Elevated BP180 ELISA at Diagnosis Correlates with Disease Severity and Relapse in Oral Mucous Membrane Pemphigoid: Preliminary Results from a Retrospective Monocentric Italian Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. ODSS Analysis
3.3. Therapy and Analysis of Predictors of Relapse
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.-H.; Werth, V.P.; Parisi, E.; Sollecito, T.P. Mucous Membrane Pemphigoid. Dent. Clin. N. Am. 2013, 57, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.; Setterfield, J. Mucous Membrane Pemphigoid and Oral Blistering Diseases. Clin. Exp. Dermatol. 2019, 44, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Furumura, M.; Fukano, H.; Li, X.; Ishii, N.; Hamada, T.; Ohata, C.; Tsuruta, D.; Shimozato, K.; Hashimoto, T. Diagnosis of Oral Mucous Membrane Pemphigoid by Means of Combined Serologic Testing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Setterfield, J.F.; Powell, A.M.; Sakuma-Oyama, Y.; Albert, S.; Bhogal, B.S.; Vaughan, R.W.; Kaneko, F.; Challacombe, S.J.; Black, M.M. Bullous Pemphigoid Antigen II (BP180) and Its Soluble Extracellular Domains Are Major Autoantigens in Mucous Membrane Pemphigoid: The Pathogenic Relevance to HLA Class II Alleles and Disease Severity. Br. J. Dermatol. 2006, 154, 90–98. [Google Scholar] [CrossRef]
- Schmidt, E.; Skrobek, C.; Kromminga, A.; Hashimoto, T.; Messer, G.; Bröcker, E.B.; Yancey, K.B.; Zillikens, D. Cicatricial Pemphigoid: IgA and IgG Autoantibodies Target Epitopes on Both Intra- and Extracellular Domains of Bullous Pemphigoid Antigen 180. Br. J. Dermatol. 2001, 145, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Tampoia, M.; Giavarina, D.; Di Giorgio, C.; Bizzaro, N. Diagnostic Accuracy of Enzyme-Linked Immunosorbent Assays (ELISA) to Detect Anti-Skin Autoantibodies in Autoimmune Blistering Skin Diseases: A Systematic Review and Meta-Analysis. Autoimmun. Rev. 2012, 12, 121–126. [Google Scholar] [CrossRef]
- Schmidt, E.; Rashid, H.; Marzano, A.V.; Lamberts, A.; Di Zenzo, G.; Diercks, G.F.H.; Alberti-Violetti, S.; Barry, R.J.; Borradori, L.; Caproni, M.; et al. European Guidelines (S3) on Diagnosis and Management of Mucous Membrane Pemphigoid, Initiated by the European Academy of Dermatology and Venereology—Part II. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1926–1948. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, M.; Tampoia, M.; Serpico, R.; Lauritano, D.; Lajolo, C.; Lucchese, A.; Della Vella, F. Evaluation of BP180-NC16A ELISA in Exclusive Oral Pemphigoid Diagnosis. A Comparative Study. Oral Dis. 2020, 27, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, E.; Di Zenzo, G.; Calabresi, V.; Carrozzo, M.; Burlando, M.; Longanesi, L.; Cerri, A.; Caproni, M.; Sera, F.; Antiga, E.; et al. Autoantibody Profile of a Cohort of 78 Italian Patients with Mucous Membrane Pemphigoid: Correlation Between Reactivity Profile and Clinical Involvement. Acta Derm. Venereol. 2016, 96, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Endo, H. BP180NC16a Autoantibody Positivity May Predict Low-Risk Mucous Membrane Pemphigoid. Oral Dis. 2023, 29, 2224–2229. [Google Scholar] [CrossRef]
- Joly, P.; Horvath, B.; Patsatsi, A.; Uzun, S.; Bech, R.; Beissert, S.; Bergman, R.; Bernard, P.; Borradori, L.; Caproni, M.; et al. Updated S2K Guidelines on the Management of Pemphigus Vulgaris and Foliaceus Initiated by the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1900–1913. [Google Scholar] [CrossRef]
- Delavarian, Z.; Layegh, P.; Pakfetrat, A.; Zarghi, N.; Khorashadizadeh, M.; Ghazi, A. Evaluation of Desmoglein 1 and 3 Autoantibodies in Pemphigus Vulgaris: Correlation with Disease Severity. J. Clin. Exp. Dent. 2020, 12, e440–e445. [Google Scholar] [CrossRef]
- Murrell, D.F.; Marinovic, B.; Caux, F.; Prost, C.; Ahmed, R.; Wozniak, K.; Amagai, M.; Bauer, J.; Beissert, S.; Borradori, L.; et al. Definitions and Outcome Measures for Mucous Membrane Pemphigoid: Recommendations of an International Panel of Experts. J. Am. Acad. Dermatol. 2015, 72, 168–174. [Google Scholar] [CrossRef]
- Ormond, M.; McParland, H.; Thakrar, P.; Donaldson, A.N.A.; Andiappan, M.; Cook, R.J.; Escudier, M.E.; Higham, J.; Hullah, E.; McMillan, R.; et al. Validation of an Oral Disease Severity Score (ODSS) Tool for Use in Oral Mucous Membrane Pemphigoid. Br. J. Dermatol. 2020, 183, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, N.; Korgaonkar, S.; Pradhan, V.; Khopkar, U.S. A Cross-Sectional Study to Correlate Disease Severity in Bullous Pemphigoid Patients with Serum Levels of Autoantibodies Against BP180 and BP230. Indian Dermatol. Online J. 2021, 12, 696–700. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, X.; Jin, H.; Li, L. Factors Associated with the Activity and Severity of Bullous Pemphigoid: A Review. Ann. Med. 2020, 52, 55–62. [Google Scholar] [CrossRef]
- Bernard, P.; Antonicelli, F.; Bedane, C.; Joly, P.; Le Roux-Villet, C.; Duvert-Lehembre, S.; Rousselle, P.; Prost-Squarcioni, C. Prevalence and Clinical Significance of Anti-Laminin 332 Autoantibodies Detected by a Novel Enzyme-Linked Immunosorbent Assay in Mucous Membrane Pemphigoid. JAMA Dermatol. 2013, 149, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-C.; Sun, Y.-J.; Ng, P.P.-L.; Tan, S.-H. Comparison of Immunofluorescence Microscopy, Immunoblotting and Enzyme-Linked Immunosorbent Assay Methods in the Laboratory Diagnosis of Bullous Pemphigoid. Clin. Exp. Dermatol. 2003, 28, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Balding, S.D.; Prost, C.; Diaz, L.A.; Bernard, P.; Bedane, C.; Aberdam, D.; Giudice, G.J. Cicatricial Pemphigoid Autoantibodies React with Multiple Sites on the BP180 Extracellular Domain. J. Investig. Dermatol. 1996, 106, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Nishie, W.; Mai, Y.; Ujiie, H.; Iwata, H.; Natsuga, K.; Shimizu, H. Detection of Mucous Membrane Pemphigoid Autoantibodies by Full-Length BP180 Enzyme-Linked Immunosorbent Assay. J. Dermatol. Sci. 2017, 88, 247–248. [Google Scholar] [CrossRef]
- Smith, E.P.; Taylor, T.B.; Meyer, L.J.; Zone, J.J. Identification of a Basement Membrane Zone Antigen Reactive with Circulating IgA Antibody in Ocular Cicatricial Pemphigoid. J. Investig. Dermatol. 1993, 101, 619–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zone, J.J.; Pazderka Smith, E.; Powell, D.; Taylor, T.B.; Smith, J.B.; Meyer, L.J. Antigenic Specificity of Antibodies from Patients with Linear Basement Membrane Deposition of IgA. Dermatology 1994, 189 (Suppl. S1), 64–66. [Google Scholar] [CrossRef] [PubMed]
- Setterfield, J.; Shirlaw, P.J.; Kerr-Muir, M.; Neill, S.; Bhogal, B.S.; Morgan, P.; Tilling, K.; Challacombe, S.J.; Black, M.M. Mucous Membrane Pemphigoid: A Dual Circulating Antibody Response with IgG and IgA Signifies a More Severe and Persistent Disease. Br. J. Dermatol. 1998, 138, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, M.; Cozzani, E.; Broccoletti, R.; Carbone, M.; Pentenero, M.; Arduino, P.; Parodi, A.; Gandolfo, S. Analysis of Antigens Targeted by Circulating IgG and IgA Antibodies in Patients with Mucous Membrane Pemphigoid Predominantly Affecting the Oral Cavity. J. Periodontol. 2004, 75, 1302–1308. [Google Scholar] [CrossRef]
- Cozzani, E.; Drosera, M.; Parodi, A.; Carrozzo, M.; Gandolfo, S.; Rebora, A. Frequency of IgA Antibodies in Pemphigus, Bullous Pemphigoid and Mucous Membrane Pemphigoid. Acta Derm. Venereol. 2004, 84, 381–384. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shimoda, M.; Ono, Y.; Villalobos, I.B.; Mitra, A.; Konia, T.; Grando, S.A.; Zone, J.J.; Maverakis, E. Persistence of Autoreactive IgA-Secreting B Cells Despite Multiple Immunosuppressive Medications Including Rituximab. JAMA Dermatol. 2015, 151, 646–650. [Google Scholar] [CrossRef]
- Li, X.; Qian, H.; Sogame, R.; Hirako, Y.; Tsuruta, D.; Ishii, N.; Koga, H.; Tsuchisaka, A.; Jin, Z.; Tsubota, K.; et al. Integrin Β4 Is a Major Target Antigen in Pure Ocular Mucous Membrane Pemphigoid. Eur. J. Dermatol. 2016, 26, 247–253. [Google Scholar] [CrossRef] [PubMed]
- van Beek, N.; Kridin, K.; Bühler, E.; Kochan, A.S.; Ständer, S.; Ludwig, R.J.; Zillikens, D.; Schmidt, E.; Günther, C. Evaluation of Site- and Autoantigen-Specific Characteristics of Mucous Membrane Pemphigoid. JAMA Dermatol. 2022, 158, 84–89. [Google Scholar] [CrossRef]
- Koga, H.; Teye, K.; Ishii, N.; Ohata, C.; Nakama, T. High Index Values of Enzyme-Linked Immunosorbent Assay for BP180 at Baseline Predict Relapse in Patients with Bullous Pemphigoid. Front. Med. 2018, 5, 139. [Google Scholar] [CrossRef]
- Bohelay, G.; Alexandre, M.; Le Roux-Villet, C.; Sitbon, I.; Doan, S.; Soued, I.; Shourick, J.; Rousset, L.; Mellottee, B.; Heller, M.; et al. Rituximab Therapy for Mucous Membrane Pemphigoid: A Retrospective Monocentric Study with Long-Term Follow-Up in 109 Patients. Front. Immunol. 2022, 13, 915205. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, A.; Euverman, H.I.; Terra, J.B.; Jonkman, M.F.; Horváth, B. Effectiveness and Safety of Rituximab in Recalcitrant Pemphigoid Diseases. Front. Immunol. 2018, 9, 248. [Google Scholar] [CrossRef]


| Clinical Variable | ODSS | Univariate Analysis | Multivariable Analysis | |
|---|---|---|---|---|
| N. | (Mean ± SD) | p-Value | p-Value | |
| Sex | 0.86 | |||
| Male | 10/44 | 14.5 ± 6.16 | ||
| Female | 34/44 | 15.35 ± 7.06 | ||
| Age (mean 64.36 ± 15.44 yrs) | 0.28 | |||
| <65 yrs | 21/44 | 16.28 ± 7.05 | ||
| >65 yrs | 23/44 | 14.13 ± 6.56 | ||
| Eosinophils | 0.74 | |||
| Absent | 31/44 | 15.41 ± 6.98 | ||
| Detectable | 13/44 | 14.53 ± 6.60 | ||
| Band-like infiltrate | 0.12 | 0.17 | ||
| Absent | 40/44 | 14.62 ± 6.44 | ||
| Detectable | 4/44 | 20.5 ± 9.11 | ||
| IgA deposit | 0.001 * | 0.035 * | ||
| Absent | 35/44 | 16.48 ± 6.96 | ||
| Detectable | 9/44 | 10 ± 2.39 | ||
| IgM deposit | ||||
| Absent | 40/44 | 15.61 ± 7.05 | 0.30 | |
| Detectable | 4/44 | 11.5 ± 3.41 | ||
| C3 deposit | ||||
| Absent | 4/44 | 16.5 ± 5.74 | 0.55 | |
| Detectable | 40/44 | 15.02 ± 6.95 | ||
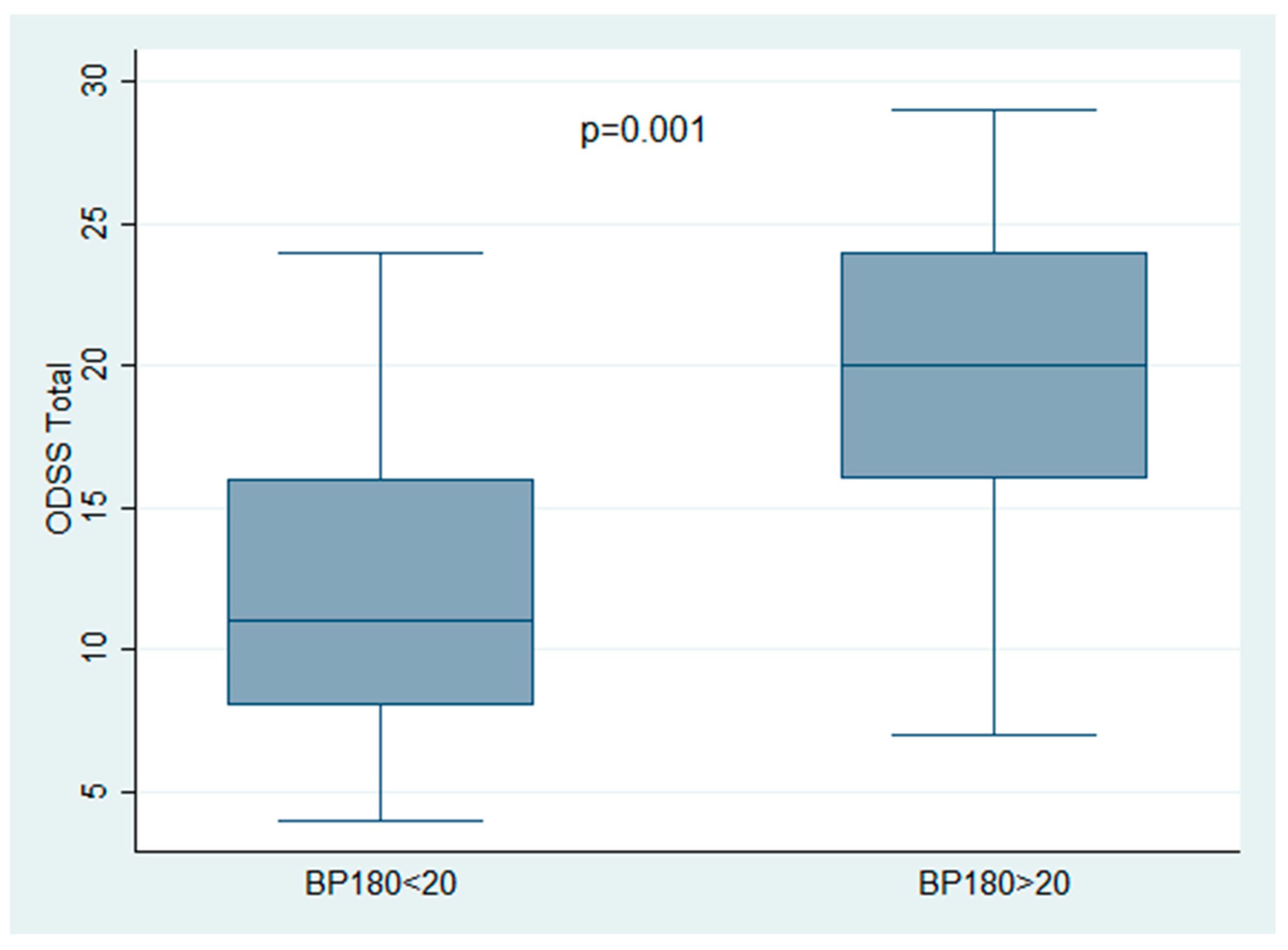
| BP180 ELISA (mean 25.81 ± 29.29 U/mL) | 0.001 * | 0.002 * | ||
| BP180 < 20 | 27/44 | 12.44 ± 5.40 | ||
| BP180 > 20 | 17/44 | 19.47 ± 6.70 | ||
| Total | 44 | 15.15 ± 6.81 |
| Clinical Variable | In Remission (Any Type) | Time to Remission (Months) | Relapse | Time to Relapse | p-Value |
|---|---|---|---|---|---|
| n | Mean ± SD | Mean ± SD | |||
| Sex | 0.89 | ||||
| F | 30/44 | 11.6 ± 11.53 | 13/39 | 10.84 ± 9.27 | |
| M | 9/44 | 9.88 ± 10.04 | 4/39 | 12.5 ± 9.94 | |
| Age | 0.38 | ||||
| <65 | 19/44 | 11.94 ± 13.46 | 8/39 | 13.87 ± 10.57 | |
| >65 | 20/44 | 10.5 ± 8.58 | 9/39 | 8.88 ± 7.49 | |
| Eosinophils | 0.22 | ||||
| Absent | 30/44 | 12.66 ± 11.85 | 16/39 | 11.18 ± 9.43 | |
| Detectable | 9/44 | 6.33 ± 6.42 | 1/39 | 12 | |
| Band-like infiltrate | 0.77 | ||||
| Absent | 36/44 | 11.11 ± 11.09 | 16/39 | 11.31 ± 9.43 | |
| Detectable | 3/44 | 12.33 ± 13.65 | 1/39 | 10 | |
| IgA deposit | 0.40 | ||||
| Absent | 31/44 | 9.77 ± 8.49 | 14/39 | 10.35 ± 8.69 | |
| Detectable | 8/44 | 16.75 ± 17.77 | 3/39 | 15.33 ± 12.09 | |
| IgM deposit | 0.60 | ||||
| Absent | 35/44 | 11.11 ± 11.59 | 15/39 | 10.4 ± 9.34 | |
| Detectable | 4/44 | 13.75 ± 7.5 | 2/39 | 17.5 ± 4.94 | |
| C3 deposit | 0.91 | ||||
| Absent | 4/44 | 16.25 ± 8.42 | 2/39 | 9.0 ± 7.07 | |
| Detectable | 35/44 | 10.62 ± 11.32 | 15/39 | 11.53 ± 9.54 | |
| BP180 ELISA | 0.009 * | ||||
| BP180 < 20 | 24/44 | 9.04 ± 8.45 | 8/39 | 13.0 ± 9.35 | |
| BP180 > 20 | 15/44 | 11.64 ± 7.97 | 9/39 | 9.66 ± 9.20 | |
| ODSS | 0.13 | ||||
| ODSS < 20 | 28/44 | 11.39 ± 12.04 | 11/39 | 13.72 ± 10.36 | |
| ODSS > 20 | 11/44 | 14.66 ± 14.0 | 6/39 | 6.66 ± 3.72 | |
| Therapy | 0.51 | ||||
| Local therapy | 17/44 | 7.76 ± 7.61 | 6/39 | 8.0 ± 7.07 | |
| Local and Systemic | 22/44 | 13.86 ± 12.72 | 11/39 | 13.0 ± 9.94 | |
| Type of Clinical remission | 0.35 | ||||
| PRmT | 16/44 | 5.31 ± 4.89 | 5/39 | 10.00 ± 12.02 | |
| CRmT | 21/44 | 16.42 ± 12.40 | 12/39 | 11.75 ± 8.24 | |
| PRoT | 2/44 | 3.50 ± 0.70 | 0/39 | NA | |
| CRoT | 0/44 | NA | NA | NA | |
| Total | 39/44 | 11.20 ± 11.10 | 17/39 | 11.23 ± 9.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabusi, A.; Gissi, D.B.; Rossi, R.; Filippi, F.; Loi, C.; Misciali, C.; Clarizio, G.; La Placa, M.; Bardazzi, F. Elevated BP180 ELISA at Diagnosis Correlates with Disease Severity and Relapse in Oral Mucous Membrane Pemphigoid: Preliminary Results from a Retrospective Monocentric Italian Study. Appl. Sci. 2025, 15, 689. https://doi.org/10.3390/app15020689
Gabusi A, Gissi DB, Rossi R, Filippi F, Loi C, Misciali C, Clarizio G, La Placa M, Bardazzi F. Elevated BP180 ELISA at Diagnosis Correlates with Disease Severity and Relapse in Oral Mucous Membrane Pemphigoid: Preliminary Results from a Retrospective Monocentric Italian Study. Applied Sciences. 2025; 15(2):689. https://doi.org/10.3390/app15020689
Chicago/Turabian StyleGabusi, Andrea, Davide B. Gissi, Roberto Rossi, Federica Filippi, Camilla Loi, Cosimo Misciali, Giacomo Clarizio, Michelangelo La Placa, and Federico Bardazzi. 2025. "Elevated BP180 ELISA at Diagnosis Correlates with Disease Severity and Relapse in Oral Mucous Membrane Pemphigoid: Preliminary Results from a Retrospective Monocentric Italian Study" Applied Sciences 15, no. 2: 689. https://doi.org/10.3390/app15020689
APA StyleGabusi, A., Gissi, D. B., Rossi, R., Filippi, F., Loi, C., Misciali, C., Clarizio, G., La Placa, M., & Bardazzi, F. (2025). Elevated BP180 ELISA at Diagnosis Correlates with Disease Severity and Relapse in Oral Mucous Membrane Pemphigoid: Preliminary Results from a Retrospective Monocentric Italian Study. Applied Sciences, 15(2), 689. https://doi.org/10.3390/app15020689







