1-Carboxy-2-phenylethan-1-aminium Iodide 2-Azaniumyl-3-phenylpropanoate Crystals: Properties and Its Biochar-Based Application for Iodine Enrichment of Parsley
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Study of the Physico-Chemical and Technological Properties of PPI
- ρb is the bulk density of the sample, kg/m3;
- m is the mass of the sample, kg;
- V is the volume of sample in the cylinder after pre–sealing, m3.
2.2. Obtaining Biochar from Walnut Shell
2.3. Study of the Physico-Chemical and Technological Properties of WS Biochar
2.4. Study of the Sorption Capacity of WS Biochar to Iodine
2.5. Production of WS Biochar-Based Fertilizer Under Laboratory Conditions
2.6. Plants Planting
- Control without processing.
- Pure biochar.
- KI at a concentration of 15 mg/kg of soil.
- Biochar with a total iodine content of 1.5% in the sample (15 mg/kg of iodine or 51 mg of PPI).
2.7. Sample Preparation of Plant Specimens
2.8. Determination of Ascorbic Acid Concentration, Polyphenols in Plants
2.9. Determination of the Antioxidant Activity of Samples (AOA)
2.10. Determination of the Total Iodine Content
2.11. Determination of the Organic Nitrogen
2.12. Statistical Analysis
3. Results and Discussion
3.1. Determination of Physico-Chemical Parameters of PPI
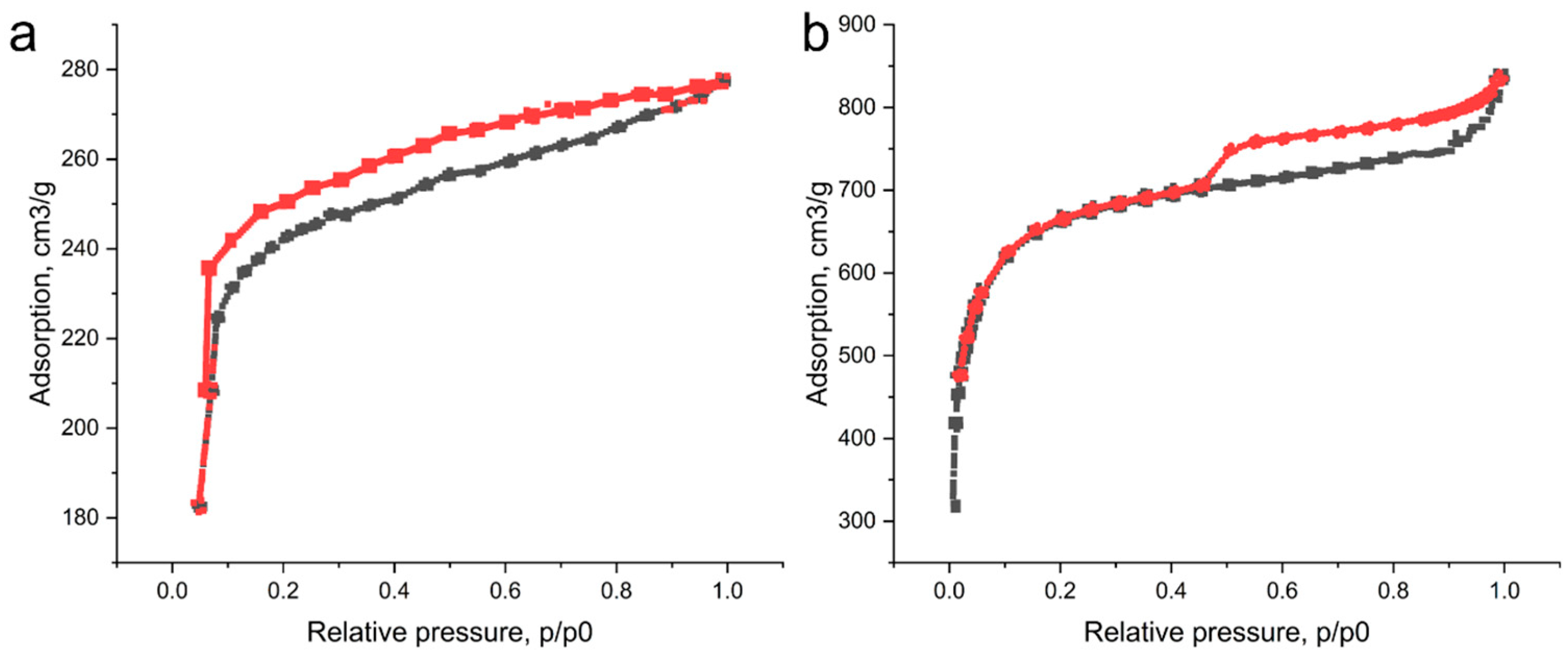
3.2. Investigation of the Characteristics of Biochar from Walnut Shells
- Electrostatic Interactions: The negatively charged surface of the carbon interacts with positively charged amino-groups of phenylalanine from PPI via electrostatic forces in aqueous solutions. This process plays a significant role in determining the charge distribution on the carbon surface during adsorption.
- Hydrogen Bonding/Van der Waals connections: Numerous hydrogen bond reactions take place between hydroxyl (OH) and carboxyl (COOH) groups on activated by KOH carbon and PPI molecules during the adsorption process. These interactions create additional binding sites and enhance adsorption capacity.
- Pore Filling: The hierarchical porous structure of activated carbon, comprising both micropores and mesopores, allows for the diffusion and trapping of PPI molecules. Micropores can adsorb small molecules, while mesopores offer additional surface area for adsorption, resulting in high adsorption efficiency.
3.3. Study of the Effect of Fertilizers on the Physiological Properties of Plants
3.4. The Effect of BIOF on the Content of Iodine and Organic Nitrogen in a Plant
3.5. The Effect of BIOF on the Antioxidant Activity of Plants
3.6. Economic Efficiency of BIOF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPI | 1-carboxy-2-phenylethan-1-aminium iodide 2-azaniumyl-3-phenylpropanoate |
| BIOF | The granulated composition PPI + biochar |
| DSC | Differential scanning calorimetry |
| WS | Walnut shell |
| XRD | X-ray diffraction analysis |
| AOA | Antioxidant activity |
References
- Ilin, A.I. Experimental Crystal Structure Determination (CCDC 1036670); Cambridge Crystallographic Data Centre: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Paretskaya, N.A.; Sabitov, A.N.; Islamov, R.A.; Tamazyan, R.A.; Tokmoldin, S.Z.; Ilin, A.I.; Martirosyan, K.S. Phenylalanine—Iodine Complex and its Structure; Physico-Mathematical Series; News of The National Academy of Sciences of The Republic of Kazakhstan: Almaty, Kazakhstan, 2017; Volume 2, pp. 5–9. Available online: https://pps.kaznu.kz/kz/Main/FileShow2/106653/114/446/8679/ (accessed on 10 February 2020).
- Grzanka, M.; Smoleń, S.; Skoczylas, Ł.; Grzanka, D. Synthesis of Organic Iodine Compounds in Sweetcorn under the Influence of Exogenous Foliar Application of Iodine and Vanadium. Molecules 2022, 27, 1822. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of Iodine to Biofortify and Promote Growth and Stress Tolerance in Crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radkowski, A.; Radkowska, I. Influence of foliar fertilization with amino acid preparations on morphological traits and seed yield of timothy. Plant Soil Environ. 2018, 64, 209–213. [Google Scholar] [CrossRef]
- GOST EN 1236-2013; Fertilizers. Method for Determining Bulk Density Without Compaction. Interstate Standard (GOST): Moscow, Russia, 2013; p. 7. Available online: https://files.stroyinf.ru/Data/563/56357.pdf (accessed on 22 April 2025).
- Wolthuis, E.; Pruiksma, A.B.; Heerema, R.P. Determination of solubility: A laboratory experiment. J. Chem. Educ. 1960, 37, 137. [Google Scholar] [CrossRef]
- Doszhanov, E.O.; Sabitov, A.N.; Mansurov, Z.A.; Doszhanov, O.M.; Zhandosov, Z.M.; Rakhymzhan, N. Method of Obtaining Sorbent from Vegetable Raw Materials. Utility Model Patent No. 8681, 1 December 2023. [Google Scholar]
- Naderi, M. Surface Area: Brunauer–Emmett–Teller (BET). In Progress in Filtration and Separation; Tarleton, S., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 585–608. ISBN 9780123847461. [Google Scholar] [CrossRef]
- GOST 33618-2015; Activated Carbon. Standard Method for Determination of Iodine Value. Interstate Standard: Moscow, Russia, 2016; p. 8. Available online: https://files.stroyinf.ru/Data2/1/4293756/4293756365.pdf (accessed on 22 April 2025).
- Nurbolatovich, S.A.; Ospanovich, D.Y.; Seitzhan, T.; Didar, N. A Method for Obtaining Granular Fertilizer Based on Pyrocarbon. Bulletin No. 3. IPC C05G 3/00 C05D 9/02. RK Patent for Utility Model No. 8791, 19 January 2024. [Google Scholar]
- GOST 31640-2012; Feeds. Methods for Determination of Dry Matter Content. Interstate Standard: Moscow, Russia, 2012; p. 11. Available online: https://files.stroyinf.ru/Data2/1/4293787/4293787414.pdf (accessed on 22 April 2025).
- Kharchenko, V.A.; Moldovan, A.I.; Golubkina, N.A.; Gins, M.S.; Shafigullin, D.R. Comparative evaluation of several biologically active compounds content in Anthriscus sylvestris (L.) Hoffm. and Anthriscus cerefolium (L.) Hoffm. Veg. Russ. 2020, 5, 81–87. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plant Antioxidants and Methods for Their Determination; Infra-M Academic Publishing House: Moscow, Russia, 2020; Volume 1, p. 181. [Google Scholar] [CrossRef]
- Doszhanov, Y.; Atamanov, M.; Jandosov, J.; Saurykova, K.; Bassygarayev, Z.; Orazbayev, A.; Turganbay, S.; Sabitov, A. Preparation of Granular Organic Iodine and Selenium Complex Fertilizer Based on Biochar for Biofortification of Parsley. Scientifica 2024, 2024, 6601899. [Google Scholar] [CrossRef]
- Ramachandran, E.; Natarajan, S. XRD, thermal and FTIR studies on gel grown DL-Phenylalanine crystals. Cryst. Res. Technol. 2007, 42, 617–620. [Google Scholar] [CrossRef]
- Tomar, D.; Chaudhary, S.; Jena, K.C. Self-assembly of l-phenylalanine amino acid: Electrostatic induced hindrance of fibril formation. RSC Adv. 2019, 9, 12596–12605. [Google Scholar] [CrossRef]
- Max, J.J.; Chapados, C. Infrared spectroscopy of acetone–water liquid mixtures. II. Molecular model. J. Chem. Phys. 2004, 120, 6625–6641. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Tang, X.; Zhang, F.; Wang, B. Study on the shift of ultraviolet spectra in aqueous solution with variations of the solution concentration. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118259. [Google Scholar] [CrossRef]
- Gogoi, P.; Mohan, U.; Borpuzari, M.P.; Boruah, A.; Baruah, S.K. UV-Visible spectroscopy and density functional study of solvent effect on halogen bonded charge-transfer complex of 2-Chloropyridine and iodine monochloride. Arab. J. Chem. 2019, 12, 4522–4532. [Google Scholar] [CrossRef]
- ChemicalBook. D-Phenylalanine—Product Chemical Properties. 2025. Available online: https://www.chemicalbook.com/ProductChemicalPropertiesCB9773699_EN.htm (accessed on 20 April 2025).
- Moon, C.Y.; Kim, Y.S.; Lee, E.C.; Jin, Y.G.; Chang, K.J. Mechanism for oxidative etching in carbon nanotubes. Phys. Rev. B 2002, 65, 155401. [Google Scholar] [CrossRef]
- Zhang, K.; Teng, J.; Ding, L.; Tang, X.; Huang, J.; Liu, S.; Cao, S.; Li, H.; Li, J. Establishing structure-property relationships grounded in oxidation strategy engineering to unravel the triggering mechanism of Na+ filling in Hard Carbon closed pore. Electrochim. Acta 2025, 538, 146970. [Google Scholar] [CrossRef]
- Burgess, C.G.; Everett, D.H.; Nuttall, S. Adsorption hysteresis in porous materials. Pure Appl. Chem. 1989, 61, 1845–1852. [Google Scholar] [CrossRef]
- Halka, M.; Klimek-Chodacka, M.; Smoleń, S.; Baranski, R.; Ledwożyw-Smoleń, I.; Sady, W. Organic iodine supply affects tomato plants differently than inorganic iodine. Physiol Plant. 2018, 164, 290–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Wang, M.; Zou, Z.; Zhou, P.; Wang, X.; Jin, J. A review of iodine in plants with biofortification: Uptake, accumulation, transportation, function, and toxicity. Sci. Total Environ. 2023, 878, 163203. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Y.; Ma, C.; Qin, J.; Nguyen, T.H.N.; Liu, D.; Gan, H.; Ding, S.; Luo, Z.B. Phenylalanine as a nitrogen source induces root growth and nitrogen-use efficiency in Populus× canescens. Tree Physiol. 2018, 38, 66–82. [Google Scholar] [CrossRef]
- Grey, C.B.; Cowan, D.P.; Langton, S.D.; Watkins, R.W. Systemic Application of L-Phenylalanine Increases Plant Resistance to Vertebrate Herbivory. J. Chem. Ecol. 1997, 23, 1463–1470. [Google Scholar] [CrossRef]
- Dobosy, P.; Vetési, V.; Sandil, S.; Endrédi, A.; Kröpfl, K.; Óvári, M.; Takács, T.; Rékási, M.; Záray, G. Effect of irrigation water containing iodine on plant physiological processes and elemental concentrations of cabbage (Brassica oleracea L. var. capitata L.) and tomato (Solanum lycopersicum L.) cultivated in different soils. Agronomy 2020, 10, 720. [Google Scholar] [CrossRef]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a nutritional role of iodine in plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayındır, Ü.; Küçükyumuk, Z. The Effects of Potassium on Plant Nutrient Concentration, Plant Development, and Rhizoctonia Rot (Rhizoctonia solani) in Pepper. Horticulturae 2025, 11, 516. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C: N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Ibrar, D.; et al. Biochar production and characteristics, its impacts on soil health, crop production, and yield enhancement: A review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Liu, X.; Huang, Y.; Cai, A.; Xu, H.; Ran, J.; Xiao, J.; Zhang, W. A global dataset of biochar application effects on crop yield, soil properties, and greenhouse gas emissions. Sci. Data 2024, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Ndede, E.O.; Kurebito, S.; Idowu, O.; Tokunari, T.; Jindo, K. The potential of biochar to enhance the water retention properties of sandy agricultural soils. Agronomy 2022, 12, 311. [Google Scholar] [CrossRef]
- Lustosa Carvalho, M.; Tuzzin de Moraes, M.; Cerri, C.E.P.; Cherubin, M.R. Biochar amendment enhances water retention in a tropical sandy soil. Agriculture 2020, 10, 62. [Google Scholar] [CrossRef]
- Sharma, M.; Kaushik, R.; Pandit, M.K.; Lee, Y.H. Biochar-induced microbial shifts: Advancing soil sustainability. Sustainability 2025, 17, 1748. [Google Scholar] [CrossRef]
- Kracmarova-Farren, M.; Alexova, E.; Kodatova, A.; Mercl, F.; Szakova, J.; Tlustos, P.; Demnerova, K.; Stiborova, H. Biochar-induced changes in soil microbial communities: A comparison of two feedstocks and pyrolysis temperatures. Environ. Microbiome 2024, 19, 87. [Google Scholar] [CrossRef]
- Barbosa, F.L.; Santos, J.M.; Mota, J.C.; Costa, M.C.; Araujo, A.S.; Garcia, K.G.; Almeida, M.S.; Nascimento, Í.V.; Medeiros, E.V.; Pereira, A.P.D.A.; et al. Potential of biochar to restoration of microbial biomass and enzymatic activity in a highly degraded semiarid soil. Sci. Rep. 2024, 14, 26065. [Google Scholar] [CrossRef]
- Vanapalli, K.R.; Samal, B.; Dubey, B.K.; Bhattacharya, J. Biochar for sustainable agriculture: Prospects and implications. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 221–262. ISBN 9780128201787. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Cezar, J.V.D.C.; Morais, E.G.D.; Lima, J.D.S.; Benevenute, P.A.N.; Guilherme, L.R.G. Iodine-enriched urea reduces volatilization and improves nitrogen uptake in maize plants. Nitrogen 2024, 5, 891–902. [Google Scholar] [CrossRef]
- Smolen, S.; Sady, W.; Rozek, S.; Ledwozyw-Smolen, I.; Strzetelski, P. Preliminary evaluation of the influence of iodine and nitrogen fertilization on the effectiveness of iodine biofortification and mineral composition of carrot storage roots. J. Elem. 2011, 16, 613–622. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Huang, Y.-Z.; Hu, Y.; Liu, Y.-X. Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: Effects of iodine species and solution concentrations. Environ. Int. 2003, 29, 33–37. [Google Scholar] [CrossRef]
- Tiyogi Nath, T.N.; Priyankar Raha, P.R.; Amitava Rakshit, A.R. Sorption and desorption behaviour of iodine in alluvial soils of Varanasi, India. Agricultura 2010, 7, 9–14. [Google Scholar]
- Shalaby, O.A. Iodine application induces the antioxidant defense system, alleviates salt stress, reduces nitrate content, and increases the nutritional value of lettuce plants. Funct. Plant Biol. 2025, 52, FP24273. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Święciło, A.; Zych-Wężyk, I. The antioxidant properties and biological quality of radish seedlings biofortified with iodine. Agronomy 2021, 11, 2011. [Google Scholar] [CrossRef]
- Liu, W.; Muzolf-Panek, M.; Kleiber, T. Effect of Nitrogen Nutrition and Planting Date on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy 2022, 12, 2869. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Nai, G.; Ma, L.; Lu, X.; Yan, H.; Gong, M.; Li, Y. Nitrogen application regulates antioxidant capacity and flavonoid metabolism, especially quercetin, in grape seedlings under salt stress. J. Integr. Agric. 2024, 23, 4074–4092. [Google Scholar] [CrossRef]
- Nista, F.; Bagnasco, M.; Gatto, F.; Albertelli, M.; Vera, L.; Boschetti, M.; Musso, N.; Ferone, D. The effect of sodium restriction on iodine prophylaxis: A review. J. Endocrinol. Investig. 2022, 46, 1121–1138. [Google Scholar] [CrossRef]
- Bath, S.C. The effect of iodine deficiency during pregnancy on child development. Proc. Nutr. Soc. 2019, 78, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Turganbay, S.; Kenesheva, S.; Jumagaziyeva, A. Synthesis, physicochemical properties and antimicrobial activity of a di-aminopropionic acid hydrogen tri-iodide coordination compound. BMC Res. Notes 2024, 17, 384. [Google Scholar] [CrossRef] [PubMed]
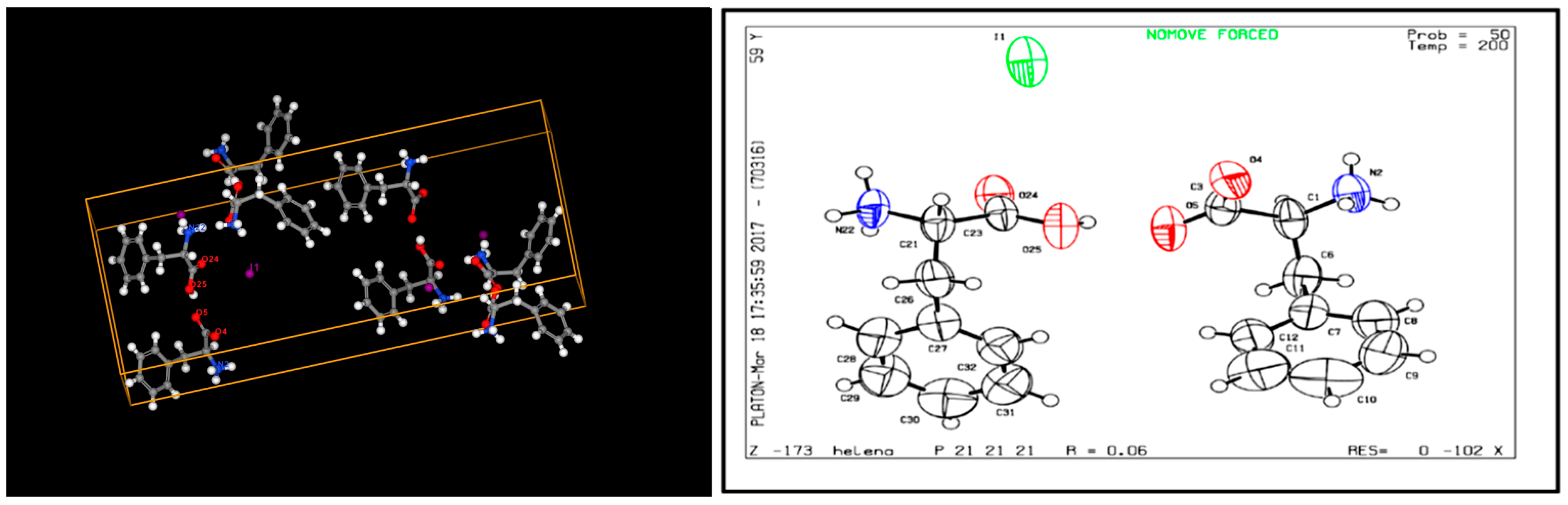
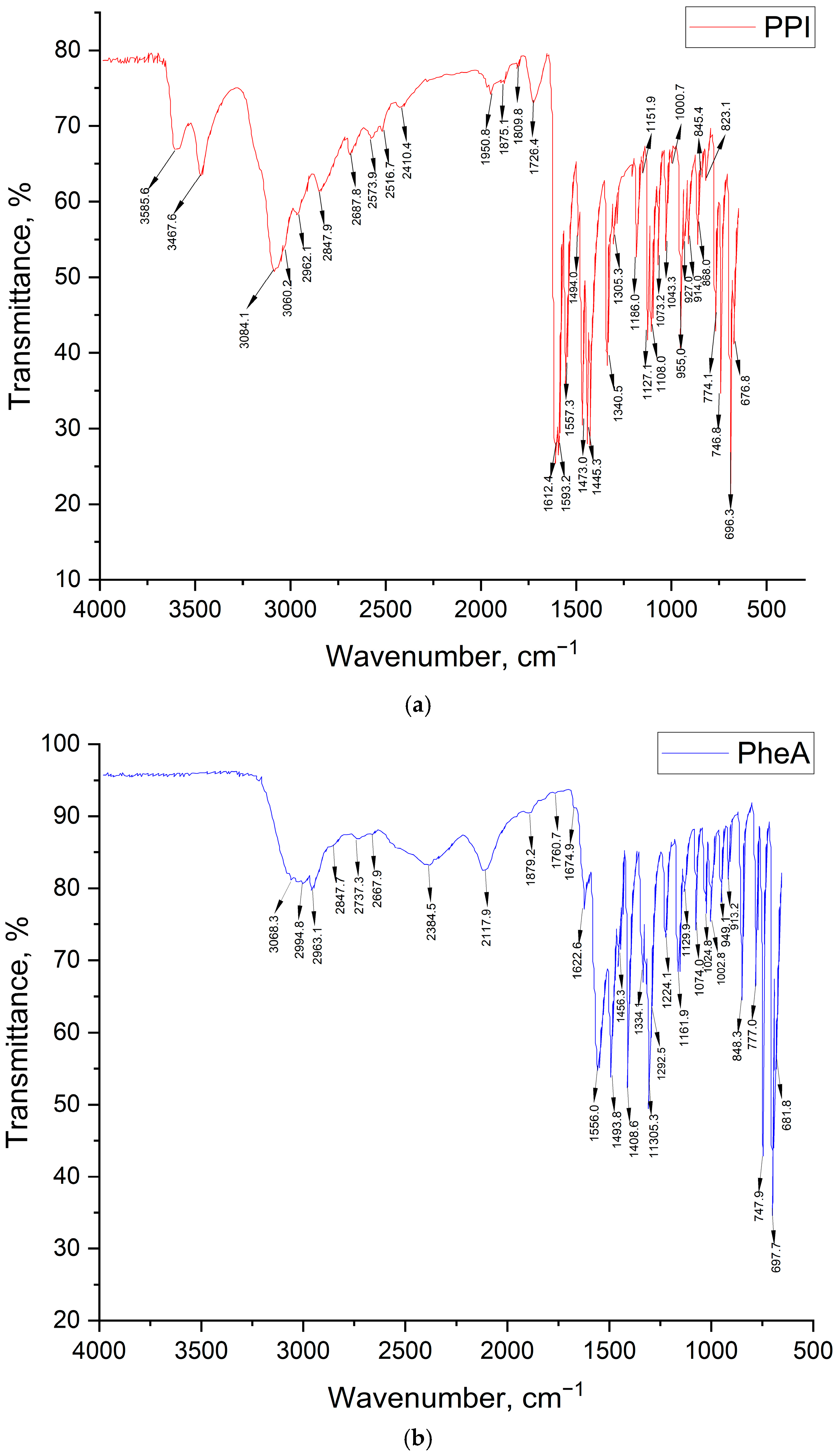
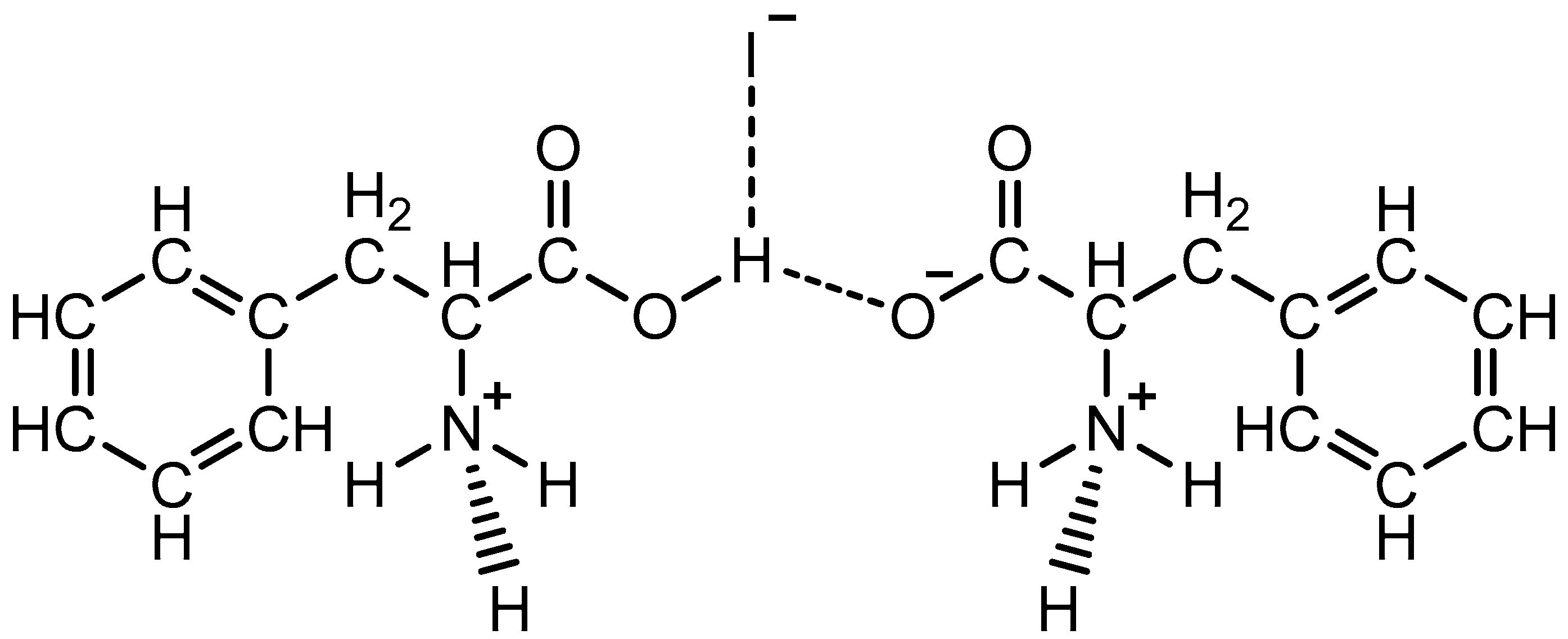
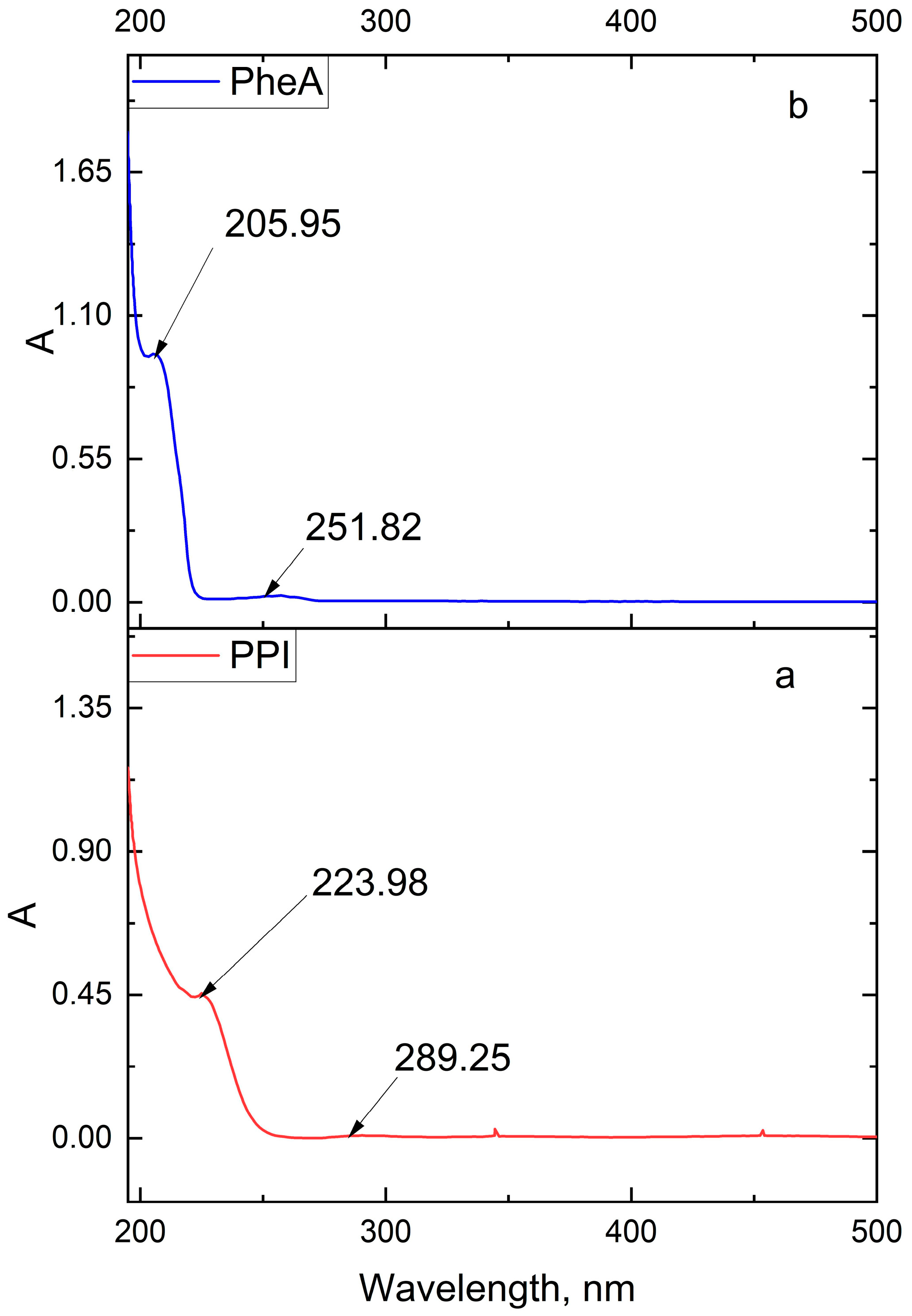
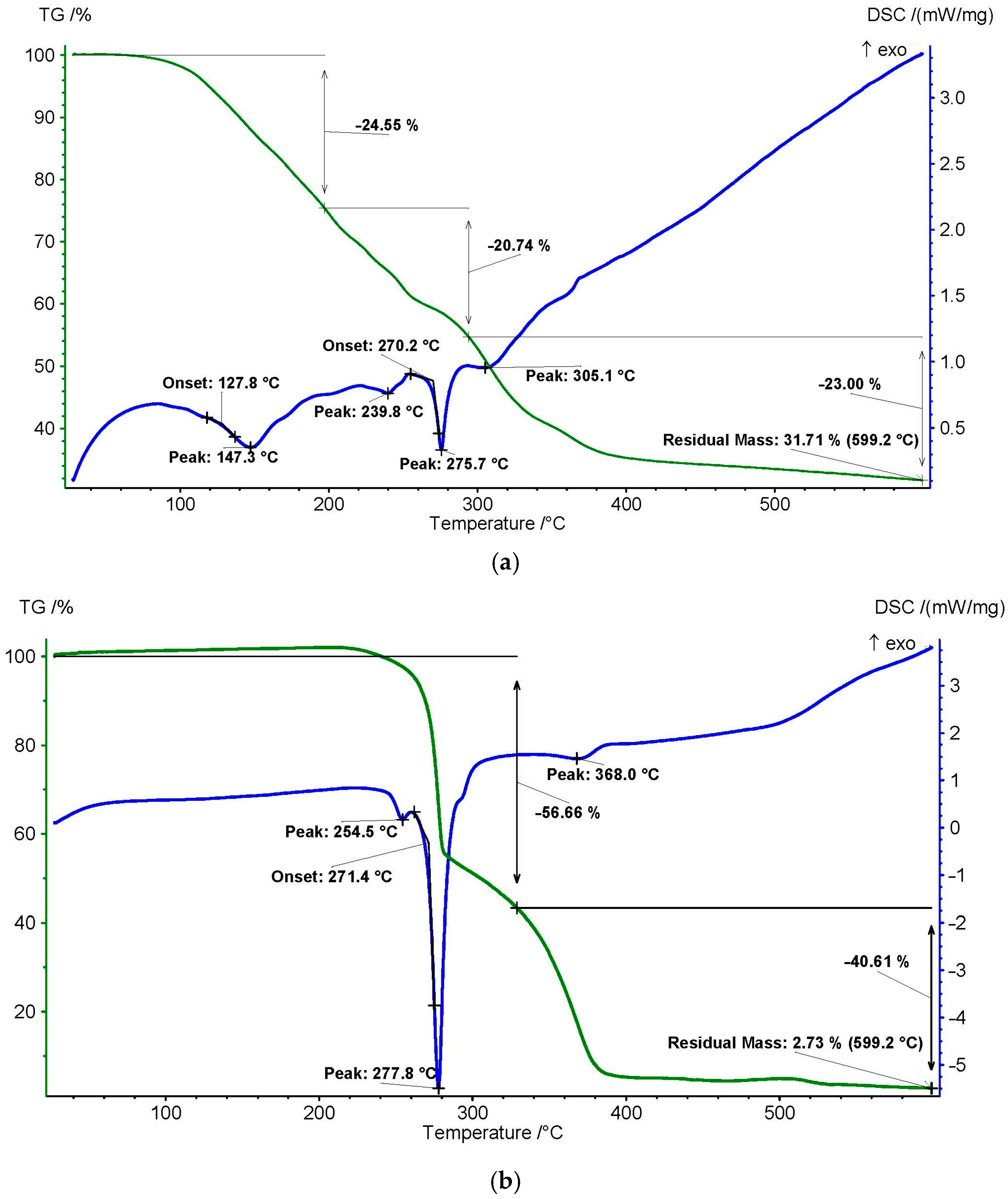
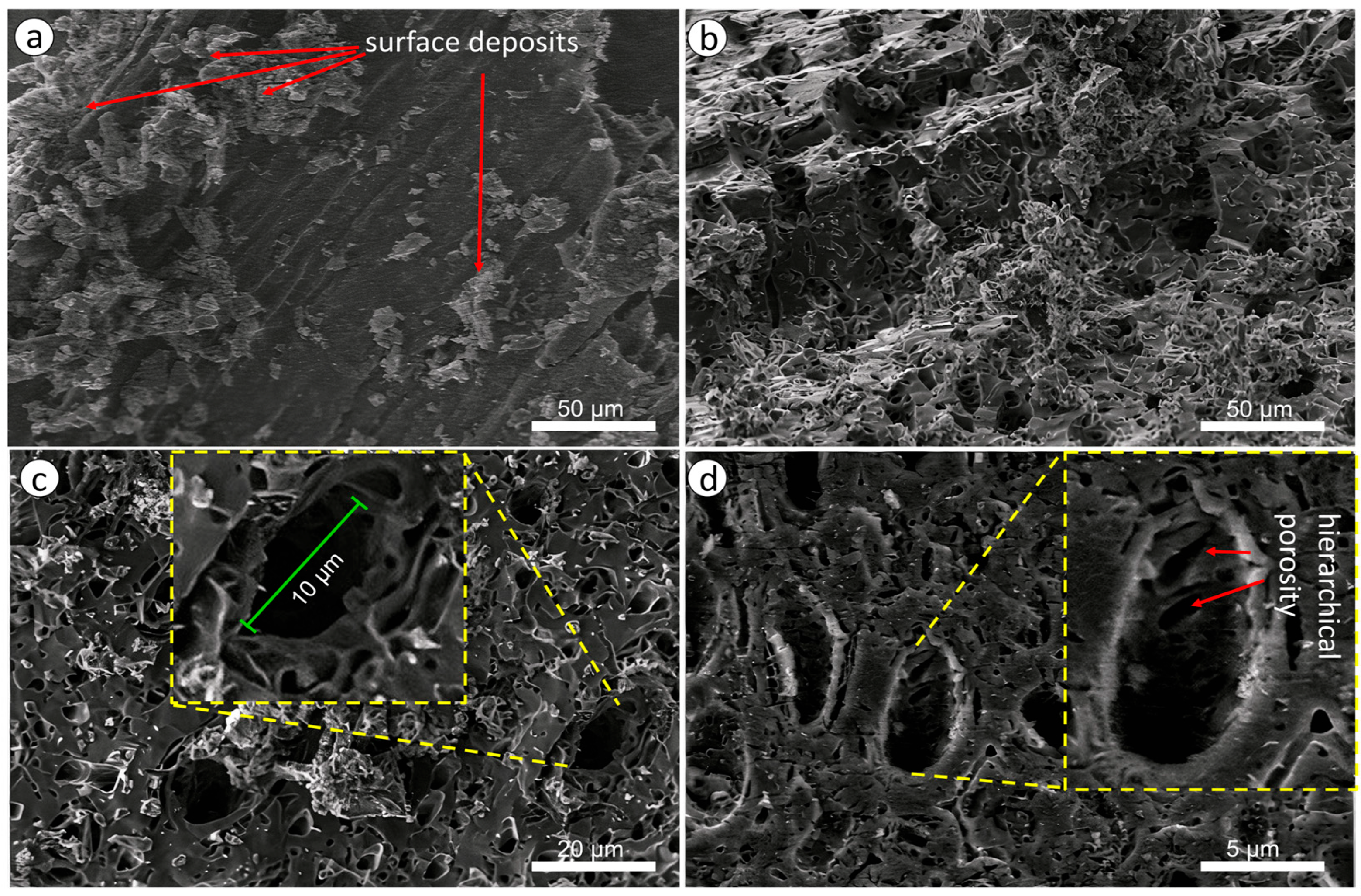

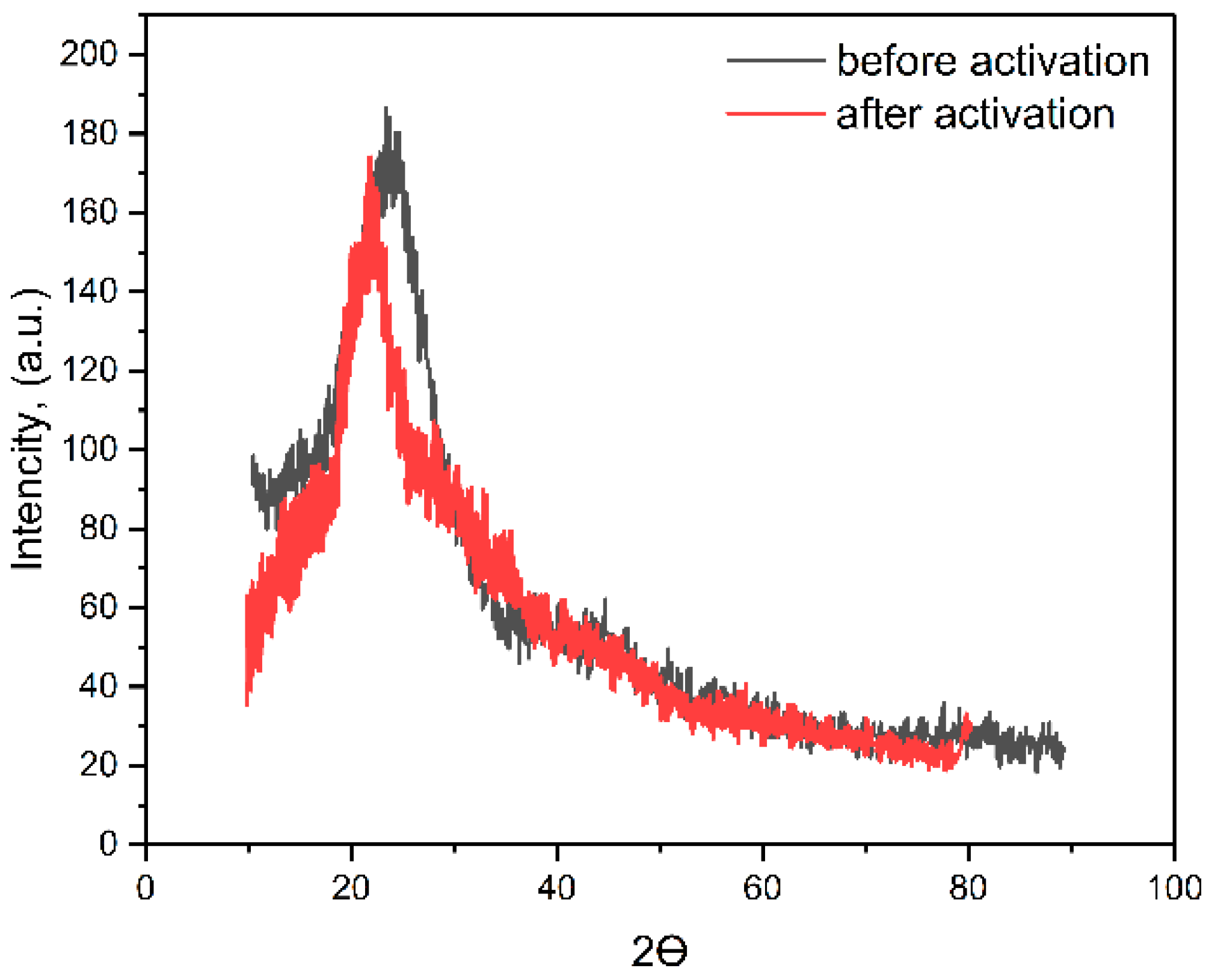
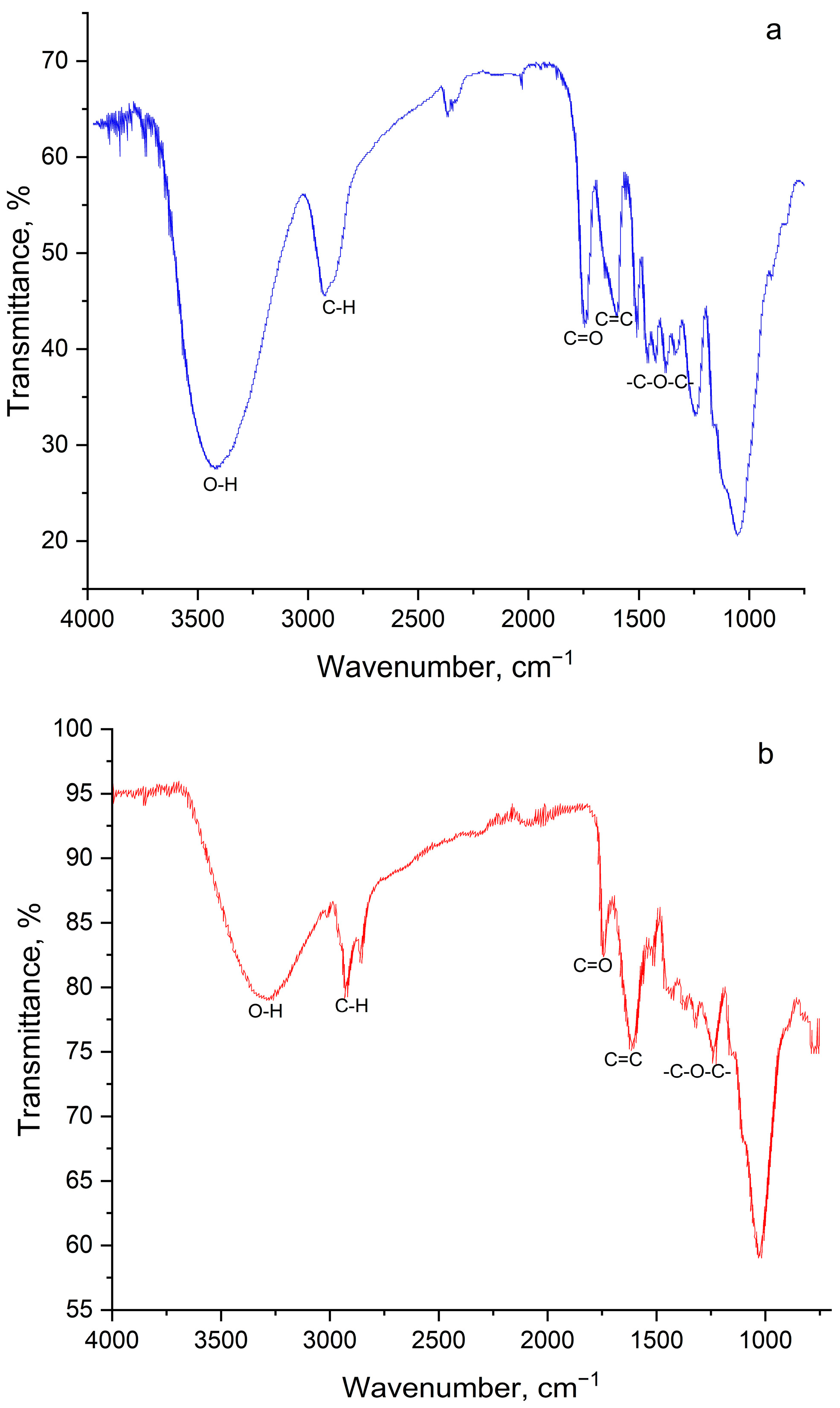
| Band Number | Phenylalanine Band, cm−1 | Acetone Band, cm−1 | PPI Band, cm−1 | Band Description |
|---|---|---|---|---|
| 1 | - | - | 3585.6 | 3602–3544, ν –OH, (intramolecular hydrogen bonds) |
| 2 | - | - | 3467.6 | 3470–3410, ν –NH2 |
| 3 | 3068.3 | - | - | ν –N–H |
| 4 | - | - | 3084.1 | 3100–3070, –NH3+ |
| 5 | - | - | 3060.2 | 3080–3030, ν –CH (aromatic) |
| 6 | 2994.8 | 3015.0 | - | ν –CH2, –CH3 |
| 7 | 2963.1 | 2966.0 | 2962.1 | 2988–2949, νs –CH2 |
| 8 | - | 2942.0 | - | |
| 9 | 2847.7 | - | 2847.9 | 3300–2500, ν –OH, ν –NH+, –NH2+, –NH3+ |
| 10 | - | - | 2687.8 | 2700–2250, ν –NH+, –NH2+ |
| 11 | - | - | 2573.9 | |
| 12 | - | - | 2516.7 | |
| 13 | 2384.5 | - | - | ν –C–NH+ |
| 14 | 2117.9 | - | - | |
| 15 | - | - | 1950.3 | 2000–1650, overtones of aromatic groups |
| 16 | - | - | 1875.1 | |
| 17 | - | - | 1809.8 | |
| 18 | - | 1730.0 | 1726.4 | 1730–1710, ν –C=O |
| 19 | 1622.6 | - | - | ν –C–NH3+ |
| 20 | - | - | 1612.4 | 1640–1530, ν –C=O |
| 21 | - | - | 1593.2 | |
| 22 | 1556.0 | - | 1557.3 | |
| 23 | 1493.8 | - | 1494.0 | 1520–1490, δs NH3+, ν –COO– |
| 24 | - | - | 1473.0 | 1525–1475, aromatic ring oscillation |
| 25 | 1456.3 | 1456.0 | 1445.3 | 1465–1440, aromatic ring oscillation, δ –CH2, –CH3 |
| 26 | - | 1434.0 | - | δ –CH2, –CH3 |
| 27 | 1408.6 | - | - | ν –COO−…HOOC– (dimers) |
| 28 | - | 1365.0 | 1340.5 | 1350–1280, ν –C–N, δ –CH-C=O |
| 29 | 1334.1 | - | - | ν C6H5–CH2–CH–NH (aromatic amines) |
| 30 | 1319.9 | - | - | |
| 31 | 1305.3 | - | 1305.3 | 1335–1300, fluctuations of ionic carboxyl in amino acids |
| 32 | 1292.5 | - | - | 1350–1280, ν –C–N |
| 33 | 1224.1 | 1227.0 | - | δ –CH2, –CH3 |
| 34 | - | 1215.0 | - | |
| 35 | 1161.9 | - | 1186.0 | 1200–1100, ν –C–N– |
| 36 | 1129.9 | 1090.0 | 1127.1 | |
| 37 | - | - | 1108.0 | |
| 38 | 1074.0 | - | 1073.2 | 1110–1070, δ –CH in aromatic |
| 39 | 1024.8 | - | 1034.3 | 1070–1000, δ –CH in aromatic |
| 40 | 1002.8 | - | - | ν C6H5– |
| 41 | 949.1 | - | 955.0 | 1000–960, δ –CH in aromatic |
| 42 | - | - | 927.1 | 955–890, δ –OH |
| 43 | 913.2 | - | 914.0 | |
| 44 | - | 892.0 | 868.0 | 900–860, δ –CH (in aromatic) |
| 45 | 848.3 | - | 845.4 | 900–650, δ –NH, ν –C–N– |
| 46 | 777.0 | 765.0 | 774.1 | 900–650, δ –C–H |
| 47 | 744.9 | - | 746.8 | |
| 48 | 697.7 | 697.0 | 696.3 | |
| 49 | 681.8 | 530.0 | 676.8 |
| Parameter | Value |
|---|---|
| Bulkdensity, g/cm3 | 0.73 |
| Particlesize, μm | 2.8–3.1 |
| Solubility | Soluble in water, DMSO, slightly soluble in ethanol, acetone and cyclohexane. |
| pH of aqueous solutions | 3.0–3.2 |
| Biochar Sample | Specific Surface Area According to BET, m2/g | Volume of Micropores, cm3/g | Mesopore Volume, cm3/g | Iodine Number, mg/g |
|---|---|---|---|---|
| WS before activation | 269.4 ± 25.8 | 0.18 ± 0.01 | 0.06 ± 0.01 | 283.8 ± 17.6 |
| WS after activation by KOH | 878.3 ± 65.4 | 0.67 ± 0.06 | 0.27 ± 0.02 | 712.3 ± 48.6 |
| Plant Processing Option | Plant Height, cm | Weight of Leaves, g/Plant | Weight of Roots, g/Plant | Dry Residue of Plant, % |
|---|---|---|---|---|
| Control | 28.33 ± 1.55 a | 65.04 ± 7.51 a | 7.12 ± 0.42 a | 10.15 ± 0.92 a |
| Pure biochar | 27.58 ± 1.36 a | 74.56 ± 7.33 b | 6.94 ± 0.35 a | 11.82 ± 1.07 a |
| KI | 28.86 ± 0.89 a | 77.72 ± 7.42 b | 7.31 ± 0.30 a | 13.27 ± 1.33 ab |
| BIOF | 28.63 ± 1.43 a | 86.55 ± 8.13 c | 8.25 ± 0.41 b | 12.35 ± 1.78 ab |
| Plant Processing Option | Iodine, mg/kg of d.w. | Organic Nitrogen, mg/kg of d.w. | ||
|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | |
| Control | - | - | 34.88 ± 2.75 a | 18.63 ± 1.64 a |
| Pure biochar | - | - | 35.25 ± 2.66 a | 17.32 ± 1.81 a |
| KI | 7.11 ± 0.72 a | 9.27 ± 0.83 a | 39.74 ± 3.41 a | 22.05 ± 1.39 b |
| BIOF | 11.86 ± 1.13 b | 13.23 ± 1.19 b | 57.37 ± 3.82 b | 36.63 ± 2.07 c |
| Plant Processing Option | Ascorbic Acid, mg/100 g d.w. (Dry Weight) | AOA, mg GA/g d.w. | Polyphenols, mg GA/g d.w. |
|---|---|---|---|
| Control | 23.31 ± 2.43 a | 29.64 ± 3.14 a | 18.62 ± 1.54 a |
| Pure biochar | 25.17 ± 2.32 a | 30.25 ± 3.03 a | 20.07 ± 1.37 a |
| KI | 23.97 ± 2.84 a | 42.04 ± 3.32 b | 21.15 ± 1.25 ab |
| BIOF | 36.46 ± 2.74 b | 44.48 ± 4.18 b | 23.79 ± 1.84 b |
| Main Indicators | BIOF | Iodized NaCl |
|---|---|---|
| Components | Low-cost biomass—walnut shell and chemicals—KOH, phenylalanine, and iodine. | Low-cost sodium iodine [51]. |
| Consumption of energy | High energy consumption during pyrolysis. | Low energy consumption during simple mixing and drying processes [52]. |
| Additional processing steps | Washing with distilled water and drying at 100–105 °C. | Additional processing steps are not required |
| Increasing the nutritional value of the treated plant | Makes agricultural crops enriched by iodine. | Iodine is primarily obtained through the consumption of salt in food. |
| Remediation of soil | Improves soil health through increased carbon sequestration and nutrient retention and protection against pathogenic microorganisms [53]. | Iodine fortification do not provides any environmental benefits. |
| Long-term financial benefits | Potential savings resulting from increased crop yields and decreased fertilizer requirements. | Long-term use of salt can lead to hypertensive diseases. |
| The cumulative effect | Although the initial investment may be greater, there are potential long-term advantages to be gained in terms of agricultural productivity and environmental sustainability. | Iodine supplementation offers a more affordable option, albeit with diminished long-term advantages. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabitov, A.; Turganbay, S.; Kerimkulova, A.; Doszhanov, Y.; Saurykova, K.; Atamanov, M.; Zhumazhanov, A.; Bolatova, D. 1-Carboxy-2-phenylethan-1-aminium Iodide 2-Azaniumyl-3-phenylpropanoate Crystals: Properties and Its Biochar-Based Application for Iodine Enrichment of Parsley. Appl. Sci. 2025, 15, 10752. https://doi.org/10.3390/app151910752
Sabitov A, Turganbay S, Kerimkulova A, Doszhanov Y, Saurykova K, Atamanov M, Zhumazhanov A, Bolatova D. 1-Carboxy-2-phenylethan-1-aminium Iodide 2-Azaniumyl-3-phenylpropanoate Crystals: Properties and Its Biochar-Based Application for Iodine Enrichment of Parsley. Applied Sciences. 2025; 15(19):10752. https://doi.org/10.3390/app151910752
Chicago/Turabian StyleSabitov, Aitugan, Seitzhan Turganbay, Almagul Kerimkulova, Yerlan Doszhanov, Karina Saurykova, Meiram Atamanov, Arman Zhumazhanov, and Didar Bolatova. 2025. "1-Carboxy-2-phenylethan-1-aminium Iodide 2-Azaniumyl-3-phenylpropanoate Crystals: Properties and Its Biochar-Based Application for Iodine Enrichment of Parsley" Applied Sciences 15, no. 19: 10752. https://doi.org/10.3390/app151910752
APA StyleSabitov, A., Turganbay, S., Kerimkulova, A., Doszhanov, Y., Saurykova, K., Atamanov, M., Zhumazhanov, A., & Bolatova, D. (2025). 1-Carboxy-2-phenylethan-1-aminium Iodide 2-Azaniumyl-3-phenylpropanoate Crystals: Properties and Its Biochar-Based Application for Iodine Enrichment of Parsley. Applied Sciences, 15(19), 10752. https://doi.org/10.3390/app151910752






