Study on Hematological and Biochemical Index of Blood and Vitreous Humor in the Celestial-Eye Goldfish
Abstract
1. Introduction
2. Methods
2.1. Experimental Animal
2.2. Experimental Design and Procedures
2.2.1. Preparation of EDTA-K2 and Heparin Sodium Anticoagulant Solution
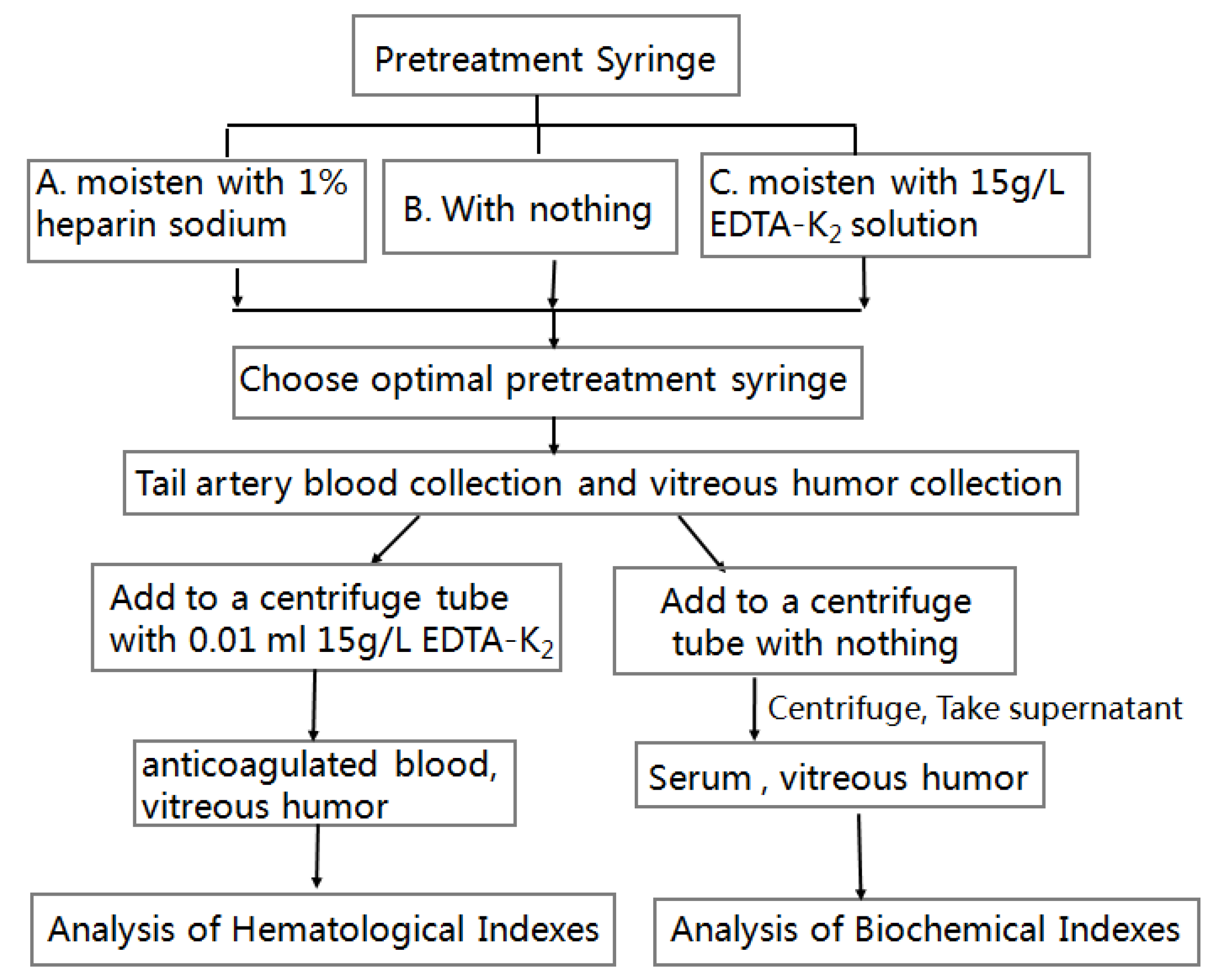
2.2.2. Effect of Three Methods of Pretreatment Syringe on Blood Sampling of Tail Vein of Goldfish and Preparation of Anticoagulant
2.2.3. Preparation of Serum from CE
2.2.4. Collection of Vitreous Humor in CE
2.2.5. Analysis of Hematological Indexes of Blood and Vitreous Humor of CE
2.2.6. Analysis of Biochemical Indexes of Blood and Vitreous Humor of CE
2.2.7. Effect of Collecting Blood and Vitreous Humor on the Follow-Up Survival Rate of CE
2.3. Calculations and Statistical Analysis
3. Results
3.1. Comparison of Three Methods of Pretreatment Syringe Collecting Blood from Arterial (Static) Pulse in the Tail of CE
3.2. Survival Statistics of CE After Blood and Vitreous Humor Collection
3.3. Analysis of Hematological Indexes of Blood and Vitreous Humor of CE
3.4. Analysis of Biochemical Indexes of Blood and Vitreous Humor of CE
4. Discussion
4.1. Exploration of Blood Collection Methods for CE
4.2. Effect on Subsequent Survival of CE After Extraction of Blood and Vitreous Humor
4.3. Analysis of the Number and Composition of Hemoglobin and Erythrocyte in CE
4.4. Analysis of the Number and Composition of White Blood Cells andPlatelets in CE
4.5. Analysis of the Characteristics of Blood Biochemical Indexes in CE
4.6. Biochemical Components in Vitreous Humor of CE
4.7. The Limiting Factors in the Study
5. Conclusions
- There was no significant difference in survival rates between celestial goldfish subjected to blood and vitreous humor extraction and those not involved in the experiment (p > 0.05). This provided a data reference for evaluating whether the experimental samples could be used for subsequent research and whether their ornamental and economic value was affected by the sampling process;
- In the celestial-eye goldfish tested, the mean value of the red blood cell count was 2.19 × 1012/L, the mean value of the white blood cell count was 62.21 × 109/L, and the mean value of hemoglobin concentration was 138.25 g/L. Neither eosinophils nor basophils were detected in the blood samples;
- The analysis of biochemical markers in the blood and vitreous humor of CE revealed that the levels of ALP, TBA, UREA, Cl−, Ca2⁺, Cre, and CHE were lower in the vitreous humor compared to serum, in contrast to the increased levels of ALT and GGT. Seven indicators showed no significant difference between the vitreous humor and serum.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C. Studies on the Karyotype of Goldfish (Carassius auratus) I. A Comparative Study of the Chromosomes in Crucian and Red Dragon-eye Goldfish. J. Genet. Genom. 1982, 9, 238–242. [Google Scholar]
- Wang, H. Atlas of Chinese Goldfish; Culture and Art Publishing House: Beijing, China, 2000. [Google Scholar]
- Li, R.; Sun, Y.; Cui, R.; Zhang, X. Comprehensive Transcriptome Analysis of Different Skin Colors to Evaluate Genes Related to the Production of Pigment in Celestial Goldfish. Biology 2023, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, R.N.; Sun, Y.S.; Tian, Z.; Wang, S.S.; Zhang, X. Effects of genetic and environmental factors on the celestial eye in Carassius auratus. Freshw. Fish. 2022, 52, 66–73. [Google Scholar]
- Li, R.N.; Sun, Y.S.; Tian, Z.; Wang, S.S.; Zhang, X. Comparative transcriptome analysis of body color change in red celestial goldfish Carassius auratus. J. Dalian Ocean. Univ. 2022, 37, 191–201. [Google Scholar]
- Sakaue, H.; Negi, A.; Matsumura, M.; Ohkuma, M.; Honda, Y. The developmental changes of ERGs on spontaneous retinal degeneration of Celestial goldfish. Adv. Ophthalmol. 1988, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Ohkuma, M.; Honda, Y. Retinal degeneration in celestial goldfish. Developmental study. Ophthalmic Res. 1982, 14, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Yoshihiro, O.; Tetsuo, K. Goldfish: An old and new model system to study vertebrate development, evolution and human disease. J. Biochem. 2019, 165, 209–218. [Google Scholar]
- Wang, Y. Expression and Significance of Necroptotic Proteins in Vitreous After Rhegmatogenous Retinal Detachment. Ph.D. Thesis, Anhui Medical University, Hefei, China, 2021. [Google Scholar]
- Rocha, A.S.; Santos, F.M.; Monteiro, J.P.; Castro-De-Sousa, J.P.; Queiroz, J.A.; Tomaz, C.T.; Passarinha, L.A. Trends in proteomic analysis of human vitreous humor samples. Electrophoresis 2014, 35, 2495–2508. [Google Scholar] [CrossRef]
- Zhou, Y.; Xing, Y.; Feng, Q. Reserch advance in haemocytes of fisher. J. Hainan Univ. Nat. Sci. Ed. 2003, 21, 171–176. [Google Scholar]
- Bahmani, M.; Oryan, S.; Pourkazemi, M.; Vosoughi, G. Effects of Ecophysiological Stress on Cellular Immunity System of Persian Sturgeon Acipenser persicus. In Proceedings of the 14th Iranian Congress of Physiology and Pharmacology, Tehran, Iran, 16–20 May 1999; pp. 16–20. [Google Scholar]
- Ren, P.; Zhang, Y.; Geng, G.; Qi, Y. Changes in morphology and quantity of peripheral blood cells in Carassius auratus collected from polluted water area. Chin. J. Zool. 2008, 43, 37–42. [Google Scholar]
- Watson, L.J.; Shechmeister, I.L.; Jackson, L.L. The hematology of goldfish Carassius auratus. Cytologia 1963, 28, 118–130. [Google Scholar] [CrossRef]
- Li, J. Effects of Different Stocking Density on Goldfish Growth, Physiological Parameters and Expression of Appetite Regulatory Factors. Ph.D. Thesis, Foshan University, Foshan, China, 2020. [Google Scholar]
- Zhao, H. Studies on Haematology of Several Economic Fish in Middle and Upper Reaches of Yangtze River. Ph.D. Thesis, Southwest Unversity, Chongqing, China, 2008. [Google Scholar]
- Mcknight, I.M. A Hematological study on the mountain whitefish, Prosopium williamsoni. South. Fish Reb. 1966, 23, 45–64. [Google Scholar] [CrossRef]
- Ning, D.; Li, X.; Wu, K. Influence of anticoagulant selection on whole blood cell analysis. Acta Med. Sin. 2004, 17, 550–551. [Google Scholar]
- Li, P. The value of heparin, sodium citrate and EDTA-K2 in blood cell analysis. Med. Lab. Sci. Clin. 2021, 32, 63–65. [Google Scholar]
- Zhang, T.; Li, K.; Li, L.; Song, T.; Ren, Z.; Fu, C.; Lan, Y.; Jiang, L. Proteome Characterization of Primary Angle-Closure Glaucoma Aqueous Humor. J. Chengdu Med. Coll. 2023, 18, 180–186. [Google Scholar]
- Huang, S.; Lu, S.; Lin, M.; Wu, S. Factors Associated with Postvitrectomy Endophthalmitis. Chin. J. Nosocomiol. 2009, 4, 401–403. [Google Scholar]
- Gao, Z.; Wang, W. Research progress on peripheral blood red blood cells of fish. Reserv. Fish. 2008, 28, 1–3. [Google Scholar]
- Wang, Y.; Liu, S.; Wang, G. Comparative Hematological Studies in Cyprinus carpio Xiangyunnensis and Cyprinus carpio Xiangjiangnensis. J. Nat. Sci. Hunan Norm. Univ. 1988, 1, 71–75. [Google Scholar]
- Mi, R. Determination of hematological indexes of grass carp, carp and abalone. Freshw. Fish. 1982, 8, 10–16. [Google Scholar]
- Cheng, C.; Ming, Q. Study on the blood physiobiochemic and hemorheologic properties of carp in Weishan Lake. Jiangsu Agric. Sci. 2005, 5, 95–97. [Google Scholar]
- Cheng, X.F.; Jiang, G.M.; Xiang, J.; Song, R.; Li, S.M.; Wu, Y.A.; Liu, L.; Wang, Z.M. Effects of dietary fishmeal repiacement with meat and bone meal on the growth performance, blood physiological and biochemical indices, muscle chemical composition and texture characteristics in juvenile furong crucian carp (fueong♀ × red crucian carp). Acta Hydrobiol. Sin. 2022, 44, 85–94. [Google Scholar]
- Lin, G. Study on Carassius auratus blood. Curr. Zool. 1979, 25, 210–219. [Google Scholar]
- Wang, D.; Deng, S.; Zou, L.; Li, S.; Jiang, G. Studied on the hematological indices of different Cyprinus carpio Koi. J. Aquac. 2016, 37, 1–5. [Google Scholar] [CrossRef]
- Yu, L.N.; Yang, D.; Liu, H.Y.; Zhang, F.R. Correlation between Hemoglobin and Asphyxiation Point in Twelve Species of Freshwater Fish. Chin. J. Zool. 2017, 52, 478–484. [Google Scholar]
- Oizaki, H. Fish Hematology and Circulatory Physiology; Shanghai Scientific and Technical Publishers: Shanghai, China, 1982; pp. 6–96. [Google Scholar]
- Tierney, K.B.; Farrell, A.P.; Kennedy, C.J. The differential leucocyte landscape of four teleosts: Juvenile Oncorhynchus kisutch, Clupea pallasi, Culaea inconstans and Pimephales promelas. J. Fish Biol. 2004, 65, 906–919. [Google Scholar] [CrossRef]
- Chen, F. Hematopathological studies in proliferative kidney disease of red grouper. Trop. Oceanol. 1997, 16, 49–53. [Google Scholar]
- Zhang, Y.; Sun, B.; Nie, P. Immune tissues and cells of fish: A review. Acta Hydrobiol. Sin. 2000, 24, 648–654. [Google Scholar]
- Chen, G.; Zhou, H.; Ye, F.; Wu, Z. A hematological study and observation on development of blood cells in American red fish (Sciaenops ocellatus). J. Trop. Oceanogr. 2006, 25, 59–66. [Google Scholar]
- Liu, Q.; Wang, Y.Q.; Liu, S.J.; Guo, X.H.; Luo, K.K.; Zhang, C.; Liu, Y. Comparison of blood and blood cells in crucian carp with different ploidy. Adv. Nat. Sci. 2004, 14, 1111–1117. [Google Scholar]
- Zhu, H.; Wang, H.; Qin, G. Studies on the blood cell morphology of crucian carp (Carassius auratus L.). Zool. Res. 1985, 6, 147–153. [Google Scholar]
- Zhou, X.; An, M.; Huang, L.; Hu, F.; Shao, Y. Hematologic Studies of Three Types of Carassius auratus. Guizhou Agric. Sci. 2012, 40, 133–138. [Google Scholar]
- Zhao, H.; Zhao, H.; Jin, L.; Zhang, Y. Microscopic structures of peripheral hematocytes in sinilabeo rendahli. Fish. Sci. 2005, 24, 24–27. [Google Scholar]
- Chen, X. The Fishes Blood. J. Chongqing Teach. Coll. 2000, 19, 70–73. [Google Scholar]
- Cheng, C. The Study of Blood Physiological and Biochemeical Parameters of Monopterus Albus. Ph.D. Thesis, Jiangxi Agricultural University, Nanchang, China, 2014. [Google Scholar]
- Han, N.; Shi, C. The Application of Blood Indexes in Ichthyological Research. J. Anhui Agric. Sci. 2010, 38, 18877–18878. [Google Scholar]
- Dong, S.; Miao, J.; Zhao, K. Study on Physiological and Biochemical Index of Blood in Leiocassis longirostri. Hubei Agric. Sci. 2016, 55, 3690–3693. [Google Scholar]
- Qian, Y.; Chen, H.; Sun, J. Effects of starvation on hematological and blood biochemical indices in cultured Lateolabrax japonicus. J. Fish. Sci. China 2002, 2, 133–137. [Google Scholar]
- Gu, L.F.; Hou, Y.Q.; Ding, B.Y. Effects of Several Plant Extracts on Growth Performance and Blood Biochemical Indices in Carassius auratus gibelio. Freshw. Fish. 2008, 28, 23–26. [Google Scholar]
- Asadi, F.; Masoudifard, M.; Vajhi, A.; Lee, K.; Pourkabir, M.; Khazraeinia, P. Serum biochemical parameters of Acipenser persicus. Fish Physiol. Biochem. 2006, 32, 43–47. [Google Scholar] [CrossRef]
- Rahimikia, E. Analysis of antioxidants and serum biochemical responses in goldfish under nickel exposure by sub-chronic test. J. Appl. Anim. Res. 2017, 45, 320–325. [Google Scholar] [CrossRef][Green Version]
- Fan, H. Study on the Relationship Between Postmortem change of Chemical Composition in Vitreous Humor and Time of Death. Ph.D. Thesis, Sichuan University, Chengdu, China, 2005. [Google Scholar]
- Chen, C.H.; Chen, S.C. Studies on soluble proteins of vitreous in experimental animals. Exp. Eye Res. 1981, 32, 381. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Wang, F.; Yan, H.; Xu, C.; Wang, Y. Effects of methoxyfenozide on transaminase and alanine aminotransferase in the silkworm. Guangdong Seric. 2019, 53, 1–7. [Google Scholar]
- Liu, R. Research progress of gamma-glutamyltranspeptidase activity in mammals. Shanghai J. Prev. Med. 2002, 14, 24–25. [Google Scholar]
- Yew, D.T.; Lai, H.W.; Zhou, L.; Lam, K.W. Chromatographic identification of a biochemical alteration in the aqueous humor of megalophthalmic Black Moor goldfish. J. Chromatogr. B Biomed. Sci. Appl. 2001, 751, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Easter, S.S.; Hitchcock, P.F. The myopic eye of the Black Moor goldfish. Vis. Res. 1986, 26, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Lane, V.M.; Lincoln, S.D. Changes in urea nitrogen and creatinine concentrations in the vitreous humor of cattle after death. Am. J. Vet. Res. 1985, 46, 1550–1552. [Google Scholar]
- Coe, J.I. postmortem chemistries of human Vitreous humor. Am. J. Clin. Pathol. 1969, 51, 741–750. [Google Scholar] [CrossRef] [PubMed]

| Syringe Pretreatment Methods | Success Rate of Blood Collection |
|---|---|
| A | 80.00% A |
| B | 0.00% B |
| C | 0.00% B |
| Groups | Survival Rate% |
|---|---|
| Blood collection groups | 80 ± 5.44 a |
| Vitreous humor collection groups | 90 ± 5.44 a |
| Normal CE groups | 90 ± 5.44 a |
| Sample | WBC 109/L | RBC 1012/L | HGB g/L | HCT % | MCV fl | MCH Pg | MCHC g/L | RDW-CV % |
|---|---|---|---|---|---|---|---|---|
| blood | 62.21 ± 8.54 | 2.19 ± 0.09 | 138.25 ± 14.74 | 41.70 ± 1.58 | 187.10 ± 1.44 | 75.75 ± 1.16 | 410.50 ± 2.05 | 21.70 ± 0.95 |
| Sample | PLT 109/L | NEU 109/L | LYM 109/L | MON 109/L | EOS 109/L | BAS 109/L | NEU % | LYM % | MON % |
|---|---|---|---|---|---|---|---|---|---|
| blood | 6.00 ± 0.94 | 1.37 ± 0.10 | 59.53 ± 14.32 | 2.05 ± 0.19 | 0 | 0 | 2.05 ± 0.03 | 94.75 ± 0.26 | 2.50 ± 0.28 |
| Sample | TP g/L | ALB g/L | ALT U/L | AST U/L | ALP U/L | GGT U/L | TBA μmol/L | GLU mmol/L | UREA μmol/L |
|---|---|---|---|---|---|---|---|---|---|
| serum | 31.63 ± 0.52 ** | 14.93 ± 0.07 ** | 26.33 ± 1.09 | 235.33 ± 3.81 ** | 30.67 ± 1.19 | 1.76 ± 0.32 | 0.70 ± 0.00 | 3.75 ± 0.16 ** | 2.34 ± 0.08 |
| vitreous humor | 2.05 ± 0.05 | 2.50 ± 0.03 | 87.00 ± 4.03 ** | 114.00 ± 1.09 | 26.00 ± 0.54 | 14.89 ± 0.47 ** | 0.40 ± 0.11 | 0.70 ± 0.02 | 1.86 ± 0.02 |
| Sample | K+ mmol/L | Na+ mmol/L | Cl−mmol/L | Ca2+ mmol/L | Cre μmol/L | CHOLmmol/L | TGmmol/L | HDL-C mmol/L | LDL-C mmol/L | CHE mmol/L |
|---|---|---|---|---|---|---|---|---|---|---|
| serum | 5.17 ± 0.07 ** | 149.40 ± 0.95 ** | 162.47 ± 19.15 | 2.62 ± 0.07 | 29.67 ± 0.72 | 6.37 ± 0.16 ** | 2.42 ± 0.04 ** | 2.37 ± 0.13 ** | 1.41 ± 0.01 ** | 345.00 ± 11.43 |
| vitreous humor | 2.24 ± 0.05 | 132.85 ± 0.08 | 151.20 ± 15.05 | 2.61 ± 0.05 | 30.00 ± 2.05 | 1.13 ± 0.05 | 0.10 ± 0.02 | 0.39 ± 0.02 | 0.17 ± 0.00 | 323.00 ± 8.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Sun, Y.; Zhang, X.; Li, W. Study on Hematological and Biochemical Index of Blood and Vitreous Humor in the Celestial-Eye Goldfish. Appl. Sci. 2025, 15, 774. https://doi.org/10.3390/app15020774
Li R, Sun Y, Zhang X, Li W. Study on Hematological and Biochemical Index of Blood and Vitreous Humor in the Celestial-Eye Goldfish. Applied Sciences. 2025; 15(2):774. https://doi.org/10.3390/app15020774
Chicago/Turabian StyleLi, Rongni, Yansheng Sun, Xin Zhang, and Wentong Li. 2025. "Study on Hematological and Biochemical Index of Blood and Vitreous Humor in the Celestial-Eye Goldfish" Applied Sciences 15, no. 2: 774. https://doi.org/10.3390/app15020774
APA StyleLi, R., Sun, Y., Zhang, X., & Li, W. (2025). Study on Hematological and Biochemical Index of Blood and Vitreous Humor in the Celestial-Eye Goldfish. Applied Sciences, 15(2), 774. https://doi.org/10.3390/app15020774






