The Effects of Combining Web-Based eHealth With Telephone Nurse Case Management for Pediatric Asthma Control: A Randomized Controlled Trial. J. Med. Internet Res. 2012, 14, e101. [Google Scholar] [CrossRef]
- Araújo, L.; Jacinto, T.; Moreira, A.; Castel-Branco, M.; Delgado, L.; Costa-Pereira, A.; Fonseca, J. Clinical Efficacy of Web-Based Versus Standard Asthma Self-Management. J. Investig. Allergol. Clin. Immunol. 2012, 22, 28–34. [Google Scholar]
- Deschildre, A.; Béghin, L.; Salleron, J.; Iliescu, C.; Thumerelle, C.; Santos, C.; Hoorelbeke, A.; Scalbert, M.; Pouessel, G.; Gnansounou, M.; et al. Home Telemonitoring (Forced Expiratory Volume in 1 s) in Children with Severe Asthma Does Not Reduce Exacerbations. Eur. Respir. J. 2012, 39, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-T.; Huang, C.-D.; Wang, C.-H.; Lee, K.-Y.; Lin, S.-M.; Kuo, H.-P. A Mobile Telephone-Based Interactive Self-Care System Improves Asthma Control. Eur. Respir. J. 2011, 37, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, R.R.; Plevinsky, J.M.; Kollin, S.R.; Gibler, R.C.; Guilbert, T.W.; Hommel, K.A. Systematic Review of Digital Interventions for Pediatric Asthma Management. J. Allergy Clin. Immunol. Pract. 2020, 8, 1284–1293. [Google Scholar] [CrossRef]
- Biblowitz, K.; Bellam, S.; Mosnaim, G. Improving Asthma Outcomes in the Digital Era: A Systematic Review. Pharm. Med. 2018, 32, 173–187. [Google Scholar] [CrossRef]
- Mosnaim, G.; Safioti, G.; Brown, R.; DePietro, M.; Szefler, S.J.; Lang, D.M.; Portnoy, J.M.; Bukstein, D.A.; Bacharier, L.B.; Merchant, R.K. Digital Health Technology in Asthma: A Comprehensive Scoping Review. J. Allergy Clin. Immunol. Pract. 2021, 9, 2377–2398. [Google Scholar] [CrossRef]
- Snoswell, C.L.; Rahja, M.; Lalor, A.F. A Systematic Review and Meta-Analysis of Change in Health-Related Quality of Life for Interactive Telehealth Interventions for Patients With Asthma. Value Health 2021, 24, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Tinschert, P.; Jakob, R.; Barata, F.; Kramer, J.-N.; Kowatsch, T. The Potential of Mobile Apps for Improving Asthma Self-Management: A Review of Publicly Available and Well-Adopted Asthma Apps. JMIR Mhealth Uhealth 2017, 5, e113. [Google Scholar] [CrossRef]
- Kosse, R.C.; Bouvy, M.L.; Belitser, S.V.; de Vries, T.W.; van der Wal, P.S.; Koster, E.S. Effective Engagement of Adolescent Asthma Patients With Mobile Health-Supporting Medication Adherence. JMIR Mhealth Uhealth 2019, 7, e12411. [Google Scholar] [CrossRef]
- Merchant, R.K.; Inamdar, R.; Quade, R.C. Effectiveness of Population Health Management Using the Propeller Health Asthma Platform: A Randomized Clinical Trial. J. Allergy Clin. Immunol. Pract. 2016, 4, 455–463. [Google Scholar] [CrossRef]
- Garin, N.; Zarate-Tamames, B.; Gras-Martin, L.; Milà, R.; Crespo-Lessmann, A.; Curto, E.; Hernandez, M.; Mestres, C.; Plaza, V. Clinical Impact of Electronic Monitoring Devices of Inhalers in Adults with Asthma or COPD: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 414. [Google Scholar] [CrossRef]
- Madanian, S.; Nakarada-Kordic, I.; Reay, S.D.; Chetty, T. Patients’ Perspectives on Digital Health Tools. PEC Innov. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Marques, A.; Bosch, P.; De Thurah, A.; Meissner, Y.; Falzon, L.; Mukhtyar, C.; Bijlsma, J.W.; Dejaco, C.; Stamm, T.A. Effectiveness of Remote Care Interventions: A Systematic Review Informing the 2022 EULAR Points to Consider for Remote Care in Rheumatic and Musculoskeletal Diseases. RMD Open 2022, 8, e002290. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Rojas, A.; Perula-de Torres, L.Á.; Cruz-Mosquera, F.E.; Molina-Recio, G. Efficacy and Acceptability of a Mobile App for Monitoring the Clinical Status of Patients With Chronic Obstructive Pulmonary Disease Receiving Home Oxygen Therapy: Randomized Controlled Trial. J. Med. Internet Res. 2025, 27, e65888. [Google Scholar] [CrossRef]
- Shickh, S.; Rafferty, S.A.; Clausen, M.; Kodida, R.; Mighton, C.; Panchal, S.; Lorentz, J.; Ward, T.; Watkins, N.; Elser, C.; et al. The Role of Digital Tools in the Delivery of Genomic Medicine: Enhancing Patient-Centered Care. Genet. Med. 2021, 23, 1086–1094. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Country | Design | Subjects | Outcomes |
|---|---|---|---|---|---|
| Zairina E., et al. [28] | 2024 | Australia | RCT | Pregnant women over 18 years old with poorly controlled asthma | Asthma control, pulmonary function, HRQoL |
| Farzandipour M et al. [29] | 2024 | Iran | RCT | Adults over 18 years old with a primary diagnosis of asthma | Asthma control, HRQoL |
| Suvarna C et al. [30] | 2024 | India | RCT | Children aged 7 to 17 years with a diagnosis of asthma | Asthma control and HRQoL |
| Jeminiwa K et al. [31] | 2024 | United States | RCT | Adults aged 18 to 29 years with a diagnosis of asthma | Asthma control and treatment adherence |
| Halterman J et al. [32] | 2024 | United States | RCT | Children aged 3 to 12 years with a diagnosis of persistent asthma | Treatment adherence, symptom-free days, and HRQoL |
| Gümüs M et al. [33] | 2024 | Turkey | RCT | Children aged 7 to 17 years with a diagnosis of asthma | Asthma control and symptom-free days |
| Mahmoud R et al. [34] | 2022 | Egypt | RCT | Adults over 18 years old with a diagnosis of asthma | Asthma control and pulmonary function |
| Gupta R et al. [35] | 2021 | United States | RCT | Children aged 4 to 17 years with a diagnosis of asthma and conventional treatment | Asthma control and HRQoL |
| Iamlaor U et al. [36] | 2021 | Thailand | RCT | Adults aged 20 to 60 years with a diagnosis of mild to moderate asthma | Asthma control, pulmonary function, and HRQoL |
| Khusial R et al. [37] | 2020 | Netherlands | RCT | Adults aged 23 to 77 years with a diagnosis of asthma managed with inhaled therapy | Treatment adherence |
| Prabhakaran L et al. [38] | 2019 | Singapore | RCT | Adults over 21 years old with a primary diagnosis of asthma | Asthma control |
| Shaoxia L et al. [39] | 2019 | China | RCT | Children aged 6 to 12 years with a diagnosis of asthma | Adherence and exacerbations |
| Kosse R et al. [40] | 2019 | Netherlands | RCT | Adolescents aged 12 to 18 years with a diagnosis of asthma | Treatment adherence and HRQoL |
| Nemanic, T et al. [41] | 2018 | Slovenia | RCT | Adults aged 18 to 75 years with a diagnosis of asthma | Asthma control, exacerbations, and pulmonary function |
| Halterman J et al. [42] | 2018 | United States | RCT | Children aged 3 to 10 years with persistent asthma | Symptom-free days |
| Cao Y et al. [43] | 2018 | China | RCT | Adults aged 18 to 75 years with a diagnosis of asthma and management with inhaled therapy | Asthma control, pulmonary function, and HRQoL |
| Lara S et al. [44] | 2017 | Netherlands | RCT | Children aged 6 to 16 years with a diagnosis of asthma | Asthma control, pulmonary function, and symptom-free days |
| Portnoy M et al. [45] | 2016 | United States | RCT | Children over 12 years old with specialized asthma care | Asthma control |
| Mi-Yeong K et al. [46] | 2016 | South Korea | RCT | Adults over 19 years old with a diagnosis of asthma | Asthma control, treatment adherence, pulmonary function, and HRQoL |
| Johnson k et al. [47] | 2016 | United States | RCT | Adolescents aged 12 to 17 years with a diagnosis of asthma | Treatment adherence |
| Bender B et al. [48] | 2015 | United States | RCT | Children aged 3 to 12 years with a diagnosis of persistent asthma | Treatment adherence |
| Ryan D et al. [49] | 2012 | United Kingdom | RCT | Adolescents and adults with poorly controlled asthma | Asthma control, exacerbations, and HRQoL |
| Gustafson D et al. [50] | 2012 | United States | RCT | Children aged 4 to 12 years with poorly controlled asthma | Asthma control, adherence, and symptom-free days |
| Araujo L et al. [51] | 2012 | Portugal | RCT | Adults aged 18 to 62 years with moderate to severe asthma | Asthma control, pulmonary function, and HRQoL |
| Deschildre A et al. [52] | 2012 | France | RCT | Children aged 6 to 16 years with asthma | Exacerbations, pulmonary function, and HRQoL |
| Liu W et al. [53] | 2011 | Taiwan | RCT | Adults over 18 years old with moderate to severe persistent asthma | Pulmonary function |
| Author, Year | Patients | % Male | Tool Type | Main Supervision Mode | Follow-Up Time | Conclusions |
|---|---|---|---|---|---|---|
| Zairina E et al., 2024 [28] | n: 72, I: 36, C: 36 | 37 | Mobile Applications | Remote | 3 to 6 months | Telehealth supported by a mobile application proved to be viable for improving asthma control and HRQoL in pregnant women. |
| Farzandipour M et al., 2024 [29] | n:60, I:30, C:30 | 35 | Mobile Applications | In-person | 3 to 6 months | The use of a mobile application for asthma self-management significantly improved symptom control and HRQoL after six months. |
| Suvarna C et al., 2024 [30] | n:192, I:96, C:96 | 71 | Mobile Applications | Remote | Up to 3 months | Telemedicine follow-up was as effective as in-person care in asthma control and quality of life. |
| Jeminiwa K et al. [31] | n: 43, I:22, C:21 | 33 | Phone follow-up | Remote | Up to 3 months | Mobile messaging increased adherence and improved asthma control in young adults. |
| Halterman J et al., 2024 [32] | n:369, I:185, C:184 | 60 | Webplatform | Remote | Greater than 6 months | The TEAM-ED program successfully increased preventive care and medication use in children with persistent asthma. |
| Gümüs M et al., 2024 [33] | n:97, I:47, C:50 | 58 | Mobile Applications | Remote | 3 to 6 months | Virtual follow-up significantly improved symptom control and quality of life in children with asthma. Participants had fewer symptomatic days. |
| Mahmoud R et al., 2022 [34] | n:200, I:100, C:100 | 46 | Mobile Applications | In-person | Up to 3 months | The use of the Clip-Tone device and its mobile application improved pulmonary function and asthma control in adults. |
| Gupta R et al., 2021 [35] | n:252, I:125, C:127 | 66 | Webplatform | Remote | Greater than 6 months | Electronic monitoring with inhaler sensors significantly improved symptom control and caregivers’ quality of life in children with moderate to severe asthma. However, an increased use of health services was also observed. |
| Iamlaor U et al., 2021 [36] | n:60, I:29, C:31 | 24 | Mobile Applications | Remote | 3 to 6 months | The intervention with the Line mobile application (SALA) improved symptom control and pulmonary function in adults with mild to moderate asthma, although no significant changes were observed in HRQoL. |
| Khusial R et al., 2020 [37] | n:30, I:15, C:15 | 23 | Webplatform | Remote | 3 to 6 months | The myAirCoach system, which integrates connected devices and a mobile application, showed that patients had a high acceptance of the technology, especially in aspects related to self-control and ease of use. |
| Prabhakaran L et al., 2019 [38] | n:424, I:212, C:212 | 42 | Mobile Applications | In-person | 3 to 6 months | The eCARE program had adequate acceptance, however, text message reminders did not improve asthma control or reduce the use of emergency services. |
| Shaoxia L et al., 2019 [39] | n:152, I:77, C:75 | 50 | Mobile Applications | In-person | Greater than 6 months | The nurse-led care model assisted by a mobile application significantly improved treatment adherence and reduced exacerbations in children with asthma. |
| Kosse R et al., 2019 [40] | n:234, I:87, C:147 | 47 | Mobile Applications | Remote | 3 to 6 months | The ADAPT mobile intervention improved adherence in adolescents with asthma but did not generate significant changes in HRQoL. |
| Nemanic, T et al., 2018 [41] | n:100, I:51, C:49 | 48 | Webplatform | In-person | Greater than 6 months | Home telemonitoring showed good acceptance, with subjective benefits reported by patients, although without statistically significant differences in asthma control between the groups. |
| Halterman J et al., 2018 [42] | n:400, I:200, C:200 | 62 | Webplatform | In-person | Greater than 6 months | The asthma management program enhanced with School Telemedicine significantly improved symptom control in children with persistent asthma. |
| Cao Y et al., 2018 [43] | n:67, I:37, C:30 | 22 | Mobile Applications | Remote | Up to 3 months | The use of WeChat as educational support and therapeutic reminder improved pulmonary function and HRQoL in patients with asthma. Although both groups showed improvements in symptom control, the digital intervention offered additional benefits. |
| Lara S et al., 2017 [44] | n:210, I:105, C:105 | 59 | Mobile Applications | Remote | Greater than 6 months | The use of a virtual clinic for asthma allowed for a 50% reduction in in-person visits without worsening asthma control. In young children, it significantly improved symptom control, although there were no differences in pulmonary function or other clinical outcomes. |
| Portnoy M et al., 2016 [45] | n:169, I:69, C:100 | 65 | Webplatform | In-person | 3 to 6 months | Telemedicine and in-person care have equal clinical equivalence. Regarding asthma symptom control, it significantly improved in both groups at six months. |
| Mi-Yeong K et al., 2016 [46] | n:44, I:22, C:22 | 27 | Mobile Applications | In-person | Up to 3 months | The use of the snuCare application did not significantly improve symptom control or pulmonary function in asthmatic adults, but it did increase quality of life and treatment adherence. |
| Johnson k et al., 2016 [47] | n:89, I:46, C:43 | 51 | Mobile Applications | In-person | Up to 3 months | The MyMediHealth text messaging system improved treatment adherence in adolescents with asthma. Despite its short period of use, important benefits for self-care were perceived. |
| Bender B et al., 2015 [48] | n:899, I:452, C:447 | 64 | Webplatform | Remote | Greater than 6 months | An automated telephone call system with voice recognition, connected to the electronic medical record, notably improved treatment adherence in children with persistent asthma. Although no differences were observed in hospitalizations or emergency visits. |
| Ryan D et al., 2012 [49] | n:288, I:145, C:143 | 37 | Mobile Applications | In-person | 3 to 6 months | Asthma monitoring via mobile phones did not show significant improvements in asthma control compared to paper-based follow-up when both groups received structured clinical care. |
| Gustafson D et al., 2012 [50] | n: 301, I:148, C:153 | 61 | Webplatform | Remote | Greater than 6 months | Integrating an eHealth program with monthly nursing calls significantly improved asthma control in children, although it did not impact medication adherence or symptom-free days. |
| Araujo L et al., 2012 [51] | n:19, I:12, C:7 | 29 | Webplatform | Remote | Up to 3 months | The web strategy and the paper format improved symptom control and HRQoL in adults with asthma, without generating significant changes in pulmonary function. |
| Deschildre A et al., 2012 [52] | n:50, I:25, C:25 | Mobile Applications | Remote | Greater than 6 months | Daily monitoring with medical feedback did not improve pulmonary function or HRQoL in children with uncontrolled severe asthma, compared to conventional treatment. | |
| Liu W et al., 2011 [53] | n:331, I:140, C:191 | 37 | Mobile Applications | Remote | 3 to 6 months | The interactive self-care system via mobile telephony can enhance asthma control, surpassing a written action plan, with improvements in pulmonary function and a decrease in medical visits. |
| Outcome | Effect Size (MD or SMD) | GRADE Certainty |
|---|---|---|
| Asthma control | SMD: 0.90 (−0.04 a 1.85) | 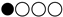 |
| Pulmonary function (FEV1) | SMD: 1.53 (−0.54 a 2.51) | 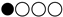 |
| Pulmonary function (FEV1/FVC) | SMD: 1.2 (0.29 a 2.10) |  |
| Pulmonary function (PEF) | SMD: 1.43 (−1.19 a 4.05) | 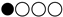 |
| Symptom-free days | MD: 0.70 (0.80 a 2.20) | 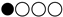 |
| HRQoL | SMD: 0.86 (−0.12 a 1.84) | 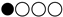 |
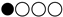 : Very low
: Very low  : low HRQoL = Health-related Quality of Life.
: low HRQoL = Health-related Quality of Life.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perlaza, C.L.; Rojas, S.M.; Choco, L.D.; González, M.P.P.; Mosquera, F.E.C.; Liscano, Y. Effectiveness of Digital Health Tools for Asthma Self-Management: A Systematic Review and Meta-Analysis of Clinical Trials. Appl. Sci. 2025, 15, 12471. https://doi.org/10.3390/app152312471
Perlaza CL, Rojas SM, Choco LD, González MPP, Mosquera FEC, Liscano Y. Effectiveness of Digital Health Tools for Asthma Self-Management: A Systematic Review and Meta-Analysis of Clinical Trials. Applied Sciences. 2025; 15(23):12471. https://doi.org/10.3390/app152312471
Chicago/Turabian StylePerlaza, Claudia Lorena, Stephania Mina Rojas, Laura Daniela Choco, María Paula Paz González, Freiser Eceomo Cruz Mosquera, and Yamil Liscano. 2025. "Effectiveness of Digital Health Tools for Asthma Self-Management: A Systematic Review and Meta-Analysis of Clinical Trials" Applied Sciences 15, no. 23: 12471. https://doi.org/10.3390/app152312471
APA StylePerlaza, C. L., Rojas, S. M., Choco, L. D., González, M. P. P., Mosquera, F. E. C., & Liscano, Y. (2025). Effectiveness of Digital Health Tools for Asthma Self-Management: A Systematic Review and Meta-Analysis of Clinical Trials. Applied Sciences, 15(23), 12471. https://doi.org/10.3390/app152312471








