Abstract
This study focuses on the sandstone of the Kizil Grottoes as the research object. Sandstone samples reinforced with barium hydroxide nanoparticle (Ba(OH)2) solutions at different concentrations were subjected to mass and deformation monitoring, wave velocity tests, triaxial shear tests, and conventional mercury intrusion porosimetry (MIP) to investigate the reinforcement mechanism and effectiveness of nano-Ba(OH)2 on Kizil sandstone. The results indicate that after treatment with nano-Ba(OH)2, the strength and wave velocity of the sandstone samples significantly increased, with the 15% concentration showing the optimal reinforcement effect. Nano-Ba(OH)2 enhances the cementation between sandstone particles, alters pore morphology and size distribution, reduces capillary water rise height, and inhibits sulfate ion crystallization and recrystallization, thereby achieving the dual effects of strength reinforcement and deterioration prevention.
1. Introduction
China’s grotto temples, as invaluable treasures of cultural heritage, are widely distributed across the country, forming a continuous historical and artistic timeline from the 3rd to the 8th century [1]. These sites are predominantly carved into sandstone or limestone cliffs, and their preservation is perpetually challenged by environmental and anthropogenic factors. Among them, the grottoes in Xinjiang, located along the ancient Silk Road, hold exceptional importance for their unique synthesis of Indian, Central Asian, and Chinese Buddhist art. The Kizil Grottoes, situated along the Kashgar-Tarim Basin axis, represent not only the earliest but also the largest and most influential Buddhist cave complex in this region [1,2].
The geological setting of the Kizil Grottoes is a primary determinant of its deterioration processes. The cliff faces into which the caves are carved comprise alternating layers of Neogene sandstone and mudstone. This lithology is inherently vulnerable due to its weak cementation, high porosity, and low mechanical strength. Consequently, the structures are highly susceptible to a range of deteriorative processes, including surface weathering, fracturing, peeling, and pigment layer oxidation. Researchers [3,4] have employed Potassium Silicate (PS) solution to reinforce the Kizil sandstone, and the strength of the sandstone was improved after reinforcement. However, field observations reveal PS reinforcement induces white crystal precipitation on sandstone surfaces, compromising grotto esthetics and structural integrity. The research on the reinforcement of Kizil Grottoes sandstone is still ongoing.
As a novel nanomaterial, nano-barium hydroxide (Ba(OH)2) addresses the limitations of conventional barium hydroxide, such as poor permeability and low solubility. In recent years, Giorgi [5,6] and Lauren R. Hall [7] have used barium hydroxide nanoparticles for mural conservation. Barium hydroxide reacts with the large amount of sulfates present in the murals to form completely insoluble barium sulfate, resulting in effective consolidation and restoration. José Delgado Rodriguesa [8,9] conducted comprehensive studies on barium hydroxide consolidation for limestone with varying porosity, encompassing both laboratory experiments and field applications. Remarkably, the consolidation effects remained significant even after six years of field exposure. At present, there is a lack of applied research on the reinforcement of sandstone cultural relics with nano-barium hydroxide in China. Considering the high concentration of SO42− ions in the groundwater at the Kizil Grottoes, this study selected nano-barium hydroxide (Ba(OH)2) as the experimental material to investigate its consolidation mechanism and effectiveness for the Kizil Grottoes sandstone.
2. Materials and Methods
2.1. Experimental Materials
The strata exposed in the area surrounding the Kizil Grottoes primarily consist of the Neogene Miocene, Pliocene, and Quaternary formations, with lithologies dominated by sandstone, mudstone, sandy conglomerate, and Quaternary unconsolidated sediments. The sandstone used in the experiment was collected from the surrounding mountains of the Kizil Grottoes. During sampling, the strongly weathered surface layer was removed to obtain fresher sandstone specimens.
2.2. Experimental Methods
The chemical composition and mineralogical characteristics of five representative sandstone samples were analyzed by X-ray diffraction (XRD) and X-ray fluorescence (XRF) techniques. XRD and XRF analyses were conducted at the Laboratory of Mineralogy and Petrology, China University of Geosciences (Wuhan). XRD was performed using a D8 Advance X-ray diffractometer from Bruker AXS GmbH (Karlsruhe, Germany) with Cu-Kα radiation (40 kV, 40 mA), and XRF was carried out using a PANalytical Axios MAX spectrometer (Malvern PANalytical, Almelo, The Netherlands).
The poor cementation, low strength, and susceptibility to water-induced dispersion of Kizil sandstone preclude direct sample cutting. For this experiment, reconstructed sandstone specimens were prepared by disaggregating natural rock samples, mixing them with water from the Kizil Thousand Tears Spring, and compacting the mixture using a multi-layer compression method to form standard cylindrical specimens measuring 100 mm in height and 50 mm in diameter. Chemical analyses of ion concentrations in the Kizil Thousand Tears Spring water were conducted by titration method, with a precision of about 0.1 mg/L.
A multi-layer compression mold was used for sample preparation. The mold consists of five separate layers. For each layer, one-fifth of the total dry sand mass (72.6 g, calculated based on the target volume (39.27 cm3) and the measured natural density of the sandstone) and one-fifth of the total spring water volume (9 mL) were added. This moisture makes the mixture reach an optimal state for molding—cohesive enough to hold shape but without yielding excess water (i.e., no slurry flow). A mechanical jack was then used to compress the mixture to the predetermined height for that layer. Prior to adding the next layer, the compacted surface was carefully roughened with a spatula to ensure optimal bonding and integration between successive layers. This method significantly enhances the homogeneity and uniformity of the reconstructed sandstone specimens.
After the mass and height of the reconstructed sandstone specimens stabilized, they were consolidated using nano-barium hydroxide aqueous solutions at mass fractions of 5%, 10%, and 15%. The untreated reconstructed sandstone specimens served as the control group. This resulted in four test groups with varying concentrations, each containing three parallel specimens.
The saturated solubility of Ba(OH)2 is approximately 3.89 g/100 g water at 20 °C [10]. Therefore, the 5% (5 g/100 g) solution was undersaturated, while the 10% and 15% solutions were supersaturated and contained nano-particulate suspensions.
Common consolidation techniques include spraying, poulticing, immersion, and grouting [11]. We combined controlled dropper application (similar to spraying and grouting) with wrapped curing (similar to poulticing) to ensure even penetration and slow reaction. The final consolidation procedure was established as follows: 30 mL of the consolidant was applied using a dropper to evenly distribute 15 mL on each surface (front and back) of the specimen. During application, the dropping rate was carefully controlled to prevent surface irregularities. After complete penetration of the solution into the sandstone specimen, the specimen was wrapped with plastic film for curing over one week. For comparative purposes, three unwrapped specimens (one per concentration group) were also prepared and treated identically but without the wrapping step. After curing, the plastic film was removed, and changes in mass and height of the consolidated specimen were recorded until stabilization was achieved. Each specimen underwent three analytical procedures: ultrasonic wave velocity tests, triaxial shear tests under different confining pressures, and conventional mercury intrusion porosimetry (MIP). While those unwrapped specimens were used solely for visual comparison of the carbonation process and were not subjected to the subsequent mass monitoring, wave velocity, or mechanical tests.
It is acknowledged that reconstructed specimens are not identical to intact rock. However, this approach is widely accepted in heritage conservation studies due to the difficulty of sampling original materials.
3. Experimental Results
3.1. Petrological Properties of Kizil Sandstone
Integrated analysis of XRD (Figure 1) and XRF (Table 1) results revealed that the sandstone is primarily composed of quartz (average content: 28.3%) and feldspar (average content: 18.8%). The cementing materials are mainly argillaceous and carbonate cements, with total clay mineral content reaching 17.4%. XRD analysis showed that the clay fraction is dominated by illite and kaolinite. Since kaolinite is non-expansive and illite exhibits very low expandability, the overall expansion potential of the clay minerals is moderate.
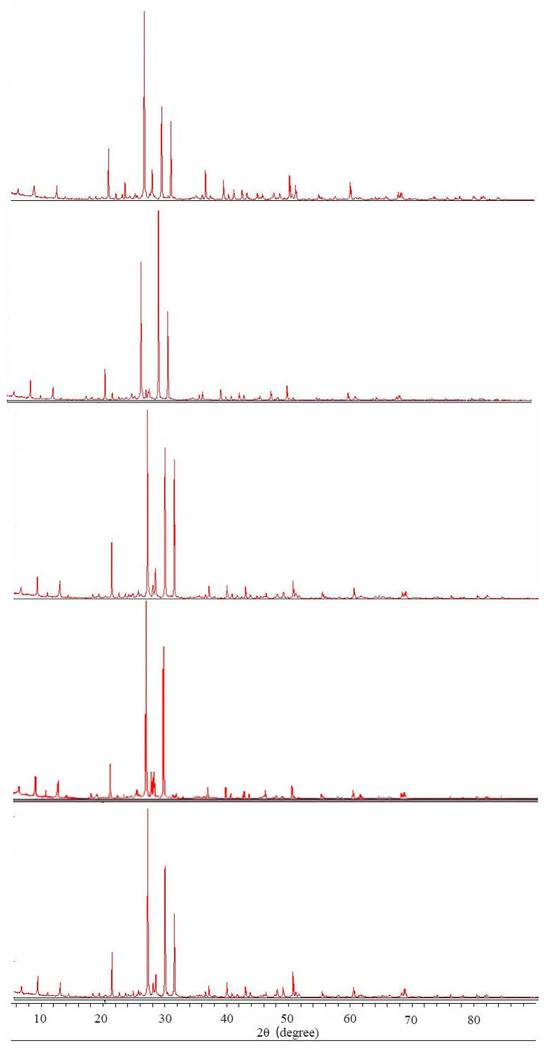
Figure 1.
XRD results of Kizil sandstone samples.

Table 1.
Chemical Composition Analysis of Kizil Sandstone by XRF.
Among carbonate minerals, calcite constitutes 23.4% while dolomite accounts for 13.1%. The rock is more accurately classified as a feldspathic sandstone with argillaceous and carbonate cement.
The ion concentrations, Total Dissolved Solids (TDS) and pH value of the Kizil Thousand Tears Spring were also tested. The results are presented in Table 2.

Table 2.
Ion concentrations, TDS and pH value of the Kizil Thousand Tears Spring water sample.
3.2. Mass and Deformation
As the study focuses on cultural heritage objects and their substrates, the consolidation of Kizil sandstone must adhere to the principles of minimal intervention and preservation of original appearance [12], with particular attention to the morphological impact of consolidants.
The mass and height changes in the consolidated specimens were measured using an electronic balance (accuracy: 0.01 g) and a vernier caliper (accuracy: 0.01 mm). The average values of mass and height for each group were plotted as curves in Figure 2 and Figure 3, where the abscissa value 0 represents the pre-consolidation state, and 1 corresponds to measurements taken immediately after dropping and curing for one week with the plastic film removed. The initial mass increase at day 1 is due to moisture retention.
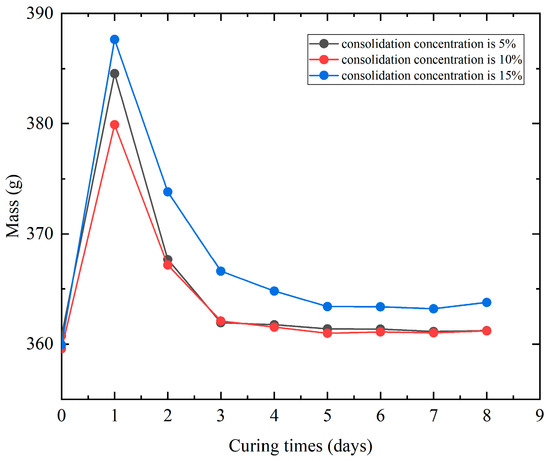
Figure 2.
Mass change after consolidation. The value 0 represents the pre-consolidation state and “day 1” measurement was taken after curing for one week and unwrapping.
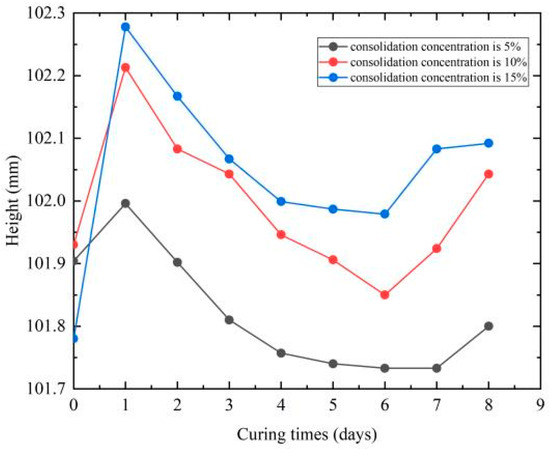
Figure 3.
Height change after consolidation. The value 0 represents the pre-consolidation state and “day 1” measurement was taken after curing for one week and unwrapping.
Figure 2 shows that the mass change-time curves exhibit similar trends across all concentrations, with specimen mass first decreasing and then slightly increasing with time. The consolidated specimens, after one week of curing, retained high moisture content with minimal water loss. Upon removal of the plastic film, rapid evaporation of free water occurred upon exposure to air, leading to significant initial mass reduction. Ultimately, the specimens exhibited a mass increase rate of 0.05–1.31%, with higher consolidant concentrations resulting in greater mass gain.
Figure 3 reveals that the height change-time curves follow similar trends across all concentrations, exhibiting consistent patterns with mass changes—an initial decrease followed by a slight increase over time. However, the 10% consolidated sample display minor erratic behavior, which is likely due to slight inhomogeneity in the reconstructed samples. Ultimately, specimens with 5% consolidation concentration showed height reduction, while those treated with 10% and 15% concentrations demonstrated height increase. Overall, the post-consolidation height variation ranged between 0.01 and 0.34%.
After PS (potassium silicate) treatment, the sandstone specimens exhibited a mass loss rate of 5.9–11.3% and a minimum volumetric shrinkage of 4.2% [2,3]. Compared to the PS treated samples, nano-barium hydroxide demonstrated significantly less impact on sandstone morphology, better complying with cultural heritage conservation principles.
3.3. Wave Velocity
Ultrasonic technology is a non-destructive testing method that reflects the structural characteristics and integrity of the sample by the change in elastic wave velocity. The Pundit Lab ultrasonic detector was used to measure the P-wave velocities across all specimen groups, with results presented in Table 3.

Table 3.
P-wave velocities of specimens under different consolidation concentrations.
Table 3 demonstrates that nano-barium hydroxide (Ba(OH)2) consolidation significantly enhanced the wave velocities of sandstone specimens. The wave velocity increased progressively with higher consolidation concentrations, indicating improved structural density and integrity. Specimens treated with 15% concentration achieved the maximum wave velocity—1.79 times that of untreated samples. Minimal variation (<2%) in repeated measurements confirmed uniform consolidation effects across all specimens.
3.4. Triaxial Shear Strength
Triaxial shear tests were conducted on each specimen group by employing the fully automated stress-path triaxial apparatus from the Institute of Rock and Soil Mechanics, Chinese Academy of Sciences. Unconsolidated undrained (UU) shear tests were performed under three confining pressures (50, 100, and 150 kPa) with a loading rate of 0.3 mm/min.
Figure 4 presents the deviator stress-axial strain curves of specimens under varying confining pressures. The curves reveal four distinct stages of deformation, namely compaction, elastic deformation, plastic deformation, and post-peak residual stage. The peak strength progressively increased with higher consolidation concentrations. Notably, specimens reinforced with 15% concentration exhibit prolonged yield stages compared to counterparts with other consolidation concentrations.
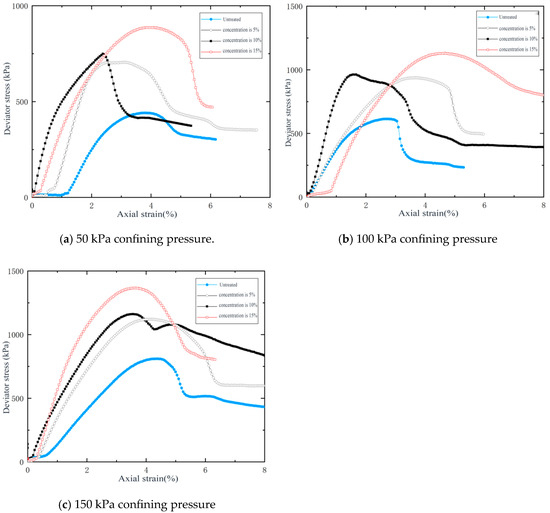
Figure 4.
Stress–strain curves under varying confining pressures.
According to the results of the triaxial shear test, the peak shear strengths and strength parameters under different confining pressures are summarized in Table 4.

Table 4.
Peak shear strengths and strength parameters of specimens under different consolidation concentrations and confining pressures.
It can be seen from Table 4 that the consolidated specimens exhibited significant improvement in peak strength, cohesion, and internal friction. The nano-barium hydroxide aqueous solution with a concentration of 15% demonstrated optimal strengthening effects. Under the confining pressure of 150 kPa, the peak strength reached 1.68 times that of the untreated specimen, while under 50 kPa, it increased to 2.01 times the untreated value. The cohesion and the internal friction angle were enhanced by factors of 2.18 and 1.13, respectively.
3.5. Pore Size Distribution and Pore Structure
Conventional mercury intrusion porosimetry (MIP) was performed on specimens consolidated with different concentrations using an AutoPore V 9620 porosimeter (Micromeritics Instrument Corporation, Norcross, GA, USA). The obtained MIP data were analyzed to generate pore size distribution curves for each treatment group, as presented in Figure 5.
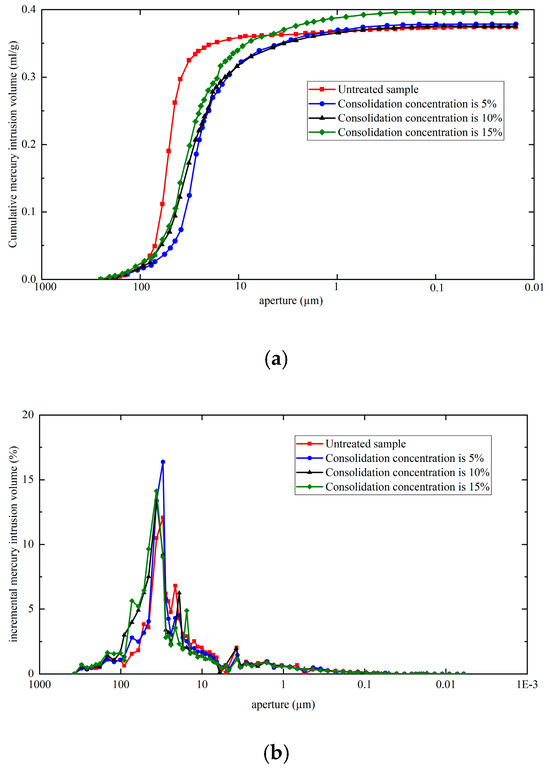
Figure 5.
Cumulative (a) and incremental (b) mercury intrusion volume of the sandstone specimens under different consolidation concentrations.
It can be seen from Figure 5 that nano-barium hydroxide treatment increased the cumulative mercury intrusion volume in sandstone specimens, indicating elevated porosity post-consolidation. The 15% concentration group showed the highest intrusion volume (0.3958 mL/g), exceeding untreated specimens by 0.0055 mL/g. The pore size distribution curves delineate three distinct regions corresponding to macro-pores, micro-pores, and sub-pores [13]. Quantitative analysis of these regions yielded the percentage pore volume distribution across different consolidation concentrations, as shown in Table 5.

Table 5.
Percentage of pore volume across different pore size ranges under different consolidation concentrations.
The pore volume distribution reveals that macro-pores (>8 μm) dominate the sandstone specimens, constituting the largest proportion of total porosity, while sub-pores (<1 μm) account for the smallest share. Nano-barium hydroxide (Ba(OH)2) significantly altered the pore size distribution characteristics, with treated specimens exhibiting reduced micro-pore and sub-pore fractions alongside increased macro-pore proportions.
4. Discussion
When nano-barium hydroxide infiltrates sandstone, multiple chemical reactions occur. Based on the experimental results and observed phenomena, the consolidation mechanism and effectiveness of nano-barium hydroxide on Kizil sandstone are systematically analyzed and summarized.
4.1. Consolidation Mechanisms
(1) Reaction of barium hydroxide with carbon dioxide:
Ba(OH)2 + CO2 → BaCO3 + H2O
Barium hydroxide reacts chemically with atmospheric carbon dioxide to form barium carbonate, a process commonly termed carbonation. The reaction between barium hydroxide and carbon dioxide is highly vigorous, rapidly precipitating abundant white barium carbonate crystals on the specimen surface (Figure 6). This may compromise the structural integrity and alter the specimen’s morphology. Consequently, post-consolidation wrapping with plastic film is essential to mitigate the carbonation rate, ensuring a controlled reaction process.
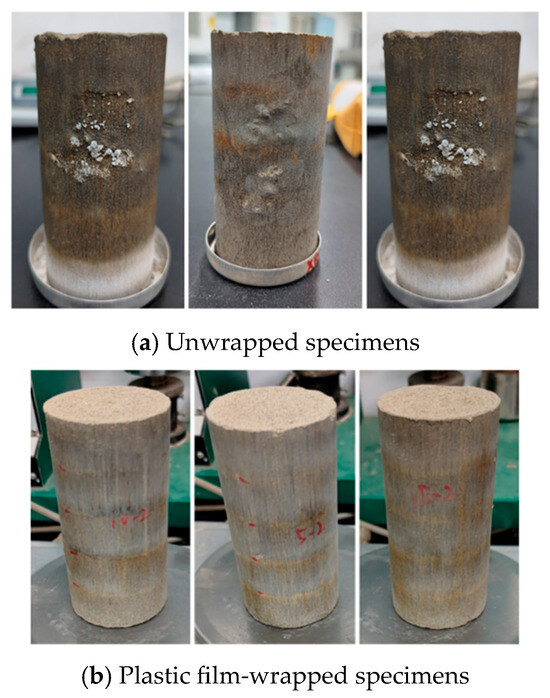
Figure 6.
Carbonation reaction of the specimens.
(2) Reaction of barium hydroxide with calcium carbonate:
CaCO3 + Ba(OH)2 → BaCO3 + Ca(OH)2
This reaction was initially proposed in the hypothesis by Lewin and Baer [14] and subsequently validated by Hansen [15]. In the short term after consolidation, barium hydroxide mainly undergoes carbonation. However, over extended periods, it participates in a double decomposition reaction with the carbonates in the sandstone, where Ca2+ ions in the calcite lattice is replaced with Ba2+ ions, ultimately forming barium carbonate and calcium hydroxide.
(3) Reaction of calcium hydroxide with carbon dioxide:
Ca(OH)2 + CO2 → CaCO3 + H2O
The newly formed calcium hydroxide in reaction (2) further reacts with atmospheric carbon dioxide to generate new calcium carbonate, which also constitutes carbonation reaction. Collectively, reactions (2) and (3) represent a process where original calcium carbonate is first decomposed, followed by the formation of new carbonate phases.
(4) Reaction of Barium hydroxide with sulfate ions:
Ba(OH)2 + SO42− → BaSO4 + 2OH−
Studies have demonstrated [14,15] that barium hydroxide nanoparticles can react with sulfate ions to form completely insoluble barium sulfate in environments with high sulfate concentrations.
4.2. Consolidation Effectiveness
(1) Mechanical Properties and Structure
In the monitoring of mass and deformation, the observed initial decrease followed by a slight increase in the mass-deformation curves is attributed to carbonation reactions. These reactions consume substantial gaseous CO2 to form solid barium carbonate. Given that CO2 has a higher relative molar mass (44 g/mol) than H2O (18 g/mol), the growth of BaCO3 crystals ultimately leads to a marginal increase in both the mass and height of the sandstone specimens.
In the wave velocity and triaxial shear tests, the consolidated specimens exhibited significant improvements in both wave velocity and strength, indicating that nano-barium hydroxide infiltration induced microstructural changes within the sandstone, resulting in denser and more intact fabric. The increase in strength stems from alterations in the internal friction angle and cohesion. The internal friction angle reflects particle interlocking and sliding resistance; And the cohesion arises from various physicochemical forces between particles, including Coulombic force, van der Waals force, cementation bonds, etc. [16]. The magnitude of these forces depends on grain spacing and cementation quality. The newly formed carbonates from nano-Ba(OH)2–sandstone reactions partially replenish weathered cementitious materials. At the same time, the nanoparticles exhibit large specific surface energy and strong cohesion [17], enabling stronger adsorption onto sandstone grains. Compared to the original cementation, these neo-formed bonds demonstrate enhanced adhesive capacity, stabilizing the granular framework and ultimately achieving effective reinforcement.
(2) Pore Size Distribution and Pore structure
The consolidation effectiveness of sandstone results from the competing effects of water-induced deterioration and nano-barium hydroxide reinforcement. The deterioration originates from water in the consolidant solution and reaction-generated water (e.g., Ba(OH)2 + CO2 → BaCO3 + H2O), while the reinforcement effect stems from the chemical reactions between nano-barium hydroxide, sandstone, and carbon dioxide. Successful consolidation requires the reinforcement effect to outweigh the deterioration effect.
From the results of wave velocity and triaxial strength tests, it can be seen that nano-barium hydroxide demonstrates effective consolidation of Kizil sandstone, with its reinforcing effect outweighing the water-induced deterioration. However, water’s detrimental impact persists. Results of MIP reveals that the porosity of the reinforced sandstone was larger than that of the untreated specimens. This phenomenon aligns with findings by Zhang [18] and Peng [19], where specimens exhibiting optimal strength enhancement paradoxically showed the highest porosity.
The observed increase in specimen porosity and pore size may be attributed to the following mechanisms: ① Under hydraulic pressure of water, pore throats expand and interconnect, enlarging pore channels [20]; ② The soluble salts (mainly gypsum and halite, which are present in the original rock due to environmental exposure) inside the sample dissolve in water, resulting in an increase in porosity; ③ High clay mineral content within the sample lead to water absorption and volumetric expansion, generating a large internal stress that disrupt pore structures, and ultimately elevating porosity.
Further analysis of MIP data enables the quantification of critical pore-throat characteristics in sandstone, as summarized in Table 6.

Table 6.
Pore throat parameters of pore structure characteristics.
Compared to untreated specimens, the 15% nano-Ba(OH)2 consolidated samples exhibit similar total pore area, but higher porosity and larger average pore diameter. For pores smaller than 6 μm, both groups show similar pore volumes (0.55 mL/g), yet the consolidated specimens display reduced pore surface area. This phenomenon confirms the established principle [21] that smaller pores generate greater surface area at equivalent volumes, thereby indicating that the nano-Ba(OH)2 treatment preferentially decreases micro- and sub-pore fractions through selective pore-filling and chemical cementation mechanisms. The resulting pore structure modification enhances macropore dominance while reducing surface-reactive microporosity, ultimately improving the sandstone’s mechanical stability despite increased overall porosity.
Compared to conventional consolidants, nano-barium hydroxide particles exhibit significantly smaller diameters, about 150 nm [22], and can penetrate into finer pores. Under the action of nano-barium hydroxide, part of the sub-pores and micro-pores are directly filled by reaction products (e.g., BaCO3), which chemically integrate with the sandstone matrix to form a consolidated monolith.
The pore size distribution fundamentally governs the height of capillary water rise. When the pore diameters fall below a critical threshold, known as the lower limit of flowing pore-throat radius [23], they lose permeability and have no effect on capillary action. This lower limit of pore-throat radius of the sandstone in the Qiuling Oilfield of the Turpan-Hami Basin is 0.4 μm [23], and that of the sandstone in the Dazu Rock Carvings is between 0.4 and 1.1 μm [24].
For Kizil sandstone, consolidation shifts the pore size distribution toward larger diameters, increasing the volume of pores exceeding the lower limit radius. Theoretically, this should enhance capillary rise. However, Peng’s research [19] on Leshan sandstone treated with nano-calcium hydroxide demonstrated the opposite trend: both capillary rise rate and height decreased This phenomenon stems from two synergistic mechanisms: (1) Physical pore-occlusion—insoluble CaCO3 precipitates selectively block critical capillary throats, disrupting hydraulic continuity; and (2) Chemical interfacial modification—the newly formed carbonate coating increases surface hydrophobicity, elevating the water contact angle by 32° and thereby weakening capillary adhesion forces.
Similarly to nano-calcium hydroxide, nano-barium hydroxide reacts with sandstone to form insoluble barium sulfate, which similarly suppresses capillary water rise and reduces the consequent deterioration of sandstone.
(3) Meso-structure
The meso-structure of the sandstone before and after consolidation was captured by an AO/CO series video microscope with a magnification of 116 times. As shown in Figure 7, Figure 7a–c are the microstructure of the fresh sandstone, the sandstone during consolidation treatment, and the surface morphology of the sandstone after consolidation, respectively, and Figure 7d is the barium carbonate crystal formed from nano-Ba(OH)2’s atmospheric carbonation.
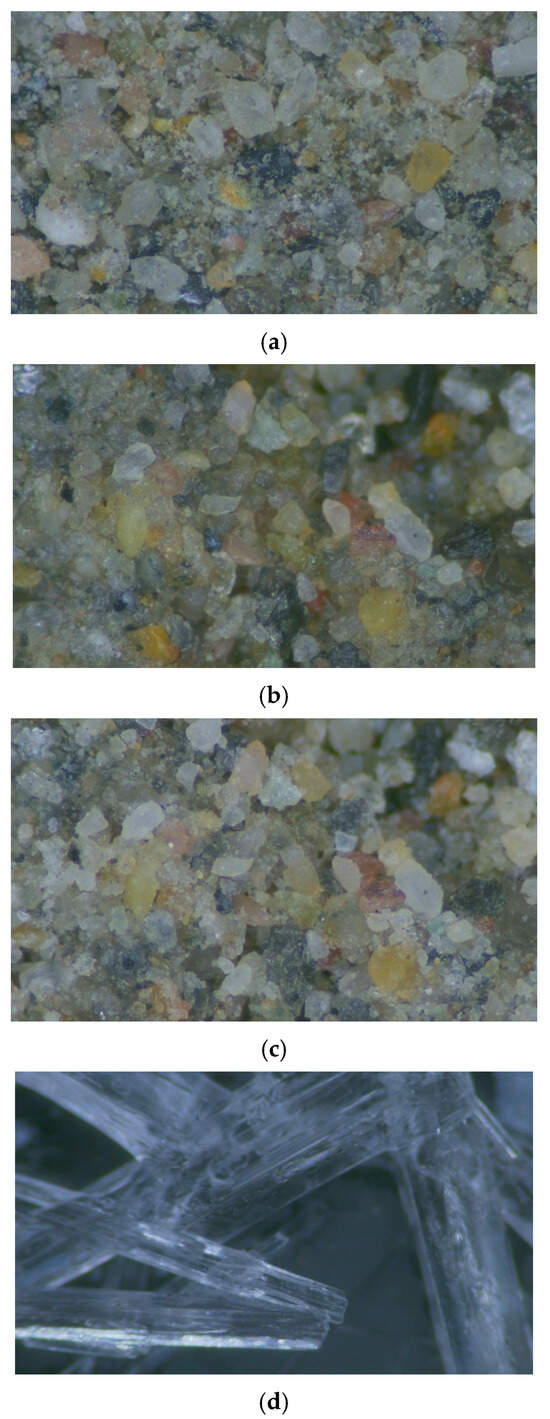
Figure 7.
Mesoscopic structural diagrams of the specimen. (a) Meso-structure of unconsolidated Kizil sandstone; (b) Meso-structure of Kizil sandstone during the consolidation process; (c) Meso-structure of the consolidated Kizil sandstone; (d) Meso-structure of barium carbonate (BaCO3) crystal.
As evidenced in Figure 7a–c, the nano-barium hydroxide (Ba(OH)2) consolidation process induces distinct microstructural transformations in sandstone. Initially, the treatment solution dissolves and flushes out pre-existing particulate materials from surface pores, resulting in enhanced pore visibility. Subsequently, chemical reactions between nano-Ba(OH)2, sandstone minerals, and atmospheric CO2 generate abundant white translucent barium carbonate (BaCO3) nanocrystals that adhere to grain surfaces. These newly formed particles exhibit irregular polygonal morphologies and demonstrate exceptional pore-filling capabilities, effectively reducing sub-micron pore volume while achieving grain contact coverage. The nanoscale cementation not only bridges micro- and sub-pores but also enhances intergranular bonding, ultimately creating a reinforced composite matrix that preserves structural integrity while maintaining vapor permeability.
Hydrochemical analysis and field investigations of the water sources near the Kizil Grottoes reveal that the high concentration of sulfate ions in the groundwater is the primary cause of salt efflorescence. In sulfate-rich environment, barium ions from nano-barium hydroxide react with sulfates to form completely insoluble barium sulfate. This reaction effectively suppresses the crystallization and recrystallization of salts, thereby mitigating salt-induced deterioration in both the grotto structure and mural paintings.
4.3. Implications for the Conservation of Kizil Grottoes
The laboratory-based results obtained from this study provide critical insights into the consolidation mechanism of nano-Ba(OH)2 and its potential applicability to the in situ preservation of the Kizil Grottoes. While the use of reconstructed samples was necessary for controlled experimentation, the fundamental chemical reactions and microstructural improvements observed are directly relevant to the natural sandstone on site.
The key consolidation mechanism—carbonation of Ba(OH)2 to form BaCO3 nanocrystals that enhance intergranular cementation and reduce harmful microporosity—is expected to function similarly in the natural sandstone of the grotto cliffs. Furthermore, the confirmed reaction between barium ions and sulfate ions present in the grotto’s environment to form insoluble BaSO4 is particularly promising. This dual function not only improves mechanical strength but also proactively inhibits salt crystallization damage, a primary threat to the wall paintings and structural integrity of the grottoes.
Translating these results to field application requires careful consideration. The low expansivity of the dominant clay minerals (illite and kaolinite) suggests that the treatment is unlikely to induce damaging stresses. However, potential differences in permeability, existing salts, and moisture content between reconstructed and natural sandstone must be addressed. Therefore, prior to any full-scale application, it is strongly recommended that in situ trials are conducted on discreet areas. These trials should monitor the penetration depth, effectiveness in strengthening, and, crucially, the long-term stability and esthetic compatibility of the treatment within the complex hygrothermal environment of the caves.
Furthermore, any conservation intervention must address potential health and safety risks. The toxicity and bio-accessibility of soluble barium compounds, particularly the initial consolidant Ba(OH)2, are recognized concerns. It is critical to emphasize that the intended final products within the treated sandstone are the highly insoluble and biologically inert barium carbonate (BaCO3) and, more importantly, barium sulfate (BaSO4)—the latter forming upon reaction with sulfates prevalent in the grotto environment. BaSO4 is widely used medically as a safe contrast agent due to its extreme inertness. For practical in situ application, stringent safety protocols for handlers (e.g., using personal protective equipment) and a post-application rinsing step to remove any soluble residues from the rock surface would be imperative to eliminate any risks to conservators and future visitors, thereby ensuring the treatment is not only effective but also safe.
In conclusion, this study establishes nano-Ba(OH)2 as a highly promising consolidant for the sustainable preservation of the Kizil Grottoes. Its ability to simultaneously reinforce deteriorated stone and mitigate sulfate-driven deterioration aligns perfectly with the principles of minimal intervention and long-term preventive conservation. The findings form a solid scientific foundation for designing effective conservation strategies for this invaluable cultural heritage site.
5. Conclusions
- (1)
- This study demonstrates that nano-barium hydroxide (Ba(OH)2) is a highly effective and compatible consolidant for the preservation of Neogene sandstone from the Kizil Grottoes. Under optimized application protocols, it induces negligible morphological alterations, with post-treatment mass change and deformation rates consistently below 1.31%, thereby adhering to the fundamental conservation principle of minimal intervention.
- (2)
- The treatment significantly enhances the mechanical integrity and structural cohesion of the sandstone. Specimens consolidated with a 15% nano-Ba(OH)2 solution exhibited the most substantial improvement, achieving a peak wave velocity increase by a factor of 1.79 and a triaxial shear strength up to 2.01 times that of untreated samples under a confining pressure of 50 kPa.
- (3)
- Beyond mechanical reinforcement, the treatment induces a favorable shift in pore structure by selectively reducing the proportion of micro- and sub-pores while increasing macro-pore dominance. This redistribution, coupled with the formation of new carbonate phases, contributes to a reduction in capillary water rise potential, thereby mitigating a key mechanism of moisture-driven deterioration.
- (4)
- The most significant contribution of this research to heritage conservation lies in its dual-function mechanism. This technology not only physically reinforces the decayed stone matrix but also provides a continuous chemical sequestration capacity for sulfate ions. This effectively inhibits the crystallization and recrystallization of soluble salts—a primary cause of deterioration at the Kizil Grottoes and many other sites worldwide. This proactive, preventive approach addresses the root cause of salt damage, offering a more sustainable conservation strategy.
- (5)
- By translating laboratory efficacy into a clear, practical framework for field application—including considerations for safety protocols and environmental control—this study provides a scientifically grounded and practically feasible protocol for mitigating sandstone deterioration in culturally significant contexts. It thus represents a meaningful advance in the toolkit for conserving earthen and sandstone heritage sites in arid and sulfate-rich environments.
Author Contributions
Conceptualization, Y.W. (Yujia Wang) and J.C.; methodology, R.G.; software, Y.W. (Yingbo Wu); validation, X.Y. and J.C.; formal analysis, G.W.; writing—original draft preparation, Y.W. (Yujia Wang); writing—review and editing, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Authors Yujia Wang, Ruitao Gao, Yingbo Wu, Xuwei Yang, Guirong Wei were employed by the company Qinghai Engineering Survey Institute Co., Ltd., China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Su, B. Studies on Chinese Grottoes; SDX Joint Publishing Company: Beijing, China, 2019. [Google Scholar]
- Yuan, H.H.; Swanson, B.F. Resolving pore space characteristics by rate—Controlled porosometry. SPE Form. Eval. 1989, 4, 17–24. [Google Scholar] [CrossRef]
- Yan, S.; Ye, M.; Chen, H.; Pi, L.; Li, W. Study on the Reinforcement Effect of PS on Kizil Sandstone. J. Yangtze River Sci. Res. Inst. 2015, 32, 55–59. [Google Scholar]
- Wang, L.; Yan, S.; Chen, J.; Dou, Y.; Ye, M. Anti-Freezing and Thawing Test of PS-Reinforced Sandstone in Kizil Grottoes. J. Yangtze River Sci. Res. Inst. 2017, 34, 130–133. [Google Scholar]
- Giorgi, R.; Ambrosi, M.; Toccafondi, N.; Baglioni, P. Nanoparticles for cultural heritage conservation: Calcium and barium hydroxide nanoparticles for wall painting consolidation. Chem.-A Eur. J. 2010, 16, 9374–9382. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, R.; Dei, L.; Baglioni, P. A New Method for Consolidating Wall Paintings Based on Dispersions of Lime in Alcohol. Stud. Conserv. 2000, 45, 154–161. [Google Scholar] [CrossRef]
- Hall, L.R.; Matero, F.G. Considerations on Complex Sequential Treatments of Gypsum Crusts: The Carrara Marble Capitals of the Philadelphia Merchants’ Exchange. J. Am. Inst. Conserv. 2011, 50, 123–148. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Pinto, A.P.F. Laboratory and onsite study of barium hydroxide as a consolidant for high porosity limestones. J. Cult. Herit. 2016, 19, 467–476. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Pinto, A.P.F.; Nogueira, R.; Gomes, A. Consolidation of lime mortars with ethyl silicate, nanolime and barium hydroxide. Effectiveness assessment with microdrilling data. J. Cult. Herit. 2018, 29, 43–53. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 4–45. [Google Scholar]
- Wang, L.; Dang, G.; Liang, G. Discussion on Weathering and Reinforcement Protection of Outdoor Stone Cultural Relics. Sci. Conserv. Archaeol. 2004, 16, 58–63. [Google Scholar] [CrossRef]
- Wang, L.; Dang, G.; Zhao, X.; Liang, G. Research Progress on Application of Reinforcement Materials in Protection of Stone Cultural Relics. J. Mater. Sci. Eng. 2004, 22, 778–782. [Google Scholar] [CrossRef]
- Chen, F. Study on Mesoscopic Pore Structure Characteristics of Salt Rock. Master’s Thesis, China University of Geosciences, Wuhan, China, 2013. [Google Scholar]
- Lewin, S.Z.; Baer, N.S. Rationale of the barium hydroxide-urea treatment of decayed stone. Stud. Conserv. 1974, 19, 24–35. [Google Scholar] [CrossRef]
- Hansen, E.; Doehne, E.; Fidler, J.; Larson, J.; Martin, B.; Matteini, M.; Rodriguez-Navarro, C.; Pardo, E.S.; Price, C.; de Tagle, A.; et al. A review of selected inorganic consolidants and protective treatments for porous calcareous materials. Stud. Conserv. 2003, 48 (Suppl. S1), 13–25. [Google Scholar] [CrossRef]
- Wang, D.; Ma, W.; Chang, X.; Sun, Z.; Feng, W.; Zhang, J. Influence of Freeze-Thaw Cycles on Physical and Mechanical Properties of Qinghai-Tibet Clay. Chin. J. Rock Mech. Eng. 2005, 23, 4313–4319. [Google Scholar]
- Zhang, L.; Mou, J. Nanomaterials and Nanostructures; Science Press: Beijing, China, 2001; p. 3. [Google Scholar]
- Zhang, X. Experimental Study on the Effect of PS Penetration Reinforcement of Earthen Relics in Humid Environment. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2011. [Google Scholar]
- Peng, L. Study on the Mechanism of Nano-Calcium Hydroxide Reinforcement and Hydraulic Property Improvement of Leshan Giant Buddha Sandstone. Master’s Thesis, China University of Geosciences, Wuhan, China, 2021. [Google Scholar]
- Su, X.; Tang, H.; Huang, L.; Shen, P.; Xia, D. The role of pH in red-stratum mudstone disintegration in the Three Gorges reservoir area, China, and the associated micromechanisms. Eng. Geol. 2020, 279, 105873. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, X.; Zhang, D. Study on Factors Affecting Capillary Water Rise Height in Coarse-Grained Soil. Site Investig. Sci. Technol. 2007, 01, 10–12+26. [Google Scholar]
- Wang, R.; Cui, X.; Wang, J.; Bai, C.; Li, Y. Preparation of Nano-Barium Hydroxide and Its Experimental Study on Reinforcing Earthen Relics. J. Nanyang Inst. Technol. 2021, 13, 69–72. [Google Scholar] [CrossRef]
- Zhao, S. Determination of the Lower Limit of Flow Pore Throat Radius by Using Pore Throat Volume-Permeability Contribution Distribution Curve. J. Southwest Pet. Inst. 1993, 03, 36–42. [Google Scholar]
- Song, J.; Yan, S.; Xiang, W.; Liu, J.; Zhao, G.; Jiang, S. Study on Influencing Factors of Capillary Water Migration Characteristics of Baoding Mountain Sandstone in Dazu Rock Carvings. Bull. Geol. Sci. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).