Ritonavir and DNA Damage: A New Perspective on an Old Drug
Abstract
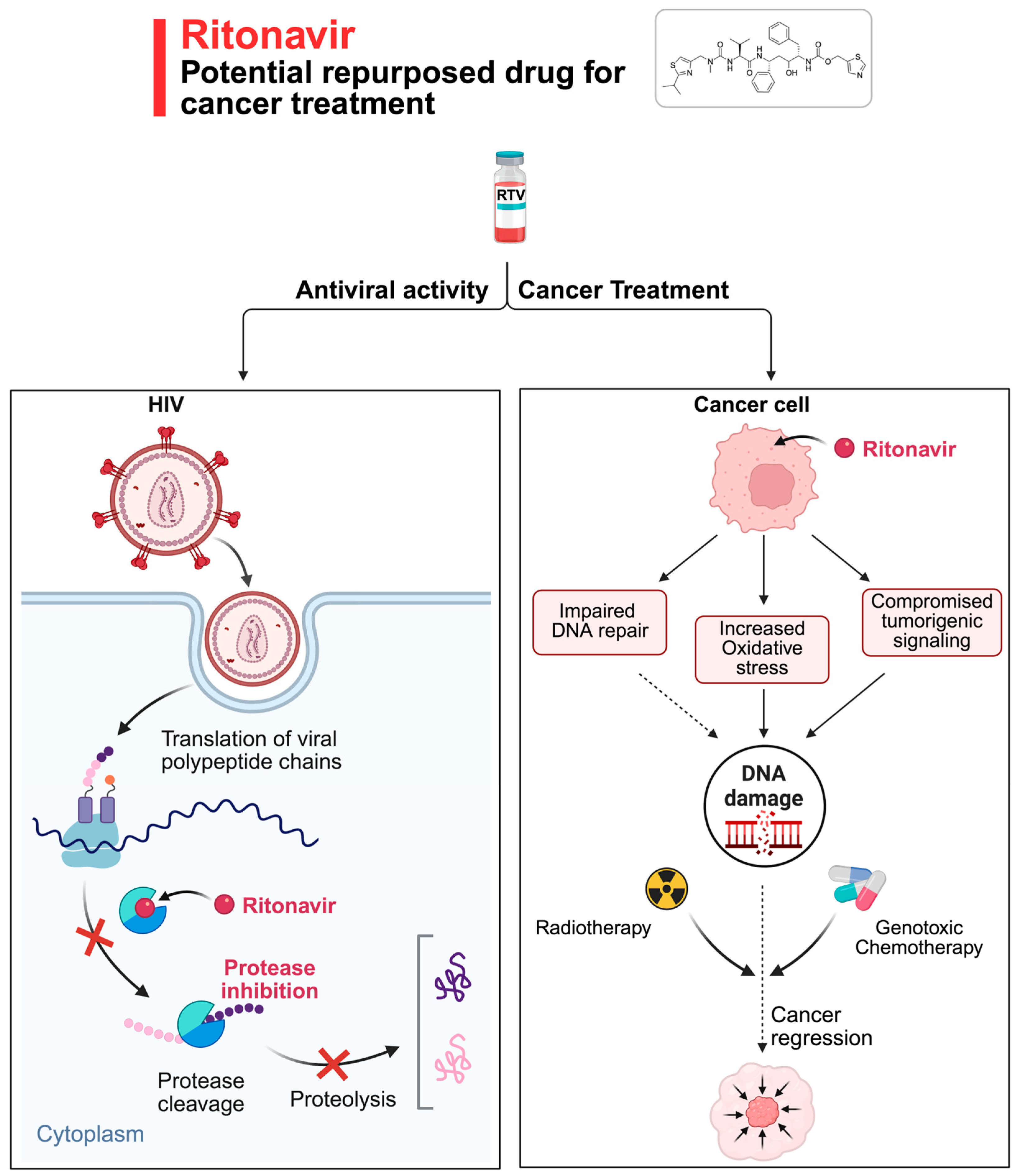
1. Introduction
2. From Antiviral to Anticancer Drug: Drug Repurposing
3. RTV and DNA Repair: Lessons from Recent Studies
4. DNA Damage Response: A Key Pathway in Cancer Therapy
5. Repurposing RTV as a Radiosensitizer and Chemosensitizer
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | phosphoinositide 3 kinase/protein kinase B |
| CYP3A4 | Cytochrome P450 3A4 |
| DDR | DNA damage response |
| EMT | epithelial-to-mesenchymal transition |
| HIV | human immunodeficiency virus |
| ROS | reactive oxygen species |
| RTV | Ritonavir |
References
- Kempf, D.J.; Marsh, K.C.; Denissen, J.F.; McDonald, E.; Vasavanonda, S.; Flentge, C.A.; Green, B.E.; Fino, L.; Park, C.H.; Kong, X.P. ABT-538 Is a Potent Inhibitor of Human Immunodeficiency Virus Protease and Has High Oral Bioavailability in Humans. Proc. Natl. Acad. Sci. USA 1995, 92, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Kempf, D.J.; Sham, H.L.; Marsh, K.C.; Flentge, C.A.; Betebenner, D.; Green, B.E.; McDonald, E.; Vasavanonda, S.; Saldivar, A.; Wideburg, N.E.; et al. Discovery of Ritonavir, a Potent Inhibitor of HIV Protease with High Oral Bioavailability and Clinical Efficacy. J. Med. Chem. 1998, 41, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.L. The Role of Pharmacological Enhancement in Protease Inhibitor-Based Highly Active Antiretroviral Therapy. Expert. Opin. Investig. Drugs 2003, 12, 401–412. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Kress, T.C.; Kennard, S.; Belin de Chantemèle, E.J. HIV Protease Inhibitor Ritonavir Impairs Endothelial Function Via Reduction in Adipose Mass and Endothelial Leptin Receptor-Dependent Increases in NADPH Oxidase 1 (Nox1), C-C Chemokine Receptor Type 5 (CCR5), and Inflammation. J. Am. Heart Assoc. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Ganta, K.K.; Chaubey, B. Endoplasmic Reticulum Stress Leads to Mitochondria-Mediated Apoptosis in Cells Treated with Anti-HIV Protease Inhibitor Ritonavir. Cell Biol. Toxicol. 2019, 35, 189–204. [Google Scholar] [CrossRef]
- Schmidtke, G.; Holzhütter, H.-G.; Bogyo, M.; Kairies, N.; Groll, M.; de Giuli, R.; Emch, S.; Groettrup, M. How an Inhibitor of the HIV-I Protease Modulates Proteasome Activity. J. Biol. Chem. 1999, 274, 35734–35740. [Google Scholar] [CrossRef]
- Marima, R.; Hull, R.; Dlamini, Z.; Penny, C. The Dual Protease Inhibitor Lopinavir/Ritonavir (LPV/r) Exerts Genotoxic Stress on Lung Cells. Biomed. Pharmacother. 2020, 132, 110829. [Google Scholar] [CrossRef]
- Chow, W.A.; Jiang, C.; Guan, M. Anti-HIV Drugs for Cancer Therapeutics: Back to the Future? Lancet Oncol. 2009, 10, 61–71. [Google Scholar] [CrossRef]
- Kumar, S.; Bryant, C.S.; Chamala, S.; Qazi, A.; Seward, S.; Pal, J.; Steffes, C.P.; Weaver, D.W.; Morris, R.; Malone, J.M.; et al. Ritonavir Blocks AKT Signaling, Activates Apoptosis and Inhibits Migration and Invasion in Ovarian Cancer Cells. Mol. Cancer 2009, 8, 26. [Google Scholar] [CrossRef]
- Srirangam, A.; Mitra, R.; Wang, M.; Gorski, J.C.; Badve, S.; Baldridge, L.; Hamilton, J.; Kishimoto, H.; Hawes, J.; Li, L.; et al. Effects of HIV Protease Inhibitor Ritonavir on Akt-Regulated Cell Proliferation in Breast Cancer. Clin. Cancer Res. 2006, 12, 1883–1896. [Google Scholar] [CrossRef]
- Pomella, S.; D’Archivio, L.; Cassandri, M.; Aiello, F.A.; Melaiu, O.; Marampon, F.; Rota, R.; Barillari, G. The HIV Protease Inhibitor Ritonavir Reverts the Mesenchymal Phenotype Induced by Inflammatory Cytokines in Normal and Tumor Oral Keratinocytes to an Epithelial One, Increasing the Radiosensitivity of Tumor Oral Keratinocytes. Cancers 2025, 17, 2519. [Google Scholar] [CrossRef] [PubMed]
- Shaji, U.P.; Tuti, N.; Alim, S.K.; Mohan, M.; Das, S.; Meur, G.; Swamy, M.J.; Anindya, R. Inhibition of Human DNA Alkylation Damage Repair Enzyme ALKBH2 by HIV Protease Inhibitor Ritonavir. DNA Repair 2024, 141, 103732. [Google Scholar] [CrossRef]
- Pati, S.; Pelser, C.B.; Dufraine, J.; Bryant, J.L.; Reitz, M.S.; Weichold, F.F. Antitumorigenic Effects of HIV Protease Inhibitor Ritonavir: Inhibition of Kaposi Sarcoma. Blood 2002, 99, 3771–3779. [Google Scholar] [CrossRef]
- Bacigalupo, I.; Palladino, C.; Leone, P.; Toschi, E.; Sgadari, C.; Ensoli, B.; Barillari, G. Inhibition of MMP-9 Expression by Ritonavir or Saquinavir Is Associated with Inactivation of the AKT/Fra-1 Pathway in Cervical Intraepithelial Neoplasia Cells. Oncol. Lett. 2017, 13, 2903–2908. [Google Scholar] [CrossRef]
- Barillari, G.; Iovane, A.; Bacigalupo, I.; Palladino, C.; Bellino, S.; Leone, P.; Monini, P.; Ensoli, B. Ritonavir or Saquinavir Impairs the Invasion of Cervical Intraepithelial Neoplasia Cells via a Reduction of MMP Expression and Activity. AIDS 2012, 26, 909–919. [Google Scholar] [CrossRef]
- Batchu, R.; Gruzdyn, O.; Bryant, C.; Qazi, A.; Kumar, S.; Chamala, S.; Kung, S.; Sanka, R.; Puttagunta, U.; Weaver, D.; et al. Ritonavir-Mediated Induction of Apoptosis in Pancreatic Cancer Occurs via the RB/E2F-1 and AKT Pathways. Pharmaceuticals 2014, 7, 46–57. [Google Scholar] [CrossRef]
- Maggiorella, L.; Wen, B.; Frascogna, V.; Opolon, P.; Bourhis, J.; Deutsch, E. Combined Radiation Sensitizing and Anti-Angiogenic Effects of Ionizing Radiation and the Protease Inhibitor Ritonavir in a Head and Neck Carcinoma Model. Anticancer. Res. 2005, 25, 4357–4362. [Google Scholar]
- Adekola, K.U.A.; Dalva Aydemir, S.; Ma, S.; Zhou, Z.; Rosen, S.T.; Shanmugam, M. Investigating and Targeting Chronic Lymphocytic Leukemia Metabolism with the Human Immunodeficiency Virus Protease Inhibitor Ritonavir and Metformin. Leuk. Lymphoma 2015, 56, 450–459. [Google Scholar] [CrossRef]
- Kraus, M.; Malenke, E.; Gogel, J.; Müller, H.; Rückrich, T.; Overkleeft, H.; Ovaa, H.; Koscielniak, E.; Hartmann, J.T.; Driessen, C. Ritonavir Induces Endoplasmic Reticulum Stress and Sensitizes Sarcoma Cells toward Bortezomib-Induced Apoptosis. Mol. Cancer Ther. 2008, 7, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Koudriakova, T.; Iatsimirskaia, E.; Utkin, I.; Gangl, E.; Vouros, P.; Storozhuk, E.; Orza, D.; Marinina, J.; Gerber, N. Metabolism of the Human Immunodeficiency Virus Protease Inhibitors Indinavir and Ritonavir by Human Intestinal Microsomes and Expressed Cytochrome P4503A4/3A5: Mechanism-Based Inactivation of Cytochrome P4503A by Ritonavir. Drug Metab. Dispos. 1998, 26, 552–561. [Google Scholar] [PubMed]
- Dewan, M.Z. Efficient Intervention of Growth and Infiltration of Primary Adult T-Cell Leukemia Cells by an HIV Protease Inhibitor, Ritonavir. Blood 2006, 107, 716–724. [Google Scholar] [CrossRef]
- Swami, D.; Mudaliar, P.; Bichu, Y.S.; Kumar Sahu, V.; Devarajan, S.; Basu, S.; Aich, J. Synergistic Combination of Ritonavir and Cisplatin as an Efficacious Therapy in Human Cervical Cancer Cells: A Computational Drug Discovery and in Vitro Insight. J. Biomol. Struct. Dyn. 2023, 41, 5802–5816. [Google Scholar] [CrossRef]
- Nugnes, R.; Orlo, E.; Russo, C.; Lavorgna, M.; Isidori, M. Comprehensive Eco-Geno-Toxicity and Environmental Risk of Common Antiviral Drugs in Aquatic Environments Post-Pandemic. J. Hazard. Mater. 2024, 480, 135947. [Google Scholar] [CrossRef]
- Baysal, M.; Karaduman, A.B.; Korkut Çelikateş, B.; Atlı-Eklioğlu, Ö.; Ilgın, S. Assessment of the Toxicity of Different Antiretroviral Drugs and Their Combinations on Sertoli and Leydig Cells. Drug Chem. Toxicol. 2024, 47, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.; Jackson, S.P. Interfaces Between the Detection, Signaling, and Repair of DNA Damage. Science (1979) 2002, 297, 547–551. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Morris, B.B.; Heeke, S.; Xi, Y.; Diao, L.; Wang, Q.; Rocha, P.; Arriola, E.; Lee, M.C.; Tyson, D.R.; Concannon, K.; et al. DNA Damage Response Signatures Are Associated with Frontline Chemotherapy Response and Routes of Tumor Evolution in Extensive Stage Small Cell Lung Cancer. Mol. Cancer 2025, 24, 90. [Google Scholar] [CrossRef]
- Pomella, S.; Cassandri, M.; Melaiu, O.; Marampon, F.; Gargari, M.; Campanella, V.; Rota, R.; Barillari, G. DNA Damage Response Gene Signature as Potential Treatment Markers for Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 2673. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science (1979) 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Shaji, U.P.; Tuti, N.; Das, S.; Anindya, R.; Mohan, M. Interactions between HIV Protease Inhibitor Ritonavir and Human DNA Repair Enzyme ALKBH2: A Molecular Dynamics Simulation Study. Mol. Divers. 2023, 27, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Shaji, U.P.; Khatua, R.R.; Rankawat, S.; Rathnam, S.S.V.; Jangra, J.; Ray, S.; Khan, F.A.; Anindya, R. A Cell-Permeable Fluorescent Probe for Imaging of DNA Repair Protein ALKBH2. Talanta 2026, 297, 128659. [Google Scholar] [CrossRef] [PubMed]
- Gratton, R.; Tricarico, P.M.; Guimaraes, R.L.; Celsi, F.; Crovella, S. Lopinavir/Ritonavir Treatment Induces Oxidative Stress and Caspaseindependent Apoptosis in Human Glioblastoma U-87 MG Cell Line. Curr. HIV Res. 2018, 16, 106–112. [Google Scholar] [CrossRef]
- Dirix, L.; Swaisland, H.; Verheul, H.M.W.; Rottey, S.; Leunen, K.; Jerusalem, G.; Rolfo, C.; Nielsen, D.; Molife, L.R.; Kristeleit, R.; et al. Effect of Itraconazole and Rifampin on the Pharmacokinetics of Olaparib in Patients with Advanced Solid Tumors: Results of Two Phase I Open-Label Studies. Clin. Ther. 2016, 38, 2286–2299. [Google Scholar] [CrossRef]
- Apostolova, N.; Blas-García, A.; Esplugues, J.V. Mitochondrial Interference by Anti-HIV Drugs: Mechanisms beyond Pol-γ Inhibition. Trends Pharmacol. Sci. 2011, 32, 715–725. [Google Scholar] [CrossRef]
- van der Putten, E.; Wosikowski, K.; Beijnen, J.H.; Imre, G.; Freund, C.R. Ritonavir Reverses Resistance to Docetaxel and Cabazitaxel in Prostate Cancer Cells with Acquired Resistance to Docetaxel. Cancer Drug Resist. 2024, 7, 3. [Google Scholar] [CrossRef]
- Overbeek, J.K.; Guchelaar, N.A.D.; Mohmaed Ali, M.I.; Ottevanger, P.B.; Bloemendal, H.J.; Koolen, S.L.W.; Mathijssen, R.H.J.; Boere, I.A.; Hamberg, P.; Huitema, A.D.R.; et al. Pharmacokinetic Boosting of Olaparib: A Randomised, Cross-over Study (PROACTIVE-Study). Eur. J. Cancer 2023, 194, 113346. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy Combined with Immunotherapy: The Dawn of Cancer Treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Subbarayan, R.; Srinivasan, D.; Balakrishnan, R.; Kumar, A.; Usmani, S.S.; Srivastava, N. DNA Damage Response and Neoantigens: A Favorable Target for Triple-Negative Breast Cancer Immunotherapy and Vaccine Development. Int. Rev. Cell Mol. Biol. 2024, 389, 104–152. [Google Scholar]
- Tang, J.-Y.; Ou-Yang, F.; Hou, M.-F.; Huang, H.-W.; Wang, H.-R.; Li, K.-T.; Fayyaz, S.; Shu, C.-W.; Chang, H.-W. Oxidative Stress-Modulating Drugs Have Preferential Anticancer Effects—Involving the Regulation of Apoptosis, DNA Damage, Endoplasmic Reticulum Stress, Autophagy, Metabolism, and Migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef]
- Zhou, H.; Gurley, E.C.; Jarujaron, S.; Ding, H.; Fang, Y.; Xu, Z.; Pandak, W.M.; Hylemon, P.B. HIV Protease Inhibitors Activate the Unfolded Protein Response and Disrupt Lipid Metabolism in Primary Hepatocytes. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 291, G1071–G1080. [Google Scholar] [CrossRef]
- Waibel, S.; Bissinger, R.; Bouguerra, G.; Abbès, S.; Lang, F. Ritonavir-Induced Suicidal Death of Human Erythrocytes. Basic. Clin. Pharmacol. Toxicol. 2016, 119, 51–57. [Google Scholar] [CrossRef]
- Wu, X.; Sun, L.; Zha, W.; Studer, E.; Gurley, E.; Chen, L.; Wang, X.; Hylemon, P.B.; Pandak, W.M.; Sanyal, A.J.; et al. HIV Protease Inhibitors Induce Endoplasmic Reticulum Stress and Disrupt Barrier Integrity in Intestinal Epithelial Cells. Gastroenterology 2010, 138, 197–209. [Google Scholar] [CrossRef]
- Zhang, S.; Carper, M.J.; Lei, X.; Cade, W.T.; Yarasheski, K.E.; Ramanadham, S. Protease Inhibitors Used in the Treatment of HIV + Induce β-Cell Apoptosis via the Mitochondrial Pathway and Compromise Insulin Secretion. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E925–E935. [Google Scholar] [CrossRef][Green Version]
- Lü, J.-M.; Jiang, J.; Jamaluddin, M.S.; Liang, Z.; Yao, Q.; Chen, C. Ginsenoside Rb1 Blocks Ritonavir-Induced Oxidative Stress and ENOS Downregulation through Activation of Estrogen Receptor-Beta and Upregulation of SOD in Human Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 294. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, X.; Weakley, S.M.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. The Soybean Isoflavonoid Equol Blocks Ritonavir-Induced Endothelial Dysfunction in Porcine Pulmonary Arteries and Human Pulmonary Artery Endothelial Cells. J. Nutr. 2010, 140, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Touzet, O.; Philips, A. Resveratrol Protects against Protease Inhibitor-Induced Reactive Oxygen Species Production, Reticulum Stress and Lipid Raft Perturbation. AIDS 2010, 24, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Hampson, L.; Maranga, I.O.; Masinde, M.S.; Oliver, A.W.; Batman, G.; He, X.; Desai, M.; Okemwa, P.M.; Stringfellow, H.; Martin-Hirsch, P.; et al. A Single-Arm, Proof-Of-Concept Trial of Lopimune (Lopinavir/Ritonavir) as a Treatment for HPV-Related Pre-Invasive Cervical Disease. PLoS ONE 2016, 11, e0147917. [Google Scholar] [CrossRef] [PubMed]
- Koolen, S.L.W.; Oostendorp, R.L.; Beijnen, J.H.; Schellens, J.H.M.; Huitema, A.D.R. Population Pharmacokinetics of Intravenously and Orally Administered Docetaxel with or without Co-administration of Ritonavir in Patients with Advanced Cancer. Br. J. Clin. Pharmacol. 2010, 69, 465–474. [Google Scholar] [CrossRef]
- Yu, H.; Janssen, J.M.; Sawicki, E.; van Hasselt, J.G.C.; de Weger, V.A.; Nuijen, B.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. A Population Pharmacokinetic Model of Oral Docetaxel Coadministered with Ritonavir to Support Early Clinical Development. J. Clin. Pharmacol. 2020, 60, 340–350. [Google Scholar] [CrossRef]
- Takahashi, S.; Karayama, M.; Takahashi, M.; Watanabe, J.; Minami, H.; Yamamoto, N.; Kinoshita, I.; Lin, C.-C.; Im, Y.-H.; Achiwa, I.; et al. Pharmacokinetics, Safety, and Efficacy of Trastuzumab Deruxtecan with Concomitant Ritonavir or Itraconazole in Patients with HER2-Expressing Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 5771–5780. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.; Durieux, V.; Auprih, M.; Fozza, A.; Dauby, N.; Cuccia, F.; Aspeslagh, S.; Verhaert, M.; Giaj-Levra, N. Systemic Treatment and Radiotherapy for Patients with Non-Small Cell Lung Cancer (NSCLC) and HIV Infection—A Systematic Review. Lung Cancer 2023, 178, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, M.; Marchetti, S.; Beijnen, J. Pharmacokinetics and Toxicities of Oral Docetaxel Formulations Co-Administered with Ritonavir in Phase I Trials. Clin. Pharmacol. 2021, 13, 21–32. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable Editing of a Target Base in Genomic DNA without Double-Stranded DNA Cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-Replace Genome Editing without Double-Strand Breaks or Donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Koblan, L.W.; Arbab, M.; Shen, M.W.; Hussmann, J.A.; Anzalone, A.V.; Doman, J.L.; Newby, G.A.; Yang, D.; Mok, B.; Replogle, J.M.; et al. Efficient C•G-to-G•C Base Editors Developed Using CRISPRi Screens, Target-Library Analysis, and Machine Learning. Nat. Biotechnol. 2021, 39, 1414–1425. [Google Scholar] [CrossRef]
- Justesen, U.S. Therapeutic Drug Monitoring and Human Immunodeficiency Virus (HIV) Antiretroviral Therapy. Basic. Clin. Pharmacol. Toxicol. 2006, 98, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U.S. Protease Inhibitor Plasma Concentrations in HIV Antiretroviral Therapy. Dan. Med. Bull. 2008, 55, 165–185. [Google Scholar]
| Refs. | Tumor Type | Cell Lines | In Vitro Range | In Vivo Range | Outcome |
|---|---|---|---|---|---|
| [9] | Ovarian cancer | MDAH-2774, SKOV-3 | 5–25 µM | NA | Inhibition of AKT, inhibition of migration, invasion, and induction of apoptosis |
| [10] | Breast cancer | MCF7, T47D, MDA-MB-231, MDA-MB-436 | 15–45 µM | 40 mg/kg daily for 52 days, IP | Inhibition of AKT, inhibition of migration, reduction in tumor growth, induction of apoptosis, S-phase cell-cycle arrest |
| [11] | Oral squamous cell carcinoma | NOK, SSC-25, Detroit 562 | 10 µM | NA | Inhibition of EMT and sensitization to IR |
| [12] | Prostate cancer | PC3 ALKBH3-KD | 1–50 µM | NA | Sensitization to alkylating agent methylmethane sulfonate |
| [13] | Kaposi sarcoma | KSIMM, HUVEC | 3–30 µM | 30 mg/kg daily for 15 days, IP | Inhibition of NF-κB, reduction in cell adhesion and tumor growth |
| [14] | Cervical intraepithelial neoplasia | W12, CIN612-7E | 10 µM | NA | Inhibition of AKT and down-regulation MMP-9 |
| [15] | Cervical intraepithelial neoplasia | CIN612–7E, CIN612–9E, NHEK, SiHa, CaSki | 10 µM | NA | Inhibition of invasion and down-regulation of MMP-2 and MMP-9 |
| [16] | Pancreatic cancer | BxPC-3, MIA PaCa-2, PANC-1 | 5–30 µM | NA | Inhibition of AKT, inhibition of invasion, cell-cycle arrest and induction of cell death |
| [17] | Head and neck carcinoma | HEP-2 | 20–2000 µM | 8 mg twice a day, 5 days a week, OG | Sensitization to IR and enhancement of IR-induced apoptosis |
| [18] | Chronic lymphocytic leukemia | Primary patient cells | 20 µM | NA | Inhibition of GLUT4 and inhibition of glucose uptake |
| [19] | Fibrosarcoma, Ewing’s sarcoma, myeloma | HT1080, RD-ES, AMO-1 | 0–15 µM | NA | Induction of ER stress and sensitization of bortezomib-resistant cells |
| [21] | Adult T-cell leukemia | MT-2, MT-4, C5/MJ SLB-1, HUT-102, MT-1, ED-40515 | 0–40 µM | 30 mg/kg daily for 30 days, IP | Inhibition of NF-κB, inhibition of cell growth and induction of apoptosis |
| [22] | Cervical cancer | HeLa | 0–50 µM | NA | Enhancement the anticancer activity of cisplatin |
| NCT Number | Conditions | Interventions | Study Status | Phase | Response Rate |
|---|---|---|---|---|---|
| NCT03150368 | Advanced Solid Tumors | ModraDoc006/r | Completed | Phase1 | NA |
| NCT01173913 | Cancer | ModraDoc001 10 mg capsules ModraDoc003 10 mg tablets and ModraDoc004 10/50 mg ModraDoc006 10 mg tablet | Completed | Phase1 | PR 11.5% SD 23% |
| NCT03136640 | Castration-resistant Prostate Cancer | ModraDoc006/r | Completed | Phase1 | PR 10% |
| NCT03890744 | Metastatic Breast Cancer Recurrent Breast Cancer | ModraDoc006/r | Completed | Phase2 | NA |
| NCT03383692 | Neoplasm Metastasis | DS-8201° Ritonavir Itraconazole | Completed | Phase1 | CR 0% PR 10% SD 7% PD 0% |
| NCT02770378 | Glioblastoma | Temozolomide Aprepitant Minocycline Disulfiram Celecoxib Sertraline Captopril Itraconazole Auranofin Ritonavir | Completed | Phase1 Phase2 | SD 60% PD 40% |
| NCT04028388 | Prostate Cancer Metastatic Castration-resistant Prostate Cancer | Docetaxel in Parenteral Dosage Form ModraDoc006/r | Completed | Phase2 | CR 0% PR 44.1% SD 44.1% PD 11.8% |
| NCT00003008 | Sarcoma | Indinavir sulfate Nelfinavir mesylate Paclitaxel Ritonavir Saquinavir mesylate | Completed | Phase2 | NA |
| NCT03147378 | Solid Tumor, Adult | ModraDoc006/r | Completed | Phase1 | NA |
| NCT01124812 | Non-Small Cell Lung Cancer | 131I-L19SIP Radioimmunotherapy in Combination with External Beam Radiotherapy and Concurrent Chemotherapy | Terminated | Phase1 | NA |
| NCT03066154 | Prostatic Neoplasms | ModraDoc/r Androgen deprivation therapy Radiation therapy | Terminated | Phase1 | NA |
| NCT05242926 | Solid Tumor, Adult | ModraDoc006/r | Terminated | Phase1 | NA |
| NCT05084456 | Solid Tumor, Adult Impaired Liver Function | ModraDoc006/r | Withdrawn | Phase1 | NA |
| NCT00637637 | Cancer | Indinavir sulfate Ritonavir Radiation therapy | Unknown | Phase2 | NA |
| NCT06710990 | Advanced Breast Cancer | SHR-A1811 Ritonavir Itraconazole | Recruiting | Phase1 | NA |
| NCT05150691 | HER2-positive Advanced Solid Tumor | DB-1303/BNT323 Pertuzumab Injection Ritonavir Itraconazole | Recruiting | Phase1 Phase2 | NA |
| NCT06428045 | High-Grade Glioma | Abacavir Lamivudine Ritonavir Temozolomide Focal Radiotherapy | Recruiting | Phase1 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomella, S.; Ferraro, E.; Marampon, F.; Barillari, G. Ritonavir and DNA Damage: A New Perspective on an Old Drug. Appl. Sci. 2025, 15, 12053. https://doi.org/10.3390/app152212053
Pomella S, Ferraro E, Marampon F, Barillari G. Ritonavir and DNA Damage: A New Perspective on an Old Drug. Applied Sciences. 2025; 15(22):12053. https://doi.org/10.3390/app152212053
Chicago/Turabian StylePomella, Silvia, Erika Ferraro, Francesco Marampon, and Giovanni Barillari. 2025. "Ritonavir and DNA Damage: A New Perspective on an Old Drug" Applied Sciences 15, no. 22: 12053. https://doi.org/10.3390/app152212053
APA StylePomella, S., Ferraro, E., Marampon, F., & Barillari, G. (2025). Ritonavir and DNA Damage: A New Perspective on an Old Drug. Applied Sciences, 15(22), 12053. https://doi.org/10.3390/app152212053







