Production, Characterization, and In Vitro Antifungal Evaluation of Itraconazole-Loaded Fibrous Sheets Prepared by Electrospinning with a Factorial Design
Abstract
1. Introduction
2. Materials and Methods
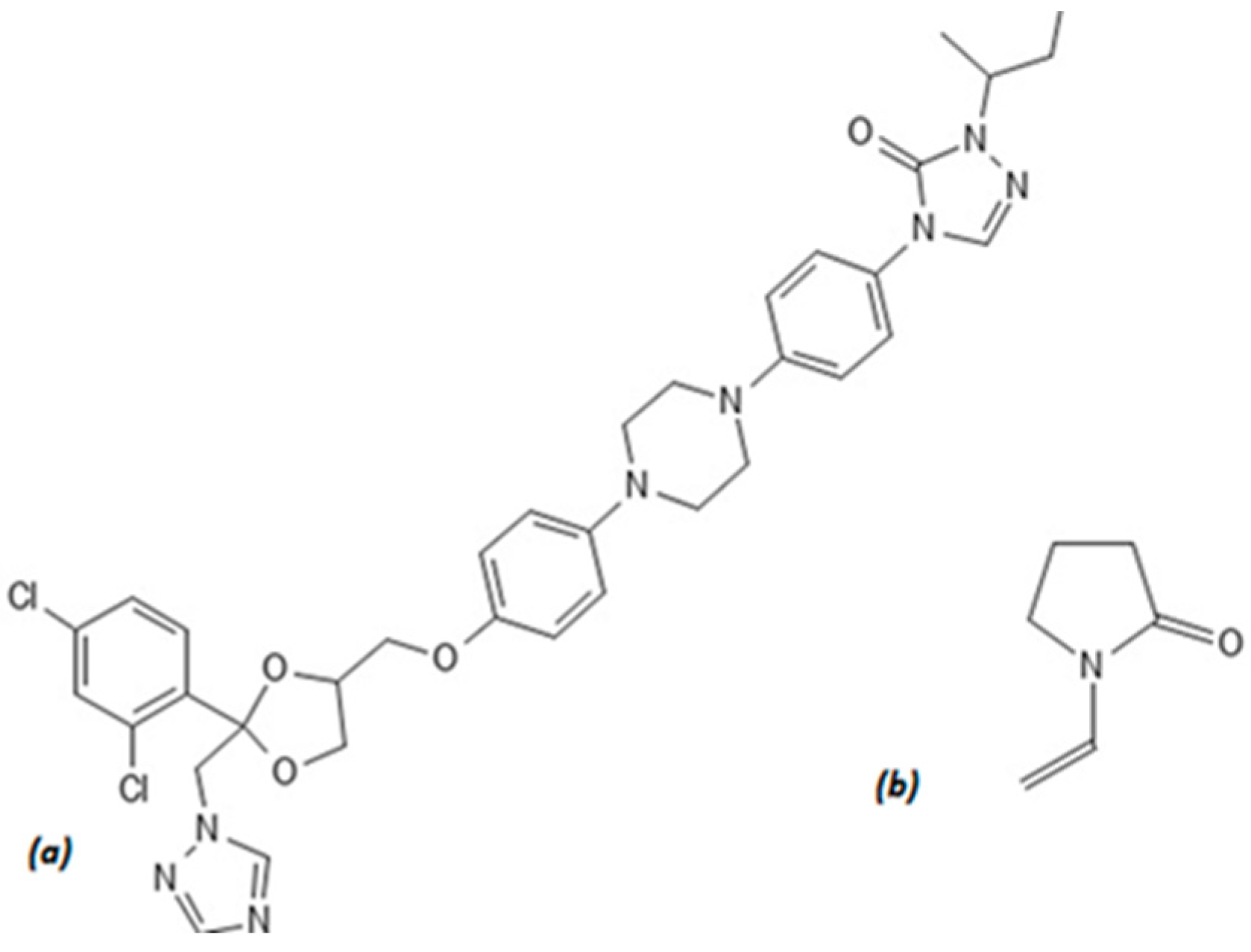
2.1. Materials
2.2. Methods
2.2.1. Fiber Preparation by Electrospinning
2.2.2. Fiber Morphology—Scanning Electron Microscopy (SEM)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.5. Determination of Drug Content
2.2.6. Disintegration Test
2.2.7. In Vitro Dissolution Test
2.2.8. In Vitro Antifungal Activity
2.2.9. Factorial Design
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Fiber Preparation by Electrospinning
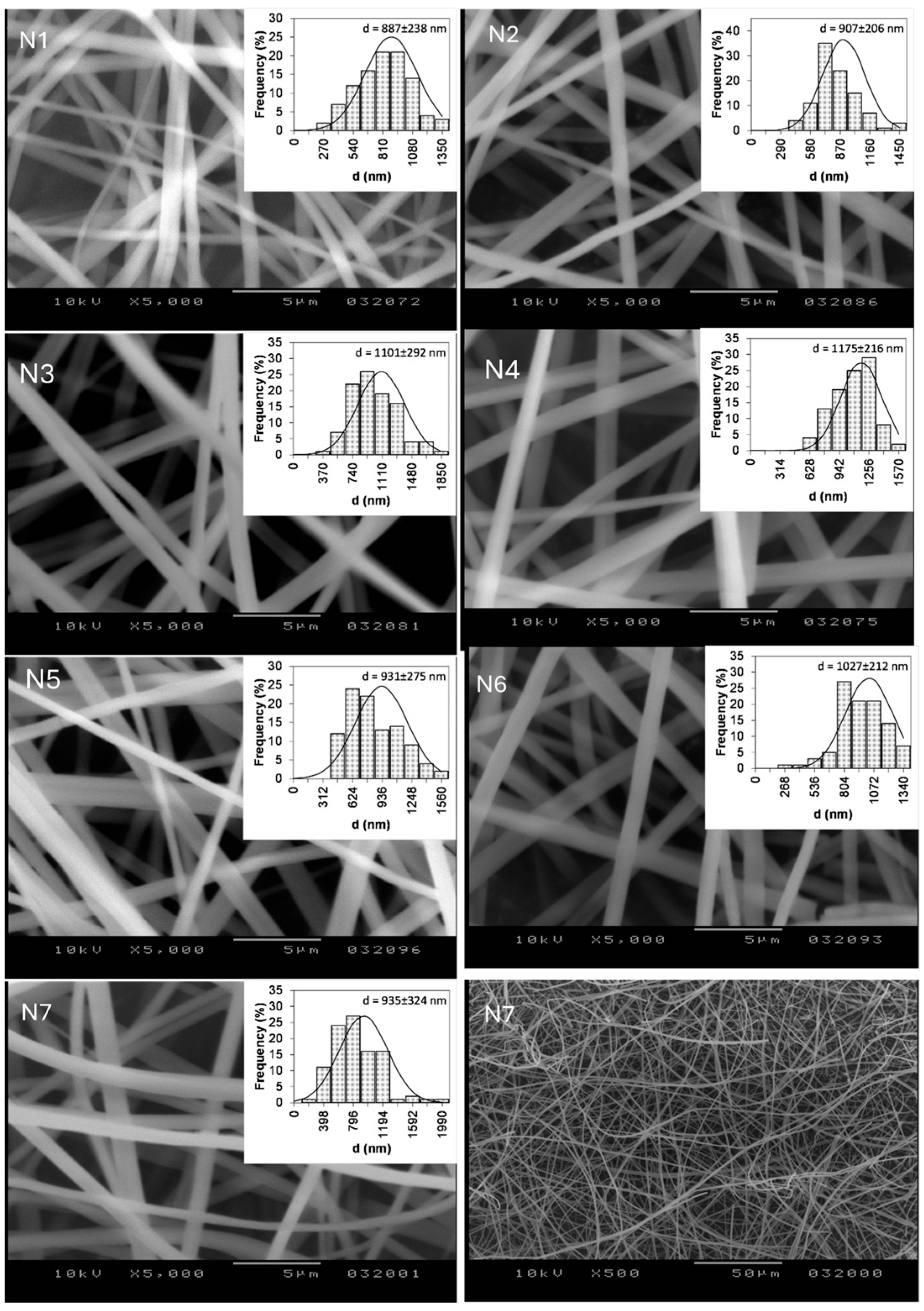
3.2. Fiber Morphology—Scanning Electron Microscopy (SEM)
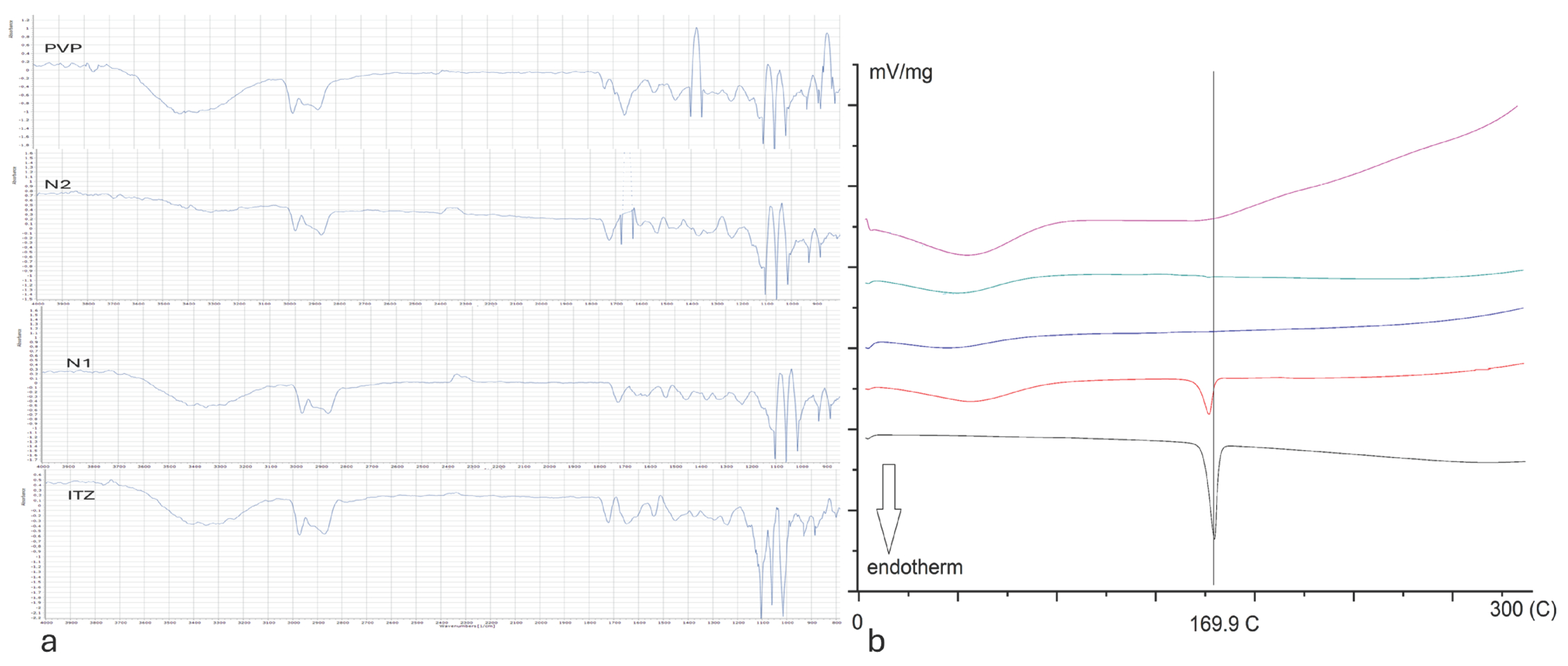
3.3. Differential Scanning Calorimetry (DSC)
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3.5. Determination of Drug Content
3.6. Disintegration
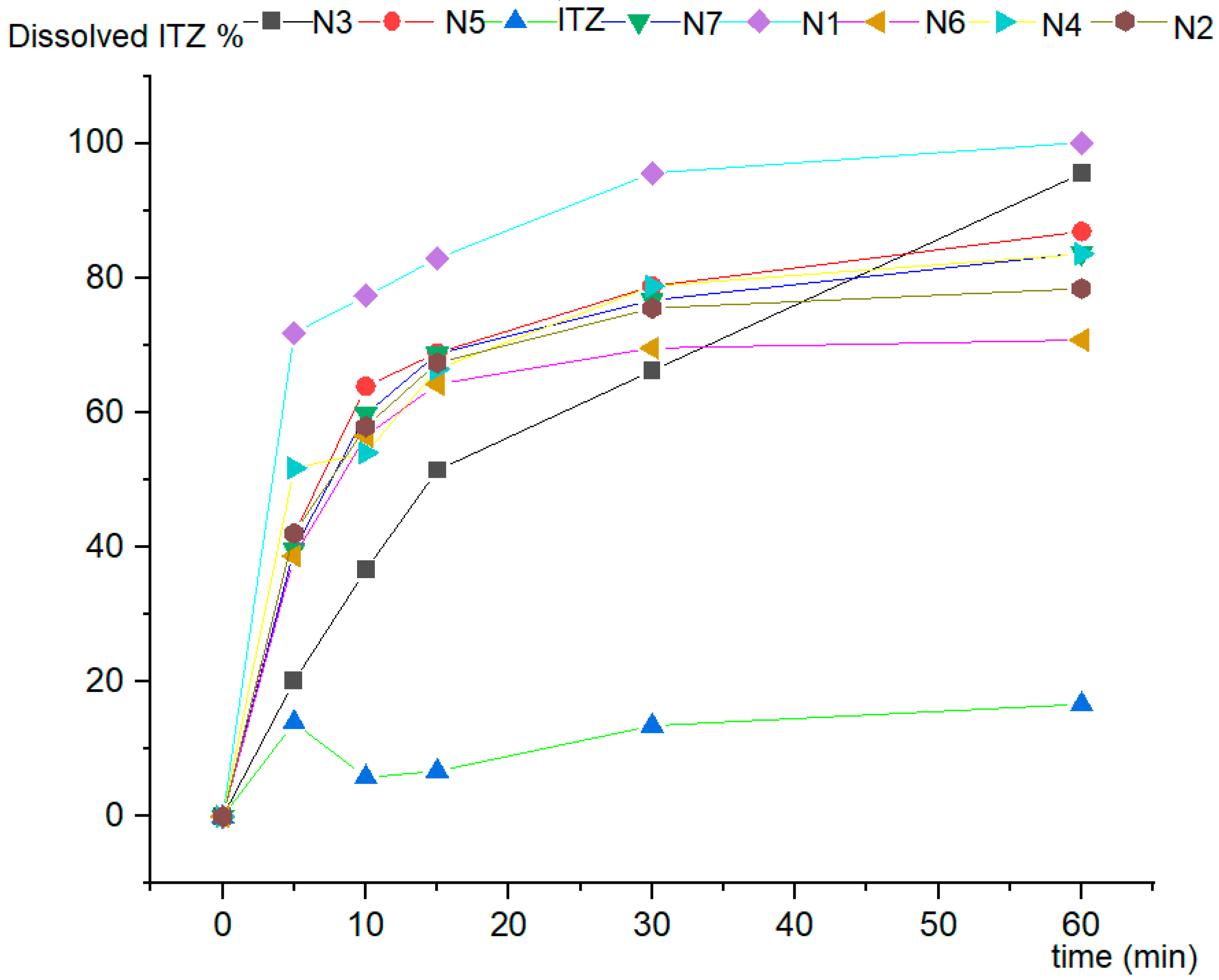
3.7. In Vitro Dissolution Studies
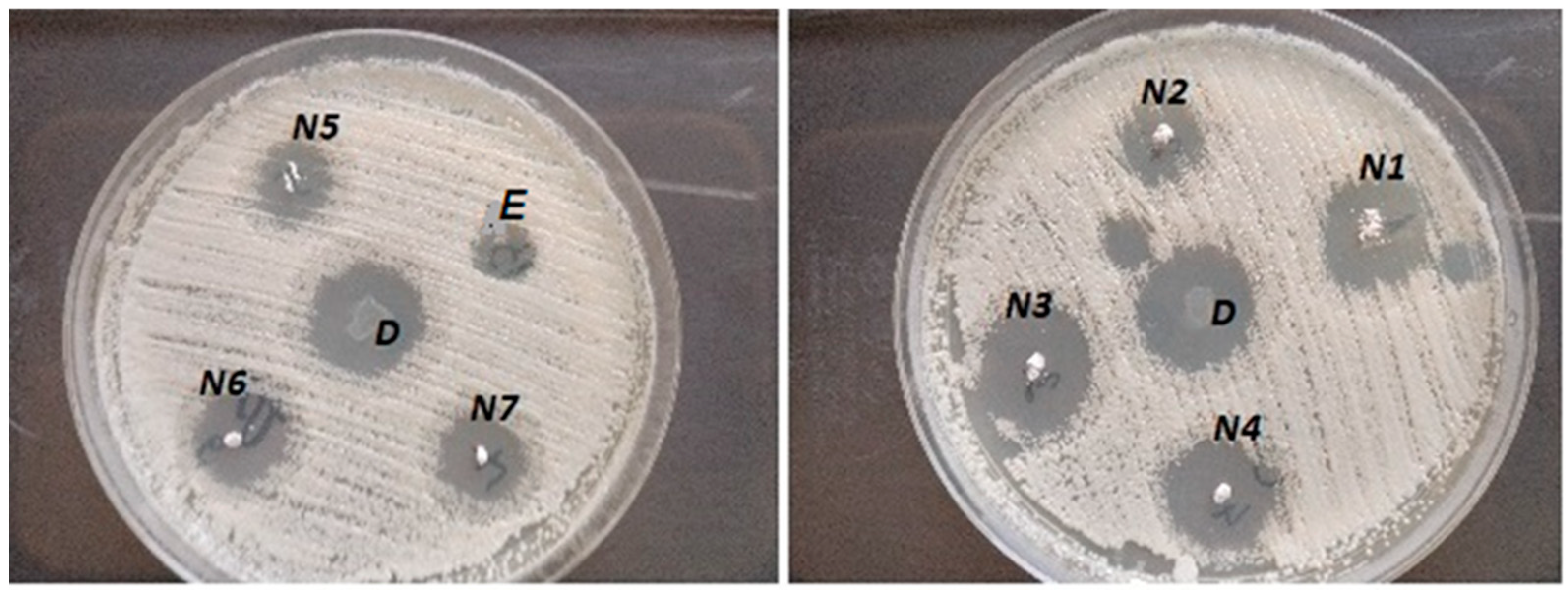
3.8. Antifungal Activity
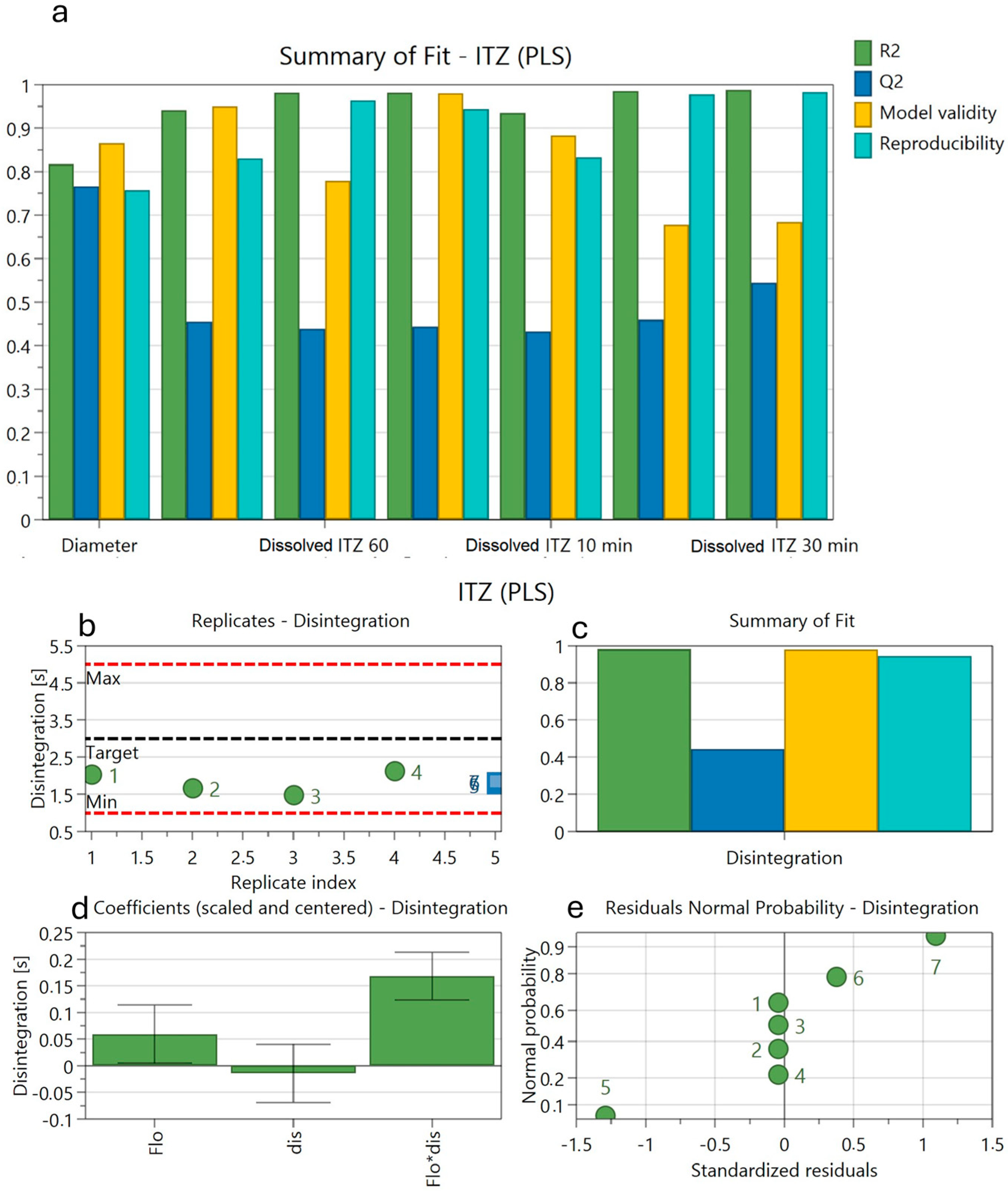
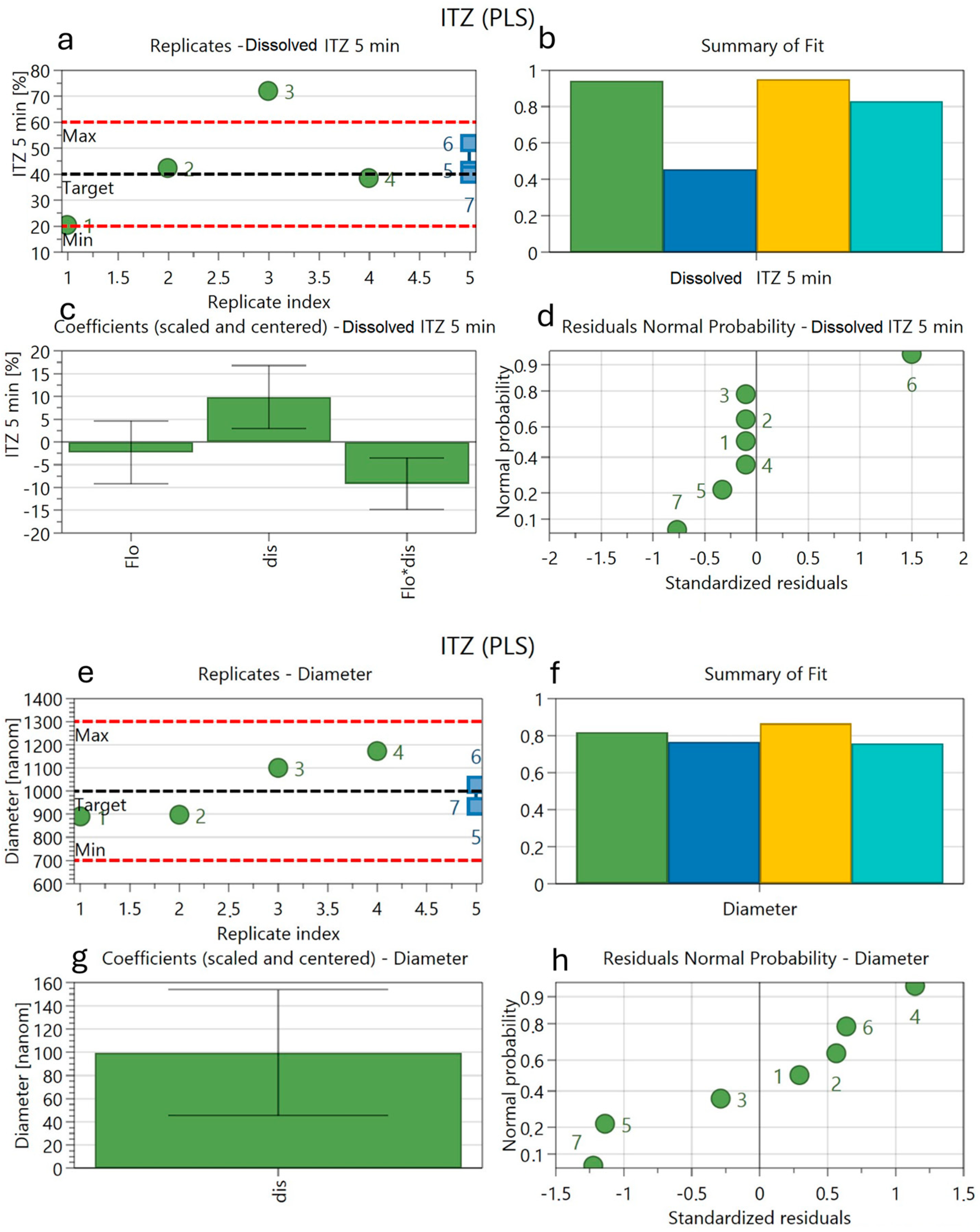
3.9. Factorial Design
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Petersen, R.V.; Feijen, J. Polymeric drug delivery systems. Drug Des. 2016, 10, 193–250. [Google Scholar]
- De Souza, R.; Zahedi, P.; Allen, C.J.; Piquette-Miller, M. Polymeric drug delivery systems for localized cancer chemotherapy. Drug Deliv. 2010, 17, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Multifunctional Role of Polyvinylpyrrolidone in Pharmaceutical Formulations. AAPS PharmSciTech 2021, 22, 34. [Google Scholar] [CrossRef]
- Kedia, A.; Kumar, P.S. Solvent-adaptable poly (vinylpyrrolidone) binding induced anisotropic shape control of gold nanostructures. J. Phys. Chem. C 2012, 116, 23721–23728. [Google Scholar] [CrossRef]
- Maertens, J.; Boogaerts, M. The place for itraconazole in treatment. J. Antimicrob. Chemother. 2005, 56, i33–i38. [Google Scholar] [CrossRef][Green Version]
- Lestner, J.; Hope, W.W. Itraconazole: An update on pharmacology and clinical use for treatment of invasive and allergic fungal infections. Expert Opin. Drug Metab. Toxicol. 2013, 9, 911–926. [Google Scholar] [CrossRef]
- De Beule, K.; Van Gestel, J. Pharmacology of itraconazole. Drugs 2001, 61, 27–37. [Google Scholar] [CrossRef]
- Aungst, B.J. Optimizing Oral Bioavailability in Drug Discovery: An Overview of Design and Testing Strategies and Formulation Options. J. Pharm. Sci. 2017, 106, 921–929. [Google Scholar] [CrossRef]
- Thompson, G.R.; Lewis, P.; Mudge, S.; Patterson, T.F.; Burnett, B.P. Open-Label Crossover Oral Bioequivalence Pharmacokinetics Comparison for a 3-Day Loading Dose Regimen and 15-Day Steady-State Administration of SUBA-Itraconazole and Conventional Itraconazole Capsules in Healthy Adults. Antimicrob. Agents Chemother. 2020, 64, 00400-20. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Park, S.J.; Shim, E.J.; Mun, J.H.; Kim, E.Y.; Shin, J.G.; Shon, J.H. Increased oral bioavailability of itraconazole and its active metabolite, 7-hydroxyitraconazole, when coadministered with a vitamin C beverage in healthy participants. J. Clin. Pharmacol. 2011, 51, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Gil Hurlé, A.; Sánchez Navarro, A.; Garcia Sanchez, M. Therapeutic drug monitoring of itraconazole and the relevance of pharmacokinetic interactions. Clin. Microbiol. Infect. 2006, 12, 97–106. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Benet, L.Z. Predicting drug disposition via application of BCS: Transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef]
- Akhgari, A.; Shakib, Z.; Sanati, S. A review on electrospun nanofibers for oral drug delivery. Nanomed. J. 2017, 4, 197–207. [Google Scholar]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Amiri, N.; Moradi, A.; Tabasi, S.A.S.; Movaffagh, J. Modeling and process optimization of electrospinning of chitosan-collagen nanofiber by response surface methodology. Mater. Res. Express 2018, 5, 045404. [Google Scholar] [CrossRef]
- Jacobs, V.; Patanaik, A.; Anandjiwala, R.D.; Maaza, M. Optimization of electrospinning parameters for chitosan nanofibres. Curr. Nanosci. 2011, 7, 396–401. [Google Scholar] [CrossRef]
- Akhgari, A.; Heshmati, Z.; Makhmalzadeh, B.S. Indomethacin electrospun nanofibers for colonic drug delivery: Preparation and characterization. Adv. Pharm. Bull. 2013, 3, 85. [Google Scholar] [PubMed]
- Verreck, G.; Chun, I.; Peeters, J.; Rosenblatt, J.; Brewster, M.E. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm. Res. 2003, 20, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Sóti, P.L.; Bocz, K.; Pataki, H.; Eke, Z.; Farkas, A.; Verreck, G.; Kiss, É.; Fekete, P.; Vigh, T.; Wagner, I.; et al. Comparison of spray drying, electroblowing and electrospinning for preparation of Eudragit E and itraconazole solid dispersions. Int. J. Pharm. 2015, 494, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Balogh, A.; Démuth, B.; Pataki, H.; Vigh, T.; Szabó, B.; Molnár, K.; Schmidt, B.T.; Horák, P.; Marosi, G. High speed electrospinning for scaled-up production of amorphous solid dispersion of itraconazole. Int. J. Pharm. 2015, 480, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Démuth, B.; Farkas, A.; Balogh, A.; Bartosiewicz, K.; Kállai-Szabó, B.; Bertels, J.; Vigh, T.; Mensch, J.; Verreck, G.; Van Assche, I.; et al. Lubricant-Induced Crystallization of Itraconazole From Tablets Made of Electrospun Amorphous Solid Dispersion. J. Pharm. Sci. 2016, 105, 2982–2988. [Google Scholar] [CrossRef]
- Mehrandish, S.; Mohammadi, G.; Mirzaeei, S. Preparation and functional evaluation of electrospun polymeric nanofibers as a new system for sustained topical ocular delivery of itraconazole. Pharm. Dev. Technol. 2022, 27, 25–39. [Google Scholar] [CrossRef]
- Wu, S.; Song, R.; Liu, T.; Li, T. Antifungal therapy: Novel drug delivery strategies driven by new targets. Adv. Drug Del. Rev. 2023, 199, 114967. [Google Scholar] [CrossRef]
- Yang, W.; Wiederhold, N.P.; Williams, R.O. Drug delivery strategies for improved azole antifungal action. Exp. Op. Drug Del. 2008, 5, 1199–1216. [Google Scholar] [CrossRef]
- Pawar, J.N.; Ali, T.M.; Moravkar, K.K.; Patole, R.K.; Jaiswar, D.R.; Amin, P.D. Recent development and achievements in solubility and dissolution enhancement of itraconazole: A review. Int. J. Pharm. Sci. Res. 2014, 5, 3096. [Google Scholar]
- Pintea, A.; Vlad, R.-A.; Antonoaea, P.; Rédai, E.M.; Todoran, N.; Barabás, E.-C.; Ciurba, A. Structural Characterization and Optimization of a Miconazole Oral Gel. Polymers 2022, 14, 5011. [Google Scholar] [CrossRef]
- Vlad, R.-A.; Antonoaea, P.; Todoran, N.; Rédai, E.-M.; Bîrsan, M.; Muntean, D.-L.; Imre, S.; Hancu, G.; Farczádi, L.; Ciurba, A. Development and evaluation of cannabidiol orodispersible tablets using a 23-factorial design. Pharmaceutics 2022, 14, 1467. [Google Scholar] [CrossRef] [PubMed]
- Fadke, J.; Desai, J.; Thakkar, H. Formulation Development of Spherical Crystal Agglomerates of Itraconazole for Preparation of Directly Compressible Tablets with Enhanced Bioavailability. AAPS PharmSciTech 2015, 16, 1434–1444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reksamunandar, R.P.; Edikresnha, D.; Munir, M.M.; Damayanti, S.; Khairurrijal. Encapsulation of β-carotene in poly(vinylpyrrolidone) (PVP) by Electrospinning Technique. Procedia Eng. 2017, 170, 19–23. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of uniform polystyrene fibers: The effect of solvent conductivity. Polymer 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Paaver, U.; Heinämäki, J.; Laidmäe, I.; Lust, A.; Kozlova, J.; Sillaste, E.; Kirsimäe, K.; Veski, P.; Kogermann, K. Electrospun nanofibers as a potential controlled-release solid dispersion system for poorly water-soluble drugs. Int. J. Pharm. 2015, 479, 252–260. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2021, 118, 111514. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; White, K.; Li, X.-L.; Zhu, L.-M. Dissolution Improvement of Electrospun Nanofiber-Based Solid Dispersions for Acetaminophen. AAPS PharmSciTech 2010, 11, 809–817. [Google Scholar] [CrossRef]
- Rane, H.S.; Bernardo, S.M.; Walraven, C.J.; Lee, S.A. In vitro analyses of ethanol activity against Candida albicans biofilms. Antimicrob. Agents Chemother. 2012, 56, 4487–4489. [Google Scholar] [CrossRef]
- Sequeira, S.O.; Phillips, A.J.; Cabrita, E.J.; Macedo, M.F. Ethanol as an antifungal treatment for paper: Short-term and long-term effects. Stud. Conservation. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- Giller, C.B.; Chase, D.B.; Rabolt, J.F.; Snively, C.M. Effect of solvent evaporation rate on the crystalline state of electrospun Nylon 6. Polymer 2010, 51, 4225–4230. [Google Scholar] [CrossRef]
- Santos, V.A.D.; Viera, P.V.A.; Oliveira, A.M.D.; Zanin, M.H.A.; Borsatti, M.A. Antifungal effect of electrospun nanofibers containing cetylpyridinium chloride against Candida albicans. Braz. Oral Res. 2014, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maha, H. Formula comparison of nanoemulsion and cream containing miconazole nitrate: Penetration test using franz diffusion cells and antifungal activity test on Tricophyton mentagrophytes, Microsporum canis and Candida albicans. Rasayan J. Chem. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Qiu, L.; Hu, B.; Chen, H.; Li, S.; Hu, Y.; Zheng, Y.; Wu, X. Antifungal efficacy of itraconazole-loaded TPGS-b-(PCL-ran-PGA) nanoparticles. Int. J. Nanomed. 2015, 10, 1415–1423. [Google Scholar] [CrossRef]
| Code | Flow Rate (mL/hour) | Level | Needle–Collector Distance (cm) |
|---|---|---|---|
| N1 | 1 | −1 | 10 |
| N2 | 2 | +1 | 10 |
| N3 | 1 | −1 | 15 |
| N4 | 2 | +1 | 15 |
| N5 | 1.5 | 0 | 12.5 |
| N6 | 1.5 | 0 | 12.5 |
| N7 | 1.5 | 0 | 12.5 |
| Codes | N1 | N2 | N3 | N4 | N5 | N6 | N7 |
|---|---|---|---|---|---|---|---|
| Drug content mg/10 mg fibers ± SD | 2.73 ± 0.21 | 2.71 ± 0.26 | 3.27 ± 0.28 | 2.89 ± 0.23 | 2.88 ± 0.17 | 2.75 ± 0.19 | 2.83 ± 0.18 |
| Code | Fiber Diameter (nm) ± SD | Disintegration Time (s) | ITZ Released | ||||
|---|---|---|---|---|---|---|---|
| 5 min (%) | 10 min (%) | 15 min (%) | 30 min (%) | 60 min (%) | |||
| N1 | 887 ± 238 | 1.47 ± 3.1 | 71.88 ± 4.65 | 77.37 ± 3.87 | 82.96 ± 6.63 | 95.65 ± 2.36 | 100.20 ± 1.99 |
| N2 | 907 ± 206 | 1.65 ± 1.2 | 42.09 ± 2.20 | 57.98 ± 3.80 | 67.45 ± 6.00 | 75.55 ± 4.30 | 78.49 ± 2.14 |
| N3 | 1101 ± 292 | 2.01 ± 0.9 | 20.17 ± 2.00 | 36.66 ± 3.01 | 51.47 ± 4.32 | 66.29 ± 5.11 | 95.60 ± 6.2 |
| N4 | 1175 ± 226 | 2.12 ± 0.6 | 38.70 ± 8.32 | 56.57 ± 2.09 | 64.28 ± 0.89 | 69.66 ± 1.11 | 70.83 ± 1.06 |
| N5 | 931 ± 275 | 1.76 ± 0.7 | 42.03 ± 3.61 | 63.95 ± 5.60 | 68.94 ± 5.27 | 78.84 ± 6.38 | 86.98 ± 5.02 |
| N6 | 1027 ± 212 | 1.83 ± 0.7 | 51.77 ± 5.25 | 54.07 ± 3.95 | 66.51 ± 2.88 | 78.85 ± 2.88 | 83.65 ± 1.77 |
| N7 | 935 ± 324 | 1.86 ± 1.4 | 39.64 ± 4.86 | 59.85 ± 3.87 | 68.77 ± 5.10 | 76.73 ± 2.99 | 83.79 ± 0.06 |
| Codes | E | D | N1 | N2 | N3 | N4 | N5 | N6 | N7 |
|---|---|---|---|---|---|---|---|---|---|
| Inhibition zone diameter (mm) | 3.0 | 7.1 | 7.1 | 6.9 | 7.5 | 7.0 | 6.6 | 6.5 | 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rédai, E.-M.; Péterfi, O.; Gergely, A.L.; Barabás, E.; Pintea, A.; Antonoaea, P.; Vlad, R.-A.; Todoran, N.; Cotoi, C.-T.; Ciurba, A.; et al. Production, Characterization, and In Vitro Antifungal Evaluation of Itraconazole-Loaded Fibrous Sheets Prepared by Electrospinning with a Factorial Design. Appl. Sci. 2025, 15, 710. https://doi.org/10.3390/app15020710
Rédai E-M, Péterfi O, Gergely AL, Barabás E, Pintea A, Antonoaea P, Vlad R-A, Todoran N, Cotoi C-T, Ciurba A, et al. Production, Characterization, and In Vitro Antifungal Evaluation of Itraconazole-Loaded Fibrous Sheets Prepared by Electrospinning with a Factorial Design. Applied Sciences. 2025; 15(2):710. https://doi.org/10.3390/app15020710
Chicago/Turabian StyleRédai, Emőke-Margit, Orsolya Péterfi, Attila Levente Gergely, Enikő Barabás, Andrada Pintea, Paula Antonoaea, Robert-Alexandru Vlad, Nicoleta Todoran, Cornelia-Titiana Cotoi, Adriana Ciurba, and et al. 2025. "Production, Characterization, and In Vitro Antifungal Evaluation of Itraconazole-Loaded Fibrous Sheets Prepared by Electrospinning with a Factorial Design" Applied Sciences 15, no. 2: 710. https://doi.org/10.3390/app15020710
APA StyleRédai, E.-M., Péterfi, O., Gergely, A. L., Barabás, E., Pintea, A., Antonoaea, P., Vlad, R.-A., Todoran, N., Cotoi, C.-T., Ciurba, A., & Sipos, E. (2025). Production, Characterization, and In Vitro Antifungal Evaluation of Itraconazole-Loaded Fibrous Sheets Prepared by Electrospinning with a Factorial Design. Applied Sciences, 15(2), 710. https://doi.org/10.3390/app15020710









