Using Organic Substances as Green Corrosion Inhibitors for Carbon Steel in HCl Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Anaysis—FTIR
2.3. Gravimetric Analysis
2.4. Electrochemical Analysis
2.5. Surface Analysis
3. Results and Discussion
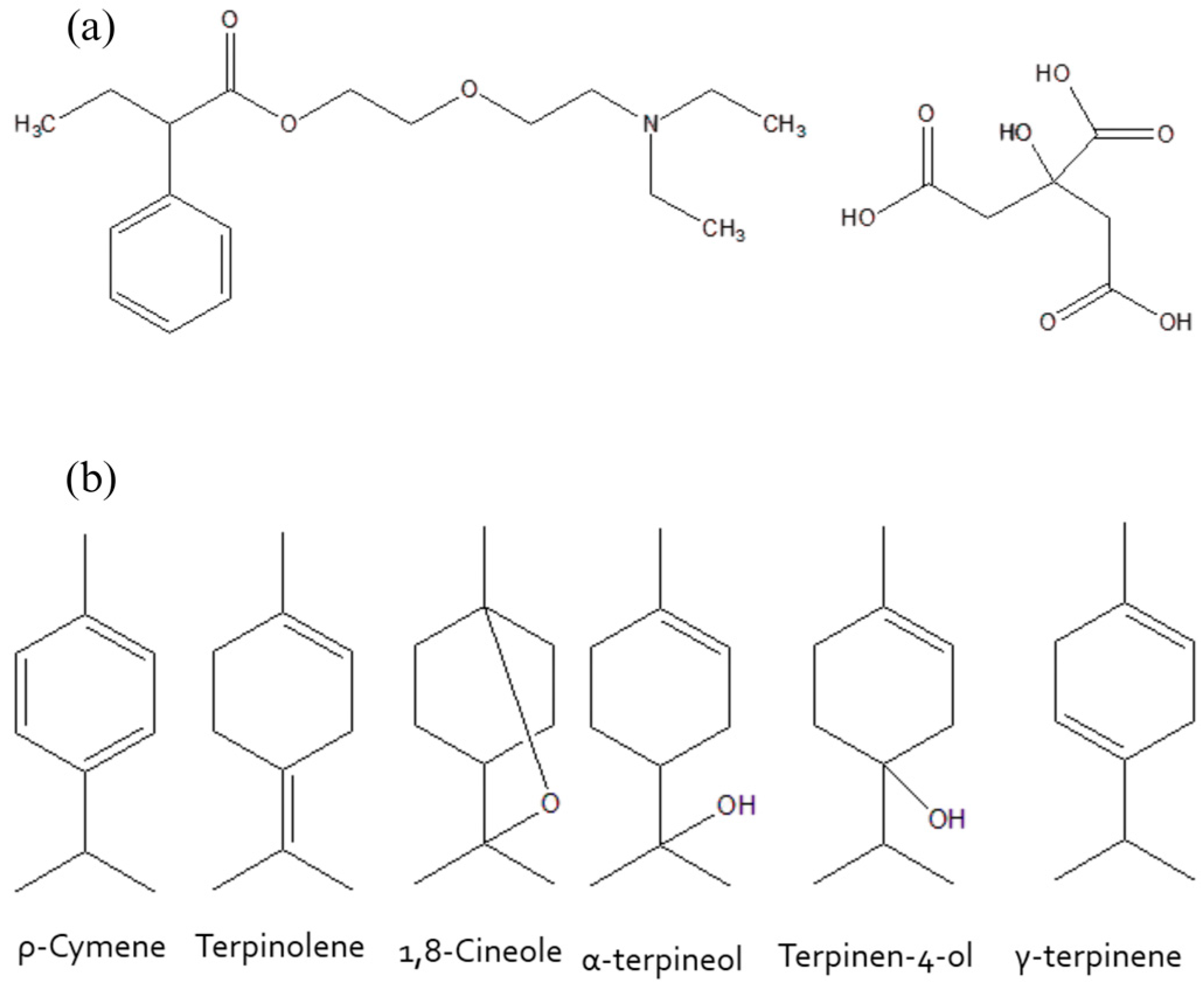
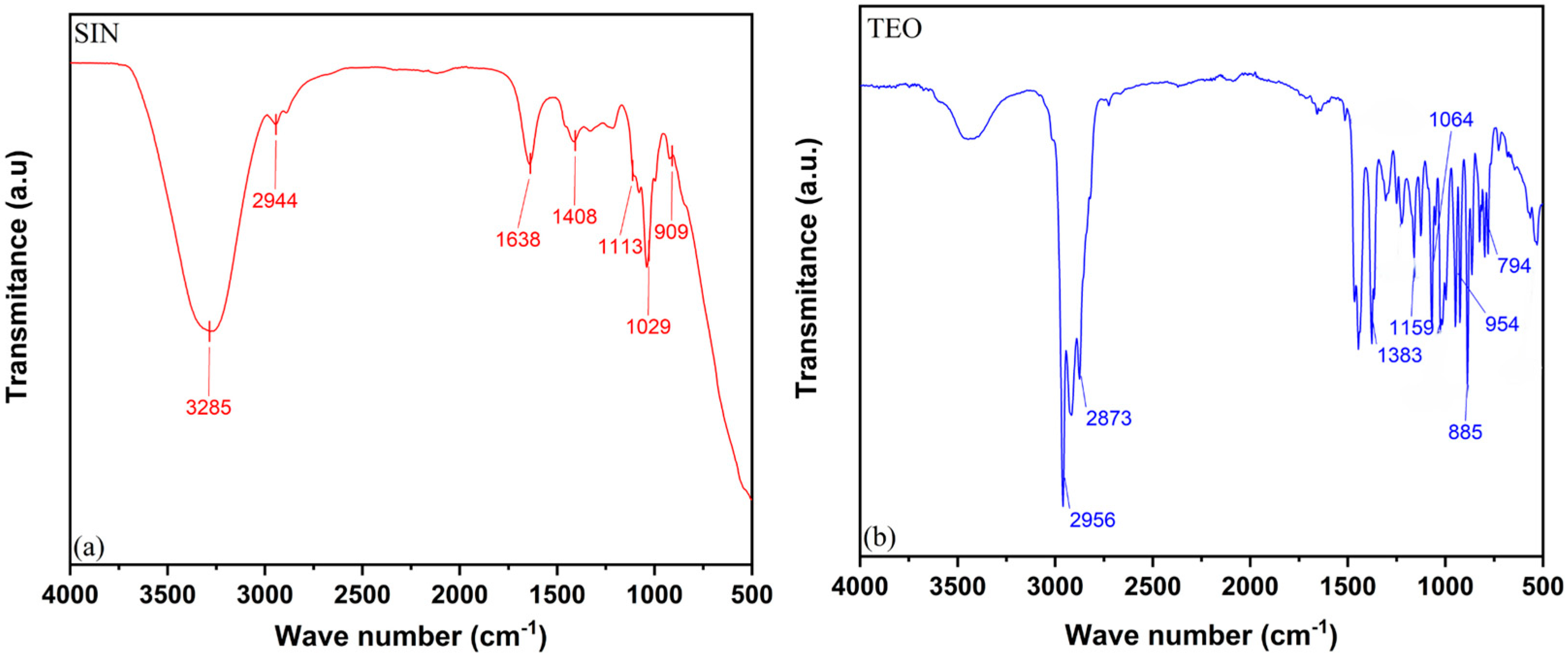
3.1. Molecular Characterization
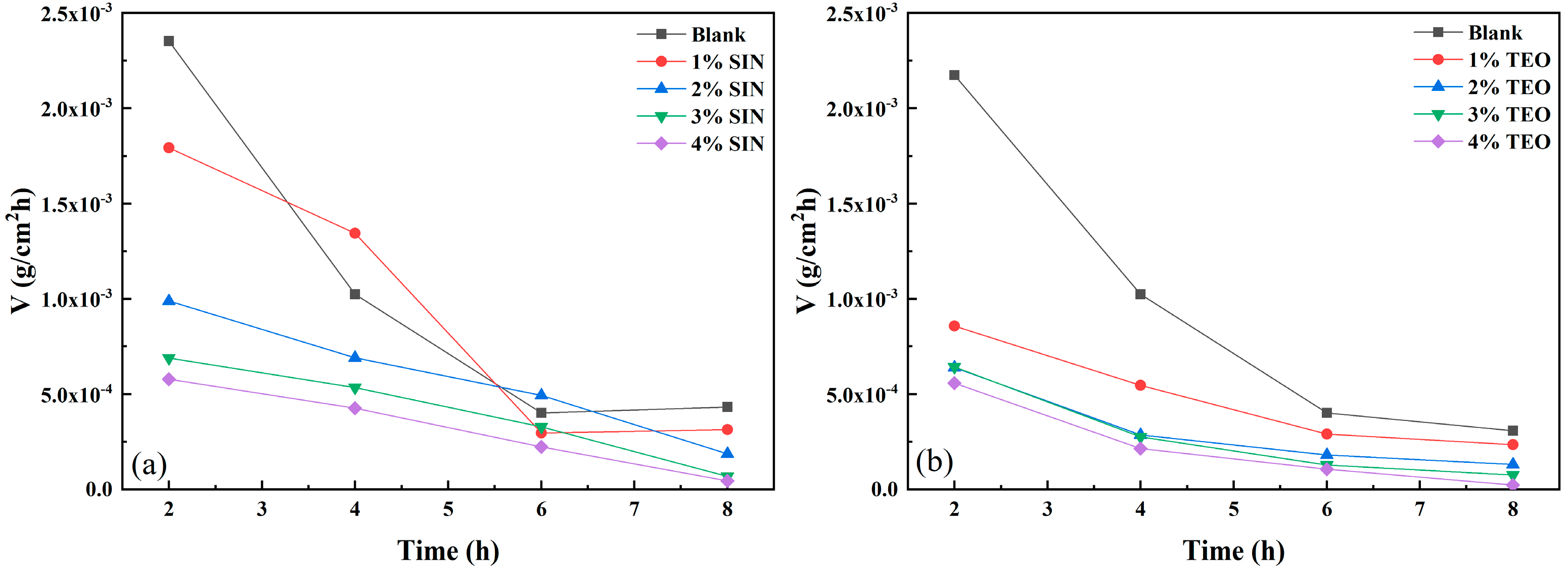
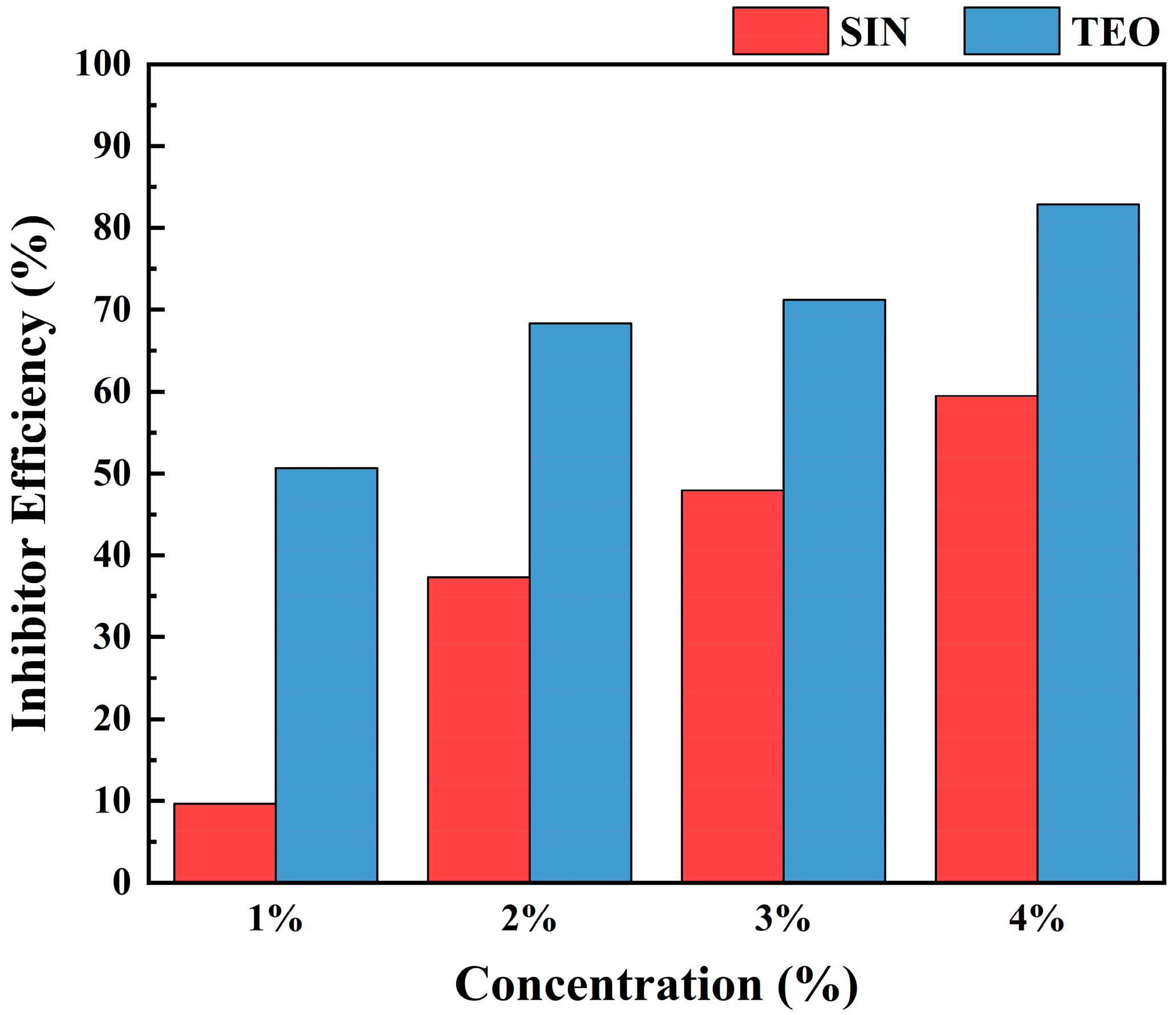
3.2. Gravimetric Data Analysis
3.3. Electrochemical Impedance Spectroscopy Data Analysis
3.4. Polarization Data Analysis
3.5. Inhibitor Efficiency in the Context of Existing Literature
3.6. Surface Analysis
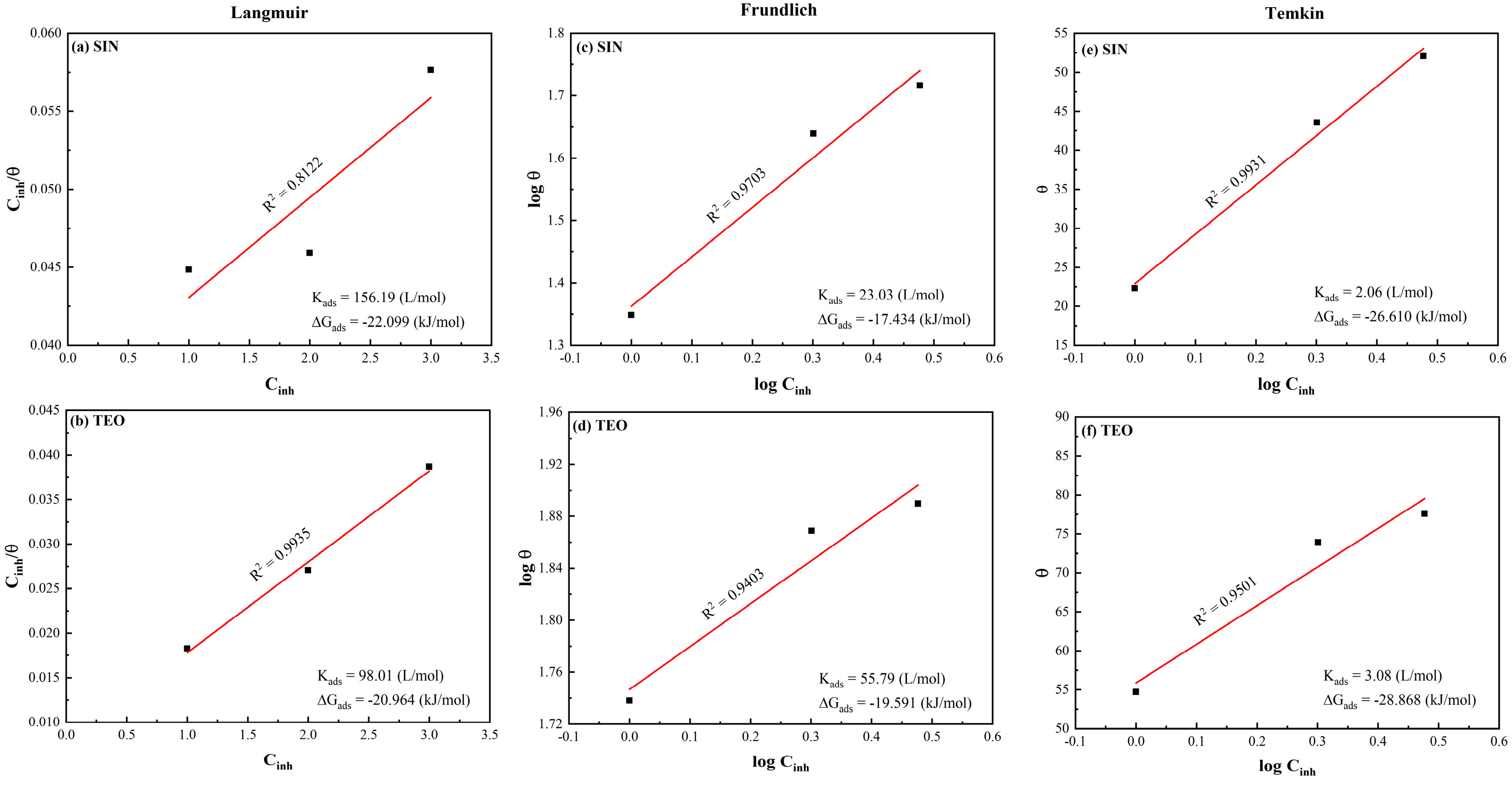
3.7. Adsorption Isotherms
3.8. Corrosion Inhibition Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huesemann, M.H. The Failure of Eco-Efficiency to Guarantee Sustainability: Future Challenges for Industrial Ecology. Environ. Prog. 2004, 23, 264–270. [Google Scholar] [CrossRef]
- Brundtland, G. Our Common Future: Report of the World Commission on Environment and Development; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Marques, C.A.; Machado, A.A.S.C. Environmental Sustainability: Implications and Limitations to Green Chemistry. Found. Chem. 2014, 16, 125–147. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, X.; Wu, J.; Zhang, Y.; Lin, L.; Yang, G.; Deng, S.; Li, L.; Yu, X.; Qi, H.; et al. Sustainability Evaluation of a Steel Production System in China Based on Emergy. J. Clean. Prod. 2016, 112, 1498–1509. [Google Scholar] [CrossRef]
- Abushama, K.; Hawkins, W.; Pelecanos, L.; Ibell, T. Optimisation of Embodied Carbon and Construction Cost of Concrete, Steel and Timber Piles. Dev. Built Environ. 2025, 22, 100656. [Google Scholar] [CrossRef]
- Kassab, L.M.; Martini, W.S.; Santos, V.T.; Lobo, F.G.; Silva, M.R.; Vital, V.G.; Lima, L.F.; de Vasconcellos, S.P.; da Silva, R.A.G.; Pellosi, D.S. Recovering ZnO-Rich Microcomposites from Metallurgic Acid-Pickling Wastewater: From Residue Processing to Material Application. J. Environ. Chem. Eng. 2024, 12, 112637. [Google Scholar] [CrossRef]
- Niecko, J. Recovery of Ferrous Sulfate and Sulfuric Acid from Spent Pickle Liquor of the Steel Industry. Conserv. Recycl. 1987, 10, 309–314. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Xu, J.; Fu, J.; Zheng, G.; Zhou, L. Modified Chemical Mineralization-Alkali Neutralization Technology: Mineralization Behavior at High Iron Concentrations and Its Application in Sulfur Acid Spent Pickling Solution. Water Res. 2022, 218, 118513. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ebrahimi, N.; Shoesmith, D.W.; Manning, P.E. Materials Selection for Use in Hydrochloric Acid. In Proceedings of the Materials Selection for Use in Hydrochloric Acid, Vancouver, BC, Canada, 6–10 March 2016. [Google Scholar]
- Miesiac, I. Removal of Zinc(II) and Iron(II) from Spent Hydrochloric Acid by Means of Anionic Resins. Ind. Eng. Chem. Res. 2005, 44, 1004–1011. [Google Scholar] [CrossRef]
- Mansur, M.B.; Rocha, S.D.F.; Magalhães, F.S.; Benedetto, J.d.S. Selective Extraction of Zinc(II) over Iron(II) from Spent Hydrochloric Acid Pickling Effluents by Liquid–Liquid Extraction. J. Hazard. Mater. 2008, 150, 669–678. [Google Scholar] [CrossRef]
- Lin, B.-L.; Shao, J.-J.; Xu, Y.-Y.; Lai, Y.-M.; Zhao, Z.-N. Adsorption and Corrosion of Renewable Inhibitor of Pomelo Peel Extract for Mild Steel in Phosphoric Acid Solution. Arab. J. Chem. 2021, 14, 103114. [Google Scholar] [CrossRef]
- Devi, A.; Singhal, A.; Gupta, R.; Panzade, P. A Study on Treatment Methods of Spent Pickling Liquor Generated by Pickling Process of Steel. Clean. Technol. Environ. Policy 2014, 16, 1515–1527. [Google Scholar] [CrossRef]
- Kamel, M.M.; Mohsen, Q.; Anwar, Z.M.; Sherif, M.A. An Expired Ceftazidime Antibiotic as an Inhibitor for Disintegration of Copper Metal in Pickling HCl Media. J. Mater. Res. Technol. 2021, 11, 875–886. [Google Scholar] [CrossRef]
- Maizia, R.; Djermoune, A.; Amoura, D.; Zaabar, A.; Thomas, A.; Dib, A.; Martemianov, S. Bidens Aurea Aiton Plant Extract as a Green and Sustainable Corrosion Inhibitor for Carbon Steel X42 in Hydrochloric Acidic Medium: Experimental and Computational Studies. Mater. Chem. Phys. 2025, 339, 130740. [Google Scholar] [CrossRef]
- Shehata, O.S.; Korshed, L.A.; Attia, A. Green Corrosion Inhibitors, Past, Present, and Future. In Corrosion Inhibitors, Principles and Recent Applications; Intech: Toulon, France, 2018. [Google Scholar]
- Huang, L.; Chen, W.-Q.; Wang, S.-S.; Zhao, Q.; Li, H.-J.; Wu, Y.-C. Starch, Cellulose and Plant Extracts as Green Inhibitors of Metal Corrosion: A Review. Environ. Chem. Lett. 2022, 20, 3235–3264. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Maghraby, Y.R.; Ibrahim, A.H.; Shouier, K.R.; Alturki, A.M.; El-Shabasy, R.M. Role of Green Chemistry in Sustainable Corrosion Inhibition: A Review on Recent Developments. Mater. Today Sustain. 2022, 20, 100242. [Google Scholar] [CrossRef]
- Verma, C.; Alfantazi, A.; Quraishi, M.A.; Rhee, K.Y. Are Extracts Really Green Substitutes for Traditional Toxic Corrosion Inhibitors? Challenges beyond Origin and Availability. Sustain. Chem. Pharm. 2023, 31, 100943. [Google Scholar] [CrossRef]
- Mungwari, C.P.; Obadele, B.A.; King’ondu, C.K. Phytochemicals as Green and Sustainable Corrosion Inhibitors for Mild Steel and Aluminium: Review. Results Surf. Interfaces 2025, 18, 100374. [Google Scholar] [CrossRef]
- Tammam, R.H.; Saleh, M.M. Corrosion Inhibition of Copper-Iron Alloy in Acid Solution Using Cetylpyridinium Bromide as Cationic Surfactant. Prot. Met. Phys. Chem. Surf. 2019, 55, 761–769. [Google Scholar] [CrossRef]
- de Souza, F.S.; Giacomelli, C.; Gonçalves, R.S.; Spinelli, A. Adsorption Behavior of Caffeine as a Green Corrosion Inhibitor for Copper. Mater. Sci. Eng. C 2012, 32, 2436–2444. [Google Scholar] [CrossRef]
- Abdallah, M. Antibacterial Drugs as Corrosion Inhibitors for Corrosion of Aluminium in Hydrochloric Solution. Corros. Sci. 2004, 46, 1981–1996. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ogbobe, O.; Okafor, P.C.; Ebenso, E.E. Polyethylene Glycol and Polyvinyl Alcohol as Corrosion Inhibitors for Aluminium in Acidic Medium. J. Appl. Polym. Sci. 2007, 105, 3363–3370. [Google Scholar] [CrossRef]
- Palumbo, G.; Berent, K.; Proniewicz, E.; Banaś, J. Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution. Materials 2019, 12, 2620. [Google Scholar] [CrossRef]
- Sharma, S.K.; Peter, A.; Obot, I.B. Potential of Azadirachta Indica as a Green Corrosion Inhibitor against Mild Steel, Aluminum, and Tin: A Review. J. Anal. Sci. Technol. 2015, 6, 26. [Google Scholar] [CrossRef]
- Okore, G.; Ejiogu, B.; Okeke, P.; Amanze, K.; Okore, S.; Oguzie, E.; Enyoh, C.E. Lawsonia Inermis as an Active Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Appl. Sci. 2024, 14, 6392. [Google Scholar] [CrossRef]
- Chang, N.; Liu, K.; Zhao, Y.; Deng, Y.; Ge, H. Inhibition Performance and Mechanism of Poly(Citric Acid–Glutamic Acid) on Carbon Steel Corrosion in Simulated Seawater. Appl. Sci. 2024, 14, 9465. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, L.; Li, Z.; Wang, Z.; Shang, B. Diphenyl Disulfide Derivatives as High-Efficiency Corrosion Inhibitors for Copper in Sulfuric Acid: Experimental and Theoretical Studies. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135751. [Google Scholar] [CrossRef]
- Vinod Kumar, K.P.; Sankara Narayanan Pillai, M.; Rexin Thusnavis, G. Green Corrosion Inhibitor from Seed Extract of Areca Catechu for Mild Steel in Hydrochloric Acid Medium. J. Mater. Sci. 2011, 46, 5208–5215. [Google Scholar] [CrossRef]
- Al-Ghaban, A.M.H.; Abdullah, H.A.; Anaee, R.A.; Naser, S.A.; Khadom, A.A. Expired Butamirate Drug as Eco-Friendly Corrosion Inhibitor for Aluminum in Seawater: Experimental and Theoretical Studies. J. Eng. Res. 2024, 12, 299–309. [Google Scholar] [CrossRef]
- Ciobotaru, I.A.; Mic, O.C.; Vaireanu, D.I. The Use of Some Natural Extracts as Environmentally Friendly Carbon Steel Corrosion Inhibitors. Appl. Sci. 2024, 14, 11528. [Google Scholar] [CrossRef]
- Pencheva, M.; Nikolova, M.; Damianova, S.; Dushkova, M.; Menkov, N.; Stoyanova, A. Inhibitory Effect of Fennel Fruit Essential Oil and Its Main Component Anethole of Corrosion on Steel Plates in 1 M HCL. Appl. Sci. 2024, 14, 7240. [Google Scholar] [CrossRef]
- Zadeh, A.S.K.; Zandi, M.S.; Kazemipour, M. Corrosion Protection of Carbon Steel in Acidic Media by Expired Bupropion Drug; Experimental and Theoretical Study. J. Indian Chem. Soc. 2022, 99, 100522. [Google Scholar] [CrossRef]
- Abdel-Hameed, R.; Qureshi, M.; Abdallah, M.; Aljuhani, E.; Alzharani, A.A.; Alfarsi, A.; Bakry, A.M.; Huwaimel, B.; Farghaly, O. Recycling of Expired Lactulose Drugs as Eco-Friendly Corrosion Inhibitor for Steel Alloys in Acidic Environment: Gravimetrical and Electrochemical Studies. Int. J. Electrochem. Sci. 2022, 17, 221270. [Google Scholar] [CrossRef]
- Salem, A.; Wahba, A.; El Hossiany, A.; Fouda, A. Experimental and Computational Chemical Studies on the Corrosion Inhibitive Properties of Metamizole Sodium Pharmaceutical Drug Compound for CS in Hydrochloric Acid Solutions. J. Indian Chem. Soc. 2022, 99, 100778. [Google Scholar] [CrossRef]
- Loto, R.T.; Alagbe, E.; Busari, A. Data Analysis of the Corrosion Protection Behavior of Ginger, Tea Tree and Grapefruit Essential Oil Extracts on Low Carbon Steel in H2SO4 Solution. Mater. Today Proc. 2023, 80, 1408–1412. [Google Scholar] [CrossRef]
- The European Comission. Best Available Techniques (BAT) Reference Document for the Ferrous Metals Processing Industry; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Verma, C. Inhibitors. In Handbook of Science & Engineering of Green Corrosion Inhibitors; Verma, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–48. ISBN 978-0-323-90589-3. [Google Scholar]
- Kong, P.; Abe, J.P.; Masuo, S.; Enomae, T. Preparation and Characterization of Tea Tree Oil-β-Cyclodextrin Microcapsules with Super-High Encapsulation Efficiency. J. Bioresour. Bioprod. 2023, 8, 224–234. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Sharifi, M.; Zaarei, D.; Shishesaz, M.R. Application of Eco-Friendly Products as Corrosion Inhibitors for Metals in Acid Pickling Processes—A Review. J. Environ. Chem. Eng. 2013, 1, 652–657. [Google Scholar] [CrossRef]
- Anderez, A.; Alguacil, F.J.; López, F.A. Acid Pickling of Carbon Steel. Rev. Metal. 2022, 58, e226. [Google Scholar] [CrossRef]
- ASTM NACE TM0169/G31—12a; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: Philadelphia, PA, USA, 2012. [CrossRef]
- Pathak, R.K.; Mishra, P. Drugs as Corrosion Inhibitors: A Review. Int. J. Sci. Res. 2016, 5, 671–677. [Google Scholar]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A Review of Surfactants as Corrosion Inhibitors and Associated Modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Verma, D.K.; Khan, F. Green Approach to Corrosion Inhibition of Mild Steel in Hydrochloric Acid Medium Using Extract of Spirogyra Algae. Green. Chem. Lett. Rev. 2016, 9, 52–60. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, J.; Li, P.; Li, R.; Tang, Q. Gleditsia Sinensic Extract as Green Corrosion Inhibitor for N80 Steel in 1 M HCl. J. Mol. Struct. 2025, 1321, 139890. [Google Scholar] [CrossRef]
- Tan, Y. Understanding the Effects of Electrode Inhomogeneity and Electrochemical Heterogeneity on Pitting Corrosion Initiation on Bare Electrode Surfaces. Corros. Sci. 2011, 53, 1845–1864. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Solmaz, R. Electrochemical Synthesis of Poly-2-Aminothiazole on Mild Steel and Its Corrosion Inhibition Performance. Prog. Org. Coat. 2011, 70, 122–126. [Google Scholar] [CrossRef]
- Dahmani, K.; Galai, M.; Rbaa, M.; Ech-Chebab, A.; Errahmany, N.; Guo, L.; AlObaid, A.A.; Hmada, A.; Warad, I.; lakhrissi, B.; et al. Evaluating the Efficacy of Synthesized Quinoline Derivatives as Corrosion Inhibitors for Mild Steel in Acidic Environments: An Analysis Using Electrochemical, Computational, and Surface Techniques. J. Mol. Struct. 2024, 1295, 136514. [Google Scholar] [CrossRef]
- Bozorg, M.; Koochakiabkenar, A.; Ashouri, Y. Combined Electrochemical and DFT Approaches to Elaeagnus Angustifolia Extract as a Green Corrosion Inhibitor for Mild Steel in HCl Solution. J. Mol. Liq. 2025, 434, 127993. [Google Scholar] [CrossRef]
- Solmaz, R.; Salci, A.; Doğrubaş, M.; Dursun, Y.A.; Kaya, S.; Berisha, A.; Kardaş, G. Comprehensive Experimental and Theoretical Study on the Adsorption and Corrosion Inhibition Efficiency of Pyronin B for Mild Steel Protection in HCl Solution. J. Phys. Chem. C 2024, 128, 21809–21825. [Google Scholar] [CrossRef]
- Altunbaş Şahin, E.; Aydın Dursun, Y.; Halil Geçibesler, İ.; Solmaz, R. Adsorption and Corrosion Inhibition Ability of Avocado Seed (Persea Americana) Extract for Copper Corrosion in 0.5 M H2SO4 Solution. Inorg. Chem. Commun. 2024, 167, 112751. [Google Scholar] [CrossRef]
- Sabiha, M.; Kerroum, Y.; El Hawary, M.; Boudalia, M.; Bellaouchou, A.; Hammani, O.; Amin, H.M.A. Investigating the Adsorption and Corrosion Protection Efficacy and Mechanism of Marjoram Extract on Mild Steel in HCl Medium. Molecules 2025, 30, 272. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, J.; Wang, B.; Yao, P. Combined Electrochemical, Surface Analysis and DFT/MD-Simulations to Evaluate Choline Histidine Ionic Liquid as a Green Corrosion Inhibitor for Mild Steel in Neutral Medium. Mater. Today Commun. 2024, 40, 109871. [Google Scholar] [CrossRef]
- Bijapur, K.; Molahalli, V.; Shetty, A.; Toghan, A.; De Padova, P.; Hegde, G. Recent Trends and Progress in Corrosion Inhibitors and Electrochemical Evaluation. Appl. Sci. 2023, 13, 10107. [Google Scholar] [CrossRef]
- Kartal, A.; Yıldız, R.; Döner, A.; Baran, M.F. Thorough Examination of Polygonum aviculare L. Plant Leaf Extract as a Green Corrosion Inhibitor for Mild Steel in 1M HCl: Synthesis, Characterization, Observations from Surface Analysis, Experimental and DFT Studies. Inorg. Chem. Commun. 2025, 176, 114241. [Google Scholar] [CrossRef]
- Kellal, R.; Zertoubi, M.; Safi, Z.S.; Wazzan, N.A.; Al-Qurashi, O.S.; Benmessaoud Left, D. Exploring the Role of Chrysanthemum Coronarium Leaves Distillation Waste as a Green Inhibitor for Carbon Steel in Acidic Environment: An Integrated Study. RSC Adv. 2024, 14, 40198–40221. [Google Scholar] [CrossRef]
- He, H.; Shi, J.; Yu, S.; Yang, J.; Xu, K.; He, C.; Li, X. Exploring Green and Efficient Zero-Dimensional Carbon-Based Inhibitors for Carbon Steel: From Performance to Mechanism. Constr. Build. Mater. 2024, 411, 134334. [Google Scholar] [CrossRef]
- Salim, R.; Ech-Chihbi, E.; Benhiba, F.; Ouakki, M.; El Kalai, F.; Benchat, N.; Ettahiri, W.; Alanazi, A.S.; Oudda, H.; Hammouti, B.; et al. Corrosion Inhibition and Adsorption Mechanism of Novel Imidazopyridine Corrosion Inhibitors: Electrochemical and Computational Studies. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]

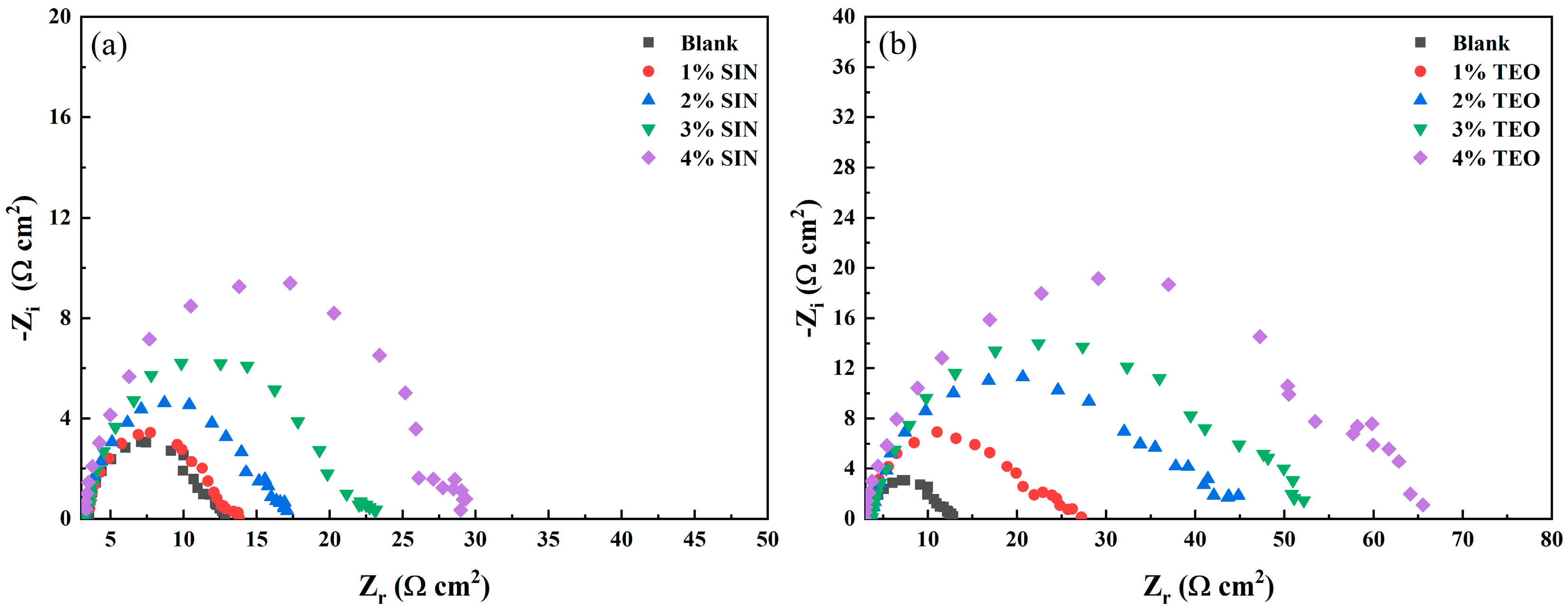
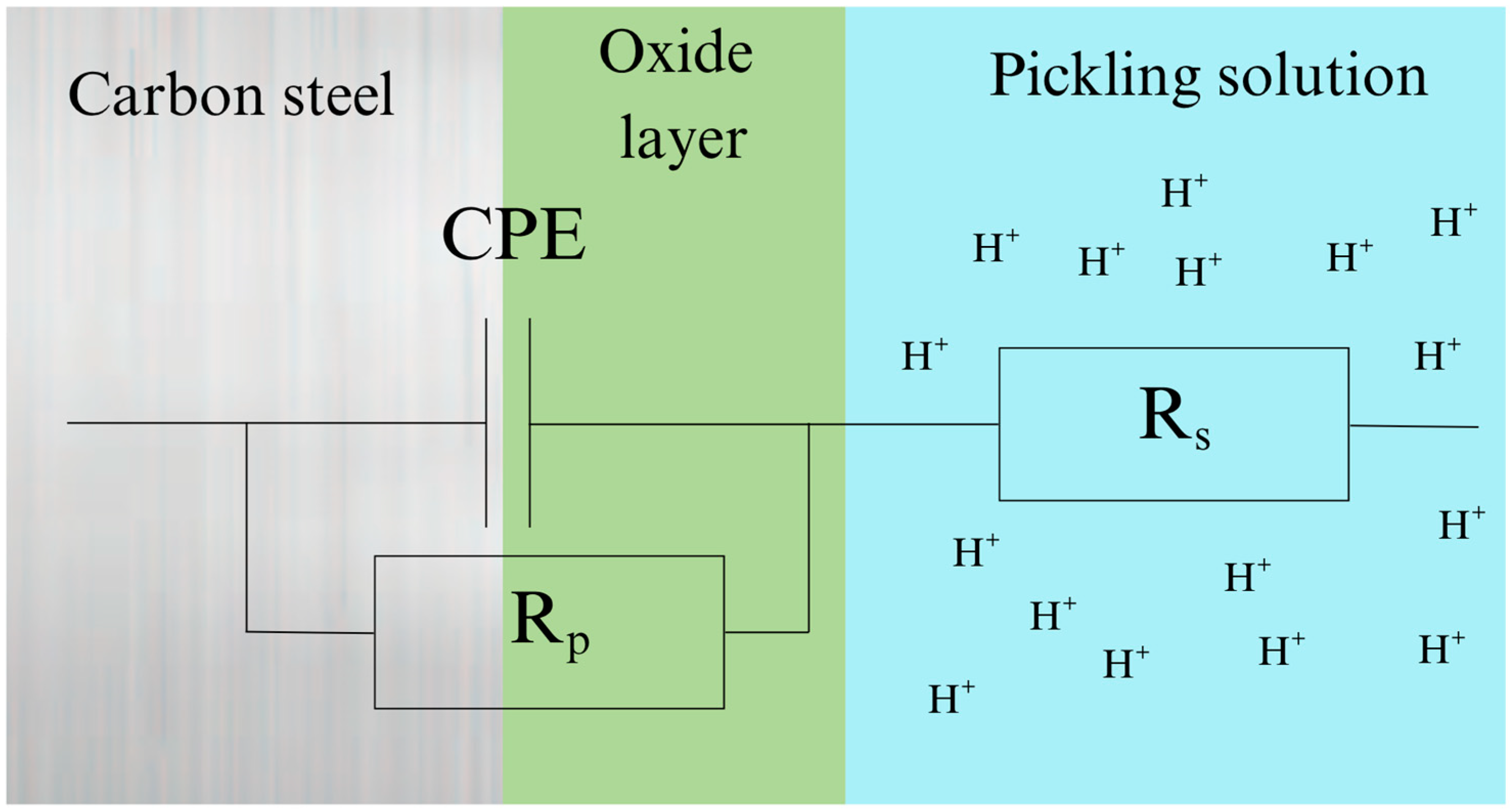

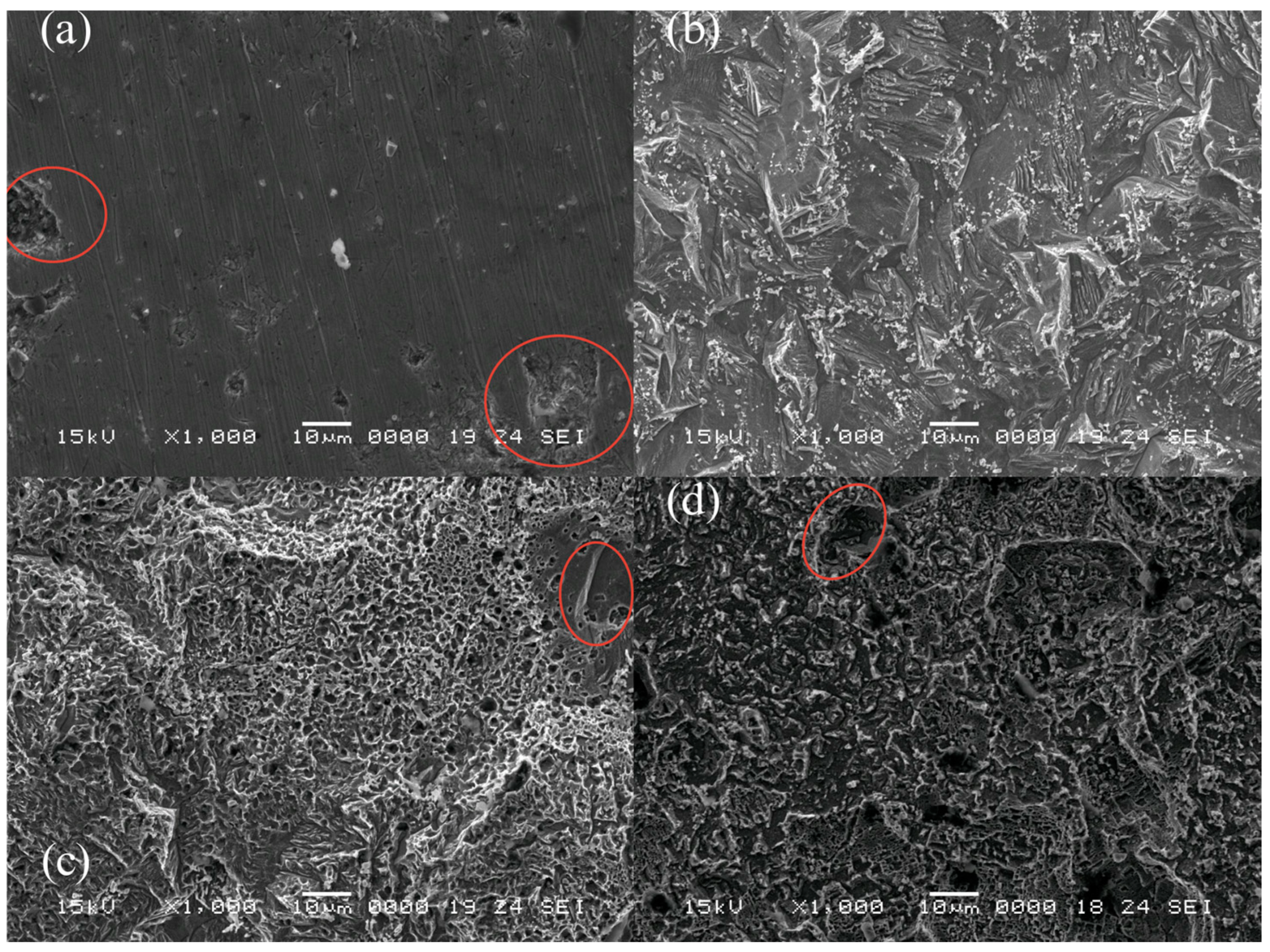

| Sample | Concentration (%) | Rs (Ω cm2) | Rp (Ω cm2) | CPE (μFsn−1 cm−2) | n | Cdl (μFcm−2) | IEEIS (%) |
|---|---|---|---|---|---|---|---|
| Blank | 3.19 | 9.86 | 903.63 | 0.73 | 2611.563 | - | |
| SIN | 1 | 3.20 | 11.26 | 549.30 | 0.81 | 425.733 | 12.43 |
| 2 | 3.27 | 17.47 | 638.95 | 0.77 | 1034.041 | 43.56 | |
| 3 | 3.20 | 20.56 | 491.13 | 0.78 | 661.594 | 52.04 | |
| 4 | 3.18 | 25.61 | 946.70 | 0.82 | 864.341 | 61.49 | |
| TEO | 1 | 2.98 | 21.78 | 660.67 | 0.75 | 1606.936 | 54.72 |
| 2 | 3.38 | 37.85 | 496.57 | 0.74 | 1576.421 | 73.94 | |
| 3 | 3.15 | 43.944 | 458.89 | 0.74 | 1493.270 | 77.56 | |
| 4 | 3.05 | 70.84 | 667.20 | 0.73 | 2282.710 | 86.08 |
| Sample | Concentration (%) | Ecorr (mV vs. Ag/AgCl) | icorr (mA/cm2) | |βc| (mV/dec) | βa (mV/dec) | IETaf (%) |
|---|---|---|---|---|---|---|
| Blank | 0 | −409.9 | 8.9812 | 444.6 | 268.8 | - |
| SIN | 1 | −427.5 | 6.7666 | 223.7 | 123.1 | 24.64 |
| 2 | −428.4 | 4.7714 | 195.5 | 105.0 | 46.86 | |
| 3 | −427.5 | 3.7849 | 216.4 | 185.5 | 57.85 | |
| 4 | −413.2 | 2.8895 | 190.4 | 126.2 | 67.82 | |
| TEO | 1 | −427.6 | 3.5047 | 207.0 | 133.3 | 60.97 |
| 2 | −433.9 | 2.4443 | 216.3 | 144.3 | 72.78 | |
| 3 | −445.1 | 1.6449 | 241.5 | 159.5 | 81.68 | |
| 4 | −497.7 | 0.9956 | 200.1 | 145.5 | 89.36 |
| Sample | Concentration (%) | IEGAV (%) | IEEIS (%) | IETaf (%) | IEavg (%) |
|---|---|---|---|---|---|
| SIN | 1 | 9.68 | 12.43 | 24.64 | 15.58 |
| 2 | 37.37 | 43.56 | 46.86 | 42.59 | |
| 3 | 47.97 | 52.04 | 57.85 | 52.62 | |
| 4 | 59.49 | 61.49 | 67.82 | 62.93 | |
| TEO | 1 | 50.68 | 54.72 | 60.97 | 55.45 |
| 2 | 68.34 | 73.94 | 72.78 | 71.68 | |
| 3 | 71.27 | 77.56 | 81.68 | 76.83 | |
| 4 | 82.92 | 86.08. | 89.36 | 86.14 |
| Isotherm Model | Linear Form | Plot |
|---|---|---|
| Langmuir | ||
| Frundlich | ||
| Temkin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crișan, C.A.; Vermeșan, H.; Ștefan-Sicoe, A.; Zdrob, N. Using Organic Substances as Green Corrosion Inhibitors for Carbon Steel in HCl Solution. Appl. Sci. 2025, 15, 9983. https://doi.org/10.3390/app15189983
Crișan CA, Vermeșan H, Ștefan-Sicoe A, Zdrob N. Using Organic Substances as Green Corrosion Inhibitors for Carbon Steel in HCl Solution. Applied Sciences. 2025; 15(18):9983. https://doi.org/10.3390/app15189983
Chicago/Turabian StyleCrișan, Claudia A., Horațiu Vermeșan, Anca Ștefan-Sicoe, and Nicoleta Zdrob. 2025. "Using Organic Substances as Green Corrosion Inhibitors for Carbon Steel in HCl Solution" Applied Sciences 15, no. 18: 9983. https://doi.org/10.3390/app15189983
APA StyleCrișan, C. A., Vermeșan, H., Ștefan-Sicoe, A., & Zdrob, N. (2025). Using Organic Substances as Green Corrosion Inhibitors for Carbon Steel in HCl Solution. Applied Sciences, 15(18), 9983. https://doi.org/10.3390/app15189983








