Abstract
Background: Postural control in healthy young adults involves complex neuromuscular processes; however, the kinematic and kinetic consequences of small, forward leg perturbations in a defined population are not fully described. This study aimed to characterize the kinematic and kinetic consequences of forward leg perturbations during quiet standing. Methods: This investigation used a quasi-experimental repeated-measures design. Sixteen healthy young women (20.1 ± 0.7 years), all right-leg dominant, were tested using the Gait Real-Time Analysis Interactive Laboratory (GRAIL) system. Forward treadmill perturbations were applied to each limb during quiet standing, and joint angles, ground reaction forces, and torques were measured across baseline, perturbation, and response phases. As the data were non-normally distributed, paired comparisons were conducted using the Wilcoxon test, with significance set at p < 0.05 (Bonferroni corrected) and effect sizes (r) reported. Results: Joint angles remained symmetrical between limbs (no significant differences after correction). In contrast, kinetic measures showed clear asymmetries: at baseline, the dominant limb produced greater knee torque (p = 0.0003, r = 0.73), ankle torque (p = 0.0003, r = 0.76), and medio-lateral GRF (p = 0.0003, r = 0.87). During perturbation, it again generated higher knee (p = 0.0036, r = 0.43) and ankle torques (p = 0.0003, r = 0.53), with larger medio-lateral GRF (p = 0.0003, r = 0.87). In the response phase, the dominant limb showed greater hip torque (p = 0.0033, r = 0.43) and a small dorsiflexion shift at the ankle (p = 0.0066, r = 0.41). Anterior–posterior GRF changes were minor and non-significant after correction. Conclusions: Induced forward leg movements caused limb-specific kinetic adjustments while maintaining overall kinematic symmetry. The dominant leg contributed more actively to balance recovery, highlighting its role in stabilizing posture under small perturbations. These findings are specific to the studied demographic and should not be generalized to males, older adults, left-dominant individuals, or clinical populations without further research.
1. Introduction
Postural control in healthy young adults is a sophisticated and adaptive process that relies on the integration of sensory information, neural coordination, and muscular activation to maintain stability during both static and dynamic states [1,2]. While traditional research emphasized static postural mechanisms, dynamic lower-limb movements, such as induced forward leg motions, have been shown to significantly influence balance and stability [3,4]. Understanding these effects is important for fall prevention, rehabilitation after lower-limb injuries, and the ergonomic design of assistive devices.
Even subtle modifications in lower-limb positioning were shown to cause measurable shifts in the center of pressure, highlighting the sensitivity of postural control systems to foot and leg configuration [5]. Recent studies confirmed that leg dominance modulates these responses, with dominant and non-dominant limbs employing distinct strategies across movement planes [6,7,8,9,10,11]. Contemporary findings also highlight that dominance-related asymmetries can impact balance control and injury risk in athletic and clinical populations [7,12,13].
Induced forward leg movements were identified as a form of dynamic perturbation requiring the musculoskeletal system to rapidly recalibrate joint angles, muscle activation, and ground reaction forces to preserve equilibrium [4]. Compensatory responses occur at the ankle, knee, and hip, involving both reflexive mechanisms and anticipatory postural adjustments (APAs), reflecting the integrated reactive and predictive control of posture [14]. Recent evidence suggests that APAs are not only robust but also adaptable to perturbation type and task context [14,15]. Nevertheless, the specific kinematic and kinetic consequences of controlled forward-directed leg movements during quiet standing remain insufficiently characterized.
New investigations have shown that perturbation-based paradigms provide valuable insight into adaptive balance strategies [16,17,18,19]. Gulatowska and Błażkiewicz [4] reported that repetitive mechanical perturbations during standing did not substantially disrupt lower-limb symmetry, as only minor asymmetries were observed in ankle angle, hip torque, and vertical ground reaction forces, indicating the robustness of neuromuscular control even under external disturbances. Similarly, Moriyama et al. [15] demonstrated that anticipatory postural adjustments preceded goal-directed lower-limb actions, such as simulated kicking, and that these adjustments could be adapted over time in response to visuomotor perturbations, highlighting the resilience and plasticity of anticipatory mechanisms. Yet, most prior studies have focused on either large external perturbations [20,21] or general sway, leaving a gap in understanding subtle but clinically relevant forward leg motions. Addressing this gap is essential, since small perturbations are more common in daily life than large disturbances, and the ability to adapt to them is closely linked to fall risk and rehabilitation outcomes [20,21,22].
The aim of this study was to evaluate the effects of induced forward leg movements on joint angles, ground reaction forces, and joint torques in healthy young right-leg-dominant women. It was expected that the dominant leg would generate higher torque and force during balance recovery. The study was conducted on a small sample of 16 participants, which limits generalizability to men, older adults, left-dominant individuals, or people with disabilities. Through this approach, deeper insights into dynamic postural control mechanisms are expected to be provided, and strategies for improving stability and fall prevention in both healthy and clinical populations are expected to be informed.
2. Materials and Methods
2.1. Participants and Selection Criteria
The sample size was estimated using GPower software (version 3.1.9.7, University of Kiel, Kiel, Germany), with an a priori Wilcoxon test. The calculation was based on the following parameters: significance level (α) set at 0.05, statistical power of 0.80, and an assumed effect size of 0.8, representing a large effect. According to these specifications, GPower indicated that a minimum of 16 participants was required.
Sixteen young women were recruited for the experiment (mean age: 20.06 ± 0.68 years; body mass: 67.75 ± 10.19 kg; body height: 168.25 ± 5.7 cm). Recruitment was carried out voluntarily through advertisements and university announcements, and participants did not receive financial or material compensation. Eligibility criteria included the absence of muscular or neurological conditions, no lower limb injuries within the previous six months, regular physical activity (at least twice per week), prior treadmill walking experience, and right-leg dominance. The requirement of engaging in regular physical activity (at least twice per week) was introduced to ensure a baseline level of motor competence and neuromuscular responsiveness among participants. Physically active individuals were less likely to exhibit confounding factors such as impaired coordination, reduced strength, or instability, which could have biased the interpretation of perturbation-related outcomes. This criterion was, therefore, intended to minimize variability and better isolate the specific effects of induced forward leg movements on postural control.
Leg dominance was verified with a ball-kicking test, during which participants identified their preferred kicking leg. All individuals demonstrated right-leg dominance, which is typical in the general population [23,24]. This ensured that the study sample reflected common motor patterns, while also facilitating protocol standardization and reducing variability related to limb dominance.
Exclusion factors involved a lack of experience with treadmill walking, impaired balance, or the use of medication affecting the nervous system. Before participation, each subject provided written informed consent. Ethical approval for the study was granted by the University Review Committee (approval no. SKE01-15/2023), and all procedures followed the principles of the Declaration of Helsinki.
This research followed a quasi-experimental repeated-measures design, in which each participant was exposed to standardized perturbations of both the dominant and non-dominant lower limb under controlled laboratory conditions.
2.2. Data Acquisition Procedure
The kinematic and kinetic variables of both perturbed and unperturbed quiet standing were assessed with the Gait Real-Time Analysis Interactive Laboratory (GRAIL, Motek Medical B.V., Amsterdam, The Netherlands). This comprehensive setup combines a dual-belt treadmill (length: 2200 mm, width: 2 × 500 mm) operating at a sampling frequency of 1000 Hz, a motion capture system (Vicon Metrics Ltd., Oxford, UK) equipped with ten Bonita cameras recording at 100 Hz, three video cameras, and synchronized virtual reality (VR) environments. The VR system consists of a circular projection screen (5 m in diameter, 3 m in height, and a 180° field of view) supported by three projectors, creating immersive and standardized experimental conditions (Figure 1A). Movement data were obtained using the Human Body Model 2 (HBM2), which relies on 25 reflective markers attached to participants for motion tracking (Figure 1B). To ensure safety, each participant was secured with a ceiling-mounted harness throughout the testing sessions. All tests were performed while participants wore their own athletic footwear, typically used for treadmill training or gym activities.
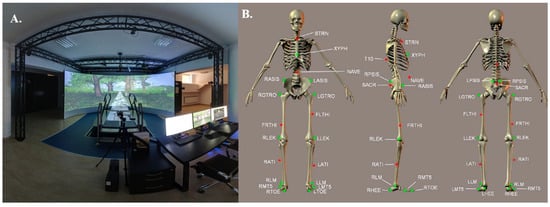
Figure 1.
(A) General view of the Gait Real-Time Analysis Laboratory (GRAIL, Motek Medical B.V., Amsterdam, The Netherlands). (B) Front, lateral, and posterior perspectives of the marker configuration applied in the Human Body Model (HBM). The green markers highlight anatomical reference points that serve to define the skeletal framework during the initialization phase. The main landmarks include: STRN (sternal notch), XYPH (xiphoid process), NAVE (umbilicus), T10 (10th thoracic vertebra), LASIS and RASIS (left and right anterior superior iliac spine), LPSIS and RPSIS (left and right posterior superior iliac spine), SACR (sacral midpoint between LPSIS and RPSIS), LGTRO and RGTRO (left and right greater trochanter), LFLTHI and RFLTHI (lateral mid-thigh between greater trochanter and lateral knee), LLEK and RLEK (lateral epicondyles of the knee), LATI and RATI (lateral mid-shank between knee and ankle), LLM and RLM (lateral malleoli), LMT5 and RMT5 (fifth metatarsals), LTOE and RTOE (toe tips), as well as LHEE and RHEE (heels) [25].
The GRAIL apparatus, managed via D-Flow software version 3.26 (Motek Medical B.V., Amsterdam, The Netherlands), was used to trigger perturbations, record data, and adjust the model parameters. Perturbations were induced by a sudden displacement of the treadmill belt beneath one of the participant’s legs. The experimental procedure involved two conditions of quiet standing: (1) standing still with eyes directed forward, during which the left treadmill belt shifted unexpectedly; (2) standing still with eyes directed forward, during which the right treadmill belt shifted unexpectedly. After each perturbation, participants were asked to return to their initial standing posture (Figure 2).
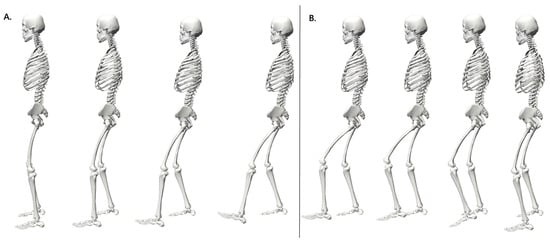
Figure 2.
Sequential representation of left lower limb response during perturbation: phases of treadmill belt acceleration (A) and recovery (B).
Each trial lasted approximately 68 s. During this time, four perturbations were applied at 10 s intervals, specifically at the 30th, 40th, 50th, and 60th seconds of the protocol (Figure 3A). Initially, the treadmill remained stationary in acceleration mode, and the perturbation intensity was set to level 5 on a five-point scale. Under this condition, the belt speed rapidly increased from ~0 m/s to 0.67 m/s. The duration of each perturbation was approximately 0.99 s (Figure 3B). This intensity was chosen because it delivers a standardized, moderate disturbance that is sufficient to elicit measurable compensatory responses while ensuring participant safety. Specifically, Siedlecki et al. [26] indicated that perturbations at treadmill belt speeds of approximately 0.62 m/s create balance disturbances in healthy adults that are larger, yet still recoverable. This magnitude simulates common, small environmental perturbations encountered in daily life, such as slips or missteps.
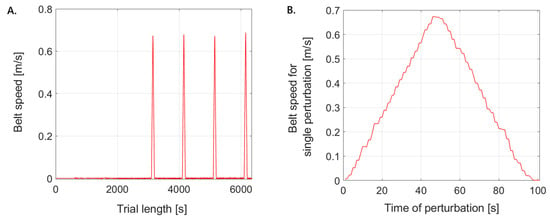
Figure 3.
Representation of treadmill speed modulation in the single-lane condition: (A) over the entire trial period and (B) at the onset of the first perturbation.
Because each leg was perturbed four times within a trial, potential habituation or learning effects were considered. To minimize this influence, the perturbations were applied with identical timing and standardized intensity for all participants. Importantly, no consistent trend suggesting adaptation between the first and last perturbation was observed in the recorded data. However, the order of perturbations was not randomized, which we acknowledge as a methodological limitation that may warrant further investigation in future studies.
Kinematic variables, including lower limb joint angles, and kinetic variables, such as joint torques and ground reaction forces, were captured with high accuracy, providing a detailed assessment of postural control in both perturbed and unperturbed states. The data were recorded using D-Flow 3.26 software, stored in .mox format, and subsequently imported into MATLAB 2021a (MathWorks, Natick, MA, USA) with the aid of a toolbox developed by Feldhege et al. [27].
For each participant, the peak values of all kinematic and kinetic measures were extracted from the two perturbation trials (left and right side), encompassing both the perturbed and contralateral limbs. Consequently, four peak values per parameter were obtained for a single lower limb (Figure 4). In addition, baseline values were determined as the mean over the interval 0–3000, i.e., prior to the first perturbation, separately for the right and left lower limb. The decision to focus on peak values was motivated by the aim of characterizing the maximal compensatory capacity of the neuromuscular system in response to perturbations. Peaks represent robust, reproducible indicators of the system’s limits in generating stabilizing forces and angles, while minimizing the influence of intra-trial noise and small baseline fluctuations.
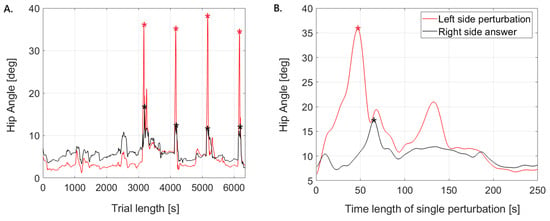
Figure 4.
Representative plots of hip joint angles. (A) shows the perturbed side (left limb) and the contralateral responding side (right limb) during a single trial; (B) illustrates the onset of the first perturbation and the corresponding response, with * marking the maximum peaks.
2.3. Statistical Analysis
Statistical analyses were conducted using Statistica v.12 software (StatSoft, Cracow, Poland), with the threshold for significance set at p < 0.05. Data normality was evaluated using the Shapiro–Wilk test. As the data were non-normally distributed, results are reported as medians with lower and upper quartiles, rather than means and standard deviations. To determine statistically significant differences, the Wilcoxon signed-rank test was applied for the following comparisons: (1) baseline kinematic and kinetic values for the left and right sides during quiet standing; (2) the maximum kinematic and kinetic peak values recorded during perturbations of the left and right lower limbs separately; (3) the maximum kinematic and kinetic peak values during the response to perturbations for each lower limb; and (4) changes between maximum kinematic and kinetic values and baseline during both perturbation and response phases.
To control for Type I error due to multiple comparisons, the significance threshold was adjusted using the Bonferroni correction. The corrected p-value threshold (p < 0.05/number of comparisons = 3) was applied for each set of related tests. Consequently, only differences with p-values below the adjusted threshold were considered statistically significant.
The effect size (r) for the Wilcoxon test was calculated for each comparison using the formula [28,29]:
where Z represents the test statistic from the Wilcoxon rank comparison, and N is the number of non-zero differences (i.e., the number of paired measurements showing a difference) [29]. Following Prajzner [30], effect sizes are interpreted as follows: small (0—0.30), medium (0.31—0.50), strong (0.51—0.70), and large (0.71—1).
All reported p-values in the Results section were compared against the Bonferroni-adjusted threshold. Marginal significances (p < 0.05 but above the corrected threshold) are interpreted with caution.
3. Results
3.1. Baseline Kinematic and Kinetic Differences Between the Dominant and Non-Dominant Lower Limb
All parameters showed non-normal distributions; therefore, non-parametric statistical tests (Wilcoxon signed-rank test with Bonferroni correction) were applied. The analysis revealed differences in kinematic and kinetic parameters between the non-dominant (left) and dominant (right) lower limbs during quiet standing.
For joint angles, hip, knee, and ankle values were highly comparable, with all differences remaining non-significant (Table 1). Although the knee showed slightly greater flexion on the non-dominant side (≈6%), the absolute difference was small and of limited practical relevance.

Table 1.
Median values (with lower and upper quartiles) of kinematic and kinetic parameters for the non-dominant (left) and dominant (right) lower limb during quiet standing. The last column reports the p-values from the Wilcoxon after Bonferroni correction, with * indicating statistically significant differences, along with the corresponding effect size.
In contrast, clear asymmetries were observed in joint torques. At the hip, the non-dominant limb generated a small extensor torque, whereas the dominant limb exhibited a small flexor torque. While the relative percentage difference appeared large, this was due to the very low absolute values; the more meaningful interpretation comes from the effect size (r = 0.44), which indicates a moderate difference. At the knee and ankle, the dominant limb produced significantly greater torques, with large effect sizes (r = 0.73 and r = 0.76, respectively), underscoring their practical importance.
Ground reaction forces (GRFs) also revealed asymmetries. The medio–lateral component differed markedly between limbs, with forces acting in opposite directions. This difference was highly significant (r = 0.87, large effect size). The proximal–distal component showed a smaller but significant asymmetry (r = 0.49, moderate effect size). By contrast, the anterior–posterior component did not remain significant after correction, and its effect size was small (r = 0.35), indicating limited practical relevance.
In summary, joint angles were largely symmetrical, with no statistically or practically meaningful differences. By contrast, kinetic parameters revealed robust asymmetries, particularly in knee and ankle torques and medio–lateral GRFs, where large effect sizes highlight the dominant limb’s more active role in stabilizing posture during quiet standing.
3.2. Comparative Study of Lower Limb Kinematics and Kinetics Under Perturbation for Dominant and Non-Dominant Leg
All variables showed non-normal distributions, with the exception of knee and ankle torque for the dominant limb and the right ankle angle. Therefore, non-parametric tests (Wilcoxon signed-rank with Bonferroni correction) were used for the main analyses. The analysis of the impact of perturbations showed that joint angles were largely symmetrical between the dominant and non-dominant limbs (Table 2). Differences in hip, knee, and ankle positions remained small in absolute terms and did not reach statistical significance after Bonferroni correction. Although some percentage differences appeared notable (e.g., hip ≈13%), they were based on small absolute values and, therefore, have limited practical relevance, as also reflected by the small effect sizes (r ≤ 0.22).

Table 2.
Median values (with lower and upper quartiles) of kinematic and kinetic parameters for the non-dominant (left) and dominant (right) lower limb during perturbations applied separately to the left and right leg. The last column reports the p-values from the Wilcoxon test after Bonferroni correction, with * indicating statistically significant differences, along with the corresponding effect size.
By contrast, kinetic parameters revealed meaningful asymmetries. At the hip, torque values were slightly higher in the dominant limb, but this difference was not statistically significant (r = 0.25, small-to-moderate effect). At the knee and ankle, the dominant limb generated significantly higher torques, and these differences remained robust after Bonferroni correction (p = 0.0036 and p = 0.0003, respectively). Importantly, both findings were associated with moderate-to-large effect sizes (r = 0.43 for the knee, r = 0.53 for the ankle), underscoring their practical significance.
The most pronounced asymmetry was observed in the medio–lateral GRF. The dominant limb produced substantially higher values, and this difference was highly significant after correction (p = 0.0003, r = 0.87, very large effect size). In contrast, differences in the proximal–distal and anterior–posterior GRF components were small in both absolute and relative terms and did not reach statistical significance.
In summary, while joint angles remained comparable between limbs during perturbations, kinetic parameters highlighted clear asymmetries. The dominant limb contributed more actively to postural stabilization, as evidenced by significantly greater knee and ankle torques and markedly higher medio–lateral GRFs. These findings emphasize that effect sizes, rather than percentage differences based on small values, provide a more reliable basis for interpreting practical significance.
3.3. Comparative Analysis of Lower Limb Kinematics and Kinetics During Perturbations Relative to Baseline
All variables had distributions different than normal; therefore, non-parametric tests (Wilcoxon signed-rank with Bonferroni correction) were applied in the main analyses. Differences between maximum values obtained during perturbations and baseline conditions were then calculated for all parameters (Table 3).

Table 3.
Median values (with lower and upper quartiles) of differences between maximum values achieved during perturbation and baseline values for the left (non-dominant) and right (dominant) lower limbs. The last column shows p-values from the Wilcoxon test after Bonferroni correction, with * indicating statistically significant differences, along with the corresponding effect size.
For joint angles, no statistically significant changes were observed after correction. Hip flexion showed a negligible decrease (≈1.7%, very small effect size), and knee flexion exhibited a small increase (≈1.2%, small effect size). Ankle dorsiflexion increased slightly (≈2%, small effect size), but this change was also non-significant. These results indicate that perturbations did not induce meaningful angular changes in the lower limb joints.
For joint torques, a significant increase was observed in hip torque in the dominant limb (p = 0.0125, small effect size), suggesting a modest but reliable contribution of the hip to counteracting the perturbation. Knee and ankle torques showed minor changes (≈0–5% differences, small effect sizes), which were not statistically significant and likely have limited practical relevance.
The medio–lateral GRF showed a significant increase in the dominant limb relative to the non-dominant limb (≈32% increase, p = 0.0001, moderate effect size). This represents a clear and practically important adjustment, indicating that medio–lateral force generation is a key mechanism for maintaining balance during perturbations. In contrast, the proximal–distal and anterior–posterior GRF components showed small, non-significant changes and small effect sizes, suggesting that these directions were less affected by the perturbations.
In summary, after Bonferroni correction, the dominant hip torque and medio–lateral GRF were the only parameters with statistically significant changes (small-to-moderate effect sizes), highlighting their primary role in stabilizing posture during perturbations. Changes in joint angles and other torques were minor and non-significant, indicating that angular excursions of the lower limb were largely preserved.
3.4. Comparative Study of Lower Limb Kinematics and Kinetics in Response to Perturbations of the Dominant and Non-Dominant Leg
In this section, all parameters had non-normal distributions, except for knee angle and torque of the dominant limb, and hip, knee, and ankle angles and torques of the non-dominant limb. The comparison of kinematic and kinetic parameters between the dominant and non-dominant leg in response to perturbations revealed both similarities and meaningful differences (Table 4).

Table 4.
Median values (with lower and upper quartiles) of kinematic and kinetic parameters for the non-dominant (left) and dominant (right) lower limb in response to perturbations applied separately to the left and right leg. The last column reports the p-values from the Wilcoxon test and after Bonferroni correction, with * indicating statistically significant differences, along with the corresponding effect size.
For joint angles, hip and knee values were highly comparable, with no statistically significant differences after Bonferroni correction. The ankle angle showed a statistically significant difference (p = 0.0066), with the dominant limb positioned in slightly greater dorsiflexion. Although the relative percentage change was small (≈2%), the effect size (r = 0.41) indicated a moderate effect, suggesting this result might have had functional relevance.
In terms of joint torques, the hip torque was significantly higher in the dominant limb (p = 0.0033, r = 0.43). This effect size pointed to a moderate practical difference in how the hip contributed to stabilization during perturbations. Knee and ankle torque differences were minimal in absolute terms and did not reach statistical significance after correction, with very small effect sizes (r ≤ 0.12), supporting the interpretation that these trends lacked practical importance.
The most pronounced difference appeared in the GRFs. The medio–lateral component was markedly greater in the dominant limb (p = 0.0003, r = 0.87), representing a very large effect size and the strongest asymmetry across all parameters. By contrast, the proximal–distal and anterior–posterior GRF components showed negligible absolute differences (<2%) and were not statistically significant after correction, with small effect sizes (r ≤ 0.17).
In summary, hip and knee kinematics remained symmetrical, while the ankle displayed a small but statistically significant dorsiflexion difference with a moderate effect size. More importantly, the dominant limb generated significantly higher hip torque and substantially greater medio–lateral GRFs, highlighting its more active role in postural stabilization during perturbations.
3.5. Comparative Analysis of Lower Limb Kinematics and Kinetics in Response to Perturbations Relative to Baseline
Differences between the maximum values during perturbations and baseline values were calculated for all parameters, with Bonferroni correction applied (Table 5). As in previous chapters, all parameters had distributions different from normal.

Table 5.
Median values (with lower and upper quartiles) of differences between maximum perturbation responses and baseline values for the left (non-dominant) and right (dominant) lower limbs. The last column shows p-values from the Wilcoxon test after Bonferroni correction, with * indicating statistically significant differences, along with the corresponding effect size.
For joint angles, no significant changes were observed in the hip (≈5.1% decrease, p = 1.0000, small effect size) or knee (≈5.8% increase, p = 0.6912, small effect size). By contrast, the ankle angle exhibited a statistically significant decrease of ≈28% (p = 0.0165, moderate effect size), very close to the corrected significance threshold. This indicates a meaningful dorsiflexion adjustment at the ankle, reflecting a relevant postural strategy during perturbations.
For joint torques, the knee torque showed the largest relative reduction (≈30%, p = 0.0186, moderate effect size). Although this change did not remain significant after Bonferroni correction, it suggests a potentially relevant adaptation in knee loading. Hip torque decreased by ≈19% (p = 0.2832, small effect size) and ankle torque by ≈15% (p = 0.636, small effect size), but these were non-significant and of limited practical relevance.
The medio–lateral GRF showed the most pronounced and statistically significant effect, shifting by ≈112% relative to baseline (p = 0.0003, large effect size). In contrast, the proximal–distal (≈3.8% decrease, p = 1.0000, small effect size) and anterior–posterior (≈5% decrease, p = 0.4014, small effect size) GRF components showed no significant changes.
In summary, after applying Bonferroni correction, only the ankle angle (≈28% decrease, moderate effect) and the medio–lateral GRF (≈112% increase, large effect) remained statistically significant (Table 5). The reduction in knee torque (≈30%) approached significance but did not survive correction. These results suggest that perturbations primarily affect ankle kinematics and medio–lateral force generation, both critical for maintaining postural stability during destabilizing events.
4. Discussion
In this study, the effects of induced forward leg perturbations on lower-limb kinematics and kinetics during quiet standing in healthy young women were examined. The findings demonstrate that kinematic symmetry was largely preserved between the dominant and non-dominant limbs, even after accounting for Bonferroni correction, while kinetic asymmetries, particularly greater hip torque, ankle dorsiflexion adjustments, and medio–lateral ground reaction forces in the dominant limb, emerged as the primary compensatory responses. Although knee torque and ankle torque showed small trends toward change, these differences were not statistically significant after correction and, thus, have limited practical importance. Likewise, anterior–posterior GRF adaptations did not remain significant and should not be interpreted as key mechanisms. Characteristic adaptations to perturbations were also observed, including reductions in ankle plantarflexion angles and large medio–lateral force shifts. Importantly, these results extend previous knowledge on limb dominance by showing that such asymmetries are apparent even under small, controlled forward treadmill perturbations, a novel disturbance paradigm compared to traditional slip or platform-shift methods. These findings suggest that dynamic stability under small perturbations is maintained primarily through kinetic modulation, especially by the dominant limb, rather than substantial changes in joint angles.
4.1. Kinematic Symmetry and Robustness of Postural Control
High levels of joint angle symmetry were observed between limbs, with no statistically significant differences at the knee and only minor changes at the ankle. Rather than focusing on small percentage changes, these results highlight the robustness of kinematic strategies, suggesting that joint configurations remain stable even under disturbance. This observation is consistent with previous findings, in which lower-limb kinematics during standing perturbations were shown to remain relatively invariant even under repeated disturbances [4]. Such invariance can be explained by neuromuscular redundancy, whereby posture is maintained through subtle adjustments without the need for large angular changes [31,32]. The small but significant reduction in ankle plantarflexion during the recovery phase observed in this study indicates that distal joints provide fine-tuning, complementing proximal stabilization strategies [33].
4.2. Kinetic Asymmetries and the Role of Leg Dominance
In contrast to kinematics, marked asymmetries were revealed in kinetic measures. These included greater torque generation and medio–lateral force production by the dominant limb. Differences in knee torque and ankle torque were not significant after Bonferroni correction, meaning they should be interpreted only as secondary trends rather than robust findings. While asymmetries in voluntary tasks such as gait [20,34,35] have been well documented, the present results demonstrate that dominance also applies to compensatory responses to externally imposed forward perturbations. This indicates that limb dominance is a general organizing principle of balance control, extending beyond voluntary movements. The dominant limb may be characterized as more mobilizing, while the non-dominant limb plays a stabilizing role [9,10,12,13]. This additionally highlights an integrated system in which force generation and stabilization are distributed asymmetrically but functionally across limbs.
4.3. Anterior–Posterior Force Modulation as a Balance Strategy
Contrary to initial expectations, anterior–posterior GRF changes did not remain statistically significant after Bonferroni correction, and effect sizes were small. Thus, anterior–posterior modulation cannot be considered a primary compensatory strategy in this paradigm. Instead, the most consistent and robust adaptation across participants was a large shift in medio–lateral ground reaction forces, underscoring their importance in postural stabilization. This finding aligns with models emphasizing the role of frontal-plane control for balance recovery, especially under perturbations where side-to-side adjustments may be more effective than anterior–posterior shifts [36]. From a neurophysiological point of view, such medio–lateral modulation likely reflects coordinated activity of ankle and hip stabilizers, allowing flexible adaptation of balance strategies [37,38].
The perturbation intensity applied in this study (belt acceleration to 0.67 m/s within ~1 s) was deliberately chosen to reflect small but meaningful disturbances. Previous research has shown that treadmill belt accelerations in the range of 0.6–0.7 m/s elicit recoverable balance disturbances without causing loss of stability in healthy young adults [26]. Comparable magnitudes have also been used in perturbation-based balance training protocols, where they successfully induced measurable postural adjustments while maintaining participant safety [39]. Moreover, Bhatt et al. [40] demonstrated that such controlled slips evoke adaptive changes in gait and posture that closely resemble natural recovery strategies after everyday balance disturbances. Taken together, these findings indicate that the intensity applied here represents a realistic and safe level of perturbation, sufficient to trigger compensatory responses while minimizing the risk of destabilization.
In situations where the amplitude or dynamics of disturbances increase, the contribution of proximal joints (especially the hip) becomes greater, while in less demanding perturbations, distal control dominates, primarily through the ankle joint [41]. The results obtained in the study suggest that balance control may be hierarchical in nature, with the neuromuscular system flexibly selecting sources of stabilising forces depending on situational requirements. This indicates that maintaining posture is not merely a matter of ‘stiffening’ structures, but rather of continuous cooperation between different segments of the body, which allows for simultaneous reduction in energy costs and maintenance of stability. In this context, force modulation in the medio–lateral axis appears to be the key mechanism integrating local muscle responses with more global postural strategies, whereas anterior–posterior adjustments showed only limited and non-significant contributions.
4.4. Clinical and Practical Implications
The predominance of kinetic over kinematic adaptations underscores the value of ground reaction forces and joint torques as sensitive indicators of postural impairments, which may not be captured by joint angle analysis alone [42,43]. However, these results are based solely on healthy participants, and direct applications in clinical populations cannot be inferred from this study. This is particularly important in populations at risk of falls, where asymmetries in torque generation have been directly linked to instability [42]. The disproportionate contribution of the dominant limb suggests that functional asymmetry may be an important factor to consider in rehabilitation, as such imbalances can intensify under disruptive conditions and lead to uneven load redistribution [44,45]. In practice, this reflects the internal functional hierarchy of the movement system, with one limb primarily responsible for generating dynamic responses and the other for stabilizing and controlling movement trajectories [45]. Training interventions designed to reduce kinetic asymmetries and promote more balanced force redistribution may, therefore, enhance resilience to perturbations in daily life, particularly in individuals recovering from unilateral injuries [46,47,48,49]. Finally, controlled forward leg perturbations provide a practical and safe method for assessing dynamic balance capacity under small but realistic disturbances [42].
4.5. Limitations and Future Directions
Several limitations of this study must be acknowledged. The sample was small and homogeneous, consisting of 16 young, healthy, right-footed women. While this homogeneity limited internal variability and allowed for consistent results, it also restricted statistical power and the generalizability of the findings. The quasi-experimental design further introduces potential biases, as randomization of limb dominance or perturbation exposure was not feasible. Only right-footed participants were included, preventing analysis of variability in left-footed individuals. Moreover, sex-related differences in postural strategies and force distribution have been documented [50], suggesting that responses may differ in male populations. An additional limitation was the lack of electromyographic (EMG) recordings, which would allow direct assessment of muscle activation patterns underlying postural control. The conclusions were, therefore, based solely on kinematic and kinetic measures, without capturing neuromuscular contributions.
Another limitation concerns the analytical scope. The study was restricted to peak values of joint angles, torques, and forces, which provide robust indicators of maximal compensatory responses. However, reaction times, response latencies, and variability measures were not included, even though they represent fundamental dimensions of dynamic postural control. These additional metrics could offer complementary insights into the temporal dynamics and consistency of balance strategies. Future studies should, therefore, integrate both peak-based and time-based parameters, along with variability indices, to deliver a more comprehensive characterization of postural adaptations.
Future studies should also expand to include males, older adults, clinical populations, and individuals with different limb dominance profiles, as well as incorporate EMG measurements to provide a more complete understanding of the mechanisms underlying observed kinetic asymmetries.
5. Conclusions
In summary, induced disturbances in the form of forward extension of the lower limb caused asymmetrical kinetic reactions while maintaining kinematic symmetry. The dominant leg played a disproportionately active role in generating stabilising moments and medio–lateral forces, which emphasises its importance in dynamic posture control. Knee torque and ankle torque showed trends toward change but were not statistically significant after Bonferroni correction, and anterior–posterior GRF adaptations were also non-significant; therefore, these parameters should not be considered primary compensatory responses.
Kinetic parameters, particularly hip torque and medio–lateral GRF, were shown to be more sensitive than kinematic parameters in detecting minor perturbations in healthy participants. Angular changes at the ankle, such as dorsiflexion adjustments, also contributed to postural stabilization, whereas hip and knee angles remained largely unchanged. However, direct application to clinical interventions cannot be inferred from this study. These results clearly emphasise the need to take into account asymmetries resulting from lateralisation in postural control research, while practical or rehabilitation implications should be interpreted with caution, limited to the context of healthy adult populations.
Author Contributions
Conceptualization, M.G. and M.B.; methodology, M.G. and M.B.; software, M.G. and M.B.; validation, M.G., M.B., A.T. and J.W.; formal analysis, M.G. and M.B.; investigation, M.G. and M.B.; resources, J.W.; data curation, M.G. and M.B.; writing—original draft preparation, M.G. and M.B.; writing—review and editing, M.G., A.T. and J.W.; visualization, M.G., M.B. and J.W.; supervision, M.B. and J.W.; project administration, M.B.; funding acquisition, M.B. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Józef Piłsudski University of Physical Education in Warsaw (protocol code SKE01-15/2023 and date of approval 24 March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The measurement data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kędziorek, J.; Błażkiewicz, M. Nonlinear Measures to Evaluate Upright Postural Stability: A Systematic Review. Entropy 2020, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Pippi, R.; Mortati, A.; Fruttini, D.; Pasqualini, L.; Gatti, A.; Vandoni, M.; Mascherini, G.; Musumeci, G.; Fanelli, C. Physical activity, sedentary time and motivation to change: An Italian survey. Phys. Act. Rev. 2024, 12, 161–175. [Google Scholar] [CrossRef]
- Comfort, P.; Jones, P.A.; Smith, L.C.; Herrington, L. Joint Kinetics and Kinematics During Common Lower Limb Rehabilitation Exercises. J. Athl. Train. 2015, 50, 1011–1018. [Google Scholar] [CrossRef]
- Gulatowska, M.; Błażkiewicz, M. The Effect of Repetitive Mechanical Perturbations on Lower Limb Symmetry in Postural Control. Symmetry 2025, 17, 245. [Google Scholar] [CrossRef]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Zhu, R.T.; Lyu, P.-Z.; Li, S.; Tong, C.Y.; Ling, Y.T.; Ma, C.Z. How Does Lower Limb Respond to Unexpected Balance Perturbations? New Insights from Synchronized Human Kinetics, Kinematics, Muscle Electromyography (EMG) and Mechanomyography (MMG) Data. Biosensors 2022, 12, 430. [Google Scholar] [CrossRef]
- Hadamus, A.; Gulatowska, M.; Ferenc, A.; Shahnazaryan, K.; Brzuszkiewicz-Kuźmicka, G.; Błażkiewicz, M. Influence of leg dominance on the symmetry in body balance measurements. Phys. Act. Rev. 2025, 13, 88–96. [Google Scholar] [CrossRef]
- Kuberski, M.; Góra, T.; Wąsik, J. Changes in selected somatic indices in 10–12 year old girls under the influence of 3-year swimming training. Phys. Act. Rev. 2024, 12, 143–149. [Google Scholar] [CrossRef]
- Promsri, A.; Longo, A.; Haid, T.; Doix, A.M.; Federolf, P. Leg Dominance as a Risk Factor for Lower-Limb Injuries in Downhill Skiers—A Pilot Study into Possible Mechanisms. Int. J. Environ. Res. Public Health 2019, 16, 3399. [Google Scholar] [CrossRef]
- Kadri, M.A.; Noé, F.; Maitre, J.; Maffulli, N.; Paillard, T. Effects of Limb Dominance on Postural Balance in Sportsmen Practicing Symmetric and Asymmetric Sports: A Pilot Study. Symmetry 2021, 13, 2199. [Google Scholar] [CrossRef]
- Sharma, S.; Szabo, I.-Z.; Danielsen, M.; Andersen, S.; Nørgaard, J.; Lord, S.; Okubo, Y.; Jorgensen, M. Perturbation-based balance training improves reactive balance and reduces falls in older people: A systematic review and meta-analysis. medRxiv 2025. [Google Scholar] [CrossRef]
- Promsri, A. Modulation of Lower-Limb Muscle Activity in Maintaining Unipedal Balance According to Surface Stability, Sway Direction, and Leg Dominance. Sports 2022, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A.; Haid, T.; Werner, I.; Federolf, P. Leg Dominance Effects on Postural Control When Performing Challenging Balance Exercises. Brain Sci. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Castellote, J.M.; Kofler, M.; Mayr, A. The benefit of knowledge: Postural response modulation by foreknowledge of equilibrium perturbation in an upper limb task. Eur. J. Appl. Physiol. 2024, 124, 975–991. [Google Scholar] [CrossRef]
- Moriyama, M.; Kouzaki, M.; Hagio, S. Anticipatory postural control in adaptation of goal-directed lower extremity movements. Sci. Rep. 2024, 14, 4142. [Google Scholar] [CrossRef]
- Temporiti, F.; Scandelli, F.; Mellina Gottardo, F.; Falco, M.; Rossi, S.; Adamo, P.; Gatti, R. Balance improvements in healthy subjects are independent to postural strategies involved in the training. Gait Posture 2023, 101, 160–165. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, H.; Chien, J.H. The ground reaction force pattern during walking under vestibular-demanding task with/without mastoid vibration: Implication for future sensorimotor training in astronauts. Front. Physiol. 2024, 15, 1325513. [Google Scholar] [CrossRef]
- Karabin, M.J.; Smith, R.W.; Sparto, P.J.; Furman, J.M.; Redfern, M.S. Balance strategies for recovery from perturbed overground walking. J. Biomech. 2024, 162, 111898. [Google Scholar] [CrossRef]
- Błażkiewicz, M.; Hadamus, A. Influence of Perturbation’s Type and Location on Treadmill Gait Regularity. Appl. Sci. 2024, 14, 493. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. 2), ii7–ii11. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. Control of rapid limb movements for balance recovery: Age-related changes and implications for fall prevention. Age Ageing 2006, 35 (Suppl. 2), ii12–ii18. [Google Scholar] [CrossRef]
- Hezel, N.; Buchner, T.; Becker, C.; Bauer, J.M.; Sloot, L.H.; Steib, S.; Werner, C. Dose-response relationship of treadmill perturbation-based balance training for improving reactive balance in older adults at risk of falling: Results of the FEATURE randomized controlled pilot trial. Eur. Rev. Aging Phys. Act. Off. J. Eur. Group Res. Elder. Phys. Act. 2025, 22, 8. [Google Scholar] [CrossRef]
- Promsri, A.; Haid, T.; Federolf, P. How does lower limb dominance influence postural control movements during single leg stance? Hum. Mov. Sci. 2018, 58, 165–174. [Google Scholar] [CrossRef]
- Barut, C.; Ozer, C.M.; Sevinc, O.; Gumus, M.; Yunten, Z. Relationships between hand and foot preferences. Int. J. Neurosci. 2007, 117, 177–185. [Google Scholar] [CrossRef]
- Ciunelis, K.; Borkowski, R.; Błażkiewicz, M. The Impact of Induced Acceleration Perturbations in Selected Phases of the Gait Cycle on Kinematic and Kinetic Parameters. Appl. Sci. 2024, 14, 4849. [Google Scholar] [CrossRef]
- Siedlecki, P.; Shoemaker, J.K.; Ivanova, T.D.; Garland, S.J. Cardiovascular response to postural perturbations of different intensities in healthy young adults. Physiol. Rep. 2022, 10, e15299. [Google Scholar] [CrossRef] [PubMed]
- Feldhege, F.; Richter, K.; Bruhn, S.; Fischer, D.C.; Mittlmeier, T. MATLAB-based tools for automated processing of motion tracking data provided by the GRAIL. Gait Posture 2021, 90, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R. Parametric measures of effect size. In The Handbook of Research Synthesis; Russell Sage Foundation: New York, NY, USA, 1994; pp. 231–244. [Google Scholar]
- Rosnow, R.L. Effect sizes for experimenting psychologists. Can. J. Exp. Psychol. Rev. Can. Psychol. Exp. 2003, 57, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Prajzner, A. Selected Indicators of Effect Size in Psychological Research. Ann. Univ. Mariae Curie-Skłodowska Sect. J Paedagog. Psychol. 2023, 35, 139–157. [Google Scholar] [CrossRef]
- Walter, J.R.; Günther, M.; Haeufle, D.F.B.; Schmitt, S. A geometry- and muscle-based control architecture for synthesising biological movement. Biol. Cybern. 2021, 115, 7–37. [Google Scholar] [CrossRef]
- Wang, S.; Pai, Y.C.; Bhatt, T. Neuromuscular mechanisms of motor adaptation to repeated gait-slip perturbations in older adults. Sci. Rep. 2022, 12, 19851. [Google Scholar] [CrossRef]
- Han, K.S.; Shin, S.H.; Yu, C.H.; Kwon, T.K. Postural responses during the various frequencies of anteroposterior perturbation. Biomed. Mater. Eng. 2014, 24, 2537–2545. [Google Scholar] [CrossRef]
- Bartosz, M.; Latocha, A.; Motowidło, J.; Krzysztofik, M.; Zajac, A. The relationship between countermovement jump performance and sprinting speed in elite sprinters. Phys. Act. Rev. 2024, 12, 29–37. [Google Scholar] [CrossRef]
- Wahb, S.; ElDeeb, A.; Kamel, H.; El-Shafei, M. Effect of Circuit Training on Calcium Level and Physical Fitness in Pregnant Women with Hypocalcemia: A randomized controlled trial. Phys. Act. Rev. 2024, 12, 22–31. [Google Scholar] [CrossRef]
- Nashner, L.M.; McCollum, G. The organization of human postural movements: A formal basis and experimental synthesis. Behav. Brain Sci. 2010, 8, 135–150. [Google Scholar] [CrossRef]
- Buchmann, A.; Kiss, B.; Badri-Spröwitz, A.; Renjewski, D. How knee muscles and ground reaction forces shape knee buckling and ankle push-off in neuromuscular simulations of human walking. Sci. Rep. 2025, 15, 2249. [Google Scholar] [CrossRef]
- Vlutters, M.; van Asseldonk, E.H.F.; van der Kooij, H. Lower extremity joint-level responses to pelvis perturbation during human walking. Sci. Rep. 2018, 8, 14621. [Google Scholar] [CrossRef]
- Liu, X.; Bhatt, T.; Pai, Y.C. Intensity and generalization of treadmill slip training: High or low, progressive increase or decrease? J. Biomech. 2016, 49, 135–140. [Google Scholar] [CrossRef]
- Bhatt, T.; Wening, J.D.; Pai, Y.C. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp. Brain Res. 2006, 170, 61–73. [Google Scholar] [CrossRef]
- Afschrift, M.; Jonkers, I.; De Schutter, J.; De Groote, F. Mechanical effort predicts the selection of ankle over hip strategies in nonstepping postural responses. J. Neurophysiol. 2016, 116, 1937–1945. [Google Scholar] [CrossRef]
- Granacher, U.; Gollhofer, A.; Hortobágyi, T.; Kressig, R.W.; Muehlbauer, T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: A systematic review. Sports Med. 2013, 43, 627–641. [Google Scholar] [CrossRef]
- Sung, P.S.; Zipple, J.T.; Andraka, J.M.; Danial, P. The kinetic and kinematic stability measures in healthy adult subjects with and without flat foot. Foot 2017, 30, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Heil, J. Load-Induced Changes of Inter-Limb Asymmetries in Dynamic Postural Control in Healthy Subjects. Front. Hum. Neurosci. 2022, 16, 824730. [Google Scholar] [CrossRef] [PubMed]
- Maly, T.; Hank, M.; Verbruggen, F.F.; Clarup, C.; Phillips, K.; Zahalka, F.; Mala, L.; Ford, K.R. Relationships of lower extremity and trunk asymmetries in elite soccer players. Front. Physiol. 2024, 15, 1343090. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Wong, J.S.; Bryce, J.; Knorr, S.; Patterson, K.K. Does perturbation-based balance training prevent falls? Systematic review and meta-analysis of preliminary randomized controlled trials. Phys. Ther. 2015, 95, 700–709. [Google Scholar] [CrossRef]
- Zwierko, M.; Rokita, A.; Jedziniak, W.; Popowczak, M. Effects of a 6-week stroboscopic training program on specific blocking reaction speed in young volleyball players. Phys. Act. Rev. 2024, 12, 1–10. [Google Scholar] [CrossRef]
- Gerards, M.; Marcellis, R.; Senden, R.; Poeze, M.; de Bie, R.; Meijer, K.; Lenssen, A. The effect of perturbation-based balance training on balance control and fear of falling in older adults: A single-blind randomised controlled trial. BMC Geriatr. 2023, 23, 305. [Google Scholar] [CrossRef]
- Unger, J.; Chan, K.; Lee, J.W.; Craven, B.C.; Mansfield, A.; Alavinia, M.; Masani, K.; Musselman, K.E. The Effect of Perturbation-Based Balance Training and Conventional Intensive Balance Training on Reactive Stepping Ability in Individuals with Incomplete Spinal Cord Injury or Disease: A Randomized Clinical Trial. Front. Neurol. 2021, 12, 620367. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Xu, K.; Xie, H.; Chen, J.; Zhu, Z.; Ji, H.; Li, D.; Sun, J. The potential of a targeted unilateral compound training program to reduce lower limb strength asymmetry and increase performance: A proof-of-concept in basketball. Front. Physiol. 2024, 15, 1361719. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).