Current Models of Transcatheter Aortic Valves: Comparative Analysis of Design, Clinical Outcomes and Development Prospects
Abstract
1. Introduction
2. Design of Modern TAVI Prostheses
2.1. Nitinol Frame: Properties and Advantages
2.2. Balloon-Expandable Valves: Alternative Materials and Designs
2.3. Valve Leaflets from Xenopericardial Tissue
2.4. Skirts and Protection Against Paravalvular Leak
3. Review of Current Models on the Global Market
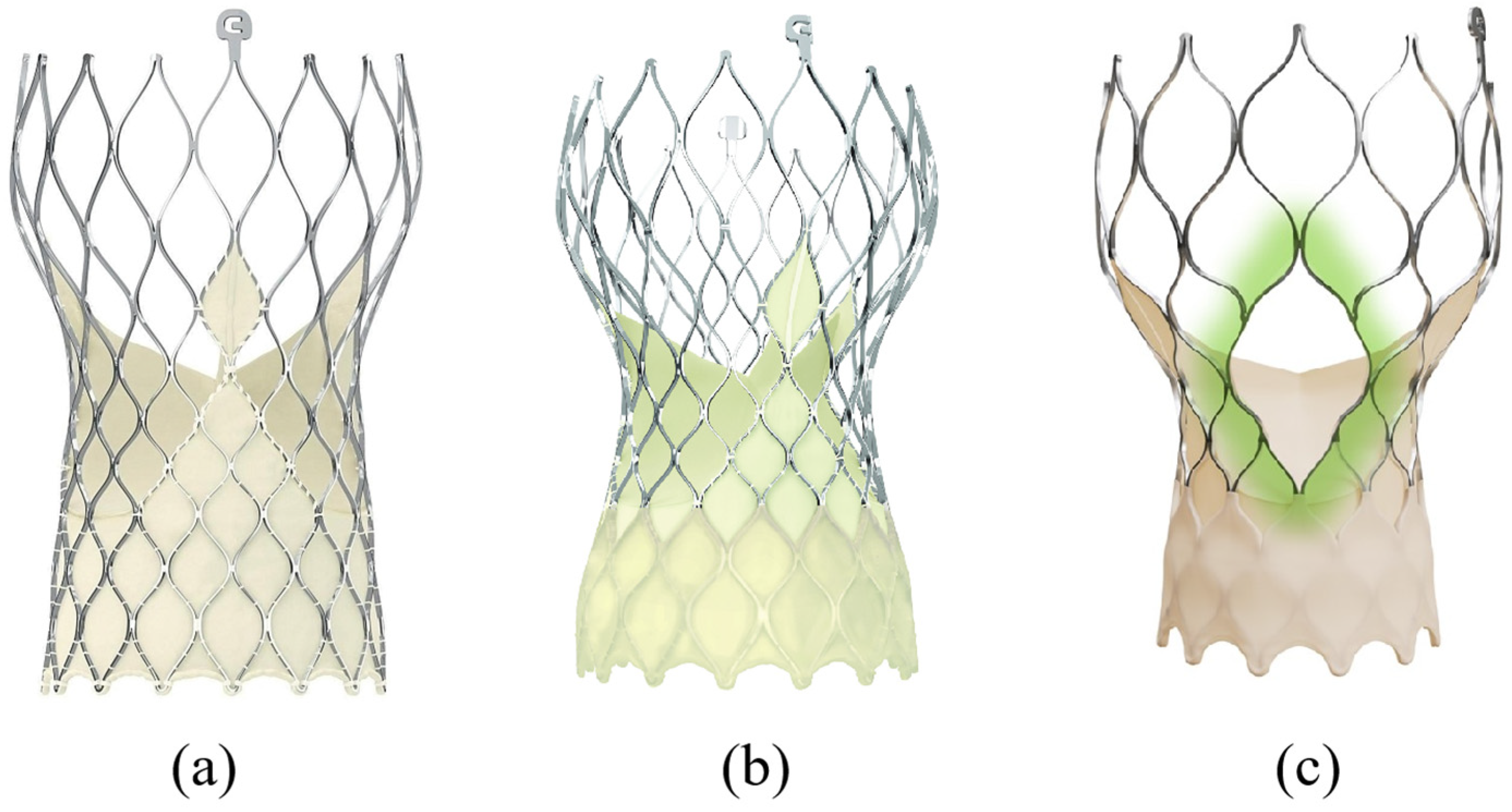
3.1. Evolut R/Pro/FX/FX+ (Medtronic—USA)
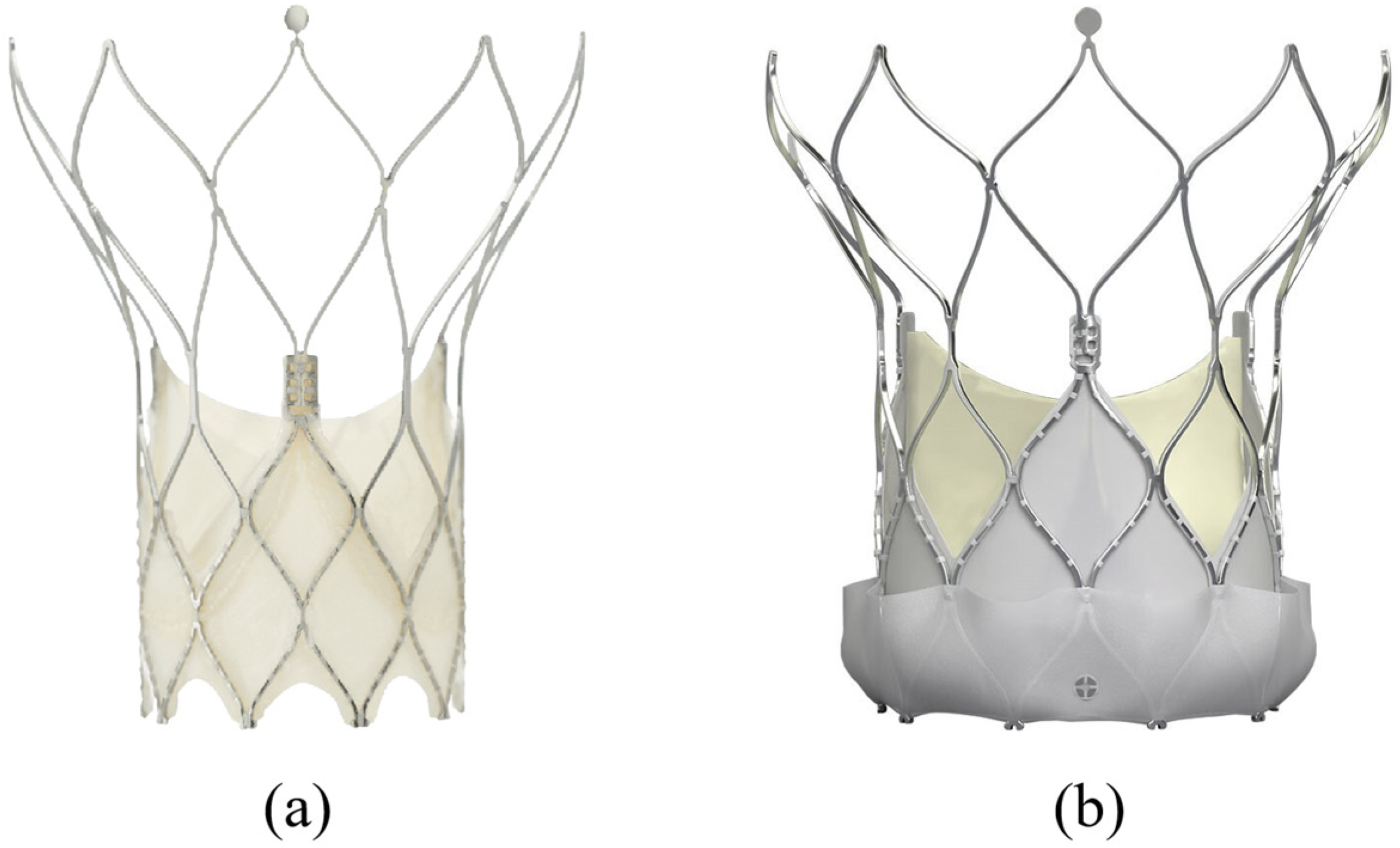
3.2. Portico/Navitor (Abbott, USA)
3.3. ACURATE Neo2 (Boston Scientific, USA)
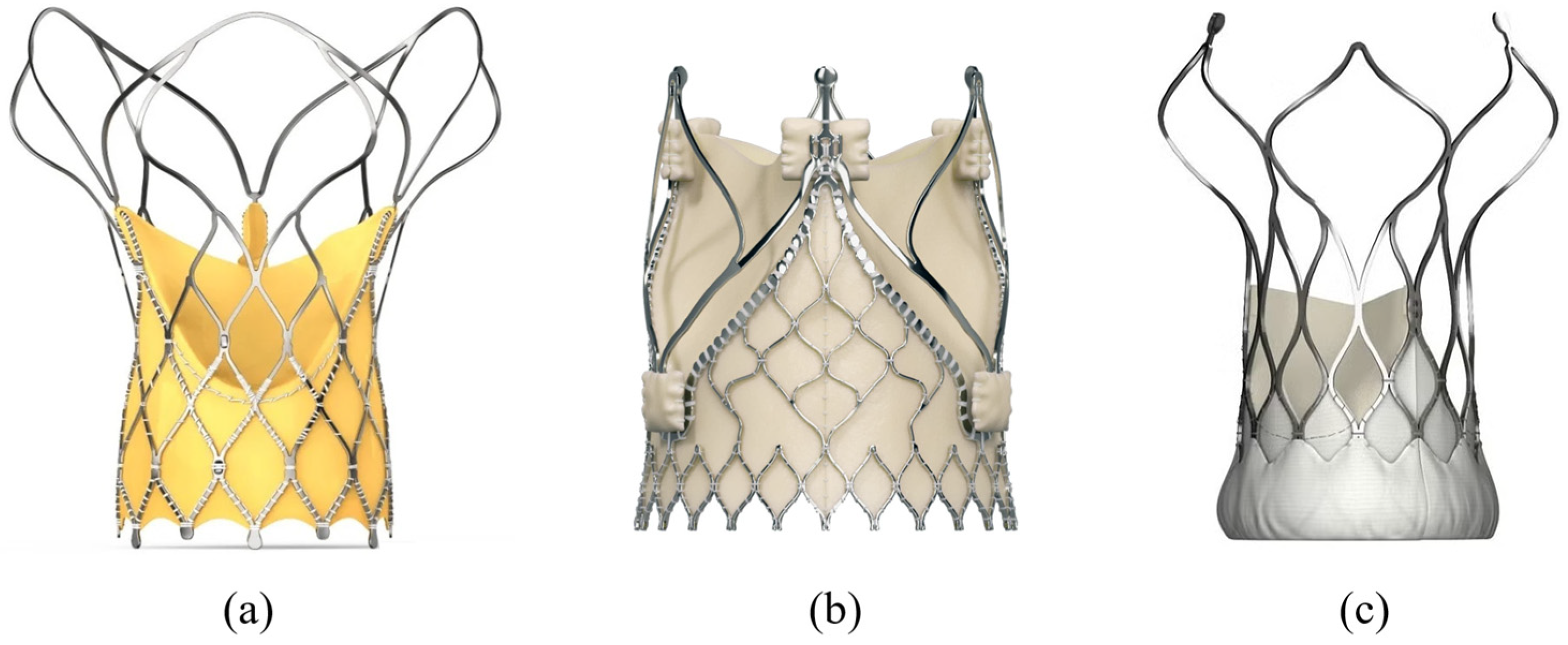
3.4. Biovalve (Biotronik, Germany)
3.5. Optimum TAV (Thubrikar Aortic Valve, Inc., USA)
3.6. Hydra, J-Valve, VitaFlow (Asia)
3.7. Analysis of Clinical and Hemodynamic Outcomes Using Different Transcatheter Valve Models
- PPM occurs more frequently in models with intra-annular leaflet placement (e.g., Portico), where the effective orifice area is limited by the size of the aortic annulus. In contrast, prostheses with supra-annular configuration (Evolut, Neo2, Navitor) provide a larger orifice and significantly reduce the risk of PPM, especially in patients with a small annulus [23].
- 30-day mortality is presented in the range (lowest 1.1%, highest 2.0%)
- ACURATE Neo2—1.1% [29]
- Navitor—1.2% [28]
- Evolut FX—1.4% [44]
- VitaFlow—1.8% [41]
- Portico—~1.8% [43]
- Optimum TAV—~2.0% (preliminary) [33]
- J-Valve—about 2.0% [39]
- Biovalve—No robust mortality data available (device in early-stage clinical trials) [31].
4. Comparative Analysis of Prostheses
5. Analysis by Key Parameters
5.1. Delivery Profile and Repositioning
5.2. Sealing Systems and PVL Protection
5.3. Access to Coronary Arteries After Implantation
6. Prospects and Development Directions
- Reducing the profile to ≤14 Fr without compromising strength and structural stability.
- Using new hybrid materials (including nanocoatings) in catheter sheaths to reduce friction and vascular wall trauma.
- Developing universal introducers with adaptive diameter and self-sealing mechanisms [12].
- Modifying xenopericardial tissues by treating tissue leaflets with anti-calcification solutions (e.g., glycerol or new-generation aldehyde stabilizers) to prevent calcium salt deposition [47].
- Developing bioinert and biocompatible coatings for metal frames to reduce the inflammatory response.
- Implementing structures with shape recovery after load (shape memory alloys) to compensate for cyclic deformations.
- Asymptomatic aortic stenosis—active clinical evaluation of the possibility of implantation before the manifestation of clinical symptoms to prevent sudden death [1].
- Patients younger than 65 years—for patients with confirmed durability of new generations of prostheses, TAVI could become an alternative to surgical intervention in the young [6].
- Aortic regurgitation—despite technical difficulties in fixing the prosthesis without calcium, new models (e.g., J-Valve, JenaValve) show promising results.
- Re-implantation (Valve-in-Valve)—experience is growing in replacing old bioprostheses with new-generation TAVI prostheses with high positioning accuracy and good hemodynamics [48].
6.1. The Challenge of Future Coronary Access and Valve-in-Valve Procedures
- As TAVI expands into younger, lower-risk patient populations with longer life expectancy, the long-term management of coronary artery disease and structural valve degeneration becomes paramount. The choice of the initial TAVI prosthesis is now recognized as a critical factor that can significantly impact the feasibility and safety of future cardiac interventions, specifically coronary angiography/percutaneous coronary intervention (PCI) and valve-in-valve (ViV) TAVI procedures.
- A key anatomical consideration is the relationship between the TAVI frame and the coronary ostia. Prostheses with tall, closed-frame designs (e.g., the Evolut series) can extend above the sinotubular junction, potentially creating a mechanical barrier that complicates or prevents selective engagement of the coronary arteries. This “coronary shadowing” effect is exacerbated in patients with shallow sinuses of Valsalva or low coronary take-off. In contrast, next-generation devices are increasingly incorporating short-frame architectures with large, open cells (e.g., the Navitor system) specifically designed to facilitate future coronary access. These designs aim to provide unimpeded pathways for coronary catheters, a feature that is transitioning from a minor advantage to an essential design criterion for younger patients [45,48].
- Similarly, the initial valve choice directly influences the outcomes of future ViV procedures for failed bioprostheses. A tall, bulky initial valve can reduce the effective orifice area after ViV implantation, leading to high gradients and patient–prosthesis mismatch. Furthermore, the risk of coronary obstruction during a ViV TAVI is substantially higher when the leaflets of the failed bioprosthesis are mounted externally (as in some older surgical valves) and are pushed against the coronary ostia by the new transcatheter valve. Therefore, pre-emptive consideration of a future ViV scenario is crucial. Selecting a contemporary TAVI prosthesis with a low-profile, intra-annular, or supra-annular leaflet position can preserve vital anatomical space and minimize risks for subsequent interventions, effectively “future-proofing” the patient’s treatment pathway.
6.2. Novel Biomaterials and the Quest for Long-Term Durability
- While current xenopericardial tissues provide excellent medium-term results, long-term structural valve deterioration (SVD) remains a concern, especially for patients under 65 years of age. The pursuit of enhanced long-term durability is driving intensive research into novel biomaterials beyond conventional glutaraldehyde-fixed pericardium. Insights from leading industry forums, such as the Medtec China 2025 exhibition, highlight several promising directions. These include advanced thermoplastic polyurethanes (TPUs) like the NEUSoft™ platform (Avient Corporation, Shanghai, China), which are engineered for superior in vivo stability and fatigue resistance, making them candidates for durable leaflet applications [49]. Furthermore, ultra-pure, biomedical-grade gelatin (e.g., X-Pure® Gelatin, Rousselot, Ghent, Belgium) and other biocompatible, absorbable polymers are being investigated as scaffolds for tissue-engineered valves that could potentially allow for host remodeling and growth [50].
- Parallel to material science, advancements in high-precision additive manufacturing (3D printing, BMF Precision Tech Inc., Boston, MA, USA) and micro–nano machining are enabling the fabrication of complex, patient-specific valve geometries with unprecedented accuracy, potentially optimizing hemodynamic performance and reducing thrombogenicity [51]. The convergence of these novel materials and advanced manufacturing technologies represents the next frontier in prosthetic valve development. The goal is to create a leaflet that is not only thromboresistant and durable but also capable of mitigating calcification and adapting to the physiological environment better than current bioprostheses. While these technologies are not yet specified for clinical use in TAVI and face significant regulatory hurdles, they hold the potential to achieve lifelong durability without the need for reintervention, a critical step for the future of valve therapy.
7. Conclusions
- Modern xenopericardial TAVI prostheses with nitinol frames provide a high degree of safety, functionality, and long-term hemodynamic efficacy.
- Most new-generation models (Evolut FX+, Navitor, ACURATE Neo2) demonstrate a significant reduction in paravalvular leakage, the possibility of precise positioning, and reliable access to coronary arteries.
- Thanks to improved delivery systems and reduced profiles, the number of patients eligible for the procedure is expanding.
- The main directions for further development include minimizing catheter profiles, extending the lifespan of biological tissues, and bioengineering solutions for adaptation to different anatomies and heart conditions.
- TAVI technologies are already transforming cardiac surgery, and their widespread use in younger patient categories, as well as in situations without calcification previously considered contraindications, is expected in the coming years.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOA | Effective Orifice Area |
| Fr | French (catheter size unit) |
| PCI | Percutaneous Coronary Intervention |
| PPI | Permanent Pacemaker Implantation |
| PPM | Patient–Prosthesis Mismatch |
| PVL | Paravalvular Leak |
| SAVR | Surgical Aortic Valve Replacement |
| SVD | Structural Valve Deterioration |
| TAVI | Transcatheter Aortic Valve Implantation |
| ViV | Valve-in-Valve |
References
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar] [CrossRef]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Cribier, A. Development of transcatheter aortic valve implantation (TAVI): A 20-year odyssey. Arch. Cardiovasc. Dis. 2012, 105, 146–152. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Neragi-Miandoab, S. Recently patented transcatheter aortic valves in clinical trials. Recent Pat. Cardiovasc. Drug Discov. 2013, 8, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Tchetche, D.; Van Mieghem, N.M. New-generation TAVI devices: Description and specifications. EuroIntervention 2014, 10 (Suppl. U), U90–U100. [Google Scholar] [CrossRef]
- Kim, W.K.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schäfer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H.; et al. The SAVI-TF registry: 1-year outcomes of the European post-market registry using the ACURATE neo™ transcatheter heart valve under real-world conditions in 1000 patients. Catheter. Cardiovasc. Interv. 2018, 91, 1290–1297. [Google Scholar] [CrossRef]
- Van Linden, A.; Blumenstein, J.; Möllmann, H.; Kim, W.K.; Hamm, C.W.; Walther, T. The ACURATE neo transcatheter heart valve: A comprehensive review. Expert Rev. Med. Devices 2018, 15, 245–252. [Google Scholar]
- van Gils, L.; Tchetche, D.; Lhermusier, T.; Abawi, M.; de Jaegere, P.P.; van Mieghem, N.M. TAVI with current CE-marked devices: Strategies for optimal sizing and valve delivery. EuroIntervention 2016, 12, Y22–Y27. [Google Scholar] [CrossRef]
- Kim, W.K.; Schäfer, U.; Tchetche, D.; Nef, H.; Arnold, M.; Avanzas, P.; Rudolph, T.K.; Scholtz, S.; Schofer, N.; Schirmer, J.; et al. The ACURATE neo Transcatheter Heart Valve: A Comprehensive Analysis of Predictors of Procedural Outcome. JACC Cardiovasc. Interv. 2018, 11, 1721–1729. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Wu, Y.; Yang, Y.; Xu, L.; Chen, M.; Wang, J.; Rocha, E.; O’Neill, W.W.; Chen, M. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc. Imaging 2016, 9, 1145–1158. [Google Scholar] [CrossRef]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef]
- Webb, J.G.; Bax, J.J.; De Bonis, M.; Delgado, V.; Tamburino, C.; Van Mieghem, N.M.; Windecker, S.; Grube, E.; Kappetein, A.P.; Mack, M.J.; et al. Transcatheter aortic valve implantation with the Edwards SAPIEN 3 valve: Impact of balloon-expandable technology on clinical outcomes. EuroIntervention 2020, 16, e757–e765. [Google Scholar]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Wenaweser, P.; Gerckens, U.; Tamburino, C.; Bosmans, J.; Bleiziffer, S.; Blackman, D.; Schäfer, U.; Müller, R.; Sievert, H.; et al. Treatment of aortic stenosis with a self-expanding transcatheter valve: The International Multi-centre ADVANCE Study. Eur. Heart J. 2014, 35, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wei, B.; Chen, L.; Yang, L.; Zheng, C.; Wang, Y. Degeneration mechanisms and advancements in optimization for preparation and crosslinking strategy of pericardium-based bioprosthetic heart valves. Acta Biomater. 2025, 201, 51–74. [Google Scholar] [CrossRef] [PubMed]
- *ISO 5840-3:2021*; Cardiovascular Implants—Cardiac Valve Prostheses—Part 3: Heart Valve Substitutes Implanted by Transcatheter Techniques. International Organization for Standardization: Geneva, Switzerland, 2021.
- Zamorano, J.L.; Badano, L.P.; Bruce, C.; Chan, K.L.; Gonçalves, A.; Hahn, R.T.; Keane, M.G.; La Canna, G.; Monaghan, M.J.; Nihoyannopoulos, P.; et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. J. Am. Soc. Echocardiogr. 2011, 24, 937–965. [Google Scholar] [CrossRef]
- Hahn, R.T.; Pibarot, P.; Stewart, W.J.; Weissman, N.J.; Gopalakrishnan, D.; Keane, M.G.; Anwaruddin, S.; Wang, Z.; Bilsker, M.; Lindman, B.R.; et al. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: A longitudinal study of echocardiography parameters in cohort A of the PARTNER trial (placement of aortic transcatheter valves). J. Am. Coll. Cardiol. 2013, 61, 2514–2521. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Windecker, S.; Manoharan, G.; Lancellotti, P.; Tamburino, C.; Kornowski, R.; Thiele, H.; Danenberg, H.; Fiorina, C.; Scholtz, W.; et al. Three-Year Outcomes with a Supra-Annular, Self-Expanding Bioprosthesis and a Pericardial Wrap-The FORWARD PRO Study. Catheter. Cardiovasc. Interv. 2025, 105, 577–587. [Google Scholar] [CrossRef]
- Généreux, P.; Head, S.J.; Hahn, R.; Daneault, B.; Kodali, S.; Williams, M.R.; van Mieghem, N.M.; Alu, M.C.; Serruys, P.W.; Kappetein, A.P.; et al. Paravalvular leak after transcatheter aortic valve replacement: The new Achilles’ heel? A comprehensive review of the literature. J. Am. Coll. Cardiol. 2013, 61, 1125–1136. [Google Scholar] [CrossRef]
- Zahr, F.; Song, H.K.; Chadderdon, S.M.; Gada, H.; Mumtaz, M.A.; Byrne, T.; Rovin, J.; Kipperman, R.M.; Spargias, K.; Kereiakes, D.J.; et al. Three-Year Outcomes from the Evolut Low Risk TAVR Trial. JACC Cardiovasc. Interv. 2024, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lenders, G.D.; Collas, V.; Hernandez, J.M.; Legrand, V.; Danenberg, H.; den Heijer, P.; Rodrigus, I.; Paelinck, B.P.; Vrints, C.J.; Bosmans, J.M. Depth of valve implantation, conduction disturbances and pacemaker implantation with CoreValve and CoreValve Accutrak system: An observational study. Int. J. Cardiol. 2014, 176, 771–775. [Google Scholar] [CrossRef]
- van der Boon, R.M.; Nuis, R.J.; Van Mieghem, N.M.; Jordaens, L.; Rodés-Cabau, J.; van Domburg, R.T.; Serruys, P.W.; Anderson, R.H.; de Jaegere, P.P. New conduction abnormalities after TAVI--frequency and causes. Nat. Rev. Cardiol. 2012, 9, 454–463. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Reardon, M.J.; Kleiman, N.S.; Adams, D.H.; Hébert, J.M.; Yakubov, S.J.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; Hermiller, J.B., Jr.; Chetcuti, S.; et al. 30-Day Outcomes of the Navitor Transcatheter Heart Valve in the NAVIGATE TAVR Study. JACC Cardiovasc. Interv. 2023, 16, 1234–1245. [Google Scholar] [CrossRef]
- Bharucha, A.H.; Sondergaard, L.; Bieliauskas, G.; De Backer, O.; Wong, I.; Tchetche, D.; Kim, W.K.; Nissen, H.; Kofoed, K.F.; Scholtz, W.; et al. Early Safety and Efficacy of the ACURATE neo2 Transcatheter Heart Valve: 30-Day Outcomes from the NEOPRO 2 Registry. Am. J. Cardiol. 2024, 195, 1–8. [Google Scholar] [CrossRef]
- BIOTRONIK Announces Successful First Implantations of Resheathable Transcatheter Aortic Valve. Building Better Healthcare. 2023. Available online: https://www.buildingbetterhealthcare.com/news/article_page/BIOTRONIK_announces_successful_first_implantations_of_Resheathable_Transcatheter_Aortic_Valve/206321 (accessed on 1 February 2025).
- Safety and Performance Outcomes Published for Biotroniks Biovalve Tavr Device. CI Today. 2024. Available online: https://citoday.com/news/safety-and-performance-outcomes-published-for-biotroniks-biovalve-tavr-device (accessed on 1 February 2025).
- Agma, H.U.; Khan, M.Z.; Khan, M.U.; Kaur, M.; Khera, R.; Balla, S. From an Unfolding Emergency Treatment to a Universal Shift in Therapy: The Story of Transcatheter Aortic Valve Implantation (TAVI). Rev. Cardiovasc. Med. 2022, 23, 349. [Google Scholar] [CrossRef]
- ResearchGate. Transcatheter Aortic Valve Replacement Technique and Current Approaches. 2023. Available online: https://www.researchgate.net/publication/375324031_Transcatheter_Aortic_Valve_Replacement_Technique_and_Current_Approaches (accessed on 1 February 2025).
- Zhao, Z.G.; Jilaihawi, H.; Feng, Y.; Chen, M. Transcatheter aortic valve implantation in bicuspid anatomy. Nat. Rev. Cardiol. 2015, 12, 123–128. [Google Scholar] [CrossRef]
- Kim, W.K.; Liebetrau, C.; Fischer-Rasokat, U.; Renker, M.; Rolf, A.; Doss, M.; Möllmann, H.; Hamm, C.W.; Walther, T. Transfemoral aortic valve implantation using a self-expanding transcatheter heart valve without pre-dilation. Int. J. Cardiol. 2017, 243, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.; Toggweiler, S.; Zuber, M.; Stortecky, S.; Huber, C.; Nietlispach, F.; Khattab, A.A.; Meier, B.; Wenaweser, P. Supra-annular sizing for transcatheter valve implantation in bicuspid aortic stenosis. Postep. Kardiol. Interwencyjnej 2018, 14, 187–190. [Google Scholar] [CrossRef]
- Aidietis, A.; Srimahachota, S.; Dabrowski, M.; Bilkis, V.; Buddhari, W.; Cheung, G.S.; Nair, R.K.; Mussayev, A.A.; Mattummal, S.; Chandra, P.; et al. 30-Day and 1-Year Outcomes with HYDRA Self-Expanding Transcatheter Aortic Valve: The Hydra CE Study. J. Am. Coll. Cardiol. Interv. 2022, 15, 93–104. [Google Scholar] [CrossRef]
- Seiffert, M.; Conradi, L.; Baldus, S.; Schirmer, J.; Knap, M.; Blankenberg, S.; Reichenspurner, H.; Diemert, P.; Treede, H. Transapical implantation of a second-generation transcatheter heart valve in patients with noncalcified aortic regurgitation. JACC Cardiovasc. Interv. 2013, 6, 590–597. [Google Scholar] [CrossRef]
- Treede, H.; Mohr, F.W.; Baldus, S.; Rastan, A.; Ensminger, S.; Arnold, M.; Bucerius, J.; Bechtel, M.; Schuler, G.; Mack, M.; et al. Transapical transcatheter aortic valve implantation using the JenaValve™ system: 30-day results of a multicentre registry. Eur. J. Cardiothorac. Surg. 2012, 41, e131–e138. [Google Scholar] [CrossRef] [PubMed]
- JenaValve Technology. Company Information, 2024. Available online: https://www.jenavalve.com/ (accessed on 1 February 2025).
- MicroPort CardioFlow. VitaFlow™ TAVI System Clinical Results, 2023. Available online: https://www.microport.com/cardioflow/en/product/vitaflow (accessed on 1 February 2025).
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef]
- Sondergaard, L.; De Backer, O.; Kofoed, K.F.; Jilaihawi, H.; Ruile, P.; Wong, I.; Mathiassen, O.N.; Olsen, P.S.; Nielsen-Kudsk, J.E. Thirty-Day and One-Year Outcomes for the Navitor Transcatheter Heart Valve in the International PORTICO NG Study. EuroIntervention 2024, 20, e122–e131. [Google Scholar] [CrossRef]
- Yashima, F.; Watanabe, Y.; Kozuma, K.; Hioki, H.; Arai, T.; Kataoka, A.; Shimizu, H.; Yamamoto, M.; Yamanaka, F.; Nara, Y.; et al. In-Hospital Outcomes of Evolut FX vs. Evolut PRO+ in the J-TVT Registry. Am. J. Cardiol. 2025, 187, 1–9. [Google Scholar] [CrossRef]
- Merdler, I.; Ben-Shoshan, J.; Danenberg, H.; Orvin, K.; Assali, A.; Vaknin-Assa, H.; Kornowski, R. Early Experience with the Evolut FX versus Evolut PRO+ Transcatheter Aortic Valve Systems. Cardiovasc. Revasc. Med. 2023, 56, 1–7. [Google Scholar] [CrossRef]
- Panagides, V.; Tang, G.H.L.; Michev, I.; Latib, A. Review of the Evolut PRO and FX Systems for Transcatheter Aortic Valve Implantation. Expert Rev. Med. Devices 2022, 19, 889–898. [Google Scholar] [CrossRef]
- Shi, S.; Hu, M.; Peng, X.; Cheng, C.; Feng, S.; Pu, X.; Yu, X. Double crosslinking decellularized bovine pericardium of dialdehyde chondroitin sulfate and zwitterionic copolymer for bioprosthetic heart valves with enhanced antithrombogenic, anti-inflammatory and anti-calcification properties. J. Mater. Chem. B 2024, 14, 3417–3435. [Google Scholar] [CrossRef] [PubMed]
- Willson, A.B.; Rodes-Cabau, J.; Wood, D.A.; Leipsic, J.; Cheung, A.; Toggweiler, S.; Binder, R.K.; Freeman, M.; DeLarochelliere, R.; Moss, R.; et al. Transcatheter aortic valve replacement with the St. Jude Medical Portico valve: First-in-human experience. J. Am. Coll. Cardiol. 2012, 60, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Hu, C. NEUSoft™ Medical Thermoplastic Polyurethane for In-Vivo solutions and Lubricious Catheters. In Proceedings of the Medtec China 2025 Conference Program, Shanghai, China, 24–26 September 2025. [Google Scholar]
- Vervust, T.; Wen, Z. Advancing Biomedical Innovation with X-Pure Ultra-Pure Gelatin. In Proceedings of the Medtec China 2025 Conference Program, Shanghai, China, 24–26 September 2025. [Google Scholar]
- Xing, Y. The Applications of Ultra-high Precision 3D Printing Technology and Materials in the Medical Industry. In Proceedings of the Medtec China 2025 Conference Program, Shanghai, China, 24–26 September 2025. [Google Scholar]


| Model | Manufacturer | Profile (Fr) | Repositioning | Sealing System | Leaflet Position |
|---|---|---|---|---|---|
| Evolut FX+ | Medtronic | 14 | Yes | Pericardial skirt | Supra-annular |
| Navitor | Abbott | 14 | Yes (Full) | NaviSeal active cuff | Intra-annular |
| ACURATE Neo2 | Boston Scientific | 14–15 | Partial | Int. and Ext. skirts | Supra-annular |
| Portico | Abbott | ~15 * | Yes (Full) | (Predecessor to Navitor) | Intra-annular |
| VitaFlow | MicroPort | 16–18 | Yes | Double-layer pericardium | Supra-annular |
| Hydra | SMT | 18 | Yes | Pericardial skirt | Supra-annular |
| J-Valve | JenaValve | ~18 * | No ** | Anchors (non-calcium dep.) | Intra-annular |
| Biovalve | Biotronik | ≤16 | Partial | Soft apposition | N/A (Trials) |
| Optimum TAV | Thubrikar Aortic | 16 | No | Pressure compensation | Intra-annular |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozyr, K.; Alexander, B.-P.; Krestyaninov, O.; Sharifulin, R.; Zalesov, A.; Mochalova, A.; Tsaroev, B.; Tamkovich, S. Current Models of Transcatheter Aortic Valves: Comparative Analysis of Design, Clinical Outcomes and Development Prospects. Appl. Sci. 2025, 15, 11997. https://doi.org/10.3390/app152211997
Kozyr K, Alexander B-P, Krestyaninov O, Sharifulin R, Zalesov A, Mochalova A, Tsaroev B, Tamkovich S. Current Models of Transcatheter Aortic Valves: Comparative Analysis of Design, Clinical Outcomes and Development Prospects. Applied Sciences. 2025; 15(22):11997. https://doi.org/10.3390/app152211997
Chicago/Turabian StyleKozyr, Konstantin, Bogachev-Prokophiev Alexander, Oleg Krestyaninov, Ravil Sharifulin, Anton Zalesov, Alexandra Mochalova, Bashir Tsaroev, and Svetlana Tamkovich. 2025. "Current Models of Transcatheter Aortic Valves: Comparative Analysis of Design, Clinical Outcomes and Development Prospects" Applied Sciences 15, no. 22: 11997. https://doi.org/10.3390/app152211997
APA StyleKozyr, K., Alexander, B.-P., Krestyaninov, O., Sharifulin, R., Zalesov, A., Mochalova, A., Tsaroev, B., & Tamkovich, S. (2025). Current Models of Transcatheter Aortic Valves: Comparative Analysis of Design, Clinical Outcomes and Development Prospects. Applied Sciences, 15(22), 11997. https://doi.org/10.3390/app152211997







