Solar-Assisted PEM Water Electrolysis with Symmetric IrO2 Electrodes for Hydrogen-Rich Water Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodeposition
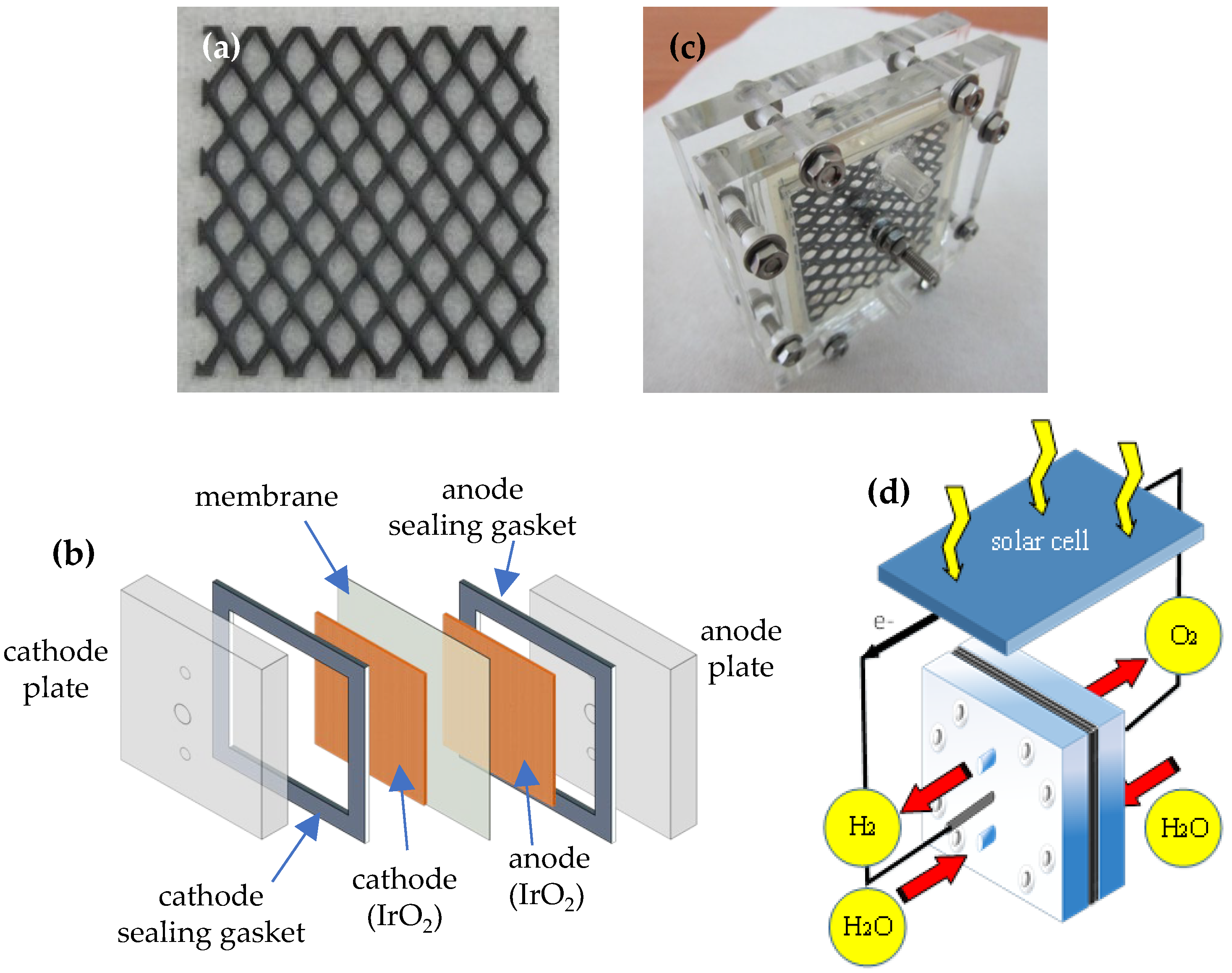
2.2. Fabrication of MEA and Water Electrolysis Cell
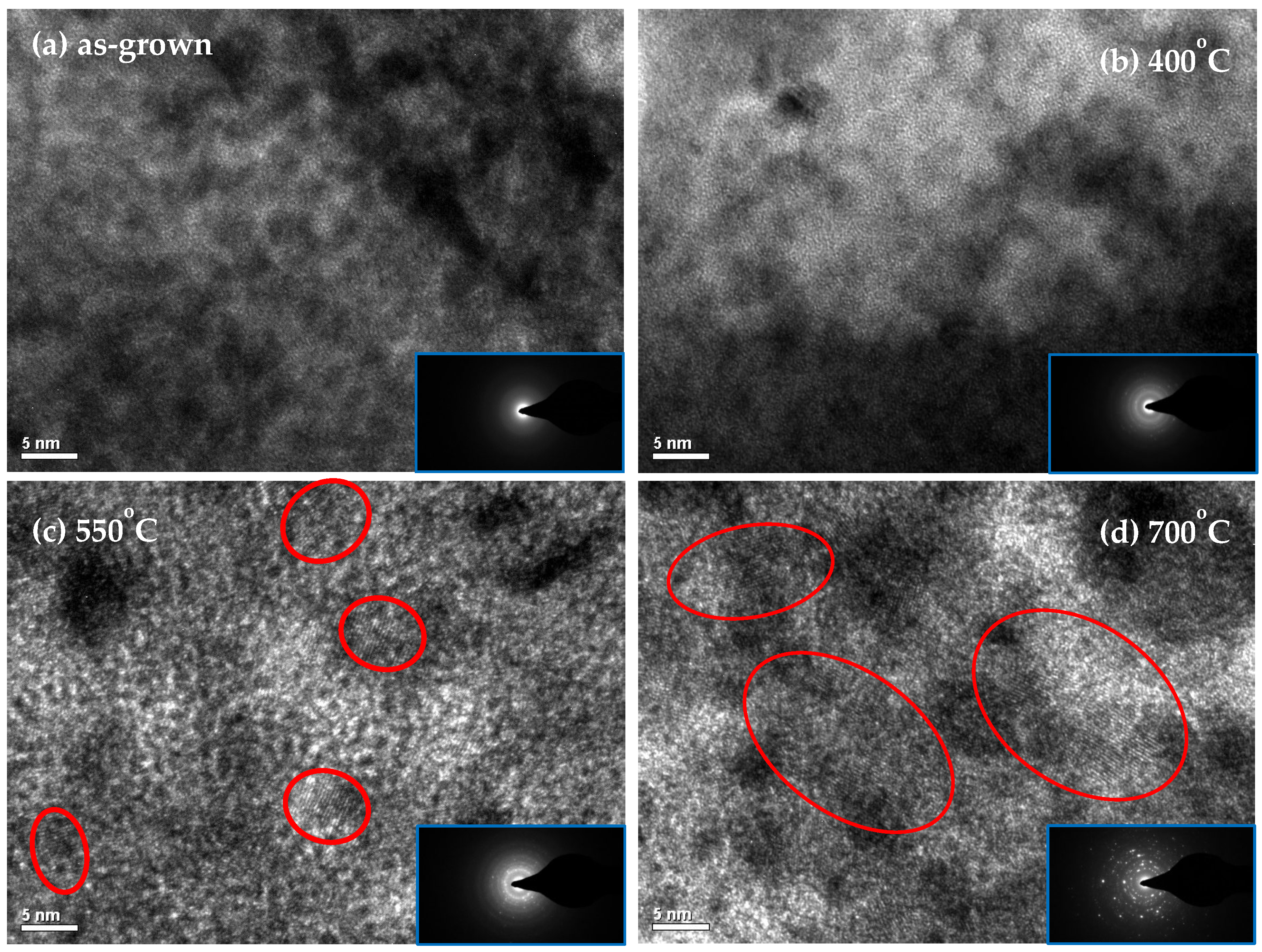
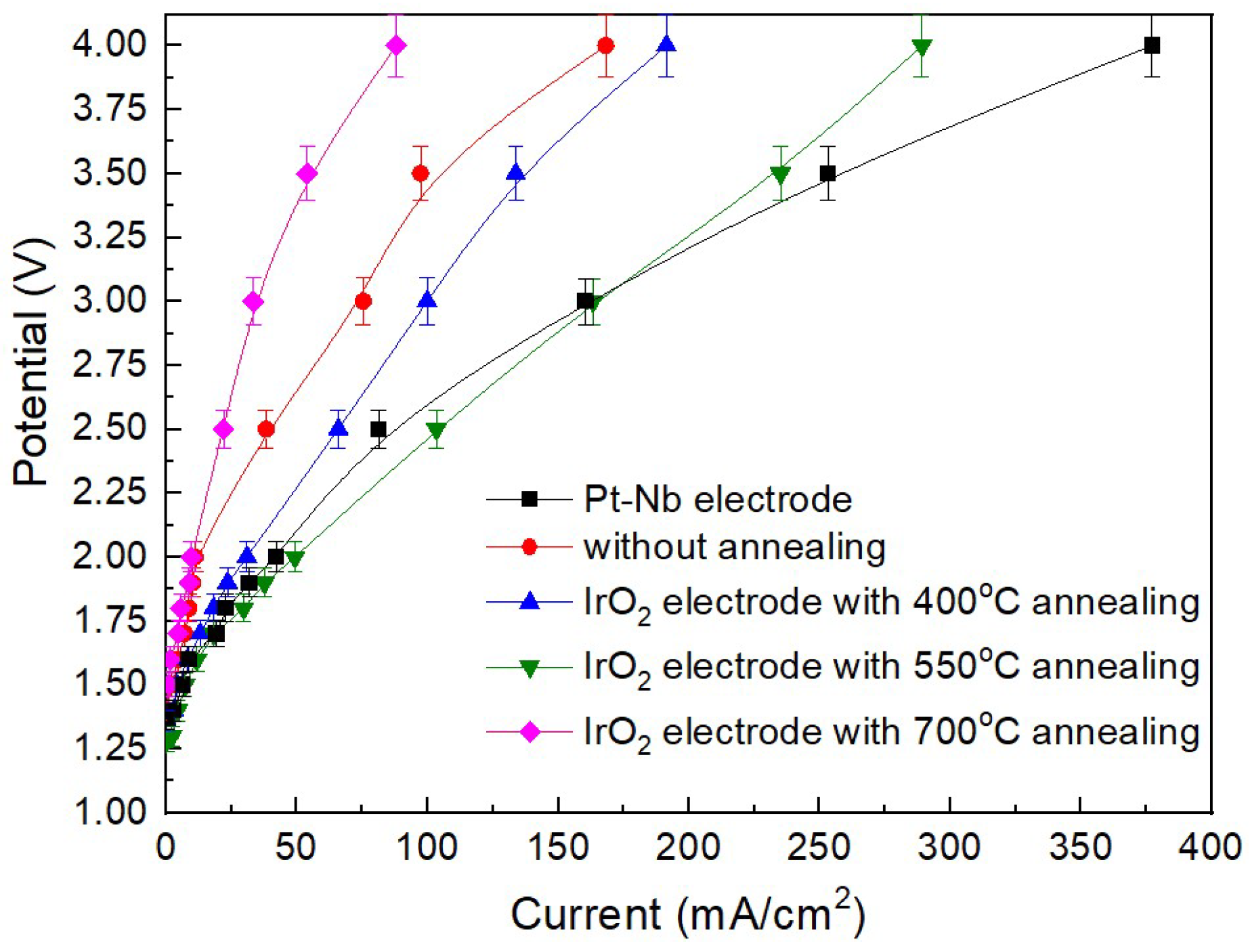
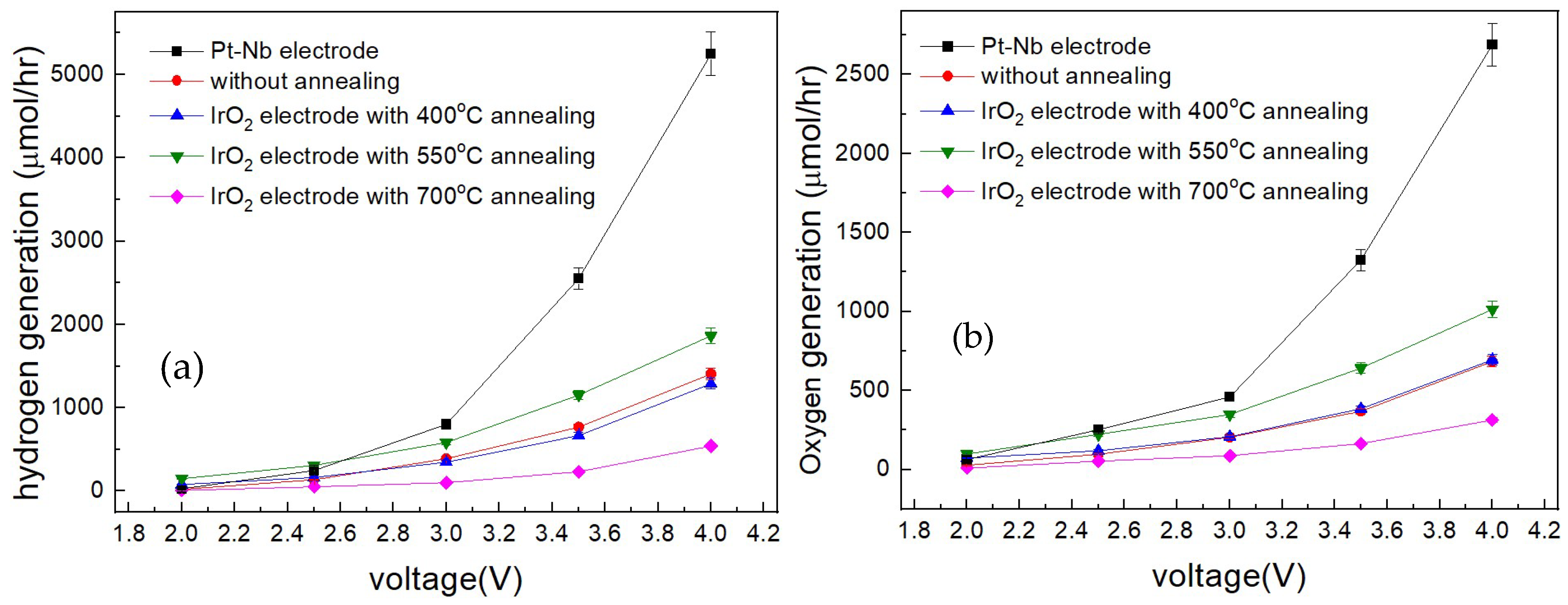
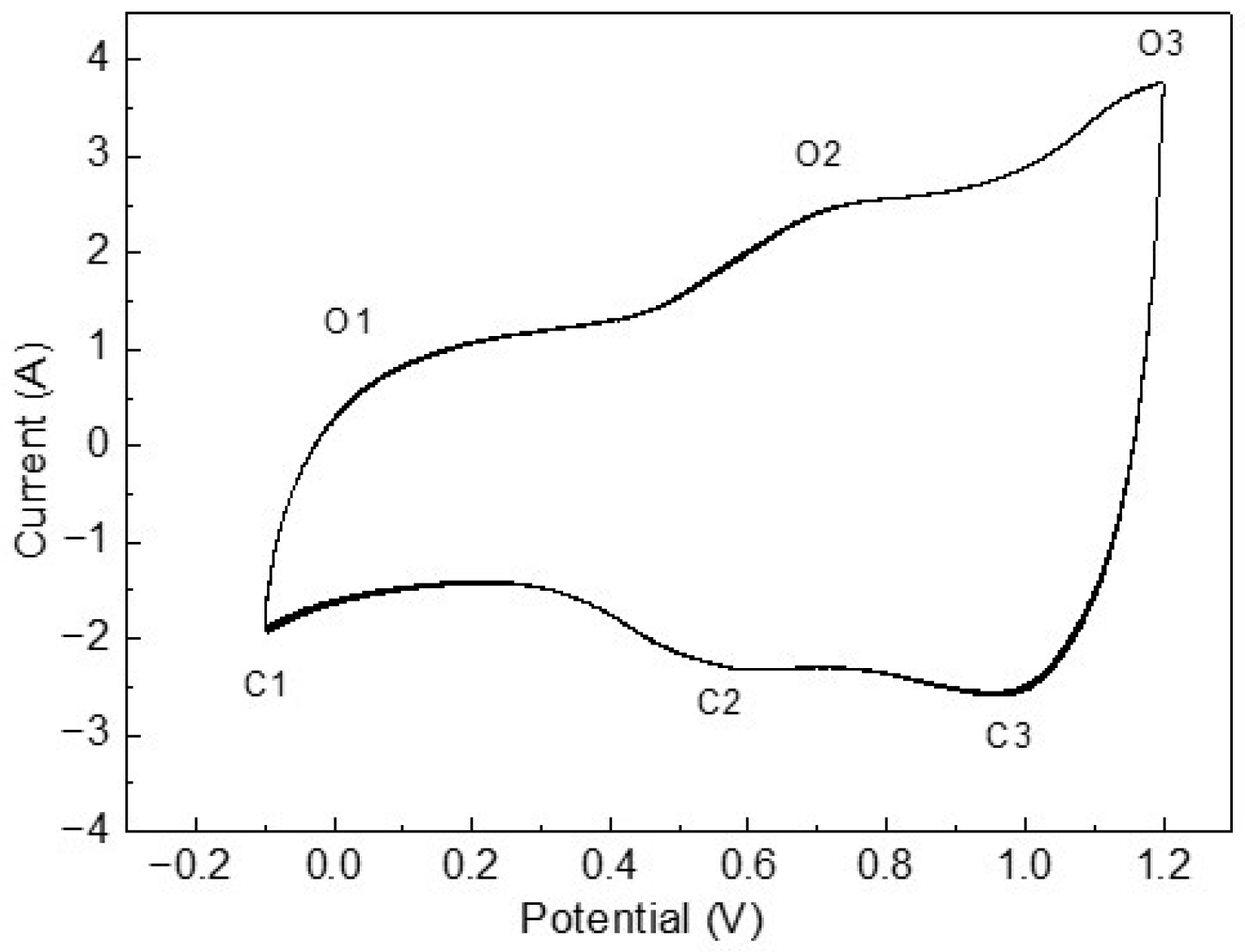
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef]
- Timón, R.; Olcina, G.; González-Custodio, A.; Camacho-Cardenosa, M.; Camacho-Cardenosa, A.; Martínez Guardado, I. Effects of 7-day intake of hydrogen-rich water on physical performance of trained and untrained subjects. Biol. Sport 2021, 38, 269–275. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The Effects of 24-Week, High-concentration hydrogen-rich water on body composition, blood lipid profiles and inflammation biomarkers in men and women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 889–896. [Google Scholar] [CrossRef]
- Vaishnavi, C.; Elangovan, G.P.; Thirumal, M.; Prashanth, S.V.; Deepika, D.; Pragathi, T.G. Comparison of the Antimicrobial Effect of Hydrogen Water and Chlorhexidine Mouth rinse in Toothbrush Disinfection Among Dental Students. J. Pharm. Bioallied Sci. 2025, 17, S528–S530. [Google Scholar] [CrossRef]
- Kuzmanovic, J.; Todorovic, N.; Ranisavljev, M.; Javorac, D.; Korovljev, D.; Tarnava, A.; Stajer, V.; Ostojica, S.M. The effects of drinking hydrogen-rich water for six weeks on exercise-related biomarkers in exercise-naïve men and women over 50 years following resistance training program: A randomized controlled pilot trial. Res. Sports Med. 2025, 33, 711–721. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Kumara, S.; Lim, H. An over view of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Mohite, S.V.; Devaraji, P.; Mao, L.; Xing, R. Ultrathin MoS2 nanosheets in situ grown on rich defective Ni0.96S as heterojunction bifunctional electrocatalysts for alkaline water electrolysis. Int. J. Hydrog. Energy 2020, 45, 29929–29937. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Zahid, M.; Tahir, N.; Afzal, A.; Lin, X.-M. Bimetallic NiCo–NiCoO2 nano-heterostructures embedded on copper foam as a self-supported bifunctional electrode for water oxidation and hydrogen production in alkaline media. Int. J. Hydrog. Energy 2021, 46, 18936–18948. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Chen, T.; Liang, J.; Zhang, Q.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Transition Metal/Metal Oxide Interface (Ni–Mo–O/Ni4Mo) Stabilized on N-Doped Carbon Paper for Enhanced Hydrogen Evolution Reaction in Alkaline Conditions. Ind. Eng. Chem. Res. 2021, 60, 5145–5150. [Google Scholar] [CrossRef]
- Lv, Z.; Ma, W.; Wang, M.; Dang, J.; Jian, K.; Liu, D.; Huang, D. Co-Constructing Interfaces of Multiheterostructure on MXene (Ti3C2Tx)-Modified 3D Self-Supporting Electrode for Ultraefficient Electrocatalytic HER in Alkaline Media. Adv. Funct. Mater. 2021, 31, 2102576. [Google Scholar] [CrossRef]
- Jang, M.J.; Yang, J.; Lee, J.; Park, Y.S.; Jeong, J.; Park, S.M.; Jeong, J.-Y.; Yin, Y.; Seo, M.-Y.; Choi, S.M.; et al. Superior performance and stability of anion exchange membrane water electrolysis: pH-controlled copper cobalt oxide nanoparticles for the oxygen evolution reaction. J. Mater. Chem. A 2022, 8, 4290. [Google Scholar] [CrossRef]
- Chen, N.; Paek, S.Y.; Lee, J.Y.; Park, J.H.; Lee, S.Y.; Lee, Y.M. High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and a durability of 1000 hours. Energy Environ. Sci. 2021, 14, 6338–6348. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, S.; Ma, X.; Li, K.; Ding, L.; Wang, W.; Cullen, D.A.; Meyer, H.M.; Yu, H.; Tong, J.; et al. MoS2 nanosheet integrated electrodes with engineered 1T-2H phases and defects for efficient hydrogen production in practical PEM electrolysis. Appl. Catal. B 2022, 313, 121458. [Google Scholar] [CrossRef]
- Jang, I.; Im, K.; Shin, H.; Lee, K.-S.; Kim, H.; Kim, J.; Yoo, S.J. Electron-deficient titanium single-atom electrocatalyst for stable and efficient hydrogen production. Nano Energy 2020, 78, 105151. [Google Scholar] [CrossRef]
- Jiang, G.; Yu, H.; Yao, D.; Li, Y.; Chi, J.; Zhang, H.; Shao, Z. Boosting the oxygen evolution stability and activity of a heterogeneous IrRu bimetallic coating on a WO3 nano-array electrode for PEM water electrolysis. J. Mater. Chem. A 2022, 10, 11893–11903. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, H.; Wang, S.; Qian, B.; Li, Q.; Ge, L.; Chen, H. Mn-doped Ruddlesden-Popper oxide La1.5Sr0.5NiO4+δ as a novel air electrode material for solid oxide electrolysis cells. Ceram. Int. 2021, 47, 1208–1217. [Google Scholar] [CrossRef]
- Vibhu, V.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Cobalt substituted Pr2Ni1−xCoxO4+δ (x = 0, 0.1, 0.2) oxygen electrodes: Impact on electrochemical performance and durability of solid oxide electrolysis cells. J. Power Sources 2021, 482, 228909. [Google Scholar] [CrossRef]
- Song, S.; Zhang, H.; Ma, X.; Shao, Z.; Baker, R.T.; Yi, B. Electrochemical investigation of electrocatalysts for the oxygen evolution reaction in PEM water electrolyzers. Int. J. Hydrog. Energy 2008, 33, 4955–4961. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Chen, H.; Gao, R.; Shi, L.; Yang, L.; Zou, X. Iridium-containing water-oxidation catalysts in acidic electrolyte. Chin. J. Catal. 2021, 42, 1054–1077. [Google Scholar] [CrossRef]
- Kim, J.-D.; Ohira, A. Water electrolysis using a porous IrO2/Ti/IrO2 catalyst electrode and Nafion membranes at elevated temperatures. Membranes 2021, 11, 330. [Google Scholar] [CrossRef]
- Bernicke, M.; Ortel, E.; Reier, T.; Bergmann, A.; Ferreira de Araujo, J.; Strasser, P.; Kraehnert, R. Iridium oxide coatings with templated porosity as highly active oxygen evolution catalysts: Structure-Activity relationships. ChemSusChem 2015, 8, 1908–1915. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, J.; Zhang, H.; Lin, B.; Lin, G. A new direct coupling method for photovoltaic module-PEM electrolyzer stack for hydrogen production. Fuel Cells 2018, 18, 543–550. [Google Scholar] [CrossRef]
- Arunachalam, M.; Han, D.S. Efficient solar-powered PEM electrolysis for sustainable hydrogen production: An integrated approach. Emerg. Mater. 2024, 7, 1401–1415. [Google Scholar] [CrossRef]
- García-Valverde, R.; Espinosa, N.; Urbina, A. Optimized method for photovoltaic-water electrolyser direct coupling. Int. J. Hydrog. Energy 2011, 36, 10574–10586. [Google Scholar] [CrossRef]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Plante, T.D.; Detlefsen, D.E. Potential-biased, asymmetric waveforms for charge-injection with activated iridium oxide (AIROF) neural stimulation electrodes. IEEE Trans. Biomed. Eng. 2006, 53, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F.; Guzelian, A.A.; Agnew, W.F.; Yuen, T.G.H.; McCreery, D.B. Over-pulsing degrades activated iridium oxide films used for intracortical neural stimulation. J. Neurosci. Methods 2004, 137, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K. Anodically electrodeposited iridium oxide films (AEIROF) from alkaline solutions for electrochromic display devices. Jpn. J. Appl. Phys. 1989, 28, 632–637. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, T.; Cai, Z.; Cao, Y.; Yang, H.; Duan, Y.Y. Anodically electrodeposited iridium oxide films microelectrodes for neural microstimulation and recording. Sen. Actuator B-Chem. 2009, 137, 334–339. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Blasi, A.D.; Briguglio, N.; Stassi, A.; Ornelas, R.; Trifoni, E.; Antonucci, V.; Arico, A.S. Electrochemical characterization of single cell and short stack PEM electrolyzers based on a nanosized IrO2 anode electrocatalyst. Int. J. Hydrogen Energy 2010, 35, 5558–5568. [Google Scholar] [CrossRef]
- Ngo, T.H.N.; Love, J.; O’Mullane, A.P. Investigating the influence of amorphous/crystalline interfaces on the stability of IrO2 for the oxygen evolution reaction in acidic electrolyte. ChemElectroChem 2023, 10, e202300438. [Google Scholar] [CrossRef]
- Jang, I.; Hwang, I.; Tak, Y. Attenuated degradation of a PEMFC cathode during fuel starvation by using carbon-supported IrO2. Electrochim. Acta 2013, 90, 148–156. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Ma, H.; Zhong, H.; Zou, Y. Study of carbon-supported IrO2 and RuO2 for use in the hydrogen evolution reaction in a solid polymer electrolyte electrolyzer. Electrochim. Acta 2010, 55, 1855–1861. [Google Scholar] [CrossRef]
- Badley, M.D.M.; Shoesmith, D.W.; Noёl, J.J. Effect of hydrogen on the dissolution of uranium dioxide in peroxide-containing environments. J. Electrochem. Soc. 2023, 170, 096506. [Google Scholar] [CrossRef]

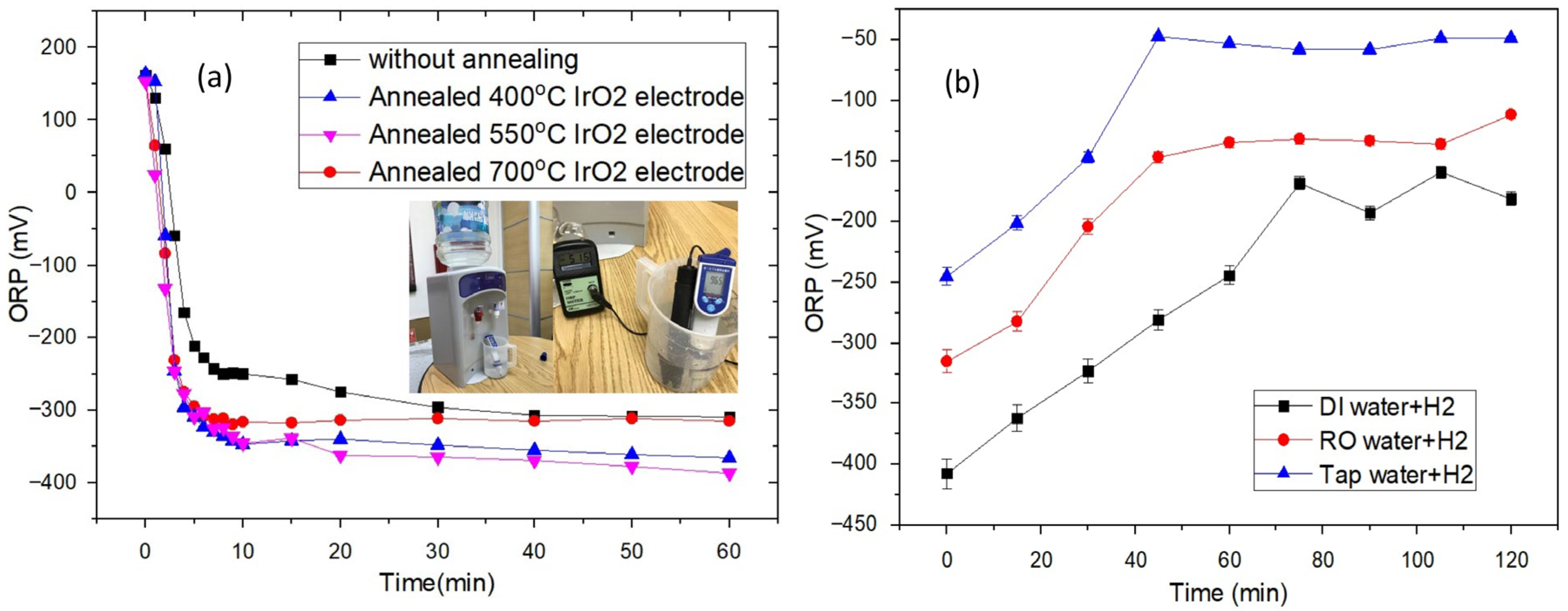
| Water Type | ORP (mV) |
|---|---|
| Tap water | 251 |
| RO water | 170 |
| DI water | 157 |
| HRW (550 °C annealed) | −332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pai, Y.-H.; Kao, C.-C.; Li, Z.-Y.; Tsai, C.-K. Solar-Assisted PEM Water Electrolysis with Symmetric IrO2 Electrodes for Hydrogen-Rich Water Production. Appl. Sci. 2025, 15, 12411. https://doi.org/10.3390/app152312411
Pai Y-H, Kao C-C, Li Z-Y, Tsai C-K. Solar-Assisted PEM Water Electrolysis with Symmetric IrO2 Electrodes for Hydrogen-Rich Water Production. Applied Sciences. 2025; 15(23):12411. https://doi.org/10.3390/app152312411
Chicago/Turabian StylePai, Yi-Hao, Chih-Cheng Kao, Zheng-Yu Li, and Cheng-Kang Tsai. 2025. "Solar-Assisted PEM Water Electrolysis with Symmetric IrO2 Electrodes for Hydrogen-Rich Water Production" Applied Sciences 15, no. 23: 12411. https://doi.org/10.3390/app152312411
APA StylePai, Y.-H., Kao, C.-C., Li, Z.-Y., & Tsai, C.-K. (2025). Solar-Assisted PEM Water Electrolysis with Symmetric IrO2 Electrodes for Hydrogen-Rich Water Production. Applied Sciences, 15(23), 12411. https://doi.org/10.3390/app152312411






