“Dissolving the Evidence”: A Forensic Experimental Study on Tissue Destruction and Trace Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Material
2.2. Study Solutions
2.3. Tool Selection
2.4. Documentation Protocol—Photography
2.5. Serological Species Identification
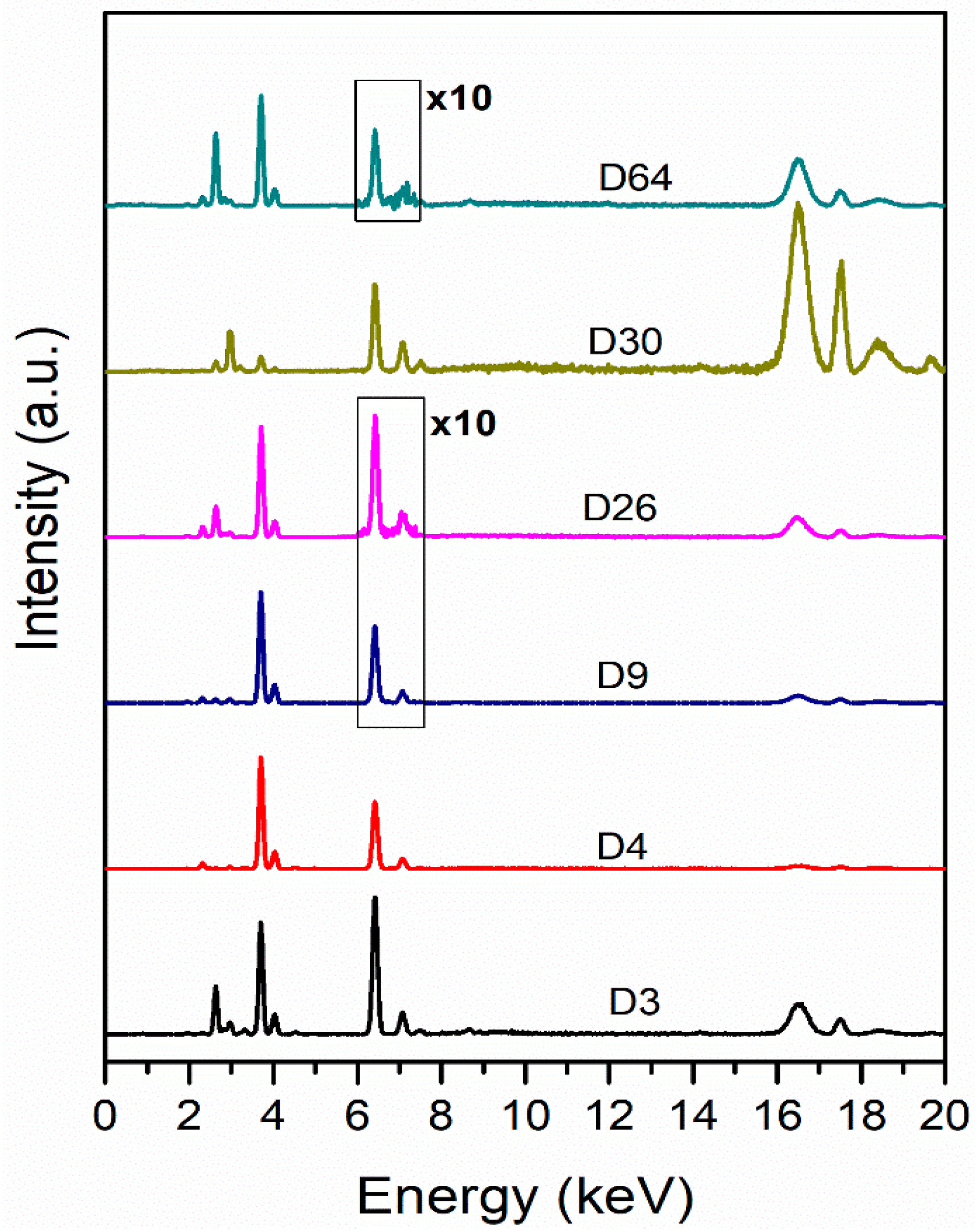
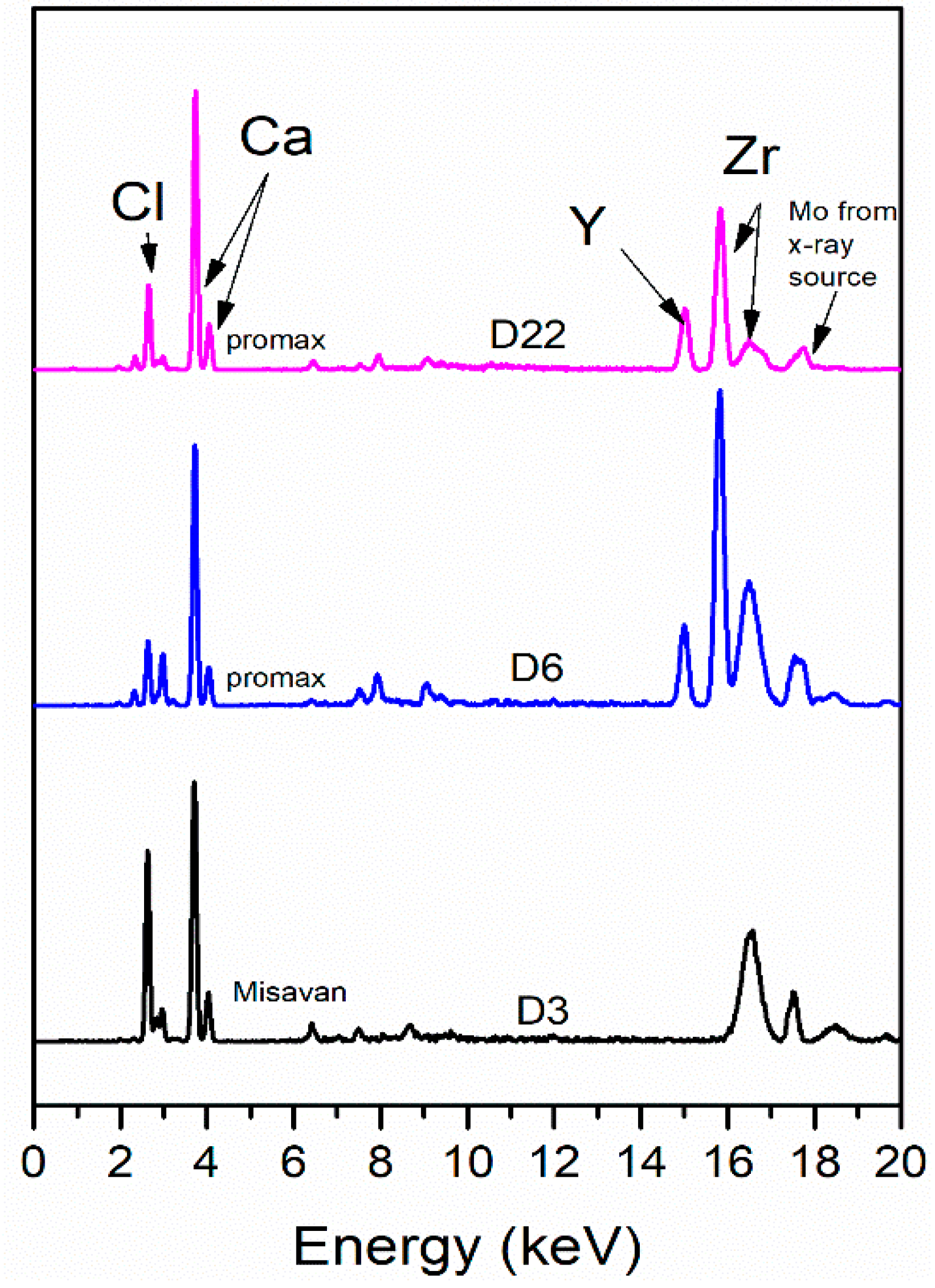
2.6. XRF Analysis Protocol
2.7. Pilot Study
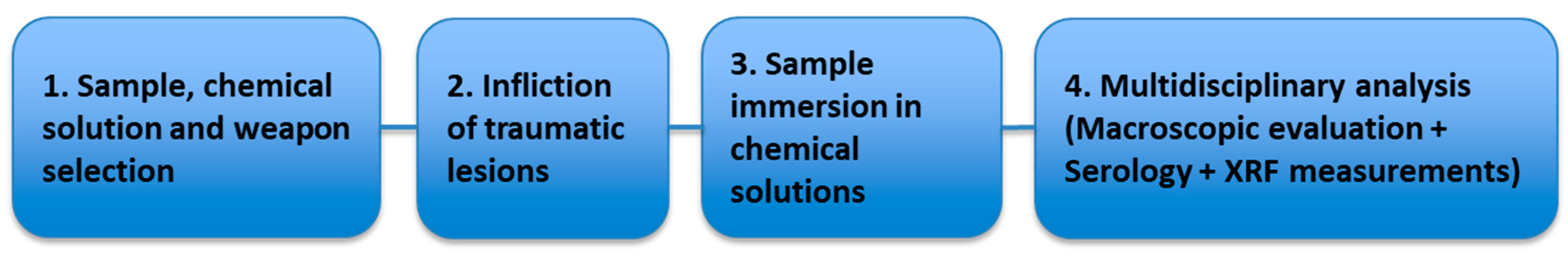
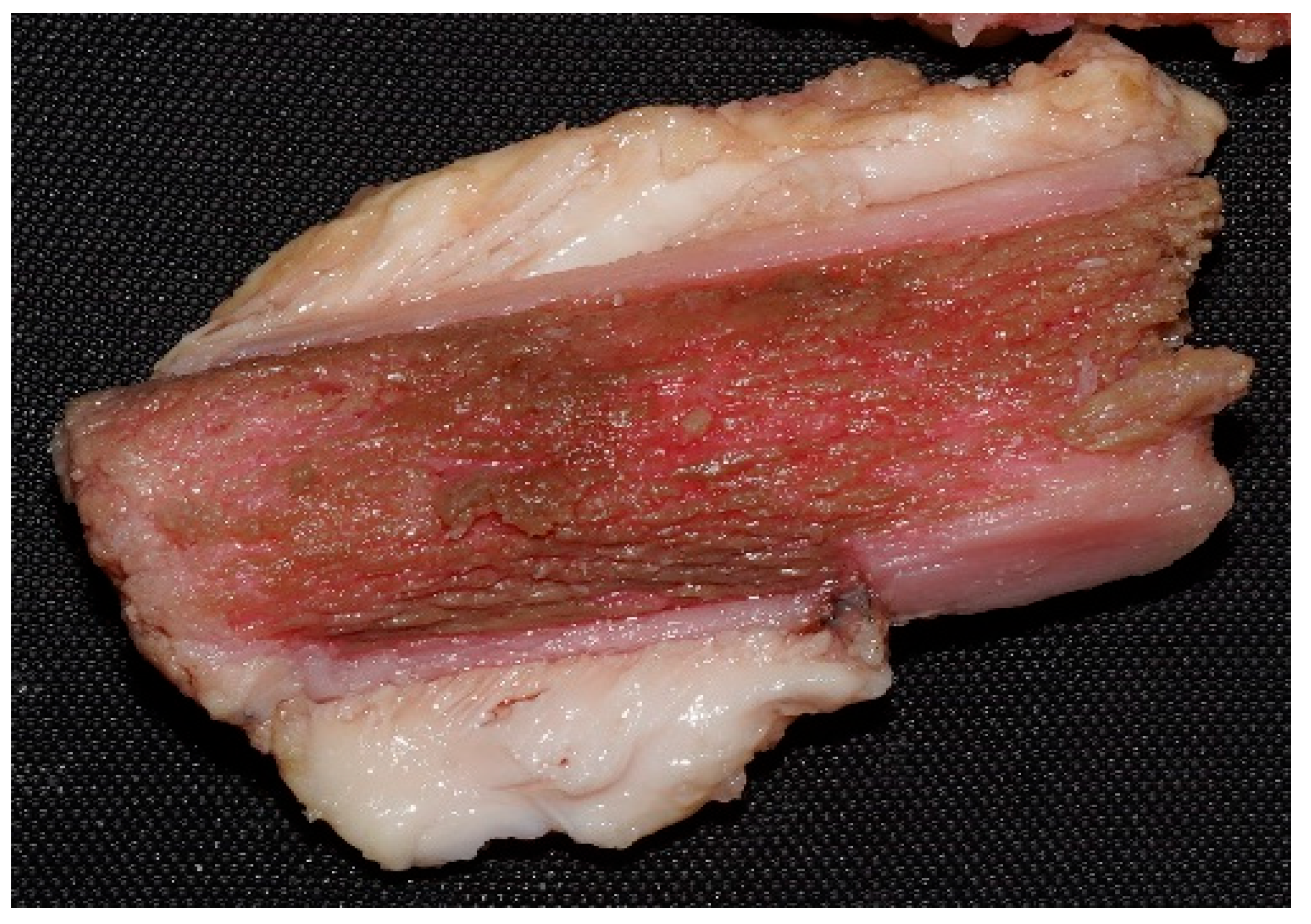
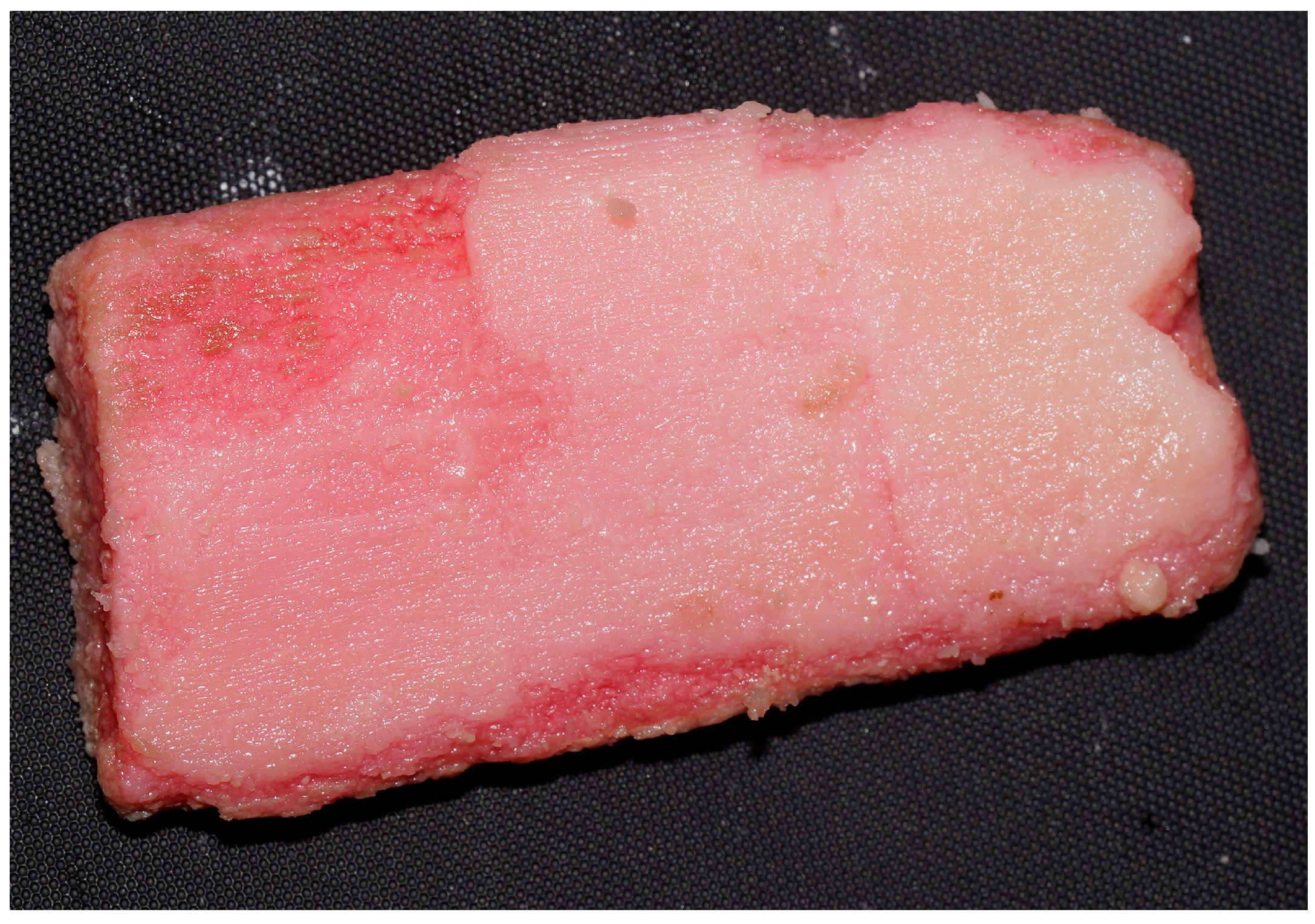
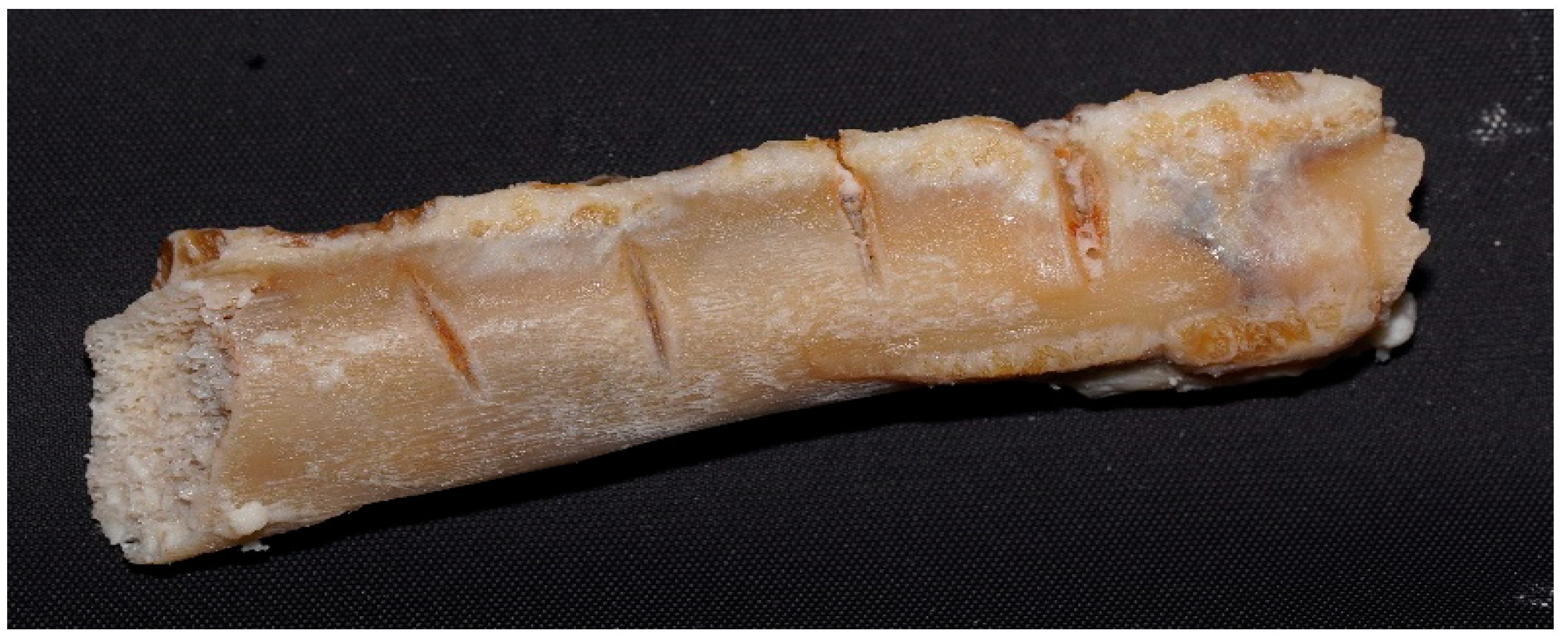
2.8. Conducting the Actual Research—Experimental Design
3. Results
3.1. Pilot Study Results (Only the Aspects Considered Relevant to the Actual Research)
3.2. Actual Research Study Results
3.2.1. Macroscopic Evolution of Samples Immersed in Chemical Solutions
Samples Freshly Extracted from Solution
- Caustic Soda
- Promax—ACB
- Promax—FL
- Misavan
- Dizol
Samples Exposed to Air
- Caustic Soda
- Promax—ACB
- Promax—FL
- Misavan
- Dizol
3.2.2. XRF Measurement Results at Lesional Level
3.2.3. Serological Results (Species Identification)
4. Discussion
4.1. Methodological Considerations and Forensic Implications
4.2. Macroscopic, Serological, and Elemental Findings: Substance-Specific Observations
4.2.1. Caustic Soda
4.2.2. Promax-ACB
4.2.3. Promax-FL
4.2.4. Misavan
4.2.5. Dizol
4.3. Molecular Considerations on Chemical-Tissue Interactions
4.4. Limitations and Further Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvestre, C.R. Looking for El Pozolero’s Traces: Identity and Liminal Condition in the War on Drug’s Disappearances. Front. Norte 2014, 26, 5–23. [Google Scholar]
- Silva, J.A.; Ferrari, M.M.; Leong, G.B. The Case of Jeffrey Dahmer: Sexual Serial Homicide from a Neuropsychiatric Developmental Perspective. J. Forensic Sci. 2002, 47, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Grillo, M.; Cascino, F.M.; Murgo, V.; Milone, L. Method of Concealment of Corpses in Mafia Related Homicides: Melting in Strong Acids. In Proceedings of the 63th Annual Scientific Meeting American Academy of Forensic Sciences, Chicago, IL, USA, 21–26 February 2011. [Google Scholar]
- Blau, S.; Ranson, D. Body in the Barrel: Complex Body Disposal and Recovery. In Case Studies in Forensic Anthropology: Bonified Skeletons; Garvin, H.M., Langley, N.R., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 91–98. [Google Scholar]
- Fulginiti, L.C.; Hartnett-McCann, K.M.; Di Monica, F. Sealed for Your Protection: A Triple Homicide Involving the Use of a Corrosive Agent to Obscure Identity. In Case Studies in Forensic Anthropology: Bonified Skeletons; Garvin, H.M., Langley, N.R., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 99–108. [Google Scholar]
- Pokines, J.T.; Springer, K. A Case of Localized Corrosion on Bone Caused by Chemical Contact. J. Forensic Identif. 2016, 66, 173–186. [Google Scholar]
- Hinkes, M.J.; Lucas, J. Body Rendering by Drug Cartels in San Diego: Case Study of Caustic Soda. Proc. Am. Acad. Sci. USA 2014, 20, 207. [Google Scholar]
- Pomara, C.; Gianpaolo, D.P.; Monica, S.; Maglietta, F.; Sessa, F.; Guglielmi, G.; Turillazzi, E. “Lupara Bianca” a Way to Hide Cadavers after Mafia Homicides. A Cemetery of Italian Mafia. A Case Study. Leg. Med. 2015, 17, 192–197. [Google Scholar] [CrossRef]
- Amadasi, A.; Camici, A.; Porta, D.; Cucca, L.; Merli, D.; Milanese, C.; Profumo, A.; Rassifi, N.; Cattaneo, C. Assessment of the Effects Exerted by Acid and Alkaline Solutions on Bone: Is Chemistry the Answer? J. Forensic Sci. 2017, 62, 1297–1303. [Google Scholar] [CrossRef]
- Amadasi, A.; Camici, A.; Sironi, L.; Profumo, A.; Merli, D.; Mazzarelli, D.; Porta, D.; Duday, H.; Cattaneo, C. The Effects of Acid and Alkaline Solutions on Cut Marks and on the Structure of Bone: An Experimental Study on Porcine Ribs. Leg. Med. 2015, 17, 503–508. [Google Scholar] [CrossRef]
- Udriştioiu, L.A.; Andrei, M.; Perde, F.; Curcă, G.C. Postmortem Tissue Alterations Induced by Corrosive Substances—A Scoping Review. J. Forensic Leg. Med. 2025, 109, 102794. [Google Scholar] [CrossRef]
- Matuszewski, S.; Hall, M.J.R.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs People: The Use of Pigs as Analogues for Humans in Forensic Entomology and Taphonomy Research. Int. J. Leg. Med. 2020, 134, 793–810. [Google Scholar] [CrossRef]
- Allen, T.; Fox, K.A. Multivariate Dimensions of Age, Gender, and Weapon Use in Spousal Homicides. Vict. Offender 2013, 8, 329–346. [Google Scholar] [CrossRef]
- Chan, H.C.O.; Beauregard, E. Choice of Weapon or Weapon of Choice? Examining the Interactions between Victim Characteristics in Single-Victim Male Sexual Homicide Offenders. J. Investig. Psychol. Offender Profiling 2016, 13, 70–88. [Google Scholar] [CrossRef]
- Stanley, S.A.; Hainsworth, S.V.; Rutty, G.N. How Taphonomic Alteration Affects the Detection and Imaging of Striations in Stab Wounds. Int. J. Leg. Med. 2018, 132, 463–475. [Google Scholar] [CrossRef]
- Komo, L.; Grassberger, M. Experimental Sharp Force Injuries to Ribs: Multimodal Morphological and Geometric Morphometric Analyses Using Micro-CT, Macro Photography and SEM. Forensic Sci. Int. 2018, 288, 189–200. [Google Scholar] [CrossRef]
- Komar, A.G.; Motronenko, V.V.; Gorshunov, Y.V. Serological Methods in Modern Biotechnologies and Their Bioanalytical Standardization. Biomed. Eng. Technol. 2019, 2, 108–116. [Google Scholar] [CrossRef]
- Dobrea, C.; Tiseanu, I.; Crăciunescu, T.; Grigore, E. Non Destructive Analysis of Large Area Metallic Coatings of Fusion Materials. Rom. J. Phys. 2011, 56, 69–73. [Google Scholar]
- Lungu, M.; Dobrea, C.; Tiseanu, I. Enhanced XRF Methods for Investigating the Erosion-Resistant Functional Coatings. Coatings 2019, 9, 847. [Google Scholar] [CrossRef]
- High Resolution Nanofocus X-Ray Facility—The Advanced X-Ray. Imagistics Lab. Available online: https://tomography.inflpr.ro/high_resolution_nanofocus_x-ray_facility/ (accessed on 23 July 2025).
- Rosa, J.; Batista de Carvalho, L.A.E.; Marques, M.P.M.; Ferreira, M.T.; Gonçalves, D.; Gil, F.P.S.C. XRF Identification of Sharp-Force Trauma in Fresh and Dry Human Bone under Varied Experimental Heat Conditions. Sci. Justice 2024, 64, 305–313. [Google Scholar] [CrossRef]
- Sabolová, V.; Brinek, A.; Sládek, V. The Effect of Hydrochloric Acid on Microstructure of Porcine (Sus Scrofa Domesticus) Cortical Bone Tissue. Forensic Sci. Int. 2018, 291, 260–271. [Google Scholar] [CrossRef]
- Cope, D.J.; Dupras, T.L. The Effects of Household Corrosive Chemicals on Human Dentition. J. Forensic Sci. 2009, 54, 1238–1246. [Google Scholar] [CrossRef]
- Dowell, T. Examining the Effects of Corrosive Household Chemicals on Bone and Tissue. Poster Presented at the Academic Excellence Showcase, Western Oregon University. 2012. Available online: https://core.ac.uk/download/pdf/228830539.pdf (accessed on 23 July 2025).
- Baglieri, G. The Effects of Household Corrosive Chemicals on Pig Bones and Human Tissue. In Proceedings of the 68th Annual Meeting of the American Academy of Forensic Sciences, Las Vegas, NV, USA, 22–27 February 2016. [Google Scholar]
- Fey, S. Examination of the Effects of Household Corrosive Substances on the Dissolution of Complete Pig (Sus Scrofa) Carcasses. Master’s Thesis, Boston University, Boston, MA, USA, 2022. [Google Scholar]
- Maki, A.G. The Effects of Standard Household Chemicals Containing Acids on Bone and Soft Tissue of Complete Pig (Sus Scrofa) Heads. Master’s Thesis, Boston University, Boston, MA, USA, 2017. [Google Scholar]
- Porta, D.; Amadasi, A.; Cappella, A.; Mazzarelli, D.; Magli, F.; Gibelli, D.; Rizzi, A.; Picozzi, M.; Gentilomo, A.; Cattaneo, C. Dismemberment and Disarticulation: A Forensic Anthropological Approach. J. Forensic Leg. Med. 2016, 38, 50–57. [Google Scholar] [CrossRef]
- Steadman, D.W.; DiAntonio, L.L.; Wilson, J.J.; Sheridan, K.E.; Tammariello, S.P. The Effects of Chemical and Heat Maceration Techniques on the Recovery of Nuclear and Mitochondrial DNA from Bone. J. Forensic Sci. 2006, 51, 11–17. [Google Scholar] [CrossRef]
- Cunha, E.; Ubelaker, D.H. Chapter 5: Processing and Examining Human Remains. Forensic Sci. Res. 2020, 5, 89–97. [Google Scholar] [CrossRef]
- Di Nunno, N.; Costantinides, F.; Vacca, M.; Di Nunno, C. Dismemberment: A Review of the Literature and Description of 3 Cases. Am. J. Forensic Med. Pathol. 2006, 27, 307–312. [Google Scholar] [CrossRef]
- Ubelaker, D.H. Taphonomic Applications in Forensic Anthropology. In Forensic Taphonomy: The Postmortem Fate of Human Remains; Haglund, W.D., Sorg, M.H., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 82–83. ISBN 0-8493-9434-1. [Google Scholar]
- Snedeker, J.; Houston, R.; Hughes, S. Twenty-Eight Days Later: The Recovery of DNA from Human Remains Submerged in Aggressive Household Chemicals. J. Forensic Sci. 2025, 70, 460–475. [Google Scholar] [CrossRef]
- van der Haas, V.M.; Garvie-Lok, S.; Bazaliiskii, V.I.; Weber, A.W. Evaluating Sodium Hydroxide Usage for Stable Isotope Analysis of Prehistoric Human Tooth Dentine. J. Archaeol. Sci. Rep. 2018, 20, 80–86. [Google Scholar] [CrossRef]
- Yadav, P.; Bishariya, N.; Lather, J.; Dhattarwal, S.K.; Sharma, N.; Lohhra, A. Assessing the Impact of Corrosive Acids on Human Bone Integrity in Forensic Context. Forensic Sci. Med. Pathol. 2025, 21, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Jose, N.; Varghese, K.A.; Venugopal, S.; Kingsley, D. A study on determining the Effect of Acid on bones. Spec. Ugdym. 2022, 1, 8141–8148. [Google Scholar]
- Vermeij, E.; Zoon, P.; van Wijk, M.; Gerretsen, R. Microscopic Residues of Bone from Dissolving Human Remains in Acids. J. Forensic Sci. 2015, 60, 770–776. [Google Scholar] [CrossRef]
- Bi, L.; Li, D.C.; Huang, Z.S.; Yuan, Z. Effects of Sodium Hydroxide, Sodium Hypochlorite, and Gaseous Hydrogen Peroxide on the Natural Properties of Cancellous Bone. Artif. Organs 2013, 37, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.; Kuzmanović, M.; Ranković, D.; Bajuk-Bogdanović, D.; Šajić, A.; Dimić, D. From Structure to Strength: Analyzing the Impact of Sulfuric Acid on Pig Bone Demineralization Through FTIR, LIBS, and AAS. Int. J. Mol. Sci. 2024, 25, 12250. [Google Scholar] [CrossRef]
- Guendalina, G.; Stefano, T.; Salvatore, A.; Paolo, B.; Giorgia, B.; Ilaria, G.; Riccardo, Z. Analysis of the Corrosive Effects of Hydrochloric Acid (HCl) on Human Bone: Preliminary Microscopic Study and Observations for Forensic Purposes. Forensic Sci. Int. 2021, 329, 111095. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Hypochlorite-Induced Oxidation of Amino Acids, Peptides and Proteins. Amino Acids 2003, 25, 259–274. [Google Scholar] [CrossRef] [PubMed]










| No. | Product (Commercial Name) | Ingredients According to the Packaging/Technical Data Sheet | pH Indicated on the Packaging | pH Measured with pH Strips * |
|---|---|---|---|---|
| 1. | Caustic Soda Flakes | Sodium Hydroxide: min. 98% + water | - | 14 |
| 2. | PROMAX Active Chlorine Bleach (PROMAX—ACB) | Sodium Hypochlorite 1–5% Stabilizers | - | 5 |
| 3. | PROMAX Floral Descaler (PROMAX—FL) | Hydrochloric Acid 15–30% Sulfuric Acid 1–5% Non-Ionic Surfactants <1% Fragrance | 2.5 | 1 |
| 4. | Misavan Descaler | Hydrochloric Acid 5–15% Fragrance Preservative | 1–2 | 1 |
| 5. | Dizol Quick Clog Remover | Sulfuric Acid 75–<100% Ethoxylated Fat Alcohol 2.5–<10% | - | 1 |
| No. | Instrument/Object | XRF Result | Degree of Use |
|---|---|---|---|
| 1 | Forged axe with wooden handle | Fe | Heavy use |
| 2 | Orthopedic saw | Fe | Limited use |
| 3 | Tefal® kitchen knife * | Fe + Cr | New |
| 4 | Hacksaw with metal blade | Fe | Heavy use, rust present |
| 5 | Metal wire | Fe | Multiple uses |
| 6 | Universal hand saw | Fe | Very limited use |
| 7 | Ceramic kitchen knife | Hf + Y + Zr | New |
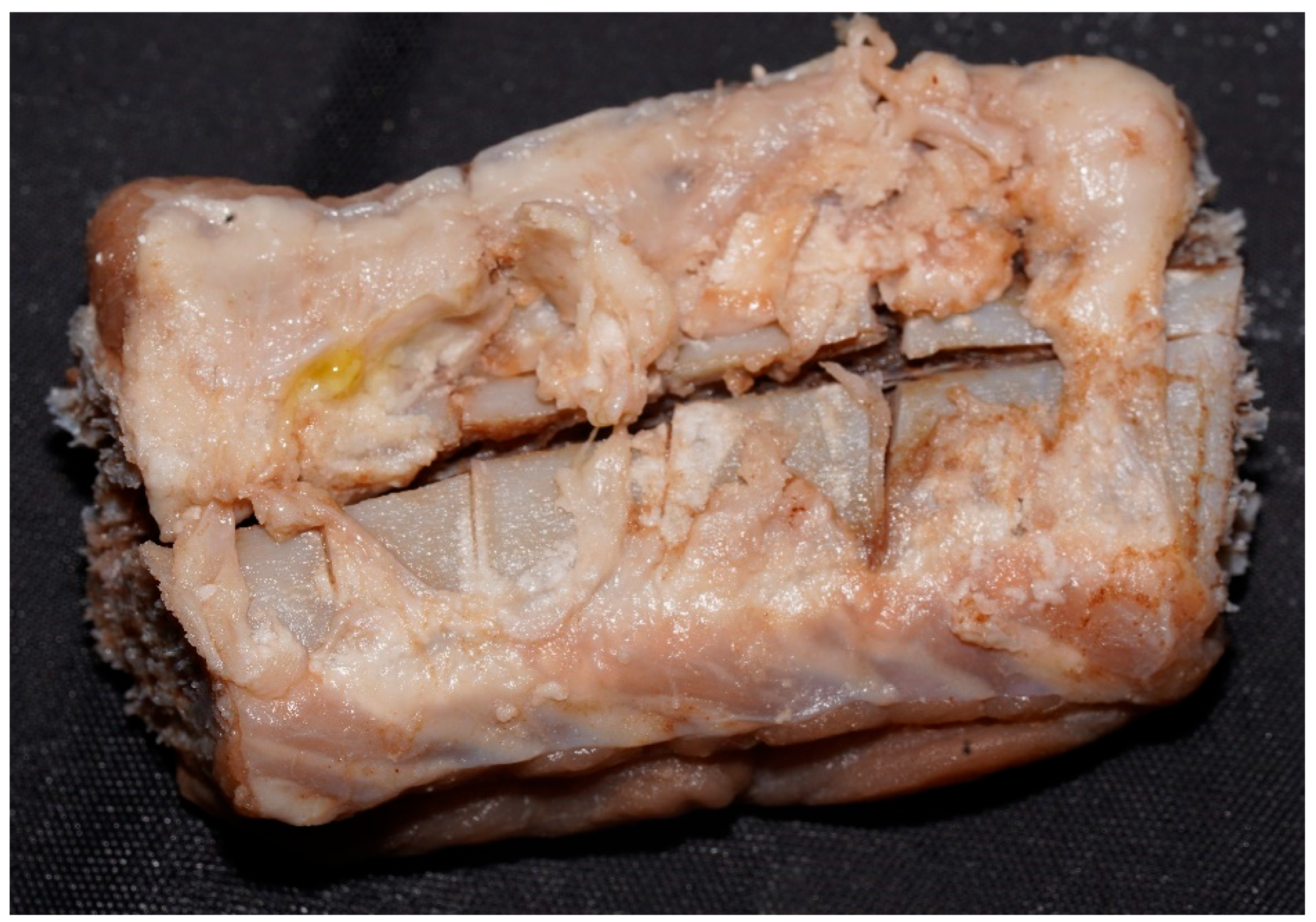
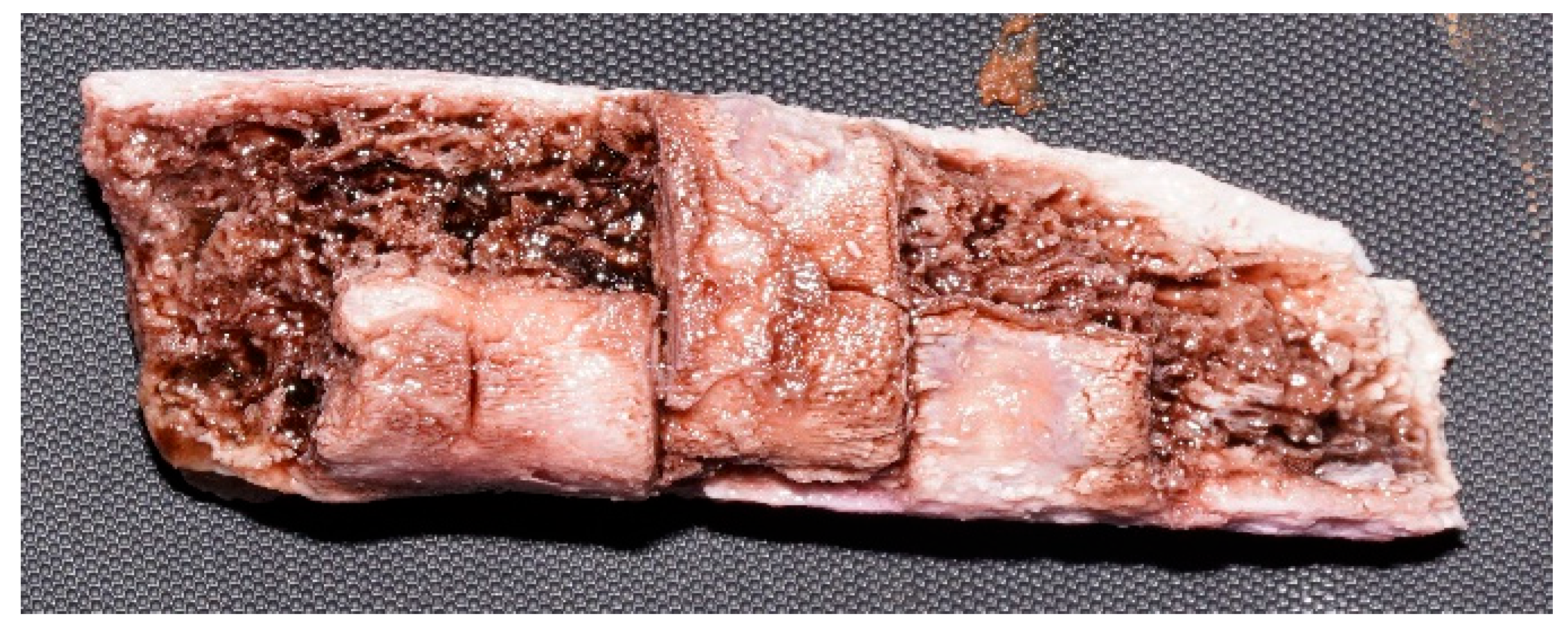
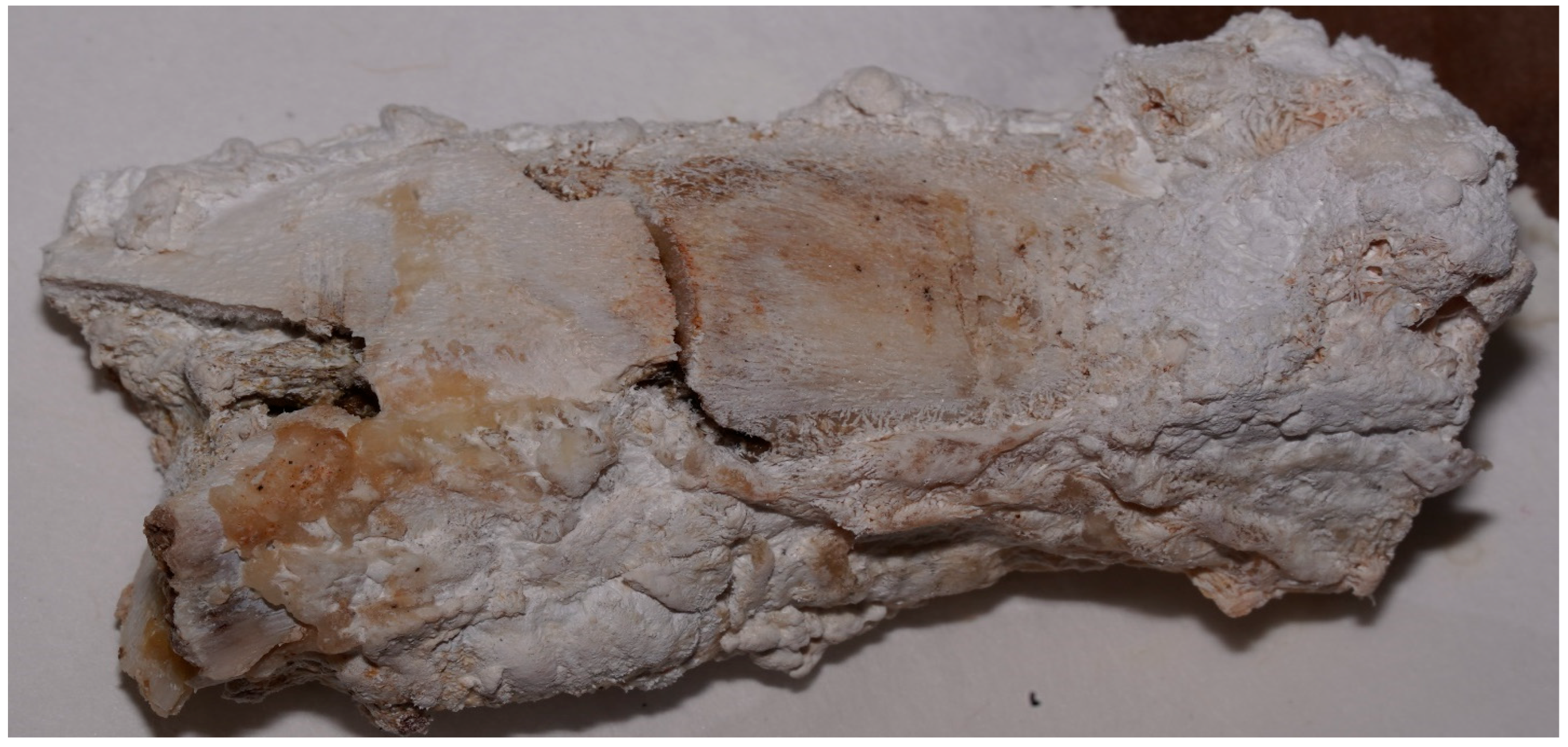
| Solution | Soft Tissue Evolution | Bone Modifications | General Appearance | Final Observation Including Lesion Evolution |
|---|---|---|---|---|
| Caustic soda | Detachment, saponification, reddish-gelatinous and later greenish aspect | Cortical detachment, emaciation, fragmentation | Pink-reddish, friable | Complete fragmentation; only traces of deep lesions remain on some fragments |
| Promax-ACB | Whitening, followed by full detachment and cleaned margins | Cortical exfoliation, white bone | Matte, firm, with clearly visible bone structure | Bone preserved, but subject to exfoliation; lesions remain well-defined |
| Promax-FL | Waterlogged, brown, with cauliflower-like fat white deposits | Accentuated cracks, marrow exposure | Elastic, dry, granular; tendency to fragmentation | Increasing fragmentation toward the end; lesions still visible |
| Misavan | Yellowish, viscous transformation, later pasty | Bone collapse, yellowish appearance | Oily, with progressive disintegration | Amorphous paste-advanced degradation; lesions no longer detectable |
| Dizol | Liquefaction, complete loss of soft tissues | Blurred lesions, bone dissolution | Brown-violet, friable, pasty | Sample “dissolution”; lesions no longer detectable |
| Solution | Axe-Induced Lesions | Ceramic-Knife Induced Lesions | General Observations |
|---|---|---|---|
| Caustic soda | Fe detected up to Day 4 | No traces detected | Limited residues, disappear rapidly |
| Promax-ACB | Fe detected up to Day 4 | No traces detected | Similar to caustic soda |
| Promax-FL | Residues detected throughout the experiment | Residues detected throughout the experiment | High persistence |
| Misavan | Residues detected up to Day 4 | Residues detected up to Day 6 | Transient presence |
| Dizol | No traces detected | No traces detected | Complete absence of residues |
| Chemical Agent | Primary Effect | Visibility of Lesions | Detectability of Residues (XRF) | Species Identification |
|---|---|---|---|---|
| Caustic soda | Fat dissolution; early saponification → progressive cortical bone degradation → complete disintegration | AL—partial contours until final stages; CKL—faded faster → absent | AL—Fe traces up to Day 4; CKL—no metallic traces after immersion | Positive throughout the experiment |
| Promax—ACB | Loss of soft tissues and marrow; later bone whitening and cortical exfoliation → nearly clean, firm white bone | AL and CKL remained visible throughout | AL—Fe traces up to Day 4; CKL—no metallic traces after immersion | Positive only on Day 1; negative afterward |
| Promax—FL | Waterlogged, cracks, deposits → drying and fissuring → partial fragmentation | AL and CKL visible until latest stages | AL and CKL traces detected throughout the experiment | Negative for all samples |
| Misavan | Soaked, viscous, greasy appearance → progressive tissue decline → collapse → amorphous paste | AL and CKL visible until later stages | Residues detectable only early: AL—up to Day 4 and CKL—Day 6 | Positive throughout the experiment |
| Dizol | Rapid tissue loss → complete disintegration | AL and CKL detectable only in early phase | No detectable elemental residues | Negative throughout the experiment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udriștioiu, L.A.; Dincă, I.; Curcă, G.C. “Dissolving the Evidence”: A Forensic Experimental Study on Tissue Destruction and Trace Detection. Appl. Sci. 2025, 15, 10347. https://doi.org/10.3390/app151910347
Udriștioiu LA, Dincă I, Curcă GC. “Dissolving the Evidence”: A Forensic Experimental Study on Tissue Destruction and Trace Detection. Applied Sciences. 2025; 15(19):10347. https://doi.org/10.3390/app151910347
Chicago/Turabian StyleUdriștioiu, Larisa Adela, Ioana Dincă, and George Cristian Curcă. 2025. "“Dissolving the Evidence”: A Forensic Experimental Study on Tissue Destruction and Trace Detection" Applied Sciences 15, no. 19: 10347. https://doi.org/10.3390/app151910347
APA StyleUdriștioiu, L. A., Dincă, I., & Curcă, G. C. (2025). “Dissolving the Evidence”: A Forensic Experimental Study on Tissue Destruction and Trace Detection. Applied Sciences, 15(19), 10347. https://doi.org/10.3390/app151910347






