Effect of a Citicoline-Containing Supplement on Lipid Profile and Redox Status in Healthy Volunteers in Relation to Lifestyle Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Supplement
2.3. Study Design and Intervention
2.4. Sample Size Calculation
2.5. Lifestyle Variables
2.6. Biochemical Analyses
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CVD | Cardiovascular disease |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| MDA | Malondialdehyde |
| NCDs | Noncommunicable diseases |
| ROS | Reactive oxygen species |
| TAG | Triacylglycerols |
| TC | Total cholesterol |
| TT | Total thiols |
References
- Dubois-deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Wazir, M.; Olanrewaju, O.A.; Yahya, M.; Kumari, J.; Kumar, N.; Singh, J.; Abbas Al-itbi, A.Y.; Kumari, K.; Ahmed, A.; Islam, T.; et al. Lipid Disorders and Cardiovascular Risk: A Comprehensive Analysis of Current Perspectives. Cureus 2023, 15, e51395. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the Management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-Optimal Lipid Levels? Curr. Atheroscler. Rep. 2021, 23, 57. [Google Scholar] [CrossRef]
- Jung, E.; Kong, S.Y.; Ro, Y.S.; Ryu, H.H.; Shin, S. Do Serum Cholesterol Levels and Risk of Cardiovascular Death: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Int. J. Environ. Res. Public Health 2022, 19, 8272. [Google Scholar] [CrossRef]
- Banach, M.; Reiner, Ž.; Surma, S.; Bajraktari, G.; Bielecka-Dabrowa, A.; Bunc, M.; Bytyçi, I.; Ceska, R.; Cicero, A.F.G.; Dudek, D.; et al. 2024 Recommendations on the Optimal Use of Lipid-Lowering Therapy in Established Atherosclerotic Cardiovascular Disease and Following Acute Coronary Syndromes: A Position Paper of the International Lipid Expert Panel (ILEP). Drugs 2024, 84, 1541–1577. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Z.; Zhang, X.; Li, Z.; Guo, X.; Xie, Y.; Sun, Y.; Zheng, L. Non-Traditional Lipid Profiles Associated with Ischemic Stroke Not Hemorrhagic Stroke in Hypertensive Patients: Results from an 8.4 Years Follow-up Study. Lipids Health Dis. 2019, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Kloska, A.; Węsierska, M.; Malinowska, M.; Gabig-Cimińska, M.; Jakóbkiewicz-Banecka, J. Lipophagy and Lipolysis Status in Lipid Storage and Lipid Metabolism Diseases. Int. J. Mol. Sci. 2020, 21, 6113. [Google Scholar] [CrossRef]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.; Gutiérrez-Rojas, L.; Carretero, M.D.; Correll, C.U. Oxidative Stress Parameters and Antioxidants in Patients with Bipolar Disorder: Results from a Meta-Analysis Comparing Patients, Including Stratification by Polarity and Euthymic Status, with Healthy Controls. Bipolar Disord. 2021, 23, 117–129. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Asfandiyar; Hadi, N.; Ali Zaidi, I.; Kamal, Z.; Ashraf; Ullah Khan, R.; Rumman; Hashim Khan, M.; Omair, F. Estimation of Serum Malondialdehyde (a Marker of Oxidative Stress) as a Predictive Biomarker for the Severity of Coronary Artery Disease (CAD) and Cardiovascular Outcomes. Cureus 2024, 16, e69756. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Zeliger, H.I. Total Oxidative Stress and Disease. In Oxidative Stress. ItsMechanisms and Impacts on Human Health and Disease Onset; Zeliger, H.I., Ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2023; Volume 16, pp. 195–210. [Google Scholar]
- Gol, M.; Özkaya, B.; Yildirim, C.; Bal, R. Regular Exercise, Overweight/Obesity and Sedentary Lifestyle Cause Adaptive Changes in Thiol–Disulfide Homeostasis. An. Acad. Bras. Cienc. 2019, 91, e20180547. [Google Scholar] [CrossRef]
- Chen, X.Y.; Fang, L.; Zhang, J.; Zhong, J.M.; Lin, J.J.; Lu, F. The Association of Body Mass Index and Its Interaction with Family History of Dyslipidemia towards Dyslipidemia in Patients with Type 2 Diabetes: A Cross-Sectional Study in Zhejiang Province, China. Front. Public Heal. 2023, 11, 1188212. [Google Scholar] [CrossRef] [PubMed]
- Madan, K.; Sawhney, J.P.S. Exercise and Lipids. Indian Heart J. 2024, 76, S73–S74. [Google Scholar] [CrossRef]
- Badri Al-mhanna, S.; Leão, C.; Wan Ghazali, W.S.; Mohamed, M.; Batrakoulis, A.; Abiola Afolabi, H.; Daku Abubakar, B.; Aldhahi, M.I.; Gülü, M.; Hilal Yagin, F.; et al. Impact of Exercise on High-Density Lipoprotein Cholesterol in Adults with Overweight and Obesity: A Narrative Review. Ann. Appl. Sport Sci. 2024, 12. [Google Scholar] [CrossRef]
- Bendtsen, M.; Seiterö, A.; Bendtsen, P.; Henriksson, H.; Henriksson, P.; Thomas, K.; Löf, M.; Müssener, U. MHealth Intervention for Multiple Lifestyle Behaviour Change among High School Students in Sweden (LIFE4YOUth): Protocol for a Randomised Controlled Trial. BMC Public Health 2021, 21, 1406. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Xiao, F.; Bhusal, C.K.; Sabapathy, P.C.; Segal, R.; Chen, J.; Bai, X. Lipids Dysregulation in Diseases: Core Concepts, Targets and Treatment Strategies. Lipids Health Dis. 2025, 24. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Kirkpatrick, C.F.; Sikand, G.; Petersen, K.S.; Anderson, C.A.M.; Aspry, K.E.; Bolick, J.P.; Kris-Etherton, P.M.; Maki, K.C. Nutrition Interventions for Adults with Dyslipidemia: A Clinical Perspective from the National Lipid Association. J. Clin. Lipidol. 2023, 17, 428–451. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Barker-Collo, S.; Jones, K.M.; Parmar, P.; Bhattacharjee, R.; Feigin, V.L. MLC901 (NeuroAiD IITM) for Cognition after Traumatic Brain Injury: A Pilot Randomized Clinical Trial. Eur. J. Neurol. 2018, 25, 1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hamidu, S.; Yang, X.; Yan, Y.; Wang, Q.; Li, L.; Oduro, P.K.; Li, Y. Dietary Supplements and Natural Products: An Update on Their Clinical Effectiveness and Molecular Mechanisms of Action During Accelerated Biological Aging. Front. Genet. 2022, 13, 880421. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F.; Dempsey, R.J. Effects of Citicoline on Phospholipid and Glutathione Levels in Transient Cerebral Ischemia. Stroke 2001, 32, 2376–2381. [Google Scholar] [CrossRef]
- Grieb, P. Neuroprotective Properties of Citicoline: Facts, Doubts and Unresolved Issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef]
- Bermejo, P.E.; Dorado, R.; Zea-Sevilla, M.A. Role of Citicoline in Patients with Mild Cognitive Impairment. Neurosci. Insights 2023, 18, 26331055231152496. [Google Scholar] [CrossRef]
- Bonvicini, M.; Travaglini, S.; Lelli, D.; Antonelli Incalzi, R.; Pedone, C. Is Citicoline Effective in Preventing and Slowing Down Dementia?—A Systematic Review and a Meta-Analysis. Nutrients 2023, 15, 386. [Google Scholar] [CrossRef]
- Synoradzki, K.; Grieb, P. Citicoline: A Superior Form of Choline? Nutrients 2019, 11, 1569. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Citicoline Decreases Phospholipase A2 Stimulation and Hydroxyl Radical Generation in Transient Cerebral Ischemia. J. Neurosci. Res. 2003, 73, 308–315. [Google Scholar] [CrossRef]
- Secades, J.J.; Gareri, P. Citicoline: Pharmacological and clinical review, 2022 update. Citicolina: Revisión farmacológica y clínica, actualización 2022. Rev Neurol. 2022, 75, S1–S89. [Google Scholar] [CrossRef]
- Buckley, A.M.; Zaidan, S.; Sweet, M.G.; Ewin, D.J.; Ratliff, J.G.; Alkazemi, A.; Davis Birch, W.; McAmis, A.M.; Neilson, A.P. Choline Metabolism to the Proatherogenic Metabolite Trimethylamine Occurs Primarily in the Distal Colon Microbiome In Vitro. Metabolites 2025, 15, 552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huo, X.; Meng, Y.; Zhao, R.; Liu, X.; Chen, J.; Mao, Z.; Li, M. The Efficacy of Different Doses of Citicoline in Improving the Prognosis of Patients with Acute Ischemic Stroke Based on Network Meta-Analysis. Front. Pharmacol. 2025, 16, 1529647. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Garcia, V.; Muñoz, M.; Gonzales, K.; Ayala, E.; del Mar Sanchez, E.; Morilla-Grasa, A. The Effect of Oral Citicoline and Docosahexaenoic Acid on the Visual Field of Patients with Glaucoma: A Randomized Trial. Life 2022, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, B.; Devkota, H.P.; Poudel, A.; Poudel, P.; Thapa, R. Rosa spp. (Rosa canina L., R. Macrophylla Lindl., R. Moschata Herrm., R. Multiflora Thunb.). In Himalayan Fruits and Berries: Bioactive Compounds, Uses and Nutraceutical Potential; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–381. ISBN 9780323855914. [Google Scholar]
- Zhou, M.; Sun, Y.; Luo, L.; Pan, H.; Zhang, Q.; Yu, C. Road to a Bite of Rosehip: A Comprehensive Review of Bioactive Compounds, Biological Activities, and Industrial Applications of Fruits. Trends Food Sci. Technol. 2023, 136, 76–91. [Google Scholar] [CrossRef]
- Negrean, O.-R.; Farcas, A.C.; Nemes, S.A.; Cic, D.-E.; Socaci, S.A. Recent Advances and Insights into the Bioactive Properties and Applications of Rosa canina L. and Its by-Products. Heliyon 2024, 10, e30816. [Google Scholar] [CrossRef]
- Winther, K.; Rein, E.; Kharazmi, A. The Anti-Inflammatory Properties of Rose-Hip. Inflammopharmacology 1999, 7, 63–68. [Google Scholar] [CrossRef]
- Andersson, U.; Berger, K.; Högberg, A.; Landin-Olsson, M.; Holm, C. Effects of Rose Hip Intake on Risk Markers of Type 2 Diabetes and Cardiovascular Disease: A Randomized, Double-Blind, Cross-over Investigation in Obese Persons. Eur. J. Clin. Nutr. 2012, 66, 585–590. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A. Rosehip Extract Decreases Reactive Oxygen Species Production and Lipid Accumulation in Hypertrophic 3T3-L1 Adipocytes with the Modulation of Inflammatory State. Nutrients 2024, 16, 3269. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Shivashankara, A.R.; Rao, S.; George, T.; Abraham, S.; Colin, M.D.; Palatty, P.L.; Baliga, M.S. Tea (Camellia sinensis L. Kuntze) as Hepatoprotective Agent: A Revisit. In Dietary Interventions in Liver Disease: Foods, Nutrients, and Dietary Supplements; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 183–192. ISBN 9780128144664. [Google Scholar] [CrossRef]
- Li, A.; Wang, Q.; Li, P.; Zhao, N.; Liang, Z. Effects of Green Tea on Lipid Profile in Overweight and Obese Women A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Vitam. Nutr. Res. 2024, 94, 239–251. [Google Scholar] [CrossRef]
- Zamani, M.; Kelishadi, M.R.; Ashtary-Larky, D.; Amirani, N.; Goudarzi, K.; Torki, I.A.; Bagheri, R.; Ghanavati, M.; Asbaghi, O. The Effects of Green Tea Supplementation on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Front. Nutr. 2023, 9, 1084455. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Rezaei Kelishadi, M.; Larky, D.A.; Bagheri, R.; Amirani, N.; Goudarzi, K.; Kargar, F.; Ghanavati, M.; Zamani, M. The Effects of Green Tea Extract Supplementation on Body Composition, Obesity-Related Hormones and Oxidative Stress Markers: A Grade-Assessed Systematic Review and Dose-Response Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2024, 131, 1125–1157. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Meng, X. Aronia melanocarpa Anthocyanin Extracts Are an Effective Regulator of Suppressor of Cytokine Signaling 3-Dependent Insulin Resistance in HepG2 and C2C12 Cells. J. Funct. Foods 2020, 75, 104258. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Effects of Aronia melanocarpa on Cardiometabolic Diseases: A Systematic Review of Quasi-Design Studies and Randomized Controlled Trials. Rev. Diabet. Stud. 2022, 18, 76. [Google Scholar] [CrossRef]
- Pei, R.; Liu, J.; Martin, D.A.; Valdez, J.C.; Jeffery, J.; Barrett-Wilt, G.A.; Liu, Z.; Bolling, B.W. Aronia Berry Supplementation Mitigates Inflammation in T Cell Transfer-Induced Colitis by Decreasing Oxidative Stress. Nutrients 2019, 11, 1316. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia Berry Polyphenol Consumption Reduces Plasma Total and Low-Density Lipoprotein Cholesterol in Former Smokers without Lowering Biomarkers of Inflammation and Oxidative Stress: A Randomized Controlled Trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef]
- Seyedsadjadi, N.; Grant, R. The Potential Benefit of Monitoring Oxidative Stress and Inflammation in the Prevention of Non-Communicable Diseases (NCDs). Antioxidants 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Hillmann, K.; Hengst, K.; Englert, H. Effects of a Lifestyle Intervention on the Biomarkers of Oxidative Stress in Non-Communicable Diseases: A Systematic Review. Front. Aging 2023, 4, 1085511. [Google Scholar] [CrossRef]
- Porter, N.A.; Nixon, J.; Isaac, R. Cyclic peroxides and the thiobarbituric assay. Biochim. Biophys. Acta 1976, 441, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rahelić, V.; Perković, T.; Romić, L.; Perković, P.; Klobučar, S.; Pavić, E.; Rahelić, D. The Role of Behavioral Factors on Chronic Diseases—Practice and Knowledge Gaps. Healthcare 2024, 12, 2520. [Google Scholar] [CrossRef]
- Fatica, M.; Çela, E.; Ferraioli, M.; Costa, L.; Conigliaro, P.; Bergamini, A.; Caso, F.; Chimenti, M.S. The Effects of Smoking, Alcohol, and Dietary Habits on the Progression and Management of Spondyloarthritis. J. Pers. Med. 2024, 14, 1114. [Google Scholar] [CrossRef]
- Ji, X.-W.; Feng, G.-S.; Li, H.-L.; Fang, J.; Wang, J.; Shen, Q.-M.; Han, L.-H.; Liu, D.-K.; Xiang, Y.-B. Gender Differences of Relationship between Serum Lipid Indices and Type 2 Diabetes Mellitus: A Cross-Sectional Survey in Chinese Elderly Adults. Ann. Transl. Med. 2021, 9, 115. [Google Scholar] [CrossRef]
- Joshi, B.R.; Yadav, T.; Amit, A.K.; Rizal, S. Gender Differences in Lipid Profile in Dyslipidemic Patients Visiting Hetauda Hospital. Med. Phoenix 2024, 9, 7–11. [Google Scholar] [CrossRef]
- Agnello, F.; Ingala, S.; Laterra, G.; Scalia, L.; Barbanti, M. Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management. J. Clin. Med. 2024, 13, 1251. [Google Scholar] [CrossRef]
- Capuozzo, M.; Ottaiano, A.; Cinque, C.; Farace, S.; Ferrara, F. Cutting-Edge Lipid-Lowering Pharmacological Therapies: Improving Lipid Control beyond Statins. Hipertens. Y Riesgo Vasc. 2025, 42, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Saber, S.; Ramadan, A.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; Youssef, M.E. Unveiling Citicoline’s Mechanisms and Clinical Relevance in the Treatment of Neuroinflammatory Disorders. FASEB J. 2024, 38. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, N.; Algeda, F.R.; Shedid, S.M. The Impact of Citicoline on Brain Mitochondrial Dysfunction Induced in Rats after Head Irradiation. Sci. Rep. 2025, 15, 25658. [Google Scholar] [CrossRef]
- Xu, R.; Yang, K.; Li, S.; Dai, M.; Chen, G. Effect of Green Tea Consumption on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2020, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Salehi-Abargouei, A.; Mozaffari, Z.; Hemayati, R. The Effect of Green Tea (Camellia sinensis) on Lipid Profiles and Renal Function in People with Type 2 Diabetes and Nephropathy: A Randomized Controlled Clinical Trial. Front. Nutr. 2023, 10, 1253275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, C.W.; Kim, J.K.; Shin, H.J.; Baik, J.H. GCG-Rich Tea Catechins Are Effective in Lowering Cholesterol and Triglyceride Concentrations in Hyperlipidemic Rats. Lipids 2008, 43, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ding, S.; Li, F.; Zhang, C.; Sun-Waterhouse, D.; Chen, Y.; Li, D. Effects of (+)-Catechin on the Differentiation and Lipid Metabolism of 3T3-L1 Adipocytes. J. Funct. Foods 2019, 62, 103558. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective Effects and Molecular Mechanisms of Tea Polyphenols on Cardiovascular Diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef]
- Basu, T.; Selman, A.; Reddy, A.P.; Reddy, P.H. Current Status of Obesity: Protective Role of Catechins. Antioxidants 2023, 12, 474. [Google Scholar] [CrossRef]
- Go, M.Y.; Kim, J.; Jeon, C.Y.; Shin, D.W. Functional Activities and Mechanisms of Aronia melanocarpa in Our Health. Curr. Issues Mol. Biol. 2024, 46, 8071–8087. [Google Scholar] [CrossRef]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and Other Dietary Polyphenols: Potential Mechanisms of Metabolic Actions and Therapy for Diabetes and Obesity. Am. J. Physiol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Suh, K.S.; Kim, Y.I.; Jang, B.-K.; Kim, B.-H.; Yim, S.-V. Bioactive Fraction of Aronia melanocarpa Fruit Inhibits Adipogenic Differentiation of Cultured 3T3-L1 Cells. Appl. Sci. 2021, 11, 9224. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Allowitz, K.; Taylor, J.; Harames, K.; Yoo, J.; Baloch, O.; Ramana, K. V Oxidative Stress-Mediated Lipid Peroxidation-Derived Lipid Aldehydes in the Pathophysiology of Neurodegenerative Diseases. Curr. Neuropharmacol. 2025, 23, 671–685. [Google Scholar] [CrossRef]
- Shawki, H.A.; Elzehery, R.; Shahin, M.; Abo-hashem, E.M.; Youssef, M.M. Evaluation of Some Oxidative Markers in Diabetes and Diabetic Retinopathy. Diabetol. Int. 2020, 12, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Shaafi, S.; Hadisi, F.; Mahmoudinezhad, M.; Razmi, H.; Nejadghaderi, S.A.; Khalili, M. The Significance of the Oxidative Stress Markers in the One-Year Prognosis of Patients with Acute Ischemic Stroke: A Case-Control Study. BMC Neurol. 2021, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Merino de Paz, N.; García-González, M.; Gómez-Bernal, F.; Quevedo-Abeledo, J.C.; de Vera-González, A.; López-Mejias, R.; Abreu-González, P.; Martín-González, C.; González-Gay, M.; Ferraz-Amaro, I. Relationship between Malondialdehyde Serum Levels and Disease Features in a Full Characterized Series of 284 Patients with Systemic Lupus Erythematosus. Antioxidants 2023, 12, 1535. [Google Scholar] [CrossRef]
- Merino de Paz, N.; Carrillo-Palau, M.; Hernández-Camba, A.; Abreu-González, P.; de Vera-González, A.; González-Delgado, A.; Martín-González, C.; González-Gay, M.; Ferraz-Amaro, I. Association of Serum Malondialdehyde Levels with Lipid Profile and Liver Function in Patients with Inflammatory Bowel Disease. Antioxidants 2024, 13, 1171. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I. Citicoline Improves Human Vigilance and Visual Working Memory: The Role of Neuronal Activation and Oxidative Stress. Basic. Clin. Neurosci. J. 2020, 11, 423–432. [Google Scholar] [CrossRef]
- Chang, K.; Cheng, M.; Tang, H.; Lin, C.; Chen, C. Dysregulation of Choline Metabolism and Therapeutic Potential of Citicoline in Huntington’s Disease. Aging Cell 2024, 23, e14302. [Google Scholar] [CrossRef]
- Golenia, A.; Olejnik, P. The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota. Antioxidants 2025, 14, 542. [Google Scholar] [CrossRef]
- Yu, J.; Li, W.; Xiao, X.; Huang, Q.; Yu, J.; Yang, Y.; Han, T.; Zhang, D.; Niu, X. (−)-Epicatechin Gallate Blocks the Development of Atherosclerosis by Regulating Oxidative Stress in Vivo and in Vitro. Food Funct. 2021, 12, 8715–8727. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Li, Z.; Zhang, P.; Song, H.; Yao, D.; Cao, J.; Zhang, J. Green Tea Improves Cognitive Function through Reducing AD-Pathology and Improving Anti-Oxidative Stress Capacity in Chinese Middle-Aged and Elderly People. Front. Aging Neurosci. 2022, 14, 919766. [Google Scholar] [CrossRef]
- Konca, Y.; Kaliber, M.; Uzkulekci, H.H.; Cimen, B.; Yalcin, H. The Effect of Rosehip (Rosa canina L.) Supplementation to Diet on the Performance, Egg and Meat Quality, Antioxidant Activity in Laying Quail. Sains Malays. 2021, 50, 3617–3629. [Google Scholar] [CrossRef]
- Adnan, M.T.; Amin, M.N.; Uddin, M.G.; Hussain, M.S.; Sarwar, M.S.; Hossain, M.K.; Uddin, S.M.N.; Islam, M.S. Increased Concentration of Serum MDA, Decreased Antioxidants and Altered Trace Elements and Macro-Minerals Are Linked to Obesity among Bangladeshi Population. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 933–938. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Nakadate, Y.; Sato, H.; Sato, T.; Codere-Maruyama, T.; Matsukawa, T.; Schricker, T. Body Mass Index Predicts Insulin Sensitivity during Cardiac Surgery: A Prospective Observational Study. Can. J. Anesth. Can. D’anesthésie 2018, 65, 551–559. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, R.; Wang, B.; Chen, K.; Shi, L.-Y.; Zhu, J.-D.; Mi, M.-T. Effect of Green Tea on Glucose Control and Insulin Sensitivity: A Meta-Analysis of 17 Randomized Controlled Trials. Am. J. Clin. Nutr. 2013, 98, 340–348. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Juan, C.-C.; Ho, L.-T.; Hsu, Y.-P.; Hwang, L.S. Effect of Green Tea Supplementation on Insulin Sensitivity in Sprague−Dawley Rats. J. Agric. Food Chem. 2004, 52, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Badia, R.R.; Pradhan, R.V.; Ayers, C.R.; Chandra, A.; Rohatgi, A. The Relationship of Alcohol Consumption and HDL Metabolism in the Multiethnic Dallas Heart Study. J. Clin. Lipidol. 2023, 17, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.; Dries, S.S.; Seibert, B.S.; Linden, R.; Perassolo, M.S. Evaluation of Oxidative Stress Markers in Ethanol Users. Braz. J. Med. Biol. Res. 2023, 56, e12465. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.W. Pathophysiologic Mechanisms of Tobacco Smoke Producing Atherosclerosis. Curr. Cardiol. Rev. 2022, 18, e110422203389. [Google Scholar] [CrossRef]
- Momayyezi, M.; Jambarsang, S.; Fallahzadeh, H.; Sefidkar, R. Association between Lipid Profiles and Cigarette Smoke among Adults in the Persian Cohort (Shahedieh) Study. BMC Public Health 2024, 24. [Google Scholar] [CrossRef]
- Hallit, S.; Zoghbi, M.; Hallit, R.; Youssef, L.; Costantine, R.; Kheir, N.; Salameh, P. Effect of Exclusive Cigarette Smoking and in Combination with Waterpipe Smoking on Lipoproteins. J. Epidemiol. Glob. Health 2017, 7, 269. [Google Scholar] [CrossRef]
- van der Plas, A.; Antunes, M.; Pouly, S.; de La Bourdonnaye, G.; Hankins, M.; Heremans, A. Meta-Analysis of the Effects of Smoking and Smoking Cessation on Triglyceride Levels. Toxicol. Rep. 2023, 10, 367–375. [Google Scholar] [CrossRef]
- Ahmadkhaniha, R.; Yousefian, F.; Rastkari, N. Impact of Smoking on Oxidant/Antioxidant Status and Oxidative Stress Index Levels in Serum of the University Students. J. Environ. Health Sci. Eng. 2021, 19, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Foronjy, R.; D’Armiento, J. The Effect of Cigarette Smoke–Derived Oxidants on the Inflammatory Response of the Lung. Clin. Appl. Immunol. Rev. 2006, 6, 53–72. [Google Scholar] [CrossRef]
- Buzdagli, Y.; Tekin, A.; Eyipinar, C.D.; Öget, F.; Siktar, E. The Effect of Different Types of Exercise on Blood Lipid Profiles: A Meta-Analysis of Randomized Controlled Studies. Sci. Sport 2022, 37, 675–687. [Google Scholar] [CrossRef]
- Kükürt, A.; Gelen, V.; Faruk Başer, Ö.; Ahmet Deveci, H.; Karapehlivan, M. Thiols: Role in Oxidative Stress-Related Disorders. In Accenting Lipid Peroxidation; IntechOpen: London, UK, 2021; ISBN 9781839688263. [Google Scholar]
- Carter, S.; Hartman, Y.; Holder, S.; Thijssen, D.H.; Hopkins, N.D. Sedentary Behavior and Cardiovascular Disease Risk: Mediating Mechanisms. Exerc. Sport Sci. Rev. 2017, 45, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mengen, E.; Uçaktürk, S.A.; Kocaay, P.; Kaymaz, Ö.; Neşelioğlu, S.; Erel, Ö. The Significance of Thiol/Disulfide Homeostasis and Ischemia-Modified Albumin Levels in Assessing Oxidative Stress in Obese Children and Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lessiani, G.; Santilli, F.; Boccatonda, A.; Iodice, P.; Liani, R.; Tripaldi, R.; Saggini, R.; Davì, G. Arterial Stiffness and Sedentary Lifestyle: Role of Oxidative Stress. Vascul. Pharmacol. 2016, 79, 1256. [Google Scholar] [CrossRef]
- Fasipe, B.; Li, S.; Laher, I. Exercise and Vascular Function in Sedentary Lifestyles in Humans. Pflügers Arch.-Eur. J. Physiol. 2023, 475, 845–856. [Google Scholar] [CrossRef] [PubMed]

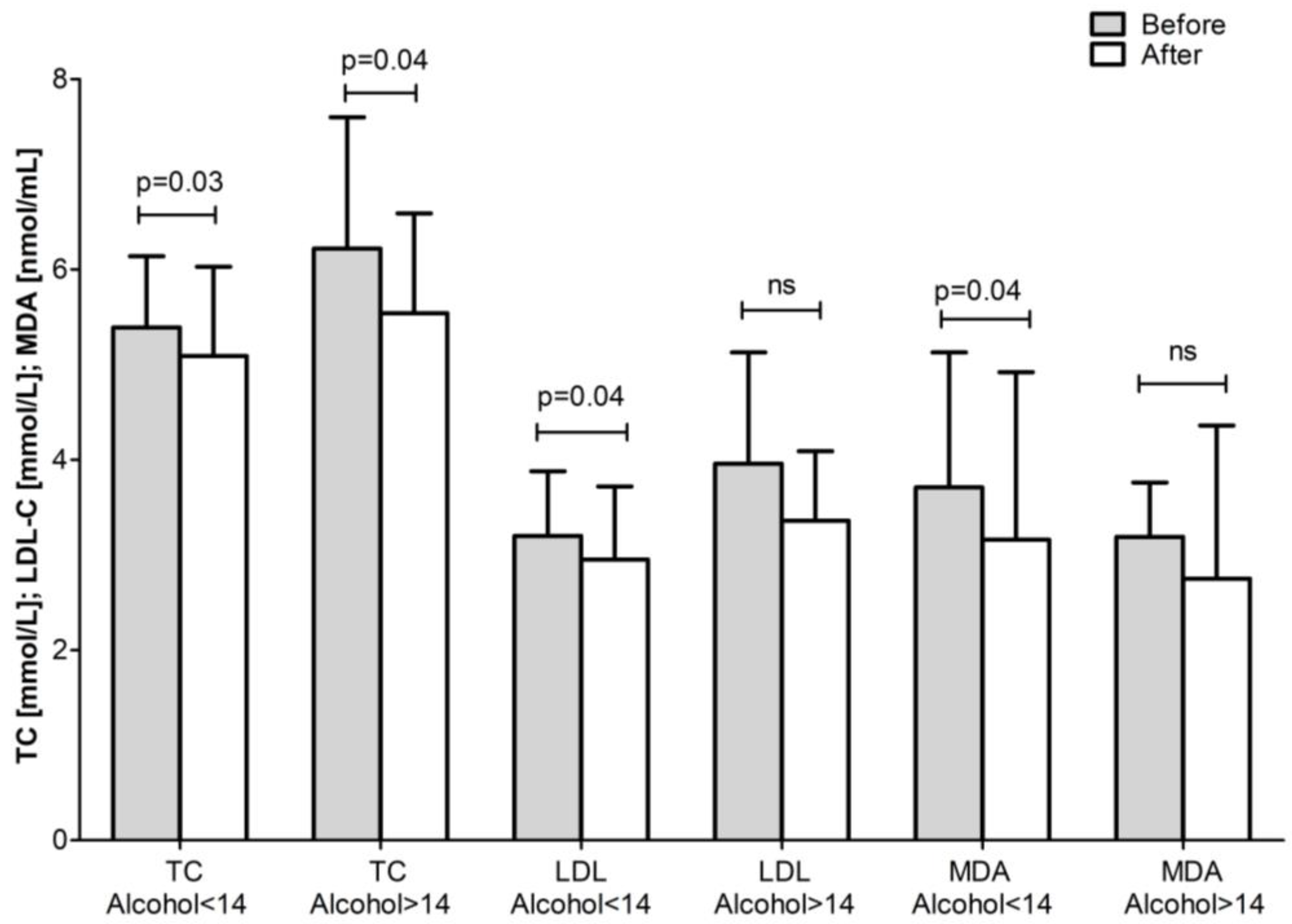
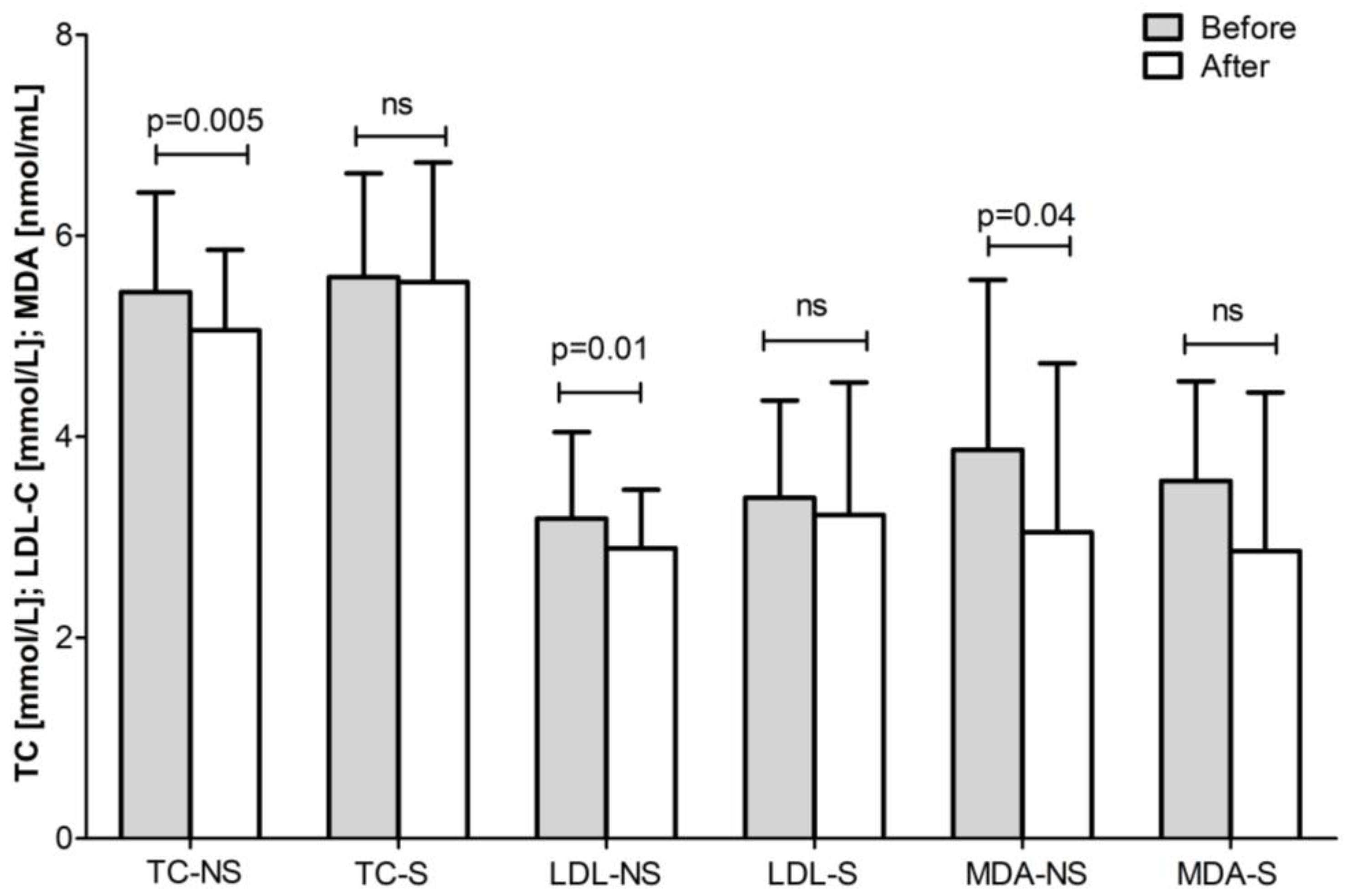
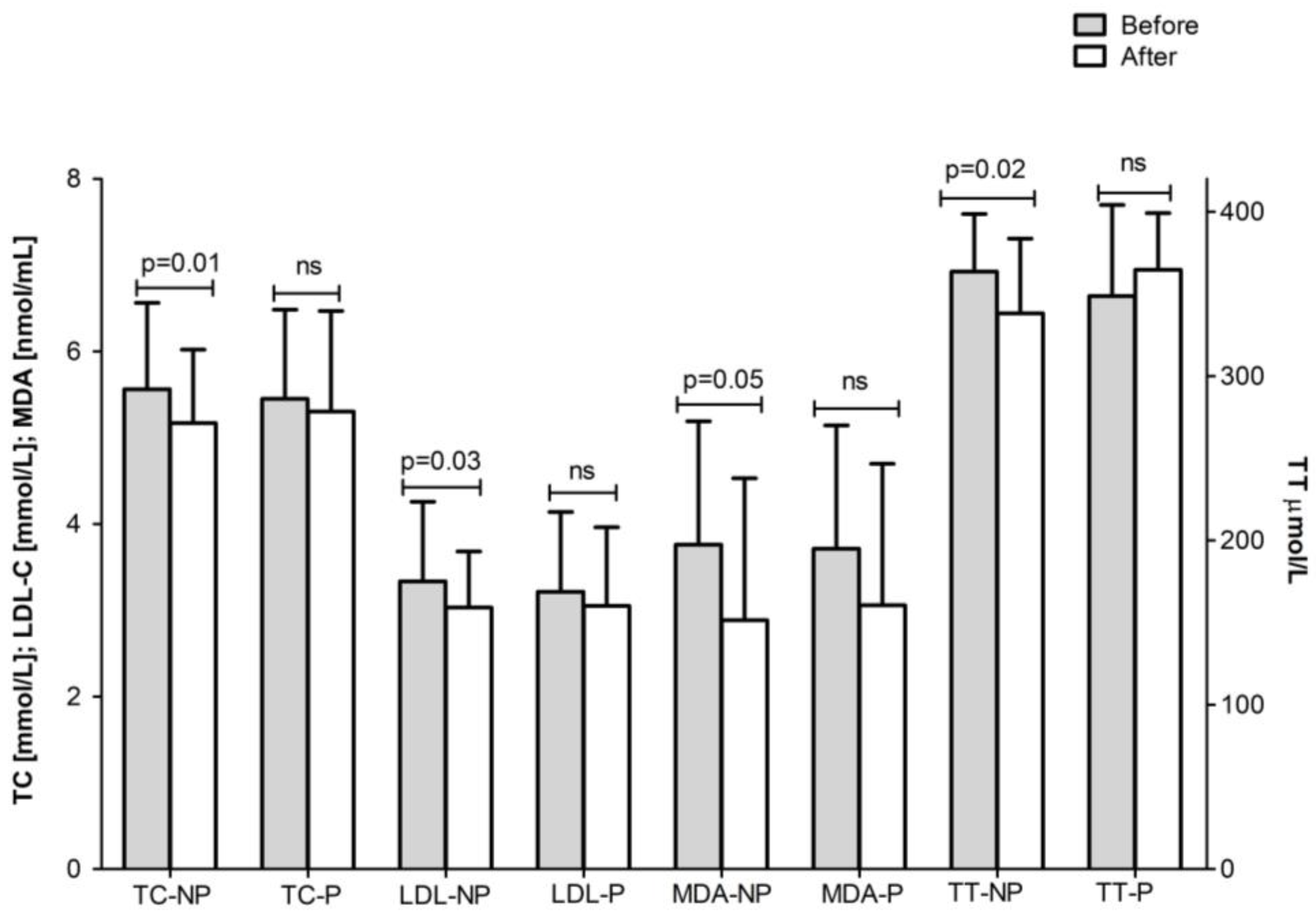
| Total Group (n = 43) | ||||
|---|---|---|---|---|
| Markers | Before Intervention (Mean ± SD) | After Intervention (Mean ± SD) | p Value | Statistical Method |
| TC (mmol/L) | 5.6 ± 1.1 | 5.3 ± 1.1 * | 0.02 | Paired t-test |
| LDL-C (mmol/L) | 3.4 ± 1 | 3.2 ± 0.9 * | 0.02 | Wilcoxon |
| HDL-C (mmol/L) | 1.7 ± 0.5 | 1.6 ± 0.5 | ns | Wilcoxon |
| TC/HDL-C | 3.6 ± 1.2 | 3.5 ± 1.1 * | 0.02 | Wilcoxon |
| LDL-C/HDL-C | 2.2 ± 0.9 | 2.1 ± 0.8 | 0.04 | Paired t-test |
| TAG (mmol/L) | 1.2 ± 0.8 | 1.2 ± 0.6 | ns | Wilcoxon |
| Glucose (mmol/L) | 5.3 ± 0.5 | 5.2 ± 0.6 | ns | Paired t-test |
| MDA (nmol/mL) | 3.6 ± 1.4 | 2.9 ± 1.5 * | 0.003 | Wilcoxon |
| TT (µmol/L) | 357.4 ± 45.9 | 348.5 ± 41.5 | ns | Paired t-test |
| Men (n = 12) | ||||
| TC (mmol/L) | 5.7 ± 1.2 | 5.5 ± 1.1 | ns | Paired t-test |
| LDL-C (mmol/L) | 3.7 ± 1.1 | 3.6 ± 0.9 * | 0.03 | Paired t-test |
| HDL-C (mmol/L) | 1.4 ± 0.2 | 1.3 ± 0.2 | ns | Paired t-test |
| TC/HDL-C | 4.4 ± 1.3 | 4.2 ± 1 | ns | Wilcoxon |
| LDL-C/HDL-C | 2.8 ± 1 | 2.6 ± 0.8 | ns | Wilcoxon |
| TAG (mmol/L) | 1.5 ± 1 | 1.3 ± 0.6 | ns | Wilcoxon |
| Glucose (mmol/L) | 5.6 ± 0.6 | 5.5 ± 0.5 | ns | Paired t-test |
| MDA (nmol/mL) | 3.4 ± 1.2 | 2.7 ± 1.2 * | 0.04 | Wilcoxon |
| TT (µmol/L) | 355.2 ± 51.2 | 360.5 ± 31.4 | ns | Paired t-test |
| Women (31) | ||||
| TC (mmol/L) | 5.5 ± 1.1 | 5.3 ± 1.3 | ns | Paired t-test |
| LDL-C (mmol/L) | 3.2 ± 1 | 3 ± 0.9 * | 0.05 | Wilcoxon |
| HDL-C (mmol/L) | 1.8 ± 0.5 | 1.76 ± 0.57 | ns | Wilcoxon |
| TC/HDL-C | 3.3 ± 1.1 | 3.2 ± 1 * | 0.05 | Wilcoxon |
| LDL-C/HDL-C | 2 ± 0.8 | 1.8 ± 0.7 | ns | Wilcoxon |
| TAG (mmol/L) | 1.2 ± 0.7 | 1.1 ± 0.6 | ns | Wilcoxon |
| Glucose (mmol/L) | 5.2 ± 0.4 | 5.2 ± 0.7 | ns | Wilcoxon |
| MDA (nmol/mL) | 3.8 ± 1.5 | 3.1 ± 1.7 * | 0.02 | Wilcoxon |
| TT (μmol/L) | 356.8 ± 44.8 | 344.1 ± 44.3 | ns | Paired t-test |
| TC/HDL-C | LDL-C/HDL-C | |||||
|---|---|---|---|---|---|---|
| Lifestyle Variable Groups | Before | After | p | Before | After | p |
| BMI < 25 | 3.27 ± 1.32 | 3.18 ± 1.08 | ns | 1.99 ± 1.10 | 1.77 ± 0.76 | ns |
| BMI > 25 | 3.88 ± 1.14 | 3.71 ± 1.02 * | 0.02 | 2.37 ± 0.83 | 2.26 ± 0.78 | ns |
| Alcohol <14 units/week | 3.54 ± 0.93 | 3.41 ± 0.90 | 0.09 | 2.11 ± 0.64 | 2.00 ± 0.65 | ns |
| Alcohol >14 units/week | 4.11 ± 1.63 | 3.58 ± 1.25 * | 0.03 | 2.67 ± 1.32 | 2.22 ± 0.99 * | 0.04 |
| Non-smokers | 3.19 ± 0.79 | 3.10 ± 0.70 | ns | 1.90 ± 0.69 | 1.81 ± 0.59 | ns |
| Smokers | 3.83 ± 1.39 | 3.59 ± 1.14 | ns | 2.34 ± 1.02 | 2.14 ± 0.84 | ns |
| Non-physical activity | 3.64 ± 1.01 | 3.50 ± 0.89 * | 0.03 | 2.17 ± 0.72 | 2.08 ± 0.64 | ns |
| Physical activity | 3.31 ± 1.26 | 3.12 ± 0.98 | ns | 2.01 ± 1.06 | 1.82 ± 0.82 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roussev, B.; Sokrateva, T.; Vankova, D.; Nikolova, M.N.; Ivanova, D.; Nashar, M. Effect of a Citicoline-Containing Supplement on Lipid Profile and Redox Status in Healthy Volunteers in Relation to Lifestyle Factors. Appl. Sci. 2025, 15, 10512. https://doi.org/10.3390/app151910512
Roussev B, Sokrateva T, Vankova D, Nikolova MN, Ivanova D, Nashar M. Effect of a Citicoline-Containing Supplement on Lipid Profile and Redox Status in Healthy Volunteers in Relation to Lifestyle Factors. Applied Sciences. 2025; 15(19):10512. https://doi.org/10.3390/app151910512
Chicago/Turabian StyleRoussev, Bogdan, Todorka Sokrateva, Daniela Vankova, Miglena N. Nikolova, Diana Ivanova, and Milka Nashar. 2025. "Effect of a Citicoline-Containing Supplement on Lipid Profile and Redox Status in Healthy Volunteers in Relation to Lifestyle Factors" Applied Sciences 15, no. 19: 10512. https://doi.org/10.3390/app151910512
APA StyleRoussev, B., Sokrateva, T., Vankova, D., Nikolova, M. N., Ivanova, D., & Nashar, M. (2025). Effect of a Citicoline-Containing Supplement on Lipid Profile and Redox Status in Healthy Volunteers in Relation to Lifestyle Factors. Applied Sciences, 15(19), 10512. https://doi.org/10.3390/app151910512







