Abstract
This article reveals the nature, causes, and main stages of occurrence and development of endogenous fires in coal mines. It is emphasized that one of the key tasks of fire protection specialists is the most accurate determination of the stage of oxidation and self-heating of coal. A review of existing gas analysis methods for identifying the initial and subsequent stages of endogenous fire development is conducted. Particular attention is focused on the importance of obtaining prompt reliable information on the self-heating temperature of coal and the dynamics of its change in the early stages of the process. Since self-heating zones are usually inaccessible for direct instrumental control, the main source of information is the gas analysis of air samples. The authors present the results of research on the dependence of the indicator gas content on the coal self-heating temperature. Based on the Graham criterion, the stages of thermal development of the process are predicted. Correlation dependencies between temperature and integral parameters of indicator gas concentrations are developed, allowing for a sufficient degree of reliability in determining the stages of coal self-heating and spontaneous combustion. Based on the results of the work, methodological recommendations for the prevention and warning of endogenous fires in coal mines and opencasts are proposed. They are based on the most informative and accessible signs suitable for quantitative assessment. The implementation of these recommendations will improve the level of industrial safety and reduce the risks of fires and explosions during mining operations.
1. Introduction
Unlike existing approaches that are mainly based on the use of individual indicator gases, this study proposes a model that incorporates integral criteria and applies the Graham criterion. This approach provides a more reliable assessment of the stages of coal self-heating and reduces the probability of false alarms in spontaneous combustion prediction. The scientific novelty of the research lies in establishing quantitative correlations between coal self-heating temperature and the concentrations of indicator gases, which form the basis for developing practical methodological recommendations for diagnosing endogenous fire hazards. Although the experiments were carried out on coals of the Karaganda basin, the proposed method has a universal character. Its adaptation to other coal-mining regions is possible through local calibration of empirical coefficients, taking into account coal composition, degree of metamorphism, and gas content, which ensures the scalability and broad applicability of the proposed model.
The problem of coal spontaneous combustion has been observed since the earliest mining operations and remains one of the most critical industrial safety challenges. It occurs not only in underground mines but also in storage facilities, external and internal dumps, and open pits. Endogenous fires pose a serious hazard to miners’ health and safety, cause substantial economic losses, and reduce coal production efficiency.
Global mining practice demonstrates the catastrophic consequences of such fires. Numerous incidents in Kazakhstan and other coal-producing countries confirm that issues of fire and explosion safety remain highly relevant [1,2,3,4,5]. In the Karaganda coal basin, several thick seams show an increased propensity for self-heating, creating severe safety concerns [6,7,8]. Many mine explosions traditionally attributed to methane accumulation [1,2,3,4,5,6] may in fact be triggered by hidden endogenous fires, where coal self-heating generates combustible gases and creates conditions for secondary explosions [9,10].
Endogenous fires are characterized by specific patterns of initiation and development. They frequently occur in inaccessible zones, including mined-out or isolated areas, coal pillars, and satellite seams [11]. Coal in these locations is often mechanically deformed and fractured, which increases its reactivity and risk of self-heating. Suppression of such fires is particularly difficult, resulting in prolonged accidents with high remediation costs. In gassy mines, which dominate in Kazakhstan and globally, endogenous fires can further escalate into methane–air explosions with catastrophic consequences.
The most typical source of spontaneous combustion in mined-out areas is the residual crushed coal or damaged edge portions of pillars. Geological disturbances exacerbate the problem by creating unstable, highly fractured zones with increased coal activity. Preventing self-heating in such environments is extremely challenging due to persistent air leaks. Therefore, research on early detection and preventive measures receives significant international attention.
The oxidation mechanism of coal remains complex and not fully explained. Thermal modeling studies describe the temperature field in mined-out areas using the principles of heat conduction [12]. Among existing hypotheses, the adsorption theory is most widely accepted: self-heating begins with oxygen adsorption on active surface sites of coal particles, releasing heat. With insufficient heat dissipation, the temperature rises, triggering exothermic oxidation reactions and ultimately leading to spontaneous combustion.
The main factors influencing coal oxidation include particle size distribution, porosity and fracturing, moisture content, mineral impurities, and climatic conditions. Finer particles and highly porous coal exhibit higher sorption capacity. Moisture plays a dual role: evaporation enhances gas exchange, replacing oxygen-depleted air with oxygen-rich inflow, thus accelerating oxidation [13,14]. However, articles in the literature provide contradictory evidence. Some studies [15] report that moisture catalyzes oxidation and accelerates combustion, while others [16,17] indicate that increased water content reduces oxygen uptake and slows self-heating. Similarly, the effect of air filtration rate through coal accumulations remains disputed.
In summary, coal spontaneous combustion is a multifactorial process strongly dependent on physical, chemical, and environmental parameters. Its prevention requires a deeper understanding of oxidation mechanisms, continuous monitoring of indicator gases, and development of reliable predictive models tailored to specific geological conditions.
The aim of the study is to conduct a comprehensive investigation of coal self-heating processes in order to develop a scientifically grounded model that enables reliable identification of self-heating stages and prevention of endogenous fires in coal mines. To achieve this aim, the study establishes quantitative correlations between coal temperature and the content of indicator gases, applies integral criteria and the Graham criterion to improve prediction accuracy, and develops methodological recommendations together with an information–analytical system for mine atmosphere monitoring. The implementation of this aim will not only enhance industrial safety but also ensure the adaptability of the proposed methodology to various geological and mining conditions.
Experimental studies and natural observations of the oxidation process and self-heating of coal have established that several characteristic stages (or phases) are noticeably distinguished in this process. The division and study of the stages of self-heating of coal pursues a very specific practical goal—to recognize the signs of a possible endogenous fire and take preventive measures. The most practical significance for preventing endogenous fires is their detection during the initial period, which, according to research [18,19], includes [20,21] three stages (Figure 1) that differ in the composition of gaseous products, the rate of oxidation of coal, and the release of heat.
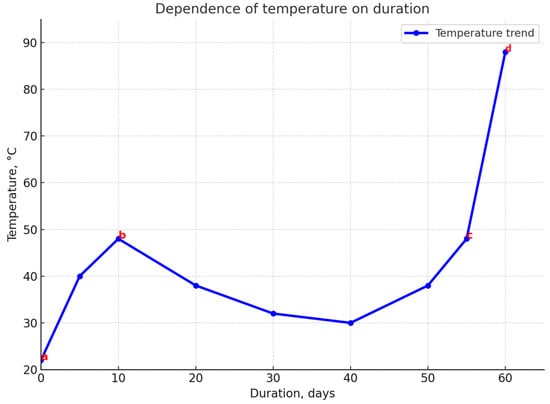
Figure 1.
The main stages in the spontaneous combustion process of coal. a–b—self-heating; b–c—moisture evaporation; c–d—intense oxidation until spontaneous combustion.
Knowledge and clear definition of the stages of oxidation and spontaneous combustion processes are necessary for miners to take precautionary measures and to prevent endogenous fires.
For example, in one article [22], a method for assessing the parameters of the risk of endogenous fire occurrence based on the duration of the incubation period is presented. The method is based on the laws of heat exchange accompanying the process of self-heating of coal, taking into account the range of the heat release characteristic for the incubation stage.
In their scientific work, the authors of [23] believe that it is advisable to use the method of multiple reversal of the ventilation stream to extinguish fires. However, issues related to the safety and effectiveness of its use, as well as the nature and duration of transient aerodynamic processes that occur in the accident zone when implementing this method, remain insufficiently studied.
Unlike existing approaches, which are mainly based on the analysis of individual indicator gases, the proposed model employs integral criteria and the Graham criterion, ensuring higher accuracy and stability in diagnosing the stages of self-heating. Experimentally established correlations between coal temperature and indicator gas concentrations make it possible to develop a methodology that demonstrates greater reliability compared to traditional gas analysis methods. An important outcome of this work is the demonstration of the universality of the proposed model: although the experiments were carried out on coals from the Karaganda basin, its application is also feasible for other coal-mining regions. For this purpose, local calibration of empirical coefficients is required, taking into account variations in composition, degree of metamorphism, and gas content of coals, which ensures the scalability of the methodology and makes it applicable in diverse mining and geological conditions.
2. Materials and Methods
Complex experimental studies of the thermal and thermodynamic properties of coals from the Karaganda basin were carried out in an accredited specialized laboratory, the Scientific and Engineering Center GeoMark LLC. The Karaganda basin was chosen as the study area due to its geological uniqueness, high industrial importance, and the known increased propensity of local coal seams to self-heating and the development of endogenous fires. In particular, the research focused on coal samples extracted from the K10, K12, and d6 seams, which belong to the category of coking fat coals. According to numerous studies, including both national and international reports [1,2,3], coking fat coals exhibit a high degree of reactivity, increased porosity, and elevated volatile matter content, which significantly enhances their susceptibility to spontaneous combustion.
During the experiment, the coal sample was heated in an electric heating furnace (rather than self-heating); the temperature of the gas–air flow was measured before entering the electric heating furnace and at the outlet after the reaction with coal. The laboratory equipment used in the study is shown in Figure 2.
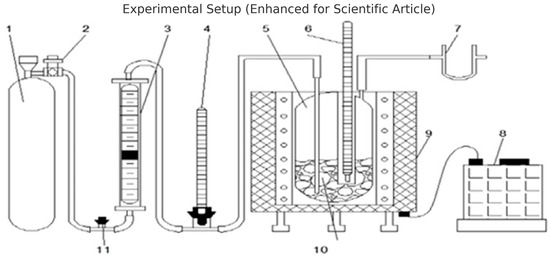
Figure 2.
Schematic diagram showing the coal heating installation: 1—air cylinder, 2—reducer, 3—rotameter, 4, 6—thermometers, 5—glass, 7—filter, 8—transformer, 9—electric heating furnace, 10—coal, 11—safety valve.
In a special glass 5 with gas-exhaust tubes and thermometers, a sample of 1–3 mm fraction coal was heated in a water bath. Periodically, at certain temperature intervals, samples of the gas–air mixture were taken from the tube in the glass chamber. The samples were analyzed by chromatography for the volume content of carbon monoxide CO, carbon dioxide CO2, and hydrogen H2.
For research and establishing correlation dependencies of the calculated temperature of the atmosphere within the controlled area, a special package of applied MATLAB programs (https://www.mathworks.com/products/matlab.html), intended for scientific research, was used.
2.1. Object of the Study and Sampling Strategy
Sampling procedures were performed in compliance with established standards (ISO 18283:2006 [24] “Hard coal and coke—Manual sampling” and GOST 10742-71 [25] “Coal sampling”). Representative coal samples were collected at working faces under controlled conditions to minimize contamination and ensure statistical validity of the obtained results. The bulk samples were subjected to preliminary preparation in the laboratory, which included drying at 105 °C until constant weight, homogenization, and sieving to obtain fractions of the desired particle size.
For the experimental series, particle size 1–3 mm coal samples were selected. The choice of this fraction was deliberate: it provides an optimal balance between sufficient surface area for oxygen adsorption and preservation of physicochemical properties representative of natural conditions. As reported in previous studies, smaller fractions (below 0.5 mm) tend to accelerate oxidation unnaturally, while larger lumps (>10 mm) hinder heat and mass transfer, making the process unrepresentative of in situ conditions.
2.2. Experimental Setup and Heating Procedure
The core of the experimental setup consisted of two heating systems: (1) an electric heating furnace for controlled heating of samples, and (2) a water-bath heating system with a glass chamber, equipped with exhaust tubes and thermometers.
In the first configuration, the coal sample was exposed to a heated gas–air stream. The temperature of the incoming gas–air flow was measured prior to entering the furnace, and the outlet temperature was recorded after its interaction with the coal mass. This approach allowed assessment of the heat exchange between the coal sample and the flowing medium, simulating the conditions of ventilation air interacting with heated coal in underground workings. The second configuration employed a specially designed glass vessel (volume 250 mL) equipped with gas-exhaust tubes and precision mercury thermometers (accuracy ±0.1 °C). A weighed sample of coal (50 g, particle size 1–3 mm) was placed in the chamber, which was immersed in a thermostatically controlled water bath. This design enabled gradual and uniform heating of the coal sample, excluding the influence of uncontrolled self-heating. The water bath ensured temperature stability within ±0.5 °C across the experimental series.
The heating rate was maintained at 0.5–1 °C/min, in line with previously validated experimental protocols, which allow simulation of low-temperature oxidation processes relevant to endogenous fire development. Rapid heating was intentionally avoided, since it can cause thermal degradation effects uncharacteristic of natural self-heating processes.
Gas Sampling and Chromatographic Analysis
At predetermined temperature intervals (typically every 10 °C, starting from 30 °C up to 200 °C), samples of the gas–air mixture were collected through the exhaust tube into pre-evacuated gas-tight syringes (volume 50 mL). The collected samples were analyzed immediately using a gas chromatograph (Crystal 5000, JSC SDO “Chromatec”, Yoshkar-Ola, Russia), equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID).
Chromatographic conditions were optimized for the separation and quantification of carbon monoxide (CO), carbon dioxide (CO2), and hydrogen (H2). The choice of these indicator gases was based on extensive literature data [9,10,11], demonstrating that their concentrations show the most consistent correlation with coal self-heating stages. Calibration of the chromatograph was performed daily using certified standard gas mixtures with accuracy better than 2%.
The volume concentrations of gases were calculated with corrections for baseline drift and detector sensitivity. For quality assurance, each experimental point was replicated three times, and the mean value was reported. The overall reproducibility of the gas concentration measurements was within ±5%, which is consistent with international practice in coal fire studies.
2.3. Methodological Justification
The choice of experimental design and data analysis methods was dictated by both scientific and practical considerations. Relevance of coking fat coals: Coals of the Karaganda basin are known for their heightened tendency to self-heating, which makes them a priority for fire hazard studies. Controlled heating: The use of an electric furnace and a thermostatic water bath eliminated the stochastic variations typical in uncontrolled self-heating experiments, thereby improving reproducibility. Indicator gases: Focusing on CO, CO2, and H2 is consistent with established international practice, as these gases are recognized as the most sensitive indicators of low-temperature oxidation processes. Chromatography: Gas chromatography remains the gold standard for precise quantitative determination of gas mixtures, ensuring accuracy and traceability. MATLAB-based modeling: The use of high-precision computational tools ensured rigorous statistical validation of results, making the developed correlations applicable for practical forecasting in mine safety systems.
2.4. Applicability and Transferability
Although the experiments were conducted on coals of the Karaganda basin, the methodology has a universal character. Adaptation to other coal-mining regions is possible through local calibration of empirical coefficients, taking into account variations in coal composition, rank, and gas content. This scalability is particularly important for developing unified approaches to fire hazard assessment across different geological settings.
Moreover, the developed methodological framework serves as the foundation for “Methodological Recommendations for Assessing Endogenous Fire Hazard Based on the Results of Indicator Gas Analysis in the Atmosphere of the Controlled Area,” which have been tailored for practical use by mine safety services.
To confirm the reliability of the obtained data and to provide a comprehensive interpretation of coal self-heating processes, graphical dependencies were constructed to illustrate the changes in gas composition and the application of integral criteria. Figure 3a–c presents the results of experimental studies, including the dynamics of major gas concentrations during heating of bituminous coal from the Karaganda basin; a comparative analysis of different coal types; and the application of the CO/ΔO2 integral criterion to diagnose the transition to the stage of intensive self-heating.
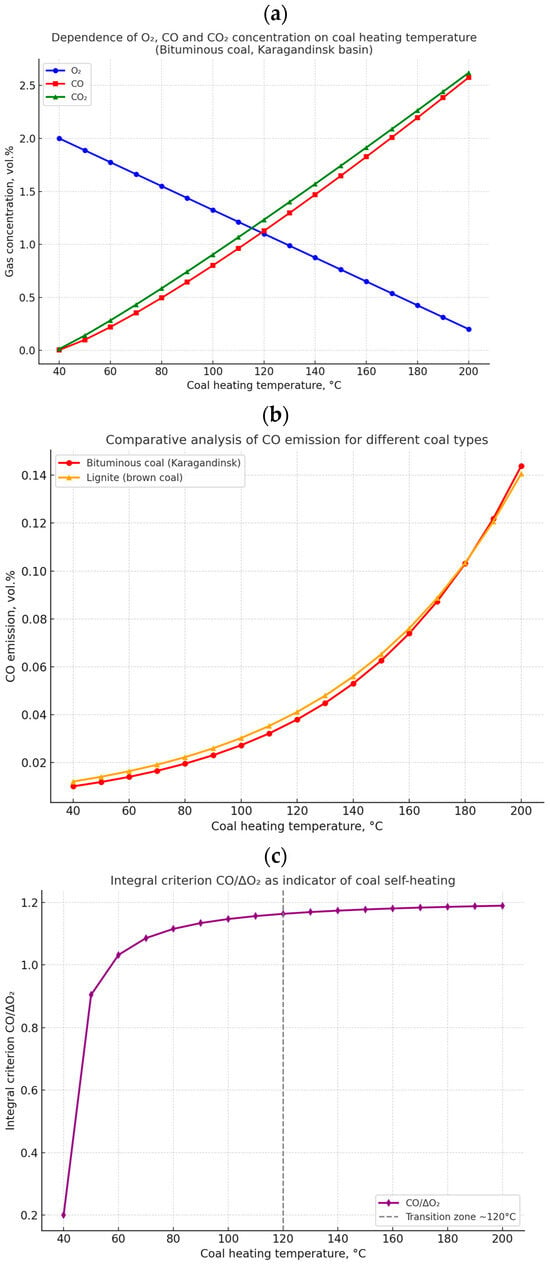
Figure 3.
(a) Dependence of O2, CO, and CO2 concentrations on coal heating temperature; (b) Comparative analysis of gas emissions for bituminous and lignite coal; (c) Integral criterion CO/ΔO2 as an indicator of the self-heating stage.
Figure 3a presents the dynamics of changes in the gas composition during the heating of bituminous coal from the Karaganda basin within the temperature range of 40–200 °C. The concentration of oxygen (O2) decreases consistently, reflecting its intensive consumption in oxidation reactions of the coal organic matter. Simultaneously, an exponential increase in carbon monoxide (CO) and carbon dioxide (CO2) concentrations is observed. The shape of the curves indicates the transition of the system from the stage of low-temperature oxidation (up to ~100 °C) to the phase of intensive self-heating, accompanied by accelerated release of incomplete (CO) and complete (CO2) oxidation products.
Figure 3b shows a comparison of gas emission processes for bituminous coal from the Karaganda basin and lignite. Despite the differences in absolute CO concentrations, the curve patterns demonstrate an identical trend: a gradual increase in carbon monoxide release with increasing heating temperature. The obtained results confirm the universal nature of the identified regularities, applicable to different coal types. Thus, the revealed dynamics of gas composition changes can be considered a general indicator of the self-heating process, regardless of the genetic type and degree of coal metamorphism.
Figure 3c shows the results of applying the integral criterion based on the ratio of carbon monoxide concentration (CO) to the decrease in oxygen content (ΔO2). In the temperature range up to ~100 °C, the criterion value changes insignificantly, corresponding to the stage of weak oxidation. Starting from about 120 °C, a steady increase in the CO/ΔO2 value is observed, coinciding with the transition to the active phase of self-heating. This result confirms the high diagnostic significance of the CO/ΔO2 criterion, which increases the accuracy of identifying critical stages of oxidative processes in coal masses.
In this work, a set of experimental methods characterized by relative simplicity was used, which was determined by the need to ensure high reproducibility and comparability of results when modeling the processes of coal self-heating. The simplicity of the methodology does not reduce its scientific value; on the contrary, it ensures analytical transparency and guarantees the correctness of data interpretation. To confirm the reliability of the results and provide mutual verification of methodological approaches, comparative studies were carried out, including the following analyses:
2.5. Main Experiment on Hard Coal from the Karaganda Basin
The dependencies of oxygen (O2), carbon monoxide (CO), and carbon dioxide (CO2) concentrations on coal heating temperature in the range of 40–200 °C were recorded. The obtained curves demonstrate a regular decrease in O2 content with an exponential increase in CO and CO2 concentrations, reflecting the transition from initial oxidation to the stage of intensive self-heating.
2.6. Comparative Analysis for Different Coal Types
Additional tests were conducted on lignite samples, the results of which showed a similar pattern of changes in the gas composition of the atmosphere during heating. Despite differences in the absolute values of gas emissions, the trends fully coincide with those observed for hard coal, confirming the universality of the identified regularities.
Application of integral criteria (CO/ΔO2).
To verify the model, an integral criterion was applied that considers the ratio between carbon monoxide release and oxygen consumption. The results showed a stable increase in CO/ΔO2 values at temperatures above 120 °C, which corresponds to the transition to the stage of active self-heating. Thus, this criterion serves as a confirming indicator of the reliability of the results and allows for improving diagnostic accuracy.
The obtained data indicate that even with the use of relatively simple methods, a high degree of reliability and reproducibility is achieved. Additional comparative experiments and integral analysis confirm the correctness of the chosen approach and enhance the scientific and practical significance of the proposed methodology.
3. Results and Discussion
3.1. Study of the Dependence of the Indicator Gas Content on the Self-Heating Temperature of Coal
The current state of investigation into the issue of the reliability of establishing the stages of low-temperature oxidation of coal, self-heating, smoldering and active flame combustion, based on the results of gas-temperature analysis, still does not allow for obtaining a clear answer: whether an endogenous fire occurs or not. The importance of the correctness of this position is very important, especially for underground conditions of coal mining or other combustible minerals.
Of great importance for substantiating the development and implementation of preventive measures used in almost all coal basins with underground mining is the detection of signs that indicate intensive self-heating with the transition to an endogenous fire at an early stage of its development. Timely implementation of fire prevention measures allows for localizing and eliminating fire outbreaks at an early stage with the lowest risk, effort, and costs.
The actual process of spontaneous combustion of coal begins in mines at 12–15 °C; therefore, the most important direction is the study of the initial stage of the process.
During the first stage, it is recommended to begin studying the coal surface to determine its characteristics and their influence on the chemical activity of coal at the usual mine or surface temperature. The most important aspect is to study the kinetics of coal oxidation at low temperatures. Apart from the known theoretical chemical reactions of the release of various gases during self-heating of coal, this must be verified experimentally. The parameters that affect the oxidation rate—oxygen concentration, temperature, relative humidity, the rate of air flow to the sample, and air pressure—are easily modeled using modern laboratory equipment.
Following this classical theoretical concept, in the practice of preventing and warning underground endogenous fires in coal mines, the content of fire indicator gases released during the oxidation of coal in the mine atmosphere is accepted as the most important informative sign [23,26,27,28,29,30,31]. The presence of such gases is due to the chemical reaction of the solid component of coal, carbon, with atmospheric oxygen.
Carbon monoxide and hydrogen are accepted and used as reliable informative indicator gases. The increase in the reliability of control for these gases is due to their inertness at low temperatures, typical for the stages of self-heating and combustion of coal, their low sorption by loosened coal and rocks in the mined-out space and low solubility in water.
To confirm the theoretical representativeness and actual informativeness of the gases accepted as fire indicators, it is necessary to study their occurrence and dynamics in natural conditions. Since it is practically impossible to perform such studies in production conditions, they are usually performed in laboratory conditions that are as close as possible to natural production conditions. The obtained confirmatory results on the release of specific gases in certain temperature ranges of oxidation and self-heating of coal can be used for objective classification of an endogenous fire at different stages of its origin and development.
Since the material composition of coals from various deposits shows that fossil coals have a fairly wide range in composition and quantitative ratios of elements, it can be expected that this will be reflected in the chemical reaction of coal oxidation and, as a consequence, in the composition and ratio of the gases released. Since this rather complex analysis requires time and resources, it is mainly performed for coals prone to spontaneous combustion.
To determine the fire hazardous properties of coals of the Dolinskaya suite of the Karaganda basin (KZh coals of this suite are the most fire hazardous in the basin), comprehensive experimental studies of their thermophysical and thermodynamic properties were performed, and a technical analysis of the coals carried out.
As a result of a series of experiments, empirical dependencies of the content (in vol.%) of fire indicator gases on the heating temperature of coal for the most fire-hazardous seams, K12 and d6, of the Karaganda basin were obtained, the formulas for which are given in Table 1.

Table 1.
Formulas of empirical dependencies for layers K12 and d6 based on the results of laboratory studies.
The obtained dependencies were analyzed to study the temperature limits of the emission of fire indicator gases, and visually represent the type of dependency graphs. These dependencies were used in several cases to assess the state of self-heating of coal and justify the development of preventive measures in the mines of the Karaganda basin.
According to Formulas (1) and (3) in Table 1, Figure 3 shows the graphical representations of experimental dependencies and provides calculated values of emission of fire indicator gases—carbon monoxide CO and dioxide CO2—based on experiments on heating coal from seam K12.
Similar results of calculations using Formula (2) in Table 1 are shown in Figure 4, characterizing the dependence of the release of hydrogen H2 during heating of coal from seam K12.
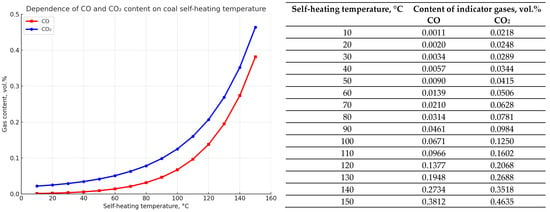
Figure 4.
Graphs showing the dependence of the emission amount of indicator fire gases—carbon monoxide CO and dioxide CO2 (in vol.%)—on the self-heating temperature of coal (°C) for seam K12.
Analysis of the calculation results for the established experimental dependencies of the emission of indicator fire gases during heating of seam K12 coal shows the following. CO monoxide and carbon dioxide CO2 begin to be emitted when the heating temperature of seam K12 coal reaches about 50 °C, which corresponds to approximately half the critical temperature of spontaneous combustion of coal from this seam under normal conditions. Further spontaneous heating leads to a steady increase in the content of these gases to 0.4–0.5 vol.%, which is characteristic for the beginning of the spontaneous combustion stage.
The release of hydrogen H2 with increasing coal temperature is characterized by a more complex dependence, as can be seen from the graph in Figure 5. The volume content of hydrogen, about 0.0006–0.001% in experiments, is observed at a coal heating temperature of 100–120 °C, which is consistent with the data provided in measures to prevent endogenous fires in mines. An increase in temperature from 120 to 300 °C, which characterizes the beginning of the spontaneous combustion process (combustion of volatile products of thermal decomposition of coal), causes a sharp increase in the hydrogen content in the gas sample.
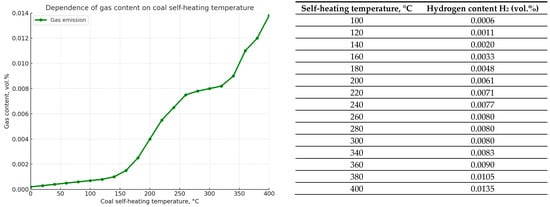
Figure 5.
Graph showing the dependence of hydrogen emission H2 (in vol.%) on the coal self-heating temperature (°C) for seam K12.
The same calculations were performed using the dependencies obtained in experimental studies of self-heating of coal from seam d6, one of the most fire-hazardous and outburst-hazardous seams in the Karaganda basin. The results of calculations using Formulas (4)–(6) of Table 1 are shown in Figure 6 and Figure 7. Qualitatively, the dynamics of indicator fire gases emission during coal self-heating of seam d6 is similar to the dynamics for seam K12, although there is some quantitative difference in the emission of carbon monoxide and carbon dioxide.
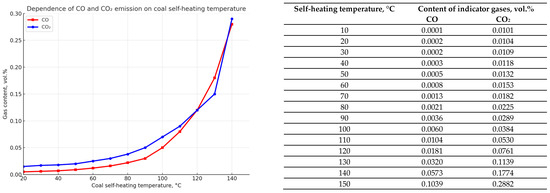
Figure 6.
Graphs showing the dependence of the emission amount of indicator fire gases—carbon monoxide CO and dioxide CO2 (in vol.%)—on the self-heating temperature of coal (°C) for seam d6.
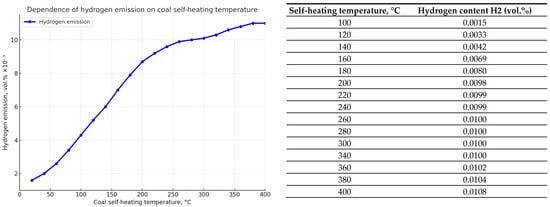
Figure 7.
Graph showing the dependence of hydrogen emission H2 (in vol.%) on the coal self-heating temperature (°C) for seam d6.
The presented results of experimental studies form the basis of regulatory and methodological documents for mine fire safety services, design organizations, scientific workers, etc.
However, in our opinion, it is necessary to solve the inverse problem on this basis, which is to evaluate the temperature and determine the stages of coal self-heating based on the quantitative content of established fire indicator gases in gas analysis samples of the mine atmosphere in the controlled area or object. This is what should be recommended to mine fire protection specialists in conditions where it is impossible to perform direct instrumental measurements.
3.2. Investigation of the Stages of Coal Self-Heating and Spontaneous Combustion Based on the Analysis of the Composition of Indicator Gases in the Mine Atmosphere
The research and analysis by scientists and specialists in fire protection of coal mines, opencasts, underground mines into the practice of applied methods and techniques for establishing and determining the stages of self-heating and spontaneous combustion of combustible solid minerals lead to the conclusion about the need to search for and develop more reliable methods that account for specific conditions.
Historically, a practice has developed for determining the signs of spontaneous combustion of coal by recognizing the content of carbon monoxide and hydrogen in samples of the mine atmosphere from the area of the supposed source that occur in quantities significantly exceeding background values. In compliance with the established rules for determining the background values of the specified indicator fire gases, this gas analysis method, relatively simple to execute, gives acceptable results for practical conclusions about the presence or absence of sources of spontaneous heating and spontaneous combustion in the controlled area of preparatory and mining work in the mine.
In the scientific work of V.I. Igishev [32], it was established that the content of fire indicator gases, in particular hydrogen, characterizes the stages of heating and cooling of the coal massif in the controlled area. It is noted that this fact of close coincidence of hydrogen concentrations during heating and cooling of coal makes it difficult to unambiguously establish the stage of an endogenous fire. Therefore, the author suggests assessing the stages of intensive self-heating (early stage of spontaneous combustion) and cooling of a heated coal accumulation by the ratio of carbon monoxide to hydrogen.
The importance of this problem for coal mine fire protection specialists is reflected in the criticism of existing and proposals for new reliable methods or techniques in the practice of preventing endogenous fires. Thus, the author of the scientific work on this issue [33] believes that “detection of the early stage of spontaneous combustion by the concentration of fire gases (carbon monoxide, hydrogen, hydrocarbons) is difficult due to their formation during low-temperature oxidation and mechanical destruction of coal. Intensive emission of carbon monoxide, hydrogen and unsaturated hydrocarbons begins at a temperature of 150–200 °C. At such a temperature, the rate of spontaneous combustion is very high, and extinguishing of the source begins at the stage of flame combustion, which is the most dangerous in mine conditions”.
In the development of the gas analytical method for recognizing the stage in the beginning and development of endogenous fires, another method has been developed, based on the changing concentration ratio of unsaturated hydrocarbons–ethylene and acetylene [34,35]. Subsequently, on the basis of these developments, a manual was created and is used in the Karaganda coal basin [36]. The initial prerequisites for the creation of this gas analytical method were the following properties and observation results.
Under normal conditions, in the absence of intense self-heating centers, the content of unsaturated hydrocarbons in the mine atmosphere is about (3–5) × 10−7 vol.%, and the ratio of ethylene to acetylene is close to unity. With increasing temperature, the content of these gases in the atmosphere rises exponentially, but the rates of increase are different, which causes a corresponding growth in their ratios. In addition, it was established and is important that the dilution of ethylene and acetylene with distance from the coal self-heating center occurs proportionally; i.e., the stability of their ratios is maintained.
It was established that the volume fraction of ethylene and acetylene at the stage of self-heating exceeds their background values by an order of magnitude and is equal to 10-3% and 10-5%, respectively. At the same time, the temperature of coal at the stage of spontaneous combustion is not determined by the ratio of unsaturated hydrocarbons.
In accordance with [36], the temperature of the coal mass in the self-heating center is established on the basis of the ratios of ethylene and acetylene actually emitted from the controlled area, which are determined from the expression
where and , respectively, are the concentrations of ethylene and acetylene in the outcoming jet of the controlled section, vol.%; and , respectively, are the concentrations of ethylene and acetylene in the incoming jet, vol.%.
The heating temperature of coal is determined by the calculated value of the ratio of ethylene and acetylene concentrations using special tables. By comparing the calculated temperature values with the critical temperature of spontaneous combustion of coal, it is proposed to determine the stage in the spontaneous combustion process. Thus, in accordance with [36], Figure 8 shows the graphs of change dependencies in the ethylene to acetylene ratio for the K12, K10, and d6 seams. As can be seen from the graphs, the most intensive increase in the ratio of unsaturated hydrocarbons corresponds to the temperature range from 100 to 250 °C, i.e., the stage of transition from low-temperature oxidation to spontaneous combustion [36].
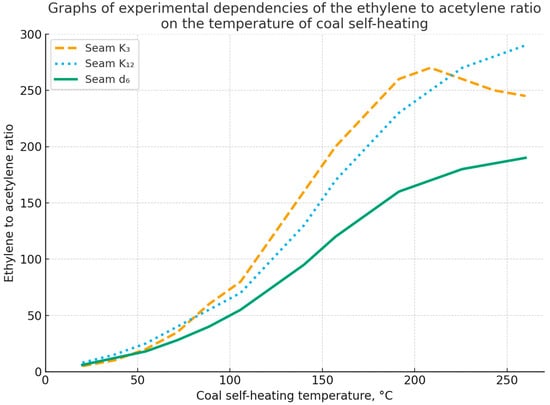
Figure 8.
Graphs of experimental dependencies of the change in the ethylene to acetylene ratio on the coal self-heating temperature for seams K12, K10, and d6 in the Karaganda basin.
Practical use of the provisions in the manual [36] for the mines in the Karaganda basin showed difficulties caused by the following reasons:
- -
- Difficulty in collecting high-quality samples of the mine atmosphere in special concentrator tubes;
- -
- Requirement for high precision of gas analysis instruments to obtain concentrations of unsaturated hydrocarbons in the order of 10-7 vol.%;
- -
- Frequent cases of absence of one of the gases, which leads to the use of average values and distortion of results and conclusions.
In addition, the depth of the coal seam changes the critical temperature of spontaneous combustion, which results from variations in the physical and chemical properties of the coal, metamorphism and methane content, and chemical activity indicators, all of which also affect the tendency toward spontaneous combustion. In this regard, it is necessary to periodically (at certain depths) conduct experimental studies to obtain more reliable estimates and make decisions based on the results of air gas analysis.
However, chemical analytical methods for monitoring self-heating and spontaneous combustion processes remain the most accessible and proven approach.
According to experts, a decrease in the oxygen content in the air and an increase in the amount of carbon dioxide can be caused, in addition to the action of a spontaneous combustion source, by a general deterioration in the mine ventilation, and sometimes by an increased emission of some gases from the rocks. Therefore, the fact of a decrease in the content of oxygen O2 and an increase in CO2 dioxide in the mine atmosphere, without accounting for local conditions and the dynamics of the process, cannot always be considered an undoubted sign of a spontaneous combustion source. If CO and CO2 are detected in the air of the mine workings as permanent components, this indicates a serious danger of a spontaneous combustion source.
It is known that during the development of an endogenous fire, in addition to a change in temperature at the fire site, significant changes in the air composition of the emergency area occur; the oxygen content decreases and the carbon oxides emission increases, the concentrations of which significantly exceed background values. Occasionally this is used and such a criterion is applied that a sign of coal self-heating in the controlled area is a stable excess of the volume fraction of CO and H2 over their background content [32]. It is assumed that at the self-heating stage the ratio of CO to H2 exceeds 10, and at the combustion stage it is less than 10. Continuous monitoring equipment is used to determine the microfractions of carbon monoxide, and a chromatographic gas analyzer is used for determining the proportion of hydrogen fractions.
However, as practice has shown [29,31], based on the results of determining the excess of carbon monoxide and hydrogen over their background content and the ratio\CO-CObH2-H2b < 10, it is impossible to distinguish the stage of self-heating from the early stage of spontaneous combustion. Furthermore, it is the determination of the stage of endogenous fire development that is of great importance, since the early stage of spontaneous combustion of coal under favorable conditions can last only a few hours.
The authors of [37] propose replacing the traditional gas analysis method (based on the content of CO, CO2, H2, and hydrocarbons) with a method for detecting the early stage of spontaneous combustion of coal based on measuring the total amount of steam and liquid aerosol in the air that has passed through the mined-out space. The essence of the method is that the air in the coal self-heating zone is saturated with steam. When it cools to the temperature of the surrounding rocks, some of the steam condenses in the form of an aerosol, which can be used as a sign of the initial stage of spontaneous combustion. To detect the process, it is sufficient to use 10 g of silica gel and an air sample with a volume of 0.008–0.01 m3, passed through a sorbent at a flow rate of less than 1.2 × 10−3 m3/min.
Usually, according to established practice, to control coal self-heating, the content of five gases in the working air is quantitatively determined—carbon dioxide CO2, oxygen O2, carbon monoxide CO, hydrogen H2, and methane CH4—with sulfur dioxide SO2 and nitrogen oxides NO included less often. For the early stage of coal spontaneous combustion, the accuracy of determining carbon monoxide indicators should be at least 0.001–0.002% by volume. As noted earlier, it is most important to obtain reliable information on the coal self-heating temperature in the controlled area and the dynamics of its change. Most often, however, these locations are inaccessible for direct instrumental temperature measurement, and the only viable method is an assessment based on the results of gas analysis of air samples.
Considering that in most coal mining companies, specialists in the prevention of endogenous fires are guided by quantitative indicators of the content of carbon monoxide (CO) and oxygen in air samples, studies were conducted to predict the stage of coal self-heating based on the Graham criterion, which includes the quantitative content of fire indicator gases: 100 × CO/−∆O2, where −∆O2 is the loss of oxygen compared to its normal content (vol.%) [28,37].
Oxygen loss can be calculated in two ways. The first is based on the calculated amount of oxygen when it mechanically replaces all other gases in the atmosphere (from those measured during sampling):
The brackets in Expression (8) represent the calculated amount of oxygen that should have been present during mechanical replacement of oxide, carbon dioxide, and methane. The number 0.209 is the normal oxygen ratio in the air (20.9:100 = 0.209), and is the actual oxygen content in the sample, vol.%.
The second method is to determine the decrease in oxygen content in air sample in relation to nitrogen:
where 0.265 is the normal ratio of oxygen to nitrogen content in the air (20.9:79 = 0.265).
The amount of nitrogen N2 in the sample is determined by the difference: 100 − (CO + CO2 + O2 + CH4), vol.%.
The studies of the relationship between these criteria and the coal self-heating temperature were carried out in the following methodological sequence.
At the first stage, by mathematical processing of experimental studies on coal self-heating, empirical dependencies of quantitative indicators of emitted gases on the coal heating temperature were established.
For experimental studies, samples were selected and prepared from almost all of the major coal seams in the Karaganda basin prone to spontaneous combustion. Experiments on coal self-heating by oxidation with atmospheric oxygen passed through a coal sample were conducted on special laboratory installations. Periodically selected gas samples for the content of oxygen, carbon monoxide and dioxide, and methane were analyzed by the chromatographic method. The average results of experiments on gas emission during heating to 200 °C prone to spontaneous combustion coals are given in Table 2.

Table 2.
Content of gases emitted in air samples depending on coal self-heating temperature.
Figure 9 shows graphical representations of the change dependence in the concentration of oxygen, carbon monoxide, and carbon dioxide on coal self-heating temperature based on the results of the experimental studies. Analytical expressions of the dependencies were obtained by mathematical processing of data using a special software package, MATLAB, the results of which are presented in Table 3.
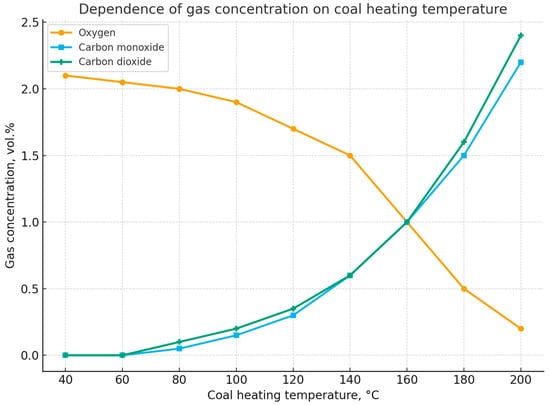
Figure 9.
Change in the concentration of oxygen, carbon monoxide, and carbon dioxide depending on coal self-heating temperature. Note: Oxygen concentration on the graph has been reduced by 10 times.

Table 3.
Results of mathematical and statistical processing of the experimental data.
The graph shows the dependence of the concentrations of the main gases (oxygen O2, carbon monoxide CO, and carbon dioxide CO2) on the coal heating temperature in the range of 40–200 °C.
Oxygen (O2). The curve demonstrates a gradual decrease in the oxygen concentration in the gas mixture as the coal heating temperature increases. At around 40–60 °C, the O2 content is about 2 vol.%, but as the temperature rises to 200 °C, it sharply decreases, approaching 0.2 vol.%. This trend reflects the intensive consumption of oxygen in coal oxidation processes.
Carbon monoxide (CO). The CO concentration begins to rise at temperatures above 80–100 °C and continues to increase with further heating. At 200 °C, the CO content exceeds 2 vol.%. This indicates the transition of the process from weak oxidation to active self-heating and the release of incomplete coal oxidation products.
Carbon dioxide (CO2). The CO2 concentration also increases with rising temperature. However, its growth is smoother at the early stages (up to 140–150 °C) and becomes more intense after 160 °C, reaching about 2 vol.% at 200 °C. This reflects the intensification of complete oxidation reactions of the coal organic matter.
Interpretation.
During the early stage (up to ~100 °C), the main process is the initial oxidation of coal, accompanied by minor release of CO and CO2. In the range of 120–160 °C, oxidation reactions intensify: the rate of O2 consumption and the release of CO/CO2 increase sharply. At temperatures above 160 °C, the process becomes intensive, corresponding to the transition from low-temperature oxidation to the stage of self-heating and the onset of conditions for an endogenous fire.
Thus, the graph illustrates the typical dynamics of gaseous products during coal self-heating and confirms the possibility of using CO and CO2 as indicator gases, while the drop in O2 concentration serves as an additional diagnostic criterion.
The high value of the correlation estimate R2 in Table 3, close to one, shows that the change in gas concentration is almost entirely due to coal self-heating temperature. The reverse transformation (for carbon oxide and dioxide) allows for obtaining estimates of coal self-heating temperature based on the results of measurements of oxygen, oxide, or carbon dioxide concentration in the atmosphere of the controlled area, which is especially important in operational actions to assess the situation and take reasonable preventive measures.
It should also be noted that the experiments were conducted under adiabatic conditions, i.e., without the removal of thermal energy from the chemical oxidation reaction into the external environment. The use of the results of such experiments is justified by the fact that in mine conditions it is often unknown under what conditions oxidation processes occur in isolated spaces with coal accumulations. Therefore, obtaining slightly overestimated calculated temperatures based on the percentage of indicator gases in air samples will be more useful for taking timely preventive measures than underestimated ones.
During the second stage, the results of chromatographic analysis of control samples of the mine atmosphere were processed for research and obtaining quantitative values of the previously specified criteria. The results of laboratory analysis of mine atmosphere samples for monitoring the atmosphere behind the bulkhead in the field drift of the mine for 187 days were accepted for analysis and mathematical–statistical processing.
The main objective of this second stage was to calculate, using the formulas in Table 3, the temperature of the atmosphere in the controlled area depending on the percentage of indicator gases in the air samples taken.
During the next, third stage of the research, the goal was to establish and obtain correlation dependencies of the calculated atmospheric temperature in the controlled area from the previously specified integrated criteria representing certain aggregate ratios of indicator gases. These calculations were performed for each of the observations using the MATLAB software environment, specially developed and intended for scientific and technical research. For each field observation, the following criteria were assessed:
- -
- dO2_1 (−∆O2)—oxygen loss calculated using the first method;
- -
- dO2_2 (−∆O2)—oxygen loss calculated using the second method;
- -
- K1—the ratio of carbon monoxide concentration CO to the loss in oxygen concentration −∆O2_1 (CO/−∆O2);
- -
- KG1—Graham criterion (100 × CO/−∆O2_1);
- -
- KG2—Graham criterion (100 × CO/−∆O2_2).
Dependence equations for the calculations were taken in the form of exponential functions. Table 4 presents a summary of the formulas of empirical dependencies with estimates of equation coefficients and the tightness of the correlation between the accepted criteria and the calculated atmospheric temperatures in the controlled areas.

Table 4.
Empirical dependencies and statistical estimates of the relationship.
The following notations are used in the formulas of Table 4:
TR1—calculated coal self-heating temperature based on carbon monoxide content in the sample (CO);
TR2—calculated temperature based on carbon dioxide content (CO2);
TR3—calculated temperature based on oxygen content (O2);
TSR is the average temperature of three calculated values (TR1, TR2, TR3).
Figure 10 and Figure 11 present the protocols generated and issued by the program from mathematical and statistical processing of field observations of the dynamics of changes in quantitative values of indicator gas content in air samples. As can be seen from the figures, the program allows for visually assessing the tightness of the relationship and obtaining numerical values of the coefficients of empirical equations.
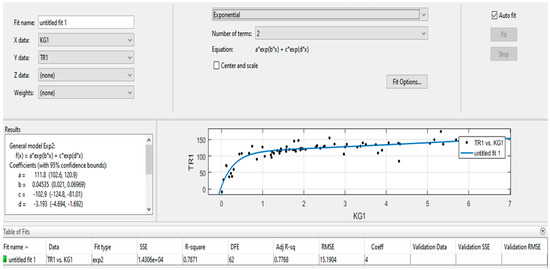
Figure 10.
Protocols of processing results and obtaining empirical dependence TR1 = a×exp(b×d_KG1) + c×exp(d×KG1).
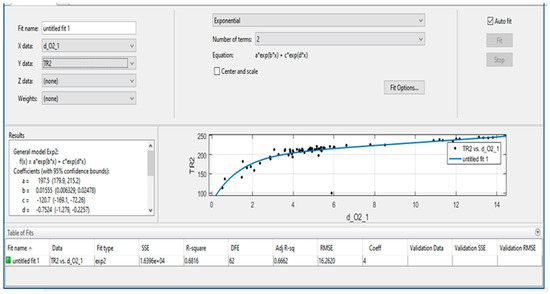
Figure 11.
Protocols of processing results and obtaining the empirical dependence TR2 = a×exp(b×d_O2_1) + c×exp(d×d_O2_1).
To evaluate the reliability of the proposed model and verify the experimental results, an exponential approximation of the relationship between TR1 and KG1 was performed. The graphical representation allows for a clear comparison between experimental data and the fitted curve, demonstrating both the adequacy of the applied regression model and the reproducibility of the observed trends. Figure 10 and Figure 11 illustrates the obtained results, highlighting the consistency between measured values and theoretical dependence.
Figure 12a presents the approximation of the dependence of parameter TR1 on KG1 using an exponential model of the form:
where the model coefficients were determined through regression analysis as follows:
f(x) = a⋅ebx + c⋅edx, f(x) = a \cdot e^{b x} + c \cdot e^{d x}, f(x) = a⋅ebx + c⋅edx,
a = 111.6a = 111.6 (95% CI: 102.8–120.2),
b = −0.1029b = −0.1029 (95% CI: −0.1248–−0.0811),
c = 0.04535c = 0.04535 (95% CI: 0.0211–0.0697),
d = −3.119d = −3.119 (95% CI: −4.649–−1.589).
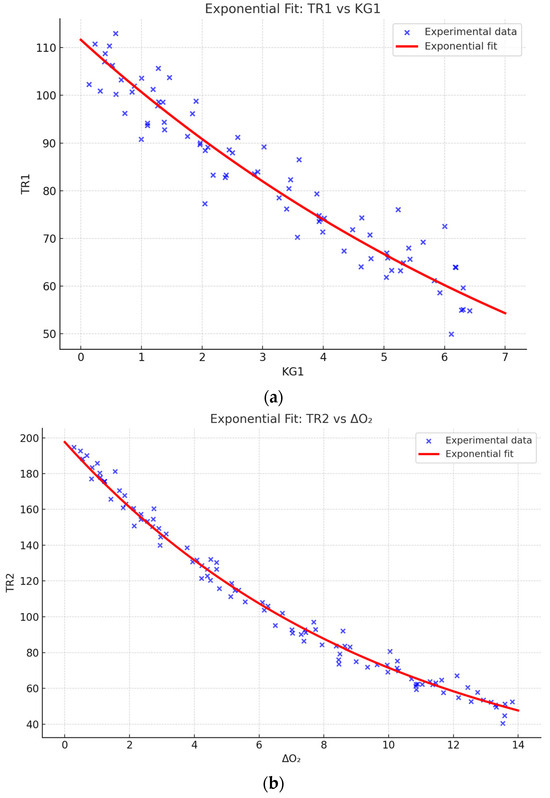
Figure 12.
(a) Approximation of the dependence of parameter TR1 on KG1 using an exponential model. (b) Approximation of the dependence of parameter TR2 on the change in oxygen concentration (ΔO2) using an exponential model.
The experimental data (blue markers) clearly demonstrate a decreasing trend in TR1 with increasing KG1. The constructed approximation curve (red line) provides a good fit to the general trend, which is confirmed by the high coefficient of determination (R2 = 0.7871R^2 = 0.7871R2 = 0.7871), the adjusted coefficient (Adj.R2 = 0.7768Adj.R^2 = 0.7768Adj.R2 = 0.7768), and the relatively low root mean square error (RMSE = 15.19RMSE = 15.19RMSE = 15.19).
Thus, the obtained results indicate the exponential nature of the TR1–KG1 relationship. The applied model not only confirms the reliability of the experimental data but also allows the use of the derived equation for predicting system behavior under varying parameters. This significantly enhances the diagnostic value of the methodology and its applicability in practical research.
Figure 12b shows the approximation of the dependence of parameter TR2 on the change in oxygen concentration (ΔO2) using an exponential model. The experimental data (blue markers) demonstrate a consistent decrease in TR2 values with increasing ΔO2, which reflects the regular reduction in the system’s reactivity as the degree of oxidation increases.
The constructed approximation curve (red line) shows good agreement with the experimental points and reliably describes the overall trend. The high coefficient of determination (R2 ≈ 0.68) and the relatively low root mean square error (RMSE ≈ 16.3) confirm the adequacy of the chosen model and the correctness of its application for analyzing gas exchange dynamics.
Thus, the obtained results confirm the exponential nature of the TR2–ΔO2 relationship and indicate the possibility of using this model to predict critical states during coal self-heating.
As follows from the formulas and estimates given in Table 4, the tightness of the relationships between the accepted criteria and the calculated temperatures is quite high; the value of the determination coefficient R2 is 0.68 and higher, which indicates a sufficient degree of correspondence between the model and the experimental data. This allows the use of the models for forecasting the atmospheric temperature of an emergency or controlled area based on the results of operational air sampling with an acceptable level of accuracy.
The value of the determination coefficient R2 is 0.68 and higher, which indicates a sufficient degree of conformity of the model to the experimental data. This means that the model explains at least 60% of the variation in the dependent variable, which allows it to be used for analysis and forecasting with an acceptable level of accuracy. In the absence of direct instrumental temperature measurement capabilities in the supposed source of spontaneous combustion of coal, the use of the obtained and tested empirical dependencies can provide practical assistance in making informed decisions and development of effective measures to prevent emergency situations.
3.3. Practical Application of Research Results
The empirical dependencies of the integrated criteria obtained for the atmospheric temperature in the controlled area formed the basis for the report titled, “Methodological recommendations for assessing endogenous fire hazard based on the results of analyzing the content of indicator gases in the atmosphere of the controlled area”. The methodological recommendations do not exclude previously developed and practically applied methods prescribed by current basin regulatory documents for predicting and preventing endogenous fires in mines, but to a certain extent complement them. It is known that additional information in emergency situations strengthens the validity of preventive measures taken related to underground fires and explosions.
Analysis of actual cases of intensive low-temperature oxidation, coal self-heating with the emission of indicator gases exceeding maximum allowable concentration (MAC), and spontaneous combustion in various coal basins of Kazakhstan and other countries confirms the correctness of the idea regarding the stages of spontaneous combustion and their assessment based on the composition of the mine atmosphere.
Figure 13 shows the diagram and sequence of procedures described in the methodological recommendations, the high-quality implementation of which can more effectively justify the measures taken to prevent endogenous fires.
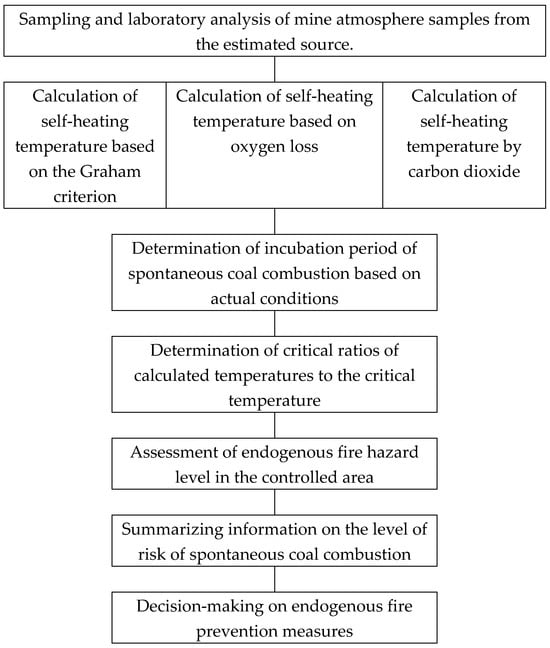
Figure 13.
Flow chart of procedures for assessing endogenous fire hazard based on the results of content analysis of indicator gases in the atmosphere within the controlled area.
In block 1, a mandatory procedure for sampling the mine atmosphere from certain places in the controlled area is performed, and laboratory analysis for gas content is carried out in accordance with the measurement methodology available in gas analysis laboratories.
In blocks 2, 3, and 4, calculations are performed for the predictive determination of coal self-heating temperature based on the calculated criteria of quantitative ratios of indicator gases using the empirical dependencies obtained within the framework of this study.
In block 5, the critical temperature of spontaneous combustion of coal (CTSC) is established. This value is either already known or can be re-determined or specified by laboratory testing of coal samples. It should be noted that CTSC does not depend on the operating conditions of the site, as the incubation period, but depends entirely on the coal material itself, its material composition, degree of metamorphism, etc.
Block 6 calculates the ratio of the estimated temperatures calculated in blocks 2, 3, and 4 to the critical temperature of spontaneous coal combustion.
Block 7 evaluates the stage of coal self-heating and the possibility of an endogenous fire. Practical experience and experimental studies give grounds to believe that an excess of the measured or calculated temperature exceeding the critical temperature by 20–30% may indicate the beginning of the transition from low-temperature oxidation to spontaneous coal combustion.
In block 8, based on available data on spontaneous combustion events in the controlled or similar areas, it is recommended that the results of the previous steps be summarized to assess the risk of spontaneous combustion.
In block 9, the commission decides whether measures to prevent endogenous fire are necessary.
It is recommended that all information obtained during the analysis and decision-making process on the investigated case be entered into the knowledge base that should be in the coal mine safety system.
4. Discussion and Results
Correlation equations of the criteria relationships, including certain quantitative ratios of indicator gases in the mine atmosphere, are more informative and can be used in the practice of assessing the stages of coal self-heating (spontaneous combustion) in order to prevent endogenous fires in mines.
Testing of mathematical and statistical models for predicting the stage of coal self-heating at actual sites of mines in the Karaganda basin confirmed their suitability for assessing the temperature accompanying oxidation processes. The empirical dependencies developed for the atmosphere temperature in the controlled area from the integrated criteria formed the basis for the report titled, “Methodological recommendations for assessing endogenous fire hazard based on the results of analyzing the content of indicator gases in the atmosphere of the controlled area”.
This report, including sequential procedures, allows for a more systematic and reasoned investigation and analysis of emergency situations related to spontaneous combustion of coal, and for taking timely measures to prevent endogenous fires in coal mines. Methodological recommendations for assessing endogenous fire hazard, which supplement known methods of predicting and preventing spontaneous combustion of coal, are included in the basin instructions. These instructions specify the stage of the oxidation process and are based on the results of the studies performed. The recommended procedures for processing data from sampling the mine atmosphere in the controlled area and analyzing the results regulate the actions of the mine fire service personnel in emergency situations to a certain extent.
Correlation equations based on quantitative ratios of indicator gases (i.e., CO, CO2, C2H4, and others) are highly informative tools for diagnosing the stages of coal self-heating and spontaneous combustion. These models make it possible to assess the dynamics of temperature changes in hidden zones of mines where direct temperature measurement is difficult. Indicator gases are closely related to coal temperature during oxidation processes, and their concentrations reflect the intensity of thermal reactions and the stages of self-heating development. For example, studies show that the index gas analysis method, including CO, C2H4, and C2H6, is widely applied because of its high sensitivity, regularity, and convenience in underground
Models employing correlation equations are particularly effective in cases where direct collection of temperature data is complicated by blockages, gas–air mixtures, or inaccessible zones. Indirect methods such as index gas analysis have become the basis of predictive methodologies, enabling continuous and reliable monitoring of the thermal state of coal.
In particular, the exponential growth of CO and CO2 concentrations with increasing temperature during low-temperature oxidation has been confirmed by experimental studies for different coal types.
Practical testing of mathematical and statistical models based on such correlations and applied to actual mine conditions in the Karaganda basin demonstrated their high reliability in assessing temperature changes associated with oxidation processes. We confirmed that empirical dependencies between the air temperature in the monitored zone and integral criteria (for example, combinations of concentrations of different gases or their ratios) can forecast thermal fields and oxidation stages of coal where direct measurement is not possible.
Figure 14 presents a comparative characteristic of the diagnostic reliability of different methods for predicting coal self-heating. Traditional methods, based on the analysis of individual indicator gases (e.g., CO, CO2, C2H4), provide an approximate diagnostic accuracy of about 60%. These methods are sensitive to variations in individual parameters and are often affected by local geological and technical conditions, which increase the likelihood of false alarms and reduce the accuracy of predictions.
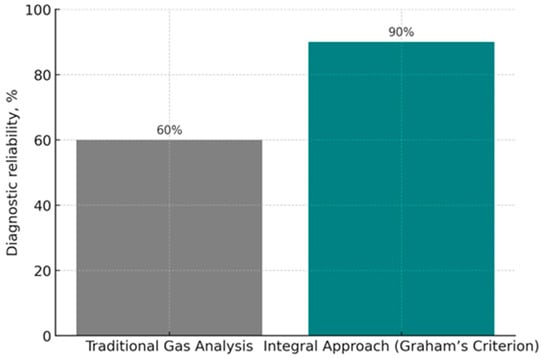
Figure 14.
Comparative assessment of the reliability of traditional gas analysis and the integral approach (Graham’s criterion).
The integral approach, based on the use of generalized criteria, in particular Graham’s criterion, demonstrates much higher effectiveness: the level of diagnostic reliability reaches 90%. This is explained by the fact that the model takes into account the interrelation of several parameters simultaneously and allows minimizing the influence of random fluctuations of individual indicators.
Thus, the comparative analysis confirms that integral criteria have significant advantages over traditional methods, providing higher accuracy in predicting the stages of coal self-heating, reducing the probability of false signals, and improving the effectiveness of early warning systems against endogenous fires.
Figure 15 shows the dynamics of changes in the concentrations of oxygen (O2), carbon monoxide (CO), and carbon dioxide (CO2) depending on the coal heating temperature in the range of 40–200 °C. The graph illustrates the patterns of chemical and thermal processes occurring in the coal mass during gradual heating and makes it possible to distinguish characteristic stages of self-heating.
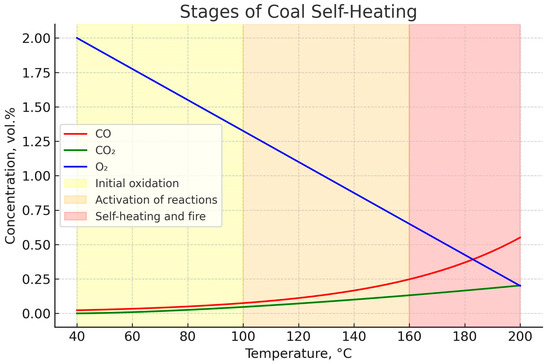
Figure 15.
Dependence of indicator gas concentrations on temperature and the stages of coal self-heating.
The decrease in oxygen concentration reflects the intensive consumption of O2 in coal oxidation reactions. At the same time, an exponential increase in the concentrations of oxidation products—CO and CO2—is observed. In the low-temperature region (up to ~100 °C), the process is limited to surface adsorption oxidation, accompanied by only minor gas release. At this stage, defined as the initial oxidation stage, the risk of self-heating is minimal, but the prerequisites for heat accumulation are already being formed.
In the temperature range of 100–160 °C, the reaction rates increase sharply, which is expressed by the intensive decline in O2 content and the rapid rise in CO and CO2 concentrations. This interval corresponds to the activation stage of reactions, characterized by the transition from slow surface oxidation processes to deeper exothermic reactions that generate significant heat accumulation.
At temperatures above 160 °C, a critical acceleration of oxidation processes occurs, accompanied by an avalanche-like growth in CO and CO2 concentrations. This interval corresponds to the intensive self-heating stage, which under industrial conditions leads to the formation of an endogenous fire. At this stage, the development of the process becomes practically uncontrollable, posing a serious threat to mining safety.
Thus, the obtained dependencies confirm the high informativeness of indicator gases as a diagnostic tool for the early detection of hazardous trends in the mine atmosphere. A comprehensive analysis of O2, CO, and CO2 concentrations makes it possible not only to identify the presence of oxidation processes but also to differentiate the stages of their development, which enables timely preventive measures to avoid endogenous fires.
Figure 16 shows the dependence of the ratio of carbon monoxide (CO) concentration to the decrease in oxygen content (ΔO2) on coal heating temperature in the range of 40–200 °C. This parameter serves as an integral criterion characterizing the balance between the rate of release of incomplete oxidation products and the degree of oxidizer consumption in the system.
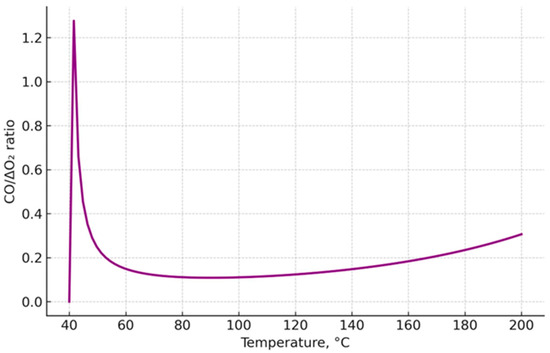
Figure 16.
Correlation CO/ΔO2 as an integral criterion of coal self-heating.
In the low-temperature region (40–100 °C), the values of the criterion remain relatively low, reflecting the weak intensity of oxidation processes. At temperatures above 100–120 °C, a steady increase in CO/ΔO2 is observed, associated with the activation of exothermic reactions and enhanced CO release, accompanied by intensive oxygen consumption.
Further temperature rise above 160 °C is accompanied by a critical increase in the criterion value, indicating the transition of the process into the self-heating stage and the formation of conditions for an endogenous fire. Thus, the CO/ΔO2 value acts as a reliable indicator of the coal self-heating stage, allowing indirect assessment of the thermal state of the coal mass under conditions where direct temperature measurements are difficult or impossible.
The use of this integral criterion in combination with other indicator parameters can significantly improve diagnostic accuracy and reduce the probability of false predictions, making it a promising tool for mine atmosphere monitoring and the prevention of endogenous fires.
The development of the “Methodological Recommendations for Assessing Endogenous Fire Hazard Based on the Results of Indicator Gas Analysis in the Atmosphere of the Controlled Area” is grounded in these empirical dependencies. These recommendations include sequential procedures for data collection, statistical processing, and interpretation of indicator gases. They are designed to systematize the analysis of emergency situations associated with coal spontaneous combustion and ensure timely preventive actions to avoid endogenous fires.
It is important to emphasize that modern studies are actively developing prediction methods using machine learning and advanced algorithms. For example, a 2025 article in Process Safety and Environmental Protection introduced an intelligent index gas modeling approach incorporating algorithms such as XGBoost, Random Forest, and AdaBoost. Indicator gases (O2, CO, C2H4) and ratios such as CO/ΔO2 and C2H4/C2H6 were used. The XGBoost model achieved the best performance in terms of MAE, RMSE, and R2, while SHAP (Shapley additive explanations) analysis revealed that ethylene (C2H4) concentration and the C2H4/C2H6 ratio contributed most significantly to predicting coal self-heating temperature.
Another advanced approach is presented in a study on a multilayer hierarchical model based on the NSGA-II-RF algorithm (Multiobjective Genetic Optimization + Random Forest). This model classifies spontaneous combustion stages using multiple indicator gases (CO, CO2, CH4, C2H6, C2H2, and gas ratios). It improved prediction accuracy by 3–9% compared with other algorithms and achieved better metrics such as precision, recall, and the F1 score.
Although our methodological recommendations do not employ such advanced algorithms, they complement existing basin instructions and forecasting methodologies through their simplicity, clarity, and suitability for practical use. Unlike high-tech AI-driven models, our recommendations can be easily implemented by mine personnel and adapted to local coal characteristics, including composition, degree of metamorphism, and gas content.
Thus, the developed correlation equations based on integral criteria of indicator gases ensure the following features:
Reliability of prediction—the strong correlation between gas parameters and temperature (confirmed under experimental conditions, including in the Karaganda basin) demonstrates the suitability of the model for practical use.
Stage differentiation—the ability to distinguish between self-heating stages and the transition to spontaneous combustion, enabling timely response.
Practical application—the recommendations are aimed at direct use by mine fire safety services, from sampling to analysis and decision-making.
Adaptability—the models can be calibrated for other coal-mining regions, taking into account their specific geological and technical conditions.
Complementarity—the recommendations supplement existing forecasting approaches, enhancing their validity.
Correlation equations based on indicator gases are thus a critically important tool for early warning systems against endogenous fires in mines. Modern machine learning methods confirm the potential of gas analysis and highlight the importance of developing clear, practical methodologies like our recommendations, which can function effectively under resource-constrained conditions and the urgent need for rapid response.
Although the presented experimental correlations were established primarily for the K12 and d6 seams of the Karaganda coal basin, it should be emphasized that their direct extrapolation to other seams in the basin, and especially to different coal-mining regions, is limited. The variability of coal composition—including the content of volatile matter, ash, inherent moisture, sorption capacity, gas content, and degree of metamorphism—has a decisive influence on oxidation kinetics and the dynamics of indicator gas emission. These differences can significantly shift the critical self-heating temperature and alter the quantitative parameters of the established dependencies.
In order to ensure the applicability of the developed methodology under diverse geological and mining conditions, local calibration of empirical coefficients is required. Such calibration involves laboratory testing of representative samples from the target seams, determination of seam-specific critical self-heating temperatures, and refinement of gas–temperature correlation equations. This approach allows the predictive model to account for local coal properties while preserving its methodological universality.
Thus, the results obtained for the Karaganda basin represent a reliable case study that validates the proposed approach under conditions of high fire hazard. Additionally, the procedure of local recalibration ensures that the methodology can be adapted for broader application across other seams and coal basins without loss of diagnostic accuracy.
In addition to the obtained empirical correlations, it is essential to provide practical threshold values that can guide mine operators in assessing the stage of coal self-heating. Based on the experimental results for the K12 and d6 seams and supported by previously reported data [18,28,32], the following indicative thresholds may be proposed:
Carbon monoxide (CO): detectable concentrations above 0.01 vol.% (~100 ppm) indicate the onset of active low-temperature oxidation; values approaching 0.3–0.5 vol.% are characteristic of the transition to the spontaneous combustion stage.
Carbon dioxide (CO2): steady increase above background values (0.02–0.05 vol.%) signals enhanced oxidation; concentrations exceeding 0.5–1.0 vol.% reflect intensive heating and progression toward ignition.
Hydrogen (H2): concentrations above 0.001 vol.% (10 ppm), typically recorded in the range of 100–120 °C, mark the onset of volatile matter release; a sharp rise to >0.005–0.01 vol.% corresponds to the stage of active thermal decomposition and imminent spontaneous combustion.
These thresholds should be interpreted in conjunction with oxygen depletion (ΔO2) and integrated indices such as the Graham criterion (100·CO/ΔO2), which enhance diagnostic reliability under mine conditions. While the exact quantitative values may vary with coal rank and geological setting, the proposed ranges provide a practical basis for early recognition of hazardous stages and for the timely implementation of preventive measures.
The experimental results (Figure 4 and Figure 6) demonstrate that hydrogen emission increases sharply only within the range of 120–300 °C, in contrast to CO and CO2, which appear at much lower temperatures (40–60 °C). This behavior reflects the more complex nature of hydrogen formation. At initial stages of oxidation, coal–oxygen interactions mainly generate CO and CO2 through surface reactions, while H2 release is negligible. Noticeable hydrogen emission begins only when structural decomposition of macromolecular coal components occurs, leading to the release of volatile matter and secondary thermal reactions.
Such delayed appearance of hydrogen implies that it is not a reliable indicator for the earliest stages of self-heating, when preventive measures are most effective. However, the sharp increase in H2 concentration above ~0.001–0.005 vol.% in the 120–300 °C interval is a critical diagnostic signal marking the transition from low-temperature oxidation to active pyrolysis and imminent spontaneous combustion. Therefore, hydrogen should be interpreted not as an early-warning indicator, but as a complementary parameter that confirms the escalation of the process.
From a practical standpoint, this specificity affects monitoring strategies: CO and CO2 remain primary markers for early detection, whereas hydrogen is more suitable for identifying the acceleration phase of self-heating and for distinguishing advanced stages of fire development. Integrating all three gases, along with oxygen depletion indices, enhances the robustness of diagnostic systems and minimizes the probability of false or delayed alarms.
A comparative evaluation of the Graham criterion with other classical diagnostic approaches reveals its relative advantages. When relying solely on CO concentration, early detection is often complicated by the overlap of background and oxidation-induced values, which may result in delayed recognition of self-heating onset. Similarly, the ethylene/acetylene ratio method, although widely applied in practice, requires highly sensitive chromatographic analysis at trace levels (10−7–10−5 vol.%), and its reliability decreases when one of the gases is absent or below the detection threshold.
In contrast, the Graham criterion (100·CO/ΔO2), by incorporating both the increase in CO concentration and the simultaneous depletion of oxygen, reflects the intensity of oxidation more robustly. Statistical analysis of the experimental data obtained in this study showed that correlation coefficients (R2 > 0.7) for Graham-based dependencies exceed those derived from CO concentration alone (R2 ≈ 0.6). This indicates improved predictive accuracy and reduced sensitivity to background fluctuations.
Thus, while individual indicators (CO, H2, or ethylene/acetylene ratio) remain useful under specific conditions, the Graham criterion provides a more integrated and stable diagnostic tool. Its application allows for earlier recognition of hazardous stages and strengthens the methodological basis for practical fire-prevention strategies in coal mines.
To improve the statistical robustness of the obtained correlations, each experimental point was measured in triplicate. The reproducibility of gas concentration measurements, evaluated as the relative standard deviation, did not exceed ±5%, which is consistent with international standards for coal fire studies. Confidence intervals (95%) for the regression equations were calculated, confirming that the empirical dependencies presented in Table 3 and Table 4 fall within narrow error margins.
It should also be noted that potential error sources include baseline drift of chromatographic detectors, slight variations in coal sample mass and particle size, and thermal stability of the water-bath system. These systematic factors were minimized by daily calibration with certified gas mixtures and by maintaining constant experimental conditions (heating rate 0.5–1 °C/min, fraction size 1–3 mm).
The inclusion of statistical uncertainty analysis indicates that the high coefficients of determination (R2 ≥ 0.7–0.99) reported in this study not only reflect strong correlations but are also supported by repeatable and reproducible experimental evidence. This enhances the reliability of the proposed predictive models for practical application in mine fire safety.
The developed information–analytical system (IAS) for assessing endogenous fire hazard has been implemented in the mines of the Karaganda coal basin, which is confirmed by an official Act of Implementation. The system is designed to integrate laboratory and field gas analysis data into a unified monitoring and decision-support framework.
Input data requirements include the concentrations of major indicator gases (CO, CO2, H2, O2, CH4) obtained by chromatographic or continuous gas monitoring systems, together with metadata on sampling location and time. These parameters are entered into the IAS either manually (from laboratory protocols) or via automated sensors.
Data processing algorithms are based on the empirical correlations developed in this study, including exponential relationships for CO and CO2, and the Graham criterion (100·CO/ΔO2). The software automatically calculates the predicted self-heating temperature of the coal mass and determines the corresponding stage of the oxidation process.
Output is presented in the following formats:
- Numerical values of predicted temperature (°C);
- Diagnostic indices (e.g., CO/ΔO2, Graham criterion);
- Categorical classification of the self-heating stage (incipient oxidation, active self-heating, transition to spontaneous combustion);
- Risk levels expressed in a color-coded scale for operational clarity.
User recommendations generated by the IAS include threshold-based alerts, suggested timing for additional sampling, and preventive actions to be initiated by mine fire-safety services.
The system is implemented with a simple graphical interface, making it accessible to operational personnel without advanced statistical training. Its practical use in production conditions has demonstrated improved accuracy in early detection and allowed timely decision-making for fire-prevention strategies.
Prospects for further research:
Future work may focus on the following features:
1. Expansion of the database by including coals from other basins in Kazakhstan and neighboring countries in order to refine and calibrate empirical coefficients.
2. The use of machine learning and artificial intelligence methods to build more complex predictive models that take into account nonlinear relationships and multiparametric dependencies.
The developed methodology can be integrated into automated mine monitoring systems, which will allow for real-time early diagnosis of endogenous fires.
3. Investigation of additional indicator gases and their ratios (for example, C2h4, ch4, h2/CO), which can increase the sensitivity and reliability of diagnostics.
4. Development of recommendations for practical operation in conditions associated with various geological and technical features, including complex ventilation systems and the presence of methane-hazardous areas.
5. Thus, the presented research is the basis for further improvement in the methods for assessing and predicting endogenous fire hazards, and for the development of integrated industrial safety management systems in coal mines.
5. Conclusions
This article, based on the research conducted, provides a solution to the current problem of improving methods for preventing endogenous fires in coal mines by developing a theoretical and experimental method for assessing the stages of coal self-heating by analyzing gas composition of mine air.
Using modern mathematical and statistical methods and internationally recognized software tools, specifically focused on scientific research, processing of natural and experimental data, for the first time for the conditions of the Karaganda basin, new correlation dependencies have been established between coal self-heating temperature and integrated criteria from the quantitative ratios of indicator gases during coal heating.
Criteria that include quantitative relationships between indicator gases in mine atmosphere samples allow more accurate prediction of coal self-heating temperature compared to individual gas concentration indicators.
Quantitative estimates of the tightness of the correlation equations, close to one, prove that the dynamics of change in the self-heating temperature of coal is completely characterized by a change in the quantitative characteristics of the criteria containing the percentage ratios of indicator gases in the mine atmosphere.
Predictive correlation equations obtained on the basis of known experimental studies and tested in actual mine conditions make it possible to identify the stage of coal self-heating in order to take timely measures to prevent endogenous fires in coal mines.
The research results obtained within the framework of this investigation formed the basis for the methodological recommendations and were used to develop the information and analytical system for forecasting and preventing endogenous fires. The software package was transferred to the mines of the Karaganda coal basin.
Author Contributions
Conceptualization, N.S. (Nurlan Suleimenov) and G.S.; methodology, N.S. (Nurlan Suleimenov); software, N.S. (Nursultan Sarsenbekov); validation, N.S. (Nurlan Suleimenov), G.S. and A.A.; formal analysis, V.P. and F.B.; investigation, N.Z.; resources, S.I.; data curation, N.E.; writing—original draft preparation, N.S. (Nurlan Suleimenov); writing—review and editing, G.S.; visualization, A.Z.; supervision, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan under grant funding (Grant No. BR24993009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are grateful to the researchers whose work was cited in this article for their contribution to the study of coal self-heating problems, and for their efforts to reduce the associated risks and develop scientific understanding of this complex area.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jordan. Top 30 Most Astonishing Mining Disasters in History. Available online: https://www.ftmmachinery.com/ru/blog/top-30-most-astonishing-mining-disasters-in-history.html (accessed on 15 May 2025).
- The Biggest Disasters in the History of the Mining Industry. Available online: https://www.prometall.info/intrigi/proishesviya/krupneyshie_katastrofy_v_istorii_gornodobyvayushchey_promyshlennosti (accessed on 15 May 2025).
- ULYSMEDIA Official Website. Available online: https://ulysmedia.kz/analitika/22358-besposhchadnaia-professiia-ili-pochemu-nikto-ne-ostanovil-gibel-kazakhstanskikh-shakhtiorov/ (accessed on 7 February 2025).
- TENGRINEWS Official Website. Available online: https://tengrinews.kz/kazakhstan_news/krupneyshie-avarii-arselormittal-temirtau-poslednie-15-let-515044/ (accessed on 7 February 2025).
- Baimukhametov, S.K.; Imashev, A.Z.; Mullagaliyev, F.A.; Mullagaliyeva, L.F.; Kolikov, K.S. Low-permeable gas-bearing and outburst-hazardous coal seam mining in the Karaganda Coal Basin. MIAB. Min. Informa. Anal. Bull. 2021, 10-1, 124–136. [Google Scholar] [CrossRef]
- Spatayev, N.D.; Sattarova, G.S.; Nurgaliyeva, A.D.; Balabas, L.K.; Batessova, F.K. Ensuring healthy and safe working conditions in breakage face with direct-flow ventilation scheme. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Technol. Sci. 2023, 2, 177–187. [Google Scholar] [CrossRef]
- Suleimenov, N.M.; Shapalov, S.K.; Naukenova, A.S.; Khodzhayev, R.R.; Huangan, N.; Rakhimberlina, A.A.; Altybayev, Z.M. Cumulative influence of informative features on the assessment of the condition of the fire situation in the sealed areas of coal mines. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Chem. Technol. 2018, 2, 56–60. Available online: https://journals.nauka-nanrk.kz/chemistry-technology/issue/view/259/231 (accessed on 10 May 2025).
- Sofranko, M. Methodology of Risk Analysis of Endogenous Fire in Coal Mines. Adv. Mater. Res. 2014, 962–965, 1153–1157. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smolinski, A. Study of the Hazard of Endogenous Fires in Coal Mines—A Chemometric Approach. Energies 2018, 11, 3047. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, T.; Liang, Y.; Wang, Z. A Review on numerical solutions to self-heating of coal stockpile: Mechanism, theoretical basis, and variable study. Fuel 2016, 182, 80–109. [Google Scholar] [CrossRef]
- Suleimenov, N.M.; Shapalov, S.K.; Sattarova, G.S.; Sapargaliyeva, B.O.; Imanbayeva, S.B.; Bosak, V.N. Numerical simulation modeling of temperature distribution in the process of coal self-heating in the mined-out spaces. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. 2021, 2, 167–173. [Google Scholar] [CrossRef]
- Lugovtcova, N.Y.; Portola, V.A. Effect of pre-cooling coal on the development of auto-ignition. Mod. Probl. Sci. Educ. 2013, 6. Available online: https://science-education.ru/ru/article/view?id=10985 (accessed on 20 May 2025).
- Voroshilov, S.P.; Voroshilov, Y.S.; Voroshilov, A.S.; Uvarova, V.A. The influence of moisture on the oxidation of coals. Bull. Sci. Cent. 2008, 2, 68–82. Available online: https://cyberleninka.ru/article/n/vliyanie-vlagi-na-okislenie-kamennyh-ugley (accessed on 10 May 2025).
- Lindenau, N.I.; Maevskaya, V.M.; Krylov, V.F. Origin, Prevention and Extinguishing of Endogenous Fires; Nedra: Moscow, Russia, 1977; p. 319. [Google Scholar]
- Saranchuk, V.I. Oxidation and Spontaneous Combustion of Coal; Naukova Dumka: Kyiv, Ukraine, 1982; p. 166. [Google Scholar]
- Portola, V.A.; Cherskikh, O.I.; Protasov, S.I.; Seregin, E.A.; Shvakov, I.A. Evaluation of the effectiveness of aqueous compositions for cooling the centers of spontaneous combustion of brown coal in open pits. Min. Ind. 2023, 3, 89–94. [Google Scholar]
- Aliyev, S.B.; Khodjaev, R.R.; Kenzhin, B.M.; Smirnov, Y.M.; Grechishkin, N.V.; Asainovo, S.N. Determination of temperature boundaries of coal spontaneous combustion stages. Coal 2022, 9, 61–66. [Google Scholar] [CrossRef]
- Suksova, S.A.; Timofeeva, Y.V.; Dolkan, A.A.; Popov, E.V. Review of methods for identifying spontaneous combustion of coal. Eurasian Sci. J. 2021, 13, 1. Available online: https://esj.today/PDF/19NZVN121.pdf (accessed on 20 May 2025).
- Engineering Research Center “GeoMark” LLP. Instructions on Prevention and Extinguishing of Endogenous Fires in the Coal Enterprises of the Coal Department of “ArcelorMittal Temirtau” JSC; Engineering Research Center “GeoMark” LLP: Karaganda, Kazakhstan, 2018; p. 108. (In Russian) [Google Scholar]
- Kazakh State Research Institute for Mining Safety. Guidelines for Monitoring the Occurrence of Endogenous Fires in the Mines of the Coal Department of ArcelorMittal Temirtau JSC; Kazakh State Research Institute for Mining Safety: Karaganda, Kazakhstan, 2009; p. 36. (In Russian) [Google Scholar]
- Novoselov, S.V.; Popov, V.B.; Golik, A.S. Assessment of the risk of endogenous fires in coal mines. Coal 2020, 5, 21–25. [Google Scholar] [CrossRef]
- Mineev, S.P.; Smolanov, S.N.; Samopalenko, P.M.; Belikov, I.B. On some questions of extraction of complex fires in coal mines. Wschod. Czas. Nauk. East Eur. Sci. J. 2018, 9, 52–57. Available online: https://cyberleninka.ru/article/n/o-nekotoryh-voprosah-tusheniya-slozhnyh-pozharov-v-ugolnyh-shahtah (accessed on 10 May 2025).
- Igishev, V.G.; Shlapakov, P.A.; Haimin, S.A.; Sin, S.A. Fire indicator gases liberation at coal oxidation at the stage of self-heating and flameless combustion. Bull. Sci. Cent. Saf. Work. Coal Ind. 2015, 4, 55–59. [Google Scholar]
- ISO 18283:2006; Hard Coal and Coke—Manual Sampling. International Organization for Standardization: Geneva, Switzerland, 2006.
- GOST 10742-71; Hard Coal and Brown Coal. Methods for Manual Sampling. Standard Publishing House: Moscow, Russia, 1971.
- Sloss, L.L. Assessing and Managing Spontaneous Combustion of Coal; IEA Clean Coal Centre: London, UK, 2015; p. 55. [Google Scholar]
- Moria, R.; Balusu, R.; Tanguturi, K.; Khanal, M. Prediction and control of spontaneous combustion in thick coal seams. In Proceedings of the 13th Coal Operators’ Conference, Wollongong, NSW, Australia, 14–15 February 2013; University of Wollongong: Wollongong, NSW, Australia, 2013; The Australasian Institute of Mining and Metallurgy & Mine Managers Association of Australia: Carlton, VIC, Australia, 2013; pp. 232–239. [Google Scholar]
- Xu, T. Heat effect of the oxygen-containing functional groups in coal during spontaneous combustion processes. Adv. Powder Technol. 2017, 28, 1841–1848. [Google Scholar] [CrossRef]
- Rules for Ensuring Industrial Safety for Hazardous Production Facilities Conducting Mining and Geological Exploration Work; Ministry of Investment and Development of the Republic of Kazakhstan: Astana, Kazakhstan, 2014; No. 352.
- Portola, V.A.; Khramtsov, V.I.; Shcherbakova, V.A.; Bobrovnikova, A.A. Influence of coal chemical activity on gas emission at low-temperature oxidation. Bull. Kuzbass State Tech. Univ. 2017, 5, 63–67. [Google Scholar]
- Igishev, V.I. Fighting Spontaneous Combustion of Coal in Mines; Nedra: Moscow, Russia, 1987; p. 165. [Google Scholar]
- Labukin, S.N. Research and Development of a Method for Detecting the Early Stage of Spontaneous Combustion of Coal in the Mined-Out Space of Coal Mines. Ph.D. Thesis, Kuzbass State Technical University, Kemerovo, Russia, 2010. [Google Scholar]
- Alperovich, V.I.; Koshovsky, V.I.; Chuntu, G.I.; Pashkovsky, P.S. Temperature control in fire sites based on the ratio of ethylene and acetylene concentrations in gases. Ugol Ukrainy 1977, 1, 46–47. [Google Scholar]
- Alperovich, V.Y.; Pashkovsky, P.S.; Koshovsky, B.I.; Zagoruiko, O.S. Improvement of the method for detecting early stages of spontaneous combustion and coal self-heating by unsaturated hydrocarbons. In Methods and Means of Fighting Underground Fires; Collection of Scientific Papers: Donetsk, Ukraine, 1981; pp. 91–95. [Google Scholar]
- Karaganda Department of All-Union Research Institute of Mine Rescue Work. Guidelines for Monitoring the Development of Endogenous Fires in Mined-Out Spaces of Mines in the Karaganda Basin (Based on the Ratio of Ethylene to Acetylene); Karaganda Department of All-Union Research Institute of Mine Rescue Work: Karaganda, Kazakhstan, 1988; p. 33. [Google Scholar]
- Portola, V.A.; Labukin, S.N. Method and device for identifying coal self-heating in mines. Bull. KuzSTU 2009, 6, 42–45. Available online: https://cyberleninka.ru/article/n/sposob-i-ustroystvo-identifikatsii-samonagrevaniya-uglya-v-shahtah (accessed on 20 May 2025).
- Grekov, S.P.; Pashkovskiy, P.S.; Orlikova, V.P. Identification of temperature for self-heating of coal caused by the ratio of carbon oxide and decreasing oxygen levels along a section exposed to a catastrophe. Bezp. Tech. Pozar. 2015, 3, 119–127. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).