Comparative Analysis of the Chelating Capacity of Two Solutions Activated with Sonic and Ultrasonic Systems: HEBP Versus EDTA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
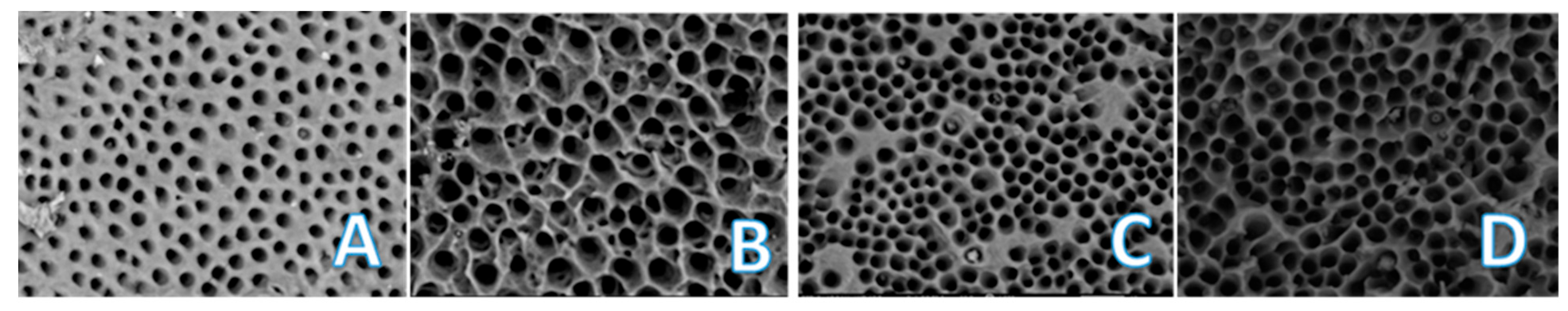
2.2. Evaluation Using Carvalho’s Quantitative Method
2.3. Tubule Size Assessment
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDT: | clean dentinal tubules |
| DDT: | dirty dentinal tubules |
| EDTA: | 17% ethylenediaminetetraacetic acid |
| HEBP: | 9% hydroxyethylidene bisphosphonate |
| NaOCl: | sodium hypochlorite |
| SEM: | scanning electron microscopy |
References
- Zehnder, M. Root Canal Irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moliz, M.T.; Ordinola-Zapata, R.; Baca, P.; Ruiz-Linares, M.; Ferrer-Luque, C.M. Antimicrobial activity of a sodium hypochlorite/etidronic acid irrigant solution. J. Endod. 2014, 40, 1999–2002. [Google Scholar] [CrossRef] [PubMed]
- Morago, A.; Ruiz-Linares, M.; Ferrer-Luque, C.M.; Baca, P.; Rodríguez Archilla, A.; Arias-Moliz, M.T. Dentine tubule disinfection by different irrigation protocols. Microsc. Res. Tech. 2019, 82, 558–563. [Google Scholar] [CrossRef]
- Tonini, R.; Salvadori, M.; Audino, E.; Sauro, S.; Garo, M.L.; Salgarello, S. Irrigating Solutions and Activation Methods Used in Clinical Endodontics: A Systematic Review. Front. Oral Health 2022, 3, 838043. [Google Scholar]
- Gomes, B.P.F.A.; Aveiro, E.; Kishen, A. Irrigants and irrigation activation systems in Endodontics. Braz. Dent. J. 2023, 34, 1–33. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions–irrigants and irrigation methods. Int. Endod. J. 2022, 55 (Suppl. 3), 588–612. [Google Scholar] [CrossRef]
- Emre Erik, C.; Onur Orhan, E.; Maden, M. Qualitative analysis of smear layer treated with different etidronate concentrations: A scanning electron microscopy study. Microsc. Res. Tech. 2019, 82, 1535–1541. [Google Scholar] [CrossRef]
- Gómez-Delgado, M.; Camps-Font, O.; Luz, L.; Sanz, D.; Mercade, M. Update on citric acid use in endodontic treatment: A systematic review. Odontology 2023, 111, 1–19. [Google Scholar] [CrossRef]
- Ali, A.; Bhosale, A.; Pawar, S.; Kakti, A.; Bichpuriya, A.; Agwan, M.A. Current Trends in Root Canal Irrigation. Cureus 2022, 14, e24833. [Google Scholar] [CrossRef]
- Morago, A.; Ordinola-Zapata, R.; Ferrer-Luque, C.M.; Baca, P.; Ruiz-Linares, M.; Arias-Moliz, M.T. Influence of Smear Layer on the Antimicrobial Activity of a Sodium Hypochlorite/Etidronic Acid Irrigating Solution in Infected Dentin. J. Endod. 2016, 42, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Balkaya, H.; Çakir, N. Efficacy of different endodontic irrigation protocols on shear bond strength to coronal dentin. J. Conserv. Dent. 2019, 22, 223–227. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef]
- Busanello, F.H.; Petridis, X.; So, M.V.R.; Dijkstra, R.J.B.; Sharma, P.K.; Van Der Sluis, L.W.M. Chemical biofilm removal capacity of endodontic irrigants as a function of biofilm structure: Optical coherence tomography, confocal microscopy and viscoelasticity determination as integrated assessment tools. Int. Endod. J. 2019, 52, 461–474. [Google Scholar] [CrossRef]
- Borges, M.M.B.; Dijkstra, R.J.B.; de Andrade, F.B.; Duarte, M.A.H.; Versluis, M.; van der Sluis, L.W.M.; Petridis, X. The response of dual-species bacterial biofilm to 2% and 5% NaOCl mixed with etidronic acid: A laboratory real-time evaluation using optical coherence tomography. Int. Endod. J. 2022, 55, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Cerroni, L.; Iorio, L.; Armellin, E.; Conte, G.; Cianconi, L. Smear layer removal and canal cleanliness using different irrigation systems (EndoActivator, EndoVac, and passive ultrasonic irrigation): Field emission scanning electron microscopic evaluation in an in vitro study. J. Endod. 2013, 39, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Cerroni, L.; Iorio, L.; Dall’Asta, L.; Cianconi, L. FESEM evaluation of smear layer removal using different irrigant activation methods (EndoActivator, EndoVac, PUI and LAI). An in vitro study. Clin. Oral Investig. 2018, 22, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Gu Lsha Schoeffel, G.J.; Wimmer, C.; Susin, L.; Zhang, K.; Arun, S.N.; Kim, J.; Looney, S.W.; Pashley, D.H. Effect of vapor lock on root canal debridement by using a side-vented needle for positive-pressure irrigant delivery. J. Endod. 2010, 36, 745–750. [Google Scholar] [CrossRef]
- De Gregorio, C.; Arias, A.; Navarrete, N.; Del Rio, V.; Oltra, E.; Cohenca, N. Effect of apical size and taper on volume of irrigant delivered at working length with apical negative pressure at different root curvatures. J. Endod. 2013, 39, 119–124. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Camargo, C.H.R.; Valera, M.C.; Camargo, S.E.A.; Mancini, M.N.G. Smear layer removal by auxiliary chemical substances in biomechanical preparation: A scanning electron microscope study. J. Endod. 2008, 34, 1396–1400. [Google Scholar] [CrossRef]
- Mayer, B.E.; Peters, O.; Barbakow, F. Effects of rotary instruments and ultrasonic irrigation on debris and smear layer scores: A scanning electron microscopic study. Int. Endod. J. 2002, 35, 582–589. [Google Scholar] [CrossRef]
- Giardino, L.; Ambu, E.; Becce, C.; Rimondini, L.; Morra, M. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J. Endod. 2006, 32, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Zmener, O.; Pameijer, C.H.; Alvarez Serrano, S.; Hernandez, S.R. Cleaning efficacy using two engine-driven systems versus manual instrumentation in curved root canals: A scanning electron microscopic study. J. Endod. 2011, 37, 1279–1282. [Google Scholar] [CrossRef]
- Scha, E.; Zapke, K. Investigation of the Efficacy of Manual and Automated Instrumentation of Root Canals. J. Endod. 2000, 26, 660–664. [Google Scholar] [CrossRef]
- Hartmann, M.S.M.; Barletta, F.B.; Camargo Fontanella, V.R.; Vanni, J.R. Canal transportation after root canal instrumentation: A comparative study with computed tomography. J. Endod. 2007, 33, 962–965. [Google Scholar] [CrossRef]
- Soares Grecca, F.; Brandao García, R.; Monteiro Bramante, C.; Gomez de Moraes, I.; Bernarduneli, N. A quantitative analysis of rotary, ultrasonic and manual techniques to treat proximally. J. Appl. Oral Sci. 2007, 15, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.G.; Rahim, N.; Bath, K.S.; Mathew, J. Comparison of removal of endodontic smear layer using NaOCl, EDTA, and different concentrations of maleic acid—A SEM study. Endodontol. 2003, 15, 20–25. [Google Scholar]
- Teixeira, C.S.; Felippe, M.C.S.; Felippe, W.T. The effect of application time of EDTA and NaOCl on intracanal smear layer removal: An SEM analysis. Int. Endod. J. 2005, 38, 285–290. [Google Scholar] [CrossRef]
- Violich, D.R.; Chandler, N.P. The smear layer in endodontics-A review. Int. Endod. J. 2010, 43, 2–15. [Google Scholar] [CrossRef]
- De Gregorio, C.; Estevez, R.; Cisneros, R.; Heilborn, C.; Cohenca, N. Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: An in vitro study. J. Endod. 2009, 35, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu, E.; Aktemur, S.; Uyanik, M.O.; Durmaz, V.; Nagas, E. Effect of ethylenediaminetetraacetic acid on root fracture with respect to concentration at different time exposures. J. Endod. 2012, 38, 1110–1113. [Google Scholar] [CrossRef]
- Sayin, T.C.; Serper, A.; Cehreli, Z.C.; Kalayci, S. Calcium loss from root canal dentin following EDTA, EGTA, EDTAC, and tetracycline-HCl treatment with or without subsequent NaOCl irrigation. J. Endod. 2007, 33, 581–584. [Google Scholar] [CrossRef]
- Khedmat, S.; Shokouhinejad, N. Comparison of the efficacy of three chelating agents in smear layer removal. J. Endod. 2008, 34, 599–602. [Google Scholar] [CrossRef]
- Mello, I.; Robazza, C.R.C.; Antoniazzi, J.H.; Coil, J. Influence of different volumes of EDTA for final rinse on smear layer removal. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 40–43. [Google Scholar] [CrossRef]
- Zehnder, M.; Schmidlin, P.; Sener, B.; Waltimo, T. Chelation in Root Canal Therapy Reconsidered. J. Endod. 2005, 31, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.E.-D.; Hashem, A.A.R. Efficacy of different final irrigation activation techniques on smear layer removal. J. Endod. 2011, 37, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Estevez, R. Efficacy of Different Irrigation and Activation Systems on the Penetration of Sodium Hypochlorite into Simulated Lateral Canals and up to Working Length: An In Vitro Study. J. Endod. 2010, 36, 1216–1221. [Google Scholar] [CrossRef]
- González-López, S.; Camejo-Aguilar, D.; Sanchez-Sanchez, P.; Bolaños-Carmona, V. Effect of CHX on the decalcifying effect of 10% citric acid, 20% citric acid, or 17% EDTA. J. Endod. 2006, 32, 781–784. [Google Scholar] [CrossRef]
- Blank-Gonçalves, L.M.; Nabeshima, C.K.; Martins, G.H.R.; Machado, M.E.D.L. Qualitative analysis of the removal of the smear layer in the apical third of curved roots: Conventional irrigation versus activation systems. J. Endod. 2011, 37, 1268–1271. [Google Scholar] [CrossRef]
- da Silva, L.A.B.; Sanguino, A.C.M.; Rocha, C.T.; Leonardo, M.R.; Silva, R.A.B. Scanning electron microscopic preliminary study of the efficacy of SmearClear and EDTA for smear layer removal after root canal instrumentation in permanent teeth. J. Endod. 2008, 34, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.M.; Silveira, A.; Santos, E.; Prado, L.; Pessoa, O.F. Efficacy of sodium hypochlorite, ethylenediaminetetraacetic acid, citric acid and phosphoric acid in calcium hydroxide removal from the root canal: A microscopic cleanliness evaluation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 820–824. [Google Scholar] [CrossRef]
- Mello, I.; Kammerer, B.A.; Yoshimoto, D.; Macedo, M.C.S.; Antoniazzi, J.H. Influence of final rinse technique on ability of ethylenediaminetetraacetic acid of removing smear layer. J. Endod. 2010, 36, 512–514. [Google Scholar] [CrossRef]
- Lima Nogueira, B.M.; da Costa Pereira, T.I.; Pedrinha, V.F.; de Almeida Rodrigues, P. Effects of Different Irrigation Solutions and Protocols on Mineral Content and Ultrastructure of Root Canal Dentine. Iran. Endod. J. 2018, 13, 209–215. [Google Scholar] [PubMed]
- Pedrinha, V.F.; Cardenas Cuellar, M.R.; Velásquez-Espedilla, E.G.; Duarte, M.A.H.; Andrade, F.B.; Rodrigues, P.A. Impact of irrigation protocols with some chelators and mechanical agitation on intratubular decontamination. Braz. Oral. Res. 2021, 35, e127. [Google Scholar] [CrossRef]
- Ulusoy, Ö.İ.; Mantı, A.Ş.; Çelik, B. Nanohardness reduction and root dentine erosion after final irrigation with ethylenediaminetetraacetic, etidronic and peracetic acids. Int. Endod. J. 2020, 53, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Shalavi, S.; Yaripour, S.; Kinoshita, J.I.; Manabe, A.; Kobayashi, M.; Giardino, L.; Palazzi, F.; Sharifi, F.; Jafarzadeh, H. Smear Layer Removing Ability of Root Canal Irrigation Solutions: A Review. J. Contemp. Dent. Pract. 2019, 20, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Mjör, I.A.; Nordal, I. The density and branching of dentinal tubules in human teeth. Arch. Oral Biol. 1996, 41, 401–412. [Google Scholar] [CrossRef]
- Ferrari MMannocci, F.; Vichi, A.; Cagidiaco, M.C.; Mjör, I.A. Bonding of root canal: Structural characteristics of the substrate. Am. J. Dent. 2000, 13, 255–260. [Google Scholar]
- Mjör, I.A.; Smith, M.R.; Ferrari, M.; Manocci, F. The structure of dentine in the apical región of human teeth. Int. Endod. J. 2001, 34, 346–353. [Google Scholar] [CrossRef]
- Garbergolio, R.; Brännström, M. Scanning electron microscopic investigation of human dentinal tubules. Arch. Oral Biol. 1976, 21, 355–362. [Google Scholar] [CrossRef]
- Dineshkumar, M.K.; Vinothkumar, T.S.; Arathi, G.; Shanthisree, P.; Kandaswamy, D. Effect of ethylene diamine tetra-acetic acid, MTAD™, and HEBP as a final rinse on the microhardness of root dentin. J. Conserv. Dent. 2012, 15, 170–173. [Google Scholar]
- Kadulkar, N.; Kataki, R.; Deka, A.; Medhi, H.; Chakraborty, S.; Singh, A. Comparative Evaluation of the Effect of Different Chelating Agents on Mineral Content and Erosion of Radicular Dentine: A FESEM-EDS Analysis. Eur. Endod. J. 2024, 9, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Tartari, T.; Duarte Junior, A.P.; Silva Júnior, J.O.; Klautau, E.B.; Silva ESouza Junior, M.H.; Silva ESouza Junior, P.d.A. Etidronate from medicine to endodontics: Effects of different irrigation regimes on root dentin roughness. J. Appl. Oral Sci. 2013, 21, 409–415. [Google Scholar] [CrossRef] [PubMed]



| Final Irrigation | ||||
|---|---|---|---|---|
| Control Group (n = 5) | Tooth 1 y 2: Without Final Irrigation Tooth 3: HEBP 9% Irrigation Tooth 4 y 5: EDTA 17% Irrigation | |||
| Activation Time | ||||
| Group 1: EDTA 17% + Sonic activation (Smart Lite Pro Endo Activator® [Dentsply Sirona. Ballaigues. Switzerland]) n = 25 | 2 mL | NaOCl 5.25% | 1 min | 20 s |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | EDTA 17% | 1 min | 20 s | |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | NaOCl 5.25% | 1 min | 20 s | |
| Group 2: HEBP + Sonic activation (SmartLite Pro EndoActivator® [Denstply Sirona. Ballaigues, Switzerland]) n = 25 | 2 mL | NaOCl 5.25% | 1 min | 20 s |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | HEBP 9% | 1 min | 20 s | |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | NaOCl 5.25% | 1 min | 20 s | |
| Group 3: EDTA 17% + Ultrasonic activation (IRRI S 21 mm No 25–VDW, Munchen, Germany]) n = 25 | 2 mL | NaOCl 5.25% | 1 min | 20 s |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | EDTA 17% | 1 min | 20 s | |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | NaOCl 5.25% | 1 min | 20 s | |
| Group 4: HEBP 9% + Ultrasonic activation (IRRI S 21 mm No 25–VDW, Munchen, Germany]) | 2 mL | NaOCl 5.25% | 1 min | 20 s |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | HEBP 9% | 1 min | 20 s | |
| 2 mL | Sodium chloride 0.9% | 1 min | - | |
| 2 mL | NaOCl 5.25% | 1 min | 20 s | |
| Ultrasonic Coronal | Ultrasonic Medium | Ultrasonic Apical | Sonic Coronal | Sonic Medium | Sonic Apical | |
|---|---|---|---|---|---|---|
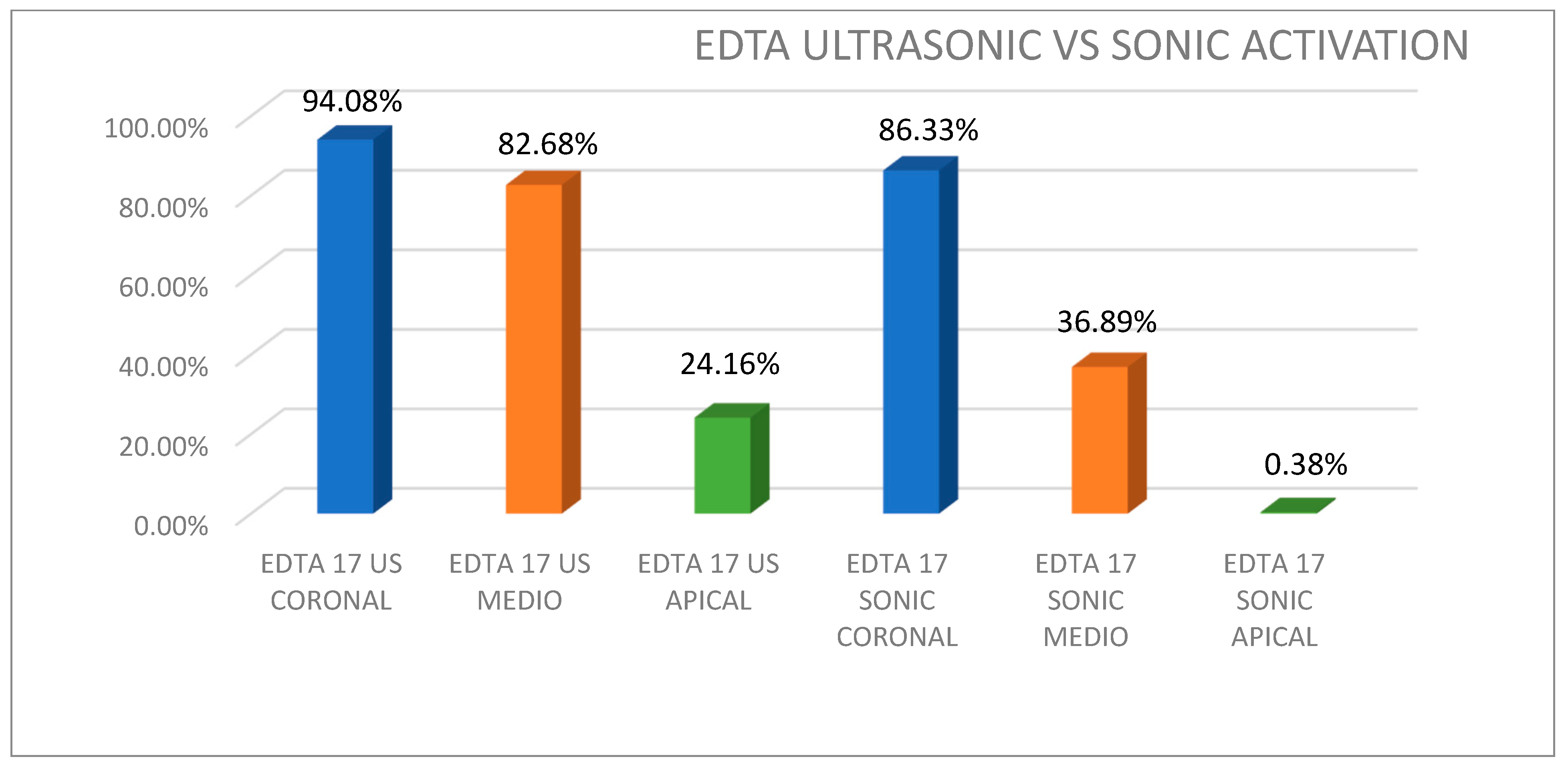
| EDTA | 94.08% (94.27) | 82.68% (84.51) | 24.16% (10.34) | 86.33% (80.00) | 36.89% (35.45) | 0.38% (0.23) |
| HEBP | 56.63% (65.04) | 15.95% (16.80) | 7.93% (4.65) | 97.43% (97.96) | 68.94% (68.96) | 39.84% (40.37) |
| Sig | 0.003 | 0.000397 | 0.299 | 0.0040 | 0.0891 | 0.000014 |
| Ultrasonic Coronal | Ultrasonic Medium | Ultrasonic Apical | Sonic Coronal | Sonic Medium | Sonic Apical | |
|---|---|---|---|---|---|---|
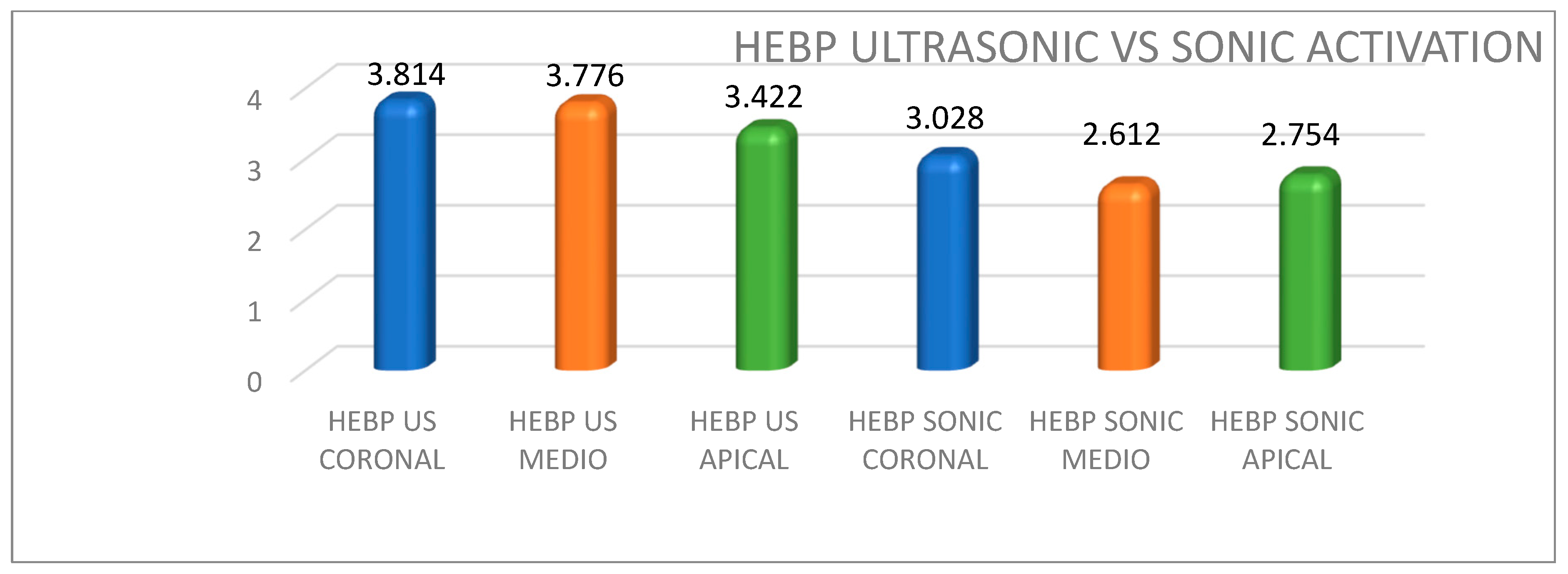
| EDTA | 3.932 (3.43) | 3.394 (2.85) | 1.254 (1.68) | 3.028 (2.8) | 2.318 (2.11) | 0.206 (0.216) |
| HEBP | 3.814 (3.5) | 3.776 (3.9) | 3.422 (2.68) | 3.028 (3.14) | 2.612 (2.71) | 2.754 (2.61) |
| Sig | 0.17 | 0.65 | 0.0002 | 1 | 0.614 | 0.0035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefevre, C.; Mena-Gómez, J.; Martin-Vacas, A.; Vera-Gónzalez, V.; Mena-Álvarez, J. Comparative Analysis of the Chelating Capacity of Two Solutions Activated with Sonic and Ultrasonic Systems: HEBP Versus EDTA. Appl. Sci. 2025, 15, 9993. https://doi.org/10.3390/app15189993
Lefevre C, Mena-Gómez J, Martin-Vacas A, Vera-Gónzalez V, Mena-Álvarez J. Comparative Analysis of the Chelating Capacity of Two Solutions Activated with Sonic and Ultrasonic Systems: HEBP Versus EDTA. Applied Sciences. 2025; 15(18):9993. https://doi.org/10.3390/app15189993
Chicago/Turabian StyleLefevre, Chloé, Julia Mena-Gómez, Andrea Martin-Vacas, Vicente Vera-Gónzalez, and Jesús Mena-Álvarez. 2025. "Comparative Analysis of the Chelating Capacity of Two Solutions Activated with Sonic and Ultrasonic Systems: HEBP Versus EDTA" Applied Sciences 15, no. 18: 9993. https://doi.org/10.3390/app15189993
APA StyleLefevre, C., Mena-Gómez, J., Martin-Vacas, A., Vera-Gónzalez, V., & Mena-Álvarez, J. (2025). Comparative Analysis of the Chelating Capacity of Two Solutions Activated with Sonic and Ultrasonic Systems: HEBP Versus EDTA. Applied Sciences, 15(18), 9993. https://doi.org/10.3390/app15189993






