1. Introduction
The rapid advancement of integrated circuit and microelectronics manufacturing technologies has driven the miniaturization of electronic chips, achieving an increase in transistor count exceeding seven orders of magnitude in less than 50 years [
1,
2,
3]. This has led to higher power and heat flux densities [
4]. High heat flux density causes uneven temperature distribution, resulting in frequent local hotspots. This poses a severe challenge to thermal control technology. The resulting temperature issues significantly impact the performance and lifespan of electronic chips, becoming one of the primary causes of electronic device failures [
5,
6,
7]. Therefore, addressing thermal control issues in electronic devices urgently requires a material with high thermal capacity and excellent heat spreading capability as a passive cooling phase-change medium. Enhancing the thermal control capabilities of materials and investigating their reinforcement mechanisms are essential to address the increasingly stringent thermal challenges in electronic devices.
Hydrogels, as a novel phase-change energy storage material widely adopted in recent years, offer advantages such as no sedimentation, non-corrosiveness, low cost, and easy availability [
8]. They form through the cross-linking of hydrophilic polymer chains in an aqueous microenvironment. Gelation can be achieved via multiple mechanisms, including physical entanglement of polymer chains, electrostatic interactions, and covalent chemical cross-linking [
9,
10]. Their network backbone contains abundant hydrophilic groups such as -OH, -COOH, and/or -NH
2 [
11]. The water state can be altered by adjusting water-polymer interactions, offering significant advantages in mechanical strength, water activation, and dehydration processes. The heating dehydration process primarily involves the evaporation of bound water, intermediate water, and free water. Compared to free water, intermediate water requires less energy to evaporate [
12,
13,
14]. By adjusting the density of its internal molecular network and selecting polymer backbones with hydratable functional groups, the water state within its structure can be effectively altered. This significantly reduces the enthalpy of water evaporation while increasing vapor generation, thereby sustaining water absorption and transport. The result is a low thermal conductivity material suitable for engineering thermal protection applications [
15]. However, due to its unsatisfactory thermal conductivity, poor fluidity, lack of support, and the fact that the dehydration rate is severely affected by temperature, the higher the temperature, the faster the dehydration rate, and the water transport rate is much lower than the evaporation rate [
16], its thermal protection performance is difficult to meet the current environmental requirements, and there is an urgent need for structural optimization and improvement.
Current structural optimization approaches for hydrogel-based phase-change materials include incorporating nanoparticles such as ultrafine nanoparticles, carbon, metals, and oxides to form nano-reinforced phase-change materials, or embedding them into porous media to create CPCM [
17,
18,
19]. Composite phase-change materials can be obtained by mixing multiple PCMs through methods such as adsorption onto porous matrices, microencapsulation, or solid solution casting. These composites effectively combine the properties of various materials [
20,
21,
22]. Among these approaches, simply dispersing high-thermal-conductivity nanoparticles (e.g., carbon nanotubes, metal/metal oxide nanoparticles) into phase-change materials (PCMs) to enhance thermal conductivity yields limited results. Such simple dispersion methods fail to significantly improve thermal conductivity and cannot form effective heat transfer or wicking networks [
23,
24]. Conversely, thermal control systems demand phase-change materials with substantial latent heat capacity. This necessitates a trade-off between high thermal conductivity and large latent heat. Therefore, materials that offer controllable thermal conductivity while maintaining high phase-change material mass fraction represent the optimal choice. Consequently, materials with continuous porous structures are well-suited as composite porous framework in composite phase-change materials [
25]. Simultaneously, porous matrix adsorption methods are widely adopted due to their simplicity and high feasibility.
Current research on hydrogel composite phase-change materials primarily focuses on enhancing thermal conductivity by utilizing porous carrier materials with high thermal conductivity [
26]. While experimental analyses of material properties have been conducted, the main emphasis remains on comparing the cooling efficiency of composite phase-change materials and improving thermal conductivity. For instance, Kang Qian et al. [
27] conducted evaporative cooling experiments using porous flat-plate specimens in a hot-air tunnel, analyzing both the evaporative cooling effect and the operational duration of the coolant. X Chen et al. [
28] proposed a hydrophobic flexible CPCM exhibiting excellent thermal flexibility, reliability, and cycling stability. Their CPCM demonstrated a 106% increase in thermal conductivity, reducing battery temperature rise during operation by 19.2 °C and 8.9 °C compared to natural convection cooling, respectively. This demonstrates the CPCM’s significant potential for heat dissipation in lithium batteries and electronic components under humid conditions. Tingting Qian et al. [
29] embedded hydrogels into a porous medium made from diatomaceous earth powder, increasing its thermal conductivity by nearly 50% while maintaining good chemical stability. G Zhu et al. [
30] innovatively combined gel-like materials with porous copper foam and polyimide tape to create a composite phase-change material featuring high thermal conductivity and excellent electrical insulation. This material demonstrated superior thermal control capabilities, significantly reducing the temperature rise rate of a simulated CPU. Sijie Zhang et al. [
31] proposed a thermal control system based on water-based sodium polyacrylate hydrogel to address thermal shocks during lithium-ion battery pack operation. This system effectively reduces the temperature rise rate of the battery pack at different discharge rates and minimizes internal temperature differences during operation, thereby enhancing stability and safety during continuous charge–discharge cycles. Mingyu Song et al. [
32] developed a composite phase-change hydrogel using sodium acetate trihydrate as the phase-change material, graphene oxide as the photothermal agent, and acrylamide and konjac glucomannan as the supporting structure. Its three-dimensional network structure can control supercooling to 1.2–3.1 °C, and due to the high light absorption of graphene oxide, the photothermal conversion efficiency reaches 89.7%. However, from a fundamental mechanism perspective, the core reason for the overall performance change in composites with a porous framework during thermal evaporation lies in the pore-scale structural alterations. Therefore, investigating the mechanism by which a porous framework enhances hydrogel thermal control capabilities and visualizing this process are crucial for CPCM’s engineering applications and further optimization.
This study investigates the mechanism by which porous frameworks enhance hydrogel thermal control performance. By establishing a visualization experimental platform, we conducted multi-scale visual studies. We compared the thermal control performance of hydrogels and CPCM under heating and evaporation conditions, analyzing differences in their heat and mass transfer characteristics. The objective was to explore a mechanism for enhancing hydrogel thermal control capabilities, providing a foundation and reference for subsequent in-depth research.
2. Experimental Materials and Experimental Setup
2.1. Hydrogels and Composite Phase-Change Materials
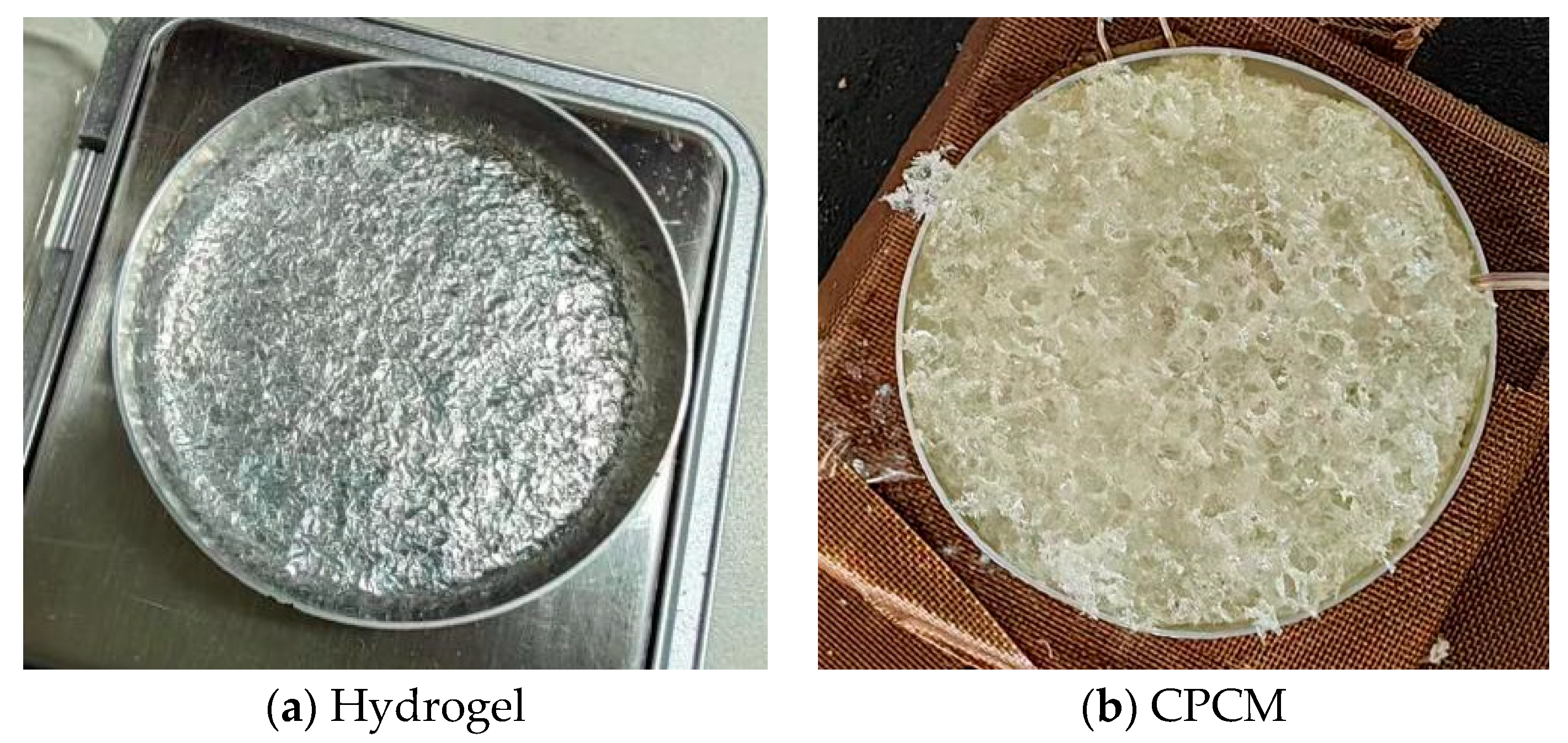
The hydrogel used in this experiment was of the super absorbent polymer type. After hydration, this material forms a typical gel state shown in
Figure 1a. The construction of CPCM adopts a multi-step composite process: Firstly, a porous frame is constructed through polyurethane foaming. Deionized water is precisely mixed with dry hydrogel material in a mass ratio of 17.7:1. Then, both the hydrogel and the porous framework are placed simultaneously in a sealed container, and the air is slowly extracted to create a negative pressure environment. As the pressure gradually decreases, the hydrogel will slowly embed into the porous skeleton, eventually forming the composite system as shown in
Figure 1b, in which the porous skeleton and the hydrogel phase form a filled network structure.
The hydrogel and CPCM used in the experiment were both controlled to have identical dimensions of 4 cm diameter and 2.5 cm thickness to eliminate the influence of thickness and heat dissipation area on the results. Additionally, hydrogel materials are reusable, with the processes of water absorption and desorption being relatively similar.
2.2. Visual Testing System
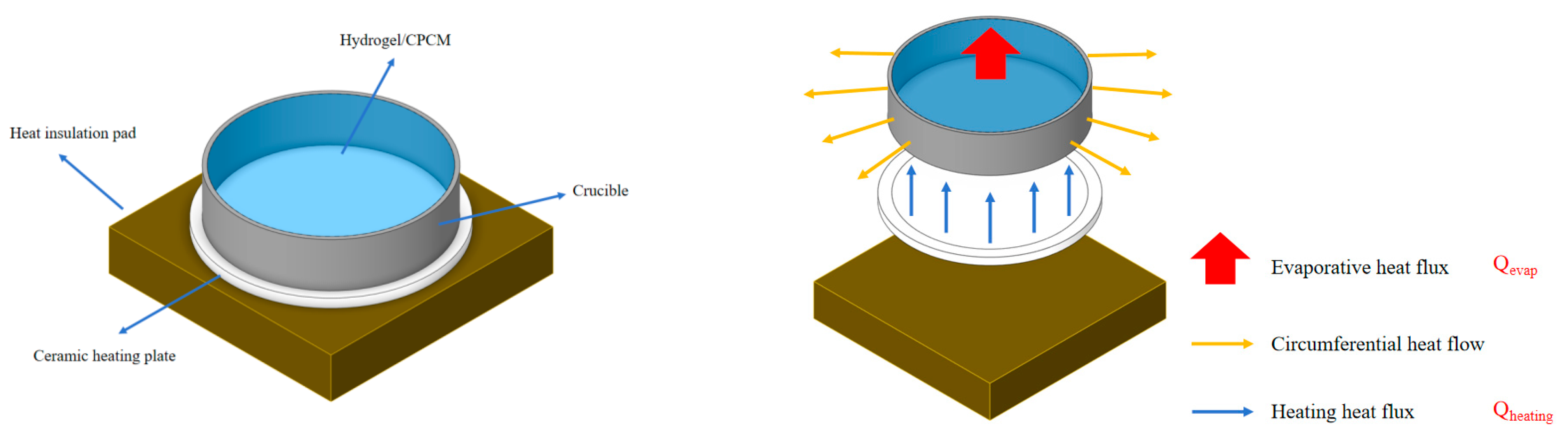
The visualization experimental platform for heat and mass transfer behavior in gel materials developed in this study comprises three components: a thermal field control system, an observation system, and a mass analysis system. The thermal field system integrates a 4 cm diameter ceramic heating plate with high heat flux density and a high-temperature resistant insulating substrate. Surface temperature is monitored in real time via armored thermocouples, and high-precision temperature control is achieved through a closed-loop system consisting of a temperature controller and a speed regulator. During experiments, a quartz Petri dish loaded with CPCM is placed at the heating element’s center for controlled heating. While maintaining constant temperature, the system records heat flux density and material surface temperature. The observation system combines an optical microscope with electronic control (MSD-4KMPA, Murzider Company, China) with high-speed camera (XM5 and, Fujifilm, China) equipment to capture micro-evaporation behavior within hydrogel pores and mass transfer structural changes within porous media, respectively. The mass analysis system, equipped with a microbalance (6003, Xi Niu Lai Bo, China), continuously records mass change data across varying temperature gradients and time series to decipher the material’s heat and mass transfer characteristics. This platform enables multi-scale dynamic correlation analysis—from microstructural evolution to macroscopic mass loss—through simultaneous thermal-mass-optical multimodal measurements. A schematic of the visualization experimental platform is shown in
Figure 2.
A calibrated K-type thermocouple was used to measure temperatures at the ceramic heating element and the top surface of the heated material. Due to the small volume of the material, the thermocouple has a high measurement accuracy (±0.2%), and only one thermocouple is used for temperature reading at each measurement point. Testing was conducted in a constant-temperature laboratory with ambient conditions of 22 °C and 45% humidity.
In the visualization experiment, the water absorption of hydrogels and the phase transition dehydration phenomenon of CPCM were observed. In the water absorption experiment of hydrogels, gel particles were placed on quartz observation glass plates, and water was added to the particles with a 5 μL injector until the material absorbed water and no longer expanded. In the phase-change dehydration experiment of CPCM, it was placed in an aluminum crucible for heating, and then placed on high-temperature resistant quartz observation glass for observation after heating for different periods of time. In infrared thermal imager experiments, it is necessary to perform central cutting on CPCM to better observe the internal temperature of the material. In the heat and mass transfer behavior experiment, the heating method of the material is consistent with that in the visualization method. The material was heated by controlling the ceramic heating plate at a constant temperature of 100 °C. The heat of the heating plate is transferred to the material through an aluminum crucible. The thermocouple can monitor the temperature more accurately and record it through a data acquisition instrument. In the experiment, the heating was carried out at 100 °C for 75 min, and the heating voltage and current were recorded through the temperature controller. The weight of the material should be measured and recorded every 5 min during the heating process.
2.3. Formula Explanations
The unit mass dehydration rate serves as a crucial parameter for evaluating dehydration efficiency within porous media or materials. It describes the ratio of mass reduction over a given time period to the unit sample mass. Its calculation formula is as follows:
In the formula: is the dehydration rate per unit mass, with the unit of 1/time; is the mass change within a certain period of time, usually representing the amount of water loss, with the unit of mass; represents the time interval, with the unit of time; is the total mass of the sample at the initial moment.
In practical applications, evaluating a material’s thermal control performance also involves assessing how effectively it dissipates heat from the heat source through evaporation rather than transferring it to the surrounding environment. Understanding energy efficiency further enables investigation into the mechanism behind the enhanced thermal control properties of CPCM-reinforced hydrogels, allowing for deeper exploration of their heat flux and energy efficiency. A schematic illustration is shown in
Figure 3.
According to its conduction path, the heat flow is divided into three components: (1) The input heat flow from the heater to the sample (Qheating); (2) The heat dissipated from the sample to the surrounding environment is undesirable as it heats the nearby components. (3) The useful heat removal by water evaporation (Qevap). Effective thermal control is achieved when a maximum portion of the heat is dissipated through the Qevap path, thereby minimizing heat accumulation. The more heat flow is conducted through this path, the better the material’s heat dissipation ability can be, and more heat will not be conducted to the wrong path, resulting in heat accumulation.
By comparing the heat flow carried away by water evaporation
during the heating process of different materials with the heating heat flow conducted by the ceramic heating plate to the heated object
, the energy efficiency of different materials can be obtained:
3. Results and Discussion
3.1. Thermal Control Performance of Hydrogel Composite Porous Framework
The different thermal control properties of the two materials can be measured by comparing the upper surface temperatures of the hydrogel and CPCM through constant-temperature heating, as shown in
Figure 4.
As shown in
Figure 4, the porous framework has a significant enhancing effect on the thermal control ability of hydrogel materials. Under the constant temperature heating condition of 100 °C, the upper surface temperature of pure hydrogel remains at a relatively high level and shows a monotonous and rapid upward trend over time in the later stage of heating. This indicates that its heat dissipation capacity is insufficient and its self-thermal regulation ability is limited. Heat continuously accumulates and it is very easy to enter a thermal saturation state, making it difficult to effectively cope with the continuous heat load. In contrast, CPCM demonstrates excellent and stable thermal control performance under various heating conditions. Under the same 100 °C heating condition, the surface temperature of CPCM is always significantly lower than that of pure hydrogel, and the heating curve is more gentle, eventually stabilizing, which reflects the effective heat conduction and temperature platform stabilization effect of the porous framework structure. When heated at 120 °C, CPCM still keeps the upper surface temperature at the lowest level all the time. Even under a higher heat load of 150 °C, CPCM can still quickly reach the same temperature platform as under heating conditions of 100 °C and 120 °C and maintain stability for a period of time, further demonstrating its excellent thermal adaptability and thermal shock resistance. This indicates that the introduction of a porous framework not only enhances the thermal control performance of the material, but more importantly, it can actively adjust the temperature under relatively low heating conditions, thereby significantly improving the thermal control reliability, stability and overall thermal control performance of the material. However, under relatively high-temperature heating conditions, the thermal control ability of the material can only be maintained for a relatively short period of time.
With the help of thermal imagers, the heat distribution of the material itself can be further analyzed, and the thermal control performance of the material can be better understood. The results obtained by cutting the material from the center and observing it are shown in
Figure 5.
By heating the same CPCM at different temperatures, it can be seen that after increasing the heating temperature by about 20 °C, the temperature of the upper surface and the center position of the material remained almost unchanged. This is consistent with the test data of the constant temperature heating test, further proving that CPCM has the ability to actively regulate temperature when facing different heating temperatures. The temperature was maintained within a relatively fixed range. Moreover, the temperature can still be controlled at a good level within a relatively close distance from the heating surface, indicating that the material still has excellent thermal control performance even when the thickness was relatively small. The heat distribution within the material presents a pyramid-shaped pattern. While maintaining the temperature in the central area of the material at a reasonable level, the temperature at the edges of the material will be even lower.
3.2. Visualization of Water Absorption and Phase Transformation in Hydrogels and CPCMs
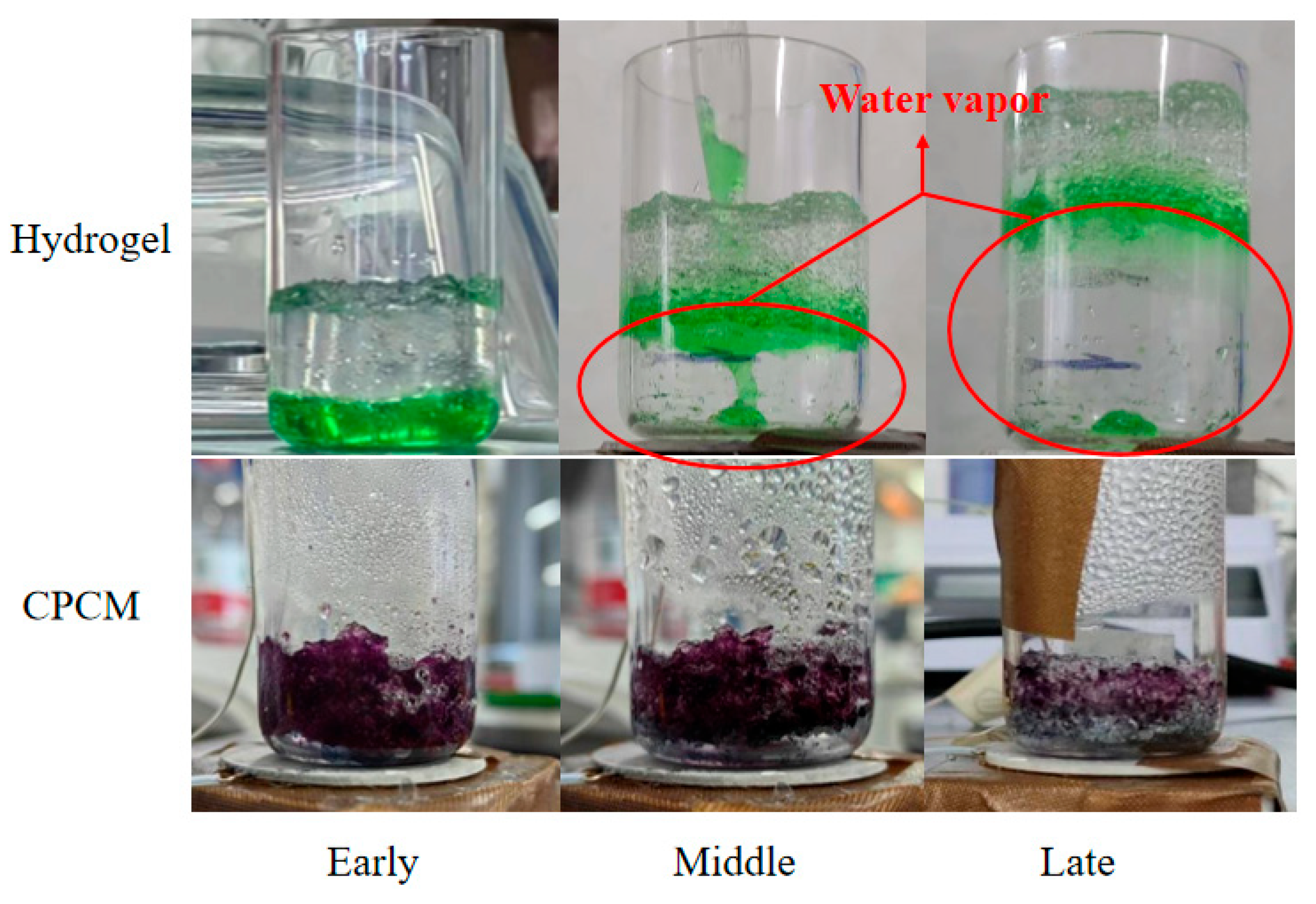
To investigate the mechanism by which a porous framework significantly enhances the thermal control capabilities of hydrogels, heating the hydrogel after staining it with CPCM and observing the results yields the phenomena depicted in
Figure 6.
After being heated for a period of time, the bottom surface of the hydrogel rapidly evaporates and dehydrates due to contact with the heating surface. However, the hydrogel’s overall low thermal conductivity and relatively dense structure make it difficult for heat to be evenly distributed throughout the material. Consequently, as heating continues, gas gradually accumulates at the bottom of the hydrogel, lifting the entire hydrogel to a certain height, as indicated by the red circle in the figure. CPCM continuously and steadily evaporates. Although it is also affected by the direct contact of the bottom with the heating surface and evaporates more thoroughly, the water vapor inside the structural material relying on the porous framework can be smoothly discharged to the external environment. The fact that there is always a large amount of condensate water above the beaker wall during the heating process indicates that the process of water evaporation inside the material turning into water vapor is stable and continuous. This to some extent explains why CPCM has more stable, long-lasting and excellent thermal control capabilities compared to hydrogels.
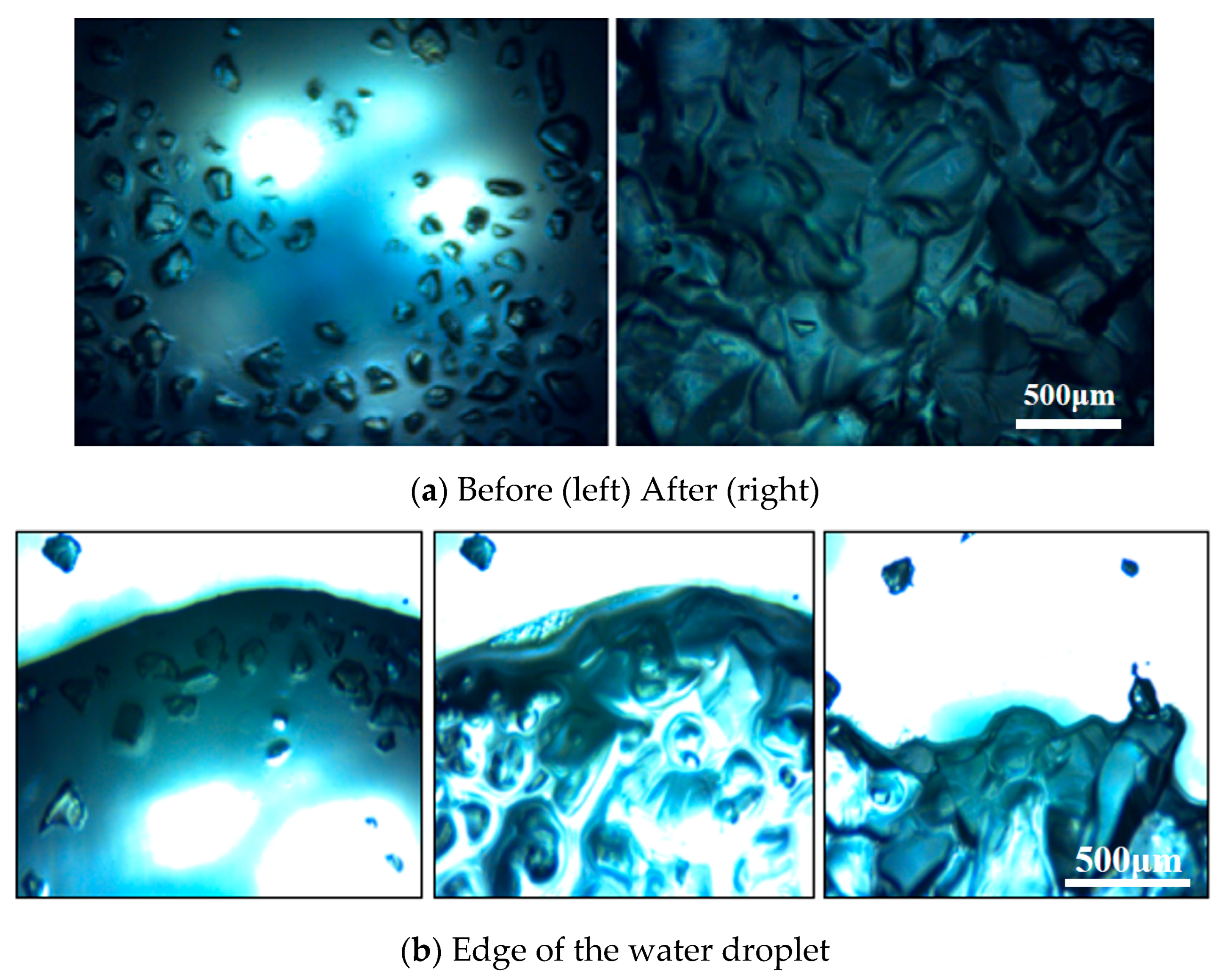
To better investigate the mechanism by which porous framework enhances the thermal control capabilities of hydrogels, it is necessary to observe the internal changes in the material at different scales. In visualization experiments, electron microscopy revealed morphological changes in multiple hydrogel particles before and after water addition, as shown in
Figure 7a. The comparison of hydrogel changes at the water droplet edge before and after water absorption is depicted in
Figure 7b.
As shown in
Figure 7, the hydrogel particles exhibit typical three-dimensional cross-linked network behavior during the swelling process. In the initial state, the particles present a dense polyhedral shape with low transparency. After coming into contact with water, the carboxylate ions on the sodium polyacrylate chain rapidly combine with water molecules under the drive of osmotic pressure difference, undergoing rapid volume expansion and first forming a semi-transparent gel block. As the water absorption process continues, the transparency of the particles in the saturated state increases significantly, their volume expands noticeably, and they gradually aggregate into larger gel clusters under the action of water’s surface tension. During the water absorption process, the droplet form is gradually replaced by gel, eventually forming a form similar to a “solidified liquid”, demonstrating its unique liquid fixation function. By observing the morphological changes in the hydrogel before and after water absorption, it can be seen that in the saturated state of water absorption, the material structure is dense and the air permeability is poor. When the moisture content is relatively low, the hydrogel undergoes coagulation and contraction, and its air permeability remains poor. This explains why water vapor cannot escape smoothly during the heating process and lifts the entire hydrogel.
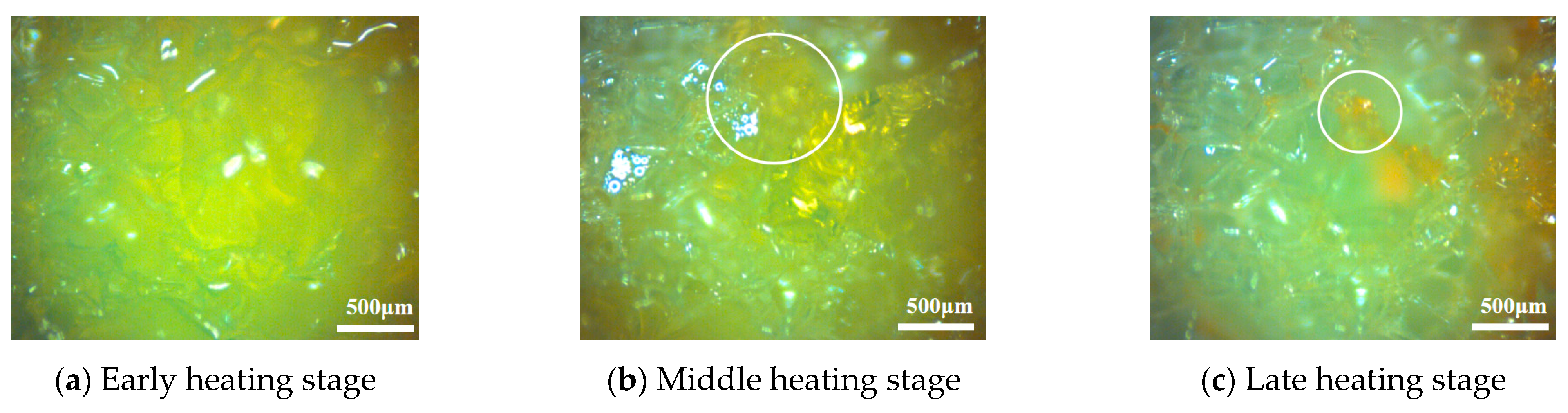
To explore the strengthening mechanism of CPCM, the internal structural changes in hydrogel and CPCM in the experiment were observed by electron microscopy. To achieve a significant effect, the hydrogel embedded with a porous framework was stained orange and subjected to a heating test. The observed test phenomena are shown in
Figure 8:
As shown in
Figure 8, the morphological changes in the hydrogel during different heating stages clearly reveal its interactions with the porous framework and the evolution of heat and mass transfer pathways during water evaporation. During the initial heating phase (
Figure 8a), the hydrogel fully expands after absorbing water, completely filling the pores within the porous framework. Its dense structure effectively retains water and maintains close contact with the framework material. At this stage, the porous framework uniformly transfers heat throughout the material. As heating progresses (
Figure 8b), the hydrogel gradually dehydrates and shrinks, causing its internal structure to collapse. Interparticle connections weaken, and the hydrogel particles gradually adsorb onto the scaffold surface, as indicated by the circle in
Figure 8b. Simultaneously, distinct cavities form within the scaffold pores. At this stage, the material’s air permeability and convective heat transfer capacity significantly increase. The porous framework provides excellent heat distribution while also offering escape pathways for water vapor. By the end of heating (
Figure 8c), the hydrogel is nearly completely dehydrated. The particles further shrink into dry solids adhering to the inner surface of the porous framework, as indicated by the circle in
Figure 8c, losing their water-trapping capability. These observations comprehensively document the dynamic process of hydrogel swelling to dehydration and shrinkage, elucidating the direct impact of phase transition behavior and structural evolution within the porous framework on heat and mass transfer. This provides a basis for subsequent quantitative analysis of the mechanism by which a porous framework enhances the thermal control properties of hydrogels.
3.3. The Influence of Porous Frameworks on the Dehydration Behavior of Hydrogels
Observations of the material at different scales reveal that the enhancement of hydrogel thermal control properties by the porous framework primarily relies on its porous structure optimizing internal water and heat transfer within the material. To better elucidate this enhancement mechanism, heating dehydration experiments were conducted on pure hydrogels and CPCM test samples to determine the weight loss of water over different heating durations. Since the initial masses of equal volumes of hydrogel and CPCM differ, the mass-specific dehydration rate was employed for analysis to eliminate mass-related effects.
After heating the two materials, the unit mass dehydration rate is shown in
Figure 9:
As shown in the figure, CPCM exhibits significantly different dehydration behavior compared to pure hydrogel. Without the thermal regulation provided by a porous framework, the dehydration rate of the pure hydrogel increases rapidly in Part 1 and Part 2, exhibiting a sustained upward trend. In Part 3, its dehydration flux per unit mass surges dramatically from 0.038 s−1 to 0.454 s−1, representing an increase of nearly 11-fold. This indicates that there is an obvious out-of-control phenomenon of water evaporation in the material, making it difficult to maintain a stable state under long-term heating. In contrast, CPCM exhibits a two-stage dehydration profile under the thermal regulation of its framework: During Part 1, the framework uniformly dissipates heat, enabling stable evaporation at a rate slightly higher than the hydrogel. Subsequently, as the hydrogel dehydrates and contracts, it forms interconnected pores. Although the dehydration rate in Part 2 slowly increases to 0.053 s−1, it remains lower than that of the hydrogel. This demonstrates that the porous framework significantly alters hydrogel dehydration behavior and breathability by uniformly dispersing and transferring heat. Simultaneously, CPCM maintains a lower dehydration rate and surface temperature throughout the heating cycle, indicating that the porous framework enhances hydrogel thermal conduction and moisture diffusion while providing stability. This further highlights CPCM’s superior sustained thermal control performance. Quantitative results show CPCM’s average dehydration rate throughout the cycle was 0.031 s−1, representing a 65% reduction compared to hydrogel’s 0.089 s−1. This indicates the porous framework enhances hydrogel thermal control primarily by optimizing internal heat and mass transfer through its porous structure.
To validate the strengthening mechanism, heating tests were conducted on CPCM at different temperatures, as shown in
Figure 10.
At 120 °C heating, CPCM maintains a dehydration rate similar to that observed at 100 °C heating, exhibiting stable internal evaporation and smooth water vapor release. This aligns with the temperature behavior measured during heating: increased temperature accelerates the phase-change contraction of the internal hydrogel, enhancing convective heat transfer efficiency and thereby maintaining temperature at a lower level. At 150 °C, the hydrogel undergoes rapid contraction. While it initially maintains a relatively stable dehydration rate and controls temperature at a low level during the early heating phase, the accelerated evaporation of internal moisture leads to severe water loss. The remaining moisture content becomes insufficient to sustain its thermal control properties. Consequently, both the upper surface temperature and dehydration rate exhibit a degree of uncontrolled variation during the later heating stages.
3.4. Characterization and Mechanism of Energy Efficiency Enhancement
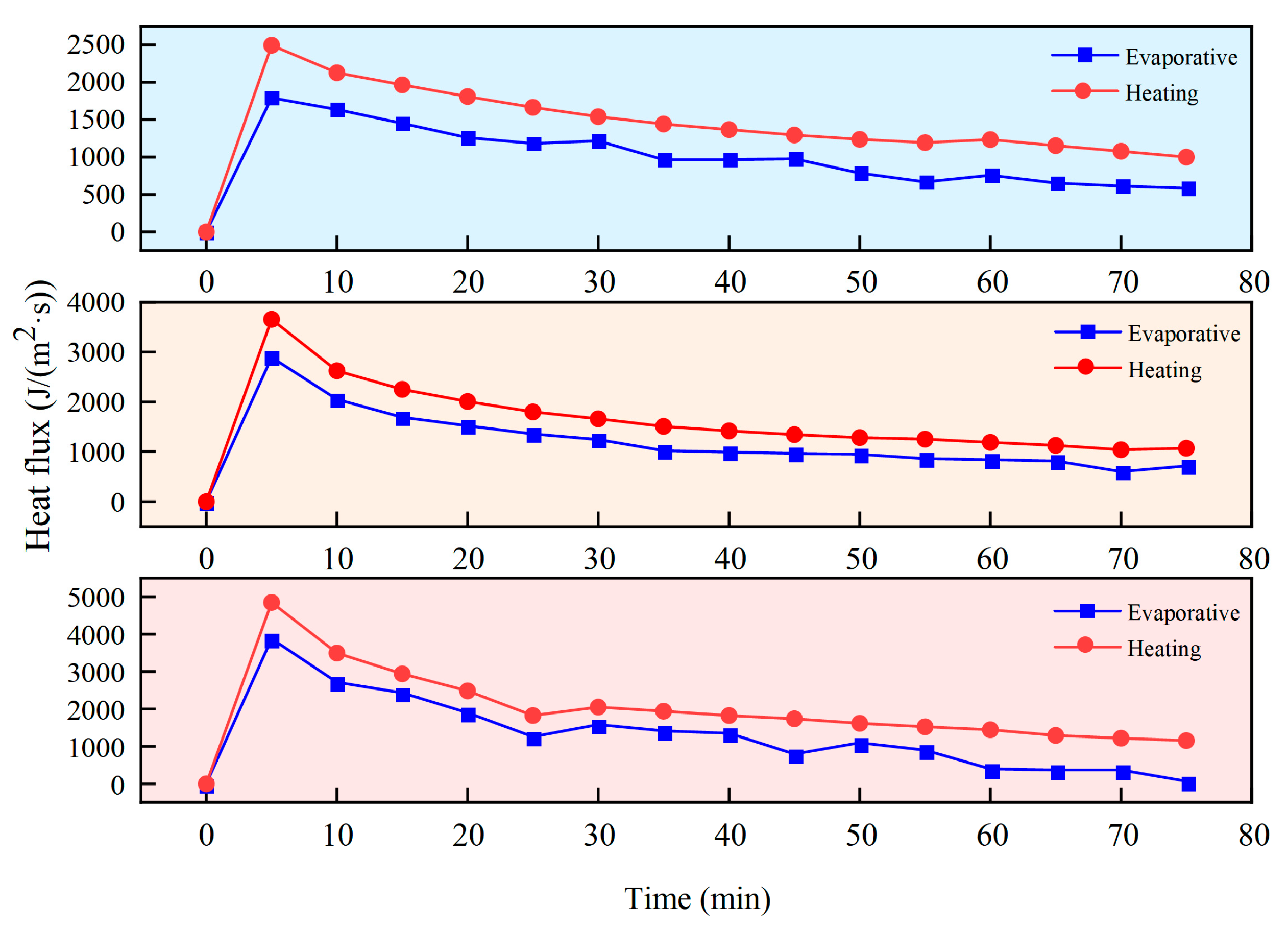
The heat flux calculation results are shown in
Figure 11. By comparing the evaporation heat flux and heating heat flux between pure hydrogel and the porous framework composite material, the differences in thermal response and thermal insulation capabilities between CPCM and hydrogel can be identified, thereby better explaining the causes for the variation in unit mass dehydration rate mentioned above.
Figure 11 shows that under identical constant-temperature heating conditions, CPCM experiences a significantly lower heating heat flux of only 2500 J/(m
2·s) compared to hydrogel, which undergoes a maximum heat flux approaching 4000 J/(m
2·s). This demonstrates that the low thermal conductivity of the porous framework material in CPCM enhances its ability to confine heat away from the heated surface, thereby better isolating external heat ingress. Simultaneously, the evaporation heat flux trend closely aligns with the heating heat flux trend in CPCM, confirming that the porous framework enables hydrogels to respond more effectively and promptly to heat flux changes, facilitating efficient heat dissipation. In contrast, the evaporation heat flux trend of the pure hydrogel deviates significantly from the heating heat flux trend, even diverging at certain time points. This inability to respond promptly to heat flux changes prevents efficient heat removal, leading to continuous heat accumulation during heating. These phenomena also explain why, compared to CPCM, the upper surface temperature of the hydrogel increases substantially while its mass-specific dehydration rate rises rapidly during the final heating stage.
Heating of CPCM at different temperatures yielded the results shown in
Figure 12.
At different heating temperatures, CPCM maintained relatively stable and timely heat flux response capabilities. As heating progressed, the slope of the material’s heat flux curve exhibited a consistent decreasing trend across all heating temperatures. In contrast, the slope of the hydrogel’s heat flux curve gradually increased during the later stages, further demonstrating that the porous framework endows the material with more enduring thermal control capabilities during prolonged heating.
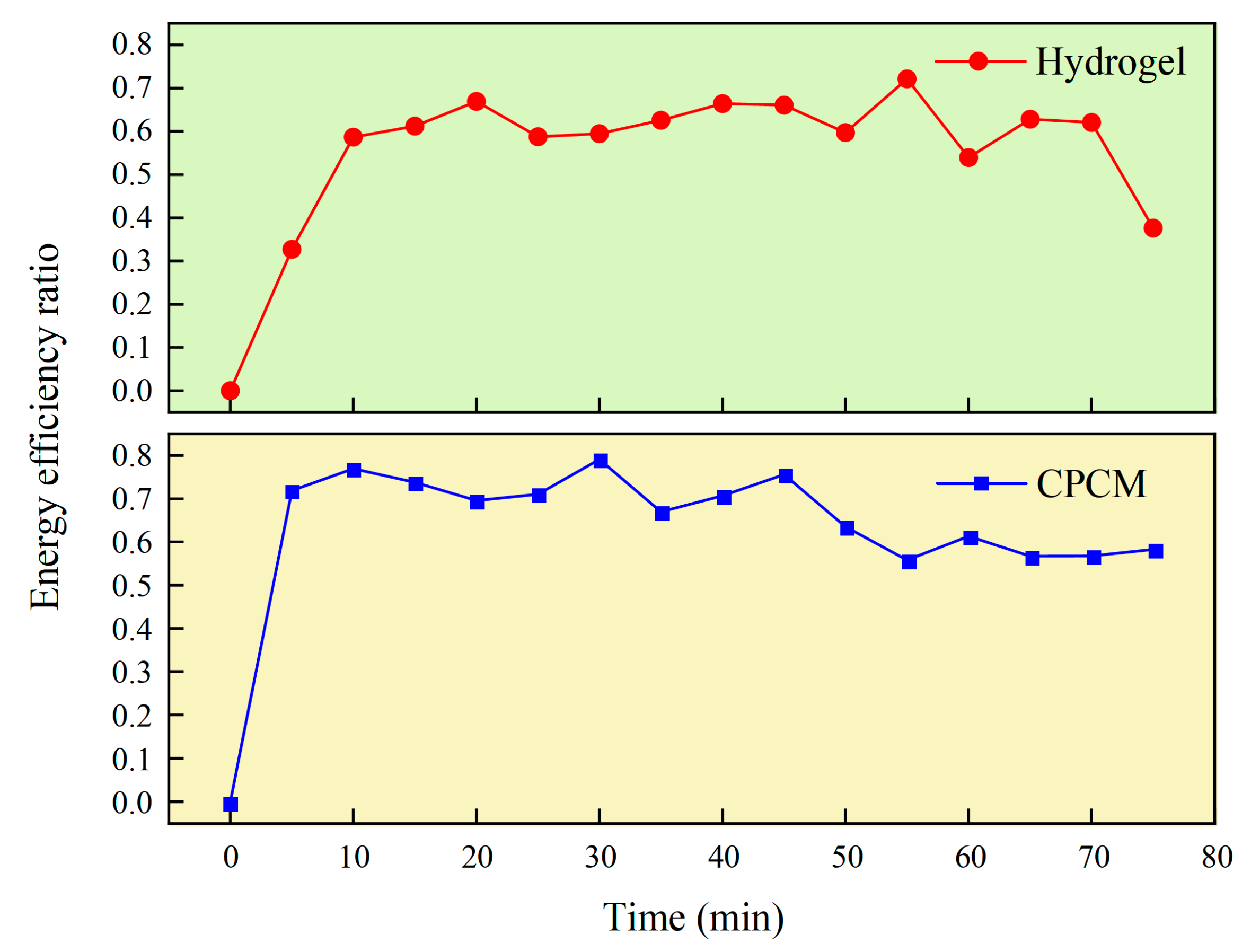
By comparing hydrogels and CPCM, the synergistic regulation of heat transfer and water evaporation by porous framework structures can be further elucidated, as shown in
Figure 13.
As heating progresses, CPCM exhibits excellent stability. During the first 25 min, its energy efficiency (η) remains essentially stable at approximately 0.71. Between 25 and 50 min, η fluctuates slightly before gradually decreasing to 0.63. This variation stems from the dehydration shrinkage of CPCM’s internal hydrogel occurring as a progressive process: initial heat accumulation during heating has minimal impact on efficiency. However, as temperature rises, localized heat accumulation before full hydrogel shrinkage causes fluctuations in evaporative heat flux, leading to changes in η. Only in the later heating stage does complete hydrogel shrinkage significantly optimize CPCM’s heat and mass transfer performance, causing the η curve to gradually stabilize. Additionally, the porous framework exerts dual regulatory effects during this process: First, capillary forces generated by the framework drive directional water transport toward the evaporation interface, preventing increased mass transfer resistance due to localized drying. Second, the framework structure stabilizes the material’s effective thermal conductivity, suppressing spatial inhomogeneity in heat flux density and thereby reducing ineffective heat dissipation.
In contrast, the pure hydrogel exhibited irregular η variation: it rapidly increased from 0.33 to 0.67 within the first 20 min and barely maintained stability. After 75 min, η abruptly dropped to 0.37, a 44.8% decrease. Simultaneous heat flux monitoring revealed that during this phase, the evaporation heat flux density decreased by 67.3%, while the mass dehydration rate increased by 23 times. This significant mismatch indicates that the internal structure of the hydrogel collapsed, allowing accumulated water vapor to escape rapidly. Further statistical analysis revealed that CPCM exhibited an average η value of 0.67, representing a 13.5% improvement over the hydrogel. Its coefficient of variation for η was 11.8%, a 34.1% reduction compared to the pure hydrogel’s 17.9%. This further confirms that the enhanced heat and mass transfer capacity enabled by the porous framework is the primary factor behind the material’s superior thermal control performance.
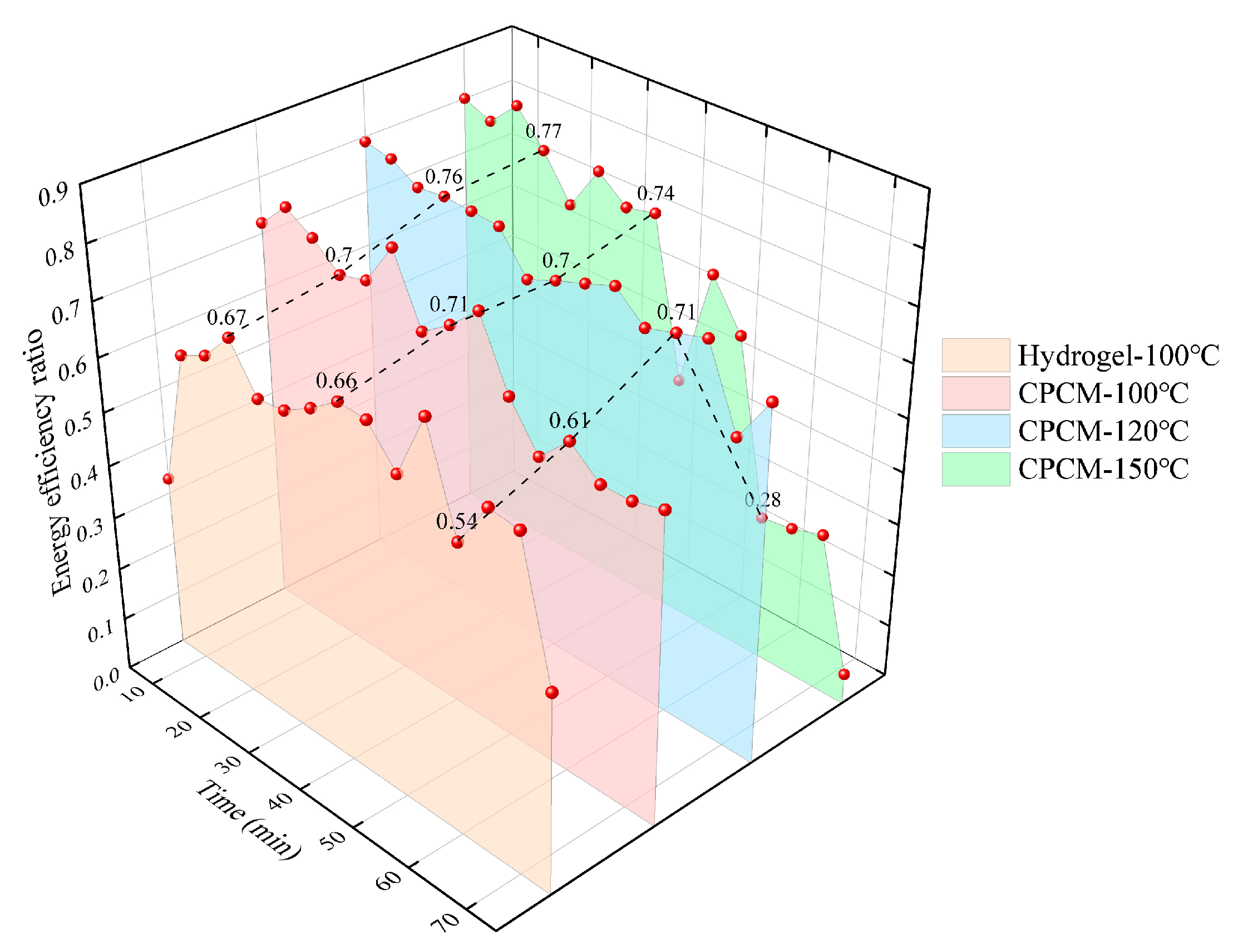
The effect of different heating temperatures on the energy efficiency of CPCM is shown in
Figure 14.
CPCM exhibits superior and more stable energy efficiency compared to hydrogels across different heating temperatures. As the heating temperature increases, the hydrogel within the material undergoes rapid evaporation phase-change due to the rapid thermal response induced by its porous framework, promptly dissipating the heat flux and thereby enhancing the material’s inherent energy efficiency. However, when subjected to both high temperatures and prolonged heating durations, CPCM’s energy efficiency rapidly declines if internal moisture levels are insufficient.
4. Limitations and Suggestions
This study primarily focuses on the performance of specific types of hydrogel and polyurethane foam scaffolds under controlled laboratory conditions. Consequently, the conclusions drawn are most directly applicable to this particular material system. The impact of environmental factors such as ambient humidity and airflow velocity on thermal control performance has not been systematically investigated, which may affect the practical applicability of the results.
The presence of a porous framework in CPCM significantly enhances the material’s thermal responsiveness. During the evaporative phase-change, the hydrogel gradually adsorbs onto the porous framework, creating numerous interconnected spaces that improve the material’s overall thermal control performance. Within this process, the internal moisture content is a critical factor in maintaining thermal control capabilities. Under extreme heating conditions involving high temperatures and prolonged exposure, the internal hydrogel rapidly contracts under heat to maintain a lower temperature. This substantially increases convective heat transfer within a short timeframe, further accelerating water evaporation. As moisture levels decrease during the later stages of heating, the material’s thermal control performance is significantly diminished. Therefore, future research should focus on increasing the water absorption ratio of materials, enhancing water content within the same volume, or employing methods to control the shrinkage rate of hydrogels. This approach aims to achieve superior and stable thermal control performance over extended periods. Concurrently, future research should explore broader porous framework structures—such as varying porosity, pore size, and thermal conductivity of the framework material—to uncover more universal enhancement mechanisms. Evaluating the long-term cyclic stability and reliability of composite phase-change materials under repeated thermal loading is essential to gauge their durability in practical applications. Furthermore, establishing empirical or numerical models that correlate framework properties—such as porosity and permeability—with overall thermal control performance holds significant value for optimizing material selection and design.