Chitosan Enhanced Polymers for Active Packaging: Intelligent Moisture Regulation and Non-Invasive Assessment
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Determination of Raw Material Thermal Stability
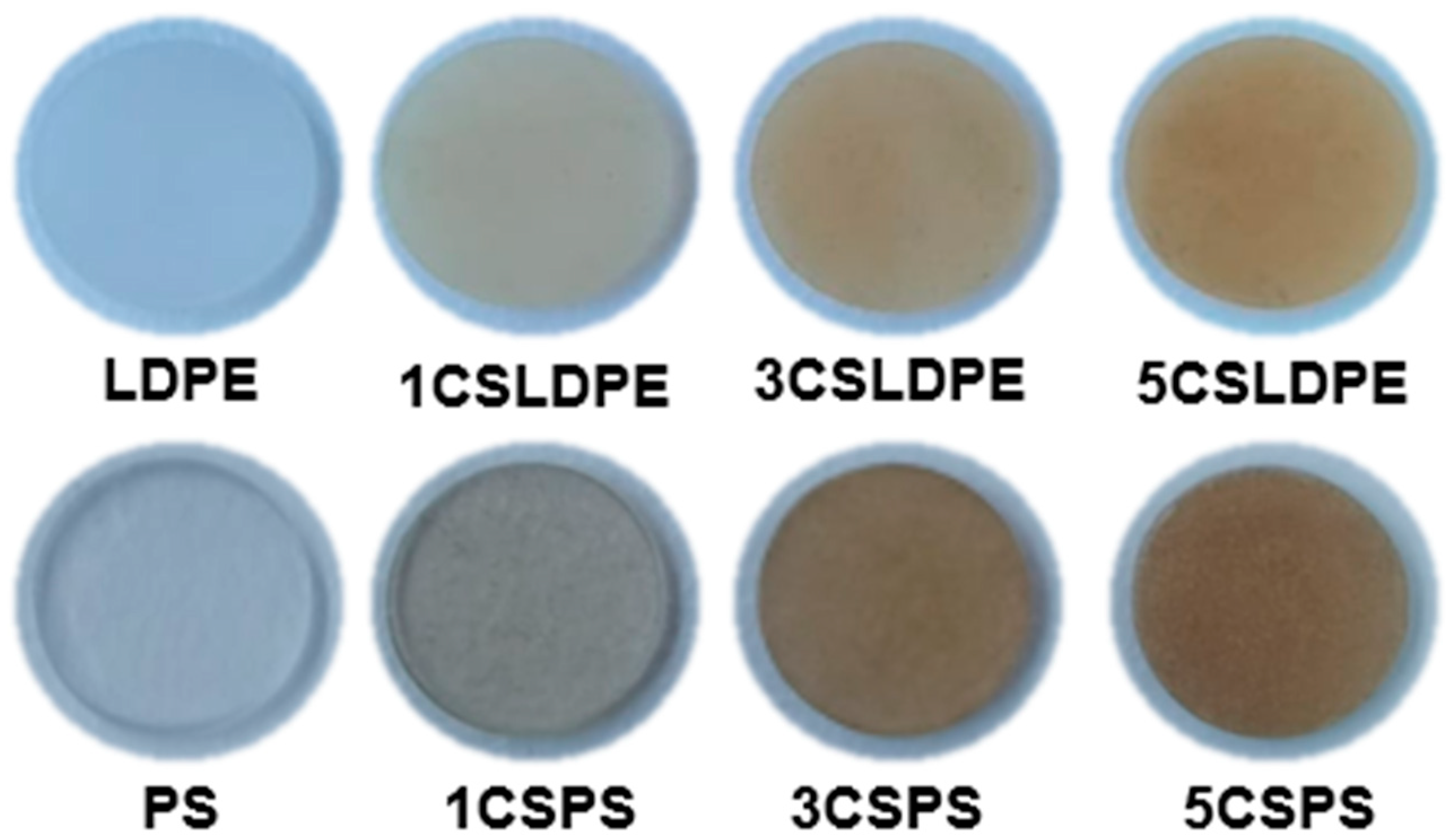
3.2. Morphological Analysis
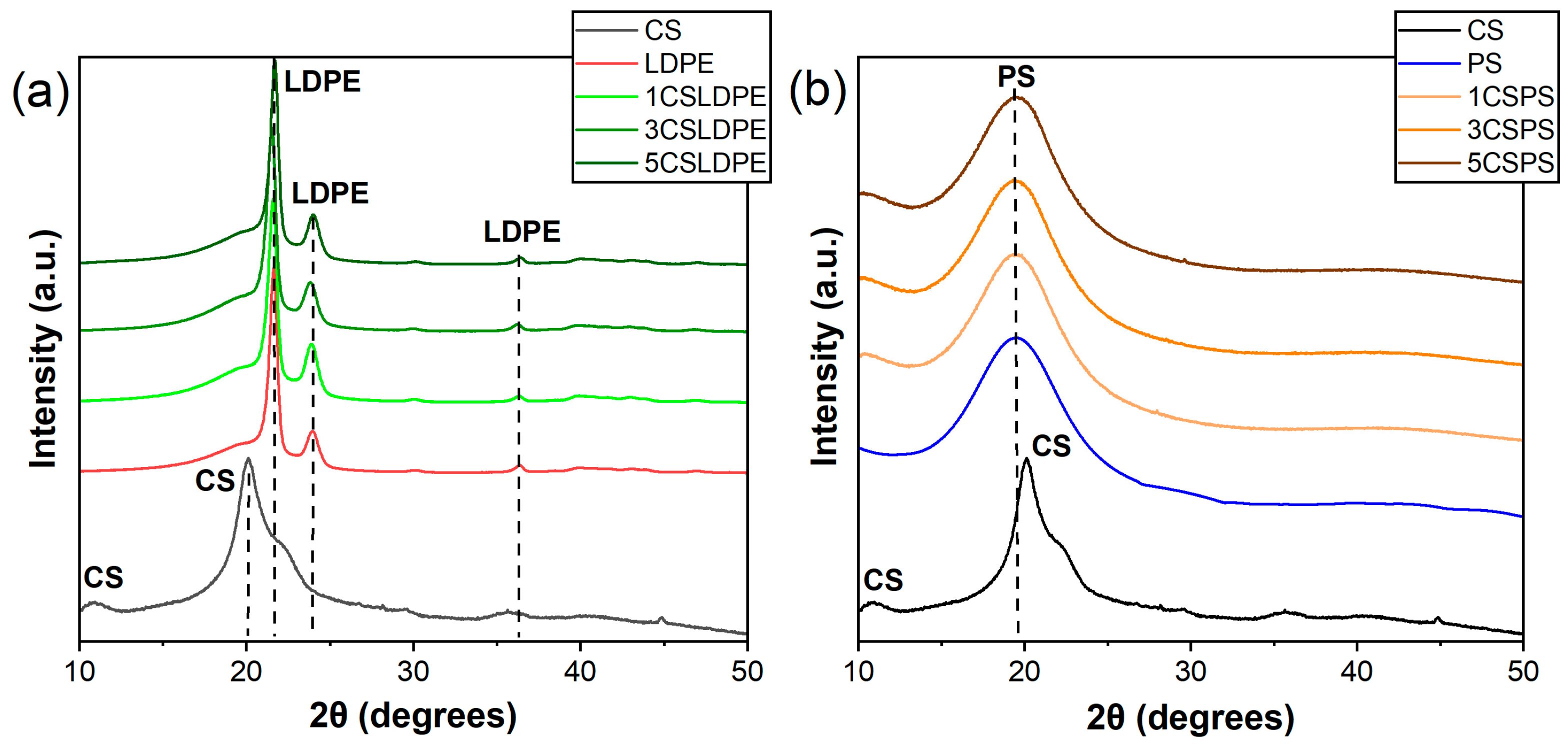
3.3. Crystal Phases Determination
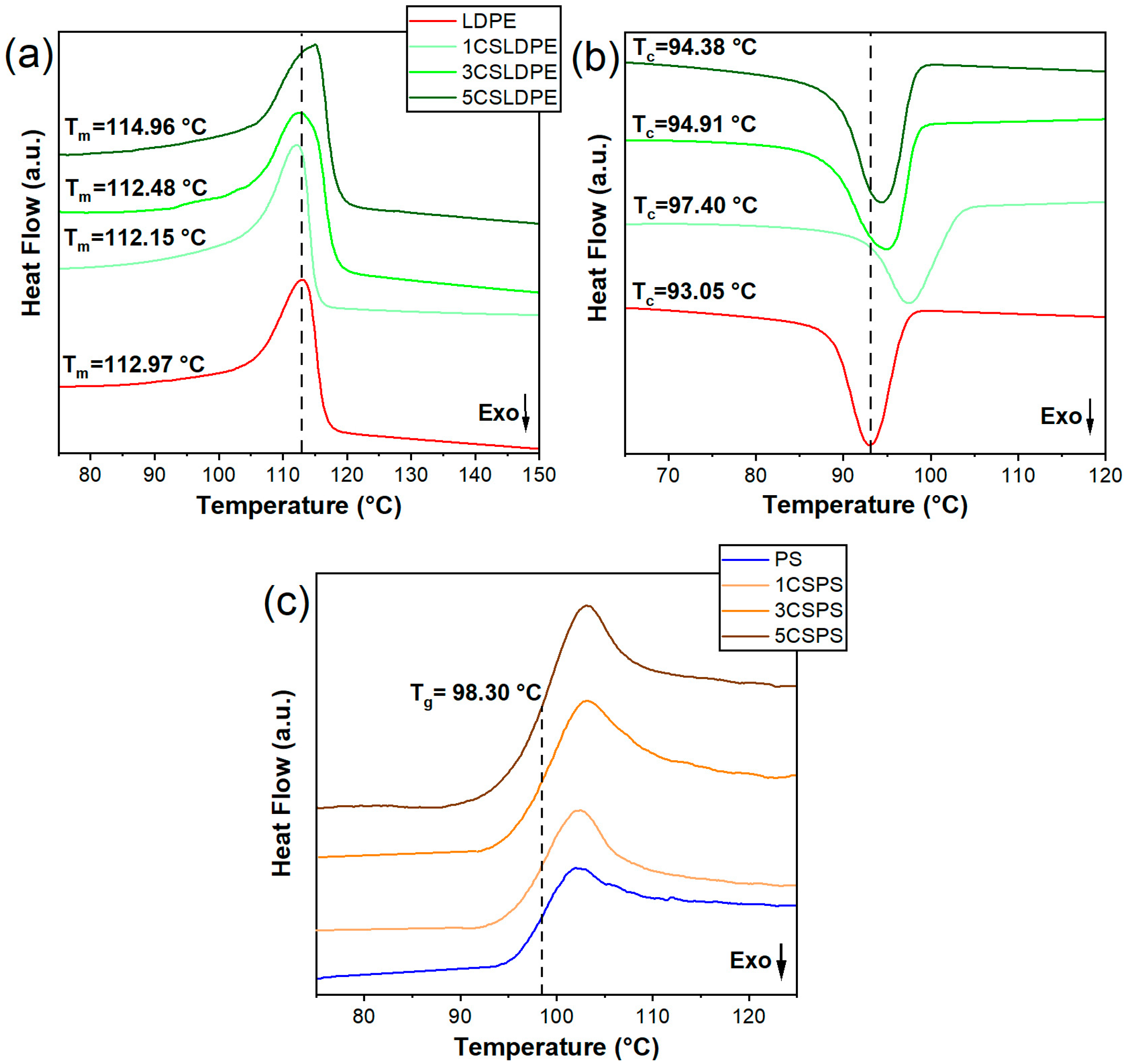
3.4. Thermal Analysis
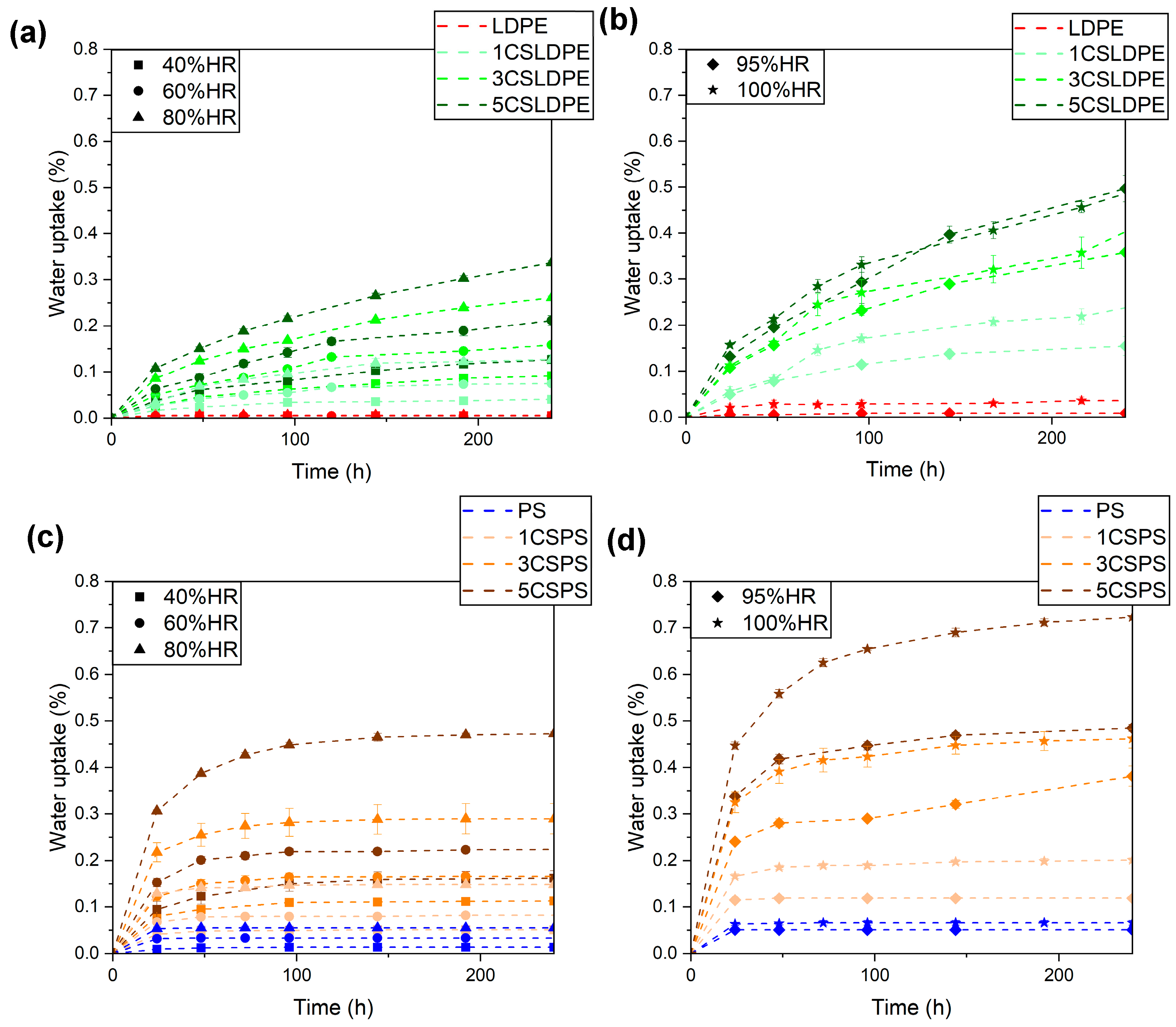
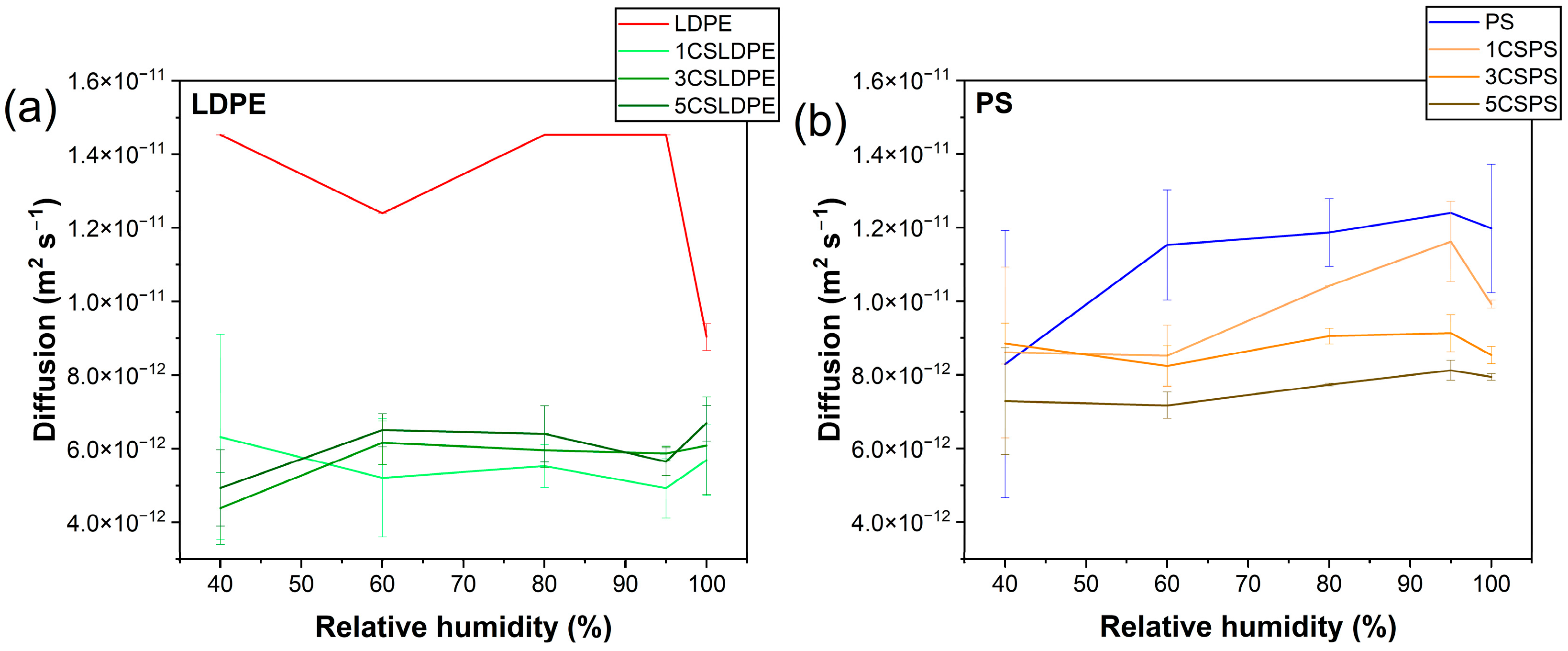
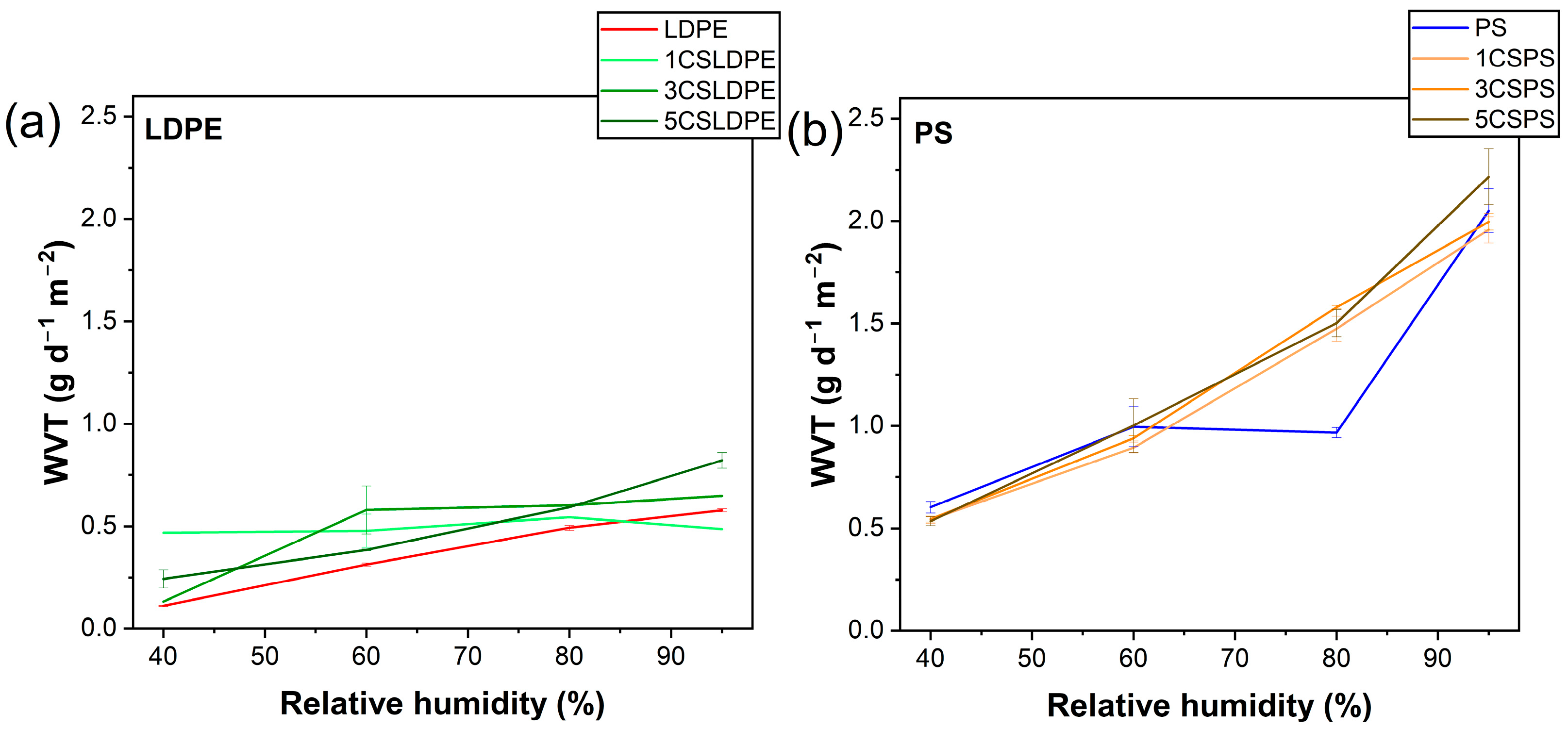
3.5. Water Barrier Properties
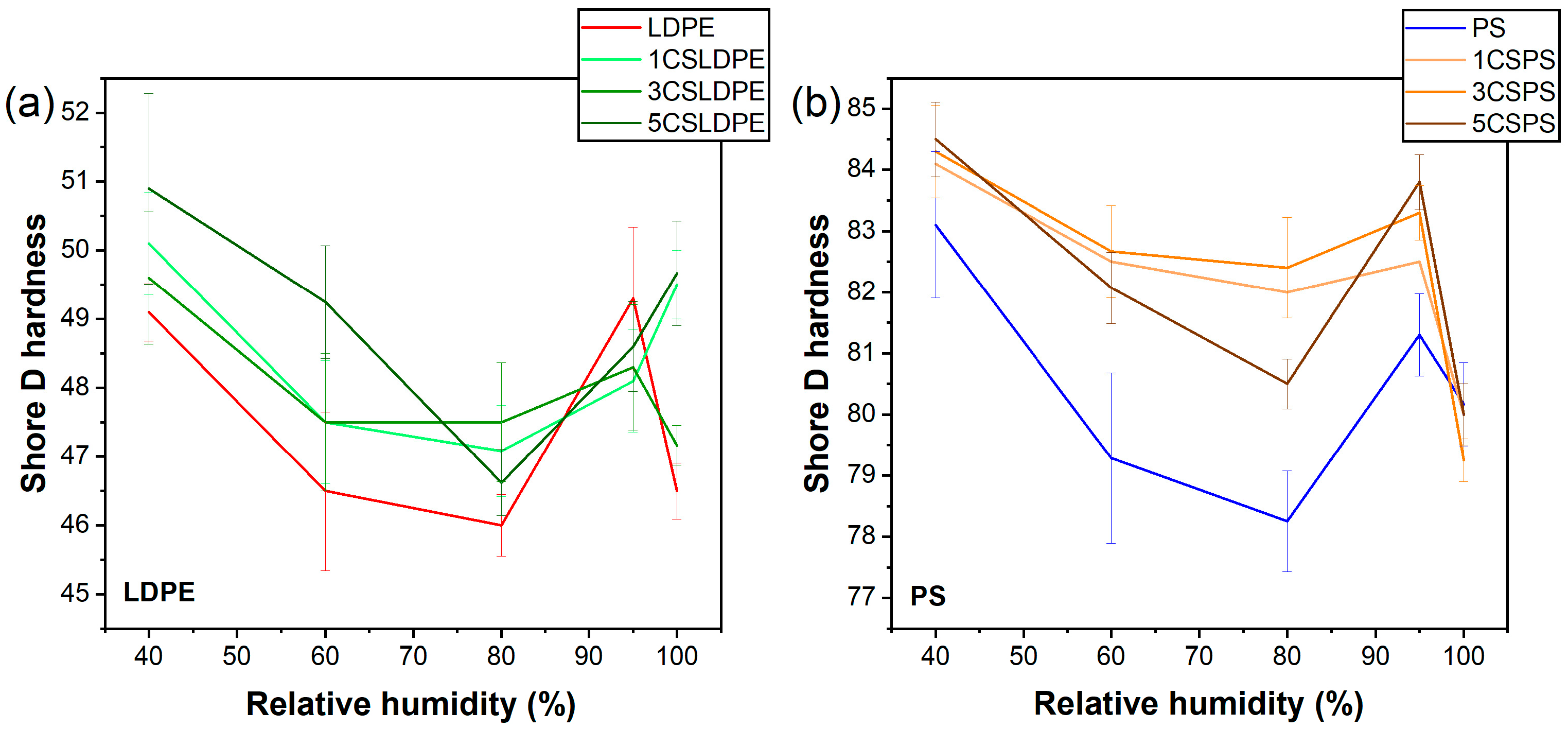
3.6. Hardness Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RH | Relative Humidity |
| CS | Chitosan |
| PLA | Polylactic Acid |
| PCL | Polycaprolactone |
| LDPE | Low Density Polyethylene |
| PS | Polystyrene |
| PVA | Poly (Vinyl Alcohol) |
| XRD | X-ray Diffraction |
| DSC | Differential Scanning Calorimetry |
| WVT | Water Vapor Transmission |
| MFI | Melt Flow Index |
| RPM | Revolutions Per Minutes |
| PHR | Per Hundred Resins |
| TGA | Thermogravimetry |
| kV | Kilovolts |
| mA | Milliamperes |
| ICDD | International Centre for Diffraction Data |
| Tg | Glass Transition Temperature |
| Tm | Melting Temperature |
| Tc | Crystallization Temperature |
| ΔHm | Melting enthalpy |
| ΔHc | Crystallization enthalpy |
| χc | Percentage of crystallinity |
| ΔH100 | 100% crystalline polyethylene enthalpy |
| %Abs | Percentage of absorption |
| D | Water Diffusion Coefficient |
| %SML | Percentage of Soluble Materia Loss |
| ASTM | American Standard for Testing and Materials |
| WW | Wet Weight |
| DW | Dry Weight |
| IW | Initial Weight |
| D0 | Uncorrected water diffusion coefficient |
| H | Height |
| %M | Percentage of Moisture |
| T | Time |
| L | Length |
| W | Wide |
| WG | Weight Gained |
| A | Area |
| HD | Hardness |
| Tonset | Initial Degradation Temperature |
| Tmax | Maximum Degradation Temperature |
| Tp | Processing Temperature |
References
- Oliviero, M.; Lamberti, E.; Cafiero, L.; Pace, B.; Cefola, M.; Gorrasi, G.; Sambandam, A.; Sorrentino, A. Biodegradable cellulose acetate/layered double-hydroxide composite film for active packaging of fresh food. Mater. Chem. Phys. 2023, 310, 128469. [Google Scholar] [CrossRef]
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Deen, M.J. Fruit Quality Monitoring with Smart Packaging. Sensors 2021, 21, 1509. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Kumar, L.; Gaikwad, K.K. Halloysite nanotubes for food packaging application: A review. Appl. Clay Sci. 2023, 234, 106856. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly(lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. LWT 2021, 135, 110034. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, J.; Huang, S.; Duan, F. High oxygen barrier property of polyethylene composite films with bilayer polyvinyl alcohol coating for emergency foods in high-humidity environments. J. Appl. Polym. Sci. 2024, 141, e55251. [Google Scholar] [CrossRef]
- Nong, W.; Luo, H.; Wang, G.; Chen, Q.; Zou, X.; Miao, W.; Wu, J.; Guan, W.; Qu, S. β-CD-MOF-based edible antimicrobial packaging film with humidity-controlled carvacrol release for preserving fresh strawberry. Carbohydr. Polym. 2025, 351, 123133. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhu, M.; Zhang, L.; Zhu, L. Development of cinnamaldehyde/aminated gelatin film as pH-responsive controlled-release packaging for cherry preservation: Effects of CO2 and humidity in the microenvironment. Food Packag. Shelf Life 2025, 48, 101457. [Google Scholar] [CrossRef]
- Dhalsamant, K.; Dalai, A.; Pattnaik, F.; Acharya, B. Biodegradable Carbohydrate-Based Films for Packaging Agricultural Products—A Review. Polymers 2025, 17, 1325. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Properties of active packaging of PLA-PCL film integrated with chitosan as an antibacterial agent and syzygium cumini seed extract as an antioxidant agent. Heliyon 2024, 10, 23952. [Google Scholar] [CrossRef]
- Suryani, S.; Rihayat, T.; Fitria, F.; Sariadi, S.; Yunus, M.; Hasanah, U.; Safitri, A. The Impact of Chitosan Incorporation on the Mechanical Characteristics of Biodegradable Packaging based on PLA/PCL Blend. E3S Web Conf. 2024, 503, 08004. [Google Scholar] [CrossRef]
- Fiallos-Núñez, J.; Cardero, Y.; Cabrera-Barjas, G.; García-Herrera, C.M.; Inostroza, M.; Estevez, M.; España-Sánchez, B.L.; Valenzuela, L.M. Eco-Friendly Design of Chitosan-Based Films with Biodegradable Properties as an Alternative to Low-Density Polyethylene Packaging. Polymers 2024, 16, 2471. [Google Scholar] [CrossRef]
- Gautam, S.; Kumari, K.; Sonowal, L. Chitosan Composite Biofilms for Active and Smart Food Packaging. ACS Symp. Ser. 2025, 2, 1–24. [Google Scholar] [CrossRef]
- Kusumastuti, Y.; Putri, N.R.E.; Timotius, D.; Syabani, M.W. Effect of chitosan addition on the properties of low-density polyethylene blend as potential bioplastic. Heliyon 2020, 6, e05280. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, Z.; Ye, H.; Li, W.; Shi, W.; Liu, Y.; Zhang, Y. Waste polystyrene foam—Chitosan composite materials as high-efficient scavenger for the anionic dyes. Colloids Surf. A Physicochem. Eng. 2021, 627, 127155. [Google Scholar] [CrossRef]
- Dai, L.; Liu, Q.; Li, M.; Wu, X.; Zheng, W.; Weng, W.; Zhang, Y. Water-resistant and antibacterial food packaging films prepared from bamboo shoot shell cellulose nanofibers and cinnamaldehyde/chitosan emulsion. Food Hydrocoll. 2025, 168, 111512. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Li, C.; Zheng, Y.; Xu, J. A strong, excellent water resistance, and anti-ultraviolet poly(vinyl alcohol)/lignocellulose/poly(butylene adipate-co-terephthalate) composite with “sandwich” structure. Int. J. Biol. Macromol. 2025, 289, 138779. [Google Scholar] [CrossRef]
- Papapetros, K.; Mathioudakis, G.N.; Vroulias, D.; Koutroumanis, N.; Voyiatzis, G.A.; Andrikopoulos, K.S. Structure-Properties Correlations of PVA-Cellulose Based Nanocomposite Films for Food Packaging Applications. Polymers 2025, 17, 1911. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, Z.; Jin, H. Nondestructive testing and evaluation techniques of defects in fiber-reinforced polymer composites: A review. Front. Mater. 2022, 9, 986645. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Pan, Z.; Liu, J.; Ma, L.; Zhou, J.; Su, Y. Review on optimization design, failure analysis and non-destructive testing of composite hydrogen storage vessel. Int. J. Hydrogen Energy. 2022, 47, 38862–38883. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Javed, S.; Khan, T.M.Y.; Baig, R.U. Assessment of Destructive and Nondestructive Analysis for GGBS Based Geopolymer Concrete and Its Statistical Analysis. Polymers 2022, 14, 3132. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 4, 793. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, X.; Cao, L.; Chen, Y. Water-resistant and barrier properties of poly(vinyl alcohol)/nanocellulose films enhanced by metal ion crosslinking. Int. J. Biol. Macromol. 2024, 277, 134245. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Xie, X. Water vapor sorption properties of cellulose nanocrystals and nanofibers using dynamic vapor sorption apparatus. Sci. Rep. 2017, 7, 14207. [Google Scholar] [CrossRef]
- Mulla, M.F.Z.; Ahmed, J.; Vahora, A.; Pathania, S.; Rashed, M.S. Characterization of biopolymers based antibacterial films enriched with thyme essential oil and their application for milk cake preservation. Front. Food Sci. Technol. 2024, 4, 1356582. [Google Scholar] [CrossRef]
- ASTM D1238–22; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2022.
- ICDD. PDF-4/Organics 2025 (Database); Kabekkodu, S.N., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA.
- Poh, L.; Wu, Q.; Chen, Y.; Narimissa, E. Characterization of industrial low-density polyethylene: A thermal, dynamic mechanical, and rheological investigation. Rheol. Acta 2022, 61, 701–720. [Google Scholar] [CrossRef]
- ASTM D570–22; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Almudaihesh, F.; Holford, K.; Pullin, R.; Eaton, M. A comparison study of water diffusion in unidirectional and 2D woven carbon/epoxy composites. Polym. Compos. 2022, 43, 118–129. [Google Scholar] [CrossRef]
- ASTM E96–22; Standard Test Method for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D2240–22; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2022.
- Sorolla-Rosario, D.; Llorca-Porcel, J.; Pérez-Martínez, M.; Lozano-Castelló, D.; Bueno-López, A. Study of microplastics with semicrystalline and amorphous structure identification by TGA and DSC. J. Environ. Chem. Eng. 2021, 10, 106886. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, R.; Hassan, S.M.M.; Karim, M.R.; Rahman, M.M.; Rahman, S.; Hossain, M.N.; Islam, D.; Shaikh, M.A.A.; Georghiou, P.E. Carboxymethyl chitin and chitosan derivatives: Synthesis, characterization and antibacterial activity. Carbohydr. Polym. Technol. Appl. 2023, 5, 100283. [Google Scholar] [CrossRef]
- Budi, S.; Suliasih, B.A.; Rahmawati, I. Size-controlled chitosan nanoparticles prepared using ionotropic gelation. ScienceAsia 2020, 46, 457. [Google Scholar] [CrossRef]
- Porkar, B.; Atmianlu, P.A.; Mahdavi, M.; Baghdadi, M.; Farimaniraad, H.; Abdoli, M.A. Chemical modification of polystyrene foam using functionalized chitosan with dithiocarbamate as an adsorbent for mercury removal from aqueous solutions. Korean J. Chem. Eng. 2023, 40, 892–902. [Google Scholar] [CrossRef]
- Popyrina, T.N.; Khavpachev, M.A.; Ivanov, P.L.; Monakhova, K.Z.; Kuchkina, I.O.; Evtushenko, Y.M.; Goncharuk, G.P.; Zelenetskii, A.N. Morphology and Physical-Chemical Properties of Composite Materials Based on Polyolefins and Chitosan. Polym. Sci. Ser. C 2024, 66, 46–54. [Google Scholar] [CrossRef]
- Bekhit, M.; Fathy, E.S.; Sharaf, A.; Shiple, M. Impact of gamma irradiation on physico-chemical and electromagnetic interference shielding properties of Cu2O nanoparticles reinforced LDPE nanocomposite films. Sci. Rep. 2024, 14, 4144. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Pashameah, R.A.; Saeed, A.; Alwafi, R.; Alzahrani, E.; AlSubhi, S.A.; Yassin, A.Y. Boosting the optical, structural, electrical, and dielectric properties of polystyrene using a hybrid GNP/Cu nanofiller: Novel nanocomposites for energy storage applications. J. Mater. Sci. Mater. Electron. 2023, 34, 678. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Agnes, E.A.; Hillig, E.; Zattera, A.J.; Beltrami, L.R.; Covas, J.A.; Hilliou, L.; Sousa, J.D.; Calado, L.; Pinto, M.; de Andrade Lucas, A. Potentialities of cellulose nanofibers (CNFs) in low density polyethylene (LDPE) composites. Eur. J. Wood Prod. 2024, 82, 1501–1510. [Google Scholar] [CrossRef]
- Tuna, S.; Şen, İ. Characterization of Olive Seed Powder Incorporated Low Density Polyethylene Composites. Sak. Univ. J. Sci. 2025, 29, 71–82. [Google Scholar] [CrossRef]
- Xu, J.; Reiter, G.; Alamo, R.G. Concepts of Nucleation in Polymer Crystallization. Crystals 2021, 11, 304. [Google Scholar] [CrossRef]
- Defelice, J.; Lipson, J.E.G. The Influence of Additives on Polymer Matrix Mobility and the Glass Transition. Soft Matter. 2021, 17, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ruan, Y.; Wang, H.; Zhao, Y.; Bi, D. Studies on glass transition temperature of chitosan with four techniques. J. Appl. Polym. Sci. 2004, 93, 1553–1558. [Google Scholar] [CrossRef]
- Han, X.; Xue, Y.; Lou, R.; Ding, S.; Wang, S. Facile and efficient chitosan-based hygroscopic aerogel for air dehumidification. Int. J. Biol. Macromol. 2023, 251, 126191. [Google Scholar] [CrossRef] [PubMed]
- Sugiman, S.; Setyawan, P.D.; Anshari, B. Effect of fiber length on the mechanical properties and water absorption of bamboo fiber/polystyrene-modified unsaturated polyester composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 532, 012008. [Google Scholar] [CrossRef]
- Kamaludin, N.H.I.; Ismail, H.; Rusli, A.; Ting, S.S. Thermal behavior and water absorption kinetics of polylactic acid/chitosan biocomposites. Iran. Polym. J. 2021, 30, 135–147. [Google Scholar] [CrossRef]
- Du, H.; Sun, X.; Chong, X.; Yang, M.; Zhu, Z.; Wen, Y. A review on smart active packaging systems for food preservation: Applications and future trends. Trends Food Sci. Technol. 2023, 141, 10420. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.; Amaraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications: A Review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, W.; Zhang, J.; Li, Q.; Guo, Z. Water-soluble amino functionalized chitosan: Preparation, characterization, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2022, 217, 969–978. [Google Scholar] [CrossRef]
- Seid, A.M.; Adimass, S.A.; Salilew, W.M.; Vignesh, K.; Paramasivam, V.; Fentaw, B.A. Recent Progress on the Physical, Thermal, and Mechanical Properties of Expanded Polystyrene Waste–Based Composites. Int. J. Polym. Sci. 2025, 1, 9285040. [Google Scholar] [CrossRef]
- Barros, C.; Miranda, S.; Castro, O.; Carneiro, O.S.; Machado, A.V. LDPE-Nanoclay films for food packaging with improved barrier properties. J. Plast. Film Sheeting 2023, 39, 304–320. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abd El-Aziz, M.E.; Morsi, S.M.M. Development and evaluation of antimicrobial LDPE/TiO2 nanocomposites for food packaging applications. Polym. Bull. 2023, 80, 5417–5431. [Google Scholar] [CrossRef]
- Heydari, H.; Salehian, S.; Amiri, S.; Soltanieh, M.; Musavi, S.A. UV-cured polyvinyl alcohol-MXene mixed matrix membranes for enhancing pervaporation performance in dehydration of ethanol. Polym. Test. 2023, 123, 108046. [Google Scholar] [CrossRef]
- Turan, D. Water Vapor Transport Properties of Polyurethane Films for Packaging of Respiring Foods. Food Eng. Rev. 2021, 1, 54–65. [Google Scholar] [CrossRef]
- Duan, Z.; Thomas, N.L. Water vapour permeability of poly(lactic acid): Crystallinity and the tortuous path model. J. Appl. Phys. 2014, 6, 115. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Baikousi, M.; Moschovas, D.; Asimakopoulos, G.; Zafeiropoulos, N.E.; Avgeropoulos, A. Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules 2021, 26, 1585. [Google Scholar] [CrossRef]
- Long, J.; Zhang, W.; Zhao, M.; Ruan, C.Q. The reduce of water vapor permeability of polysaccharide-based films in food packaging: A comprehensive review. Carbohydr. Polym. 2023, 321, 121267. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, J.; Kandukuri, V.A.; Chidambaram, R. A critical review on various treatment, conversion, and disposal approaches of commonly used polystyrene. Polym. Bull. 2024, 81, 2819–2845. [Google Scholar] [CrossRef]
- Trinh, B.M.; Chang, B.P.; Mekonnen, T.H. The barrier properties of sustainable multiphase and multicomponent packaging materials: A review. Prog. Mater. Sci. 2023, 133, 101071. [Google Scholar] [CrossRef]
- Charles, A.P.R.; Rajasekaran, B.; Awasti, N.; Choudhary, P.; Khanashyam, A.C.; Majumder, K.; Wu, Y.; Pandiselvam, R.; Jin, T.Z. Emerging chitosan systems incorporated with polyphenols: Their applications in intelligent packaging, active packaging, and nutraceutical systems—A comprehensive review. Int. J. Biol. Macromol. 2025, 308, 142714. [Google Scholar] [CrossRef]
- Shi, B.; Hao, Z.; Du, Y.; Jia, M.; Xie, S. Chitosan composite films; Review. Bioresources 2024, 19, 4001–4014. [Google Scholar] [CrossRef]
- Despond, S.; Espuche, E.; Domard, A. Water sorption and permeation in chitosan films: Relation between gas permeability and relative humidity. J. Polym. Sci. B Polym. Phys. 2001, 39, 3114–3127. [Google Scholar] [CrossRef]
- Wason, S.; Verma, T.; Subbiah, J. Validation of process technologies for enhancing the safety of low-moisture foods: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4950–4992. [Google Scholar] [CrossRef]
- Jiménez-Regalado, E.J.; Caicedo, C.; Fonseca-García, A.; Rivera-Vallejo, C.C.; Aguirre-Loredo, R.Y. Preparation and Physicochemical Properties of Modified Corn Starch–Chitosan Biodegradable Films. Polymers 2021, 13, 4431. [Google Scholar] [CrossRef]
- Solomon, S.; Hall, R.; He, J.; John, V.; Pesika, N. Enhancing the Tribological Properties of Low-Density Polyethylene Using Hard Carbon Microfillers. Materials 2024, 17, 1536. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodriguez-Hernandez, A.I.; Velazquez, G. Modelling the effect of temperature on the water sorption isotherms of chitosan films. Food Sci. Technol. 2016, 37, 112–118. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef]
- Caicedo, C.; Díaz-Cruz, C.A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films. Materials 2022, 15, 1274. [Google Scholar] [CrossRef] [PubMed]
- Varyan, I.; Tyubaeva, P.; Kolesnikova, N.; Popov, A. Biodegradable Polymer Materials Based on Polyethylene and Natural Rubber: Acquiring, Investigation, Properties. Polymers 2022, 14, 2457. [Google Scholar] [CrossRef] [PubMed]
- Cherkashina, N.I.; Pavlenko, Z.V.; Pushkarskaya, D.V.; Denisova, L.V.; Domarev, S.N.; Ryzhikh, D.A. Synthesis and Properties of Polystyrene Composite Material with Hazelnut Shells. Polymers 2023, 15, 3212. [Google Scholar] [CrossRef] [PubMed]
- Fila, K.; Podkościelna, B.; Szymczyk, K. The application of chitosan as an eco-filler of polymeric composites. Adsorption 2024, 30, 157–165. [Google Scholar] [CrossRef]



| Technique/Method | Parameters/Standard |
|---|---|
| TGA | Heating rate of 10 °C min−1, ranging from 30 °C to 600 °C. |
| XRD | CuKα radiation at 40 kilovolts (kV) and 30 milliamperes (mA). The scan range covered 2θ angles from 10° to 80°, with a scan rate of 0.026° s−1. |
| DSC | Temperature ranges from 30 °C to 160 °C, with a heating rate of 10 °C min−1, under a nitrogen atmosphere |
| Water absorption/diffusion | ASTM D570 |
| WVT | ASTM E96 |
| Hardness | ASTM D2240 |
| Tm | ΔHm | Tc | Hc | χc | |
|---|---|---|---|---|---|
| Composite | (°C) | (J g−1) | (°C) | (J g−1) | |
| LDPE | 113.3 | 45 | 93 | 67.6 | 15.3 |
| 1CSLDPE | 112.1 | 42.3 | 97.4 | 65.4 | 14.4 |
| 3CSLDPE | 112.4 | 40.1 | 94.9 | 64.3 | 13.6 |
| 5CSLDPE | 115.1 | 45.7 | 94.3 | 62.6 | 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas Méndez, J.R.; Téllez Rosas, M.M.; Aguirre Flores, R.; Avalos Belmontes, F.; González, F.J.; Hoyos, M. Chitosan Enhanced Polymers for Active Packaging: Intelligent Moisture Regulation and Non-Invasive Assessment. Appl. Sci. 2025, 15, 11744. https://doi.org/10.3390/app152111744
Villegas Méndez JR, Téllez Rosas MM, Aguirre Flores R, Avalos Belmontes F, González FJ, Hoyos M. Chitosan Enhanced Polymers for Active Packaging: Intelligent Moisture Regulation and Non-Invasive Assessment. Applied Sciences. 2025; 15(21):11744. https://doi.org/10.3390/app152111744
Chicago/Turabian StyleVillegas Méndez, Jesús R., María Maura Téllez Rosas, Rafael Aguirre Flores, Felipe Avalos Belmontes, Francisco J. González, and Mario Hoyos. 2025. "Chitosan Enhanced Polymers for Active Packaging: Intelligent Moisture Regulation and Non-Invasive Assessment" Applied Sciences 15, no. 21: 11744. https://doi.org/10.3390/app152111744
APA StyleVillegas Méndez, J. R., Téllez Rosas, M. M., Aguirre Flores, R., Avalos Belmontes, F., González, F. J., & Hoyos, M. (2025). Chitosan Enhanced Polymers for Active Packaging: Intelligent Moisture Regulation and Non-Invasive Assessment. Applied Sciences, 15(21), 11744. https://doi.org/10.3390/app152111744







