Occurrence of Aflatoxin M1 in Raw and Processed Milk: A Contribution to Human Exposure Assessment After 12 Years of Investigation
Abstract
1. Introduction
2. Materials and Methods
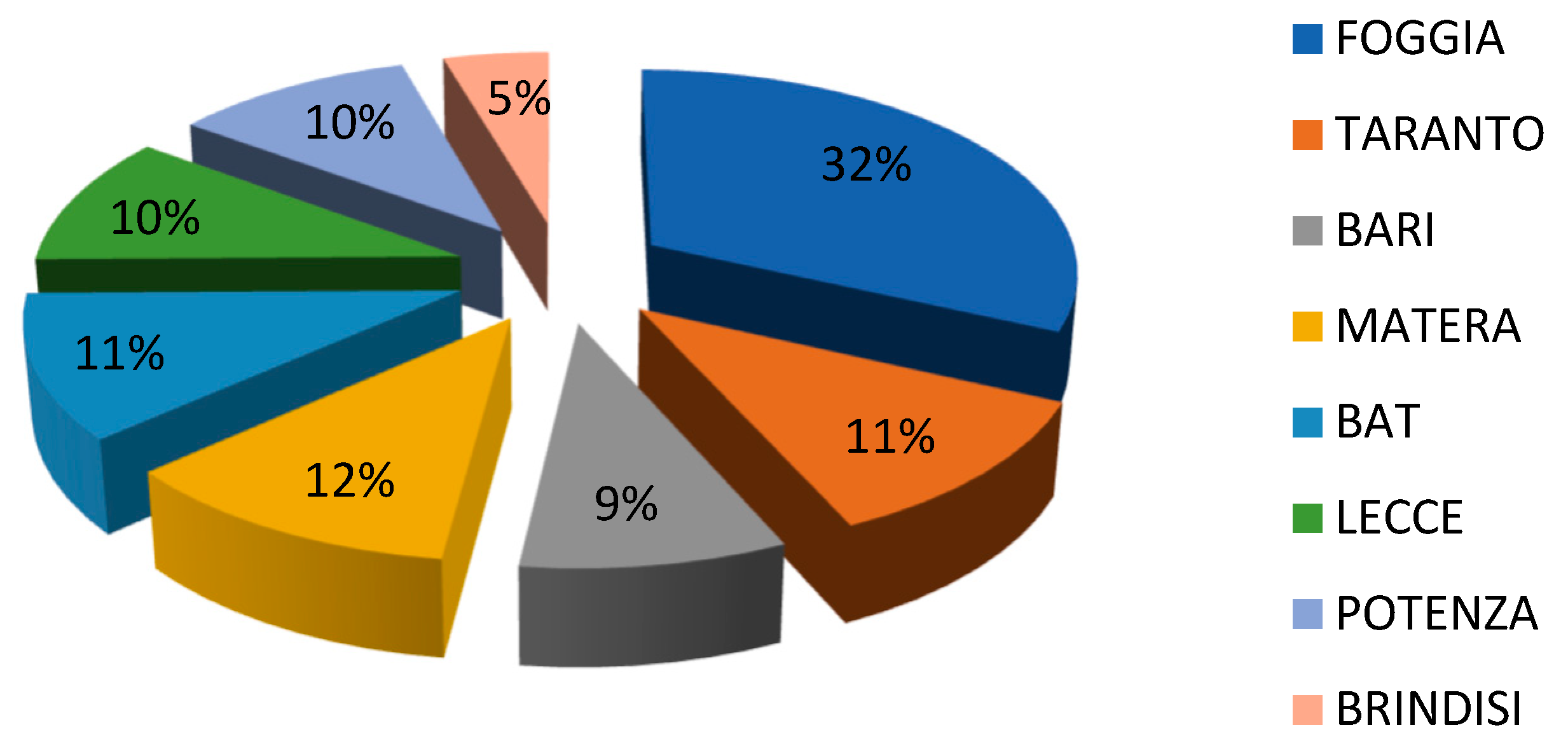
2.1. Sample Collection
2.2. Chemicals and Analytical Methods
2.2.1. Sample Pre-Treatment
2.2.2. Immunoassay Reagents, Equipment and Procedure
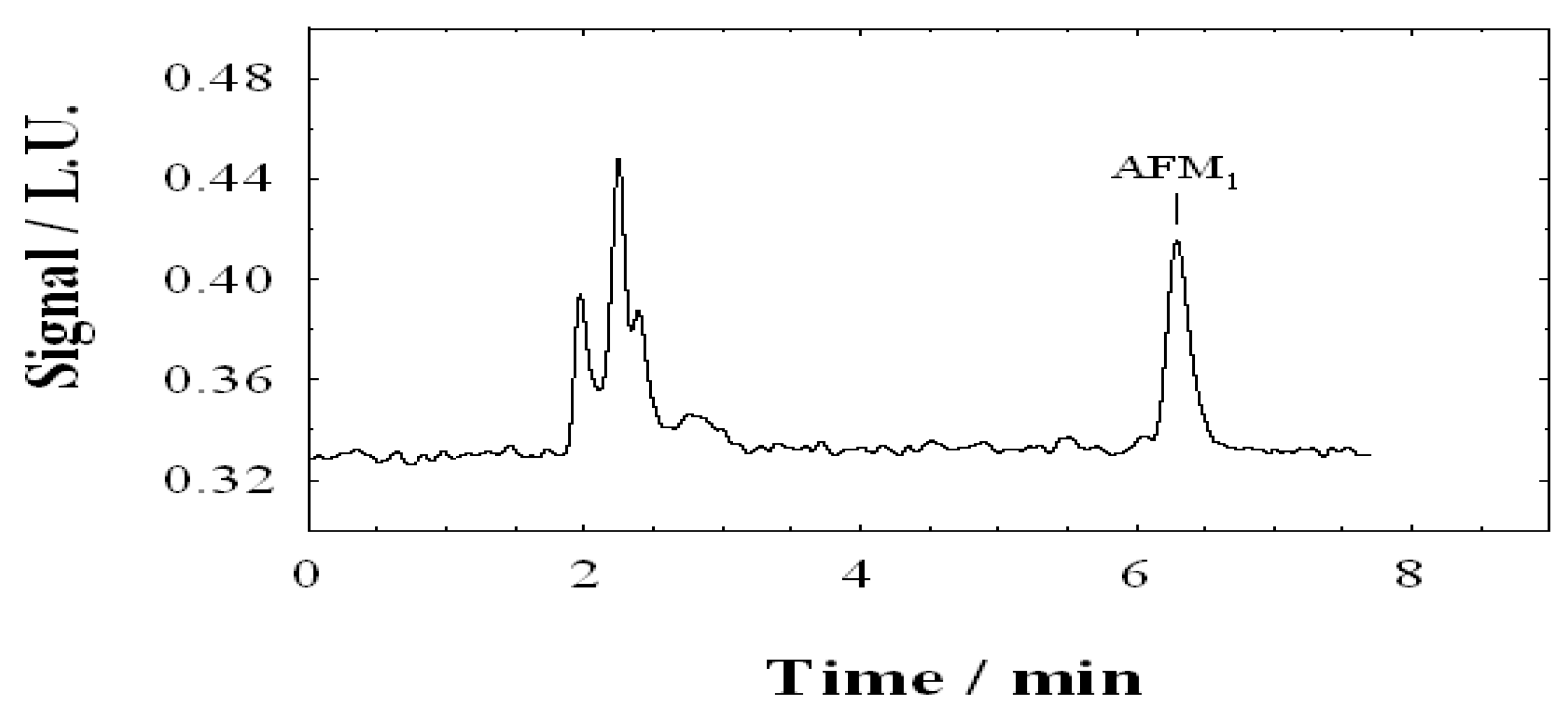
2.2.3. HPLC Reagents, Equipment and Procedure
2.3. Risk Exposure
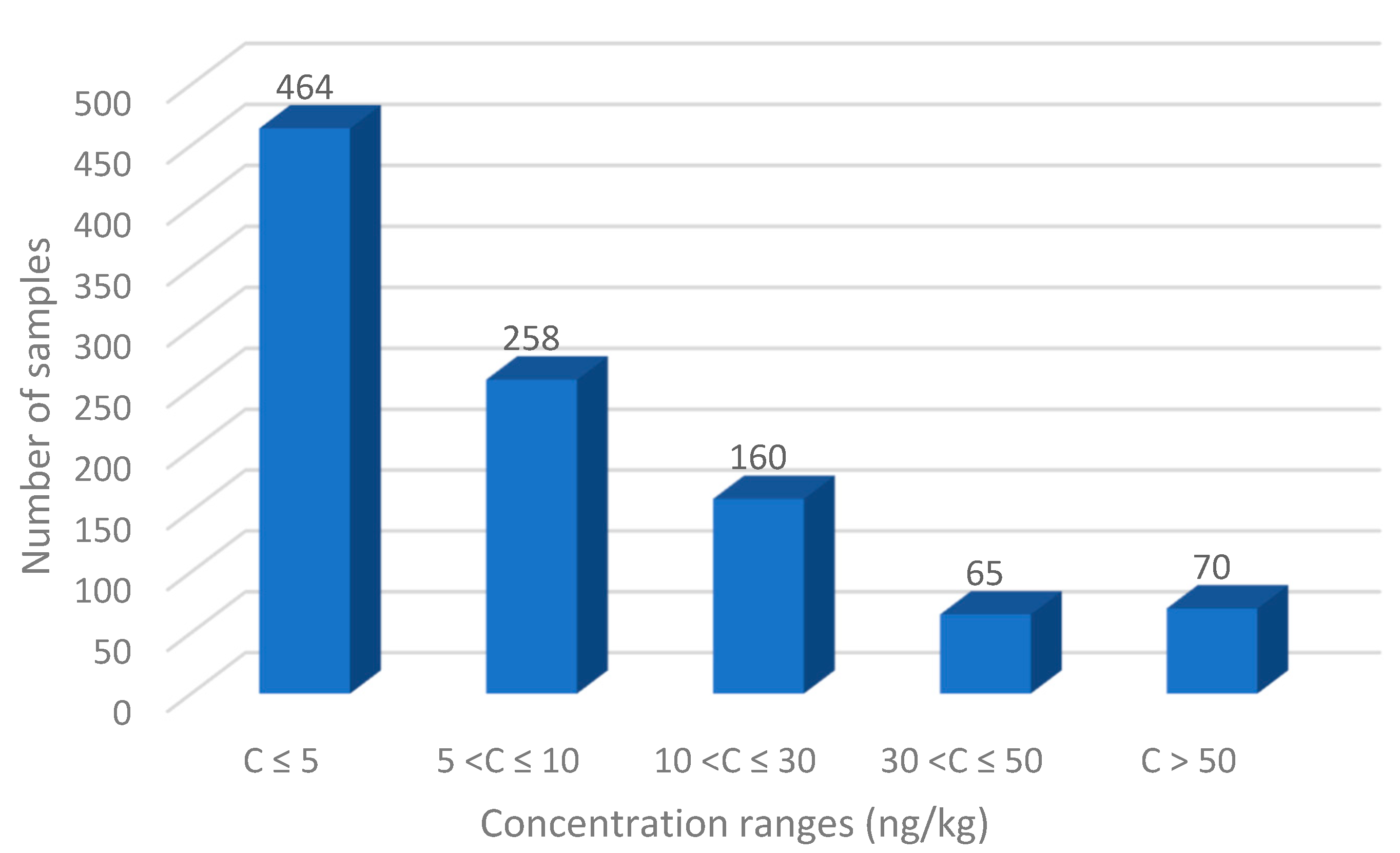
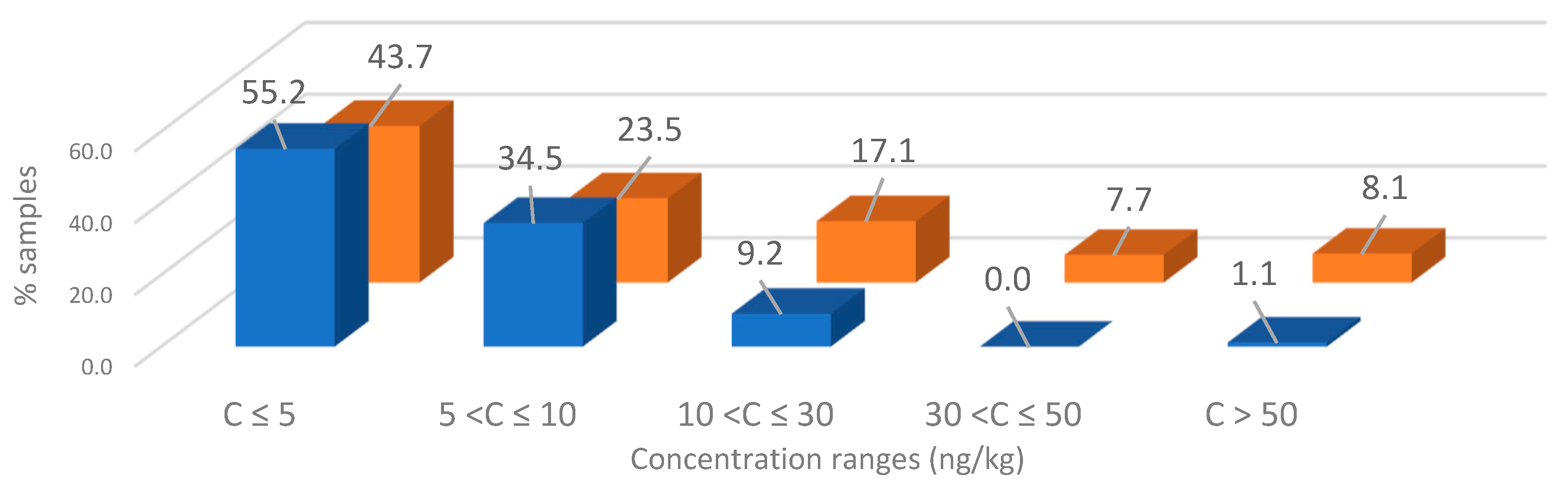
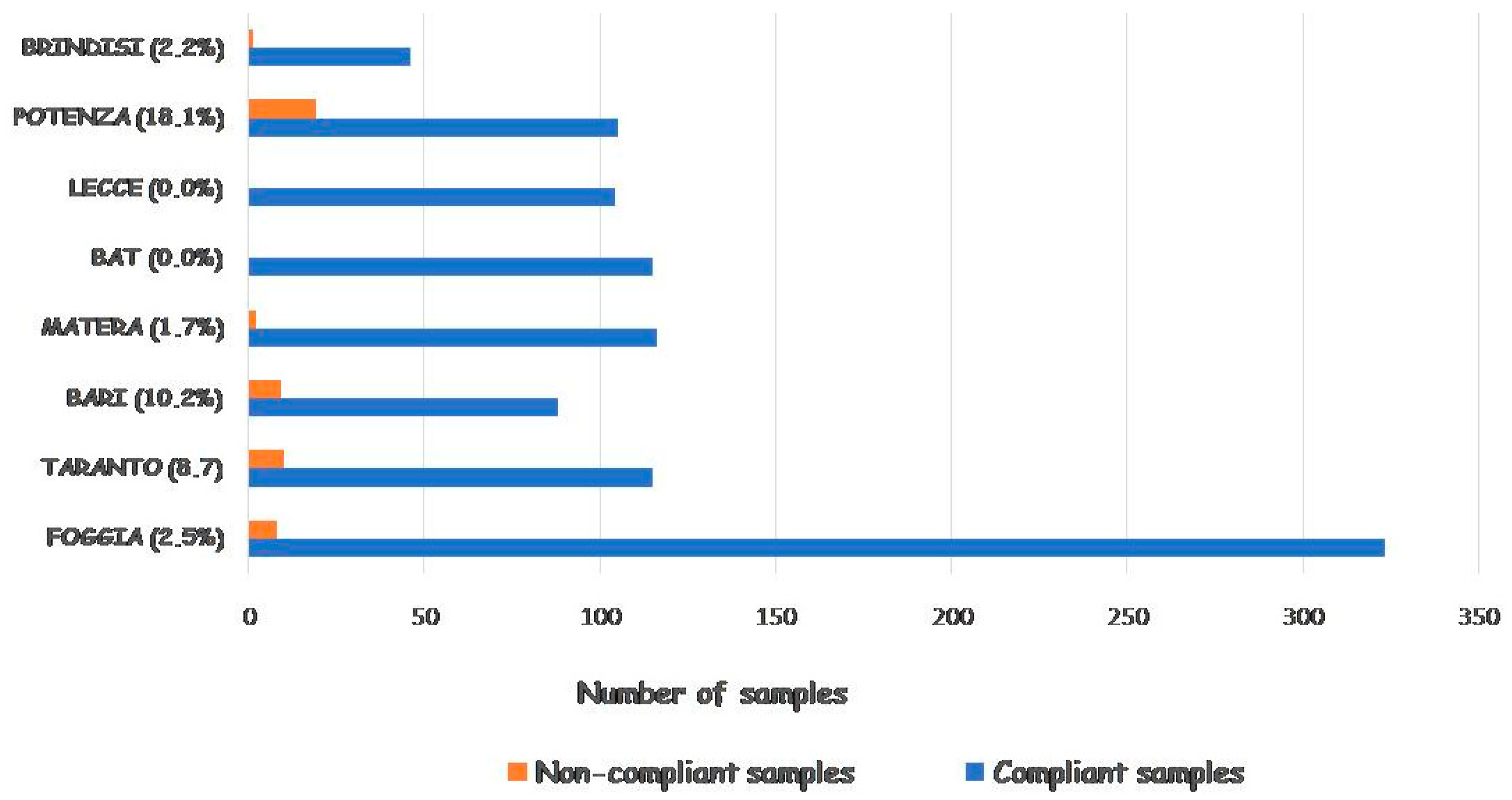
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). Climate change and food security: Risks and responses. In Food and Agriculture Organization of the United Nations (FAO) Report; FAO: Rome, Italy, 2016. [Google Scholar]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Gonzalez-Jartín, J.M.; Sainz, M.J. Impact of global warming on mycotoxins. Curr. Opin. Food Sci. 2017, 18, 76–81. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Trucksess, M.W.; Diaz-Amingo, C. Mycotoxins in Foods. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 888–897. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, H.; Bhadauria, S. Aflatoxin contamination in food crops: Causes, detection, and management: A review. Food Prod. Process. Nutr. 2021, 3, 17–26. [Google Scholar] [CrossRef]
- Khayoon, W.S.; Saad, B.; Yan, C.B.; Hashim, N.H.; Ali, A.S.M.; Salleh, M.I.; Salleh, B. Determination of aflatoxins in animal feeds by HPLC with multifunctional column clean-up. Food Chem. 2010, 118, 882–886. [Google Scholar] [CrossRef]
- Giray, B.; Girgin, G.; Engin, A.B.; Aydin, S.; Sahin, G. Aflatoxin levels in wheat samples consumed in some regions of Turkey. Food Control 2007, 18, 23–29. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kralj Cigić, I.; Prosen, H. An overview of conventional and emerging analytical methods for the determination of Mycotoxins. Int. J. Mol. Sci. 2009, 10, 62–115. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Aflatoxins. In Chemical Agents and Related Occupations; Agency for Research on Cancer: Lyon, France, 2012; pp. 225–248. [Google Scholar]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Vaz, A.; Cabral Silva, A.C.; Rodrigues, P.; Venâncio, A. Detection Methods for Aflatoxin M1 in Dairy Products. Microorganisms 2020, 8, 246. [Google Scholar] [CrossRef]
- Asi, M.R.; Iqbal, S.Z.; Ariño, A.; Hussain, A. Effect of seasonal variations and lactation times on aflatoxin M1 contamination in milk of different species from Punjab, Pakistan. Food Control 2012, 25, 34–38. [Google Scholar] [CrossRef]
- Zentai, A.; Jóźwiak, Á.; Süth, M.; Farkas, Z. Carry-Over of Aflatoxin B1 from Feed to Cow Milk—A Review. Toxins 2023, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Serraino, A.; Bonilauri, P.; Kerekes, K.; Farkas, Z.; Giacometti, F.; Canever, A.; Zambrini, A.V.; Ambrus, À. Occurence of Aflatoxin M1 in raw milk marketed in Italy: Exposure assessment and risk characterization. Front. Microbiol. 2019, 10, 2516. [Google Scholar] [CrossRef] [PubMed]
- Girolami, F.; Barbarossa, A.; Badino, P.; Ghadiri, S.; Cavallini, D.; Zaghini, A.; Nebbia, C. Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels. Toxins 2022, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Topi, D.; Spahiu, J.; Rexhepi, A.; Marku, N. Two-year survey of aflatoxin M1 in milk marketed in Albania, and human exposure assessment. Food Control 2022, 136, 108831. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2002; Volume 56, pp. 171–176. [Google Scholar]
- Ibáñez-Vea, M.; Corcuera, L.N.; Remiro, R.; Murillo-Arbizu, M.T.; González-Peñas, E.; Lizarraga, E. Validation of a UHPLC-FLD method for the simultaneous quantification of aflatoxins, ochratoxin A and zearalenone in barley. Food Chem. 2011, 127, 351–358. [Google Scholar] [CrossRef]
- Cullen, J.M.; Newberne, P.M. Acute Hepatotoxicity of Aflatoxins. In The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: San Diego, CA, USA, 1993; Volume 129, pp. 3–26. [Google Scholar]
- Ferreira, R.G.; Cardoso, M.V.; de Souza Furtado, K.M.; Espíndola, K.M.M.; Amorim, R.P.; Monteiro, M.C. Epigenetic alterations caused by aflatoxin b1: A public health risk in the induction of hepatocellular carcinoma. Transl. Res. 2019, 204, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, A.D.W. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of Aflatoxin Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Boudra, H.; Barnouin, J.; Dragacci, S.; Morgavi, D.P. Aflatoxin M1 and Ochratoxin A in Raw Bulk Milk from French Dairy Herds. J. Dairy Sci. 2007, 90, 3197–3201. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Aliabadi-Sh, F.; Salah Nejad, A.; Poursoltani, H.; Motallebi, A.A.; Mahdavi, H. Aflatoxin M1 contamination in winter and summer milk in 14 states in Iran. Food Control 2008, 19, 1033–1036. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No. 915/2023 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No. 1881/2006. Off. J. Eur. Union 2023, L119, 103–157. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 13 January 2025).
- FAO (Food and Agriculture Organization of the United Nations). Worldwide Regulations for Mycotoxins in Food and Feed in 2003; Food and Agriculture Organization, FAO Food and Nutrition: Rome, Italy, 2004; Available online: https://www.fao.org/4/y5499e/y5499e02.htm (accessed on 7 November 2024).
- Womack, E.D.; Sparks, D.L.; Brown, A.E. Aflatoxin M1 in milk and milk products: A short review. World Mycotoxin J. 2016, 9/2, 305–315. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 786/2015 of 19 May 2015 Defining Acceptability Criteria for Detoxification Processes Applied to Products Intended for Animal Feed as Provided for in Directive 2002/32/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, L125, 10–14. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R0786 (accessed on 7 November 2024).
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted Methods for Decontamination of AflatoxinM1 in Milk Using Microbial Adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Carraro, A.; De Giacomo, A.; Giannossi, M.L.; Medici, L.; Muscarella, M.; Palazzo, L.; Quaranta, V.; Summa, V.; Tateo, F. Clay minerals as adsorbents of aflatoxin M1 from contaminated milk and effects on milk quality. Appl. Clay Sci. 2014, 88/89, 92–99. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyamb, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Kos, J.; Janić Hajnal, E.; Jajić, I.; Krstović, S.; Mastilović, J.; Šarić, B.; Jovanov, P. Comparison of ELISA, HPLC-FLD and HPLC-MS/MS methods for determination of aflatoxin M1 in natural contaminated milk samples. Acta Chim. Slov. 2016, 63, 747–756. [Google Scholar] [CrossRef]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Development of a multiresidue method for analysis of major Fusarium mycotoxins in corn meal using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Soleimany, F.; Jinap, S.; Abas, F. Determination of Mycotoxins in Cereals by Liquid Chromatography Tandem Mass Spectrometry. Food Chem. 2012, 130, 1055–1060. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0401 (accessed on 7 November 2024).
- Muscarella, M.; Lo Magro, S.; Palermo, C.; Centonze, D. Validation according to European Commission Decision 2002/657/EC of a confirmatory method for aflatoxin M1 in milk based on immunoaffinity columns and high performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 2007, 594, 257–264. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Scientific opinion—Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Kortei, N.K.; Annan, T.; Kyei-Baffour, V.; Ken Essuman, E.; Boakye, A.A.; Tettey, C.O.; Boadi, N.O. Exposure assessment and cancer risk characterization of aflatoxin M1 (AFM1) through ingestion of raw cow milk in southern Ghana. Toxicol. Rep. 2022, 9, 1189–1197. [Google Scholar] [CrossRef]
- Djekic, I.; Petrovic, J.; Jovetic, M.; Redzepovic-Djordevic, A.; Stulic, M.; Lorenzo, J.M.; Iammarino, M.; Tomasevic, I. Aflatoxins in Milk and Dairy Products: Occurrence and Exposure Assessment for the Serbian Population. Appl. Sci. 2020, 10, 7420. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A. The Italian national food consumption survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Guidance of EFSA—Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J. 2011, 9, 2097. [Google Scholar] [CrossRef]
- Lo Magro, S.; Summa, S.; Iammarino, M.; D’antini, P.; Marchesani, G.; Chiaravalle, A.E.; Muscarella, M. A 5-Years (2015–2019) Control Activity of an EU Laboratory: Contamination of Histamine in Fish Products and Exposure Assessment. Appl. Sci. 2020, 10, 8693. [Google Scholar] [CrossRef]
- Italian National Institute of Health. Trattamento dei Dati Inferiori al Limite di Rivelabilità nel Calcolo dei Risultati Analitici—Rapporti ISTISAN 04/15. 2004. Available online: https://www.iss.it/documents/20126/955767/0415.1106219644.pdf/51c15924-7b63-07ca-cce4-9a37d772190d?t=1575578775857 (accessed on 30 November 2024).
- Li, S.; Min, L.; Wang, P.; Zhang, Y.; Zheng, N.; Wang, J. Aflatoxin M1 contamination in raw milk from major milk-producing areas of China during four seasons of 2016. Food Control 2017, 82, 121–125. [Google Scholar] [CrossRef]
- Bilandzic, N.; Varga, I.; Varenina, I.; Kolanovic, B.S.; Luburic, D.B.; Dokic, M.; Sedak, M.; Cvetnic, L.; Cvetnic, Z. Seasonal Occurrence of Aflatoxin M1 in Raw Milk during a Five-Year Period in Croatia: Dietary Exposure and Risk Assessment. Foods 2022, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Fumagalli, F.; Rizzi, N.; Grandi, E.; Vailati, S.; Manoni, M.; Ottoboni, M.; Cheli, F.; Pinotti, L. An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy. Toxins 2022, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Rizzi, N.; Grandi, E.; Clerici, E.; Tirloni, E.; Stella, S.; Bernardi, C.E.M.; Pinotti, L. Compliance between Food and Feed Safety: Eight-Year Survey (2013–2021) of Aflatoxin M1 in Raw Milk and Aflatoxin B1 in Feed in Northern Italy. Toxins 2023, 15, 168. [Google Scholar] [CrossRef]
| Time (min) | Water% | Acetonitrile% | Methanol% |
|---|---|---|---|
| 0.0 | 55 | 40 | 5 |
| 6.0 | 55 | 40 | 5 |
| 7.0 | 15 | 80 | 5 |
| 10.0 | 15 | 80 | 5 |
| 11.0 | 55 | 40 | 5 |
| 17.0 | 55 | 40 | 5 |
| Sample n | Milk Type | AFM1 Concentration Values (µg/kg ± Measurement Uncertainty) | Province of Origin | Year of Sampling/Analysis |
|---|---|---|---|---|
| 1 | RAW | 0.216 +/− 0.030 | FOGGIA | 2012 |
| 2 | RAW | 0.062 +/− 0.010 | BARI | 2012 |
| 3 | RAW | 0.552 +/− 0.070 | FOGGIA | 2012 |
| 4 | RAW | 0.065 +/− 0.009 | BARI | 2012 |
| 5 | RAW | 0.099 +/− 0.014 | TARANTO | 2012 |
| 6 | RAW | 0.162 +/− 0.021 | TARANTO | 2012 |
| 7 | RAW | 0.095 +/− 0.013 | FOGGIA | 2012 |
| 8 | PASTEURIZED | 0.162 +/− 0.022 | FOGGIA | 2012 |
| 9 | RAW | 0.070 +/− 0.009 | BARI | 2012 |
| 10 | RAW | 0.162 +/− 0.022 | POTENZA | 2012 |
| 11 | RAW | 0.134 +/− 0.018 | POTENZA | 2012 |
| 12 | RAW | 0.126 +/− 0.017 | POTENZA | 2012 |
| 13 | RAW | 0.134 +/− 0.018 | POTENZA | 2012 |
| 14 | RAW | 0.103 +/− 0.014 | POTENZA | 2012 |
| 15 | RAW | 0.091 +/− 0.013 | POTENZA | 2012 |
| 16 | RAW | 0.167 +/− 0.022 | POTENZA | 2012 |
| 17 | RAW | 0.097 +/− 0.013 | POTENZA | 2012 |
| 18 | RAW | 0.173 +/− 0.023 | POTENZA | 2012 |
| 19 | RAW | 0.158 +/− 0.022 | TARANTO | 2012 |
| 20 | RAW | 0.154 +/− 0.021 | POTENZA | 2012 |
| 21 | RAW | 0.162 +/− 0.022 | POTENZA | 2012 |
| 22 | RAW | 0.148 +/− 0.020 | POTENZA | 2012 |
| 23 | RAW | 0.107 +/− 0.014 | TARANTO | 2012 |
| 24 | RAW | 0.077 +/− 0.010 | TARANTO | 2012 |
| 25 | RAW | 0.154 +/− 0.021 | TARANTO | 2012 |
| 26 | RAW | 0.085 +/− 0.012 | TARANTO | 2012 |
| 27 | RAW | 0.106 +/− 0.015 | POTENZA | 2012 |
| 28 | RAW | 0.142 +/− 0.019 | POTENZA | 2012 |
| 29 | RAW | 0.090 +/− 0.012 | TARANTO | 2012 |
| 30 | RAW | 0.270 +/− 0.036 | FOGGIA | 2013 |
| 31 | RAW | 0.084 +/− 0.011 | FOGGIA | 2013 |
| 32 | RAW | 0.061+/− 0.009 | MATERA | 2013 |
| 33 | RAW | 0.109 +/− 0.015 | POTENZA | 2013 |
| 34 | RAW | 0.086 +/− 0.012 | POTENZA | 2013 |
| 35 | RAW | 0.066 +/− 0.009 | POTENZA | 2013 |
| 36 | RAW | 0.099 +/− 0.013 | BARI | 2013 |
| 37 | RAW | 0.073 +/− 0.010 | BARI | 2013 |
| 38 | RAW | 0.080 +/− 0.011 | BRINDISI | 2013 |
| 39 | RAW | 0.110 +/− 0.015 | POTENZA | 2013 |
| 40 | RAW | 0.095 +/− 0.014 | POTENZA | 2013 |
| 41 | RAW | 0.071 +/− 0.010 | TARANTO | 2013 |
| 42 | RAW | 0.061 +/− 0.009 | TARANTO | 2013 |
| 43 | RAW | 0.061 +/− 0.009 | BARI | 2013 |
| 44 | RAW | 0.062 +/− 0.009 | BARI | 2013 |
| 45 | RAW | 0.110 +/− 0.015 | FOGGIA | 2014 |
| 46 | PASTEURIZED | 0.401 +/− 0.055 | FOGGIA | 2015 |
| 47 | RAW | 0.067 +/− 0.009 | BARI | 2017 |
| 48 | RAW | 0.066 +/− 0.009 | BARI | 2019 |
| 49 | RAW | 0.182 +/− 0.025 | MATERA | 2023 |
| Margin of Exposure (P95) | |||||
|---|---|---|---|---|---|
| Children | Adolescents | Adults | Elderly | ||
| Most-likely exposure scenario | Raw milk | 37,142 | 52,000 | 65,000 | 52,000 |
| Processed milk | 65,000 | 86,667 | 130,000 | 86,000 | |
| High-exposure scenario (all samples) | 5098 | 6667 | 7879 | 6667 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summa, S.; Lo Magro, S.; Vita, V.; Franchino, C.; Scopece, V.; D’Antini, P.; Iammarino, M.; De Pace, R.; Muscarella, M. Occurrence of Aflatoxin M1 in Raw and Processed Milk: A Contribution to Human Exposure Assessment After 12 Years of Investigation. Appl. Sci. 2025, 15, 853. https://doi.org/10.3390/app15020853
Summa S, Lo Magro S, Vita V, Franchino C, Scopece V, D’Antini P, Iammarino M, De Pace R, Muscarella M. Occurrence of Aflatoxin M1 in Raw and Processed Milk: A Contribution to Human Exposure Assessment After 12 Years of Investigation. Applied Sciences. 2025; 15(2):853. https://doi.org/10.3390/app15020853
Chicago/Turabian StyleSumma, Simona, Sonia Lo Magro, Valeria Vita, Cinzia Franchino, Valentina Scopece, Pasqualino D’Antini, Marco Iammarino, Rita De Pace, and Marilena Muscarella. 2025. "Occurrence of Aflatoxin M1 in Raw and Processed Milk: A Contribution to Human Exposure Assessment After 12 Years of Investigation" Applied Sciences 15, no. 2: 853. https://doi.org/10.3390/app15020853
APA StyleSumma, S., Lo Magro, S., Vita, V., Franchino, C., Scopece, V., D’Antini, P., Iammarino, M., De Pace, R., & Muscarella, M. (2025). Occurrence of Aflatoxin M1 in Raw and Processed Milk: A Contribution to Human Exposure Assessment After 12 Years of Investigation. Applied Sciences, 15(2), 853. https://doi.org/10.3390/app15020853







