Non-Redox-Based Electrochemical Detection of Adrenaline: A Simple and Reliable Approach Using Glass Nanopipets
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fabrication of Nanopipets
2.3. Electrochemical Measurements
2.4. Mathematical Determination of Potential Shift
3. Results and Discussion
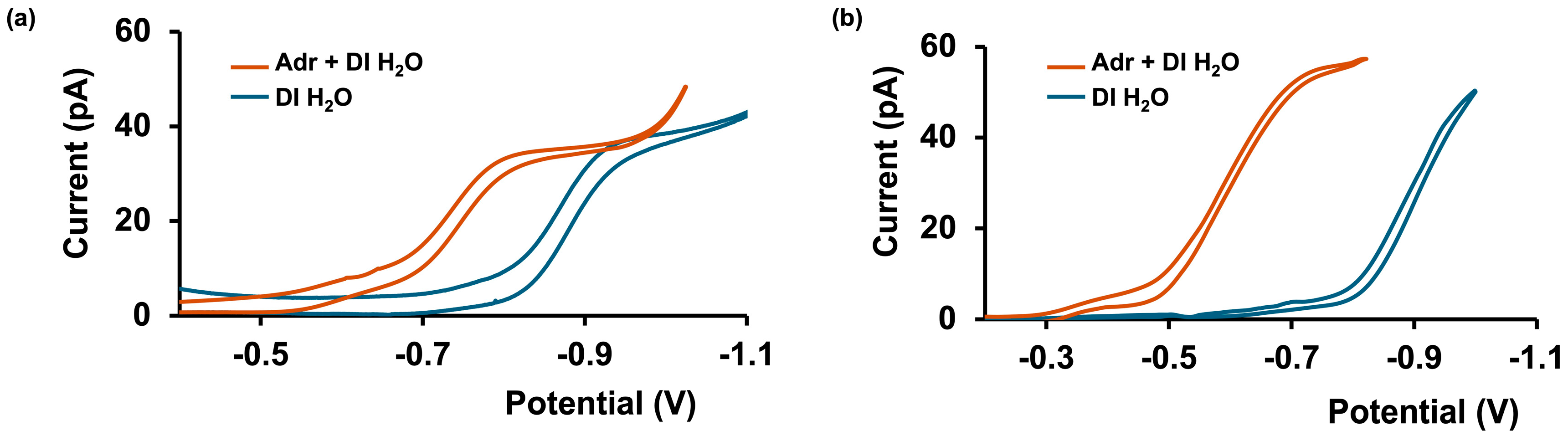
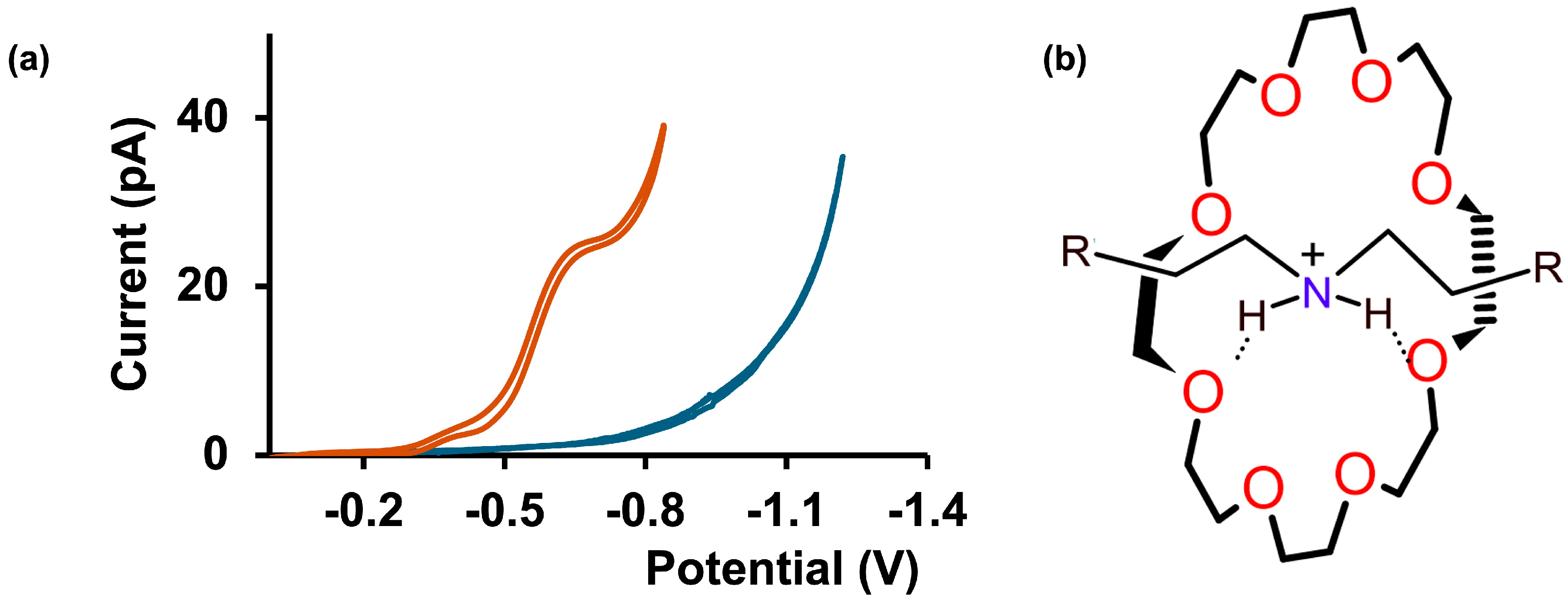
3.1. Adrenaline Transfer Across Aqueous/DCE Interface in Simple Matrices
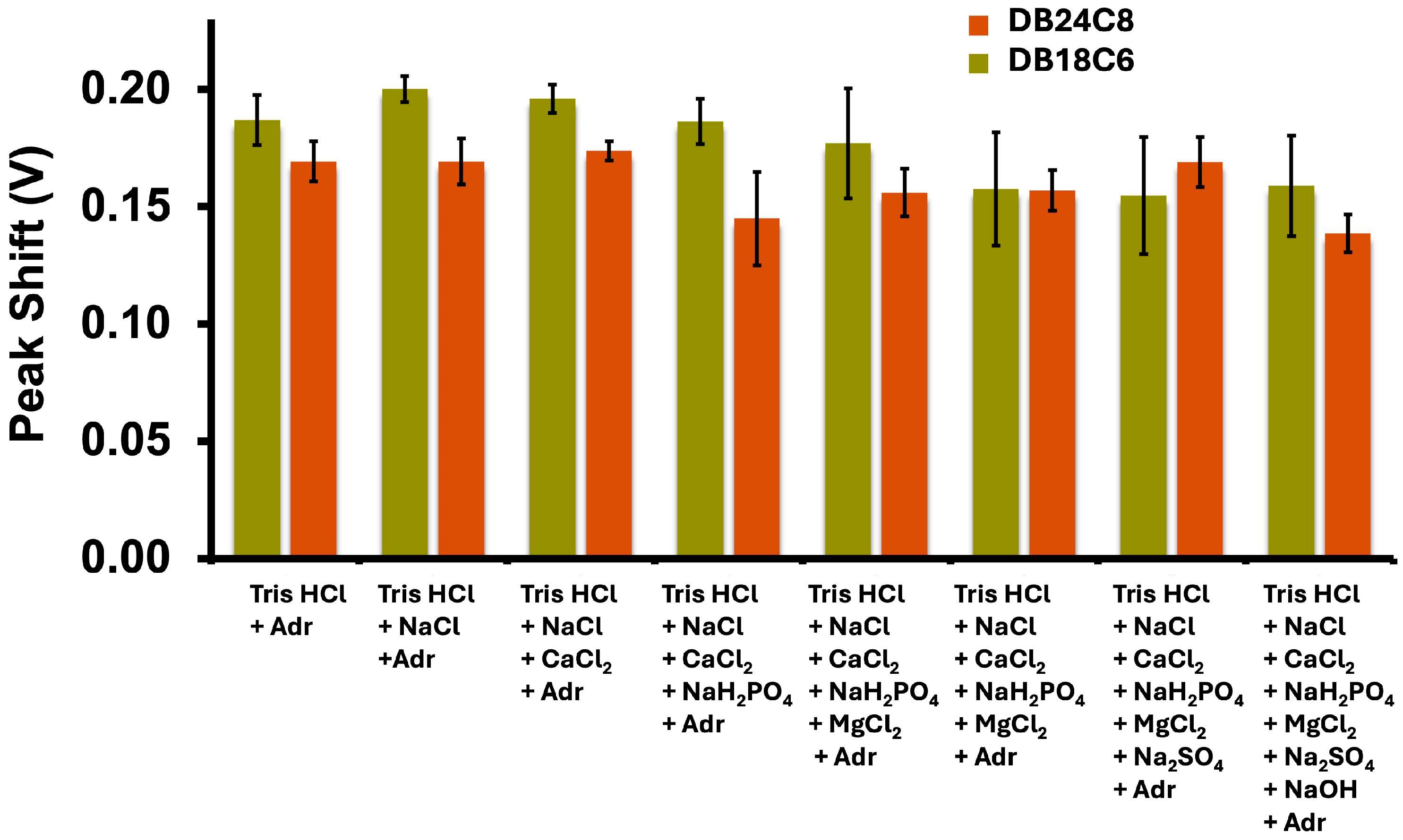
3.2. Adrenaline Transfer in Complex Matrices
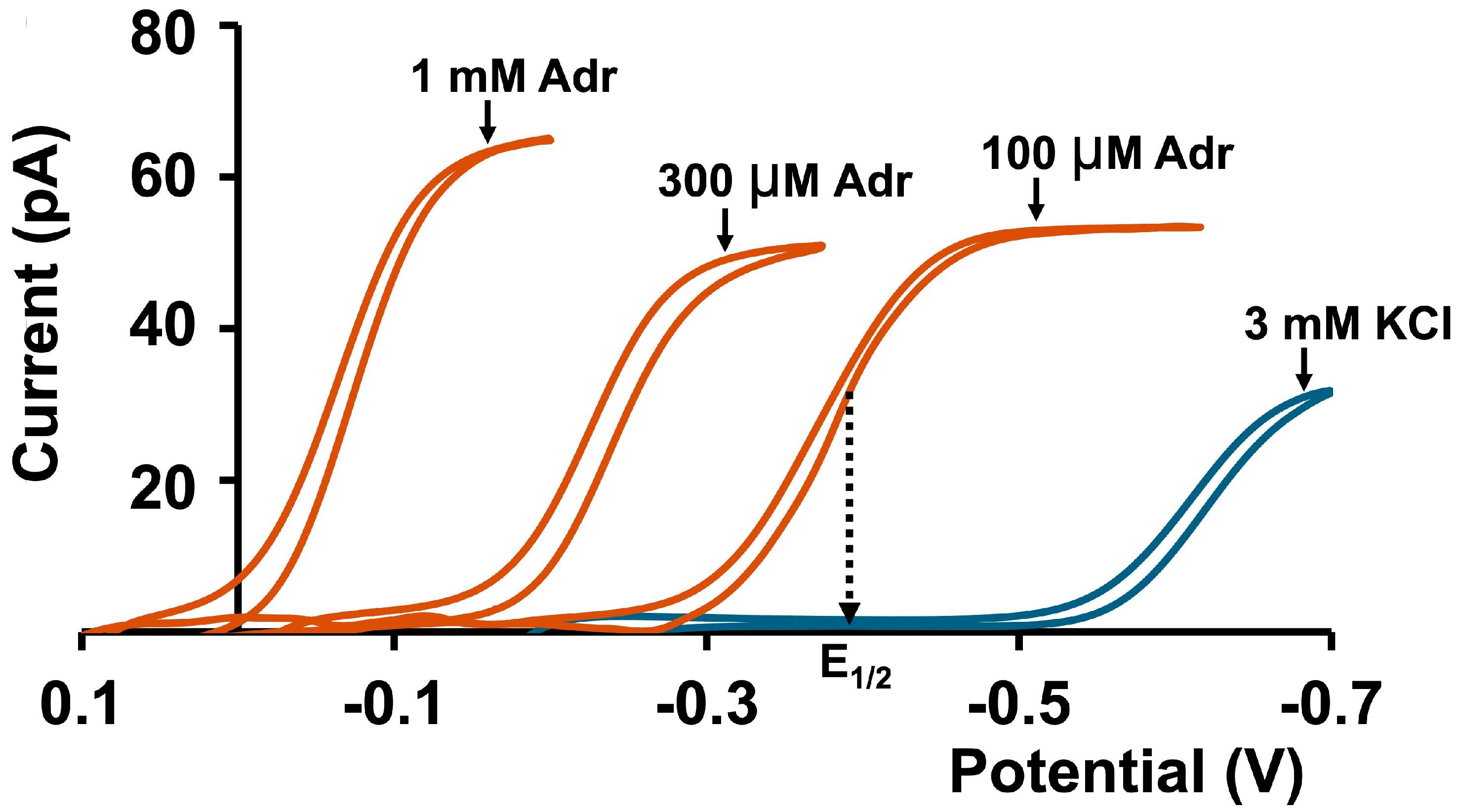
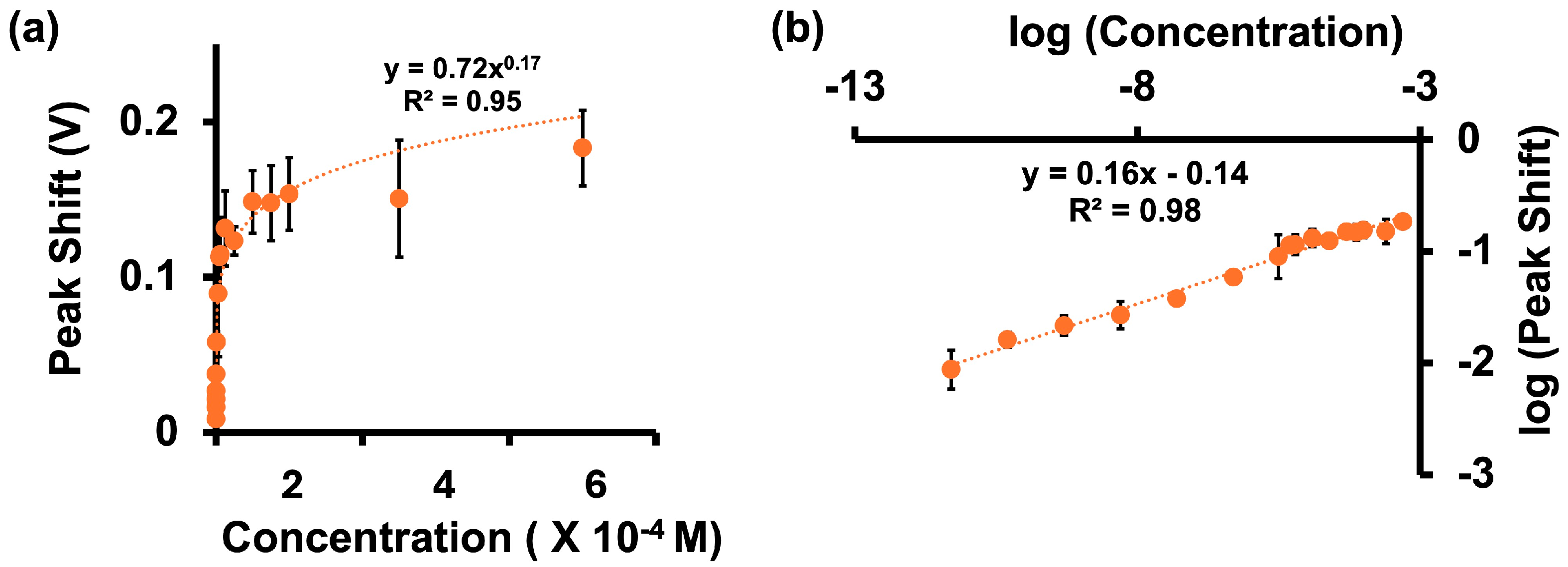
3.3. Calibration Curves for Adr in Tris Buffer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Werdermann, M.; Bornstein, S.R.; Steenblock, C. The Adrenal Gland in Stress–Adaptation on a Cellular Level. J. Steroid Biochem. Mol. Biol. 2019, 190, 198–206. [Google Scholar] [CrossRef]
- Schmidt, M.V.; Sterlemann, V.; Müller, M.B. Chronic Stress and Individual Vulnerability. Ann. N. Y. Acad. Sci. 2008, 1148, 174–183. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Berger, I.; Scriba, L.; Santambrogio, A.; Steenblock, C. Adrenal Cortex–Medulla Interactions in Adaptation to Stress and Disease. Curr. Opin. Endocr. Metab. Res. 2019, 8, 9–14. [Google Scholar] [CrossRef]
- Dayas, C.V.; Buller, K.M.; Crane, J.W.; Xu, Y.; Day, T.A. Stressor Categorization: Acute Physical and Psychological Stressors Elicit Distinctive Recruitment Patterns in the Amygdala and in Medullary Noradrenergic Cell Groups. Eur. J. Neurosci. 2001, 14, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Aich, P.; Potter, A.A.; Griebel, P.J. Modern Approaches to Understanding Stress and Disease Susceptibility: A Review with Special Emphasis on Respiratory Disease. Int. J. Gen. Med. 2009, 2, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.; Grujic, D. Epinephrine; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Farrugia, F.; Martikos, G.; Tzanetis, P.; Charalampopoulos, A.; Misiakos, E.; Zavras, N.; Sotiropoulos, D. Pheochromocytoma, Diagnosis and Treatment: Review of the Literature. Endocr. Regul. 2017, 51, 168–181. [Google Scholar] [CrossRef] [PubMed]
- ESLER, M. Sympathetic Nervous System and Insulin Resistance: From Obesity to Diabetes. Am. J. Hypertens 2001, 14, S304–S309. [Google Scholar] [CrossRef]
- Guest, F.L.; Martins-de-Souza, D.; Rahmoune, H.; Bahn, S.; Guest, P.C. The Effects of Stress on Hypothalamic-Pituitary-Adrenal (HPA) Axis Function in Subjects with Schizophrenia. Arch. Clin. Psychiatry (São Paulo) 2012, 40, 20–27. [Google Scholar] [CrossRef]
- Rahi, V.; Kumar, P. Animal Models of Attention-deficit Hyperactivity Disorder (ADHD). Int. J. Dev. Neurosci. 2021, 81, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Diego, A.M.G.; Ortega-Cruz, D.; García, A.G. Disruption of Exocytosis in Sympathoadrenal Chromaffin Cells from Mouse Models of Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 1946. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, K.; Brabant, S.; Prie, D.; Piketty, M.-L. Hormone Immunoassay Interference: A 2021 Update. Ann. Lab. Med. 2022, 42, 3–23. [Google Scholar] [CrossRef]
- Gorbunova, M.V.; Gutorova, S.V.; Berseneva, D.A.; Apyari, V.V.; Zaitsev, V.D.; Dmitrienko, S.G.; Zolotov, Y.A. Spectroscopic Methods for Determination of Catecholamines: A Mini-Review. Appl. Spectrosc. Rev. 2019, 54, 631–652. [Google Scholar] [CrossRef]
- Kirkpatrick, D.; Yang, J.; Trehy, M. Determination of the Enantiomeric Purity of Epinephrine by HPLC with Circular Dichroism Detection. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 556–563. [Google Scholar] [CrossRef]
- Bertler, Å.; Carlsson, A.; Rosengren, E. A Method for the Fluorimetric Determination of Adrenaline and Noradrenaline in Tissues. Acta Physiol. Scand. 1958, 44, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Saraf, N.; Bosak, A.; Willenberg, A.; Das, S.; Willenberg, B.J.; Seal, S. Colorimetric Detection of Epinephrine Using an Optimized Paper-Based Aptasensor. RSC Adv. 2017, 7, 49133–49143. [Google Scholar] [CrossRef]
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Anik, Ü. Electrochemical Medical Biosensors for POC Applications. In Medical Biosensors for Point of Care (POC) Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–292. [Google Scholar]
- Zhang, H. Studies of the Electrochemical Behavior of Epinephrine at a Homocysteine Self-Assembled Electrode. Talanta 2002, 56, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Silva, F.; Pereira, C.M. Electrochemical Sensing of Catecholamines at the Water/1,6-Dichlorohexane Interface. Electroanalysis 2013, 25, 2331–2338. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Miranda, I.M.; Silva, F.; Pereira, C.M. Electrochemical Study of Dopamine and Noradrenaline at the Water/1,6-Dichlorohexane Interface. Phys. Chem. Chem. Phys. 2010, 12, 15190. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Buchanan, A.M.; Witt, C.E.; Hashemi, P. Frontiers in Electrochemical Sensors for Neurotransmitter Detection: Towards Measuring Neurotransmitters as Chemical Diagnostics for Brain Disorders. Anal. Methods 2019, 11, 2738–2755. [Google Scholar] [CrossRef]
- Ahmed, M.M.N.; Bodowara, F.S.; Zhou, W.; Penteado, J.F.; Smeltz, J.L.; Pathirathna, P. Electrochemical Detection of Cd(II) Ions in Complex Matrices with Nanopipets. RSC Adv. 2022, 12, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Bai, S.; Ma, Y.; Liu, C.; Wang, L. A Nanopipette Supported Oil/Water Interface Sensor for the Kinetics Analysis and Determination of Phenothiazine Derivatives. Electrochim. Acta 2022, 423, 140568. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Radhapyari, K.; Jadon, N.; Agarwal, S. Voltammetric Techniques for the Assay of Pharmaceuticals—A Review. Anal. Biochem. 2011, 408, 179–196. [Google Scholar] [CrossRef]
- Pathirathna, P.; Balla, R.J.; Jantz, D.T.; Kurapati, N.; Gramm, E.R.; Leonard, K.C.; Amemiya, S. Probing High Permeability of Nuclear Pore Complexes by Scanning Electrochemical Microscopy: Ca 2+ Effects on Transport Barriers. Anal. Chem. 2019, 91, 5446–5454. [Google Scholar] [CrossRef]
- Koryta, J. Electrochemical Polarization Phenomena at the Interface of Two Immiscible Electrolyte Solutions. Electrochim. Acta 1979, 24, 293–300. [Google Scholar] [CrossRef]
- Guo, J.; Amemiya, S. Voltammetric Heparin-Selective Electrode Based on Thin Liquid Membrane with Conducting Polymer-Modified Solid Support. Anal. Chem. 2006, 78, 6893–6902. [Google Scholar] [CrossRef]
- Sochr, J.; Švorc, Ľ.; Rievaj, M.; Bustin, D. Electrochemical Determination of Adrenaline in Human Urine Using a Boron-Doped Diamond Film Electrode. Diam. Relat. Mater. 2014, 43, 5–11. [Google Scholar] [CrossRef]
- Sukanya, S.D.; Swamy, B.E.K.; Shashikumara, J.K.; Sharma, S.C.; Hariprasad, S.A. A Novel, Extreme Low-Cost Poly (Erythrosine) Modified Pencil Graphite Electrode for Determination of Adrenaline. Sci. Rep. 2023, 13, 4523. [Google Scholar] [CrossRef] [PubMed]
- Charithra, M.M.; Manjunatha, J.G. Electrochemical Sensing of Adrenaline Using Surface Modified Carbon Nanotube Paste Electrode. Mater. Chem. Phys. 2021, 262, 124293. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Fayemi, O.E.; Adekunle, A.S.; Sherif, E.-S.M.; Ebenso, E.E. Electrochemical Sensor for the Detection of Adrenaline at Poly(Crystal Violet) Modified Electrode: Optimization and Voltammetric Studies. Heliyon 2022, 8, e10835. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Pereira, C.M. Applications of Electrochemistry at the ITIES in Drug Discovery and Development—A Review. ChemElectroChem 2024, 11, e202400134. [Google Scholar] [CrossRef]
- Späth, A.; König, B. Molecular Recognition of Organic Ammonium Ions in Solution Using Synthetic Receptors. Beilstein J. Org. Chem. 2010, 6, 32. [Google Scholar] [CrossRef]
- Vizi, E.S.; Orsó, E.; Osipenko, O.N.; Haskó, G.; Elenkov, I.J. Neurochemical, Electrophysiological and Immunocytochemical Evidence for a Noradrenergic Link between the Sympathetic Nervous System and Thymocytes. Neuroscience 1995, 68, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Levey, A. Immunohistochemical Localization of Proteins in the Nervous System. Curr. Protoc. Neurosci. 2003, 25, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.C.; Rossolini, G.; Piantanelli, L.; Migani, P. Neurochemical Parameters of the Main Neurotransmission Systems in Aging Mice. Arch. Gerontol. Geriatr. 2000, 30, 269–279. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, R.J.; Koifman, G.; Manring, N.; Smeltz, J.L.; Pathirathna, P. Non-Redox-Based Electrochemical Detection of Adrenaline: A Simple and Reliable Approach Using Glass Nanopipets. Appl. Sci. 2025, 15, 869. https://doi.org/10.3390/app15020869
Page RJ, Koifman G, Manring N, Smeltz JL, Pathirathna P. Non-Redox-Based Electrochemical Detection of Adrenaline: A Simple and Reliable Approach Using Glass Nanopipets. Applied Sciences. 2025; 15(2):869. https://doi.org/10.3390/app15020869
Chicago/Turabian StylePage, Ralph J., Gene Koifman, Noel Manring, Jessica L. Smeltz, and Pavithra Pathirathna. 2025. "Non-Redox-Based Electrochemical Detection of Adrenaline: A Simple and Reliable Approach Using Glass Nanopipets" Applied Sciences 15, no. 2: 869. https://doi.org/10.3390/app15020869
APA StylePage, R. J., Koifman, G., Manring, N., Smeltz, J. L., & Pathirathna, P. (2025). Non-Redox-Based Electrochemical Detection of Adrenaline: A Simple and Reliable Approach Using Glass Nanopipets. Applied Sciences, 15(2), 869. https://doi.org/10.3390/app15020869





