From Vines to Ecosystems: Understanding the Ecological Effects of Grapevine Leafroll Disease
Abstract
1. Introduction
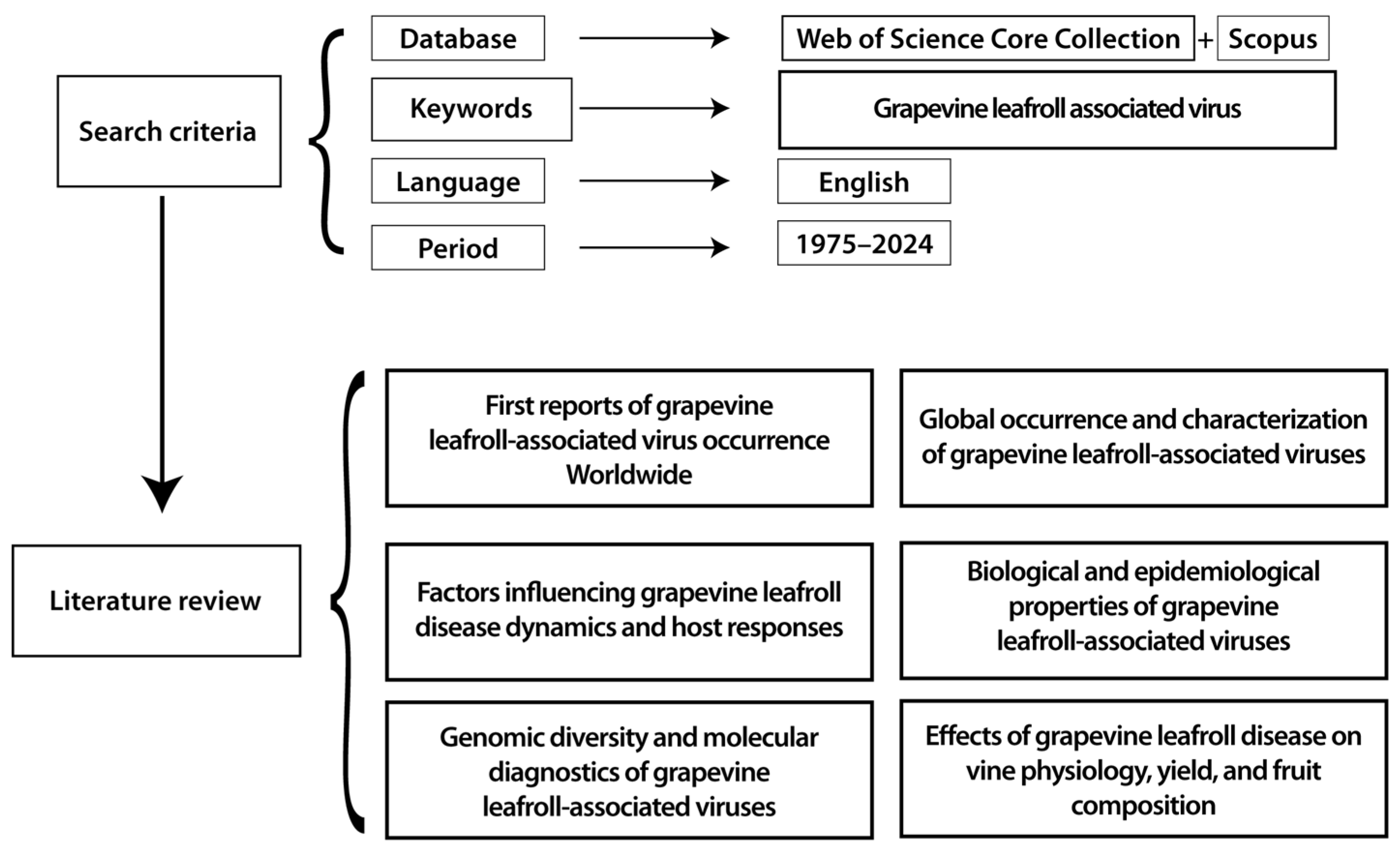
2. Materials and Methods
3. Results
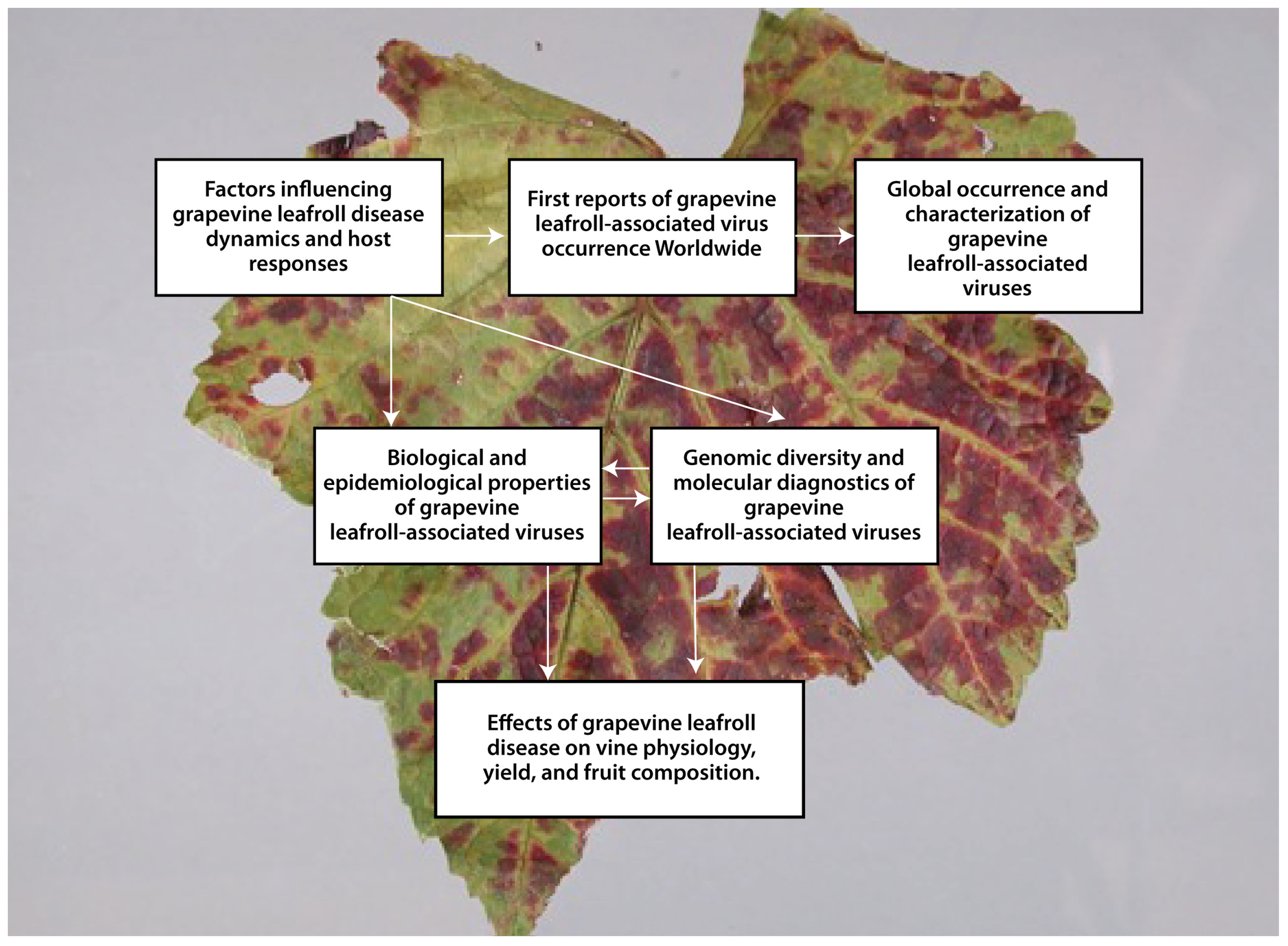
3.1. First Reports of Grapevine Leafroll-Associated Virus Occurrence Worldwide
3.2. Global Occurrence and Characterization of Grapevine Leafroll-Associated Viruses
3.3. Factors Influencing Grapevine Leafroll Disease Dynamics and Host Responses
3.3.1. Interaction Between GLRaV-3 Infection and Abiotic Stress Tolerance
3.3.2. Effectiveness of Spatial Roguing in Virus Incidence Reduction
3.3.3. Seasonal Patterns of Virus Detection and Distribution
3.4. Biological and Epidemiological Properties of Grapevine Leafroll-Associated Viruses
3.4.1. Characterization of GLRaV-2-H4 Isolate
3.4.2. Use of Foliar Symptoms for Field Identification of GLRaV-3
3.4.3. Seasonal Progress and Tissue Distribution of GLRaV-3 in Different Cultivars
3.4.4. Transmission of Leafroll-Associated Viruses
3.5. Genomic Diversity and Molecular Diagnostics of Grapevine Leafroll-Associated Viruses
3.6. Effects of Grapevine Leafroll Disease on Vine Physiology, Yield, and Fruit Composition
4. Discussion
4.1. First Reports of Grapevine Leafroll-Associated Virus Occurrence Worldwide
4.2. Global Occurrence and Characterization of Grapevine Leafroll-Associated Viruses
4.2.1. Regional Patterns and Epidemiological Contexts
- (1)
- Vector ecology plays a dominant role. The abundance and species composition of mealybugs and soft scales strongly correlate with disease prevalence, with warmer climates favoring rapid population growth and year-round transmission cycles.
- (2)
- Propagation practices are a second determinant. Regions relying heavily on uncertified or locally propagated plant material tend to accumulate mixed infections over time.
- (3)
- Climatic constraints, particularly cold winters or high summer temperatures, can suppress both vectors and virus survival, as seen in Canada and Tunisia.
- (4)
- Regulatory and management frameworks, including certification schemes, monitoring programs, and coordinated vine removal, further modulate transmission dynamics, as illustrated by the contrasting outcomes between New York and Washington State.
4.2.2. Genetic Diversity and Its Implications
4.2.3. Management Challenges and Strategies
4.3. Factors Influencing Grapevine Leafroll Disease Dynamics and Host Responses
4.3.1. Interaction Between GLRaV-3 Infection and Salt Stress Tolerance
4.3.2. Effectiveness of Spatial Roguing in Virus Incidence Reduction
4.3.3. Seasonal Patterns of Virus Detection and Distribution
4.4. Biological and Epidemiological Properties of Grapevine Leafroll-Associated Viruses
4.4.1. Characterization of GLRaV-2-H4 Isolate
4.4.2. Use of Foliar Symptoms for Field Identification of GLRaV-3
4.4.3. Seasonal Progress and Distribution of GLRaV-3 in Different Cultivars
4.5. Genomic Diversity and Molecular Diagnostics of Grapevine Leafroll-Associated Viruses
4.6. Effects of Grapevine Leafroll Disease on Vine Physiology, Yield, and Fruit Composition
4.6.1. Gaps and Limitations
- Incomplete understanding of disease etiology: The exact role of individual GLRaVs in symptom development and yield loss is not fully elucidated, particularly in cases of mixed infections or latent infections where symptoms are absent or mild.
- Regional biases in research: Most studies have been conducted in well-established viticulture regions (Europe, North America, South Africa, China), while data from emerging wine-growing regions (e.g., Africa beyond South Africa, Central Asia, parts of South America) remain limited.
- Variability in detection methods: Although molecular diagnostics have advanced, inconsistencies remain in assay sensitivity and specificity, especially for genetically diverse or newly emerging GLRaV variants.
- Limited ecological perspective: While physiological and yield impacts are documented, the ecological consequences of GLRaVs on vineyard ecosystems—such as interactions with microbial communities, insect vectors, or wild V. species—are underexplored.
- Management challenges: Existing control strategies (use of certified material, vector control, roguing) are not universally effective, and the long-term sustainability of these measures under changing climate and globalized plant trade is uncertain.
4.6.2. Future Research Directions
- Clarifying virus–host interactions: Multi-omics approaches (genomics, transcriptomics, metabolomics) should be used to dissect host responses to single and mixed GLRaV infections, linking molecular changes to physiological and agronomic outcomes.
- Expanding geographical coverage: Systematic surveys and molecular characterization of GLRaVs in underrepresented viticultural areas are needed to capture global diversity and track viral spread.
- Improving diagnostics: Development of broad-spectrum, multiplex, and portable diagnostic tools (e.g., CRISPR-based assays, nanopore sequencing) could enhance early and field-level detection, including in vectors.
- Understanding ecological impacts: Studies should investigate how GLRaV infections affect vineyard biodiversity, soil microbiota, vector dynamics, and potential virus reservoirs in wild grapevine populations.
- Innovative management strategies: Exploration of novel approaches such as RNA interference (RNAi), biological control of vectors, or breeding for virus-resistant/tolerant grapevine cultivars could provide more sustainable solutions.
- Socioeconomic assessments: Economic studies should quantify the cost–benefit of different control strategies across diverse production systems, helping growers adopt regionally adapted management approaches.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martelli, G.P.; Boudon-Padieu, E. (Eds.) Options Méditerranéennes. Serie B: Studies and Research; CIHEAM (Centre International de Hautes Etudes Agronomiques Méditerranéen): Bari, Italy, 2006; Volume 55, ISSN 1016-1228. [Google Scholar]
- Boudon-Padieu, É.; Maixner, M. Potential effects of climate change on distribution and activity of insect vectors of grapevine pathogens. In Proceedings of the International and Multi-Disciplinary “Global Warming, Which Potential Impacts on the Vineyards?”, Beaune, France, 28–30 March 2007; p. 23. [Google Scholar]
- Fuchs, M.; Martinson, T.E.; Loeb, G.M.; Hoch, H.C. Survey for the three major leafroll disease-associated viruses in Finger Lakes vineyards in New York. Plant Dis. 2009, 93, 395–401. [Google Scholar] [CrossRef]
- Atallah, S.S.; Gómez, M.I.; Conrad, J.M.; Nyrop, J.P. A plant-level, spatial, bioeconomic model of plant disease diffusion and control: Grapevine leafroll disease. Am. J. Agric. Econ. 2015, 97, 199–218. [Google Scholar] [CrossRef]
- Mannini, F.; Digiaro, M. The effects of viruses and viral diseases on grapes and wine. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G., Golino, D., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Alabi, O.J.; Casassa, L.F.; Gutha, L.R.; Larsen, R.C.; Henick-Kling, T.; Harbertson, J.F.; Naidu, R.A. Impacts of Grapevine leafroll disease on fruit yield and grape and wine chemistry in a wine grape (Vitis vinifera L.) cultivar. PLoS ONE 2016, 11, e0149666. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.A.; Rowhani, A.; Fuchs, M.; Golino, D.; Martelli, G.P. Grapevine leafroll: A complex viral disease affecting a high-value fruit crop. Plant Dis. 2014, 98, 1172–1185. [Google Scholar] [CrossRef]
- Constable, F.E.; Connellan, J.; Nicholas, P.; Rodoni, B.C. The reliability of woody indexing for detection of grapevine virus-associated diseases in three different climatic conditions in Australia. Aust. J. Grape Wine Res. 2012, 19, 74–80. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A.; García-Berrios, J.J. Effects of Grapevine leafroll-associated virus 3 on the physiology and must of Vitis vinifera L. cv. Albariño following contamination in the field. Am. J. Enol. Vitic. 1999, 50, 40–44. [Google Scholar] [CrossRef]
- Reynard, J.S.; Brodard, J.; Zufferey, V.; Rienth, M.; Gugerli, P.; Schumpp, O.; Blouin, A.G. Nuances of Responses to Two Sources of Grapevine Leafroll Disease on Pinot Noir Grown in the Field for 17 Years. Viruses 2022, 14, 1333. [Google Scholar] [CrossRef]
- Cui, Z.-H.; Bi, W.-L.; Pan, C.; Xu, Y.; Wang, Q.-C. Abiotic stress improves in vitro biological indexing of Grapevine leafroll-associated virus-3 in red grapevine cultivars. Aust. J. Grape Wine Res. 2015, 21, 490–495. [Google Scholar] [CrossRef]
- Mannini, F.; Mollo, A.; Credi, R. Field performance and wine quality modification in a clone of Vitis vinifera cv. Dolcetto after GLRaV-3 elimination. Am. J. Enol. Vitic. 2012, 63, 144–147. [Google Scholar] [CrossRef]
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Plant Pathol. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Prajapati, M.R.; Gupta, N.; Shimray, M.; Gehlot, J.; Tiwari, A.; Thapa, P.; Diksha, D.; Holkar, S.K.; Mahajan, P.J.; Saha, S.; et al. Genome characterization of a newly discovered grapevine leafroll-associated virus S, in the genus ampelovirus by high-throughput sequencing. J. Genet. Eng. Biotechnol. 2025, 23, 100494. [Google Scholar] [CrossRef]
- Sharma, A.M.; Wang, J.; Duffy, S.; Zhang, S.; Wong, M.K.; Rashed, A.; Cooper, M.L.; Daane, K.M.; Almeida, R.P. Occurrence of grapevine leafroll-associated virus complex in Napa Valley. PLoS ONE 2011, 6, e26227. [Google Scholar] [CrossRef]
- Sultanova, N.; Rastgou, M.; Huseynova, I. Occurrence of Single and Mixed Viral Infections of Grapevine (Vitis Spp.) in Azerbaijan. Pol. J. Environ. Stud. 2024, 33, 4345–4353. [Google Scholar] [CrossRef]
- Schoelz, J.; Volenberg, D.; Adhab, M.; Fang, Z.; Klassen, V.; Cpinka, V.; Al Rwahnih, M. A Survey of Viruses Found in Grapevine Cultivars Grown in Missouri. Am. J. Enol. Vitic. 2021, 72, 73–84. [Google Scholar] [CrossRef]
- Hančević, K.; Čarija, M.; Radić Brkanac, S.; Gaši, E.; Likar, M.; Zdunić, G.; Regvar, M.; Radić, T. Grapevine Leafroll-Associated Virus 3 in Single and Mixed Infections Triggers Changes in the Oxidative Balance of Four Grapevine Varieties. Int. J. Mol. Sci. 2022, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine leafroll disease and associated viruses: A unique pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef]
- Charles, J.G.; Cohen, D.; Walker, J.T.S.; Forgie, S.A.; Bell, V.A.; Breen, K.C. A review of the ecology of Grapevine leafroll-associated virus type 3 (GLRaV-3). N. Z. Plant Prot. 2006, 59, 330–337. [Google Scholar]
- Almeida, R.P.; Daane, K.M.; Bell, V.A.; Blaisdell, G.K.; Cooper, M.L.; Herrbach, E.; Pietersen, G. Ecology and management of grapevine leafroll disease. Front. Microbiol. 2013, 4, 94. [Google Scholar] [CrossRef]
- Dincă, L.; Crisan, V.; Ienaşoiu, G.; Murariu, G.; Drăşovean, R. Environmental Indicator Plants in Mountain Forests: A Review. Plants 2024, 13, 3358. [Google Scholar] [CrossRef]
- Bratu, I.; Dinca, L.; Constandache, C.; Murariu, G. Resilience and decline: The impact of climatic variability on temperate oak forests. Climate 2025, 13, 119. [Google Scholar] [CrossRef]
- Dincă, L.; Constandache, C.; Postolache, R.; Murariu, G.; Tupu, E. Timber Harvesting in Mountainous Regions: A Comprehensive Review. Forests 2025, 16, 495. [Google Scholar] [CrossRef]
- Budău, R.; Timofte, C.S.C.; Mirisan, L.V.; Bei, M.; Dincă, L.; Murariu, G.; Racz, K.A. Living landmarks: A review of monumental trees and their role in ecosystems. Plants 2025, 14, 2075. [Google Scholar] [CrossRef]
- Dinca, L.; Murariu, G.; Lupoae, M. Understanding the ecosystem services of riparian forests: Patterns, gaps, and global trends. Forests 2025, 16, 947. [Google Scholar] [CrossRef]
- Enescu, C.M.; Mihalache, M.; Ilie, L.; Dinca, L.; Constandache, C.; Murariu, G. Agricultural benefits of shelterbelts and windbreaks: A bibliometric analysis. Agriculture 2025, 15, 1204. [Google Scholar] [CrossRef]
- Dinca, L.; Coca, A.; Tudose, N.C.; Marin, M.; Murariu, G.; Munteanu, D. The Role of Trees in Sand Dune Rehabilitation: Insights from Global Experiences. Appl. Sci. 2025, 15, 7358. [Google Scholar] [CrossRef]
- Murariu, G.; Dinca, L.; Munteanu, D. Trends and applications of principal component analysis in forestry research: A literature and bibliometric review. Forests 2025, 16, 1155. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Clarivate. Web of Science Core Collection. Available online: https://clarivate.com/academia-government/lp/pivot-rp/?campaignname=UL_PivotRP_LeadGen_AG_Global&campaignid=701QO00000BvPD5YAN&utm_campaign=UL_PivotRP_LeadGen_AG_Global&utm_source=Google&utm_medium=Paid_Search&utm_content=&utm_term=&gad_source=1&gad_campaignid=22932413470&gbraid=0AAAAACv9_uM80D8RkAoKAvA66B-nwF5dA&gclid=CjwKCAiAzrbIBhA3EiwAUBaUdcYek4E9ZI2G8vm6Q6B1NvmDgkQ1cExBwVa15FjHNVq8QdS5kvMxshoCo1AQAvD_BwE (accessed on 10 June 2025).
- Elsevier; Scopus. Available online: https://www.elsevier.com/products/scopus (accessed on 11 June 2025).
- Microsoft Corporation. Microsoft Excel. Available online: https://www.microsoft.com/en-us/microsoft-365/excel?legRedir=true&CorrelationId=3bb60ab0-fe13-41a4-812b-2627667cf346 (accessed on 16 July 2025).
- Geochart. Available online: https://developers.google.com/chart/interactive/docs/gallery/geochart (accessed on 23 July 2025).
- VOSviewer. Available online: https://www.vosviewer.com/ (accessed on 5 July 2025).
- Kumar, S.; Sawant, S.D.; Sawant, I.S.; Prabha, K.; Jain, R.K.; Baranwal, V.K. First report of Grapevine leafroll-associated virus 1 infecting grapevines in India. Plant Dis. 2012, 96, 1828. [Google Scholar] [CrossRef]
- Zongoma, A.M.; Dangora, D.B.; Al Rwahnih, M.; Bako, S.P.; Alegbejo, M.D.; Alabi, O.J. First report of Grapevine leafroll-associated virus 1 infecting grapevines (Vitis spp.) in Nigeria. Plant Dis. 2018, 102, 258. [Google Scholar] [CrossRef]
- Gazel, M.; Caglayan, K.; Elçi, E.; Öztürk, L. First report of Grapevine Pinot gris virus in grapevine in Turkey. Plant Dis. 2016, 100, 657. [Google Scholar] [CrossRef]
- Immanuel, T.M.; Delmiglio, C.; Ward, L.I.; Denton, J.O.; Clover, G.R.G. First Reports of Grapevine virus A, Grapevine fleck virus, and Grapevine leafroll-associated virus 1 in the United Kingdom. Plant Dis. 2015, 99, 898. [Google Scholar] [CrossRef]
- Crnogorac, A.; Panno, S.; Mandić, A.; Gašpar, M.; Caruso, A.G.; Noris, E.; Davino, S.; Matić, S. Survey of five major grapevine viruses infecting Blatina and Žilavka cultivars in Bosnia and Herzegovina. PLoS ONE 2021, 16, e0245959. [Google Scholar] [CrossRef]
- Elbeaino, T.; Kontra, L.; Demian, E.; Jaksa-Czotter, N.; Slimen, A.B.; Fabian, R.; Lazar, J.; Tamisier, L.; Digiaro, M.; Massart, S.; et al. Complete sequence, genome organization and molecular detection of grapevine line pattern virus, a new putative anulavirus infecting grapevine. Viruses 2020, 12, 602. [Google Scholar] [CrossRef]
- Pop, I.; Gugerli, P.; Banu, E.; Tomoioaga, L. Results regarding the identification of closteroviruses associated with the leafroll disease of some grapevine varieties grown in Romania. In Proceedings of the Extended Abstracts 11th ICVG Meeting, Montreux, Switzerland, 5–10 September 1993; pp. 123–124. [Google Scholar]
- Bertazzon, N.; Angelini, E.; Signorotto, M.; Genov, N. First report of grapevine Pinot gris virus and grapevine leafroll-associated virus 2 in Bulgarian vineyards. J. Plant Dis. Prot. 2021, 128, 597–599. [Google Scholar] [CrossRef]
- Afechtal, M.; Bibi, I.; Aarabe, A.; Sbaghi, M.; Ouantar, M.; Faddoul, Z. First report of grapevine leafroll-associated virus 2 in Moroccan vineyards. J. Plant Pathol. 2017, 29, 533–543. [Google Scholar]
- Porotikova, E.V.; Dmitrenko, U.D.; Yurchenko, E.G.; Vinogradova, S.V. First Report of Grapevine leafroll-associated virus 2 in Russian Grapevines (Vitis vinifera). Plant Dis. 2019, 103, 164. [Google Scholar] [CrossRef]
- Aboughanem-Sabanadzovic, N.; Sabanadzovic, S. First report of Grapevine leafroll-associated virus 2 infecting muscadine (Vitis rotundifolia) and summer grape (Vitis aestivalis) in the United States. Plant Dis. 2015, 99, 163. [Google Scholar] [CrossRef]
- Jones, T.J.; Westover, F.; Nita, M. First Report of Grapevine leafroll-associated virus-2 and -3 in Texas Vineyards. Plant Dis. 2014, 98, 1592. [Google Scholar] [CrossRef]
- Lunden, S.; Qiu, W. First report of Grapevine leafroll-associated virus 2 in a hybrid grape ‘Vidal Blanc’ in Missouri. Plant Dis. 2012, 96, 462. [Google Scholar] [CrossRef]
- Gugerli, P.; Ramel, M.E. Grapevine leafroll associated virus II analyzed by monoclonal antibodies. In Proceedings of the 11th Congress of ICVG, Montreux, Switzerland, 5–10 September 1993; pp. 23–24. [Google Scholar]
- Ribeiro, G.P.; Saldarelli, P.; Hong, N.; Xiang, B.C.; Zhang, X.L.; Wang, G.P.; Martelli, G.P. First record of three grapevine viruses in the Chinese Province of Sinkiang. J. Plant Pathol. 2004, 86, 152. [Google Scholar]
- Anfoka, G.H.; Shahrour, W.; Nakhla, M.K. Detection and molecular characterization of Grapevine fanleaf virus and Grapevine leafroll-associated virus 3 in Jordan. J. Plant Pathol. 2004, 86, 203–207. [Google Scholar]
- Kostadinovska, E.; Mitrev, S.; Bianco, P.A.; Casati, P.; Bulgari, D. First report of Grapevine virus A and Grapevine fleck virus in the Former Yugoslav Republic of Macedonia. Plant Dis. 2014, 98, 1747. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Selmi, I.; Elair, M.; Garfi, G.; Pasta, S.; Carimi, F.; Pacifico, D. First report of grapevine leafroll-associated virus 3 in wild vines (Vitis vinifera subsp. sylvestris) in Tunisia. J. Plant Pathol. 2021, 103, 1039. [Google Scholar] [CrossRef]
- Mishchenko, L.T.; Konup, L.O.; Dunich, A.A.; Gorobets, V.F.; Konup, A.I.; Zaimenko, N.V.; Kozub, N.O.; Dashchenko, A.V.; Chistyakova, V.L.; Shcherbakova, T.O.; et al. First report of grapevine leafroll-associated virus-3 on peony plants in Ukraine. J. Plant Dis. Prot. 2023, 130, 189–198. [Google Scholar] [CrossRef]
- Hoffmann, M.; Talton, W.; Nita, M.; Jones, T.; Al Rwahnih, M.; Sudarshana, M.; Almeyda, C. First report of grapevine leafroll-associated virus 3 in Vitis vinifera in North Carolina. J. Plant Pathol. 2021, 103, 385–386. [Google Scholar] [CrossRef]
- Mekuria, T.A.; Karasev, A.V.; Martin, R.R.; Naidu, R.A. First report of Grapevine leafroll-associated virus-3 in six wine grape cultivars in Idaho. Plant Dis. 2009, 93, 1218. [Google Scholar] [CrossRef]
- Soule, M.J.; Eastwell, K.C.; Naidu, R.A. First report of Grapevine leafroll associated virus-3 in American Vitis spp. Grapevines in Washington State. Plant Dis. 2006, 90, 1461. [Google Scholar] [CrossRef]
- Pietersen, G.; Spreeth, N.; Oosthuizen, T.; van Rensburg, A.; van Rensburg, M.; Lottering, D.; Rossouw, N.; Tooth, D. Control of grapevine leafroll disease spread at a commercial wine estate in South Africa: A case study. Am. J. Enol. Vitic. 2013, 64, 296–305. [Google Scholar] [CrossRef]
- Martelli, G.P. (Ed.) Directory of Infectious Diseases of Grapevines and Viroses and Virus-like Diseases of the Grapevine: Bibliographic Report 1998–2004; Ciheam: Paris, France, 2006. [Google Scholar]
- Gugerli, P.; Brugger, J.J.; Bovey, R. L’enroulement de la vigne: Mise en evidence de particules virales et developpement d’une methode immuno-enzymatique pour le diagnostic rapide. Rev. Suisse Vitic. Arboric. Hortic. 1984, 16, 299–304. [Google Scholar]
- Kuhn, G.B. Identificação, incidencia e controle do virus do enrolamento da folha da videira no Estado do Rio Grande do Sul. Fitopatol. Bras. 1989, 14, 220–226. [Google Scholar]
- Minafra, A.; Hadidi, A. Sensitive detection of grapevine virus A, B, or leafroll-associated III from viruliferous mealybugs and infected tissue by cDNA amplification. J. Virol. Methods 1994, 47, 175–187. [Google Scholar] [CrossRef]
- Escobar, P.F.; Fiore, N.; Valenzuela, P.D.T.; Engel, E.A. First Detection of Grapevine leafroll-associated virus 4 in Chilean Grapevines. Plant Dis. 2008, 92, 1474. [Google Scholar] [CrossRef]
- Pei, G.Q.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D. First Report of Grapevine leafroll-associated virus 4 and 5 in Grapevines in China. Plant Dis. 2010, 94, 130. [Google Scholar] [CrossRef] [PubMed]
- Olah, R.; Turcsan, M.; Jaksa-Czotter, N.; Galbacs, Z.N.; Olah, K.; Sardi, D.N.; Plesko, I.M.; Varallyay, E. First report of Grapevine leafroll-associated virus 4 infecting grapevine in Hungary. Plant Dis. 2024, 108, 2245. [Google Scholar] [CrossRef]
- Rizzo, D.; Luvisi, A.; Stefani, L.; Paoli, M.; Marchi, G.; Panattoni, A.; Materazzi, A. First report of Grapevine leafroll associated virus-4 strain 5 in Italy. J. Plant Pathol. 2014, 96, 129. [Google Scholar]
- Choueiri, E.; Jreijiri, F.; Habib, W.; Abou Kubaa, R.; Elbeaino, T. First report on the occurrence of Grapevine leafroll-associated virus-4 strain 6 in Lebanon. Plant Dis. 2017, 101, 1066. [Google Scholar] [CrossRef]
- Štrukelj, M.; Pleško, I.M.; Urek, G. Molecular characterization of a grapevine leafroll-associated virus 4 from Slovenian vineyards. Acta Virol. 2016, 60, 174–180. [Google Scholar] [CrossRef]
- Kaya, A.; Erilmez, S.; Paylan, I.C.; Erkan, S. First report of grapevine leafroll-associated virus 4 in vineyards of Turkey. Plant Dis. 2012, 96, 1230. [Google Scholar] [CrossRef]
- Padilla, C.V.; Cretazzo, E.; Hita, I.; López, N.; Padilla, V.; Velasco, L. First report of Grapevine leafroll-associated virus 5 in Spain. Plant Dis. 2010, 94, 1507. [Google Scholar] [CrossRef]
- Gómez Talquenca, S.; Muñoz, C.; Grau, O.; Gracia, O. First description of Grapevine leafroll-associated virus 5 in Argentina and partial genome sequence. Virus Genes 2009, 38, 184–186. [Google Scholar] [CrossRef]
- Engel, E.A.; Escobar, P.; Montt, C.; Gómez-Talquenca, S.; Valenzuela, P.D.T. First report on the occurrence of Grapevine leafroll-associated virus 7 and 9 in Chilean grapevines. Plant Dis. 2008, 92, 1252. [Google Scholar] [CrossRef]
- Padilla, V.; Cretazzo, E.; Alcalá, M.J.; Hita, I.; Lopez, N.; Padilla, V.; Velasco, L. First report of Grapevine leafroll-associated virus 9 in Spain. J. Plant Pathol. 2013, 95, 662. [Google Scholar] [CrossRef]
- Buzkan, N.; Karadağ, S.; Kaya, A.; Baloğlu, S.; Minafra, A.; Ben-Dov, Y. First report of the occurrence of Grapevine leafroll-associated virus-5 in Turkish vineyards. J. Phytopathol. 2010, 158, 448–449. [Google Scholar] [CrossRef]
- Lyu, M.D.; Li, M.J.; Li, J.; Li, X.M.; Cheng, Y.Q. First report of Grapevine leafroll-associated virus 7 in two native grape varieties in China. Plant Dis. 2013, 97, 150. [Google Scholar] [CrossRef]
- Morales, R.Z.; Monis, J. First detection of Grapevine leafroll-associated virus-7 in California vineyards. Plant Dis. 2007, 91, 465. [Google Scholar] [CrossRef]
- Peake, B.K.; Mackie, A.E.; Sivasithamparam, K.; Habili, N.; McKirdy, S.J. First report of grapevine leafroll associated virus 9 (GLRaV-9) in Western Australia. Australas. Plant Pathol. 2004, 33, 445–446. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Chiumenti, M.; Roberto, R.; Pirolo, C.; Minafra, A.; Saldarelli, P. First detection of Grapevine leafroll-associated virus 9 in Italy. J. Plant Pathol. 2011, 93, S4. [Google Scholar]
- Padilla, C.V.; Cretazzo, E.; López, N.; de Rosa, B.G.; Padilla, V.; Velasco, L. First report of Grapevine leafroll-associated virus 4 (GLRaV-4) in Spain. New Dis. Rep. 2010, 21, 21. [Google Scholar] [CrossRef]
- Jarugula, S.; Soule, M.J.; Rowhani, A.; Naidu, R.A. First report of Grapevine leafroll-associated virus-9 in Washington state vineyards. Plant Dis. 2008, 92, 485. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Digiaro, M.; Dhouibi, M.H. Transmission of Grapevine leafroll viruses by Planococcus ficus (Hemiptera: Pseudococcidae) and Ceroplastes rusci (Hemiptera: Coccidae). Plant Dis. 2009, 93, 999–1002. [Google Scholar] [CrossRef]
- Avgelis, A.; Boscia, D. Grapevine leafroll-associated closterovirus 7 in Greece. Phytopathol. Mediterr. 2001, 40, 289–292. [Google Scholar]
- Čarija, M.; Radić, T.; Černi, S.; Mucalo, A.; Zdunić, G.; Vončina, D.; Jagunić, M.; Hančević, K. Prevalence of virus infections and GLRaV-3 genetic diversity in selected clones of Croatian indigenous grapevine cultivar Plavac Mali. Pathogens 2022, 11, 176. [Google Scholar] [CrossRef]
- Zindović, J.; Viršček Marn, M.; Mavrič Pleško, I. Phytosanitary status of grapevine in Montenegro. EPPO Bull. 2014, 44, 60–64. [Google Scholar] [CrossRef]
- Tomić, L.; Štajner, N.; Jovanović-Cvetković, T.; Cvetković, M.; Javornik, B. Identity and genetic relatedness of Bosnia and Herzegovina grapevine germplasm. Sci. Hortic. 2012, 143, 122–126. [Google Scholar] [CrossRef]
- Merkuri, J.; Martelli, G.P.; Boscia, D.; Savino, V. Viruses of grapevine in Albania. EPPO Bull. 1994, 24, 215–220. [Google Scholar] [CrossRef]
- Cseh, E.; Palkovics, L.; Apró, M.; Gáborjányi, R.; Kocsis, L.; Takács, A.P. Occurrence and evolutionary relationships of Grapevine leafroll-associated virus 3 isolates in Hungary. J. Plant Pathol. 2013, 95, S1.51. [Google Scholar]
- Komínek, P. Distribution of grapevine viruses in vineyards of the Czech Republic. J. Plant Pathol. 2008, 90, 357–358. [Google Scholar]
- Messmer, N.; Bohnert, P.; Schumacher, S.; Fuchs, R. Studies on the occurrence of viruses in planting material of grapevines in southwestern Germany. Viruses 2021, 13, 248. [Google Scholar] [CrossRef]
- Komorowska, B.; Berniak, H.; Golis, T. Detection of grapevine viruses in Poland. J. Phytopathol. 2014, 162, 326–331. [Google Scholar] [CrossRef]
- Credi, R.; Giunchedi, L. Grapevine leafroll-associated viruses and Grapevine virus A in selected Vitis vinifera cultivars in northern Italy. Plant Pathol. 1996, 45, 1110–1116. [Google Scholar] [CrossRef]
- Esteves, F.; Teixeira Santos, M.; Eiras-Dias, J.E.; Fonseca, F. Occurrence of grapevine leafroll-associated virus 5 in Portugal: Genetic variability and population structure in field-grown grapevines. Arch. Virol. 2012, 157, 1747–1765. [Google Scholar] [CrossRef]
- Grecu, C.; Buciumeanu, E.; Dumitraşcu, M. Purification du clostérovirus du type III associé à l’enroulement de la vigne [Purification of closterovirus type III associated with grapevine leafroll disease]. Rev. Roum. Virol. 1994, 45, 19–23. [Google Scholar]
- Guță, I.C.; Buciumeanu, E.C. Grapevine Pinot gris virus infecting grapevine in Romania. Hort. Sci. 2021, 48, 47–50. [Google Scholar] [CrossRef]
- Kyrychenko, A.M.; Hrynchuk, K.V.; Antipov, I.O.; Konup, A.I. A survey of grapevine leafroll-associated virus 1 and 3 in the south of Ukraine and development of primers for GLRaV-3 identification. Mikrobiol. Zhurnal 2022, 84, 82–91. [Google Scholar] [CrossRef]
- Fan, X.; Hong, N.; Dong, Y.; Ma, Y.; Zhang, Z.P.; Ren, F.; Wang, G. Genetic diversity and recombination analysis of grapevine leafroll-associated virus 1 from China. Arch. Virol. 2015, 160, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Li, M.J.; Qi, H.H.; Guo, R.; Liu, X.M.; Wang, Q.; Cheng, Y.Q. Occurrence of grapevine leafroll-associated viruses in China. Plant Dis. 2013, 97, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Brannen, P.M.; Deom, C.M.; Alabi, O.J.; Naidu, R.A. Prevalence of viruses in commercial wine grape vineyards in Georgia. Plant Health Prog. 2018, 19, 342–346. [Google Scholar] [CrossRef]
- Naderpour, M.; Shahbazi, R.; Alizadeh, A.; Karimi, S.; Tabei, M.; Hajizadeh, M.; Hosseinibay, K. The status of Grapevine leafroll-associated viruses in Iran. Acta Hortic. 2020, 1269, 113–118. [Google Scholar] [CrossRef]
- Rai, R.; Khurana, S.P.; Kumar, S.; Sharma, S.K.; Watpade, S.; Baranwal, V.K. Characterization of Grapevine leafroll-associated virus 4 from Indian vineyards. J. Plant Pathol. 2017, 99, 255–259. [Google Scholar]
- Rasool, S.; Naz, S.; Rowhani, A.; Diaz-Lara, A.; Golino, D.A.; Farrar, K.D.; Al Rwahnih, M. Survey of grapevine pathogens in Pakistan. J. Plant Pathol. 2019, 101, 725–732. [Google Scholar] [CrossRef]
- Akbaş, B.; Kunter, B.; Ilhan, D. Occurrence and distribution of grapevine leafroll-associated viruses 1, 2, 3 and 7 in Turkey. J. Phytopathol. 2007, 155, 122–124. [Google Scholar] [CrossRef]
- Mslmanieh, T.; Digiaro, M.; Elbeaino, T.; Boscia, D.; Martelli, G.P. Viruses of grapevine in Syria. EPPO Bull. 2006, 36, 523–528. [Google Scholar] [CrossRef]
- Ben Salem-Fnayou, A.; Gugerli, P.; Zemni, H.; Mliki, A.; Ghorbel, A. Decreased detectability of Grapevine leafroll-associated virus 3 in Sakasly grapevines cultivated under the Sahara conditions. J. Phytopathol. 2006, 154, 528–533. [Google Scholar] [CrossRef]
- Lehad, A.; Selmi, I.; Louanchi, M.; Aitouada, M.; Mahfoudhi, N. Occurrence and diversity of Grapevine leafroll-associated virus 1 in Algeria. Phytopathol. Mediterr. 2019, 58, 277–282. [Google Scholar]
- Aldrich, D.J.; Bester, R.; Burger, J.; Maree, H.J. Characterisation of different GLRaV-3 variant infections by determining virus concentration ratios and miRNA expression profiles. Vitis 2019, 58, 79–86. [Google Scholar]
- Allsopp, E. Transmission of grapevine leafroll-associated virus 3 by vine mealybug, Planococcus ficus (Signoret), to grapevines treated with imidacloprid. S. Afr. J. Enol. Vitic. 2015, 36, 252–255. [Google Scholar] [CrossRef]
- Poojari, S.; Moreau, D.L.; Kahl, D.; Ritchie, M.; Ali, S.; Úrbez-Torres, J.R. Disease incidence and genetic variability of economically important grapevine viruses in Nova Scotia. Can. J. Plant Pathol. 2020, 42, 584–594. [Google Scholar] [CrossRef]
- Fiore, N.; Zamorano, A.; Rivera, L.; González, F.; Aballay, E.; Montealegre, J.; Pino, A.M. Grapevine Viruses in the Atacama Region of Chile. J. Phytopathol. 2011, 159, 743–750. [Google Scholar] [CrossRef]
- Adiputra, J.; Kesoju, S.R.; Naidu, R.A. The relative occurrence of Grapevine leafroll-associated virus 3 and Grapevine red blotch virus in Washington State vineyards. Plant Dis. 2018, 102, 2129–2135. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Aguilar-Molina, V.H.; Monjarás-Barrera, J.I.; Vončina, D.; Erickson, T.M.; Al Rwahnih, M. Potential implications and management of grapevine viruses in Mexico: A review. Int. J. Plant Biol. 2023, 14, 177–189. [Google Scholar] [CrossRef]
- de Borbón, C.M.; Gracia, O.; Talquenca, G.S.G. Mealybugs and grapevine leafroll-associated virus 3 in vineyards of Mendoza, Argentina. Am. J. Enol. Vitic. 2004, 55, 283–285. [Google Scholar] [CrossRef]
- Habili, N.; Fazeli, C.F.; Ewart, A.; Hamilton, R.; Cirami, R.; Saldarelli, P.; Rezaian, M.A. Natural spread and molecular analysis of grapevine leafroll-associated virus 3 in Australia. Phytopathology 1995, 85, 1418–1422. [Google Scholar] [CrossRef]
- Hao, X.; Jiao, B.; Liu, Z.; Wang, X.; Wang, J.; Zhang, J.; Wang, Q.C. Crosstalk between grapevine leafroll-associated virus-3 (GLRaV-3) and NaCl-induced salt stress in in vitro cultures of the red grape ‘Cabernet Sauvignon’. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 649–660. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Apostol, E.N.; Stuparu, E.; Scarlatescu, V.; Budeanu, M. Testing Hungarian oak (Quercus frainetto Ten.) provenances in Romania. iForest 2020, 13, 9–15. [Google Scholar] [CrossRef]
- Besliu, E.; Curtu, A.L.; Apostol, E.N.; Budeanu, M. Using adapted and productive European beech (Fagus sylvatica L.) provenances as future solutions for sustainable forest management in Romania. Land 2024, 13, 183. [Google Scholar] [CrossRef]
- Budeanu, M.; Şofletea, N.; Petriţan, I.C. Among-population variation in quality traits in two Romanian provenance trials with Picea abies L. Baltic For. 2014, 20, 37–47. [Google Scholar]
- Budeanu, M.; Popescu, F.; Beşliu, E.; Apostol, N.E. Diallel crossing (10x10) in Swiss stone pine. Juvenile-adult correlations and genetic gain for predicting forward selection. Ann. For. Res. 2024, 67, 109–120. [Google Scholar] [CrossRef]
- Budeanu, M.; Beşliu, E.; Pepelea, D. Testing the radial increment and climate–growth relationship between Swiss stone pine European provenances in the Romanian Carpathians. Forests 2025, 16, 391. [Google Scholar] [CrossRef]
- Zhu, J. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Mostert, I.; Bester, R.; Burger, J.T.; Maree, H.J. Investigating protein–protein interactions between Grapevine leafroll-associated virus 3 and Vitis vinifera. Phytopathology 2023, 113, 1994–2005. [Google Scholar] [CrossRef]
- Hesler, S.; Cox, R.; Bhandari, R.; Loeb, G.; Martinson, T.; Fuchs, M. Spatial roguing reduces the incidence of leafroll disease and curtails its spread in a Finger Lakes Cabernet franc vineyard. Am. J. Enol. Vitic. 2022, 73, 227–236. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Fuchs, M.F.; Martinson, T.; Hesler, S.; Loeb, G.M. Slowing the spread of grapevine leafroll-associated viruses in commercial vineyards with insecticide control of the vector, Pseudococcus maritimus (Hemiptera: Pseudococcidae). J. Insect Sci. 2015, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, G.K.; Cooper, M.L.; Kuhn, E.J.; Taylor, K.A.; Daane, K.M.; Almeida, R.P. Disease progression of vector-mediated Grapevine leafroll-associated virus 3 infection of mature plants under commercial vineyard conditions. Eur. J. Plant Pathol. 2016, 146, 105–116. [Google Scholar] [CrossRef]
- Bell, V.A.; Hedderley, D.I.; Pietersen, G.; Lester, P.J. Vineyard-wide control of grapevine leafroll-associated virus 3 requires an integrated response. J. Plant Pathol. 2018, 100, 399–408. [Google Scholar] [CrossRef]
- Abou-Ghanem, N.; Sabanadzovic, S.; Castellano, M.A.; Boscia, D.; Martelli, G.P. Properties of a new isolate of grapevine leafroll-associated virus 2. Vitis 2000, 39, 119–121. [Google Scholar]
- Jooste, A.E.C.; Maree, H.J.; Bellstedt, D.U.; Goszczynski, D.E.; Pietersen, G.; Burger, J.T. Three genetic grapevine leafroll-associated virus 3 variants identified from South African vineyards show high variability in their 5′ UTR. Arch. Virol. 2010, 155, 1997–2006. [Google Scholar] [CrossRef]
- Komar, V.; Vigne, E.; Demangeat, G.; Fuchs, M. Beneficial effect of selective virus elimination on the performance of Vitis vinifera cv. Chardonnay. Am. J. Enol. Vitic. 2007, 58, 202–210. [Google Scholar] [CrossRef]
- Maree, H.J.; Almeida, R.P.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.; Martelli, G.P.; et al. Grapevine leafroll-associated virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Real-time RT-PCR (TaqMan®) assays for the detection of Grapevine leafroll-associated viruses 1–5 and 9. J. Virol. Methods 2007, 141, 22–29. [Google Scholar] [CrossRef]
- Alabi, O.J.; Al Rwahnih, M.; Karthikeyan, G.; Poojari, S.; Fuchs, M.; Rowhani, A.; Naidu, R.A. Grapevine leafroll-associated virus 1 occurs as genetically diverse populations. Phytopathology 2011, 101, 1446–1456. [Google Scholar] [CrossRef]
- Maree, H.J.; Pirie, M.D.; Oosthuizen, K.; Bester, R.; Rees, D.J.G.; Burger, J.T. Phylogenomic analysis reveals deep divergence and recombination in an economically important grapevine virus. PLoS ONE 2015, 10, e0126819. [Google Scholar] [CrossRef]
- Turturo, C.; Saldarelli, P.; Yafeng, D.; Digiaro, M.; Minafra, A.; Savino, V.; Martelli, G.P. Genetic variability and population structure of Grapevine leafroll-associated virus 3 isolates. J. Gen. Virol. 2005, 86, 217–224. [Google Scholar] [CrossRef]
- Chooi, K.M.; Bell, V.A.; Blouin, A.G.; Cohen, D.; Mundy, D.; Henshall, W.; MacDiarmid, R.M. Grapevine leafroll-associated virus 3 genotype influences foliar symptom development in New Zealand vineyards. Viruses 2022, 14, 1348. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro, C.; Pesqueira, A.M.; García-Berrios, J.J. Assessment of symptoms of grapevine leafroll disease and relationship with yield and quality of Pinot Noir grape must in a 10-year study period. Plants 2023, 12, 2127. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hanner, R.H.; Meng, B. Probing into the Effects of Grapevine Leafroll-Associated Viruses on the Physiology, Fruit Quality and Gene Expression of Grapes. Viruses 2021, 13, 593. [Google Scholar] [CrossRef]
- Donda, B.P.; Kesoju, S.R.; Arnold, K.; McRoberts, N.; Naidu, R.A. Spatio-Temporal Spread of Grapevine Leafroll Disease in Washington State Vineyards. Plant Dis. 2023, 107, 1471–1480. [Google Scholar] [CrossRef]
- Tanne, E.; Spiegel-Roy, P.; Shlamovitz, N. Rapid in vitro indexing of grapevine viral diseases: The effect of stress-inducing agents on the diagnosis of leafroll. Plant Dis. 1996, 80, 972–974. [Google Scholar] [CrossRef]
- Marin, M.; Clinciu, I.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L.; Mărțoiu, N.E.; Tudose, O.N. Assessment of seasonal surface runoff under climate and land use change scenarios for a small forested watershed: Upper Tarlung Watershed (Romania). Water 2022, 14, 2860. [Google Scholar] [CrossRef]
- Davidescu, S.O.; Clinciu, I.; Tudose, N.C.; Ungurean, C. An evaluating methodology for hydrotechnical torrent-control structures condition. Ann. For. Res. 2012, 55, 125–143. [Google Scholar] [CrossRef]
- Mihalache, A.L.; Marin, M.; Davidescu, Ș.O.; Ungurean, C.; Adorjani, A.; Tudose, N.C.; Davidescu, A.A.; Clinciu, I. Physical status of torrent control structures in Romania. Environ. Eng. Manag. J. 2020, 19, 861–872. [Google Scholar] [CrossRef]
- Marin, M.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L. Application of life cycle assessment for torrent control structures: A review. Land 2024, 13, 1956. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Saldarelli, P.; Rowhani, A. Grapevine leafroll-associated virus 7. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 221–228. [Google Scholar]
- Tsai, C.W.; Daugherty, M.P.; Almeida, R.P.P. Seasonal dynamics and virus translocation of Grapevine leafroll-associated virus 3 in grapevine cultivars. Plant Pathol. 2012, 61, 977–985. [Google Scholar] [CrossRef]
- Douglas, N.; Krüger, K. Transmission efficiency of Grapevine leafroll-associated virus 3 (GLRaV-3) by the mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae). Eur. J. Plant Pathol. 2008, 122, 207–212. [Google Scholar] [CrossRef]
- Herrbach, E.; Alliaume, A.; Prator, C.A.; Daane, K.M.; Cooper, M.L.; Almeida, R.P.P. Vector Transmission of Grapevine Leafroll-Associated Viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G., Golino, D., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Mahfoudhi, N.; Habili, N.; Masri, S.A.; Dhouibi, M.H. First report on the occurrence of Grapevine leafroll-associated viruses 5 and 9 in Tunisian grapevines. Plant Dis. 2007, 91, 1359. [Google Scholar] [CrossRef]
- Hommay, G.; Beuve, M.; Herrbach, E. Transmission of Grapevine Leafroll-Associated Viruses and Grapevine Virus A by Vineyard-Sampled Soft Scales (Parthenolecanium corni, Hemiptera: Coccidae). Viruses 2022, 14, 2679. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A. Some characteristics of the transmission of Grapevine leafroll-associated virus 3 by Planococcus citri Risso. Eur. J. Plant Pathol. 1997, 103, 373–378. [Google Scholar] [CrossRef]
- Chooi, K.M.; Bell, V.A.; Blouin, A.G.; Sandanayaka, M.; Gough, R.; Chhagan, A.; MacDiarmid, R.M. The New Zealand perspective of an ecosystem biology response to grapevine leafroll disease. Adv. Virus Res. 2024, 118, 213–272. [Google Scholar]
- Habili, N.; Wu, Q.; Rinaldo, A.; Constable, F. A Chronological Study on Grapevine Leafroll-Associated Virus 2 in Australia. Viruses 2023, 15, 1105. [Google Scholar] [CrossRef]
- Mikona, C.; Jelkmann, W. Replication of Grapevine leafroll-associated virus-7 (GLRaV-7) by Cuscuta species and its transmission to herbaceous plants. Plant Dis. 2010, 94, 471–476. [Google Scholar] [CrossRef]
- Sforza, R.; Boudon-Padieu, E.; Greif, C. New Mealybug Species Vectoring Grapevine Leafroll-Associated Viruses-1 and -3 (GLRaV-1 and -3). Eur. J. Plant Pathol. 2003, 109, 975–981. [Google Scholar] [CrossRef]
- Zhang, C.W.; Huang, H.Q.; Huang, W.T.; Li, H.W.; Chi, H.; Cheng, Y.Q. Grapevine leafroll-associated virus 2 and grapevine ‘Pinot gris’ virus are present in seedlings developed from seeds of infected grapevine plants. Vitis 2022, 61, 21–25. [Google Scholar]
- Donda, B.P.; Jarugula, S.; Naidu, R.A. An analysis of the complete genome sequence and subgenomic RNAs reveals unique features of the Ampelovirus, Grapevine leafroll-associated virus 1. Phytopathology 2017, 107, 1069–1079. [Google Scholar] [CrossRef]
- Little, A.; Fazeli, C.F.; Rezaian, M.A. Hypervariable genes in Grapevine leafroll associated virus 1. Virus Res. 2001, 80, 109–116. [Google Scholar] [CrossRef]
- Sefc, K.M.; Leonhardt, W.; Steinkellner, H. Partial sequence identification of grapevine-leafroll-associated virus-1 and development of a highly sensitive IC-RT-PCR detection method. J. Virol. Methods 2000, 86, 101–106. [Google Scholar] [CrossRef]
- Bertazzon, N.; Borgo, M.; Vanin, S.; Angelini, E. Genetic variability and pathological properties of Grapevine leafroll-associated virus 2 isolates. Eur. J. Plant Pathol. 2010, 127, 185–197. [Google Scholar] [CrossRef]
- Jarugula, S.; Alabi, O.J.; Martin, R.R.; Naidu, R.A. Genetic variability of natural populations of Grapevine leafroll-associated virus 2 in Pacific Northwest vineyards. Phytopathology 2010, 100, 698–707. [Google Scholar] [CrossRef]
- Fonseca, F.; Esteves, F.; Teixeira Santos, M.; Brazão, J.; Eiras-Dias, J.E. Genetic variants of Grapevine leafroll-associated virus 2 infecting Portuguese grapevine cultivars. Phytopathol. Mediterr. 2016, 55, 73–88. [Google Scholar]
- Fust, C.; Lameront, P.; Shabanian, M.; Song, Y.; Abou Kubaa, R.; Bester, R.; Meng, B. Grapevine leafroll-associated virus 3: A global threat to grapevine and wine industries but a gold mine for scientific discovery. J. Exp. Bot. 2025, 76, eraf039. [Google Scholar] [CrossRef]
- Bester, R.; Jooste, A.E.; Maree, H.J.; Burger, J.T. Real-time RT-PCR high-resolution melting curve analysis and multiplex RT-PCR to detect and differentiate grapevine leafroll-associated virus 3 variant groups I., II, III and VI. Virol. J. 2012, 9, 219. [Google Scholar] [CrossRef]
- Chooi, K.M.; Cohen, D.; Pearson, M.N. Generic and sequence-variant specific molecular assays for the detection of the highly variable Grapevine leafroll-associated virus 3. J. Virol. Methods 2013, 189, 20–29. [Google Scholar] [CrossRef]
- Fei, F.; Lyu, M.D.; Li, J.; Fan, Z.F.; Cheng, Y.Q. Complete nucleotide sequence of a Chinese isolate of Grapevine leafroll-associated virus 3 reveals a 5′ UTR of 802 nucleotides. Virus Genes 2013, 46, 182–185. [Google Scholar] [CrossRef]
- Glasa, M.; Predajna, L. Partial sequence analysis of a Grapevine leafroll-associated virus 3 isolate from Slovakia. J. Plant Pathol. 2012, 94, 675–679. [Google Scholar]
- Jooste, A.E.; Pietersen, G.; Burger, J.T. Distribution of grapevine leafroll associated virus-3 variants in South African vineyards. Eur. J. Plant Pathol. 2011, 131, 371–381. [Google Scholar] [CrossRef]
- Jooste, A.E.C.; Goszczynski, D.E. Single-strand conformation polymorphism (SSCP), cloning and sequencing reveals two major groups of divergent molecular variants of grapevine leafroll-associated virus 3 (GLRaV-3). Vitis 2005, 44, 39–43. [Google Scholar]
- Jooste, A.E.C.; Molenaar, N.; Burger, J.T.; De Koker, W.C.; Bester, R.; Morey, L.; Maree, H.J. Identification and distribution of multiple virus infections in Grapevine leafroll diseased vineyards. Eur. J. Plant Pathol. 2015, 142, 363–375. [Google Scholar] [CrossRef]
- Jooste, A.E.C.; Pietersen, G.; Burger, J.T. Distribution of grapevine leafroll associated virus-3 variants in South African vineyards. Eur. J. Plant Pathol. 2011, 131, 371–381. [Google Scholar] [CrossRef]
- Abou-Ghanem, N.; Sabanadzovic, S.; Minafra, A.; Saldarelli, P.; Martelli, G.P. Some properties of Grapevine leafroll-associated virus 2 and molecular organization of the 3′ region of the viral genome. J. Plant Pathol. 1998, 80, 37–46. [Google Scholar]
- Adiputra, J.; Jarugula, S.; Naidu, R.A. Intra-species recombination among strains of the ampelovirus Grapevine leafroll-associated virus 4. Virol. J. 2019, 16, 139. [Google Scholar] [CrossRef]
- Good, X.; Monis, J. Partial genome organization, identification of the coat protein gene, and detection of Grapevine leafroll-associated virus-5. Phytopathology 2001, 91, 274–281. [Google Scholar] [CrossRef]
- Thompson, J.R.; Fuchs, M.; Perry, K.L. Genomic analysis of Grapevine leafroll associated virus-5 and related viruses. Virus Res. 2012, 163, 19–27. [Google Scholar] [CrossRef]
- Ito, T.; Nakaune, R. Molecular characterization of a novel putative ampelovirus tentatively named grapevine leafroll-associated virus 13. Arch. Virol. 2016, 161, 2555–2559. [Google Scholar] [CrossRef]
- Osman, F.; Golino, D.; Hodzic, E.; Rowhani, A. Virus distribution and seasonal changes of grapevine leafroll-associated viruses. Am. J. Enol. Vitic. 2018, 69, 70–76. [Google Scholar] [CrossRef]
- Morán, F.; Olmos, A.; Glasa, M.; Silva, M.B.D.; Maliogka, V.; Wetzel, T.; Ruiz-García, A.B. A novel and highly inclusive quantitative real-time rt-pcr method for the broad and efficient detection of grapevine leafroll-associated virus 1. Plants 2023, 12, 876. [Google Scholar] [CrossRef]
- Beuve, M.; Sempé, L.; Lemaire, O. A sensitive one-step real-time RT-PCR method for detecting Grapevine leafroll-associated virus 2 variants in grapevine. J. Virol. Methods 2007, 141, 117–124. [Google Scholar] [CrossRef]
- Acheche, H.; Fattouch, S.; M’hirsi, S.; Marzouki, N.; Marrakchi, M. Use of optimised PCR methods for the detection of GLRaV-3: A closterovirus associated with grapevine leafroll in Tunisian grapevine plants. Plant Mol. Biol. Rep. 1999, 17, 31–42. [Google Scholar] [CrossRef]
- Bester, R.; Burger, J.T.; Maree, H.J. Transcriptome analysis reveals differentially expressed small RNAs and genes associated with grapevine leafroll-associated virus 3 infections. Physiol. Mol. Plant Pathol. 2017, 100, 220–236. [Google Scholar] [CrossRef]
- López-Fabuel, I.; Wetzel, T.; Bertolini, E.; Bassler, A.; Vidal, E.; Torres, L.B.; Yuste, A.; Olmos, A. Real-time multiplex RT-PCR for the simultaneous detection of the five main grapevine viruses. J. Virol. Methods 2013, 188, 21–24. [Google Scholar] [CrossRef]
- Atallah, S.S.; Gomez, M.I.; Fuchs, M.F.; Martinson, T.E. Economic impact of Grapevine leafroll disease on Vitis vinifera cv. Cabernet Franc in Finger Lakes vineyards of New York. In Working Papers 2011; Cornell Univ., Dept. of Applied Economics and Management, No. 126597: Ithaca, NY, USA, 2011. [Google Scholar]
- Martínez, L.; Miranda, C.; Royo, J.B.; Urrestarazu, J.; Martínez de Toda, F.; Balda, P.; Santesteban, L.G. Direct and indirect effects of three virus infections on yield and berry composition in grapevine (Vitis vinifera L.) cv. ‘Tempranillo’. Sci. Hortic. 2016, 212, 20–28. [Google Scholar] [CrossRef]
- Salo, W.; Considine, J.A.; Considine, M.J. Influence of mixed and single infection of grapevine leafroll-associated viruses and viral load on berry quality. Tree Physiol. 2024, 44, tpae035. [Google Scholar] [CrossRef]
- Bertamini, M.; Malossini, U.; Muthuchelian, K.; Nedunchezhian, N. Physiological response of field grown grapevine (Vitis vinifera L. cv. Marzemino) to grapevine leafroll-associated virus (GLRaV-1). Phytopathol. Mediterr. 2005, 44, 256–265. [Google Scholar]
- Cabaleiro, C.; Pesqueira, A.M.; Barrasa, M.; García-Berrios, J.J. Analysis of the losses due to grapevine leafroll disease in Albariño vineyards in Rias Baixas (Spain). Ciência Téc. Vitiv. 2013, 28, 43–50. [Google Scholar]
- El Aou-ouad, H.; Montero, R.; Medrano, H.; Bota, J. Interactive effects of grapevine leafroll-associated virus 3 (GLRaV-3) and water stress on the physiology of Vitis vinifera L. cv. Malvasia de Banyalbufar and Giro-Ros. J. Plant Physiol. 2016, 196, 106–115. [Google Scholar] [CrossRef]
- Endeshaw, S.T.; Sabbatini, P.; Romanazzi, G.; Schilder, A.C.; Neri, D. Effects of grapevine leafroll associated virus 3 infection on growth, leaf gas exchange, yield and basic fruit chemistry of Vitis vinifera L. cv. Cabernet Franc. Sci. Hortic. 2014, 170, 228–236. [Google Scholar] [CrossRef]
- Montero, R.; Mundy, D.; Albright, A.; Grose, C.; Trought, M.C.T.; Cohen, D.; Chooi, K.M.; MacDiarmid, R.; Flexas, J.; Bota, J. Effects of Grapevine leafroll-associated virus 3 (GLRaV-3) and duration of infection on fruit composition and wine chemical profile of Vitis vinifera L. cv. Sauvignon blanc. Food Chem. 2016, 197, 1177–1183. [Google Scholar] [CrossRef]
- Vega, A.; Gutiérrez, R.A.; Pena-Neira, A.; Cramer, G.R.; Arce-Johnson, P. Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol. Biol. 2011, 77, 261–274. [Google Scholar] [CrossRef]
- Tsai, C.W.; Rowhani, A.; Golino, D.A.; Daane, K.M.; Almeida, R.P. Mealybug transmission of grapevine leafroll viruses: An analysis of virus–vector specificity. Phytopathology 2010, 100, 830–834. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Daubert, S.; Urbez-Torres, J.R.; Cordero, F.; Rowhani, A. Deep sequencing evidence from single grapevine plants reveals a virome dominated by mycoviruses. Arch. Virol. 2011, 156, 397–403. [Google Scholar] [CrossRef]
- Petter, F.; Giovani, B.; Trontin, C. International Cooperation to Support the Diagnosis of Forestry Pests: The Role of EPPO and Euphresco. Forests 2023, 14, 1461. [Google Scholar] [CrossRef]
- Rubio, R.O.; Rubio, E.O.; Pérez, C.O.; Izquierdo, M.A.P.; Rustioni, L.; Failla, O.; Chipashvili, R.; Maghradze, D. Ecological and sanitary characteristics of the Eurasian wild grapevine (Vitis vinifera L. ssp. sylvestris (Gmelin) Hegi) in Georgia (Caucasian region). Plant Genet. Resour. 2012, 10, 155–162. [Google Scholar]
- Pietersen, G.; Bell, V.A.; Krüger, K. Management of grapevine leafroll disease and associated vectors in vineyards. In Grapevine viruses: Molecular Biology, Diagnostics and Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 531–560. [Google Scholar]
- Jung, Y. Re-creating economic and cultural values in Bulgaria’s wine industry: From an economy of quantity to an economy of quality? Econ. Anthropol. 2016, 3, 280–292. [Google Scholar] [CrossRef]
- Overton, J.; Murray, W.E. Playing the scales: Regional transformations and the differentiation of rural space in the Chilean wine industry. J. Rural. Stud. 2011, 27, 63–72. [Google Scholar] [CrossRef]
- Laimer, M.; Barba, M. Elimination of systemic pathogens by thermotherapy, tissue culture, or in vitro micrografting. In Virus and Virus-like Diseases of Pome and Stone Fruits; American Phytopathological Society: St. Paul, MN, USA, 2011; pp. 389–393. [Google Scholar]
- Bhat, A.I.; Rao, G.P. Virus elimination by meristem-tip culture. In Characterization of Plant Viruses: Methods and Protocols; Springer: New York, NY, USA, 2020; pp. 465–477. [Google Scholar]
- Bell, V.A.; Blouin, A.G.; Cohen, D.; Hedderley, D.I.; Oosthuizen, T.; Spreeth, N.; Lester, P.J.; Pietersen, G. Visual symptom identification of grapevine leafroll-associated virus 3 in red berry cultivars supports virus management by roguing. J. Plant Pathol. 2017, 99, 477–482. [Google Scholar]
- Fuller, K.B.; Alston, J.M.; Golino, D.A. The benefits from certified virus-free nursery stock: A case study of Grapevine leafroll-3. In The North Coast Region of California; Robert Mondavi Institute-Center for Wine Economics Working Paper Number 1306; UC-Davis: Davis, CA, USA, 2013; p. 35. [Google Scholar]
- Sun, Y.-D.; Folimonova, S.Y. Location matters: From changing a presumption about the citrus tristeza virus tissue tropism to understanding the stem pitting disease. New Phytol. 2022, 233, 631–638. [Google Scholar] [CrossRef]
- Olmos, A.; Bertolini, E.; Ruiz-García, A.B.; Martínez, C.; Peiró, R.; Vidal, E. Modeling the accuracy of three detection methods of Grapevine leafroll-associated virus 3 during the dormant period using a Bayesian approach. Phytopathology 2016, 106, 510–518. [Google Scholar] [CrossRef]
- Martelli, G.P. Directory of virus and virus-like diseases of the grapevine and their agents. J. Plant Pathol. 2014, 96, 1–136. [Google Scholar]
- Burger, J.T.; Maree, H.J.; Gouveia, P.; Naidu, R.A. Grapevine leafroll-associated virus 3. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 167–195. [Google Scholar]
- Bertazzon, N.; Angelini, E. Advances in the detection of Grapevine leafroll-associated virus 2 variants. J. Plant Pathol. 2004, 86, 283–290. [Google Scholar]
- Goszczynski, D.E.; Kasdorf, G.G.F.; Pietersen, G.; van Tonder, H. Detection of two strains of grapevine leafroll-associated virus 2. Vitis 1996, 35, 133–135. [Google Scholar]
- Martelli, G.P.; Gallitelli, D. Emerging and re-emerging viruses of plants. In Encyclopedia of Virology; Bamford, D.H., Zuckerman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 86–92. [Google Scholar]
- Galvan, F.E.R.; Pavlick, R.; Trolley, G.; Aggarwal, S.; Sousa, D.; Starr, C.; Forrestel, E.; Bolton, S.; Alsina, M.D.M.; Dokoozlian, N.; et al. Scalable Early Detection of Grapevine Viral Infection with Airborne Imaging Spectroscopy. Phytopathology 2023, 113, 1439–1446. [Google Scholar] [CrossRef]
- Bendel, N.; Kicherer, A.; Backhaus, A.; Köckerling, J.; Maixner, M.; Bleser, E.; Klück, H.-C.; Seiffert, U.; Voegele, R.T.; Töpfer, R. Detection of Grapevine leafroll-associated virus 1 and 3 in white and red grapevine cultivars using hyperspectral imaging. Remote Sens. 2020, 12, 693. [Google Scholar] [CrossRef]
- Shabanian, M.; Xiao, H.; Meng, B. Seasonal dynamics and tissue distribution of two major viruses associated with grapevine Leafroll under cool climate condition. Eur. J. Plant Pathol. 2020, 158, 1017–1031. [Google Scholar] [CrossRef]
- Fazeli, C.F.; Habili, N.; Rezaian, M.A. Efficient cloning of cDNA from grapevine leafroll-associated virus 4 and demonstration of probe specificity by the viral antibody. J. Virol. Methods 1998, 70, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Abou Ghanem-Sabanadzovic, N.; Sabanadzovic, S.; Gugerli, P.; Rowhani, A. Genome organization, serology and phylogeny of Grapevine leafroll-associated viruses 4 and 6: Taxonomic implications. Virus Res. 2012, 163, 120–128. [Google Scholar] [CrossRef]
- Ling, K.S.; Zhu, H.Y.; Drong, R.F.; Slightom, J.L.; McFerson, J.R.; Gonsalves, D. Nucleotide sequence of the 3′-terminal two-thirds of the grapevine leafroll-associated virus-3 genome reveals a typical monopartite closterovirus. J. Gen. Virol. 1998, 79, 1299–1307. [Google Scholar] [CrossRef]
- Alkowni, R.; Rowhani, A.; Daubert, S.; Golino, D. Partial characterization of a new ampelovirus associated with grapevine leafroll disease. J. Plant Pathol. 2004, 86, 123–133. [Google Scholar]
- Pesqueira, A.M.; Cabaleiro, C.; Velasco, L. Genetic analysis of Grapevine leafroll-associated virus 3 population from Galicia, Spain. Plant Pathol. 2015, 65, 310–321. [Google Scholar] [CrossRef]
- Vondras, A.M.; Lerno, L.; Massonnet, M.; Minio, A.; Rowhani, A.; Liang, D.; Garcia, J.; Quiroz, D.; Figueroa-Balderas, R.; Golino, D.A.; et al. Rootstock influences the effect of grapevine leafroll-associated viruses on berry development and metabolism via abscisic acid signalling. Mol. Plant Pathol. 2021, 22, 984–1005. [Google Scholar] [CrossRef]
- Halldorson, M.M.; Keller, M. Grapevine leafroll disease alters leaf physiology but has little effect on plant cold hardiness. Planta 2018, 248, 1201–1211. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A. Temporal analysis of Grapevine leafroll-associated virus 3 epidemics. Eur. J. Plant Pathol. 2006, 114, 441–446. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Pesqueira, A.; García-Berrios, J. Influence of Grapevine leafroll-associated virus 3 in mature plants of Vitis vinifera L. cv Albariño on 110R and 196.17C rootstocks. S. Afr. J. Enol. Vitic. 2021, 42, 165–174. [Google Scholar] [CrossRef]
- Montero, R.; El Aou Ouad, H.; Pacifico, D.; Marzachì, C.; Castillo, N.; García, E.; Del Saz, N.F.; Florez-Sarasa, I.; Flexas, J.; Bota, J. Effects of Grapevine leafroll-associated virus 3 on physiology in asymptomatic plants of Vitis vinifera. Ann. Appl. Biol. 2017, 171, 155–171. [Google Scholar] [CrossRef]
- Moutinho-Pereira, J.; Correia, C.M.; Gonçalves, B.; Bacelar, E.A.; Coutinho, J.F.; Ferreira, H.F.; Lousada, J.L.; Cortez, M.I. Impacts of leafroll-associated viruses (GLRaV-1 and -3) on the physiology of the Portuguese grapevine cultivar ‘Touriga Nacional’ growing under field conditions. Ann. Appl. Biol. 2012, 160, 237–249. [Google Scholar] [CrossRef]
- Repetto, O.; Bertazzon, N.; De Rosso, M.; Miotti, L.; Flamini, R.; Angelini, E.; Borgo, M. Low susceptibility of grapevine infected by GLRaV-3 to late Plasmopara viticola infections: Towards understanding the phenomenon. Physiol. Mol. Plant Pathol. 2012, 79, 55–63. [Google Scholar] [CrossRef]
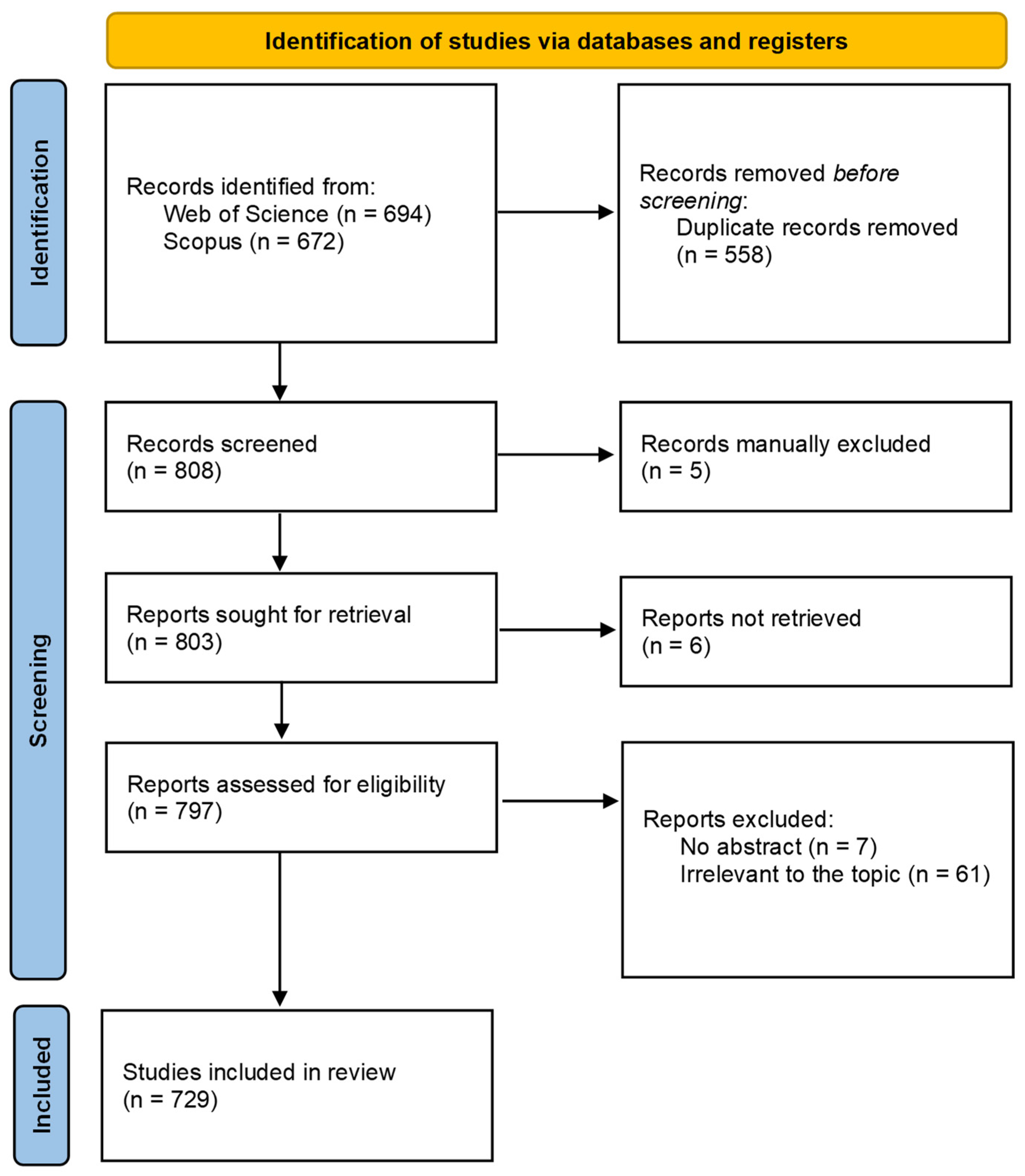
| Cur. No. | Virus (GLRaV) | Host (Reported) | Country/Area | Cited by/Earliest Representative Citation |
|---|---|---|---|---|
| 1 | GLRaV-1 | general | India | Kumar et al., 2012 [36] |
| 2 | general | Nigeria | Zongoma et al., 2018 [37] | |
| 3 | Chardonnay, Emir, Kadın parmağı, Muscat of Hamburg, and Pinot noir | Turkey | Gazel et al., 2016 [38] | |
| 4 | V. vinifera cv. Queen of Esther and Theresa | United Kingdom | Immanuel et al., 2015 [39] | |
| 5 | Blatina, Žilavka and other local cultivars | Bosnia and Herzegovina | Crnogorac et al., 2021 [40] | |
| 6 | V. vinifera cv. Mavro | Cyprus | Elbeaino et al., 2020 [41] | |
| 7 | general | Romania | Pop et al., 1993 [42] | |
| 8 | GLRaV-2 | Pinot gris | Bulgaria | Bertazzon et al., 2021 [43] |
| 9 | Red Globe | Marocco | Afechtal et al., 2017 [44] | |
| 10 | Landish, Uybilei Novocherkasska, Kolobok, Livia, and Veles | Russia | Porotikova et al., 2019 [45] | |
| 11 | V. rotundifolia and V. aestivalis | USA, Mississippi | Aboughanem-Sabanadzovic and Sabanadzovic, 2015 [46] | |
| 12 | Lenoir and cv. Blanc du Bois | USA, Texas | Jones et al., 2014 [47] | |
| 13 | Norton, Chambourcin, Chardonel, Vignoles, Vidal Blanc, Traminette, and Cayuga White | USA, Missouri | Lunden and Qiu, 2012 [48] | |
| 14 | general | Switzerland | Gugerli and Ramel, 1993 [49] | |
| 15 | GLRaV-3 | general | China, Sinkiang | Ribeiro et al., 2004 [50] |
| 16 | general | Jordan | Anfoka et al., 2004 [51] | |
| 17 | Vranec, Francovka, and Pinot noir | Republic of Macedonia | Kostadinovska et al., 2014 [52] | |
| 18 | V. vinifera subp. sylvestris | Tunisia | Mahfoudhi et al., 2021 [53] | |
| 19 | Chardonnay, Emir, Kadın parmağı, Muscat of Hamburg, and Pinot noir | Turkey | Gazel et al., 2016 [38] | |
| 20 | general | Ukraine | Mishchenko et al., 2023 [54] | |
| 21 | V. vinifera cv. Cabernet franc, Nero D’Avola, Malbec, Merlot, Chambourcin | USA, North Carolina | Hoffmann et al., 2021 [55] | |
| 22 | Lenoir and Blanc du Bois | USA, Texas | Jones et al., 2014 [47] | |
| 23 | Cabernet Sauvignon, Merlot, Syrah, and Petit Syrah | USA, Idaho | Mekuria et al., 2009 [56] | |
| 24 | V. labruscana cv. Concord and V. labrusca cv. Niagara | USA, Washington | Soule et al., 2006 [57] | |
| 25 | V. vinifera (various cultivars) | South Africa | Pietersen et al., 2013 [58] | |
| 26 | Yemen collection isolates | Yemen | Martelli, 2006 [59] | |
| 27 | V. vinifera | Switzerland | Gugerli et al., 1984 [60] | |
| 28 | General | Brazil | Kuhn, 1989 [61] | |
| 29 | general | Italy | Minafra and Hadidi, A., 1994 [62] | |
| 30 | GLRaV-4 | general | Chile | Escobar et al., 2008 [63] |
| 31 | general | China | Pei et al., 2010 [64] | |
| 32 | general | Hungary | Olah et al., 2024 [65] | |
| 33 | Sangiovese and Canaiolo | Italy | Rizzo et al., 2014 [66] | |
| 34 | Red Globe, Black Pearl, and Crimson | Lebanon | Choueiri et al., 2017 [67] | |
| 35 | general | Slovenia | Strukelj et al., 2016 [68] | |
| 36 | V. vinifera L. | Turkey | Kaya et al., 2012 [69] | |
| 37 | Estaladina and Tempranillo | Spain | Padilla et al., 2010 [70] | |
| 38 | GLRaV-5 | V. vinifera cv. Red Globe | Argentina | Gomez Talquenca et al., 2009 [71] |
| 39 | general | Chile | Engel et al., 2010 [72] | |
| 40 | general | China | Pei et al., 2010 [64] | |
| 41 | Sangiovese and Canaiolo | Italy | Rizzo et al., 2014 [66] | |
| 42 | Tempranillo | Spain | Padilla et al., 2010 [73] | |
| 43 | general | Turkey | Buzkan et al., 2010 [74] | |
| 44 | GLRaV-7 | general | Chile | Engel et al., 2008 [72] |
| 45 | Cabernet Sauvignon, Centennial Seedless, and Semillon | China | Lyu et al., 2013 [75] | |
| 46 | Cabernet-Sauvignon | Marocco | Afechtal et al., 2018 [41] | |
| 47 | Chardonnay, Merlot, Pinot noir, and Sauvignon blanc | USA, California | Morales and Monis, 2007 [76] | |
| 48 | GLRaV-9 | Cabernet Franc | Australia | Peake et al., 2004 [77] |
| 49 | general | Chile | Engel et al., 2008 [72] | |
| 50 | general | Italy | Giampetruzzi et al., 2011 [78] | |
| 51 | clone of Mantúa | Spain | Padilla et al., 2013 [79] | |
| 52 | Cabernet Sauvignon, Merlot, Pinot noir, Mourvedre, and Lagrein | USA | Jarugula et al., 2008 [80] | |
| 53 | general | Tunisia | Mahfoudhi et al., 2007 [81] |
| Virus | Genome Size (nt) | Key Genomic/Biological Features | Diagnostic Methods (Main Target) | Main Outcomes |
|---|---|---|---|---|
| GLRaV-1 | 18,731–18,946 | Nine ORFs; divergent CP copies; CP-homologous domains in four genes; high variation in ORFs 3, 6, and 7 | IC-RT-PCR (HSP70h region); TaqMan® qRT-PCR (CP) | ~125× more sensitive than ELISA; highly specific vs. GLRaV-2, -3, and -4 |
| GLRaV-2 | ~16,500–17,000 | Five clades (PN, H4, RG, BD, PV20); lineage-specific evolution and recombination; variable virulence | qRT-PCR (3′ genome end); CP gene phylogenetics | PN group most widespread; RG clade symptomless but causes graft incompatibility |
| GLRaV-3 | ~18,500 | Large genome; replication linked to outer mitochondrial membrane; cultivar-specific gene expression response | Multiplex RT-PCR (HSP70h); HRM assay (variant groups I–VI); IC-RT-PCR (mealybugs) | Detection from plant tissue and vectors; variant-specific primers increase accuracy |
| GLRaV-4 complex | 13,820–13,850 | Recombinant strains (4, 5, 9); close relationship with GLRaV-6; possible reclassification | RT-PCR (CP, HSP70h) | Hybrid genomes stable in field; taxonomy under review |
| GLRaV-5 | ~13,800 | Four ORFs; conserved p5 gene; two divergent CPs; monophyletic except for p23 | RT-PCR (partial genome regions) | Shares genomic features with GLRaV-3; part of GLRaV-4-like viruses |
| GLRaV-7 | 16,404 | Nine ORFs; ORF8 and ORF9 lack homology to known viral proteins | RT-PCR (partial genome) | Distinct from other GLRaVs; classified in subgroup I |
| GLRaV-13 | 17,608 | Eleven ORFs; closest but distinct from GLRaV-1 | RT-PCR (full genome) | Proposed novel Ampelovirus species |
| Advances in diagnostics | – | – | Real-time TaqMan® RT-PCR, HRM, multiplex RT-PCR | High sensitivity and specificity; improved RNA extraction enhances reliability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buciumeanu, E.-C.; Guță, I.-C.; Vizitiu, D.-E.; Dinca, L.; Murariu, G. From Vines to Ecosystems: Understanding the Ecological Effects of Grapevine Leafroll Disease. Appl. Sci. 2025, 15, 11920. https://doi.org/10.3390/app152211920
Buciumeanu E-C, Guță I-C, Vizitiu D-E, Dinca L, Murariu G. From Vines to Ecosystems: Understanding the Ecological Effects of Grapevine Leafroll Disease. Applied Sciences. 2025; 15(22):11920. https://doi.org/10.3390/app152211920
Chicago/Turabian StyleBuciumeanu, Elena-Cocuța, Ionela-Cătălina Guță, Diana-Elena Vizitiu, Lucian Dinca, and Gabriel Murariu. 2025. "From Vines to Ecosystems: Understanding the Ecological Effects of Grapevine Leafroll Disease" Applied Sciences 15, no. 22: 11920. https://doi.org/10.3390/app152211920
APA StyleBuciumeanu, E.-C., Guță, I.-C., Vizitiu, D.-E., Dinca, L., & Murariu, G. (2025). From Vines to Ecosystems: Understanding the Ecological Effects of Grapevine Leafroll Disease. Applied Sciences, 15(22), 11920. https://doi.org/10.3390/app152211920








