Abstract
Approximate entropy (ApEn) and sample entropy (SampEn) are statistical indices designed to quantify the regularity or predictability of time-series data. Although ApEn has been a prominent choice in analyzing non-linear data, it is currently unclear which method and parameter selection combination is optimal for its application in biomechanics. This research aimed to examine the differences between ApEn and SampEn related to center-of-pressure (COP) data during tandem standing balance tasks, while also changing the tolerance window, r. Six participants completed five, 30 s trials, feet-together and tandem standing with eyes open and eyes closed. COP data (fs = 60 Hz, downsampled from 1200 Hz) from ground reaction force platforms were collected. ApEn and SampEn were calculated using a constant vector length, i.e., m = 2, but differing values of r (tolerance window). For each of the participants, four separate one-way analysis of variance analyses (ANOVA) were conducted for ApEn and SampEn along the anterior–posterior (AP) and medial–lateral (ML) axes. Dunnett’s intervals were applied to the one-way ANOVA analyses to determine which tandem conditions differed significantly from the baseline condition. ApEn and SampEn provided comparable results in the predictability of patterns for different stability conditions, with increasing instability, i.e., tandem eyes closed postures, being associated with greater unpredictability. The selection of r had a relatively consistent effect on mean ApEn and SampEn values across r = 0.15 × SD to r = 0.25 × SD, where both entropy methods tended to decrease as r increased. Mean SampEn values were generally lower than ApEn values. The results suggest that both ApEn and SampEn indices demonstrated relative consistency and were equally effective in quantifying the level of the center-of-pressure signal regularity during quiet tandem standing postural balance tests.
1. Introduction
For over 40 years, the use of various entropy methods to define periodicity or regularity in non-linear dynamic time series, e.g., human physiological and biomechanical data, has been well described [1,2]. Two commonly used methods for estimating the randomness of biological time-series data without previous knowledge about the source generating the signal are approximate entropy (ApEn) and sample entropy (SampEn). Because entropy quantifies the likelihood of the next state of a system, based on what is known about the present state of a time series, it has been used to quantify physiological changes with aging [3,4], cardiovascular status [5,6,7], and respiratory pathology [1,8,9]. Entropy calculations can take many forms, but ApEn and SampEn have been particularly useful for understanding more about the changes in postural control [10,11,12,13,14,15], human gait mechanics [16,17], and standing balance [2,18,19,20,21]. Before reviewing the details of this prior research, we present a brief treatise on the theoretical foundations upon which ApEn and SampEn are based.
Since entropy has been defined as a measure to quantify information, stochastic and deterministic processes must be differentiated. Stochastic processes are understood as a succession of random variables that are interrelated in a system that evolves, whereas a deterministic system is one without randomness in how states of the system are determined. With stochastic processes, our interest lies not with measuring total entropy but with how the entropy of the time series evolves, i.e., a measure of the entropy rate. Moreover, the analysis of randomness is a way that the complexity of a time series can be analyzed. Kolmogorov and Sinai’s concepts of complexity and entropy for dynamic systems led to a generalization of Shannon entropy for dynamic systems known as Kolmogorov–Sinai entropy (KS). KS entropic analyses were particularly applicable to chaotic systems and well-defined analytical systems; however, KS was challenging for limited and noisy measurements of a signal represented in a time series [1]. To overcome the limitations of KS entropy, several investigators [22,23,24,25,26,27] modified it, but typically examined only well-defined analytical systems, e.g., flows, Poincaré or one-time maps, Hénon maps, etc. Grassberger and Procaccia [22] described their use of Rényi entropy (a generalization of Shannon entropy) resulting in calculations more suitable for long time series. Their algorithm computed a value, termed a correlation integral, which measured with a tolerance r, the regularity (or frequency) of patterns similar to a given template of a given length (later identified as m or pattern length). They showed that the trajectory of a dynamic system did not need to follow the evolution of all degrees of freedom because it could be constructed with d measures of a single coordinate. Based on their experience investigating entropies, Grassberger and Procaccia [28] offered several comments relevant to their method: (1) the time series had to be long enough, (2) the system had to be stationary, (3) low measured correlations could be misleading, and (4) optimal embedding, e.g., the delay used in the embedding, did not exist. However, limitations were encountered when the Grassberger entropy equations were applied to experimental time series [1]. Subsequently, Takens [23] modified Grassberger and Procaccia’s [22] formula by introducing a difference function (i.e., identified as d functions) between two vectors. Eckmann and Ruelle [26] then revised Takens’ formula, which led to a more direct estimate of the KS entropy; a change that became the standard entropy measure referred to as the Eckmann–Ruelle (E-R) entropy formula.
According to [1], the formulas used to measure the complexity of a system were developed to analyze chaotic systems but were less successful in characterizing limited, noisy, and stochastically derived time series. The KS worked well for real dynamic systems, but even a small amount of noise made those algorithms meaningless, with values tending to infinity. In addition, a finite correlation dimension value could not guarantee that the process under examination was deterministic. KS was developed to classify deterministic systems depending on the rates of generation of information by determining an entropy rate. However, the former entropy formalism was unsuitable for statistical calculations. Therefore, based on the ground-breaking work of Grassberger and colleagues, later modified by others [23,26] Pincus’s extension of the E-R formulations resulted in a statistical version that could be used to characterize experimental data series. Alternatively, Pincus described the use of ApEn, which measures correlation, persistence, or regularity in a biological time series and could be used to compare systems with deterministic and stochastic components [1]. ApEn was not intended to be an approximation of the KS entropy but a statistical measure, or quantification, of the rate of regularity in a biological time series [1]. Theoretically, low ApEn values reflect a persistent, repetitive, and predictive system and high values reflect independence between data, a low number of repeated patterns, and unpredictability [29]. With biological data sets in general, but human movement in particular, quantifying levels of complexity has become important. For example, newer motor control theories, e.g., dynamical systems theory, do not consider variability in movement as an error [17]. Dynamical systems theory considers the complexity of movement patterns, i.e., variability, to be associated with system stability.
ApEn and SampEn have been shown to demonstrate the state and changes in the complexity of various physiological signals related to seated postural control in individuals with chronic stroke [30], electrocardiograms [31], electroencephalograms (EEG) [32,33], heart rate variability [34,35], and neural respiratory time series [8]. In complex systems, e.g., cardiac, respiratory, somatosensory, etc., lower ApEn values reflect persistent, repetitive, and predictive systems, with patterns that repeat themselves throughout the series [6,36]. So, it is more appropriate to use terms like probability, predictability, and regularity, when describing the nature of a measurable complex system. In summary, using ApEn and SampEn was not meant to analyze complex systems comprehensively but to statistically quantify the dynamics of time series related to complex systems [37].
Based on the work by Pincus [34,36] that characterized complex physiological systems using entropic measures, biomechanists have measured the excursions of the center of mass (COM) and center of pressure (COP) during quiet standing; the COP is the point of application of the vertical ground reaction force vector and represents a weighted average of all the pressures over the surface area in contact with the ground. The interpretation of chaotic and/or irregular excursions in the anterior–posterior and medial–lateral directions has been noted as a sign of poor balance and deficient postural control [2]. Alternatively, it has been suggested that chaotic excursions of a complex system may be interpreted as a characteristic of a successful strategy to maintain balance. It is notable that during quiet standing, the displacements of the COP display highly irregular and non-stationary fluctuations [13] that are not just the result of white noise but display non-linear dynamics that consist of an orderliness (which may appear random) that emerge over time, likely the result of the complex interactions among underlying postural control systems, i.e., visual, vestibular, and somatosensory [10]. For example, under fixed tasks, e.g., quiet standing, and environmental conditions, the non-linear properties of the postural system likely arise partly due to elastic and damping characteristics of muscles [10]. Because typical biomechanical measures of postural stability, e.g., the magnitude of the medio-lateral excursion of the COP, have been based on linear statistical models, it has been shown that they cannot detect subtle changes in postural control [18]. For example, some research found that, despite reporting concussion-related symptoms, many athletes continue to display postural instability [18].
The quantification of the non-linear dynamic nature of COP measures using entropic measures, such as ApEn and SampEn, has made it possible to sort out the issues of system complexity versus regularity. Ramdani et al. [13] tested quiet standing posture under eyes-open and eyes-closed conditions in young healthy individuals. They reported a significant reduction in the SampEn in the COP anterior–posterior and medial–lateral excursion in the eyes closed condition. A reduction in ApEn or SampEn suggests a more regular time series, indicating that the postural control system was more constrained in a potentially less stable task. Borg and Laxåback [2] compared the SampEn of the COP time series in two dimensions from quiet standing between young and elderly adults. In that study, there was greater entropy in the anterior–posterior direction in the elderly group suggesting the effect of a more impaired sensory system, which provided less precise input for balance control. Motivated by retrospective data that illustrated that COP oscillations in the anterior–posterior and medial–lateral directions became more regular (lower ApEn value) after injury despite the absence of postural instability, Cavanaugh et al. [18] reported that ApEn values for the AP and ML COP time series generally declined immediately after injury (i.e., concussion) in both steady (normal COP metrics) and unsteady injured athletes, suggesting a postural control system that was more constrained after injury. In that same study, reduced ApEn values were still evident 48 to 96 h after injury although postural instability had resolved. In a follow-up study, Cavanaugh et al. [19] further substantiated the usefulness of ApEn by examining its responsiveness to evaluate the immediate, short-term effect of secondary cognitive task performance on postural control in healthy, young adults. During dual-task performance, ApEn revealed a change in the randomness of COP oscillations in various sensory conditions, illustrating the potential of ApEn to detect subtle changes in postural control. Based on this review, it seems clear that both ApEn and SampEn methods have the potential to assist in the clarification of neuropathology, as well as their medical management.
Pincus considered ApEn (m, r) as a family of formulas and ApEn (m, r, N) as a family of statistics and suggested that system comparisons are possible with fixed m and r [34]. Yet both ApEn and SampEn methods utilize four input parameters: (1) N is the data length, (2) m is the pattern length (often referred to as the embedding dimension, vector length, segment length, or pattern window), (3) r is the tolerance window (a de facto noise filter), and (4) the delay used in the embedding. ApEn and SampEn quantify complexity in the data by looking at the difference in m point vs. m + 1 point patterns, over the N point data length. ApEn measures the logarithmic probability that nearby pattern runs remain close in the next incremental comparison [19], but allow self-counting. When dealing with stochastic processes, the analysis of conditional probabilities causes large values of m or minimal values of r to produce statistically low estimates. Ultimately, the value of the estimate depends on m and r so ApEn can vary significantly with the choice of m and r. Thus, ApEn is a relative measure where the choice of input parameters needs to consider several factors, e.g., the nature of the problem being addressed, the research question, and the characteristics of the time series.
Pincus [36] suggested that one of the advantages of ApEn is that the algorithm was finite for stochastic, noisy deterministic, and composite processes, i.e., models for complex biological systems. ApEn can differentiate between different mixed methods of deterministic and random components occurring with a different probability and is robust to outliers because the pattern formed by wild points will rarely be repeated in the waveform [7]. It has been demonstrated that greater ApEn values correspond to a more complex time series. The limitations of ApEn include that relative consistency is not guaranteed, and depending on the value of r, the ApEn values will change [1]. Additionally, the value of ApEn depends on the length of the data series. Lastly, the self-counting aspect of the algorithm creates a statistical bias that particularly impacts situations with small data sets, which is when only a few or even no matches are present, the entropic result is biased toward zero [1].
Acknowledging the ground-breaking work of Grassberger & Procaccia [22] and Eckmann & Ruelle [26] from the 1980s, Richman and Moorman [6] introduced SampEn, which differs from ApEn in the following ways: (1) SampEn does not allow self-counting, (2) SampEn takes the logarithm of all the times two sequences are similar, and (3) ApEn is dependent on the size of the series, whereas SampEn is not [1]. They believed that SampEn counteracted the limitations of ApEn, claiming that SampEn, as a statistical alternative, solved the self-counting problem eliminating the bias associated with ApEn. The use of SampEn appears to quantify regularity more effectively and eliminates many of the problems associated with ApEn [6]. SampEn maintains the relative consistency and is also mostly independent of the length of the time series [6]. SampEn was created to address the bias and inconsistencies of ApEn, yet both methods retain similarities [6], except in the case of strong multifractality. There is no consensus on which method is preferable, but one’s choice should be dependent on the research question and time series being evaluated.
When using ApEn and SampEn important consideration must be given to parameter selection, as these choices may have the greatest impact on the final entropy value even in the presence of noise [38]. Previously, we discussed how ApEn and SampEn were used to distinguish quiet standing postural control between the young and elderly [2], between healthy and concussed athletes [18,19], and between eyes-open and eyes-closed conditions [13]. However, in each case, the researchers chose specific values for parameters N, m, and r, without the examination of and prior evaluation of parameter choices. It has been shown that given a time series with N data points, the calculation of entropy requires a priori determination of two unknown parameters, embedding dimension, m, and threshold, r [39]. Multiple pairings of parameter selections allow one to examine relative consistency where a better discrimination capacity can be accomplished. Incorrect parameter choice, and lack of due diligence in selecting m, r, and N can undermine the interpretation and application of entropy results, as shown by previous investigators [8,12,15].
According to Yentes et al. [17], when the sampling rate is too high, i.e., frequency collection rates greater than 1000 Hz, redundancy likely exists within the data, which tends to artificially reduce entropy values [21]. Redundancy, i.e., repetitiveness of, or repeating, values, results in smaller entropy values and more signal regularity due to the counting of repeated matches. The redundant data problem can be solved by downsampling overly redundant time-series data sets. Although downsampling removes real data, sensitivity analyses have demonstrated that the removal of some data does not impact the subsequent application of the revised data set [12,16]. Previous studies demonstrated the link between entropy and sampling rate. Powell et al. [40] simulated different sampling rates by resampling ankle joint angle time series and found that higher sampling rates significantly reduced ApEn values. Conversely, results from Rhea et al. [41] suggest that excessive downsampling, e.g., to 25 Hz, artificially altered the standing center-of-pressure displacement and velocity SampEn values.
Few studies have examined the effect of different values of N, m, and r on the calculated values of ApEn and SampEn. Richman and Moorman [6] in their comparison of ApEn and SampEn selected constant m (of 2) and demonstrated differences in both entropies across different N’s and r’s in data unrelated to standing postural control. Yentes et al. [17] showed that both ApEn and SampEn were sensitive to short data sets (N ≤ 200) and parameter choices after subjecting theoretical and experimental (i.e., gait) data to all combinations of m = 2, 3, and 4, and r = 0.05, 0.1, 0.15, 0.20, 0.25, and 0.30 times the standard deviation of the entire time series; and N = 100, 120, 140, 160, 180, and 200. In a follow-up study on gait (over ground and treadmill conditions) data, Yentes et al. [16] again compared ApEn and SampEn using different combinations of N, m, and r, but with much larger time series (i.e., N) and r constant (in addition to r × standard deviation). They found that when r was constant, SampEn demonstrated overall excellent relative consistency for all combinations of r, m, and N and that when r constant was used, overground walking was more regular than treadmill, but treadmill walking was more regular when using r × SD for both ApEn and SampEn. According to Yentes and co-workers [16,38], care should be taken when selecting the input parameters of tolerance window, r, vector length m, and time-series length N. However, there is a paucity of research on the selection of input parameters involving the analysis of COP time series of quiet standing, particularly related to the postural control of tandem stance.
Based on our review of the literature, it is clear that the appropriate selections of N, m, and r are critical for computing ApEn and SampEn values that can enhance the interpretation of inherently non-linear biological time series. Therefore, the purposes of this research were to (1) examine and evaluate the effect of altering input tolerance, r (with the constant embedding dimension, m = 2) on ApEn and SampEn values related to the center-of-pressure time series during quiet standing postures, i.e., feet together and tandem standing, under eyes-open and eyes-closed conditions, and (2) assess which entropy measure was less biased and most consistent.
2. Materials and Methods
2.1. Participants
Six individuals (age: 24.8 ± 3.3 years; height: 171 ± 10.5 cm; body mass: 71.0 ± 13.5 kg) participated after voluntarily providing their signed informed consent. All participants were in good health, with no history of neurological or muscular disorders or injuries [42]. Before data collection commenced, foot dominance for each subject was determined based on the leg with which they preferred to kick a ball. This study was approved by the Grand Valley State University Institutional Review Board (18-246-H), and data from a previous data collection were used to extend a prior analysis.
2.2. Instrumentation
Vicon Nexus v2.8 motion capture software (Vicon Motion System Ltd., Oxford Metrics, Oxford, UK) and Vicon 16 MX T40 cameras (120 Hz) were used to track the movement trajectories of a modified Full-Body Plug-in-Gait model. Capturing motion was synchronized with the collection of ground reaction forces from floor-embedded AMTI (Advanced Mechanical Technology Inc., Watertown, MA, USA) force plates (1200 Hz). The center-of-pressure measures are reported to have a precision of <0.2 mm (AMTI General Brochure; https://www.amti.biz/support/downloads/, accessed on 30 November 2024). Surface electrodes were used to collect the electromyographical (EMG) signals (1200 Hz) from bilateral gastrocnemius, soleus, and tibialis anterior muscles using a 16-channel MA300-XVI patient unit acquisition system (Motion Lab Systems Inc., Baton Rouge, LA, USA). Only ground reaction force plate data were used for this study. The force plates were oriented with one directly in front of the other (Figure 1). Center-of-pressure data were extracted using Vicon NEXUS motion capture software v2.8 (Oxford Metrics, Oxford, UK) and exported to Excel for later analysis.
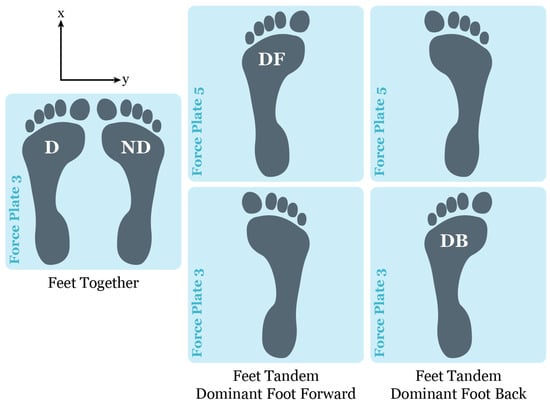
Figure 1.
Force plate foot placement for feet together and feet tandem standing balance conditions, where the x-axis (anterior–posterior (AP) direction) and y-axis (medial–lateral (ML) direction define the center-of-pressure orientation. Note: D = dominant foot; ND = nondominant foot; DF = dominant foot forward; and DB = dominant foot back [21,42].
2.3. Experimental Procedures
Ground reaction force data were collected for 30 s until five successful trials were completed per quiet standing condition (Table 1). Unsuccessful trials were defined by a major loss of balance, i.e., having to make significant changes in foot position, and/or if participants demonstrated large movements of their torso and changed arm positions. The standing postural condition of eyes open feet together (EOFT) was defined as the most stable and hence was used as a baseline for all entropy comparisons. Participants were asked to hold a quiet standing position for thirty seconds without moving their bodies or stepping out of position. Balance tasks were performed barefoot with the arms positioned with the shoulders and elbows flexed, forearms supinated, and the fingers touching the anterior aspect of the shoulder; the hips and knees were extended with the ankles maintained in neutral (i.e., neither dorsiflexed nor plantarflexed). Participants progressed through increasingly unstable balance conditions, with 2 min breaks between trials. Conditions included eyes open or closed and changing foot position, i.e., feet together on force plate 5 or tandem stance using force plates 3 and 5, as shown in Figure 1. We chose to test tandem standing because although it may be a commonly used clinical test of balance, there was a paucity of research examining the non-linear dynamics of quiet tandem standing. In the feet-together stance, the feet were as close as possible but constrained by contact between the medial malleoli of the right and left tibiae. The order of testing conditions was not randomized. The 2 min breaks between trials allowed participants to rest and change their postural stance. Consequently, each trial was assumed to be independent and could be analyzed without needing an alternative entropy computation, i.e., multiscale entropy.

Table 1.
Quiet Standing Balance Test Conditions.
2.4. Data Reduction
2.4.1. Determining Total Body Center of Pressure from Two Force Plates
In the feet-together postural stances, COP data from ground reaction forces (GRF) came from one force plate, whereas in the tandem standing trials, GRF data came from two separate force plates. The two-column tandem trial GRF data were combined into one resultant COP using Equation (4) [43,44]:
where COPL and COPR are the values of the COP signal from the left and right foot, respectively, and FzL and FzR are the vertical forces exerted on the force plates under the left and right foot, respectively. Approximate entropy (ApEn) and sample Entropy (SampEn) were determined using the data in the anterior–posterior and medial–lateral directions. See Figure 2 for the data processing functional block diagram.
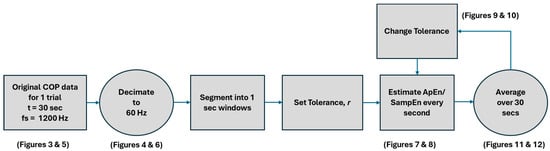
Figure 2.
Data processing functional block diagram.
2.4.2. Downsampling
Based on a preliminary analysis, the original COP data sets, recorded at 1200 Hz for 30 s by Tipton et al. [21], resulted in lower estimates of ApEn because the embedding time window was too small. Therefore, for this study, MATLAB’s built-in function was used to decimate the data from 1200 Hz to 60 Hz. The function low pass filters the data before downsampling to prevent aliasing. Figure 3, Figure 4, Figure 5 and Figure 6 show the time series before (Figure 3 and Figure 5) and after (Figure 4 and Figure 6) downsampling from a representative participant tested with EOFT and ECTanDF, respectively. We believe it was important to examine and show the results of downsampling for both the EOFT and ECanDF conditions because they were clearly different based on the subsequent entropy analyses. The figures show that downsampling did not affect the observed major trends in either of the stability conditions.
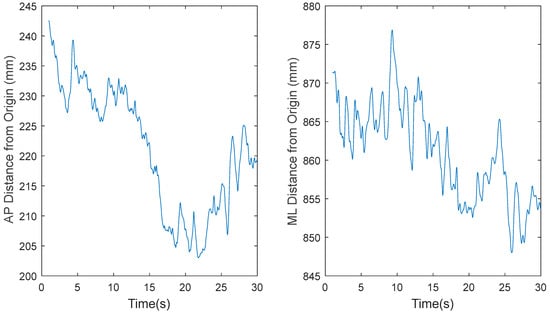
Figure 3.
Representative time series for raw center-of-pressure (COP) data of participant 1 eyes open feet together (EOFT) Trial 4, where fs = 1200 Hz, in the anterior–posterior (AP) and medial–lateral (ML) directions, respectively.
Figure 4.
Representative time series for downsampled center-of-pressure (COP) data of participant 1 eyes open feet together (EOFT) Trial 4, where fs = 60 Hz, in the anterior–posterior (AP) and medial–lateral (ML) directions, respectively.
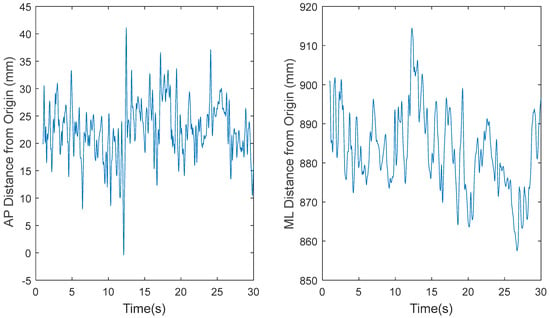
Figure 5.
Representative time series for raw center-of-pressure (COP) data of participant 1 eyes closed, feet tandem, dominant foot forward (ECTanDF) Trial 29, where fs = 1200 Hz, in the anterior–posterior (AP) and medial–lateral (ML) directions, respectively.
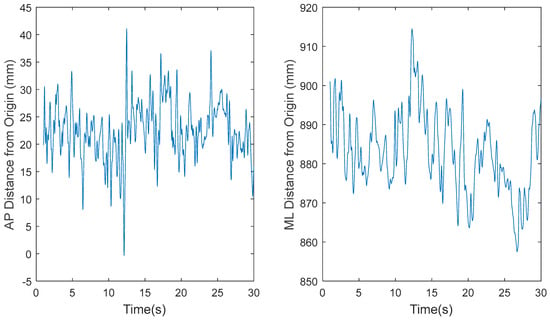
Figure 6.
Representative time series for the downsampled center-of-pressure (COP) data of participant 1 eyes closed, feet tandem, dominant foot forward (ECTanDF) Trial 29, where fs = 60 Hz, in the anterior–posterior (AP) and medial–lateral (ML) directions, respectively.
2.4.3. Determination of Approximate and Sample Entropy
Approximate and sample entropy were estimated every second and averaged over a 30 s interval for all five trials under each condition using custom MATLAB® R2021b (The MathWorks, Natick, MA, USA) code. For each COP time series, N = 60 data points, pattern length, m = 2, and tolerance window, for f = 0.05, 0.1, 0.15, 0.2, and 0.3, were used to estimate ApEn and SampEn. The choice of tolerance levels was motivated by exploring the responses of ApEn and SampEn for a range of r similar to previously reported research [16]. ApEn and SampEn were estimated as described in detail by Delgado-Bonal and Marshak [1]. For approximate entropy, given a sequence of numbers of length N, a non-negative integer m, with and a positive real number r, define the blocks and , and calculate the distance between them as . Then, calculate the value such that This counts the number of m-point patterns within tolerance r, in the data. So, computing:
we then define , with .
Sample entropy eliminates the self-counting problem and defines the total number of possible m-point pattern matches by calculating:
and then averaging:
In the same way, define the total number of matches by calculating each model vector and then adding them:
so that is the probability that two sequences are similar for m points, while is the probability that two sequences are similar for m + 1 matches. Sample entropy was then estimated as .
2.5. Statistical Analysis
Statistical analysis and graphics on entropy quantities were performed with R Statistical Software (v4.4.0; R Core Team 2024) [45] running in RStudio: Integrated Development Environment for R [46]. Descriptive graphics were generated to present an overall picture of entropy for the center-of-pressure excursions along the anterior–posterior (AP) and medial–lateral (ML) axes, overlaying the approximate and sample entropy values. Entropy quantities were derived for each r value, each Method, i.e., ApEn and SampEn, and each COP time series. The entropy values were calculated from five trials for each of the six participants in each of the five standing postural conditions, e.g., EOFT, etc., for a total of 5 × 6 × 5 (or 150) data points. Figures 9 and 10 were generated to illustrate the entropy values for each standing condition and each participant. Each cell of the graphics was derived from five COP time series, i.e., 5 trials. The graphics have 60 points in each cell from the 12 different calculations performed on each of the five time series. Lines are Loess curves and were used to provide a visual pattern for each method within each cell.
Box plots are provided to illustrate inter-trial variability. Figures 11 and 12 illustrate the entropy variability at one r-value, i.e., r = 0.25 × SD, for the medial–lateral COP excursion for ApEn and SampEn, respectively.
There were 144 one-way ANOVAs with Dunnett’s post hoc tests for the 6 participants, 6 values of r, 2 axes, and 2 methods. Each analysis was performed within a single participant with entropy calculated from a single combination of r, axis, and entropy method. The population for each statistical analysis was then entropy calculated with a particular combination of r, axis, and method for all COP time series for an individual under the five standing postural conditions. Each sample is then 25 independent trials under the 5 standing postural conditions.
Tables and graphics were generated for Shapiro and Levene’s test p-values below 0.05, indicating potential normality and heteroscedasticity issues. Note that the normality and heteroscedasticity were related to entropy values, not biomechanical measurements over time.
Nonparametric tests were considered but few of the 144 tests indicated a possible need for them. It is known that ANOVA is robust to failures of normality, especially for symmetric errors. All Shapiro test issues showed roughly symmetric errors in the Q-Q plots. ANOVA is also known to be robust to failures of equal variance in the case of equal group sizes. All group sizes were equal except for participant #5, for condition ECTDB, which did not fail either Shapiro or Levene’s tests.
All of the ANOVA F-tests except six had p-values < 0.05. Given the exploratory nature of this study and for the completeness of the graphics, Dunnett’s intervals were included in the confidence interval graphics even though the F-test was not statistically significant. We graphed 95% confidence intervals (Figures 13 and 14) in a format similar to the raw data graphics but plotted only the confidence intervals, not the raw data. Significant intervals do not contain zero (horizontal black line). The two entropy methods, i.e., ApEn and SampEn, are shown in separate rows.
For a direct comparison of ApEn and SampEn methods for each value of r, we constructed histograms (Figure 15) of their difference at each value of r. Values to the left of the vertical black line (zero) indicated that ApEn was larger, whereas values to the right of the vertical black line indicated that SampEn was larger.
3. Results
3.1. General Observations of Approximate and Sample Entropy
Approximate and sample entropy were determined for all trials and conditions, so it was important to examine the ApEn and SampEn values over the 30 s time series for m = 2, r = 0.2 × SD. Note that for this exercise, the chosen values of m and r reflected the most commonly chosen parameters in previously published studies. Figure 7 and Figure 8 illustrate these data for one trial of an eyes open feet together (EOFT) condition and one trial of an eyes closed feet tandem condition (ECTanDB) for one representative participant. Visual inspection of each plot suggests that the ApEn and SampEn magnitudes were comparable and the spikes over the 30 s time series appeared similar. Having established this for a representative participant and trial, it seemed appropriate to determine the mean ApEn and SampEn values for further statistical analysis.
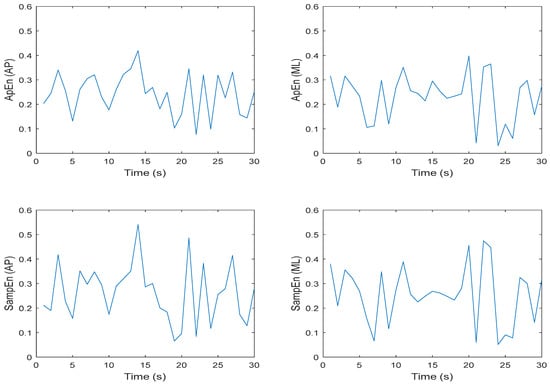
Figure 7.
Representative time series for ApEn and SampEn calculated every second for 30 s for participant 1 eyes open feet together (EOFT), Trial 03 COP data; where N = 60 data points, m = 2, and r = 0.2 × SD; in the anterior–posterior (AP) and medial–lateral (ML) directions, respectively.
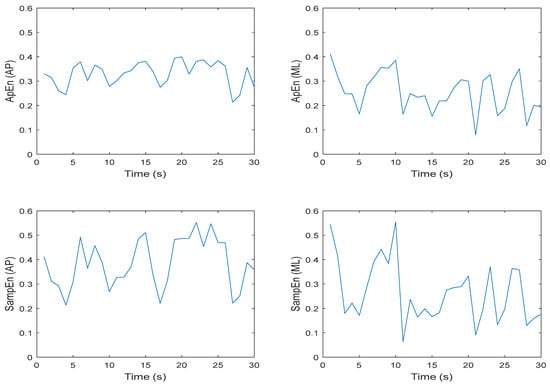
Figure 8.
Representative time series for ApEn and SampEn calculated every second for 30 s of participant 1 eyes closed, feet tandem, dominant foot back (ECTanDB), Trial 19 COP data; where N = 60 data points, m = 2, and r = 0.2 × SD; in the anterior–posterior (AP) and medial–lateral (ML) directions, respectively.
Figure 9 and Figure 10 provide a perspective of ApEn and SampEn values for each tolerance window and all participants under each standing postural test condition, except with feet together and eyes closed. See Appendix A for means and standard deviations of the approximate and sample entropy values for the anterior–posterior and medial–lateral COP excursion time series for each participant and the three representative conditions, i.e., EOFT, EOTanDB, and ECTanDB. For the COP excursion in the anterior–posterior direction, (Figure 9) entropy values suggested the following:
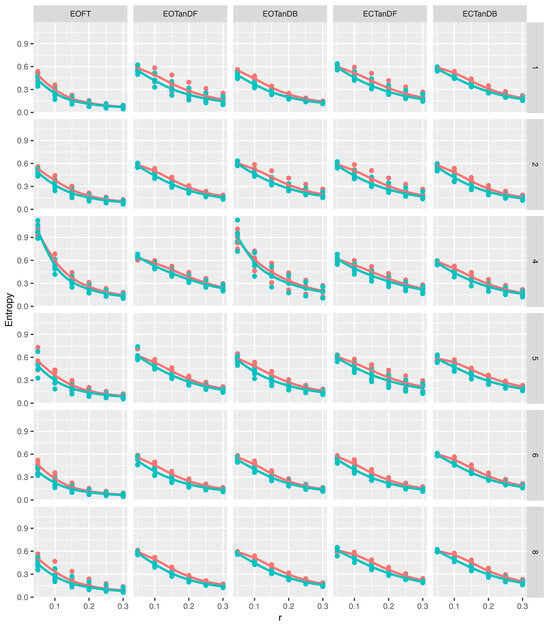
Figure 9.
Anterior–posterior (AP) direction approximate (red) and sample (blue) entropy values (vertical axis) versus six different tolerance windows (horizontal axis), i.e., r, while standing in five different postures, i.e., eyes open feet together (EOFT), etc., for each participant (rows). Note that 12 r and Method, i.e., ApEn or SampEn, quantities were derived from each COP time series. There were five time series for each cell giving 60 graphed points in each cell. Loess curves are provided to help visualize the relationship between r and entropy values for each method in each cell.
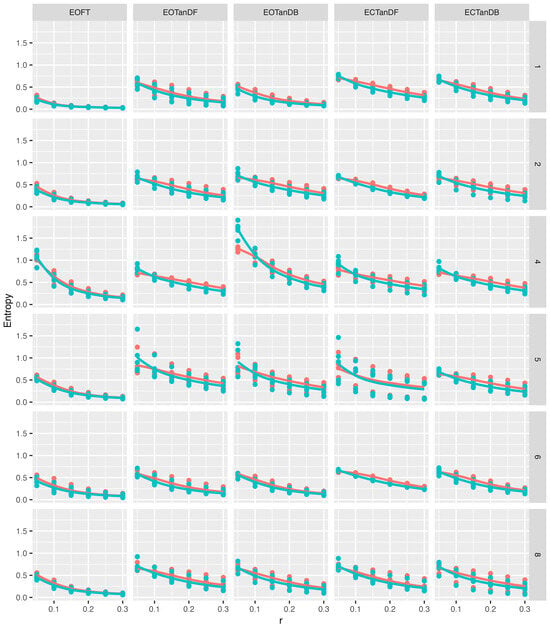
Figure 10.
Medial–lateral (ML) direction approximate (red) and sample (blue) entropy values (vertical axis) versus six different tolerance windows (horizontal axis), i.e., r, while standing in five different postures, i.e., eyes open feet together (EOFT), etc., for each participant (rows). Note that 12 r and Method, i.e., ApEn or SampEn, quantities were derived from each COP time series. There were five time series for each cell giving 60 graphed points in each cell. Loess curves are provided to help visualize the relationship between r and entropy values for each method in each cell.
- Entropy values were larger in the most stable standing condition, i.e., eyes open feet together, for r = 0.05 × SD and 0.1 × SD;
- ApEn (red) and SampEn (blue) Loess curves were similar;
- Entropy values generally decreased as r increased, leveling off after; r = 0.2 × SD;
- Entropy values were larger with tandem standing postures compared to eyes open feet together, but there did not appear to be differences in entropy values between eyes open and eyes closed for the tandem standing postures;
- Participant #4 demonstrated different entropy value patterns for the eyes open feet together and eyes open tandem dominant back conditions, compared to the other five participants.
For the medial–lateral excursion of the COP (Figure 10), first note that the entropy scale (vertical axis) is different than in Figure 9, yet patterns of entropy values were similar in Figure 10. On the whole, we observed the following:
- Entropy values were generally smaller in the eyes open feet together condition, but there do not appear to be differences in entropy values between eyes open and eyes closed for the tandem standing postures;
- ApEn (red) and SampEn (blue) Loess curves were similar;
- Entropy values generally decreased as r increased, but the decreasing slope of the Loess curves, as r increased, appeared to be reduced for medial–lateral excursions;
- Participants #4 and #5 exhibited different entropy patterns, with more variability.
Because of the apparent greater variability, among participants #4 and #5 in Figure 9 and Figure 10, we took a closer look at the variability across all participants and trials for both ApEn and SampEn for the COP excursion in the medial–lateral direction (Figure 11 and Figure 12, respectively). Overall, it appeared that ApEn and SampEn values were quite similar. In particular, we noted that the lowest entropy values were generally less variable and associated with the eyes open feet together standing posture, whereas greater values were associated with the eyes closed tandem conditions. As noted previously but more apparent in Figure 11 and Figure 12, participants #4 and #5 appeared different, i.e., larger entropy values than the other participants. Yet Figure 11 and Figure 12 also revealed that participant #6 appeared to generally have lower entropy values than all other participants and also perhaps demonstrated less trial-to-trial variability. Finally, greater variability of the entropy values was demonstrated across all participants and trials for the more challenging tandem standing postures (both eyes-open and -closed conditions).
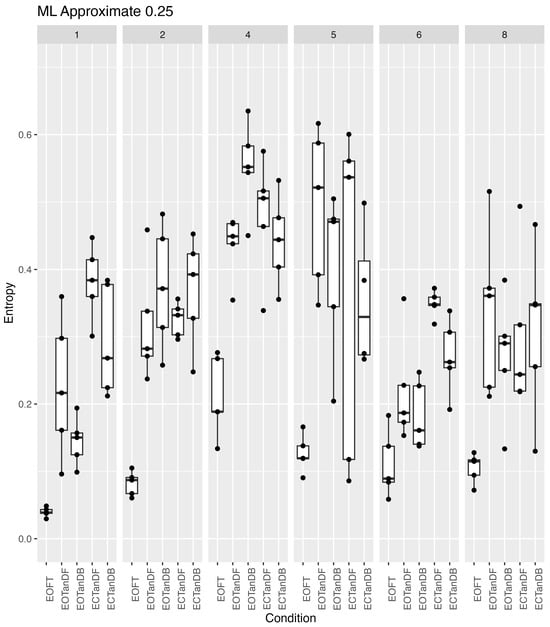
Figure 11.
Approximate entropy (vertical axis) (m = 2, r = 0.25 × SD) across six participants for all standing postural test conditions (horizontal axis). Data collected consisted of five trials for each participant (columns) at each stance condition. Each boxplot is derived from the five calculated entropy values for each condition for each participant (30 boxplots).
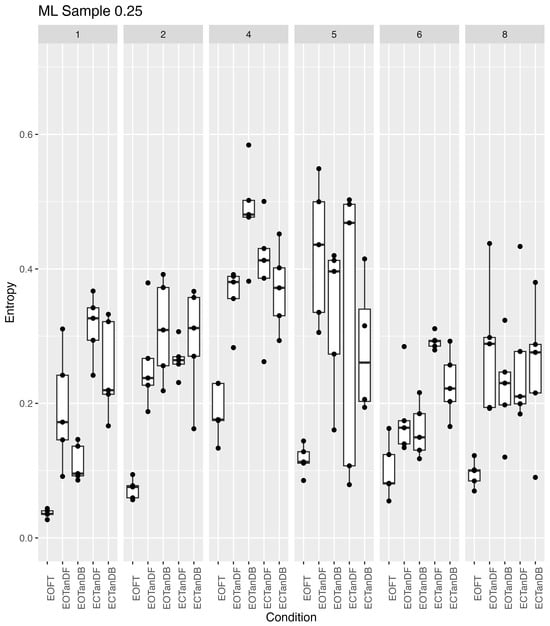
Figure 12.
Sample entropy (vertical axis) (m = 2, r = 0.25 × SD) across six participants for all standing postural test conditions (horizontal axis). Data collected consisted of five trials for each participant (columns) at each stance condition. Each boxplot is derived from the five calculated entropy values for each condition for each participant (30 boxplots).
3.2. ApEn and SampEn: Comparing Entropy Across Conditions
We chose the eyes open feet together as the baseline assuming that this postural stance would result in less postural instability (i.e., more postural or balance control) than tandem standing tasks, especially the tandem tasks with eyes closed. We compared the ApEn (Figure 13) and SampEn (Figure 14) values for each of the tandem stances with the baseline postural stance across all trials by running 144 one-way ANOVAs with Dunnett’s post hoc tests. Despite some outliers, both ApEn and SampEn significantly distinguished differences in standing postural control between the baseline posture, i.e., EOFT, and the eyes-open and eyes-closed tandem stances.
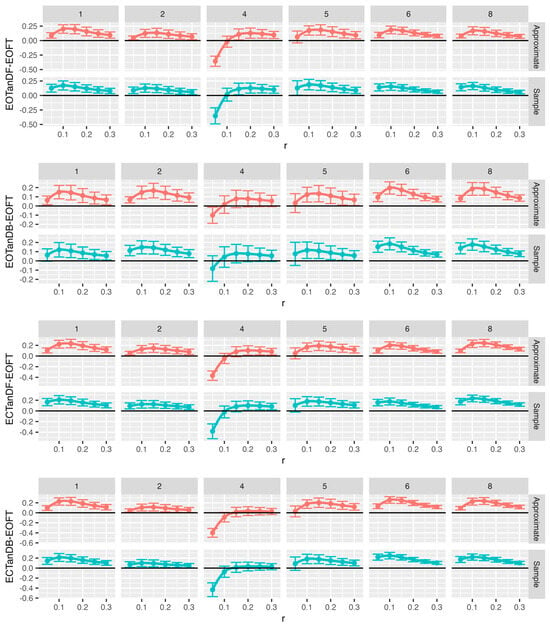
Figure 13.
Comparison of approximate (red) and sample (blue) entropy values across standing postural conditions for the center-of-pressure data in the anterior–posterior (AP) direction for each participant. Note that all F-tests, except six, had p < 0.05. What is graphed are Dunnet’s 95% confidence intervals. Loess curves through the confidence interval centers are included to aid visualization. Significant intervals can be found above the black (zero) horizontal line. Comparison pairs along the vertical axis, with r values along the horizontal axis. Note: to minimize unused white space, the vertical scaling varies for each comparison pair.
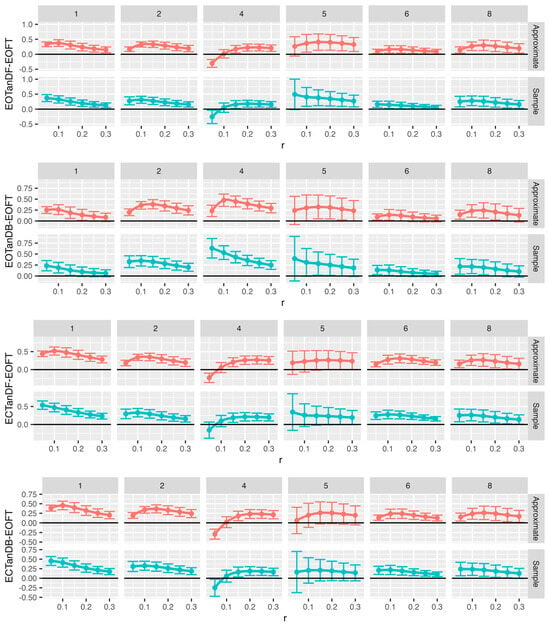
Figure 14.
Comparison of approximate (red) and sample (blue) entropy values across standing postural conditions for the center-of-pressure data in the medial–lateral (ML) direction for each participant. Note that all F-tests, except six, had p < 0.05. What is graphed are Dunnet’s 95% confidence intervals. Loess curves through the confidence interval centers are included to aid visualization Significant intervals can be found above the black (zero) horizontal line. Comparison pairs along the vertical axis, with r values along the horizontal axis. Note: to minimize unused white space, the vertical scaling varies for each comparison pair.
3.3. Comparing ApEn and SampEn Values Across Different Tolerance Windows
To make direct comparisons between ApEn and SampEn values across different tolerance windows, i.e., r values, we created histograms of their difference at each r (Figure 15). It is notable that, although in Figure 9 and Figure 10 similarities between ApEn and SampEn were observed, the significant differences between the alternative approaches to calculating entropy show that ApEn values for COP excursion in the anterior–posterior and medial–lateral directions were larger than SampEn values.
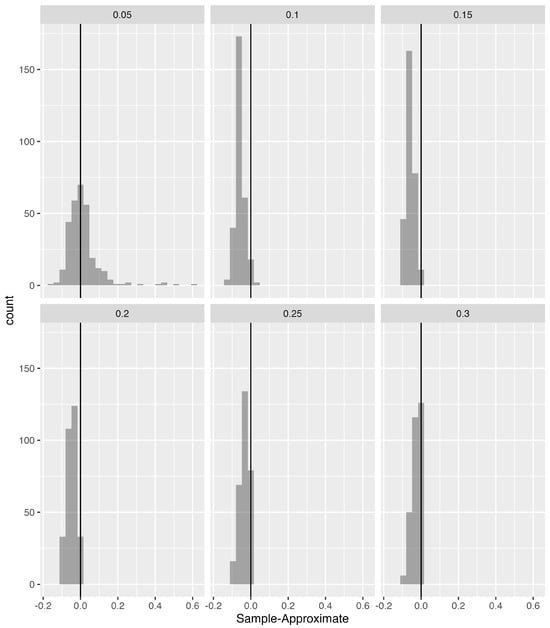
Figure 15.
Histograms, i.e., count (vertical axis) of the difference between SampEn and ApEn, i.e., Sample–Approximate (horizontal axis). Values to the left of the vertical black line indicate that ApEn was larger, and values to the right of the vertical black line indicate that SampEn was larger.
4. Discussion
Understanding how individuals respond to different static and dynamic postural conditions and utilizing the best method to measure responses is important when comparing how a healthy nervous system responds versus how a brain-injured individual responds to the same conditions. Previous research that examined children and adults with mild traumatic brain injury, e.g., concussion, has suggested that the use of the center-of-pressure (COP) data can be useful in delineating a normal from an abnormal response to the perturbations of static and dynamic balance [10,13,14,18,47,48,49,50]. Since it has been shown that the COP time series is non-linear, traditional methods of assessing various COP parameters, e.g., statistical use of means and standard deviation, have not been effective [10,48]. Previous work using non-linear metrics, such as approximate and sample entropy, to study normal and pathological balance has been more useful [3,18,19,51,52,53], with some interest in the selection of the input parameters, i.e., N, m, and r, for the calculation of ApEn and SampEn. Previous work by Yentes et al. [16,17,38] discussed parameter selection processes relative to gait data [16,38] and short data sets [17], but only Montesinos et al. [52] examined a range of input parameters related to COP time series in healthy young and older adults. In our laboratory, Tipton et al. [21] measured the center of pressure of healthy college-aged participants under various quiet standing postures and used ApEn to characterize the time series, but their method was limited by an overly redundant data set and included the determination and use of only one tolerance window, r, that was atypical. Based on the need described in the literature for more work investigating the diverse application of ApEn and SampEn analyses, and the methodological limitations of the previous research in our laboratory, the primary purpose of this study was to compare ApEn and SampEn under various difficult quiet standing conditions and examine the effects of different tolerance window values. The results revealed the following: (1) that even though SampEn tended to yield lower mean values than ApEn, both indices provided measures of unpredictability or irregularity of a COP time series in both the anterior–posterior and medial–lateral directions; (2) both ApEn and SampEn effectively differentiated a more stable quiet standing posture, i.e., eyes open feet together, from less stable standing postures, i.e., eyes-open and eyes-closed tandem standing postures; and (3), the selection of r had a relatively consistent effect with both entropic statistical analyses. Ours is the first study to examine and report ApEn and SampEn related to the non-linear dynamics of the center-of-pressure excursions for quiet tandem standing under eyes-open and -closed conditions.
It has been demonstrated that high sampling rates result in reduced estimated entropy values, likely since higher rates are well above the frequency of the tested behavior creating an artificial increase in the number of matches [12,38]. Tipton et al. [21] reported average ApEn estimates ranging from 0.005 to 0.030 for each 30 s COP time series sampled at 1200 Hz. By contrast, in this study, the estimates of average ApEn are higher and ranged from 0.1 to 0.6 for each 30 s COP time series sampled at 60 Hz. By low-pass filtering and decimating the data to 60 Hz, we minimized the effect of measurement noise above 30 Hz on the entropy estimates. Others have demonstrated the importance of how data management is handled and interpreted. For example, Rhea et al. [41] noted that downsampling from 100 Hz to 50 Hz and 25 Hz produced a data set that appeared to be linearly less regular, i.e., increasing SampEn. They cautioned that researchers must identify how much change is driven by the neuromotor system and how much is a function of the data processing technique. Lubetsky et al. [12] noted that since postural sway typically lies between 0.15 and 0.4 Hz (and as high as 3 Hz), a sampling rate of 25 Hz should be sufficient to detect time-series patterns and better reflect the underlying postural sway pattern. Thus, they were interested in evaluating how sample entropy of COP time-series data sampled at 100 Hz (N = 2000) from prolonged standing tasks on normal and compliant surfaces would be affected by downsampling by two, three, and four. They found that although downsampling increased SampEn values, it had an insignificant effect on the comparisons to the original data sets. However, they concluded that if other researchers performed such procedures, they should be well justified. Yentes et al. [17] recommended, as best practice, that practitioners not exceed sampling data beyond 1000 Hz. We found that the COP waveforms were observationally nearly identical when comparing unfiltered data to the downsampled data and that the entropy values that were subsequently determined, i.e., average ApEn and SampEn, ranged from 0.08 to 0.90; values that are consistent with other published works.
Incorrect parameter selection, i.e., vector length, m, tolerance or threshold window, r, and data length, N, regardless of the biological time series being considered, can undermine the ApEn and SampEn discrimination capacity [54]. For this study, the embedding dimension, m = 2, and data set length, N, were fixed input parameters. We focused on assessing entropy outcomes relative to changes in the tolerance window since this parameter may have the greatest influence on determining ApEn or SampEn [38] and is considered one of the most difficult to select [16]. Selecting a tolerance window too small has been shown to limit the number of matches found and selecting too large a window could lead to too many matches found and increase the probability [38]. Many approaches to calculating r have been suggested, including utilizing the standard deviation (SD) of the whole time series [34,52], the standard error of the entropy values [55], predefined tolerance levels [56,57,58], and using a heuristic stochastic model [54]. Most commonly, the tolerance window is calculated as r times the standard deviation of the time series [34]. However, many researchers have reported determining, and using, r = 0.2 × SD, with the rationale that it was commonly used in previous research. Yentes et al. [38] recommended that researchers test multiple r values, and if using the default method, i.e., r × SD, test the relative consistency of r = 0.15, 0.25, and 0.30 × SD, but this was based on both theoretical and gait data [16,17], whereas the present work examined quiet biped and tandem standing balance data. On the other hand, Yentes et al. [16] reported that as r increased, the value of SampEn decreased, which is similar to our results for both ApEn and SampEn during quiet biped and tandem standing. Montesinos et al. [52] examined changing parameters m, r, and N on ApEn and SampEn values in a COP time series, as well as the ability of these entropy measures to discriminate between young and older adults (nonfallers and fallers). They reported significant three-way interactions between m, r, and N confirming the sensitivity of ApEn and SampEn to the input parameters. Although we examined changes in entropy over a range of r values, similar to Montesinos et al., we constrained the embedding dimension to two; we did not study the interaction of multiple m’s and r’s. Our study results are limited because we did not examine the interaction between changing embedding dimensions and tolerance windows. We agree with [38,52] that looking for and using optimal, or widely used, values of m and r is not recommended, and researchers should look for how these values correlate to specific biological time series, i.e., what they mean biologically. Researchers should try different m values in combination with multiple r values that ensure relative consistency. Moreover, once the consistency of a particular combination of parameters (m, r, and N) is established for a particular type of biological time series it may effectively be used relative to longitudinal clinical testing, e.g., for pre- and post-treatment tests. Future research on the use of entropy as a relative value for testing intra-individual changes in clinical status is warranted.
We calculated ApEn and SampEn related to the anterior–posterior and medial–lateral COP excursions during a variety of quiet standing postures and used r = 0.05, 0.10, 0.15, 0.20, 0.25, and 0.30 × SD (Figure 8 and Figure 9). For COP anterior–posterior excursion, we found that entropy magnitudes (ApEn and SampEn) in the EOFT condition were larger for r = 0.05 × SD and 0.10 × SD, and values decreased as r increased, leveling off after r = 0.20 × SD. Entropy magnitudes for COP medial–lateral excursion were generally reduced in the EOFT condition compared to the AP excursion data, but decreasing entropy with increasing r mirrored the pattern seen for the AP data. Using long walking trials, Yentes et al. [16] reported a similar relationship between SampEn and changing r, but a more variable relationship between ApEn and changing r. Whereas, with quiet standing, Montesinos et al. [52] showed that for chosen m values (two, three, four, and five) both ApEn and SampEn consistently decreased as r increased for COP time series in both the anterior–posterior and medial–lateral directions, which is similar to our findings for both feet together and tandem balance tasks. In other words, the COP time series exhibited more regularity (i.e., lower entropy values) for greater similarity tolerances and greater subseries lengths. According to [52], the increase in regularity for greater r values seems to be an expected result, as it is a reasonable assumption that a greater number of subseries will meet the similarity criterion for a more relaxed tolerance. Montesinos’ results highlighted issues with relative consistency in COP time series for ApEn, as observed by the change in direction of differences between groups (known as “flips” or “crossovers”) for some combinations of m and r, which seemed to be accentuated for shorter time series (N = 600). In contrast, SampEn showed relative consistency. On the other hand, our data demonstrated relative consistency for both ApEn and SampEn with changes in the tolerance window, particularly for r = 0.20, r = 0.25, and r = 0.30 × SD. Be reminded, however, that we examined quiet feet together and tandem quiet standing positions, whereas ref. [52] tested standard feet apart stances. Based on the relative consistency of both ApEn and SampEn using a range of r’s equal to 0.20, 0.25, and 0.30 × SD, we suggest that future research investigating postural sway based on COP time series consider the method we used. Despite the consistency of the ApEn and SampEn values we report, we caution the reader that our choice for determining the tolerance window may be limited by factors, e.g., data length, non-stationarity of the data, spikes, and outliers, that affect data variance.
Outliers and spikes were identified in some of our data, particularly for participants #4 and #5 (see Figure 8, Figure 9, Figure 10 and Figure 11), in the ApEn and SampEn values for r = 0.05 and r = 0.1 × SD, likely due to the overly stringent conditions. Molina-Picó et al. [59] evaluated the impact of abnormal spikes on the interpretation of entropy results in the context of biosignal analysis and suggested removing these results, as they can misrepresent the signal regularity. We believe additional research is needed related to reproducing our findings relative to the entropy values using smaller r values. For this project, we presented the data using smaller r values, we but are unsure of their clinical interpretation and meaningfulness.
Before any measurement tool can be used for clinical purposes, its precision, i.e., reliability or consistency, should be established. Intuition suggests that an amplitude metric like the center of pressure, e.g., the magnitude of postural sway, denotes precision; that is, optimal postural control is evidenced by less movement, or error, about a target position. In the past, based on this assumption, linear modeling and averaging the COP variability, i.e., standard deviation, suggesting that COP variability was a general indicator of error, was practical and justified. However, it has been shown that the COP time series is non-linear and that its variations are neither random nor independent but have deterministic properties [10]. Thus, it appears that the COP is part of a complex of interacting systems, i.e., visual, somatosensory, and vestibular, that must be adaptable and flexible in an unpredictable environment. How might this be explained? Several neurological and biomechanical models of the postural control system have generally evolved from the groundbreaking work of Bernstein [60], who suggested that coordinated movement in general, and postural control in particular, was a “problem” that involved mastering, using a hierarchical central nervous system (CNS) model, the many redundant degrees of freedom (DoF) available to the neuro-musculoskeletal system. Berstein suggested that the CNS implemented specific control structures to limit the DoF at four levels, one of which includes muscle synergies. Muscle synergies are groups of muscles that cooperate to produce and/or control movement as part of the somatosensory system. Latash et al. [61], on the other hand, proposed an alternative model guided by a “principle of abundance” that suggested that all the elements, i.e., DoF, always participated in all the tasks, which assured both stability and flexibility, i.e., variability, of the performance. Latash et al. triggered a rethinking of how the COP time series might be examined. The successful use of ApEn by clinicians, such as Pincus, led to the introduction and use of entropic measures in biomechanical posture analysis. Generally, that work demonstrated the following: (1) reduced variability of COP time series is produced by a system under greater constraints and fewer DoF choices associated with poor postural control, and (2) more irregular output, i.e., increased variability, is produced by a less constrained system with more available DoF [10]. This brief review of coordinated movement modeling is relevant to the variability of the ApEn and SampEn values that we observed between trials of individuals, and between individuals. For example, Figure 11 (ApEn) and Figure 12 (SampEn) illustrated several common features related to the medial–lateral excursion of the COP time series among the two entropy methods: (1) reduced ApEn and SampEn average values and inter-trial variability for all participants for the EOFT, i.e., baseline, condition; (2) greater ApEn and SampEn and inter-trial variability for both the eyes-open and eyes-closed tandem stance conditions; and (3) observable differences in the magnitude and variability of ApEn and SampEn between participants #4 and #5 and the other participants. Our data suggest that the reduced average entropy and variability in the baseline condition may be associated with an environment that was more routine and less challenging for each participant, and that reduced adaptability in the use of DoF was necessary. On the other hand, more flexibility and adaptability, i.e., more choices in how patterns of the DoF were solicited and used, were necessary for the more challenging balance tasks. Finally, the differences between individuals may be consistent with the notion of general biological variability in the development of individual performances of unique tasks, i.e., standing in a bipedal posture with eyes open versus tandem standing. This is relevant information that may be clinically useful since if we can discern differences in these biosignal measures with normal healthy individuals, perhaps more telling differences will be seen when testing balance in the elderly who are at risk for falling [62] and in persons with neuro- or musculoskeletal pathology [10]. Our results suggest that inter-trial variability of entropic measures may be as important as their changes in magnitude with variation in tasks, so we believe that additional research may be warranted. In particular, future research might focus on teasing out the variation within individuals that is related to biology versus the measurement itself. This is particularly important for cases where longitudinal studies are warranted, e.g., repeated quiet standing balance tests of athletes post-concussion bi-weekly over 12 weeks.
Although we determined ApEn and SampEn for both EOFT and ECFT postures, we chose to use the EOFT posture as a relative baseline for postural stability. An additional purpose of this study was to ascertain whether these two entropies could differentiate signal predictability similarly when comparing a relatively stable quiet standing posture from postures that were more difficult to maintain over a 30 s time frame. Our data showed that ApEn and SampEn values were greater for tandem standing positions whether the eyes were open or closed, and whether the dominant foot (leg) was placed back or forward (Figure 12 and Figure 13); i.e., we did not evaluate whether ApEn and SampEn discriminated between eyes-open and eyes-closed conditions. Thus, we concluded that the tandem standing positions produced anterior–posterior and medial–lateral COP excursions associated with a system that produced a time series that was more random, less probable and predictable, and one with a greater amount of new information gained from the next data points in the time series [38]. In another study that assessed quiet standing balance and altering visual conditions, Ramdani et al. [13] used SampEn to analyze human postural sway. They reported that SampEn distinguished between the eyes-open and eyes-closed conditions of participants standing on a single force plate. Specifically, in the eyes-closed condition, SampEn was lower. Montesinos et al. [52], also testing eyes-open and eyes-closed conditions, found that for any given parameter combination, the mean SampEn value by group increased across the four testing conditions (vision-surface [rigid and foam mat]): eyes-open (EO)-rigid < eyes-closed (EC)-rigid < EO-foam < EC-foam. Other research results concur with Ramdani’s findings [56], whereas some reported contradictory results [63]. One limitation of the present study was that we did not examine whether ApEn and SampEn could distinguish the eyes open from the eyes closed in tandem standing postures. Future research should address this.
Since ApEn and SampEn values were significantly greater with tandem standing postures, compared to the eyes open feet together posture, we wondered which entropic measure was “better”. We are not aware of previous research that compared ApEn and SampEn from the COP time series for tandem standing balance by altering only the tolerance window. Yentes et al. [16] investigated the step time series of walking trials and evaluated various combinations of N, m, and r. They concluded that SampEn demonstrated excellent relative consistency for long gait data sets when using two different modes of walking, i.e., overground versus treadmill. On the other hand, our results suggest that both ApEn and SampEn demonstrated similar relative consistency for all test conditions.
In addition to reported differences in relative consistency for ApEn and SampEn between Yentes et al. [16] and our findings, there were also differences when comparing the two entropy value magnitudes. We found that, overall, ApEn magnitudes were greater than SampEn values, whereas Yentes et al. reported that generally mean ApEn values were lower than mean SampEn values in the analysis of step time gait data.
We believe our study’s results can provide more than academic interest. Although our participants were young healthy adults, we have shown that ApEn and SampEn can differentiate COP signal regularity among different quiet standing positions, i.e., EOFT and EOTanDB. Similarly, Cavanaugh et al. [19] demonstrated, with young healthy adults, the sensitivity of ApEn to COP changes related to secondary cognitive tasks during quiet standing, suggesting support for the potential of ApEn to detect subtle changes in postural control. Cavanaugh and colleagues [10,18,64] reported using ApEn to examine COP anterior–posterior and medial–lateral time series in athletes with concussion. They found, for example, that (1) concussed athletes with normal postural stability nonetheless demonstrated more regular COP oscillations, i.e., lower ApEn values; and (2) ApEn values for the medial–lateral time series remained significantly depressed among athletes whose initial postural stability had resolved. Others [65] examined the use of ApEn, SampEn, and fuzzy entropy (FuzzyEn), computed using multiple combinations of m and r, to analyze COP time series in type-2 diabetic patients with and without neuropathy during quiet trials with eyes open. Similar to our findings, they reported the consistency of entropy measures for different input values and showed significant differences between the two cohorts in terms of COP regularity in the AP and ML directions. In that study, the FuzzyEn results suggested low complexity in the postural control of neuropathic patients in the ML direction. The authors of this report indicated that measures of complexity, like FuzzyEn, could complement the use of ApEn and SampEn. Although our results suggest that the application of ApEn and SampEn may be equally effective in detecting subtle changes in COP time series from balance studies involving elderly individuals, for those with head injuries, e.g., post-concussion, or other neuropathologies, the use of additional non-linear analyses may be needed. For example, since the functions of physiological systems are deployed through the interactions between different control dynamics, across multiple spatial and temporal scales, multiscale entropy (MSE), which is a derivative of SampEn, performs an entropy analysis over multiple time scales, providing a measure of the complexity of a time series rather than only quantification of its regularity [66,67,68,69]. Previous research has demonstrated that multiscale entropy (MSE) has greater potential for use in tests of standing balance for other clinical entities, e.g., peripheral neuropathies related to diabetes and multiple sclerosis. Mengarelli et al. [70], examining COP time series during unperturbed standing in patients with and without neuropathic symptoms, reported a significant loss of complexity in the COP time series medial–lateral direction in the symptomatic neuropathic cohort. In another application, Busa and colleagues [71] aimed to identify whether the complexity index (a derivative of MSE) of the COP time series was reduced in those with multiple sclerosis (MS) compared to controls under conditions of limited vision and increasingly demanding standing tasks. They found that COP complexity was significantly reduced in the anterior–posterior and medial–lateral directions in the MS group compared to controls during the performance of maximal self-regulated leans and with reduced vision, and that complexity was correlated with cutaneous sensitivity. These data suggest that the future use of entropy measures for clinical testing consider several alternative options.
5. Limitations
The results of this project may be limited by several methodological decisions. Our sample was limited by convenience and its small size. Additional research is needed using a larger cross-sectional sample of male and female healthy individuals. However, we do not believe that attempting to develop normative ApEn and SampEn value tables is necessary, or even useful, since the best use of any entropy measure is likely in the initial and longitudinal testing of individuals with disease. On the other hand, it might be interesting to further examine the inter-trial and subject variability that was detected in our small sample with larger and more diverse samples. Results of these investigations, which may also include examining the regularity and complexity of electromyographic time series of the selective lower leg muscles involved with postural control, may provide further insight into central nervous system control of quiet standing postures. Additionally, it will be important to test our methods against different neuropathologies, e.g., post-concussed athletes, individuals with Parkinson’s disease, or persons with musculoskeletal impairments. As noted in the discussion, several methods have been described for determining the tolerance window so it may be useful to compare ApEn and SampEn values using the standard method we used and other accepted methods, e.g., a fixed tolerance window [56,57] and the method suggested by Chon et al. [54]. Our results may also have been influenced by the data resolution of the downsampling technique employed. Despite the limitations of this project, our method reports several unique features: (1) changing the tolerance window r, while constraining input parameter m (to a common previously used value); (2) comparing entropy values between a relatively stable standing posture to a significantly more unstable tandem standing posture; and (3) reporting results that appear to challenge pre-existing reports, i.e., ApEn as more biased and less relatively consistent.
6. Conclusions
The primary purpose of this study was to investigate the impact of varying only one of the input parameters, i.e., tolerance window, r, used to calculate ApEn and SampEn. We agree with previous research groups [38,52,61] who suggested that if r is multiplied by the standard deviation, then practitioners need to be aware that any factor that affects variation, e.g., data length, nonstationarity, spikes, and outliers, will likely affect the tolerance. Thus, researchers should try multiple r values, examine the relative consistency of the entropy values, and provide their findings in Supplementary Material. However, since we have shown that ApEn and SampEn values were consistent across several values of r, we are confident that the algorithms r = 0.20, r = 2.5, or r = 0.30 × SD may be equally suitable for characterizing the COP time-series excursions in the anterior–posterior and medial–lateral excursions for bipedal feet together and tandem quiet standing postures. Ours is the first study to examine the non-linear dynamics of the center of pressure for tandem standing, a test we believe might be particularly useful for testing patients with neuropathology, e.g., athletes post-concussion. This recommendation is based on the good relative consistency of the entropy values that we reported with changing tolerance windows, and the ability of both ApEn and SampEn to distinguish more from less stable quiet standing postures. Our results demonstrated that although, on average, ApEn values were larger than SampEn values, one estimation technique was not particularly “better” than the other; that is, if used in longitudinal measures of COP time series either entropy algorithm would be acceptable. In other words, ApEn did not appear more biased, and both ApEn and SampEn exhibited relative consistency. Finally, based on the consistency of our results using a COP time series that was downsampled, we suggest that collecting ground reaction forces at 60 Hz would provide a sufficient data set for future laboratory and clinical testing. Finally, based on the consistency of our results using a COP time series that was downsampled, we suggest that collecting ground reaction forces at 60 Hz would provide a sufficient data set for future laboratory and clinical testing.
Author Contributions
Conceptualization, J.W., S.R. and G.A.; Methodology, J.W., S.R. and G.A.; Software, J.W.; Validation, J.W.; Formal Analysis, J.W.; S.R., G.A. and D.W.Z.; Resources, G.A.; Data curation, J.W. and S.R.; Writing (original draft)—J.W.; Writing (review and editing), J.W., S.R., G.A. and D.W.Z.; Visualization—J.W., S.R. and D.W.Z.; Supervision, S.R., G.A. and D.W.Z.; Project administration, S.R. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki, and approved by the Institutional Review Board of Grand Valley State University (18-246-H and 9 March 2020) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to restrictions imposed by our institution on retired faculty.
Acknowledgments
We acknowledge University administrative support for the use of equipment and materials needed for motion capture in the Biomechanics and Motor Performance Laboratory and all study participants.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Approximate and sample entropy values for each participant for the anterior–posterior center-of-pressure time series for eyes open feet together.
Table A1.
Approximate and sample entropy values for each participant for the anterior–posterior center-of-pressure time series for eyes open feet together.
| Participants | Method | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
|---|---|---|---|---|---|---|---|
| 1 | Approximate | 0.49(0.053) | 0.3(0.054) | 0.18(0.037) | 0.13(0.025) | 0.094(0.019) | 0.075(0.015) |
| Sample | 0.43(0.058) | 0.25(0.052) | 0.16(0.037) | 0.11(0.027) | 0.087(0.021) | 0.07(0.017) | |
| 2 | Approximate | 0.53(0.026) | 0.39(0.059) | 0.25(0.052) | 0.18(0.036) | 0.13(0.026) | 0.1(0.02) |
| Sample | 0.48(0.039) | 0.32(0.055) | 0.21(0.045) | 0.15(0.033) | 0.12(0.025) | 0.096(0.02) | |
| 4 | Approximate | 0.98(0.059) | 0.59(0.064) | 0.37(0.047) | 0.26(0.036) | 0.19(0.028) | 0.15(0.021) |
| Sample | 1(0.09) | 0.51(0.071) | 0.32(0.052) | 0.22(0.039) | 0.17(0.03) | 0.13(0.023) | |
| 5 | Approximate | 0.55(0.11) | 0.37(0.076) | 0.24(0.06) | 0.16(0.041) | 0.12(0.03) | 0.097(0.023) |
| Sample | 0.49(0.13) | 0.29(0.068) | 0.19(0.048) | 0.14(0.034) | 0.11(0.026) | 0.087(0.02) | |
| 6 | Approximate | 0.46(0.053) | 0.28(0.064) | 0.17(0.042) | 0.12(0.028) | 0.088(0.02) | 0.071(0.017) |
| Sample | 0.37(0.049) | 0.22(0.052) | 0.14(0.036) | 0.1(0.026) | 0.08(0.02) | 0.066(0.017) | |
| 8 | Approximate | 0.51(0.039) | 0.33(0.084) | 0.22(0.074) | 0.15(0.053) | 0.11(0.039) | 0.09(0.029) |
| Sample | 0.44(0.062) | 0.27(0.066) | 0.18(0.056) | 0.13(0.043) | 0.1(0.034) | 0.082(0.027) |
Note: Mean (standard deviation) entropy values for each participant across six different threshold windows (r).

Table A2.
Approximate and sample entropy values for each participant for the anterior–posterior center-of-pressure time series for eyes open tandem dominant back.
Table A2.
Approximate and sample entropy values for each participant for the anterior–posterior center-of-pressure time series for eyes open tandem dominant back.
| Participants | Method | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
|---|---|---|---|---|---|---|---|
| 1 | Approximate | 0.55(0.0092) | 0.45(0.019) | 0.33(0.013) | 0.24(0.011) | 0.18(0.0075) | 0.14(0.0049) |
| Sample | 0.49(0.03) | 0.37(0.035) | 0.27(0.026) | 0.2(0.019) | 0.16(0.014) | 0.13(0.01) | |
| 2 | Approximate | 0.6(0.017) | 0.53(0.031) | 0.42(0.047) | 0.32(0.05) | 0.25(0.046) | 0.2(0.039) |
| Sample | 0.6(0.028) | 0.46(0.031) | 0.35(0.037) | 0.27(0.036) | 0.21(0.034) | 0.17(0.03) | |
| 4 | Approximate | 0.88(0.097) | 0.6(0.094) | 0.45(0.091) | 0.34(0.08) | 0.26(0.064) | 0.2(0.052) |
| Sample | 0.92(0.15) | 0.55(0.11) | 0.4(0.096) | 0.3(0.081) | 0.24(0.066) | 0.19(0.054) | |
| 5 | Approximate | 0.59(0.048) | 0.49(0.039) | 0.37(0.039) | 0.27(0.031) | 0.21(0.024) | 0.16(0.019) |
| Sample | 0.57(0.048) | 0.41(0.057) | 0.3(0.051) | 0.23(0.04) | 0.18(0.031) | 0.14(0.024) | |
| 6 | Approximate | 0.56(0.02) | 0.48(0.031) | 0.34(0.036) | 0.25(0.03) | 0.18(0.024) | 0.15(0.018) |
| Sample | 0.53(0.027) | 0.41(0.031) | 0.29(0.029) | 0.22(0.025) | 0.17(0.021) | 0.13(0.016) | |
| 8 | Approximate | 0.59(0.0059) | 0.52(0.02) | 0.4(0.029) | 0.3(0.026) | 0.22(0.021) | 0.18(0.017) |
| Sample | 0.57(0.011) | 0.45(0.016) | 0.34(0.022) | 0.25(0.02) | 0.2(0.016) | 0.16(0.013) |
Note: Mean (standard deviation) entropy values for each participant across six different threshold windows (r).

Table A3.
Approximate and sample entropy values for each participant for the anterior–posterior center-of-pressure time series for eyes closed tandem dominant back.
Table A3.
Approximate and sample entropy values for each participant for the anterior–posterior center-of-pressure time series for eyes closed tandem dominant back.
| Participants | Method | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
|---|---|---|---|---|---|---|---|
| 1 | Approximate | 0.58(0.0071) | 0.53(0.015) | 0.41(0.02) | 0.31(0.025) | 0.24(0.022) | 0.19(0.02) |
| Sample | 0.57(0.021) | 0.46(0.018) | 0.35(0.019) | 0.27(0.019) | 0.21(0.017) | 0.17(0.015) | |
| 2 | Approximate | 0.58(0.023) | 0.49(0.039) | 0.37(0.037) | 0.27(0.031) | 0.2(0.025) | 0.16(0.02) |
| Sample | 0.55(0.04) | 0.42(0.045) | 0.31(0.039) | 0.23(0.03) | 0.18(0.023) | 0.14(0.018) | |
| 4 | Approximate | 0.58(0.012) | 0.5(0.033) | 0.39(0.042) | 0.29(0.041) | 0.22(0.035) | 0.17(0.029) |
| Sample | 0.57(0.023) | 0.44(0.031) | 0.34(0.041) | 0.26(0.039) | 0.2(0.033) | 0.16(0.028) | |
| 5 | Approximate | 0.58(0.023) | 0.54(0.025) | 0.44(0.025) | 0.35(0.021) | 0.27(0.018) | 0.22(0.016) |
| Sample | 0.58(0.053) | 0.48(0.048) | 0.37(0.042) | 0.29(0.033) | 0.23(0.025) | 0.19(0.019) | |
| 6 | Approximate | 0.59(0.0099) | 0.54(0.026) | 0.42(0.033) | 0.31(0.032) | 0.24(0.026) | 0.19(0.021) |
| Sample | 0.59(0.014) | 0.47(0.018) | 0.36(0.021) | 0.27(0.021) | 0.21(0.018) | 0.17(0.016) | |
| 8 | Approximate | 0.6(0.0069) | 0.56(0.02) | 0.45(0.037) | 0.34(0.039) | 0.26(0.034) | 0.21(0.027) |
| Sample | 0.61(0.017) | 0.49(0.025) | 0.38(0.034) | 0.3(0.033) | 0.23(0.029) | 0.19(0.023) |
Note: Mean (standard deviation) entropy values for each participant across six different threshold windows (r).

Table A4.
Approximate and sample entropy values for each participant for the medial–lateral center-of-pressure time series for eyes open feet together.
Table A4.
Approximate and sample entropy values for each participant for the medial–lateral center-of-pressure time series for eyes open feet together.
| Participants | Method | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
|---|---|---|---|---|---|---|---|
| 1 | Approximate | 0.27(0.054) | 0.11(0.025) | 0.07(0.014) | 0.05(0.0093) | 0.039(0.0071) | 0.032(0.0059) |
| Sample | 0.22(0.05) | 0.1(0.022) | 0.063(0.012) | 0.046(0.0082) | 0.036(0.0063) | 0.029(0.0052) | |
| 2 | Approximate | 0.46(0.054) | 0.25(0.059) | 0.15(0.037) | 0.11(0.024) | 0.082(0.018) | 0.066(0.015) |
| Sample | 0.38(0.064) | 0.21(0.05) | 0.13(0.03) | 0.093(0.02) | 0.073(0.015) | 0.059(0.012) | |
| 4 | Approximate | 1(0.12) | 0.63(0.13) | 0.41(0.1) | 0.28(0.077) | 0.21(0.06) | 0.17(0.048) |
| Sample | 1.1(0.16) | 0.57(0.11) | 0.36(0.073) | 0.25(0.054) | 0.19(0.041) | 0.15(0.033) | |
| 5 | Approximate | 0.57(0.031) | 0.39(0.055) | 0.25(0.051) | 0.17(0.037) | 0.13(0.028) | 0.1(0.021) |
| Sample | 0.52(0.034) | 0.33(0.048) | 0.21(0.04) | 0.15(0.029) | 0.12(0.022) | 0.094(0.017) | |
| 6 | Approximate | 0.49(0.064) | 0.32(0.12) | 0.21(0.095) | 0.15(0.068) | 0.11(0.05) | 0.088(0.038) |
| Sample | 0.42(0.075) | 0.27(0.093) | 0.18(0.075) | 0.13(0.056) | 0.1(0.043) | 0.081(0.034) | |
| 8 | Approximate | 0.51(0.042) | 0.32(0.067) | 0.2(0.047) | 0.14(0.031) | 0.11(0.022) | 0.084(0.017) |
| Sample | 0.45(0.045) | 0.27(0.052) | 0.17(0.037) | 0.12(0.026) | 0.096(0.02) | 0.077(0.016) |
Note: Mean (standard deviation) entropy values for each participant across six different threshold windows (r).

Table A5.
Approximate and sample entropy values for each participant for the medial–lateral center-of-pressure time series for eyes open tandem dominant back.
Table A5.
Approximate and sample entropy values for each participant for the medial–lateral center-of-pressure time series for eyes open tandem dominant back.
| Participants | Method | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
|---|---|---|---|---|---|---|---|
| 1 | Approximate | 0.52(0.042) | 0.37(0.057) | 0.26(0.051) | 0.19(0.045) | 0.14(0.036) | 0.11(0.028) |
| Sample | 0.45(0.068) | 0.29(0.065) | 0.2(0.049) | 0.14(0.036) | 0.11(0.028) | 0.091(0.022) | |
| 2 | Approximate | 0.66(0.058) | 0.61(0.047) | 0.54(0.071) | 0.45(0.087) | 0.37(0.092) | 0.31(0.086) |
| Sample | 0.71(0.11) | 0.55(0.078) | 0.46(0.081) | 0.38(0.08) | 0.31(0.074) | 0.26(0.067) | |
| 4 | Approximate | 1.3(0.046) | 1.1(0.11) | 0.86(0.097) | 0.68(0.079) | 0.55(0.068) | 0.46(0.06) |
| Sample | 1.7(0.17) | 1.1(0.14) | 0.79(0.11) | 0.61(0.086) | 0.49(0.072) | 0.4(0.06) | |
| 5 | Approximate | 0.81(0.22) | 0.69(0.14) | 0.57(0.13) | 0.48(0.13) | 0.4(0.13) | 0.34(0.12) |
| Sample | 0.91(0.32) | 0.64(0.17) | 0.5(0.14) | 0.4(0.13) | 0.33(0.11) | 0.28(0.1) | |
| 6 | Approximate | 0.58(0.032) | 0.46(0.058) | 0.33(0.072) | 0.24(0.063) | 0.18(0.051) | 0.14(0.04) |
| Sample | 0.56(0.052) | 0.4(0.062) | 0.28(0.059) | 0.21(0.05) | 0.16(0.04) | 0.13(0.033) | |
| 8 | Approximate | 0.66(0.058) | 0.55(0.084) | 0.44(0.11) | 0.35(0.11) | 0.27(0.091) | 0.22(0.075) |
| Sample | 0.67(0.1) | 0.48(0.094) | 0.37(0.097) | 0.28(0.087) | 0.22(0.074) | 0.18(0.063) |
Note: Mean (standard deviation) entropy values for each participant across six different threshold windows (r).

Table A6.
Approximate and sample entropy values for each participant for the medial–lateral center-of-pressure time series for eyes closed tandem dominant back.
Table A6.
Approximate and sample entropy values for each participant for the medial–lateral center-of-pressure time series for eyes closed tandem dominant back.
| Participants | Method | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
|---|---|---|---|---|---|---|---|
| 1 | Approximate | 0.65(0.033) | 0.57(0.056) | 0.47(0.085) | 0.37(0.09) | 0.29(0.083) | 0.23(0.07) |
| Sample | 0.67(0.061) | 0.51(0.068) | 0.4(0.083) | 0.31(0.081) | 0.25(0.073) | 0.2(0.063) | |
| 2 | Approximate | 0.65(0.046) | 0.59(0.068) | 0.52(0.088) | 0.44(0.089) | 0.37(0.082) | 0.31(0.071) |
| Sample | 0.69(0.085) | 0.54(0.092) | 0.44(0.099) | 0.36(0.094) | 0.29(0.083) | 0.25(0.071) | |
| 4 | Approximate | 0.73(0.058) | 0.65(0.035) | 0.59(0.043) | 0.52(0.059) | 0.44(0.068) | 0.38(0.071) |
| Sample | 0.82(0.092) | 0.63(0.05) | 0.52(0.052) | 0.44(0.059) | 0.37(0.062) | 0.31(0.062) | |
| 5 | Approximate | 0.63(0.028) | 0.59(0.056) | 0.51(0.094) | 0.43(0.11) | 0.36(0.11) | 0.3(0.1) |
| Sample | 0.68(0.064) | 0.53(0.076) | 0.42(0.1) | 0.34(0.11) | 0.28(0.1) | 0.24(0.094) | |
| 6 | Approximate | 0.63(0.038) | 0.56(0.051) | 0.45(0.064) | 0.35(0.063) | 0.27(0.056) | 0.21(0.047) |
| Sample | 0.63(0.07) | 0.48(0.071) | 0.37(0.069) | 0.29(0.058) | 0.23(0.049) | 0.18(0.041) | |
| 8 | Approximate | 0.66(0.076) | 0.56(0.13) | 0.46(0.15) | 0.38(0.14) | 0.31(0.13) | 0.25(0.11) |
| Sample | 0.7(0.13) | 0.5(0.14) | 0.39(0.14) | 0.31(0.12) | 0.25(0.11) | 0.21(0.093) |
Note: Mean (standard deviation) entropy values for each participant across six different threshold windows (r).
References
- Delgado-Banal, A.; Marshak, A. Approximate entropy and sample entropy: A comprehensive tutorial. Entropy 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- St-Amant, G.; Rahman, T.; Polskaia, N.; Fraser, S.; Lojoie, Y. Unveiling the cerebral and sensory contributions to automatic postural control during dual-task standing. Hum. Mov. Sci. 2020, 70, 102587. [Google Scholar] [CrossRef]
- Borg, F.G.; Laxåback, G. Entropy of balance—Some recent results. J. Neuroeng. Rehabil. 2010, 7, 38. [Google Scholar] [CrossRef]
- Richer, N.; Lojoie, Y. Automaticity of postural control while dual-tasking revealed in young and older adults. Exp. Aging Res. 2020, 46, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, M.; Signorini, M.G.; Magenes, G.; Cerutti, A. Comparison of entropy-based regularity estimators: Application of the fetal heart rate signal for the identification of fetal distress. IEEE Trans. Biomed. Eng. 2006, 53, 119–125. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M.; Viscarello, R.R. Approximate entropy: A regularity measure for fetal heart rate analysis. Obstet. Gynecol. 1992, 79, 249–255. [Google Scholar] [PubMed]
- Chen, X.; Solomon, I.; Chon, K. Comparison of the use of approximate entropy and sample entropy: Applications to neural respiratory signal. In Proceedings of 27th Annual International Conference of the IEEE, Shanghai, China, 17–18 January 2006. [Google Scholar]
- Estrada, L.; Torres, A.; Sarlabous, L.; Jané, R. Influence of parameter selection in fixed sample entropy of surface diaphragm electromyography for estimating respiratory activity. Entropy 2017, 19, 460. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Stergiou, N. A nonlinear dynamic approach for evaluating postural control: New directions for the management of sport-related cerebral concussion. Sports Med. 2005, 35, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Wei, Q.; Shieh, J.S.; Fourcade, P.; Isableu, B.; Majed, L. Sample entropy, univariate, and multivariate multi-scale entropy in comparison with classical postural sway parameters in young healthy adults. Front. Hum. Neurosci. 2017, 11, 206. [Google Scholar] [CrossRef]
- Lubetzky, A.V.; Harel, D.; Lubetzky, E. On the effects of signal processing on sample entropy for postural control. PLoS ONE 2018, 13, e0193460. [Google Scholar] [CrossRef]
- Ramdani, S.; Seigle, B.; Lagarde, J.; Bouchara, F.; Bernard, P.L. On the use of sample entropy to analyze human postural sway data. Med. Eng. Phys. 2009, 31, 1023–1031. [Google Scholar] [CrossRef]
- Reilly, N.; Prebor, J.; Moxey, J.; Schusler, E. Chronic impairments of static postural stability associated with history of concussion. Exp. Brain Res. 2020, 238, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Rhea, C.K.; Silver, T.A.; Hong, S.L.; Ryu, J.H.; Studenka, B.E.; Hughes, C.M.L.; Haddad, J.M. Noise and complexity in human postural control: Interpreting the different estimations of entropy. PLoS ONE 2011, 6, e17696. [Google Scholar] [CrossRef] [PubMed]
- Yentes, J.M.; Denton, W.; McCamley, J.; Raffalt, P.C.; Schmid, K.K. Effect of parameter selection on entropy calculations for long walking trials. Gait Posture 2018, 60, 128–134. [Google Scholar] [CrossRef]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.S.; Stergiou, N. Recovery of postural control after cerebral concussion: New insights using approximate entropy. J. Athl. Train. 2006, 41, 305–313. [Google Scholar] [PubMed] [PubMed Central]
- Cavanaugh, J.T.; Mercer, V.S.; Stergiou, N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: A methodological report. J. Neuroeng. Rehabil. 2007, 4, 42. [Google Scholar] [CrossRef]
- Saraiva, M.; Vilas-Boas, J.P.; Fernandes, O.J.; Castro, M.A. Effects of motor task difficulty on postural control complexity during dual tasks in young adults: A nonlinear approach. Sensors 2023, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Tipton, N.; Alderink, G.; Rhodes, S. Approximate entropy and velocity of center of pressure to determine postural stability: A pilot study. Appl. Sci. 2023, 13, 9259. [Google Scholar] [CrossRef]
- Grassberger, P.; Procaccia, I. Estimation of the Kolmogorov entropy from a chaotic signal. Phys. Rev. A 1983, 28, 2591–2593. [Google Scholar] [CrossRef]
- Takens, F. Invariants related to dimension and entropy. In Atlas do 13 Coloquio Brasileiro de Matematica; Instituto de Matemática Pura e Aplicada: Rio de Janeiro, Brazil, 1983. [Google Scholar]
- Grasberger, P.; Procaccia, I. Dimensions and entropies of strange attractors from a fluctuating dynamics approach. Phys. D 1984, 13, 34–54. [Google Scholar] [CrossRef]
- Cohen, A.; Procaccia, I. Computing the Kolmogorov entropy from time signals of dissipative and conservative dynamical systems. Phys. Rev. A 1985, 31, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, J.P.; Ruelle, D. Ergodic theory of chaos and strange attractors. Rev. Mod. Phys. 1985, 57, 617–656. [Google Scholar] [CrossRef]
- Schouten, J.C.; Takens, F.; van den Bleek, C.M. Maximum-likelihood estimation of the entropy of an attractor. Phys. Rev. E 1994, 49, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, P.; Schreiber, T.; Schaffrath, C. Nonlinear time sequence analysis. Inc. J. Bifurcat. Chaos 1991, 1, 521–547. [Google Scholar] [CrossRef]
- Pincus, S.M. Quantifying complexity and regularity of neurological systems. Methods Neurosci. 1995, 28, 336–363. [Google Scholar] [CrossRef]
- Perlmutter, S.; Lin, F.; Makhsous, M. Quantitative analysis of static sitting posture in chronic stroke. Gait Posture 2010, 32, 53–56, Corrigendum in Gait Posture 2011, 33, 510. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, P.; Di Rienzo, M. How the threshold “r” influences approximate entropy analysis of heart-rate variability. In Proceedings of the 2008 Computers in Cardiology, Bologna, Italy, 14–17 September 2008; Volume 35, pp. 561–564. [Google Scholar]
- Tononi, G.; Edelman, G.M.; Sporns, O. Complexity and coherency: Integrating information in the brain. Trends Cogn. Sci. 1998, 2, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Lehnertz, K.; Elger, C.E. Spatio-temporal dynamics of the primary epileptogenic area in temporal lobe epilepsy characterized by neuronal complexity loss. Electroencephalogr. Clin. Neurophysiol. 1995, 95, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Pro Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Shao, P.; Li, L.; Sun, X.; Wang, X.; Liu, F. Comparison of different threshold values r for approximate entropy: Application to investigate the heart rate variability between heart failure and healthy control groups. Physiol. Meas. 2011, 32, 167–180. [Google Scholar] [CrossRef]
- Pincus, S. Approximate entropy (ApEn) as a complexity measure. Chaos 1995, 5, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Sternad, D. Complexity of human postural control in young and older adults during prolonged standing. Exp. Brain Res. 2008, 191, 265–276. [Google Scholar] [CrossRef]
- Yentes, J.M.; Raffalt, P.C. Entropy analysis in gait research: Methodological considerations and recommendations. Ann. Biomed. Eng. 2021, 49, 979–990. [Google Scholar] [CrossRef]
- Lu, S.; Chen, X.; Kanters, J.; Solomon, I.C.; Chon, K.H. Automatic selection of the threshold value r for approximate entropy. IEEE Trans. Biomed. Eng. 2008, 55, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W.; Szekely, B.; Blackmore, S.E.; Nelson, A.; Schallert, A.; Lester, D.B.; Murray, N.G.; Puppa, M. Effects of sampling rate and movement frequency on entropic measures of regularity. J. Nat. Sci. 2018, 4, e504. [Google Scholar]
- Rhea, C.K.; Kiefer, A.W.; Wright, W.G.; Raisbeck, L.D.; Haran, F.J. Interpretation of postural control may change due to data processing techniques. Gait Posture 2015, 41, 731–735. [Google Scholar] [CrossRef] [PubMed]
- McCrumb, D.D. Analysis of Connectivity in EMG Signals to Examine Neural Correlations in Muscular Activation of Lower Leg Muscles for Postural Stability. Master’s Thesis, Grand Valley State University, Grand Rapids, MI, USA, 2019. [Google Scholar]
- Combined Output from Multiple Force Plates—Nexus 2.6 Documentation—Vicon Documentation. Available online: https://docs.vicon.com/display/Nexus26/Combined+output+from+multiple+force+plates (accessed on 17 May 2021).
- Exell, T.; Kerwin, D.; Irwin, G.; Gittoes, M. Calculating Centre of Pressure from Multiple Force Plates for Kinetic Analyses of Sprint Running. Port. J. Sport Sci. 2011, 11, 4. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 10 June 2024).
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2024; Available online: https://www.posit.co/ (accessed on 10 June 2024).
- Potvin-Desrochers, A.; Richer, N.; Lajoie, Y. Cognitive tasks promote automatization of postural control in young and older adults. Gait Posture 2017, 57, 40–45. [Google Scholar] [CrossRef]
- Quatman-Yates, C.C.; Bonnette, S.; Hugentobler, J.A.; Médé, B.; Kiefer, A.W.; Kurowski, B.G.; Riley, M.A. Postconcussion postural sway variability changes in youth: The benefit of structural variability analyses. Pediatr. Phys. Ther. 2015, 27, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Senthinathan, A.; Mainwaring, L.M.; Hutchinson, M. Heart rate variability of athletes across concussion recovery milestones: A preliminary study. Clin. J. Sport. Med. 2017, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Brink, K.J.; McKenzie, K.L.; Likens, A.D. Nonlinear analyses distinguish load carriage dynamics in walking and standing: A systematic review. J. Appl. Biomech. 2022, 38, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Barbado Murillo, D.; Sabido Solana, R.; Vera-Garcia, F.J.; Gusi Fuertes, N.; Moreno, F.J. Effect of increasing difficulty in standing balance tasks with visual feedback on postural sway and EMG: Complexity and performance. Hum. Mov. Sci. 2012, 31, 1224–1237. [Google Scholar] [CrossRef]
- Montesinos, L.; Castaldo, R.; Pecchia, L. On the use of approximate entropy and sample entropy with center of pressure time-series. J. Neuroeng. Rehabil. 2018, 15, 116. [Google Scholar] [CrossRef]
- Rose, M.H.; Bandholm, T.; Jensen, B.R. Approximate entropy based on attempted steady isometric contractions with the ankle dorsal- and plantarflexors: Reliability and optimal sampling frequency. J. Neurosci. Methods 2009, 177, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.; Scully, C.G.; Lu, S. Approximate entropy for all signals. IEEE Open J. Eng. Med. Biol. 2009, 28, 18–23. [Google Scholar] [CrossRef]
- Lake, D.E.; Richman, J.S.; Griffin, M.P.; Moorman, J.R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R789–R797. [Google Scholar] [CrossRef]
- Forrest, S.M.; Challis, J.H.; Winter, S.L. The effect of signal acquisition and processing choices on ApEn values: Towards a “gold standard” for distinguishing effort levels from isometric force records. Med. Eng. Phys. 2014, 36, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Sarlabous, L.; Torres, A.; Fiz, J.A.; Gea, J.; Martinez-Llorens, J.M.; Morera, J.; Jane, R. Interpretation of the approximate entropy using fixed tolerance values as a measure of amplitude variations in biomedical signals. In Proceedings of 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5967–5970. [Google Scholar]
- Donker, S.F.; Roerdink, M.; Greven, A.J.; Beek, P.J. Regularity of center-of-pressure trajectories depends on the amount of attention invested in postural control. Exp. Brain Res. 2007, 181, 1–11. [Google Scholar] [CrossRef]
- Molina-Picó, A.; Cuesta-Frau, D.; Aboy, M.; Crespo, C.; Miró-Martinez, P.; Oltra-Crespo, S. Comparative study of approximate entropy and sample entropy robustness to spikes. Artif. Intell. Med. 2011, 53, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Berstein, N. The Coordination and Regulation of Movements, 1st ed.; Pergamon Press Ltd.: Oxford, UK, 1967. [Google Scholar]
- Latash, M.L.; Scholz, J.P.; Schöner, G. Motor control strategies revealed in the structure of motor variability. Exerc. Sport. Sci. Rev. 2002, 30, 26–31. [Google Scholar] [CrossRef]
- Markovic, G.; Mikulic, P.; Kern, H.; Sarabon, N. Intra-session reliability of traditional and non-linear time-series posturographic measures in a semi-tandem stance: A reference to age. Measurement 2014, 51, 124–132. [Google Scholar] [CrossRef]
- Sabatini, A.M. Analysis of postural sway using entropy measures of signal complexity. Med. Biol. Eng. Comput. 2000, 38, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.; Stergiou, N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br. J. Sports Med. 2005, 39, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Mengarelli, A.; Verdini, F.; Cardarelli, S.; Tigrini, A.; Strazza, A.; Di Nardo, F.; Rabini, R.A.; Mercante, O.; Fioretti, S. Complexity measures of postural control in Type-2 diabetic patients. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3527–3530. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of complex physiological time series. Phys. Rev. Lett. 2002, 89, 6–9. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E. 2005, 71, 021906. [Google Scholar] [CrossRef]
- Gow, B.J.; Peng, C.-K.; Wayne, P.M.; Ahn, A.C. Multiscale entropy analysis of center-of-pressure dynamics in human postural control: Methodological considerations. Entropy 2015, 17, 7926–7947. [Google Scholar] [CrossRef]
- Costa, M.; Priplata, A.A.; Lipsitz, L.A.; Wu, Z.; Huang, N.E.; Goldberger, A.L.; Peng, C.-K. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Eurosci Lett. 2007, 77, 68008. [Google Scholar] [CrossRef]
- Mengarelli, A.; Tigrini, A.; Verdini, F.; Rabini, R.A.; Fioretti, S. Multiscale fuzzy entropy analysis of balance: Evidence of scale-dependent dynamics on diabetic patients with and without neuropathy. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1462–1471. [Google Scholar] [CrossRef]
- Busa, M.A.; Jones, S.L.; Hamill, J.; van Emmerik, R.E.A. Multiscale entropy identifies differences in complexity in postural control in women with multiple sclerosis. Gait Posture 2016, 45, 7–11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).