From DNA Methylation and Histone Modifications to Non-Coding RNAs: Evaluating Tools for Epigenetic Research
Abstract
1. Introduction
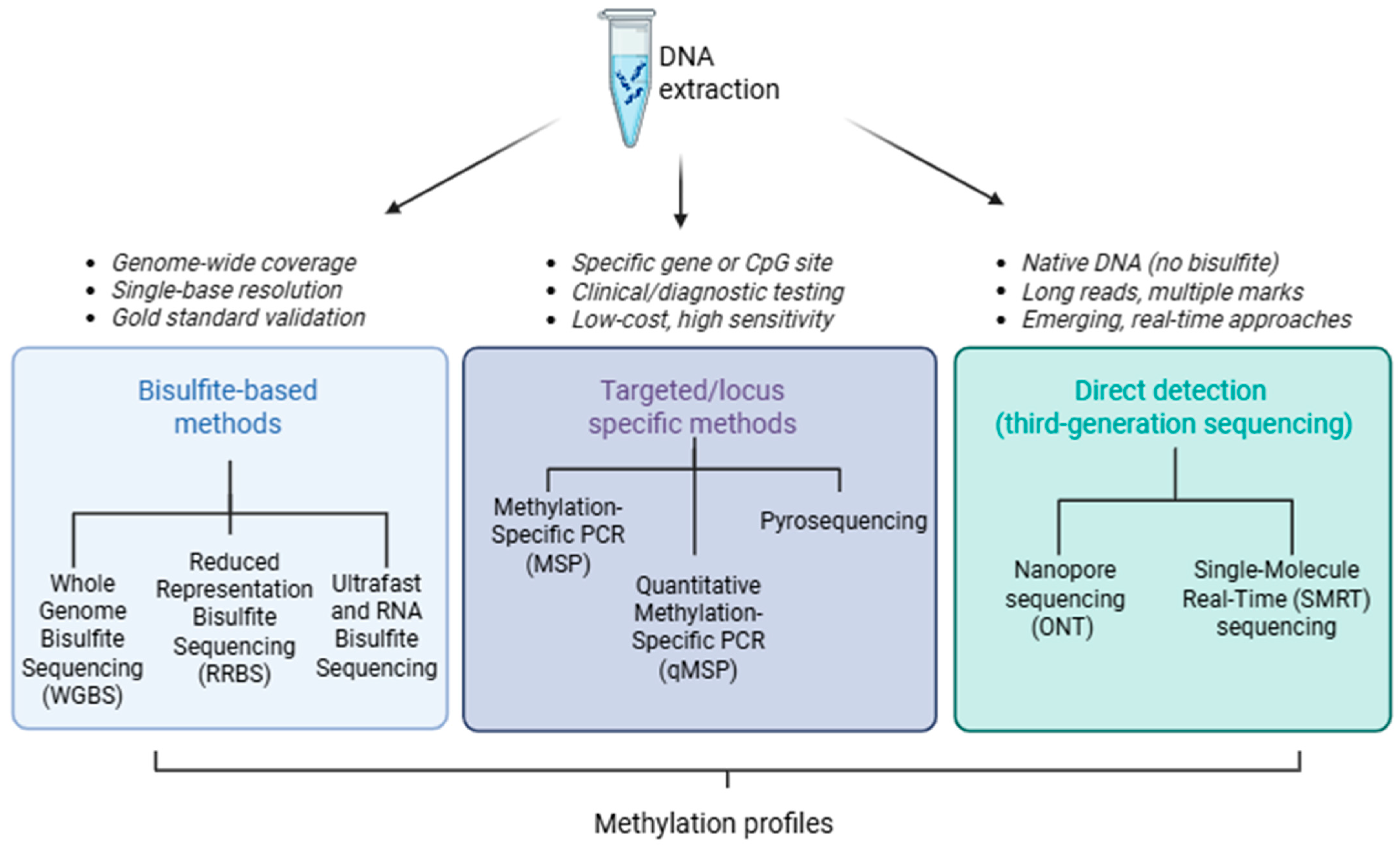
2. DNA Methylation
2.1. Bisulfite Sequencing: A Gold Standard for Base-Resolution DNA Methylation Profiling
2.1.1. Whole-Genome Bisulfite Sequencing (WGBS)
2.1.2. Reduced Representation Bisulfite Sequencing (RRBS)
2.1.3. Ultrafast and RNA Bisulfite Sequencing (UBS-Seq)
2.2. Methylation-Specific PCR (MSP): Gene-Specific Epigenetic Analysis
- ∘
- Nested MSP increases sensitivity by using two rounds of amplification—an initial outer primer set followed by methylation-specific primers, which is useful for detecting low-abundance methylation events [42].
- ∘
- ∘
- Quantitative MSP (qMSP) provides methylation quantification using real-time PCR. Dye-based (e.g., SYBR Green) or probe-based (e.g., TaqMan) detection enables relative quantification against reference standards and has been adopted in clinical diagnostics for cancer and other epigenetic disorders [44].
- ∘
- ∘
- Combined Bisulfite Restriction Analysis (COBRA) uses restriction enzymes to digest PCR amplicons post-bisulfite treatment. It enables qualitative assessment of methylation at specific restriction sites, but is limited to regions containing suitable enzyme recognition motifs [16].
- ∘
- Hairpin-Bisulfite PCR, developed by Laird et al., facilitates the study of methylation symmetry by preserving complementary strands with a covalently linked hairpin structure, allowing simultaneous analysis of both DNA strands [16].
2.3. Quantitative Methylation Detection Using Pyrosequencing Technology
2.4. Nanopore Technology for Simultaneous Basecalling and Methylation Detection
2.5. Single-Molecule Real-Time Sequencing (SMRT): Direct Epigenetic Profiling via Polymerase Kinetics
2.6. Comparative Evaluation of DNA Methylation Analysis Methods
2.7. Experimental Considerations and Pipeline Optimization
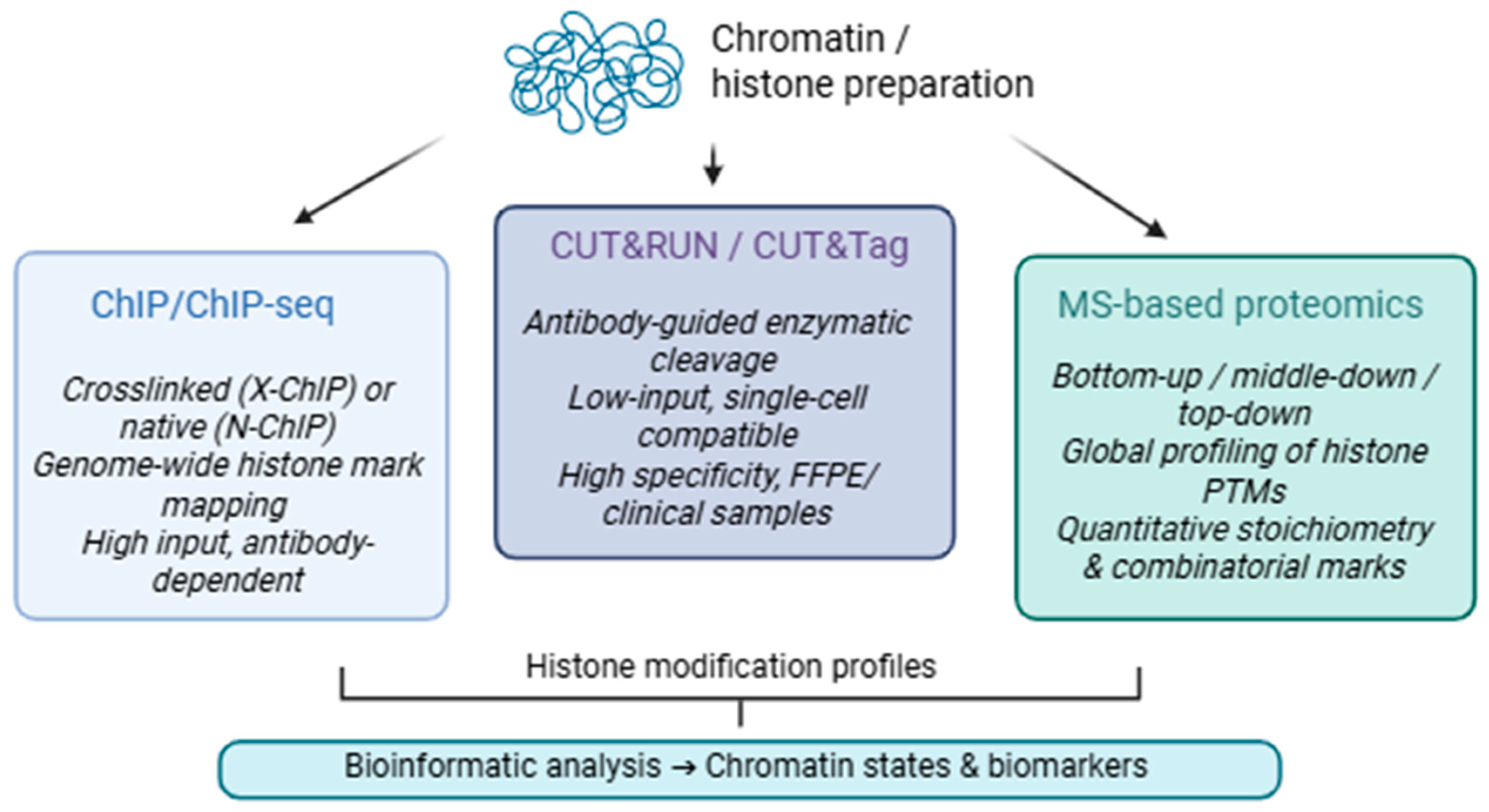
3. Histone Modifications
3.1. Chromatin Immunoprecipitation
3.2. Cleavage Under Targets (CUT&RUN) and Release Using Nuclease) and Cleavage Under Targets and Tagmentation (CUT&Tag)
3.3. Mass Spectrometry-Based Proteomics
- Bottom-up proteomics, the predominant methodology, entails trypsin digestion of chemically modified histones to produce short peptides for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. This technology is resilient and ideally suited for high-throughput operations, including the analysis of clinical samples, as evidenced in patient stratification studies for cancer and other disorders [112].
- Middle–down proteomics facilitates the examination of extended polypeptides, preserving greater information regarding concurrent post-translational modifications (PTMs) and permitting the identification of combinatorial patterns, such as bivalent marks like H3K4me3/H3K27me3. This method integrates the sensitivity and throughput of bottom-up techniques with the superior resolution of top-down procedures, albeit it necessitates sophisticated chromatographic and computational tools [111].
- Top-down proteomics directly examines intact histones without preliminary digestion, providing exceptional insight into individual proteoforms, particularly those with multiple, distant alterations. Although technically challenging and currently constrained in large-scale use, advancements in ion mobility and computational deconvolution are progressively enhancing the accessibility of this method [111].
3.4. Comparative Evaluation of Histone Modification Profiling Techniques
3.5. Experimental Considerations and Pipeline Optimization
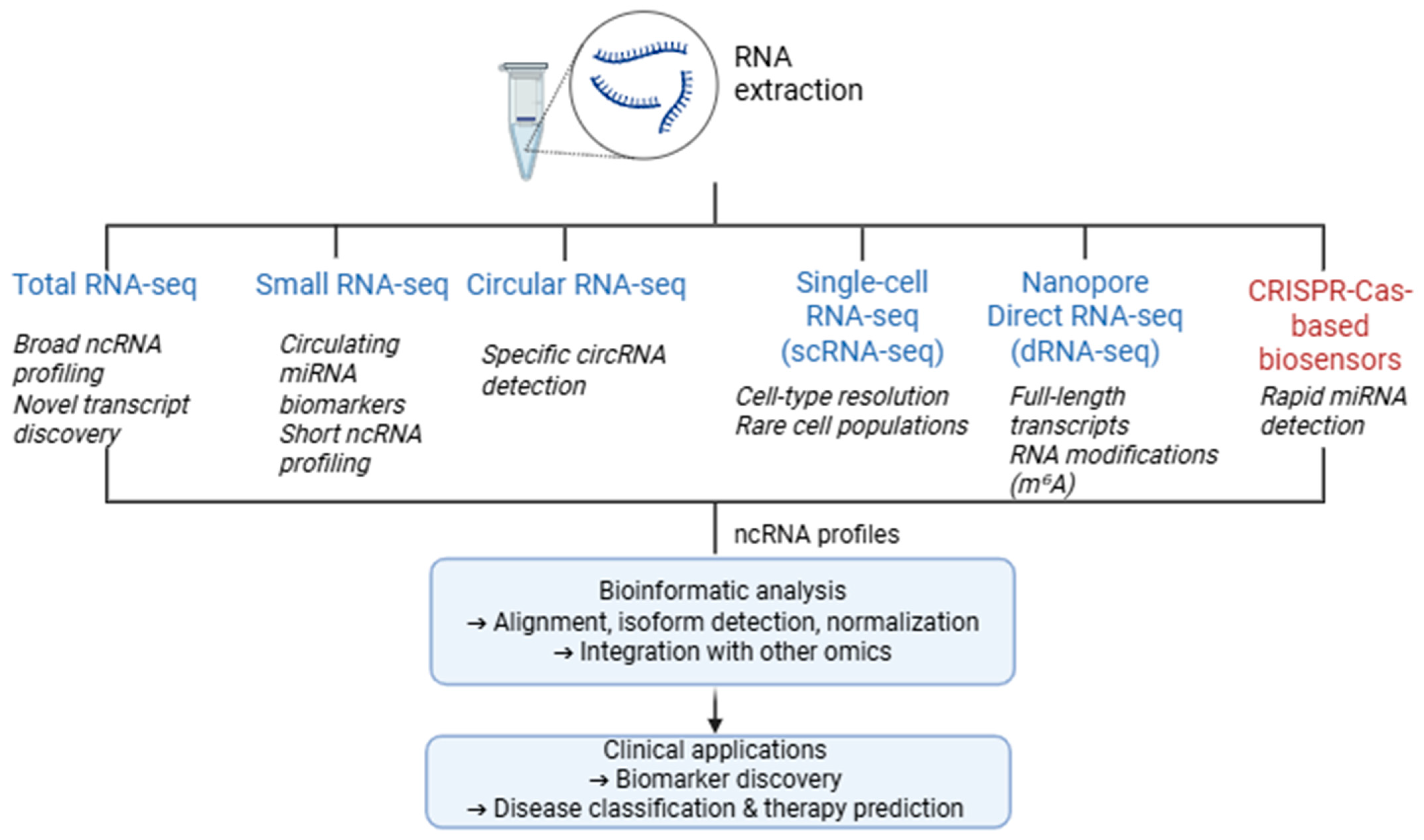
4. Non-Coding RNAs
4.1. RNA-Seq Approaches
4.1.1. Total RNA Sequencing
4.1.2. Small RNA Sequencing
4.1.3. Circular RNA Sequencing
4.1.4. Single-Cell RNA Sequencing
4.1.5. Nanopore Direct RNA Sequencing (dRNA-Seq)
4.1.6. Computational Pipelines and Reproducibility
4.2. CRISPR-Cas-Based Sensors for miRNA
4.3. Comparative Evaluation of RNA-Based Methods for Non-Coding RNA Profiling
4.4. Experimental Considerations and Pipeline Optimization
5. Artificial Intelligence Models Integrated for Epigenetics Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavalli, G.; Heard, E. Advances in Epigenetics Link Genetics to the Environment and Disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef]
- Choi, B.Y.; Han, M.; Kwak, J.W.; Kim, T.H. Genetics and Epigenetics in Allergic Rhinitis. Genes 2021, 12, 2004. [Google Scholar] [CrossRef]
- Recillas-Targa, F. Cancer Epigenetics: An Overview. Arch. Med. Res. 2022, 53, 732–740. [Google Scholar] [CrossRef]
- Prasher, D.; Greenway, S.C.; Singh, R.B. The Impact of Epigenetics on Cardiovascular Disease. Biochem. Cell Biol. 2020, 98, 12–22. [Google Scholar] [CrossRef]
- Kumar, S.; Shanker, O.R.; Banerjee, J.; Tripathi, M.; Chandra, P.S.; Dixit, A.B. Epigenetics in Epilepsy. Prog. Mol. Biol. Transl. Sci. 2023, 198, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Surace, A.E.A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Saadat, A. Neurodegeneration and Epigenetics: A Review. Neurologia 2023, 38, e62–e68. [Google Scholar] [CrossRef]
- Ho, D.H.; Burggren, W.W. Epigenetics and Transgenerational Transfer: A Physiological Perspective. J. Exp. Biol. 2010, 213, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, L.; Álvarez-Errico, D.; Esteller, M. The Contribution of Epigenetics to Cancer Immunotherapy. Trends Immunol. 2020, 41, 676–691. [Google Scholar] [CrossRef]
- Li, Y. Modern Epigenetics Methods in Biological Research. Methods 2020, 187, 104. [Google Scholar] [CrossRef]
- Comendul, A.; Ruf-Zamojski, F.; Ford, C.T.; Agarwal, P.; Zaslavsky, E.; Nudelman, G.; Hariharan, M.; Rubenstein, A.; Pincas, H.; Nair, V.D.; et al. Comprehensive Guide for Epigenetics and Transcriptomics Data Quality Control. STAR Protoc. 2025, 6, 103607. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Lv, R.; Shi, X.; Yang, G.; Jin, J. CRISPR/DCas9 Tools: Epigenetic Mechanism and Application in Gene Transcriptional Regulation. Int. J. Mol. Sci. 2023, 24, 14865. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Frommer, M.; McDonald, L.E.; Millar, D.S.; Collis, C.M.; Watt, F.; Grigg, G.W.; Molloy, P.L.; Paul, C.L. A Genomic Sequencing Protocol That Yields a Positive Display of 5-Methylcytosine Residues in Individual DNA Strands. Proc. Natl. Acad. Sci. USA 1992, 89, 1827–1831. [Google Scholar] [CrossRef]
- Harrison, A.; Parle-McDermott, A. DNA Methylation: A Timeline of Methods and Applications. Front. Genet. 2011, 2, 74. [Google Scholar] [CrossRef]
- Söderhäll, C.; Reinius, L.E.; Salmenperä, P.; Gentile, M.; Acevedo, N.; Konradsen, J.R.; Nordlund, B.; Hedlin, G.; Scheynius, A.; Myllykangas, S.; et al. High-Resolution Targeted Bisulfite Sequencing Reveals Blood Cell Type-Specific DNA Methylation Patterns in IL13 and ORMDL3. Clin. Epigenetics 2021, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.A.; Müller, S.; Hummel, E.M.; Limberg, A.S.; Dieckmann, L.; Frach, L.; Pakusch, J.; Flasbeck, V.; Brüne, M.; Beygo, J.; et al. Targeted Bisulfite Sequencing: A Novel Tool for the Assessment of DNA Methylation with High Sensitivity and Increased Coverage. Psychoneuroendocrinology 2020, 120, 104784. [Google Scholar] [CrossRef]
- Pu, W.; Qian, F.; Liu, J.; Shao, K.; Xiao, F.; Jin, Q.; Liu, Q.; Jiang, S.; Zhang, R.; Zhang, J.; et al. Targeted Bisulfite Sequencing Reveals DNA Methylation Changes in Zinc Finger Family Genes Associated with KRAS Mutated Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 759813. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Bock, C.; Mikkelsen, T.S.; Jäger, N.; Smith, Z.D.; Tomazou, E.; Gnirke, A.; Lander, E.S.; Meissner, A. Genome-Scale DNA Methylation Mapping of Clinical Samples at Single-Nucleotide Resolution. Nat. Methods 2010, 7, 133. [Google Scholar] [CrossRef]
- Doherty, R.; Couldrey, C. Exploring Genome Wide Bisulfite Sequencing for DNA Methylation Analysis in Livestock: A Technical Assessment. Front. Genet. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, F.; Li, B.; Zhang, Y.; Chen, M.; Fan, T.; Wang, T. Whole Genome Bisulfite Sequencing Methylome Analysis of Mulberry (Morus alba) Reveals Epigenome Modifications in Response to Drought Stress. Sci. Rep. 2020, 10, 8013. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.B.; Karkala, F.; Kukk, M.M.; Adams, H.H.H.; Kayser, M.; Vidaki, A. Comparative Performance Evaluation of Bisulfite- and Enzyme-Based DNA Conversion Methods. Clin. Epigenetics 2025, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Ye, C.; Irkliyenko, I.; Wang, Y.; Sun, H.L.; Gao, Y.; Liu, Y.; Beadell, A.; Perea, J.; Goel, A.; et al. Ultrafast Bisulfite Sequencing Detection of 5-Methylcytosine in DNA and RNA. Nat. Biotechnol. 2024, 42, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Kurdyukov, S.; Bullock, M. DNA Methylation Analysis: Choosing the Right Method. Biology 2016, 5, 3. [Google Scholar] [CrossRef]
- Rauluseviciute, I.; Drabløs, F.; Rye, M.B. DNA Methylation Data by Sequencing: Experimental Approaches and Recommendations for Tools and Pipelines for Data Analysis. Clin. Epigenetics 2019, 11, 193. [Google Scholar] [CrossRef]
- Khodadadi, E.; Fahmideh, L.; Khodadadi, E.; Dao, S.; Yousefi, M.; Taghizadeh, S.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Current Advances in DNA Methylation Analysis Methods. BioMed Res. Int. 2021, 2021, 8827516. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Q.; Braun, P.R.; Perzel Mandell, K.A.; Jaffe, A.E.; Tan, H.Y.; Hyde, T.M.; Kleinman, J.E.; Potash, J.B.; Shinozaki, G.; et al. Deep Learning Predicts DNA Methylation Regulatory Variants in the Human Brain and Elucidates the Genetics of Psychiatric Disorders. Proc. Natl. Acad. Sci. USA 2022, 119, e2206069119. [Google Scholar] [CrossRef]
- Dixon, G.; Matz, M. Benchmarking DNA Methylation Assays in a Reef-Building Coral. Mol. Ecol. Resour. 2021, 21, 464–477. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Zou, Q.; Xu, L. Recall DNA Methylation Levels at Low Coverage Sites Using a CNN Model in WGBS. PLoS Comput. Biol. 2023, 19, e1011205. [Google Scholar] [CrossRef]
- Simpson, D.J.; Zhao, Q.; Olova, N.N.; Dabrowski, J.; Xie, X.; Latorre-Crespo, E.; Chandra, T. Region-Based Epigenetic Clock Design Improves RRBS-Based Age Prediction. Aging Cell 2023, 22, e13866. [Google Scholar] [CrossRef] [PubMed]
- Guanzon, D.; Ross, J.P.; Ma, C.; Berry, O.; Liew, Y.J. Comparing Methylation Levels Assayed in GC-Rich Regions with Current and Emerging Methods. BMC Genom. 2024, 25, 741. [Google Scholar] [CrossRef]
- Liu, X.; Pang, Y.; Shan, J.; Wang, Y.; Zheng, Y.; Xue, Y.; Zhou, X.; Wang, W.; Sun, Y.; Yan, X.; et al. Beyond the Base Pairs: Comparative Genome-Wide DNA Methylation Profiling across Sequencing Technologies. Brief. Bioinform. 2024, 25, bbae440. [Google Scholar] [CrossRef]
- Troyee, A.N.; Peña-Ponton, C.; Medrano, M.; Verhoeven, K.J.F.; Alonso, C. Herbivory Induced Methylation Changes in the Lombardy Poplar: A Comparison of Results Obtained by EpiGBS and WGBS. PLoS ONE 2023, 18, e0291202. [Google Scholar] [CrossRef]
- Attree, E.; Griffiths, B.; Panchal, K.; Xia, D.; Werling, D.; Banos, G.; Oikonomou, G.; Psifidi, A. Identification of DNA Methylation Markers for Age and Bovine Respiratory Disease in Dairy Cattle: A Pilot Study Based on Reduced Representation Bisulfite Sequencing. Commun. Biol. 2024, 7, 1251. [Google Scholar] [CrossRef] [PubMed]
- Aroke, E.N.; Overstreet, D.S.; Penn, T.M.; Crossman, D.K.; Jackson, P.; Tollefsbol, T.O.; Quinn, T.L.; Yi, N.; Goodin, B.R. Identification of DNA Methylation Associated Enrichment Pathways in Adults with Non-Specific Chronic Low Back Pain. Mol. Pain. 2020, 16, 1744806920972889. [Google Scholar] [CrossRef] [PubMed]
- Nkongolo, K.; Michael, P. Reduced Representation Bisulfite Sequencing (RRBS) Analysis Reveals Variation in Distribution and Levels of DNA Methylation in White Birch (Betula papyrifera) Exposed to Nickel. Genome 2024, 67, 351–367. [Google Scholar] [CrossRef]
- Beck, D.; Ben Maamar, M.; Skinner, M.K. Genome-Wide CpG Density and DNA Methylation Analysis Method (MeDIP, RRBS, and WGBS) Comparisons. Epigenetics 2022, 17, 518–530. [Google Scholar] [CrossRef]
- Zhao, Y.; O’Keefe, C.M.; Hsieh, K.; Cope, L.; Joyce, S.C.; Pisanic, T.R.; Herman, J.G.; Wang, T.H. Multiplex Digital Methylation-Specific PCR for Noninvasive Screening of Lung Cancer. Adv. Sci. 2023, 10, 2206518. [Google Scholar] [CrossRef]
- Herman, J.G.; Graff, J.R.; Myohanen, S.; Nelkin, B.D.; Baylin, S.B. Methylation-Specific PCR: A Novel PCR Assay for Methylation Status of CpG Islands (DNA Methylation/Tumor Suppressor Genes/Pl6/P15). Proc. Natl. Acad. Sci. USA 1996, 93, 9821–9826. [Google Scholar] [CrossRef]
- Yoshioka, M.; Matsutani, T.; Hara, A.; Hirono, S.; Hiwasa, T.; Takiguchi, M.; Iwadate, Y.; Yoshioka, M.; Matsutani, T.; Hara, A.; et al. Real-Time Methylation-Specific PCR for the Evaluation of Methylation Status of MGMT Gene in Glioblastoma. Oncotarget 2018, 9, 27728–27735. [Google Scholar] [CrossRef]
- Wani, K.; Aldape, K.D. PCR Techniques in Characterizing DNA Methylation. Methods Mol. Biol. 2016, 1392, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.C.; de Oliveira Ganzella, F.A.; Miniskiskosky, G.; da Cunha, R.S.; de Souza Ramos, E.A. Digital Methylation-Specific PCR: New Applications for Liquid Biopsy. Biomol. Concepts 2024, 15, 20220041. [Google Scholar] [CrossRef] [PubMed]
- Santourlidis, S.; Ghanjati, F.; Beermann, A.; Hermanns, T.; Poyet, C. IDLN-MSP: Idiolocal Normalization of Real-Time Methylation-Specific PCR for Genetic Imbalanced DNA Specimens. Biotechniques 2016, 60, 84–87. [Google Scholar] [CrossRef]
- Tost, J.; Gut, I.G. Analysis of Gene-Specific DNA Methylation Patterns by Pyrosequencing® Technology. Methods Mol. Biol. 2007, 373, 89–102. [Google Scholar] [CrossRef]
- Harrington, C.T.; Lin, E.I.; Olson, M.T.; Eshleman, J.R. Fundamentals of Pyrosequencing. Arch. Pathol. Lab. Med. 2013, 137, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, M.; Tejero, N.F.; Duncan, G.; McCord, B. Pyrosequencing: Current Forensic Methodology and Future Applications—A Review. Electrophoresis 2023, 44, 298–312. [Google Scholar] [CrossRef]
- Taryma-Lesniak, O.; Kjeldsen, T.E.; Hansen, L.L.; Wojdacz, T.K. Influence of Unequal Amplification of Methylated and Non-Methylated Template on Performance of Pyrosequencing. Genes 2022, 13, 1418. [Google Scholar] [CrossRef]
- Park, J.W.; Park, I.H.; Kim, J.M.; Noh, J.H.; Kim, K.A.; Park, J.Y. Rapid Detection of FMO3 Single Nucleotide Polymorphisms Using a Pyrosequencing Method. Mol. Med. Rep. 2022, 25, 48. [Google Scholar] [CrossRef]
- De Battisti, C.; Marciano, S.; Magnabosco, C.; Busato, S.; Arcangeli, G.; Cattoli, G. Pyrosequencing as a Tool for Rapid Fish Species Identification and Commercial Fraud Detection. J. Agric. Food Chem. 2014, 62, 198–205. [Google Scholar] [CrossRef]
- Ogino, S.; Kawasaki, T.; Brahmandam, M.; Yan, L.; Cantor, M.; Nangyal, C.; Mino-Kenudson, M.; Lauwers, G.Y.; Loda, M.; Fuchs, C.S. Sensitive Sequencing Method for KRAS Mutation Detection by Pyrosequencing. J. Mol. Diagn. 2005, 7, 413. [Google Scholar] [CrossRef]
- Konrad, H.; Schäfer, L.; Sturm, H.; Hördt, L.; Bajanowski, T.; Poetsch, M. Vibration as a Pitfall in Pyrosequencing Analyses. Int. J. Legal Med. 2022, 136, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Thumbovorn, R.; Bhattarakosol, P.; Chaiwongkot, A. Detection of Global DNA Methylation in Cervical Intraepithelial Neoplasia and Cancerous Lesions by Pyrosequencing and Enzyme-Linked Immunosorbent Assays. Asian Pac. J. Cancer Prev. 2022, 23, 143–149. [Google Scholar] [CrossRef]
- Alghanim, H.; Balamurugan, K.; McCord, B. Development of DNA Methylation Markers for Sperm, Saliva and Blood Identification Using Pyrosequencing and QPCR/HRM. Anal. Biochem. 2020, 611, 113933. [Google Scholar] [CrossRef]
- Alghanim, H.; Wu, W.; McCord, B. DNA Methylation Assay Based on Pyrosequencing for Determination of Smoking Status. Electrophoresis 2018, 39, 2806–2814. [Google Scholar] [CrossRef]
- Fleckhaus, J.; Schneider, P.M. Novel Multiplex Strategy for DNA Methylation-Based Age Prediction from Small Amounts of DNA via Pyrosequencing. Forensic Sci. Int. Genet. 2020, 44, 102189. [Google Scholar] [CrossRef] [PubMed]
- Dorey, A.; Howorka, S. Nanopore DNA Sequencing Technologies and Their Applications towards Single-Molecule Proteomics. Nat. Chem. 2024, 16, 314–334. [Google Scholar] [CrossRef] [PubMed]
- Chera, A.; Stancu-Cretu, M.; Zabet, N.R.; Bucur, O. Shedding Light on DNA Methylation and Its Clinical Implications: The Impact of Long-Read-Based Nanopore Technology. Epigenetics Chromatin 2024, 17, 39. [Google Scholar] [CrossRef]
- Liu, Y.; Rosikiewicz, W.; Pan, Z.; Jillette, N.; Wang, P.; Taghbalout, A.; Foox, J.; Mason, C.; Carroll, M.; Cheng, A.; et al. DNA Methylation-Calling Tools for Oxford Nanopore Sequencing: A Survey and Human Epigenome-Wide Evaluation. Genome Biol. 2021, 22, 295. [Google Scholar] [CrossRef]
- Tourancheau, A.; Mead, E.A.; Zhang, X.S.; Fang, G. Discovering Multiple Types of DNA Methylation from Bacteria and Microbiome Using Nanopore Sequencing. Nat. Methods 2021, 18, 491–498. [Google Scholar] [CrossRef]
- Yue, X.; Xie, Z.; Li, M.; Wang, K.; Li, X.; Zhang, X.; Yan, J.; Yin, Y. Simultaneous Profiling of Histone Modifications and DNA Methylation via Nanopore Sequencing. Nat. Commun. 2022, 13, 7939. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Huang, N.; Nie, F.; Zhang, J.; Zhang, Z.; Wu, B.; Bai, L.; Liu, W.; Xiao, C.L.; Luo, F.; et al. Genome-Wide Detection of Cytosine Methylations in Plant from Nanopore Data Using Deep Learning. Nat. Commun. 2021, 12, 5976. [Google Scholar] [CrossRef]
- Yuen, Z.W.S.; Srivastava, A.; Daniel, R.; McNevin, D.; Jack, C.; Eyras, E. Systematic Benchmarking of Tools for CpG Methylation Detection from Nanopore Sequencing. Nat. Commun. 2021, 12, 3438. [Google Scholar] [CrossRef]
- Yuen, Z.W.S.; Shanmuganandam, S.; Stanley, M.; Jiang, S.; Hein, N.; Daniel, R.; McNevin, D.; Jack, C.; Eyras, E. Profiling Age and Body Fluid DNA Methylation Markers Using Nanopore Adaptive Sampling. Forensic Sci. Int. Genet. 2024, 71, 103048. [Google Scholar] [CrossRef]
- Jain, M.; Koren, S.; Miga, K.H.; Quick, J.; Rand, A.C.; Sasani, T.A.; Tyson, J.R.; Beggs, A.D.; Dilthey, A.T.; Fiddes, I.T.; et al. Nanopore Sequencing and Assembly of a Human Genome with Ultra-Long Reads. Nat. Biotechnol. 2018, 36, 338–345. [Google Scholar] [CrossRef]
- Akbari, V.; Garant, J.M.; O’Neill, K.; Pandoh, P.; Moore, R.; Marra, M.A.; Hirst, M.; Jones, S.J.M. Genome-Wide Detection of Imprinted Differentially Methylated Regions Using Nanopore Sequencing. eLife 2022, 11, e77898. [Google Scholar] [CrossRef]
- Xia, Q.; Chang, T.; Ding, T.; Liu, Z.; Liu, J.; Li, Y.; Yao, Z. Clinical Application of Nanopore Sequencing for Haplotype Linkage Analysis in Preimplantation Genetic Testing for Duchenne Muscular Dystrophy. Sci. Rep. 2025, 15, 30498. [Google Scholar] [CrossRef]
- Lau, B.T.; Almeda, A.; Schauer, M.; McNamara, M.; Bai, X.; Meng, Q.; Partha, M.; Grimes, S.M.; Lee, H.J.; Heestand, G.M.; et al. Single-Molecule Methylation Profiles of Cell-Free DNA in Cancer with Nanopore Sequencing. Genome Med. 2023, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Katsman, E.; Orlanski, S.; Martignano, F.; Fox-Fisher, I.; Shemer, R.; Dor, Y.; Zick, A.; Eden, A.; Petrini, I.; Conticello, S.G.; et al. Detecting Cell-of-Origin and Cancer-Specific Methylation Features of Cell-Free DNA from Nanopore Sequencing. Genome Biol. 2022, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Dimond, J.L.; Nguyen, N.; Roberts, S.B. DNA Methylation Profiling of a Cnidarian-Algal Symbiosis Using Nanopore Sequencing. G3 Genes Genomes Genet. 2021, 11, jkab148. [Google Scholar] [CrossRef] [PubMed]
- Drag, M.H.; Debes, K.P.; Franck, C.S.; Flethøj, M.; Lyhne, M.K.; Møller, J.E.; Ludvigsen, T.P.; Jespersen, T.; Olsen, L.H.; Kilpeläinen, T.O. Nanopore Sequencing Reveals Methylation Changes Associated with Obesity in Circulating Cell-Free DNA from Göttingen Minipigs. Epigenetics 2023, 18, 2199374. [Google Scholar] [CrossRef]
- Flusberg, B.A.; Webster, D.R.; Lee, J.H.; Travers, K.J.; Olivares, E.C.; Clark, T.A.; Korlach, J.; Turner, S.W. Direct Detection of DNA Methylation during Single-Molecule, Real-Time Sequencing. Nat. Methods 2010, 7, 461–465. [Google Scholar] [CrossRef]
- Nakano, K.; Shiroma, A.; Shimoji, M.; Tamotsu, H.; Ashimine, N.; Ohki, S.; Shinzato, M.; Minami, M.; Nakanishi, T.; Teruya, K.; et al. Advantages of Genome Sequencing by Long-Read Sequencer Using SMRT Technology in Medical Area. Hum. Cell 2017, 30, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Puchtler, T.J.; Johnson, K.; Palmer, R.N.; Talbot, E.L.; Ibbotson, L.A.; Powalowska, P.K.; Knox, R.; Shibahara, A.; Cunha, P.M.S.; Newell, O.J.; et al. Single-Molecule DNA Sequencing of Widely Varying GC-Content Using Nucleotide Release, Capture and Detection in Microdroplets. Nucleic Acids Res. 2020, 48, e132. [Google Scholar] [CrossRef]
- Ni, P.; Nie, F.; Zhong, Z.; Xu, J.; Huang, N.; Zhang, J.; Zhao, H.; Zou, Y.; Huang, Y.; Li, J.; et al. DNA 5-Methylcytosine Detection and Methylation Phasing Using PacBio Circular Consensus Sequencing. Nat. Commun. 2023, 14, 4054. [Google Scholar] [CrossRef] [PubMed]
- Coy, S.R.; Gann, E.R.; Papoulis, S.E.; Holder, M.E.; Ajami, N.J.; Petrosino, J.F.; Zinser, E.R.; Van Etten, J.L.; Wilhelm, S.W. SMRT Sequencing of Paramecium Bursaria Chlorella Virus-1 Reveals Diverse Methylation Stability in Adenines Targeted by Restriction Modification Systems. Front. Microbiol. 2020, 11, 887. [Google Scholar] [CrossRef]
- Forde, B.M.; McAllister, L.J.; Paton, J.C.; Paton, A.W.; Beatson, S.A. SMRT Sequencing Reveals Differential Patterns of Methylation in Two O111:H- STEC Isolates from a Hemolytic Uremic Syndrome Outbreak in Australia. Sci. Rep. 2019, 9, 9436. [Google Scholar] [CrossRef]
- Wang, C.; Feng, J.; Chen, Y.; Li, D.; Liu, L.; Wu, Y.; Zhang, S.; Du, S.; Zhang, Y. Revealing Mitogenome-Wide DNA Methylation and RNA Editing of Three Ascomycotina Fungi Using SMRT Sequencing. Mitochondrion 2020, 51, 88–96. [Google Scholar] [CrossRef]
- Nanda, A.S.; Wu, K.; Irkliyenko, I.; Woo, B.; Ostrowski, M.S.; Clugston, A.S.; Sayles, L.C.; Xu, L.; Satpathy, A.T.; Nguyen, H.G.; et al. Direct Transposition of Native DNA for Sensitive Multimodal Single-Molecule Sequencing. Nat. Genet. 2024, 56, 1300–1309. [Google Scholar] [CrossRef]
- Sahin, H.; Salehi, R.; Islam, S.; Müller, M.; Giehr, P.; Carell, T. Robust Bisulfite-Free Single-Molecule Real-Time Sequencing of Methyldeoxycytidine Based on a Novel HpTet3 Enzyme. Angew. Chem.—Int. Ed. 2024, 63, e202418500. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sebra, R.; Pullman, B.S.; Qiao, W.; Peter, I.; Desnick, R.J.; Geyer, C.R.; DeCoteau, J.F.; Scott, S.A. Quantitative and Multiplexed DNA Methylation Analysis Using Long-Read Single-Molecule Real-Time Bisulfite Sequencing (SMRT-BS). BMC Genom. 2015, 16, 350. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, J.; Chen, X.; Inoue, M.; Liu, Y.; Song, C.X. Whole-Genome Long-Read TAPS Deciphers DNA Methylation Patterns at Base Resolution Using PacBio SMRT Sequencing Technology. Nucleic Acids Res. 2022, 50, E104. [Google Scholar] [CrossRef] [PubMed]
- Tierling, S.; Schmitt, B.; Walter, J. Comprehensive Evaluation of Commercial Bisulfite-Based DNA Methylation Kits and Development of an Alternative Protocol with Improved Conversion Performance. Genet. Epigenetics 2018, 10. [Google Scholar] [CrossRef]
- Steiert, T.A.; Parra, G.; Gut, M.; Arnold, N.; Trotta, J.R.; Tonda, R.; Moussy, A.; Gerber, Z.; Abuja, P.M.; Zatloukal, K.; et al. A Critical Spotlight on the Paradigms of FFPE-DNA Sequencing. Nucleic Acids Res. 2023, 51, 7143. [Google Scholar] [CrossRef]
- Hansen, K.D.; Langmead, B.; Irizarry, R.A. BSmooth: From Whole Genome Bisulfite Sequencing Reads to Differentially Methylated Regions. Genome Biol. 2012, 13, R83. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Pan, X.; Xu, D.; Ji, G.; Wang, Y.; Tian, Y.; Cai, J.; Li, J.; Zhang, Z.; Yuan, X. Benchmarking DNA Methylation Analysis of 14 Alignment Algorithms for Whole Genome Bisulfite Sequencing in Mammals. Comput. Struct. Biotechnol. J. 2022, 20, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, S.L.; Grady, P.G.S.; Pauloski, N.; O’Neill, R.J.; Wegrzyn, J.L. Profiling Genome-wide Methylation in Two Maples: Fine-scale Approaches to Detection with Nanopore Technology. Evol. Appl. 2024, 17, e13669. [Google Scholar] [CrossRef]
- Amarasinghe, S.L.; Su, S.; Dong, X.; Zappia, L.; Ritchie, M.E.; Gouil, Q. Opportunities and Challenges in Long-Read Sequencing Data Analysis. Genome Biol. 2020, 21, 30. [Google Scholar] [CrossRef]
- Halliwell, D.O.; Honig, F.; Bagby, S.; Roy, S.; Murrell, A. Double and Single Stranded Detection of 5-Methylcytosine and 5-Hydroxymethylcytosine with Nanopore Sequencing. Commun. Biol. 2025, 8, 243. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Gillette, T.G.; Hill, J.A. Readers, Writers and Erasers: Chromatin as the Whiteboard of Heart Disease. Circ. Res. 2015, 116, 1245. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Wang, Y.; Gao, D.; King, J.; Xu, Y.; Liang, F. Sen Investigating Crosstalk between H3K27 Acetylation and H3K4 Trimethylation in CRISPR/DCas-Based Epigenome Editing and Gene Activation. Sci. Rep. 2021, 11, 15912. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H. Recruitment and Biological Consequences of Histone Modification of H3K27me3 and H3K9me3. ILAR J. 2012, 53, 232. [Google Scholar] [CrossRef]
- Gade, P.; Kalvakolanu, D.V. Chromatin Immunoprecipitation Assay as a Tool for Analyzing Transcription Factor Activity. Methods Mol. Biol. 2012, 809, 85–104. [Google Scholar] [CrossRef]
- Park, P.J. ChIP-Seq: Advantages and Challenges of a Maturing Technology. Nat. Rev. Genet. 2009, 10, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Wiehle, L.; Breiling, A. Chromatin Immunoprecipitation. Methods Mol. Biol. 2016, 1480, 7–21. [Google Scholar] [CrossRef]
- Nakato, R.; Sakata, T. Methods for ChIP-Seq Analysis: A Practical Workflow and Advanced Applications. Methods 2021, 187, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Landt, S.G.; Marinov, G.K.; Kundaje, A.; Kheradpour, P.; Pauli, F.; Batzoglou, S.; Bernstein, B.E.; Bickel, P.; Brown, J.B.; Cayting, P.; et al. ChIP-Seq Guidelines and Practices of the ENCODE and ModENCODE Consortia. Genome Res. 2012, 22, 1813–1831. [Google Scholar] [CrossRef]
- Carroll, T.S.; Liang, Z.; Salama, R.; Stark, R.; de Santiago, I. Impact of Artifact Removal on ChIP Quality Metrics in ChIP-Seq and ChIP-Exo Data. Front. Genet. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Farinati, S.; Forestan, C.; Joseph, J.; Bonghi, C.; Varotto, S. An Efficient Chromatin Immunoprecipitation (ChIP) Protocol for Studying Histone Modifications in Peach Reproductive Tissues. Plant Methods 2022, 18, 43. [Google Scholar] [CrossRef]
- Ranawaka, B.; Tanurdzic, M.; Waterhouse, P.; Naim, F. An Optimised Chromatin Immunoprecipitation (ChIP) Method for Starchy Leaves of Nicotiana Benthamiana to Study Histone Modifications of an Allotetraploid Plant. Mol. Biol. Rep. 2020, 47, 9499–9509. [Google Scholar] [CrossRef]
- Cejas, P.; Li, L.; O’Neill, N.K.; Duarte, M.; Rao, P.; Bowden, M.; Zhou, C.W.; Mendiola, M.; Burgos, E.; Feliu, J.; et al. Chromatin Immunoprecipitation from Fixed Clinical Tissues Reveals Tumor-Specific Enhancer Profiles. Nat. Med. 2016, 22, 685–691. [Google Scholar] [CrossRef]
- Amatori, S.; Fanelli, M. The Current State of Chromatin Immunoprecipitation (ChIP) from FFPE Tissues. Int. J. Mol. Sci. 2022, 23, 1103. [Google Scholar] [CrossRef]
- Gorkin, D.U.; Lee, D.; Reed, X.; Fletez-Brant, C.; Bessling, S.L.; Loftus, S.K.; Beer, M.A.; Pavan, W.J.; McCallion, A.S. Integration of ChIP-Seq and Machine Learning Reveals Enhancers and a Predictive Regulatory Sequence Vocabulary in Melanocytes. Genome Res. 2012, 22, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Strattan, J.S.; Hur, J.K.; Bento, J.; Urban, A.E.; Song, G.; Cherry, J.M. CNN-Peaks: ChIP-Seq Peak Detection Pipeline Using Convolutional Neural Networks That Imitate Human Visual Inspection. Sci. Rep. 2020, 10, 7933. [Google Scholar] [CrossRef]
- de Mello, F.N.; Tahira, A.C.; Berzoti-Coelho, M.G.; Verjovski-Almeida, S. The CUT&RUN Greenlist: Genomic Regions of Consistent Noise Are Effective Normalizing Factors for Quantitative Epigenome Mapping. Brief. Bioinform. 2024, 25, bbad538. [Google Scholar] [CrossRef]
- Ruiz, S.E.; Maul, R.W.; Gearhart, P.J. Optimized CUT&RUN Protocol for Activated Primary Mouse B Cells. PLoS ONE 2025, 20, e0322139. [Google Scholar] [CrossRef]
- Tao, X.Y.; Guan, X.Y.; Hong, G.J.; He, Y.Q.; Li, S.J.; Feng, S.L.; Wang, J.; Chen, G.; Xu, F.; Wang, J.W.; et al. Biotinylated Tn5 Transposase-Mediated CUT&Tag Efficiently Profiles Transcription Factor-DNA Interactions in Plants. Plant Biotechnol. J. 2023, 21, 1191–1205. [Google Scholar] [CrossRef]
- Qasim, M.N.; Valle Arevalo, A.; Paropkari, A.D.; Ennis, C.L.; Sindi, S.S.; Nobile, C.J.; Hernday, A.D. Genome-Wide Profiling of Transcription Factor-DNA Binding Interactions in Candida Albicans: A Comprehensive CUT&RUN Method and Data Analysis Workflow. J. Vis. Exp. 2022, 2022, e63655. [Google Scholar] [CrossRef]
- Thompson, M.; Byrd, A. Untargeted CUT&Tag and BG4 CUT&Tag Are Both Enriched at G-Quadruplexes and Accessible Chromatin. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lu, C.; Coradin, M.; Porter, E.G.; Garcia, B.A. Accelerating the Field of Epigenetic Histone Modification through Mass Spectrometry–Based Approaches. Mol. Cell. Proteom. 2021, 20, 100006. [Google Scholar] [CrossRef] [PubMed]
- Noberini, R.; Robusti, G.; Bonaldi, T. Mass Spectrometry-Based Characterization of Histones in Clinical Samples: Applications, Progress, and Challenges. FEBS J. 2022, 289, 1191–1213. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, S.; De Clerck, L.; Willems, S.; Van Puyvelde, B.; Daled, S.; Deforce, D.; Dhaenens, M. Comprehensive Histone Epigenetics: A Mass Spectrometry Based Screening Assay to Measure Epigenetic Toxicity. MethodsX 2020, 7, 101055. [Google Scholar] [CrossRef]
- Verhelst, S.; Van Puyvelde, B.; Willems, S.; Daled, S.; Cornelis, S.; Corveleyn, L.; Willems, E.; Deforce, D.; De Clerck, L.; Dhaenens, M. A Large Scale Mass Spectrometry-Based Histone Screening for Assessing Epigenetic Developmental Toxicity. Sci. Rep. 2022, 12, 1256. [Google Scholar] [CrossRef]
- Zhong, J.; Ye, Z.; Clark, C.R.; Lenz, S.W.; Nguyen, J.H.; Yan, H.; Robertson, K.D.; Farrugia, G.; Zhang, Z.; Ordog, T.; et al. Enhanced and Controlled Chromatin Extraction from FFPE Tissues and the Application to ChIP-Seq. BMC Genom. 2019, 20, 249. [Google Scholar] [CrossRef] [PubMed]
- Skene, P.J.; Henikoff, J.G.; Henikoff, S. Targeted in Situ Genome-Wide Profiling with High Efficiency for Low Cell Numbers. Nat. Protoc. 2018, 13, 1006–1019. [Google Scholar] [CrossRef]
- Kulej, K.; Avgousti, D.C.; Weitzman, M.D.; Garcia, B.A. Characterization of Histone Post-Translational Modifications during Virus Infection Using Mass Spectrometry-Based Proteomics. Methods 2015, 90, 8. [Google Scholar] [CrossRef]
- Scheid, R.; Dowell, J.A.; Sanders, D.; Jiang, J.; Denu, J.M.; Zhong, X. Histone Acid Extraction and High Throughput Mass Spectrometry to Profile Histone Modifications in Arabidopsis Thaliana. Curr. Protoc. 2022, 2, e527. [Google Scholar] [CrossRef]
- Maksimovic, I.; David, Y. Non-Enzymatic Covalent Modifications as a New Chapter in the Histone Code. Trends Biochem. Sci. 2021, 46, 718. [Google Scholar] [CrossRef]
- Hansen, J.C.; Maeshima, K.; Hendzel, M.J. The Solid and Liquid States of Chromatin. Epigenetics Chromatin 2021, 14, 50. [Google Scholar] [CrossRef]
- Khanduja, J.S.; Motamedi, M. Protocol for Chromatin Immunoprecipitation of Chromatin-Binding Proteins in Schizosaccharomyces Pombe Using a Dual-Crosslinking Approach. STAR Protoc. 2025, 6, 103695. [Google Scholar] [CrossRef]
- Abbasova, L.; Urbanaviciute, P.; Hu, D.; Ismail, J.N.; Schilder, B.M.; Nott, A.; Skene, N.G.; Marzi, S.J. CUT&Tag Recovers up to Half of ENCODE ChIP-Seq Histone Acetylation Peaks. Nat. Commun. 2025, 16, 2993. [Google Scholar] [CrossRef]
- Angel, T.E.; Aryal, U.K.; Hengel, S.M.; Baker, E.S.; Kelly, R.T.; Robinson, E.W.; Smith, R.D. Mass Spectrometry Based Proteomics: Existing Capabilities and Future Directions. Chem. Soc. Rev. 2012, 41, 3912. [Google Scholar] [CrossRef]
- Liu, T. Use Model-Based Analysis of ChIP-Seq (MACS) to Analyze Short Reads Generated by Sequencing Protein–DNA Interactions in Embryonic Stem Cells. Methods Mol. Biol. 2014, 1150, 81–95. [Google Scholar] [CrossRef]
- Zang, C.; Schones, D.E.; Zeng, C.; Cui, K.; Zhao, K.; Peng, W. A Clustering Approach for Identification of Enriched Domains from Histone Modification ChIP-Seq Data. Bioinformatics 2009, 25, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Orouji, E.; Raman, A.T. Computational Methods to Explore Chromatin State Dynamics. Brief. Bioinform. 2022, 23, bbac439. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.F.; Arnaudo, A.M.; Garcia, B.A. Mass Spectrometric Analysis of Histone Proteoforms. Annu. Rev. Anal. Chem. 2014, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Diermeier, S.D.; Leask, M.P. History and Definitions of NcRNAs. In Navigating Non-Coding RNA: From Biogenesis to Therapeutic Application; Academic Press: Cambridge, MA, USA, 2023; pp. 1–46. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA Sequencing Technologies and Applications: A Brief Overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Wang, S.; Sun, S.T.; Zhang, X.Y.; Ding, H.R.; Yuan, Y.; He, J.J.; Wang, M.S.; Yang, B.; Li, Y.B. The Evolution of Single-Cell RNA Sequencing Technology and Application: Progress and Perspectives. Int. J. Mol. Sci. 2023, 24, 2943. [Google Scholar] [CrossRef]
- Simoneau, J.; Dumontier, S.; Gosselin, R.; Scott, M.S. Current RNA-Seq Methodology Reporting Limits Reproducibility. Brief. Bioinform. 2021, 22, 140–145. [Google Scholar] [CrossRef]
- Pisu, D.; Huang, L.; Grenier, J.K.; Russell, D.G. Dual RNA-Seq of Mtb-Infected Macrophages In Vivo Reveals Ontologically Distinct Host-Pathogen Interactions. Cell Rep. 2020, 30, 335–350.e4. [Google Scholar] [CrossRef]
- Westermann, A.J.; Vogel, J. Cross-Species RNA-Seq for Deciphering Host–Microbe Interactions. Nat. Rev. Genet. 2021, 22, 361–378. [Google Scholar] [CrossRef]
- Smail, C.; Montgomery, S.B. RNA Sequencing in Disease Diagnosis. Annu. Rev. Genom. Hum. Genet. 2025, 25, 353–367. [Google Scholar] [CrossRef]
- Sant, P.; Rippe, K.; Mallm, J.P. Approaches for Single-Cell RNA Sequencing across Tissues and Cell Types. Transcription 2023, 14, 127–145. [Google Scholar] [CrossRef]
- Ziegenhain, C.; Vieth, B.; Parekh, S.; Reinius, B.; Guillaumet-Adkins, A.; Smets, M.; Leonhardt, H.; Heyn, H.; Hellmann, I.; Enard, W. Comparative Analysis of Single-Cell RNA Sequencing Methods. Mol. Cell 2017, 65, 631–643.e4. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, D.; Yang, P.; Guo, R.; Kong, M.; Gao, Y.; Yu, X.; Lu, X.; Fan, X. Revealing the Transcriptional Heterogeneity of Organ-specific Metastasis in Human Gastric Cancer Using Single-cell RNA Sequencing. Clin. Transl. Med. 2022, 12, e730. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.T.; Sanz-Jimenez, P.; Ning, X.T.; Tahir ul Qamar, M.; Chen, L.L. Direct RNA Sequencing in Plants: Practical Applications and Future Perspectives. Plant Commun. 2024, 5, 101064. [Google Scholar] [CrossRef]
- Walter, T.J.; Suter, R.K.; Ayad, N.G. An Overview of Human Single-Cell RNA Sequencing Studies in Neurobiological Disease. Neurobiol. Dis. 2023, 184, 106201. [Google Scholar] [CrossRef]
- Bawa, G.; Liu, Z.; Yu, X.; Qin, A.; Sun, X. Single-Cell RNA Sequencing for Plant Research: Insights and Possible Benefits. Int. J. Mol. Sci. 2022, 23, 4497. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, I.; Suva, M.L. Cancer Cell States: Lessons from Ten Years of Single-Cell RNA-Sequencing of Human Tumors. Cancer Cell 2024, 42, 1497–1506. [Google Scholar] [CrossRef]
- Jain, M.; Abu-Shumays, R.; Olsen, H.E.; Akeson, M. Advances in Nanopore Direct RNA Sequencing. Nat. Methods 2022, 19, 1160–1164. [Google Scholar] [CrossRef]
- Kim, Y.; Saville, L.; O’Neill, K.; Garant, J.M.; Liu, Y.; Haile-Merhu, S.; Ghashghaei, M.; Hoang, Q.A.; Louwagie, A.; Park, Y.P.; et al. Nanopore Direct RNA Sequencing of Human Transcriptomes Reveals the Complexity of MRNA Modifications and Crosstalk between Regulatory Features. Cell Genom. 2025, 5, 100872. [Google Scholar] [CrossRef] [PubMed]
- Pratanwanich, P.N.; Yao, F.; Chen, Y.; Koh, C.W.Q.; Wan, Y.K.; Hendra, C.; Poon, P.; Goh, Y.T.; Yap, P.M.L.; Chooi, J.Y.; et al. Identification of Differential RNA Modifications from Nanopore Direct RNA Sequencing with XPore. Nat. Biotechnol. 2021, 39, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, J.; Lam, H.M.; Chan, T.F. Nanopore Direct RNA Sequencing Reveals N6-Methyladenosine and Polyadenylation Landscapes on Long Non-Coding RNAs in Arabidopsis Thaliana. BMC Plant Biol. 2024, 24, 1126. [Google Scholar] [CrossRef]
- Hong, A.; Kim, D.; Kim, V.N.; Chang, H. Analyzing Viral Epitranscriptomes Using Nanopore Direct RNA Sequencing. J. Microbiol. 2022, 60, 867–876. [Google Scholar] [CrossRef]
- Leger, A.; Amaral, P.P.; Pandolfini, L.; Capitanchik, C.; Capraro, F.; Miano, V.; Migliori, V.; Toolan-Kerr, P.; Sideri, T.; Enright, A.J.; et al. RNA Modifications Detection by Comparative Nanopore Direct RNA Sequencing. Nat. Commun. 2021, 12, 7198. [Google Scholar] [CrossRef]
- Naarmann-de Vries, I.S.; Eschenbach, J.; Dieterich, C. Improved Nanopore Direct RNA Sequencing of Cardiac Myocyte Samples by Selective Mt-RNA Depletion. J. Mol. Cell Cardiol. 2022, 163, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.T.; Comai, A.; Bruzel, A.; Sun, G.; Dedon, P.C.; Cheung, V.G. Nanopore-Based Direct Sequencing of RNA Transcripts with 10 Different Modified Nucleotides Reveals Gaps in Existing Technology. G3 Genes Genomes Genet. 2023, 13, jkad200. [Google Scholar] [CrossRef]
- Prawer, Y.D.J.; Gleeson, J.; De Paoli-Iseppi, R.; Clark, M.B. Pervasive Effects of RNA Degradation on Nanopore Direct RNA Sequencing. NAR Genom. Bioinform. 2023, 5, lqad060. [Google Scholar] [CrossRef]
- Pust, M.-M.; Davenport, C.F.; Wiehlmann, L.; Tümmler, B. Direct RNA Nanopore Sequencing of Pseudomonas Aeruginosa Clone C Transcriptomes. J. Bacteriol. 2022, 204, e0041821. [Google Scholar] [CrossRef]
- Hussen, B.M.; Rasul, M.F.; Abdullah, S.R.; Hidayat, H.J.; Faraj, G.S.H.; Ali, F.A.; Salihi, A.; Baniahmad, A.; Ghafouri-Fard, S.; Rahman, M.; et al. Targeting MiRNA by CRISPR/Cas in Cancer: Advantages and Challenges. Mil. Med. Res. 2023, 10, 32. [Google Scholar] [CrossRef]
- Alinejad, T.; Modarressi, S.; Sadri, Z.; Hao, Z.; Chen, C.S. Diagnostic Applications and Therapeutic Option of Cascade CRISPR/Cas in the Modulation of MiRNA in Diverse Cancers: Promises and Obstacles. J. Cancer Res. Clin. Oncol. 2023, 149, 9557–9575. [Google Scholar] [CrossRef]
- Yun, D.; Jung, C. MiRNA-Responsive CRISPR-Cas System via a DNA Regulator. Biosensors 2023, 13, 975. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Zou, X.; Ma, F.; Zhang, C.Y. CRISPR/Cas-Based MicroRNA Biosensors. Chem.-Eur. J. 2023, 29, e202203412. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Reis, R.S. Using CRISPR/Cas to Enhance Gene Expression for Crop Trait Improvement by Editing MiRNA Targets. J. Exp. Bot. 2023, 74, 2208–2212. [Google Scholar] [CrossRef] [PubMed]
- McAlexander, M.A.; Phillips, M.J.; Witwer, K.W. Comparison of Methods for MiRNA Extraction from Plasma and Quantitative Recovery of RNA from Cerebrospinal Fluid. Front. Genet. 2013, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951. [Google Scholar] [CrossRef]
- Kellman, B.P.; Baghdassarian, H.M.; Pramparo, T.; Shamie, I.; Gazestani, V.; Begzati, A.; Li, S.; Nalabolu, S.; Murray, S.; Lopez, L.; et al. Multiple Freeze-Thaw Cycles Lead to a Loss of Consistency in Poly(A)-Enriched RNA Sequencing. BMC Genom. 2021, 22, 69. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A Survey of Best Practices for RNA-Seq Data Analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Venø, M.T.; Damgaard, C.K.; Kjems, J. Comparison of Circular RNA Prediction Tools. Nucleic Acids Res. 2015, 44, e58. [Google Scholar] [CrossRef]
- Ran, D.; Zhang, S.; Lytal, N.; An, L. ScDoc: Correcting Drop-out Events in Single-Cell RNA-Seq Data. Bioinformatics 2020, 36, 4233–4239. [Google Scholar] [CrossRef]
- GitHub—FaryabiLab/Dockerize-Workflows: Genomic Data Processing Pipelines Written in WDL Making Use of Docker Containers to Ensure Stability. Available online: https://github.com/faryabiLab/dockerize-workflows (accessed on 29 August 2025).
- Blischak, J.D.; Davenport, E.R.; Wilson, G. A Quick Introduction to Version Control with Git and GitHub. PLoS Comput. Biol. 2016, 12, e1004668. [Google Scholar] [CrossRef]
- Tahir, M.; Norouzi, M.; Khan, S.S.; Davie, J.R.; Yamanaka, S.; Ashraf, A. Artificial Intelligence and Deep Learning Algorithms for Epigenetic Sequence Analysis: A Review for Epigeneticists and AI Experts. Comput. Biol. Med. 2024, 183, 109302. [Google Scholar] [CrossRef] [PubMed]
- Benfatto, S.; Sill, M.; Jones, D.T.W.; Pfister, S.M.; Sahm, F.; von Deimling, A.; Capper, D.; Hovestadt, V. Explainable Artificial Intelligence of DNA Methylation-Based Brain Tumor Diagnostics. Nat. Commun. 2025, 16, 1787. [Google Scholar] [CrossRef] [PubMed]
- Guido, R.; Ferrisi, S.; Lofaro, D.; Conforti, D. An Overview on the Advancements of Support Vector Machine Models in Healthcare Applications: A Review. Information 2024, 15, 235. [Google Scholar] [CrossRef]
- Kausik, A.K.; Rashid, A.B.; Baki, R.F.; Jannat Maktum, M.M. Machine Learning Algorithms for Manufacturing Quality Assurance: A Systematic Review of Performance Metrics and Applications. Array 2025, 26, 100393. [Google Scholar] [CrossRef]
- Levy, J.J.; Titus, A.J.; Petersen, C.L.; Chen, Y.; Salas, L.A.; Christensen, B.C. MethylNet: An Automated and Modular Deep Learning Approach for DNA Methylation Analysis. BMC Bioinform. 2020, 21, 108. [Google Scholar] [CrossRef]
- Singh, R.; Lanchantin, J.; Robins, G.; Qi, Y. DeepChrome: Deep-Learning for Predicting Gene Expression from Histone Modifications. Bioinformatics 2016, 32, i639–i648. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, M.; Liu, Q.; Lv, H.; Jiang, R. DeepHistone: A Deep Learning Approach to Predicting Histone Modifications. BMC Genom. 2019, 20, 11–23. [Google Scholar] [CrossRef]
- Lanchantin, J.; Qi, Y. Graph Convolutional Networks for Epigenetic State Prediction Using Both Sequence and 3D Genome Data. Bioinformatics 2020, 36, i659–i667. [Google Scholar] [CrossRef]
- Seal, D.B.; Das, V.; Goswami, S.; De, R.K. Estimating Gene Expression from DNA Methylation and Copy Number Variation: A Deep Learning Regression Model for Multi-Omics Integration. Genomics 2020, 112, 2833–2841. [Google Scholar] [CrossRef]
- Rauschert, S.; Raubenheimer, K.; Melton, P.E.; Huang, R.C. Machine Learning and Clinical Epigenetics: A Review of Challenges for Diagnosis and Classification. Clin. Epigenetics 2020, 12, 51. [Google Scholar] [CrossRef]
- Yassi, M.; Chatterjee, A.; Parry, M. Application of Deep Learning in Cancer Epigenetics through DNA Methylation Analysis. Brief. Bioinform. 2023, 24, bbad411. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Castilho, L.S.; Palomares, B.H.; Squarize, C.H. Determinants of Chromatin Organization in Aging and Cancer—Emerging Opportunities for Epigenetic Therapies and AI Technology. Genes 2024, 15, 710. [Google Scholar] [CrossRef]
| Feature | Bisulfite Sequencing | Methylation-Specific PCR (MSP) | Pyrosequencing | Nanopore Sequencing | SMRT Sequencing |
|---|---|---|---|---|---|
| Detection Principle | Chemical conversion of unmethylated cytosines | Primer specificity to methylated/unmethylated DNA | Sequencing-by-synthesis + bisulfite conversion | Electrical current changes in native DNA | Polymerase kinetics in real-time synthesis |
| Methylation Resolution | Single-nucleotide | CpG site (qualitative/semi-quantitative) | Single CpG (quantitative) | Single base | Single base |
| 5mC vs. 5hmC Distinction | Not distinguished | Not distinguished | Not distinguished | Partially possible | Detectable Via kinetic signatures |
| Input DNA Requirement | Moderate–high (low-input possible) | Low | Low | Moderate–high | High (some low-input adaptations exist) |
| Genome Coverage | Genome-wide (WGBS); CpG-rich regions (RRBS) | Locus-specific | Locus-specific | Genome-wide (long-read) | Genome-wide or targeted (HiFi/SMRT-BS) |
| Quantitative Output | Absolute methylation % | Qualitative/semi-quantitative | Quantitative | Quantitative | Quantitative |
| Cost and Scalability | High (WGBS); moderate (RRBS) | Low | Moderate | Moderate | High |
| Equipment Requirements | NGS platform | Standard PCR + gel/qPCR | Pyrosequencer | Nanopore device | PacBio sequencer |
| Throughput | High | Low–moderate | Low | High | High |
| Special Applications | Methylome maps, cancer, development | Clinical panels, diagnostics | Forensics, exposure, aging | Long-range phasing, cfDNA, forensic ID | Methylation + genome architecture, allele phasing |
| Major Limitations | DNA degradation, no 5hmC resolution | False positives, low throughput | Short read length, low multiplexing | Lower raw accuracy, high computation | High input, expensive, size selection bias |
| Feature | ChIP-Seq | CUT&RUN/CUT&Tag | MS-Based Proteomics |
|---|---|---|---|
| Genomic resolution | 150–300 bp | 20–50 bp (narrower peaks, higher specificity) | Not applicable (no genomic localization) |
| Input requirement | 1–10 million cells | 10,000–100,000 cells (or fewer in optimized protocols) | Micrograms of protein or acid-extracted histones |
| Crosslinking required | Yes | No | No |
| Detection of PTM stoichiometry | Limited, semi-quantitative | Limited, semi-quantitative | High, quantitative (esp. middle-/top-down MS) |
| Detection of combinatorial PTMs | No | Partial (one per antibody) | Yes—multiple PTMs on same proteoform |
| Single-cell compatibility | Emerging | High (e.g., scCUT&Tag, scCUT&RUN) | Currently limited, under development |
| Sample versatility (e.g., FFPE) | Limited (requires optimization) | High (esp. CUT&Tag in clinical samples) | High (Via histone extraction from FFPE or serum nucleosomes) |
| Method | Total RNA-Seq | Small RNA-Seq | circRNA-Seq | scRNA-Seq | dRNA-Seq (Nanopore) |
|---|---|---|---|---|---|
| Target RNAs | lncRNAs, polyA−/polyA + RNAs, others | miRNAs, piRNAs, siRNAs | Circular RNAs | mRNAs, lncRNAs, some circRNAs | Full-length RNAs, isoforms, modifications |
| Input Type | Ribosomal RNA-depleted total RNA | Size-selected RNA (~18–30 nt (protocol dependent)) | RNase R-treated RNA | Single cells | Polyadenylated RNA (native) |
| Strengths | Broad RNA profiling, captures both coding and non-coding transcripts | Sensitive to short RNAs, useful for isoforms, circulating miRNAs | High specificity for circRNAs, back-splice detection, exonuclease resistance | High-resolution profiling, cell-type-specific expression, heterogeneity capture | Direct sequencing, detects modifications (e.g., m6A), isoform-level resolution |
| Limitations | High computational noise, requires deep sequencing and standardization | Bias from adapter ligation, low reproducibility across protocols | Requires special bioinformatics (e.g., CIRCexplorer, CIRI), prone to misannotation | Poor detection of small RNAs, polyA bias, dropout and amplification artifacts | High input requirement, lower accuracy, polyA bias excludes some ncRNAs |
| Key Applications | Discovery of novel lncRNAs, low-abundance ncRNAs in tissue, tumor, extracellular vesicles | Plasma/serum biomarker discovery, small RNA profiling, cancer diagnostics | Liquid biopsy, neurodegeneration, cardiovascular disease markers | Tumor heterogeneity, rare cell types, lncRNA biomarker discovery | Isoform diversity, epitranscriptomic biomarker mapping, neurodegenerative disease |
| Model | Epigenetic Input | Additional Modalities | Architecture | Output | Applications | Advantages | Ref. |
|---|---|---|---|---|---|---|---|
| MethylNet | DNA methylation (Î2-values from 450 K/EPIC arrays) | None | Variational Autoencoder + Fully Connected layers | Age, smoking status, tumor subtype, immune infiltration | Epigenetic biomarker discovery, age prediction, subtype classification | High-dimensional methylation data compression, task-agnostic embeddings | [28] |
| DeepChrome | Histone modifications (H3K4me3, H3K27ac, etc.) around TSS | None | 1D CNN + MLP | Binary classification of gene expression (high/low) | Understanding combinatorial histone code and gene expression | Visualizable model, captures mark-specific regulation | [29] |
| INTERACT | CpG methylation (target site) | Genomic sequence (2 kb, one-hot encoded) | CNN + Transformer + FC | Predicted methylation levels and variant effects | Variant prioritization, regulatory element identification | Long-range sequence dependency modeling Via self-attention | [31] |
| DeepHistone | Histone mark presence | Genomic sequence + DNase I hypersensitivity | Dual CNN branches + FC | Histone mark prediction | Cross-cell-type prediction of histone marks | Contextual prediction with chromatin accessibility | [30] |
| ChromeGCN | Histone marks, TF binding (1 kb bins) | Genomic sequence + Hi-C contact maps | CNN + Graph-CNN (on Hi-C contact map) | Multi-label prediction of chromatin state | Interpretable enhancer promoter interaction prediction | Captures 3D chromatin interactions, interpretable GCN saliency maps | [32] |
| DDAE + MLP | DNA methylation (450 K) + CNVs | Copy number variation (CNV) | Stacked Denoising Autoencoder + MLP | Gene expression levels (in liver cancer) | Multi-omics modeling of cancer transcriptomes | Integrates genetic and epigenetic drivers of expression | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benčik, I.; Saftić Martinović, L.; Mladenić, T.; Ostojić, S.; Dević Pavlić, S. From DNA Methylation and Histone Modifications to Non-Coding RNAs: Evaluating Tools for Epigenetic Research. Appl. Sci. 2025, 15, 9940. https://doi.org/10.3390/app15189940
Benčik I, Saftić Martinović L, Mladenić T, Ostojić S, Dević Pavlić S. From DNA Methylation and Histone Modifications to Non-Coding RNAs: Evaluating Tools for Epigenetic Research. Applied Sciences. 2025; 15(18):9940. https://doi.org/10.3390/app15189940
Chicago/Turabian StyleBenčik, Ines, Lara Saftić Martinović, Tea Mladenić, Saša Ostojić, and Sanja Dević Pavlić. 2025. "From DNA Methylation and Histone Modifications to Non-Coding RNAs: Evaluating Tools for Epigenetic Research" Applied Sciences 15, no. 18: 9940. https://doi.org/10.3390/app15189940
APA StyleBenčik, I., Saftić Martinović, L., Mladenić, T., Ostojić, S., & Dević Pavlić, S. (2025). From DNA Methylation and Histone Modifications to Non-Coding RNAs: Evaluating Tools for Epigenetic Research. Applied Sciences, 15(18), 9940. https://doi.org/10.3390/app15189940







