1. Introduction
Microfluidic devices are specialized systems designed to manipulate fluids at the submillimeter scale, typically through microchannels ranging in size from 10 nanometers to 500 μm. These devices operate with tiny fluid volumes, often within the nanoliter to femtoliter range, enabling precise control over fluid behavior and interactions [
1,
2]. While much of the existing literature on temperature control in microfluidic systems predominantly focuses on traditional methods such as proportional-integral-derivative (PID) control and thermoelectric cooling (TECs), this review provides a unique perspective by highlighting emerging and next-generation technologies. These include additive manufacturing, which allows for the co-fabrication of microfluidic structures and functional components like heaters and sensors, as well as nanomaterials such as quantum dots and gallium-infused carbon nanotubes for non-invasive, real-time thermal sensing. Furthermore, we explore the integration of artificial intelligence (AI)-driven feedback systems, which hold significant potential for enhancing the precision and responsiveness of temperature regulation in microfluidic devices. This review provides a comprehensive overview of established and emerging methods and situates them within a forward-looking framework of challenges and opportunities. A key advantage of microfluidic devices is their ability to harness unique fluid dynamics at the microscale, where laminar flow dominates due to low Reynolds numbers. This enhances mass and heat transfer efficiency [
2]. The predictable nature of laminar flow is essential for applications requiring precision, including chemical synthesis, molecular cell analysis, and point-of-care diagnostics [
1]. Often referred to as “lab-on-a-chip” technology, microfluidic devices integrate multiple laboratory processes into a single, compact platform. This miniaturization allows for efficient analyses while significantly reducing sample and reagent consumption [
2,
3]. Advances in manufacturing techniques have further enhanced the complexity and functionality of these devices, enabling the creation of intricate multilayered microchannel structures [
1]. The ability of microfluidic devices to facilitate rapid and efficient chemical and biological reactions has expanded their applications in areas such as drug delivery, environmental monitoring, and food safety testing [
2]. Their adoption across various industries is driven by key benefits, including cost reduction, minimal waste generation, and improved analytical performance due to enhanced mass and heat transfer capabilities [
2]. These features make microfluidic technology an essential tool for precision fluid manipulation and analytical applications.
At the microscale, unique properties emerge due to the interplay of forces and the high surface-to-volume ratio, which influences fluid dynamics, heat transfer, and chemical reactions. Microfluidic systems offer precise control over fluid motion, temperature, and mechanical forces, which is critical for biological and chemical applications [
4]. The increased surface-to-volume ratio improves mass and heat transfer, enabling rapid mixing and reaction processes essential for synthesizing monodisperse nanoparticles with controlled size and distribution [
5,
6]. Additionally, the microscale environment allows for precise manipulation of physical parameters such as temperature and chemical concentration, which is valuable in dynamic cellular microenvironment studies during live-cell imaging experiments [
7]. One example of precise control mechanisms in microfluidics includes the use of Surface Acoustic Waves (SAWs) to induce efficient fluid mixing and nanoparticle synthesis [
8]. The microscale environment also facilitates detailed studies of biological interactions, such as cell-surface adhesion and microenvironmental effects on cell growth, which are challenging to observe using traditional macroscopic techniques [
4]. Furthermore, integrating various materials such as polymers and silicon in microfluidic device fabrication enhances their biocompatibility and chemical resistance, which are crucial for biomedical applications [
9,
10]. These distinct properties make microfluidic systems valuable for technological advancements in nanomaterial synthesis, biomedical research, and environmental analysis [
11].
Temperature control is critical in microfluidic applications, influencing the efficiency and accuracy of biochemical reactions and analyses. Precise thermal regulation is significant in processes such as polymerase chain reaction (PCR), which requires rapid and accurate thermocycling between 50 °C and 100 °C [
12,
13]. Maintaining a stable temperature ensures reproducibility and prevents reaction inconsistencies observed in conventional reactor systems [
12]. Additionally, temperature gradients in microfluidic systems play a role in directing chemical movement and concentration, as seen in techniques like Temperature Gradient Focusing (TGF), which leverages temperature differentials to generate electric field gradients and electrophoretic velocities [
13]. Integrating temperature control mechanisms in microfluidic devices enables localized heating and cooling, improving the precision of chemical processes and facilitating studies on thermosensory responses in biological systems, such as neuronal activity in Caenorhabditis elegans [
14]. Temperature regulation is also crucial for preserving biological sample integrity, as cells require stable environments to maintain viability during analysis [
13]. The development of advanced thermal control strategies, including thermoelectric coolers and adaptive fuzzy PID controllers, has significantly improved the precision of microfluidic temperature management, allowing for both uniform and gradient temperature regulation under diverse experimental conditions [
15]. These technological advancements enhance microfluidic device performance and expand their applicability in biotechnology, chemistry, and medicine, where temperature-sensitive processes are critical [
16].
Now, achieving precise temperature control at the microscale involves several challenges due to the necessity for rapid and localized thermal adjustments, efficient integration of heating and cooling mechanisms, and effective management of thermal gradients. A key issue is maintaining stable and accurate temperature regulation in microfluidic systems, mainly when operating below room temperature. This challenge is further complicated by the need for fast response times and precise local temperature monitoring, which are critical for applications such as single-cell analysis and polymerase chain reaction (PCR) processes [
4,
17]. The integration of thermoelectric coolers (TECs) offers a potential solution due to their rapid response time and compact size. However, TECs are susceptible to temperature overshoot and deviation, primarily when utilized for continuous gradient temperature control [
15].
Additionally, the thermal conductivity of the substrate material plays a crucial role in regulating heat transfer. High thermal conductivity can lead to heat dissipation, reducing the efficiency of thermoelectric cooling and making it challenging to maintain consistent temperature conditions [
16]. The design of microfluidic devices must also account for the interaction between heating and cooling elements to achieve stability and uniformity. This is further complicated by the need for precise control algorithms and the constraints of microscale environments [
17]. Additionally, the thermal mass of microfabricated structures can hinder rapid cooling and contribute to temperature overshoot, necessitating advanced control strategies such as adaptive fuzzy PID control to enhance accuracy and reduce fluctuations [
15]. These factors illustrate the complexities of achieving effective thermal control in microfluidic systems. These challenges require optimized materials, refined device design, and advanced control mechanisms. Addressing these considerations is essential for improving the performance of microfluidic applications in research, diagnostics, and industrial processes.
Foundational work on lab-on-chip thermocycling and calorimetry in the 1990s established many of the principles built upon here, including early on-chip PCR in micromachined chambers, continuous-flow PCR in microstructured channels, integrated DNA analysis microsystems, and the first chip-based microcalorimeters. We refer the reader to seminal reports from that era on (i) chamber-based and continuous-flow PCR on chip, (ii) integrated microfabricated DNA analysis, and (iii) silicon-based microcalorimetry, which together defined core thermal design constraints and control strategies for the field [
18,
19,
20,
21,
22].
Therefore, this review paper explores some of the recent methods developed to control temperature in microfluidic devices, such as the proportional–integral–derivative (PID) method, Segmental and Localized Temperature Control method, Thermoelectric and Adaptive Control method, examine heat-source and evaporation methods and integrated heating elements. This review distinguishes itself by presenting a comprehensive range of temperature-control strategies but also by integrating emerging innovations such as additive manufacturing and AI-driven feedback systems. These next-generation approaches, alongside the novel use of gallium-infused carbon nanotubes and quantum dots, represent a promising future for more precise, efficient, and scalable microfluidic devices. The integration of these technologies offers an opportunity to push the boundaries of current microfluidic applications, particularly in areas such as real-time diagnostics, personalized medicine, and high-throughput assays. As we look to the future, the convergence of advanced thermal regulation, intelligent feedback mechanisms, and innovative fabrication techniques will be pivotal in overcoming existing challenges, ultimately leading to the development of robust, versatile, and cost-effective microfluidic platforms. We are also discussing its procedure, results, and challenges. By doing so, we aim to provide a comprehensive view of this advancement that might inspire readers and audiences interested in microfluidic devices.
2. Applications for Temperature Control in Microfluidics
Temperature control is a critical factor in enhancing the precision, reproducibility, and efficiency of various applications, particularly in microfluidic systems. Accurate temperature regulation is fundamental in biochemical assays to maintain reaction integrity, as seen in polymerase chain reaction (PCR) processes, where precise thermal cycling is essential for DNA amplification. Managing temperature gradients also facilitates the generation of electric field gradients, a key requirement for processes like electrophoresis, ultimately improving analytical accuracy [
23]. Furthermore, integrating temperature control mechanisms within microfluidic devices, such as thermoelectric coolers, enables precise thermal gradient management. This capability is particularly valuable in applications such as droplet microfluidics and single-cell analysis, where maintaining consistent temperature conditions is essential. These systems facilitate continuous gradient temperature regulation, mitigating temperature overshoot and enhancing system stability, thereby improving experimental reproducibility and efficiency [
15]. Advanced control strategies, such as adaptive fuzzy PID control, have demonstrated superior performance in temperature regulation compared to conventional PID control. These methods minimize temperature fluctuations, ensuring enhanced reliability and efficiency in temperature-sensitive processes [
15]. In the food processing industry, microfluidic technology enables precise thermal regulation, improving product consistency and quality while reducing energy consumption and waste [
24]. Integrating embedded heating elements and temperature sensors in microfluidic platforms further enhances responsiveness and precision, reinforcing experimental reproducibility [
23].
- A.
Proportional–integral–derivative (PID):
In the remainder of this section, we adopt a functional taxonomy to distinguish roles and avoid overlap. Actuation refers to how heat is added or removed, sensing to how temperature is measured, and control to how actuation is adjusted in real time. PID and fuzzy PID belong to the control layer and are typically paired with heaters, thermoelectric coolers (TECs), or phase-change elements. TECs, evaporation/condensation processes, and exo-/endothermic reactions provide actuation for bidirectional or spatially localized heat flow, while integrated heating elements (e.g., Ni–Cr traces) also function as actuation and can be co-located with channels to reduce thermal lag. Liquid-metal–mediated, non-contact compensation primarily supports sensing by inferring channel temperature from the substrate, and this signal can feed back into closed-loop control. Many platforms operate in hybrid configurations—for example, TECs governed by adaptive or fuzzy control for precise gradients, integrated heaters with PID for zoned PCR, or chemically driven actuation combined with optical or resistance temperature detector (RTD) feedback. In the following discussion, we map each method to its primary functional role and, where relevant, highlight its dominant contribution to the overall stack.
The PID control uses a combination of proportion, integration, and differentiation of real-time error data to adjust the actuator and maintain device operations under optimal conditions [
25]. The Proportional (P) control component of a PID controller produces an output directly proportional to the current error between the desired setpoint and the actual temperature, which causes the immediate response to the error magnitude. Besides that, the Integral (I) control component sums up past error values over time to eliminate steady-state error, providing a corrective action based on the accumulated error history. And finally, the Derivative (D) control component predicts future error trends by calculating the rate of change of the error, helping to lower the system’s response and prevent overshooting and oscillations by anticipating the error’s future behavior and adjusting the control output accordingly. These three components work together to precisely control the temperature by adjusting the power supplied to the heating element or cooling system based on the real-time error between the desired and actual temperatures [
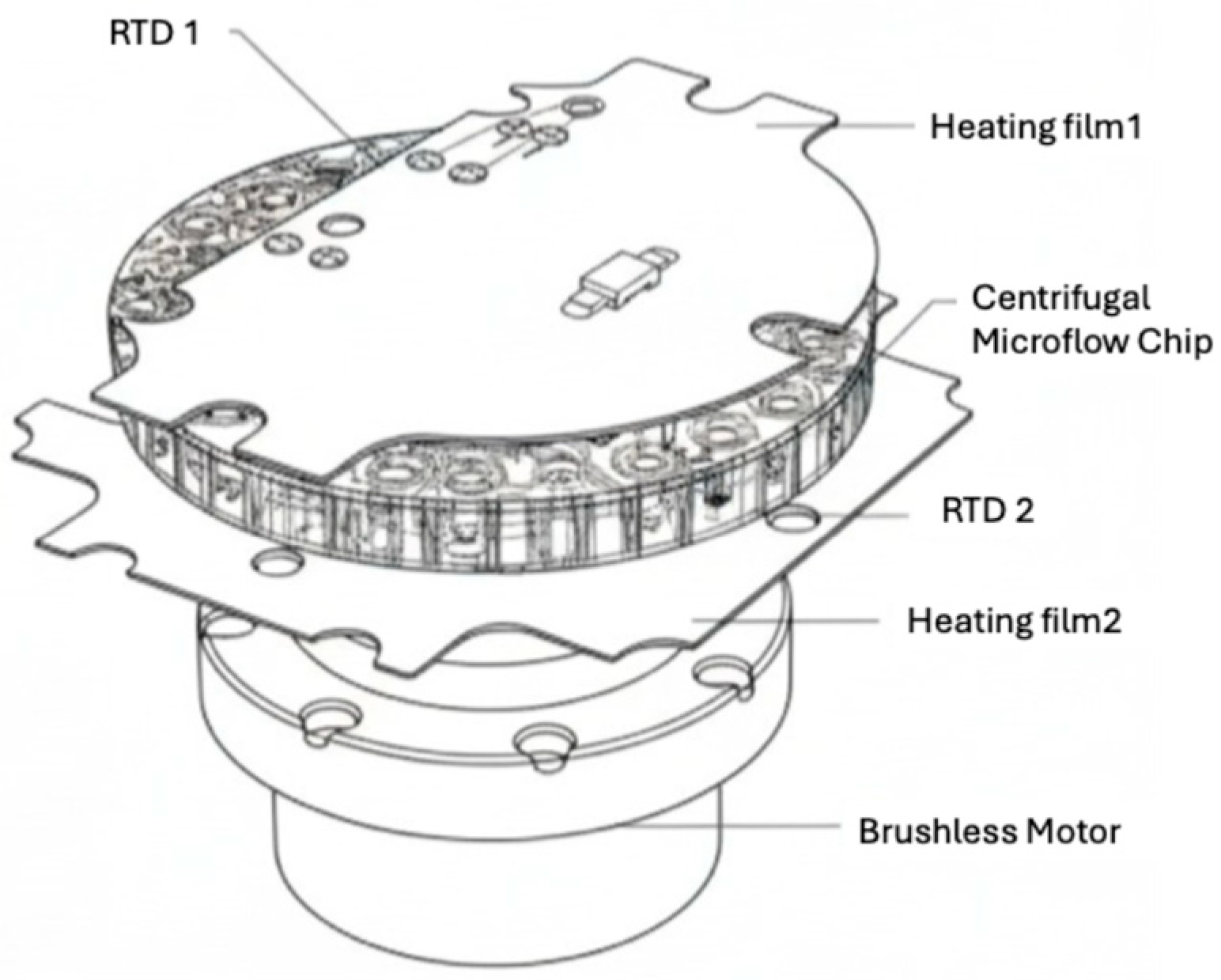
26]. Reported using the fuzzy-PID algorithm to control temperature in a liver function test system based on a centrifugal microfluidic device. The fuzzy PID algorithm combines the traditional PID control with fuzzy logic to handle the nonlinear and time-varying characteristics of the system, providing improved control accuracy and performance. As shown in
Figure 1, the temperature control system for the chip includes heating films above and below the chip, monitored by Resistance Temperature Detectors (RTDs). These RTDs and a temperature acquisition module (RTDs and LMP90100) send temperature data to a microprocessor through the SPI interface. The microprocessor uses the fuzzy PID algorithm to control the heating film’s power and the fan’s speed, ensuring the chip’s temperature is regulated. This system operates as a closed-loop negative feedback temperature control system [
27]. The fuzzy PID controller receives two inputs (error and error change rate) and provides three outputs (ΔKp, ΔKi, ΔKd). Here, ΔKp, ΔKi, and ΔKd are the incremental adjustments to the proportional, integral, and derivative gains computed by the fuzzy tuner from the instantaneous error and its rate, they update Kp, Ki, and Kd online to reduce overshoot and steady state error.
Initial measurements showed an overshoot in system temperature due to significant initial PID parameters Kp and Ki values. By adjusting these parameters using the fuzzy PID algorithm, the authors reduced the overshoot and improved temperature control. They divided the error signal into two stages and applied different control actions based on the error magnitude. Through iterative adjustments of the PID parameters, the system achieved precise temperature control with reduced overshoot and stable temperature fluctuations. The treated chip inserted into the device demonstrated stable temperature control within a narrow range, proving the fuzzy PID algorithm’s effectiveness in maintaining temperature stability. The system’s temperature control capabilities were further validated by testing liver function markers, where it accurately measured the absorbance change rate to identify AST and ALT in whole blood samples. Overall, the study demonstrated the successful application of the fuzzy PID algorithm in regulating temperature within the liver function test system, effectively controlling temperature fluctuations, minimizing overshoot, and providing stable conditions for accurate biochemical analysis, highlighting the potential of this approach for future applications in fields other than clinical diagnostics.
Also, (Kim et al.) [
28] successfully maintained constant temperature in a microfluidic PCR chip using proportional–integral–derivative (PID) control. As shown in
Figure 2, this system employs silicone rubber heater(s) for denaturation and extension zones, regulated by a PID controller. A thermocouple inserted near the polydimethylsiloxane (PDMS) microchannel provides real-time temperature feedback. The PID controller continuously adjusts heating elements based on actual versus desired temperatures, ensuring precise temperature control throughout the PCR process. Integration of the PID system enabled stable temperatures in denaturation, annealing, and extension zones, crucial for reliable DNA amplification.
The PID controller monitored temperature in real-time via a thermocouple, allowing immediate adjustments to maintain setpoint temperatures during PCR. Temperature stability across denaturation, annealing, and extension zones was achieved, which is crucial for DNA processing. The PID method ensured accuracy and stability, maintaining constant temperatures despite setpoint changes, thereby optimizing DNA amplification for genetic analysis. Overall, PID-based temperature control in microfluidic PCR chips proved effective for reliable DNA amplification in genetic research applications.
The PID control method offers precise and accurate temperature control by continuously adjusting the output based on the error signal between the desired setpoint and the actual temperature [
28]. It helps maintain a stable temperature in various zones, ensuring consistent PCR results with minimal fluctuations [
27,
28]. Moreover, because the algorithm allows practical tuning of PID parameters, this leads to improved control performance [
27]. However, properly tuning the PID controller parameters can be challenging and time-consuming [
28]. Implementing a fuzzy PID controller adds complexity to the system design, requiring more effort and expertise for parameter tuning [
27]. Improper tuning may lead to overshoot, oscillations, or instability, negatively impacting the process [
28]. Despite these challenges, the fuzzy PID algorithm significantly improves temperature regulation accuracy and stability in specialized systems [
27]. Understanding PID principles and tuning may be complex for those without a background in control systems engineering [
28]. Nonetheless, the PID control method offers enhanced control accuracy and stability, making it suitable for complex applications [
27,
28].
- B.
Segmental and Localized Temperature Control:
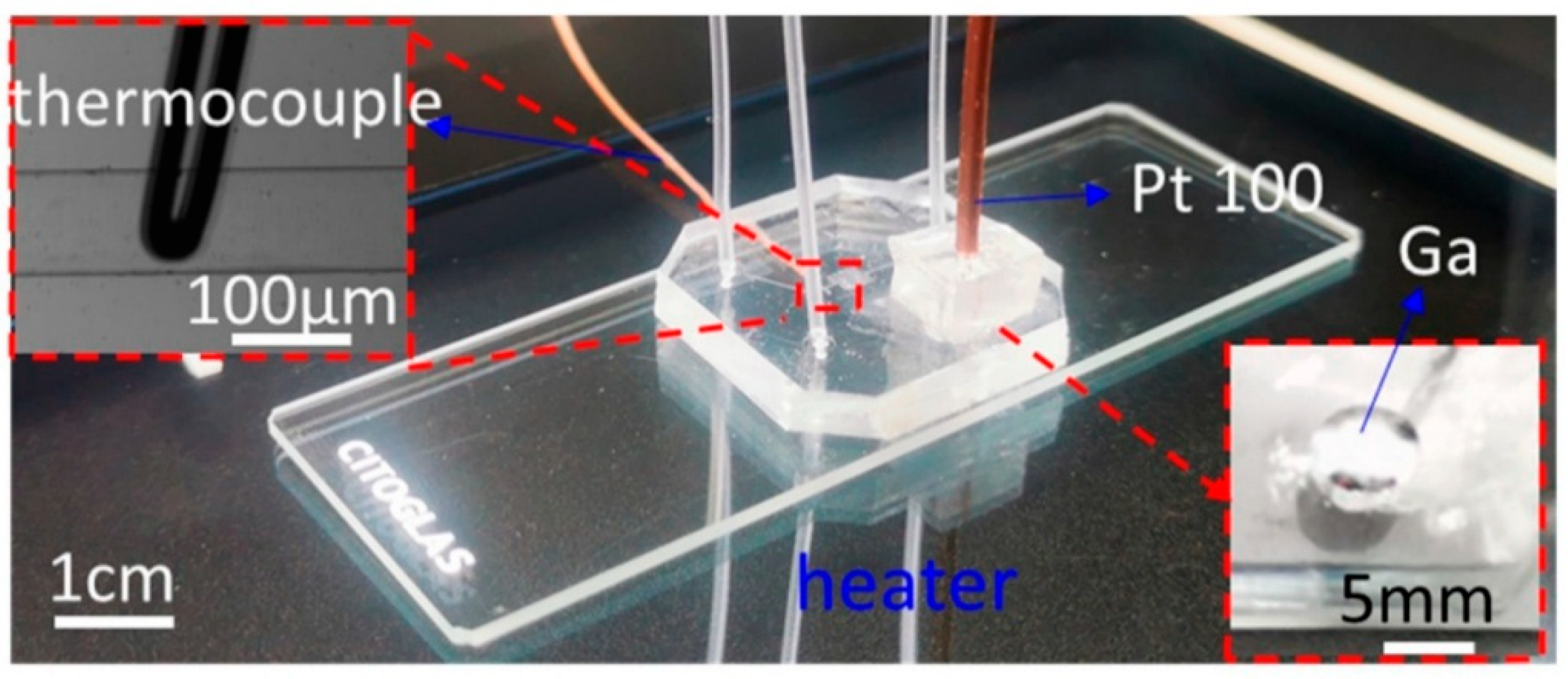
This method couples a platinum RTD to the glass substrate through a liquid-metal bridge to infer channel temperature non-invasively with ~±0.5 °C accuracy across 30–100 °C [
29]. This repeatable approach avoids direct contact between the sensor and microfluid, making it ideal for precise temperature control in microscale applications. Also, liquid metal, known for its high thermal conductivity and ease of handling, is a thermal conductive medium, facilitating temperature conversion between microscopic and macroscopic scales. Jiyu Meng and colleagues have successfully controlled temperature using the Segmental and Localized Temperature Control method by implementing a novel indirect temperature measurement approach. This approach integrated a platinum resistance millimeter-scale industrial sensor with liquid metal within a microfluidic chip channel [
29]. The method allowed precise temperature monitoring without direct contact between the sensor and the microfluid, ensuring repeatable temperature control.
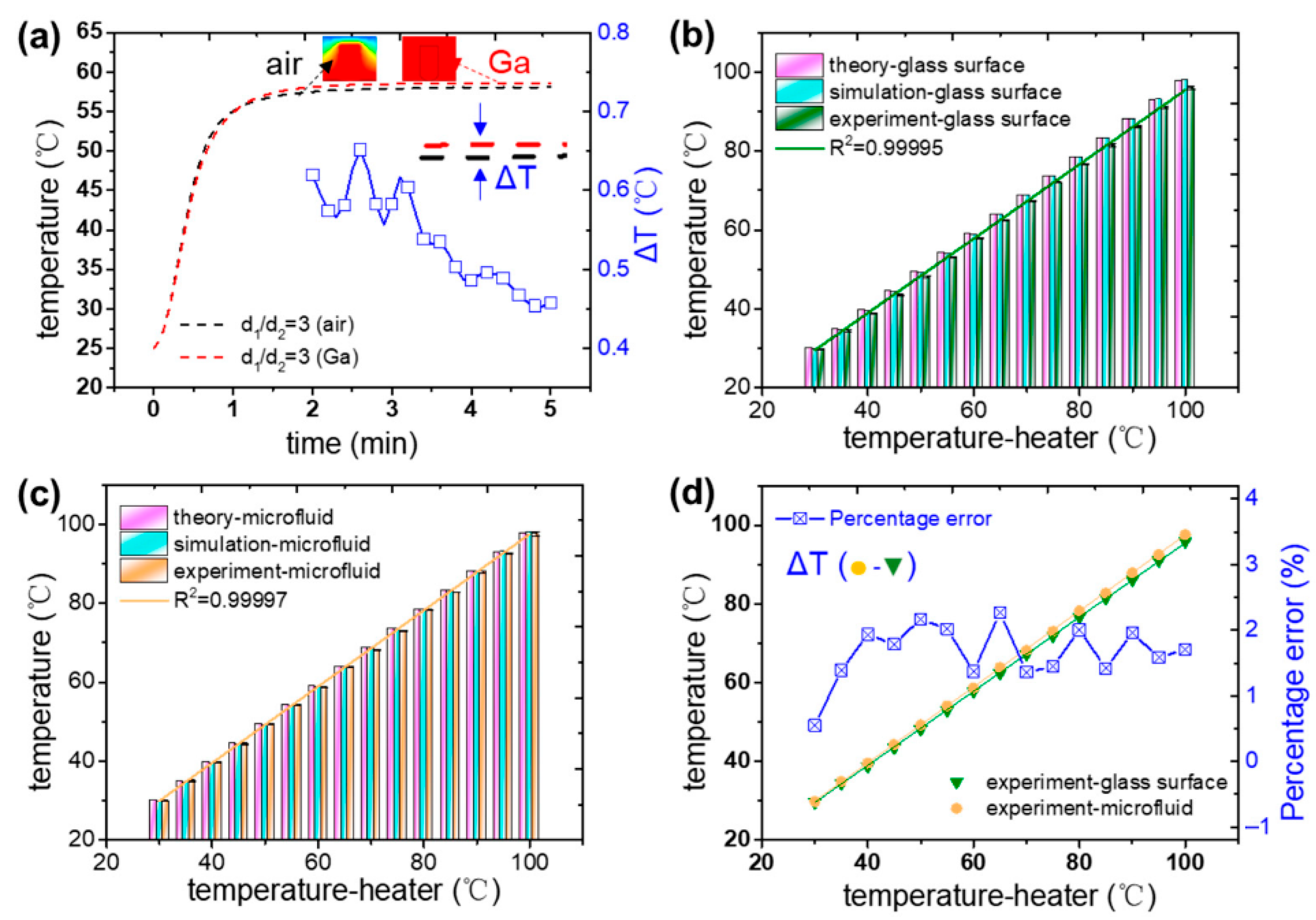
To better understand the thermal behavior within microfluidic systems, researchers first examined the thermal relationship between the fluid in microchannels and the glass substrate of standard microfluidic chips. Through numerical simulations, it was determined that the temperature at the upper surface of the glass substrate closely corresponded with the temperature of the microfluid within the channel. Leveraging this correlation, they used the glass surface temperature as an indirect fluid temperature indicator. As illustrated in
Figure 3, temperature sensors were strategically positioned on the upper surface of the glass, avoiding direct interaction with the fluidic channel. These sensors utilized liquid metal as a thermally conductive medium to facilitate efficient heat transfer. By optimizing the design of the sensor apertures, the system achieved high measurement accuracy, enabling the establishment of a compensation function that linked surface measurements to actual microfluid temperatures over a range of 30–100 °C. This approach allowed for dynamic linear temperature tracking within this range, maintaining an uncertainty margin below 0.5 °C. Further validation came from nucleic acid amplification tests, where the temperature sensitivity of the method was confirmed, underscoring its utility for both microfluidic and broader micro–macro interface sensing applications. In summary, integrating a platinum resistance industrial-grade sensor with liquid metal in a non-contact configuration enabled accurate and real-time thermal monitoring within microchannels. This demonstrates strong potential for precision temperature control in microscale systems.
The Segmental and Localized Temperature Control Method using liquid metal heat transfer offers precise temperature control in microfluidic devices, ensuring accurate monitoring of temperature changes. This approach avoids direct contact between the sensor and microfluid, potentially enhancing the efficiency of biochemical reactions through real-time temperature monitoring. Moreover, it shows promise in bridging microfluidic and macroscopic temperature sensing, making it versatile for various applications. However, implementing this method may require specialized equipment and expertise, increasing system complexity. Calibration of the compensation relationship between measured and microfluid temperatures can also be time-consuming and resource intensive. Furthermore, its calibration within a limited temperature range of 30–100 °C may restrict its applicability in other applications requiring lower or higher temperatures. Additionally, using platinum resistance sensors and liquid metal may increase the overall cost of this system. But overall, while offering precise temperature control in microfluidics, researchers must carefully understand the method’s complexity, calibration needs, temperature range limitations, and cost implications before deciding on the technique.
- C.
Thermoelectric and Adaptive Control Methods:
Thermoelectric coolers (TECs) operate on the principle of the Peltier effect [
30], where an electric current passing through P-type and N-type semiconductors generates a temperature differential across the device. This phenomenon allows TECs to function as coolers and heaters by reversing the current direction [
15]. Each TEC comprises multiple thermoelectric modules connected electrically in series and thermally in parallel, with each module containing semiconductor materials forming thermocouples. Applying a DC will transfer heat from the cool side to the hot side of the device. The cooling capacity of a TEC depends on factors like the number of modules, current intensity, and temperature differential between the hot and cool sides, enabling precise temperature management. However, effective cooling requires appropriate heat dissipation methods, such as heat sinks, fans, or liquid cooling systems, to efficiently manage heat generated on the hot side. In microfluidics, TECs regulate fluidic channel temperatures, ensuring precise control over chemical reactions, biological assays, and other processes.
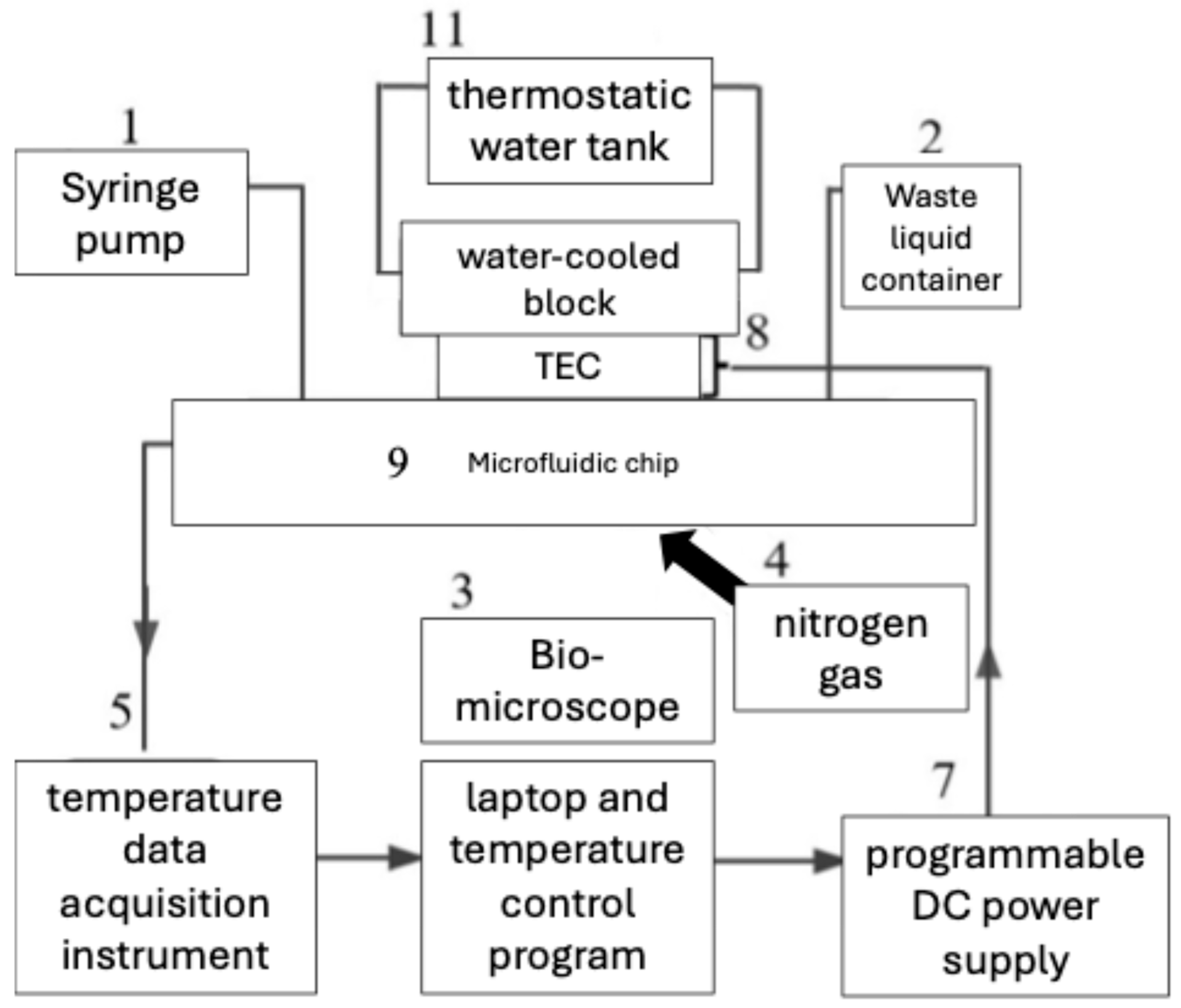
On this principle, Zhilin Liu and colleagues reported that the temperature of the microfluidic chip has been successfully controlled using a combination of a thermoelectric cooler (TEC) and an adaptive fuzzy PID control strategy [
15]. A direct current drives the TEC to achieve energy conversion for both cooling and heating functions. By adjusting the current flow through the TEC, the cooling capacity can be controlled, allowing precise temperature regulation of the microfluidic chip. The adaptive fuzzy PID control strategy combines fuzzy logic, neural networks, and PID control to dynamically adjust the control parameters based on system feedback and error signals.
Figure 4 shows the experimental set up applied, where No. 1 is a syringe pump, No. 2 is waste liquid container, No. 3 is a biomicroscope, No. 4 is a nitrogen gas, No. 5 is a temperature data acquisition instrument, No. 6 is a laptop and temperature control program, No. 7 is a programmable DC power supply, No. 8 is the TEC, No. 9 is the microfluidic chip, No. 10 is water-cooled block, and finally No. 11 is a thermostatic water tank. Heat from the TEC’s hot side is dissipated through the water-cooled block (No. 10), which is connected to the thermostatic bath (No. 11). This configuration stabilizes the hot-side temperature and preserves the desired ΔT, without sufficient heat sinking, the TEC saturates, and control accuracy deteriorates.
This adaptive control method enables the system to adapt to nonlinear and time-varying characteristics, ensuring stable and accurate temperature control of the microfluidic chip. The researchers demonstrate that this integrated approach of using TECs and adaptive control strategies effectively achieves continuous gradient temperature control in the microfluidic system, providing a reliable method for temperature management in microfluidic applications.
The researchers achieved significant results in temperature control using thermoelectric cooling coupled with adaptive control methods. They achieved precise continuous gradient cooling of the microfluidic chip, achieving a more minor deviation (σ) of 0.309 °C and a controlled steady-state error (Ess) within ±0.1 °C. Also, they have successfully maintained setpoints spanning +10 °C to −25 °C (including −15, −20, −25 °C). In extensive gradient temperature control experiments ranging from 20 °C down to 0 °C and then −20 °C, they achieved tighter control with reduced deviation. The maximum temperature deviation was 0.192 °C with a steady-state error within ±0.07 °C for small gradient control, while for considerable gradient control, the maximum deviation was 0.309 °C with a steady-state error within ±0.1 °C. These outcomes underscore the effectiveness of thermoelectric cooling and adaptive control methods, particularly adaptive fuzzy PID control, in achieving precise and stable temperature regulation in microfluidic systems, even under significant temperature fluctuations.
TEC offers several advantages and disadvantages in temperature control applications, just like any other technology. On the positive side, TEC is compact and reliable, providing precise temperature control capabilities suitable for miniaturized systems like microfluidic devices. It operates without refrigerants, making it environmentally friendly. TE devices can function in both cooling and heating modes by simply reversing the electric current, adding versatility to their applications. However, TEC has limitations. It typically offers lower cooling capacity than traditional refrigeration systems and becomes less efficient at higher temperature differentials. The initial cost of TEC devices is higher, and they require effective heat dissipation mechanisms, which can be challenging in specific applications.
- D.
Heat Source and Evaporation Method:
Heat-source inputs and evaporation/condensation are classical phase-change strategies for thermal management. This method provides the necessary thermal energy to the device, enabling efficient heat transfer and temperature control. On the other hand, the Evaporation Methods involve the conversion of the working fluid from liquid to vapor state, facilitating heat absorption and dissipation within the system. Together, these methods play a critical role in maintaining optimal operating temperatures and managing heat fluxes in electronic components, ensuring reliable performance in compact and high-heat-flux environments.
Navdeep Singh Dhillon has reported using a device that was designed for electronics thermal management [
31]. Specifically, it is an ultra-thin planar Microscale Capillary Pumped Loop (μCPL) intended for high-heat-flux thermal transport in compact electronic devices. This device addresses challenges posed by Joule heating in modern electronics by providing an efficient thermal management solution. The μCPL can be integrated into high-power stacked electronic modules where conventional cooling solutions are not feasible.
Figure 5 shows the setup used to conduct the experiment. The temperature control within the microscale capillary pumped loop (μCPL) is achieved systematically using the Heat Source and Evaporation method. Heat Source is applied to the evaporator section of the μCPL device, initiating the evaporation of the fluid by providing thermal energy. This phase change process transitions the working fluid from liquid to vapor. Casing effectively absorbs the latent heat and facilitates heat removal from electronic components. Control of the heat input from the Heat Source allows researchers to adjust the amount of thermal energy transferred to the working fluid. This regulation directly influences the evaporation rate and heat dissipation within the device, enabling precise temperature control. Optimization of the Evaporation method within the μCPL device plays a critical role in managing the evaporation process. These methods regulate the evaporation rate and ensure efficient heat transfer, contributing to stable temperature conditions crucial for reliable device operation. Continuous monitoring of the device’s temperature allows researchers to adjust the heat input and evaporation process in real-time. This monitoring ensures the maintenance of the desired operating temperature, preventing overheating and safeguarding the reliable performance of electronic components.
As a result, the evaporation methods employed within the μCPL device were carefully controlled to optimize the heat transfer process. By monitoring the liquid-vapor interface in the vapor transport channels, the researchers assessed the evaporation rate and the corresponding heat flux absorbed by the evaporator. This approach ensured effective thermal management by efficiently transferring heat from the electronic components through the evaporation and condensation cycle inherent in the μCPL design. During device startup, experimental observations focused on the behavior of the liquid-vapor meniscus and the distribution of the working fluid within the μCPL device. Optimization of the evaporation process and liquid supply mechanisms demonstrated the device’s capability to handle heat fluxes exceeding 20 W/cm2 without causing the wick to dry out, proving its effective temperature control capabilities.
Overall, the successful implementation of the heat source and evaporation methods underscored their critical role in achieving reliable and efficient thermal management for high-performance electronic devices within the μCPL framework. Researchers highlighted the importance of controlled heating and optimized evaporation strategies in enabling precise temperature control and managing high-heat-flux scenarios in microscale electronics applications. Using this method, they have achieved precise temperature control within the μCPL device by regulating heat input, ensuring safe operating temperatures for electronics. This proves that these methods manage high heat fluxes efficiently, which is crucial for thermal management in compact electronic designs with high processing speeds.
However, these methods also present challenges. The complexity and design challenges of implementing effective heat source systems can complicate heat distribution and control mechanisms within the μCPL device. Similarly, optimizing evaporation processes in microscale devices poses challenges in achieving uniform temperature distribution and maximizing heat transfer efficiency. Energy consumption is another concern, particularly in applications requiring continuous operation of heat sources to manage high heat fluxes, impacting overall energy efficiency. Maintenance and reliability issues arise from the need for periodic checks and servicing of heat source components and ensuring long-term reliability of evaporation methods in sustaining effective thermal management. After all, while the Heat Source and Evaporation Methods offer substantial benefits in terms of efficient heat transfer, precise temperature control, and high heat flux management in μCPL devices, they necessitate addressing complexities, energy consumption, and maintenance requirements for optimal thermal management in electronic systems. Achieving a balance between these advantages and challenges is crucial for enhancing the reliability and performance of thermal management solutions in modern electronics.
A practical concern with evaporation-based schemes is the risk of two-phase instabilities. In microchannels, excessive superheat can rapidly vapor-fill the channel, collapsing effective thermal conductivity and destabilizing control. Reliable operation requires several precautions: (i) maintaining inlet sub-cooling and limiting wall superheat, (ii) implementing closed-loop regulation of coolant flow and pressure, (iii) incorporating capillary wicking structures to delay dry-out, (iv) degassing to suppress premature nucleation, and (v) enforcing ramp rates below the geometry-specific onset of critical heat flux. These constraints, together with the need for accurate flow sensing, should be explicitly reported whenever evaporation-based strategies are applied in microfluidic platforms.
- E.
Integrated Heating Elements method
Integrated heating elements in microfluidic devices play a crucial role in controlling and influencing chemical and physical processes within microchannels. The integration of temperature control systems in microfluidic devices offers a simple and efficient solution without requiring additional microfabrication steps [
12]. These heating elements can be used for spatially localized cooling and heating using endothermic and exothermic processes, respectively, initiated by mixing two components through the application of a vacuum [
12]. The impact of the thermal effect can be controlled by adjusting the flow ratio of the components or selecting them based on their properties [
32]. And as a result, integrating heating elements into microfluidic devices offers an efficient method for temperature control, enabling precise manipulation of chemical and physical processes within microchannels.
Rosanne M. Guijt and colleagues have reported a successful temperature control of a microfluidic device using a novel approach that exploited endothermic or exothermic processes in microchannels to cool or heat solutions in another near microchannel [
12]. The cooling process was facilitated by an endothermic process involving acetone evaporation within the microchannel. Acetone was introduced and left to evaporate, which resulted in the microchannel cooling due to its endothermic properties. Control over cooling was managed by regulating acetone flow rates and optimizing microchannel design for enhanced cooling efficiency. On the other hand, heating was achieved by using an exothermic process where concentrated sulfuric acid dissolved in water generated heat within the microchannel. This exothermic reaction was initiated by mixing the acid with water, leading to a controlled temperature increase. Adjustment of water and sulfuric acid flow rates enabled precise control over the heating process to achieve desired temperatures within the microchannel. Temperature monitoring relied on fluorescence-based calibration methods. Rhodamine B fluorescence intensity and molecular beacons served as temperature-sensitive indicators, allowing researchers to estimate temperature changes in the microchannel. These methods effectively integrated endothermic and exothermic processes within the microfluidic device, demonstrating their capability for precise temperature control and highlighting their potential for spatially localized cooling and heating applications.
Also, Sung Yong Jung and colleagues reported successful temperature control of a microchannel heat sink device using a Ni-Cr wire heater integrated into the PDMS channel [
33]. As shown in
Figure 6, the Ni-Cr wire heater, with a diameter of 0.3 mm, is positioned within the microchannel and connected to a power source for heat control. Applying an electrical current to the Ni-Cr wire heater generates heat within the microchannel, allowing for precise temperature control during experiments. Temperature sensors may be utilized to monitor and adjust the temperature of the device as needed. This setup enables the researchers to regulate the temperature within the microchannel heat sink, facilitating the study of heat transfer characteristics under controlled thermal conditions.
The researchers achieved significant results using the Ni-Cr wire heater integration for temperature control in the PDMS microchannel heat sink. By precisely regulating the temperature within the device, they could conduct experiments to study heat transfer and flow characteristics under controlled thermal conditions. As a result, integrating the Ni-Cr wire heater allowed for targeted heating of the microchannel, enabling the researchers to analyze temperature distribution, flow behavior, and heat transfer efficiency within the microchannel heat sink. Also, this temperature control method facilitated the collection of valuable data on temperature profiles, velocity fields, and heat transfer rates, leading to a comprehensive understanding of the thermal performance of the PDMS microchannel heat sink. The controlled temperature experiments conducted using the Ni-Cr wire heater integration contributed to the successful analysis of heat transfer phenomena in microfluidic devices, providing insights to design and optimize microscale heat transfer systems.
Here, temperature control has been achieved using a Ni-Cr wire heater integrated into the PDMS channel, enabling precise control for studying heat transfer characteristics [
12]. Also, the cooling method, utilizing acetone evaporation as an endothermic process, effectively achieved localized cooling in the microchannel, demonstrating reliable cooling performance [
33]. The Ni-Cr wire heater offers advantages such as direct heating capability and precise temperature control but may pose challenges like potential for localized heating and overheating risks if not carefully managed [
12]. However, it was noted that cooling efficiency was lower in glass-glass devices compared to glass-PDMS hybrids due to acetone vapor escaping through PDMS [
33]. Challenges included viscosity differences affecting flow rate ratios and the influence of PDMS lid thickness on acetone evaporation rates, necessitating further investigation for optimized performance [
33]. However, both methods present a promising concept regarding thermal management on the microscale.
In practice, method selection is strongly application-driven. Zoned heaters with PID remain the default for PCR-class workflows that require stable isothermal and step zones with relatively simple hardware. Liquid-metal–based non-contact compensation is well suited to fragile chemistries and chip designs where sensor intrusion is unacceptable, but sub-degree accuracy is still required. TECs with adaptive control are preferred for bidirectional ramps or continuous gradients, particularly below ambient, provided that hot-side heat removal is sufficient. Phase-change approaches, such as evaporation or reaction-driven heating/cooling, are highly effective for compact, high–heat-flux applications but introduce additional fluid-management complexity. Integrated resistive elements (e.g., Ni–Cr traces) offer highly localized heating with minimal external infrastructure, making them attractive for small on-chip assays or micro–heat-sink studies, though careful design is needed to avoid hot spots. Overall, these trade-offs map directly onto assay constraints such as temperature range, spatial uniformity, ramp speed, biocompatibility, and power budget.
Table 1 below is a more concise comparative table of the primary temperature control methods. Each row covers the core principle, key advantages and disadvantages, and typical applications in microfluidics.
In practice, TECs with adaptive control are preferred for applications requiring bidirectional gradients or sub-ambient operation, liquid-metal compensation is most suitable for fragile chemistries where sensor intrusion must be avoided, and zoned resistive heaters governed by PID remain the default choice for PCR-class workflows.
Temperature control in microfluidics underpins many processes that demand consistency and reproducibility, from polymerase chain reactions (PCR) to high-throughput biochemical assays. Various control methods have emerged, each offering distinct benefits and facing particular limitations. Proportional–integral–derivative (PID) control, sometimes enhanced by fuzzy logic (Fuzzy PID), provides highly stable regulation by continuously adjusting outputs in response to the error between the setpoint and measured temperature. This approach minimizes drift and overshoot, making it especially valuable for processes like PCR, where temperature consistency directly influences reaction yields. However, tuning PID parameters requires a nuanced understanding of control theory, and improper calibration can lead to oscillations or extended settling times. Segmental and localized approaches, such as liquid metal heat transfer, circumvent direct sensor-fluid contact by measuring substrate temperatures, delivering accurate regulation without contaminating fragile samples. Though these methods achieve precise control (within ±0.5 °C), their operational temperature range is somewhat limited (around 30–100 °C) and demands specialized hardware for calibration, complicating scale-up.
Thermoelectric coolers (TECs) exhibit strong versatility for heating and cooling applications. The Peltier effect allows TECs to reverse current flow for dual heating-cooling functionalities. When paired with adaptive fuzzy or neural network controllers, TEC systems achieve excellent temperature uniformity and can compensate for nonlinearity in real time. Yet, these devices become less efficient at large temperature differentials and require robust heat dissipation. Other researchers focus on phase-change strategies like heat source and evaporation methods, which excel in dissipating high heat fluxes in compact devices. These techniques rely on fluid evaporation to absorb latent heat, effectively reducing temperature in electronics and microfluidic loops. Although proven for handling demanding thermal loads, they introduce engineering complexities in channel design, fluid maintenance, and energy consumption. Meanwhile, integrated heating elements, ranging from Ni-Cr wire resistors to exothermic reactions, offer localized temperature zones directly on-chip, reducing bulky external hardware. This localized control can be an asset in small-scale bioassays or heat sink studies, though design challenges arise in avoiding excessive localized heating and ensuring uniform heat dispersion.
Microfluidic temperature control methods must balance precision, scalability, and cost-effectiveness. PID and fuzzy PID controllers remain a gold standard for straightforward, accurate regulation, but can be time-consuming to tune. Liquid metal–based localized sensing offers high accuracy, yet it has a narrower effective temperature window. Thermoelectric modules provide compact heating-cooling versatility, requiring careful thermal management to maintain efficiency at more significant temperature gradients. Evaporation-based cooling excels under high power loads but increases design complexity, while integrated heating elements enable direct “on-chip” manipulation if coupled with meticulous thermal distribution strategies. Selecting a method ultimately hinges on application-specific goals, reaction sensitivity, temperature range, fabrication complexity, and budget constraints. These guides researchers toward the control solution best suited to sustaining stable and reproducible microfluidic processes.
To complement the method-centric comparison in
Table 1, we provide a concise set of representatives, device-level implementations that integrate thermal actuation, control logic, and sensing, feedback.
Table 2 highlights four widely used strategies, zone-based PID/fuzzy-PID regulation for PCR and clinical chips, non-contact liquid-metal-assisted compensation, thermoelectric cooling with adaptive control, and chemically driven endo/exothermic thermal zones, summarizing the essential actuation, control, feedback, and performance outcomes reported in each study [
12,
15,
21,
23,
24].
Because practical adoption depends on how well a platform handles heat flow and maintains stability, we distill the key performance figures reported by representative systems.
Table 3 summarizes four modalities, adaptive TEC control, non-contact compensation, two-phase heat transport, and zone-controlled PCR. Highlighting the essential quantitative outcomes and their implications for use in microfluidic settings [
15,
23,
24,
26].
Taken together, the device-level evidence indicates that closed-loop controllers (PID/fuzzy PID and adaptive schemes) reliably suppress overshoot and steady-state error, non-contact compensation sustains sub-degree accuracy over 30–100 °C, and two-phase loops handle > 20 W cm
−2 albeit with added design complexity. These constraints and trade-offs motivate the fabrication and materials choices elaborated in
Section 3, particularly additive manufacturing for co-locating heaters/sensors and nanomaterial-based approaches for high-resolution, non-intrusive thermometry.
While
Section 2 described how established actuation, sensing, and control stacks such as PID-regulated zoned heaters, TECs with adaptive control, liquid-metal-assisted sensing, and phase-change methods enable reliable temperature regulation for many assays, the same studies reveal persistent limitations. These include thermal lag from sensor–heater standoff, reduced efficiency at large ΔT, perturbations from contact sensors in sensitive chemistries, and difficulties in scaling up. The emerging technologies in
Section 3 directly address these gaps. Additive manufacturing reduces thermal path length by co-locating heaters and sensors at the design stage, nanomaterials provide high-resolution and minimally invasive thermometry, and AI-driven feedback enhances disturbance rejection and setpoint tracking under nonlinear loads. Taken together, these advances complement rather than replace conventional methods by reducing lag, improving spatial uniformity, and automating tuning, which is particularly valuable for portable, point-of-care, and high-throughput applications.
3. Recent Advancements in Microfluidic Devices’ Temperature Control
- A.
Additive Manufacturing (AM):
Additive manufacturing (AM) technologies, such as material extrusion (e.g., fused filament fabrication) and vat polymerization, have opened exciting new avenues for monolithic fabrication and direct integration of functional components, like heaters, sensors, and cooling elements, into microfluidic chips [
34,
35,
36,
37]. Conventional fabrication methods for incorporating active elements often involve complex multi-step procedures, including wafer-level deposition, intermediate bonding, and stringent alignment processes. By contrast, AM allows researchers to co-fabricate microfluidic structures and functional inserts in a single manufacturing cycle, effectively combining multiple materials and functional inks where necessary [
38,
39,
40].
A key advantage of this approach lies in its ability to strategically position active elements, for instance, resistive heaters or temperature sensors, anywhere within the fluidic network without having to rely on insert-molding or post-bonding integrations [
34,
41]. As a result, these functional layers can be embedded at precise depths or adjacent to channel walls, enhancing thermal management or sensing resolution. Similarly, cooling channels designed for thermal regulation of biochemical reactions can be integrated on demand, simply by defining the requisite channel geometry in computer-aided design software and printing it alongside the main flow path [
42,
43].
Moreover, the capacity for multi-material printing significantly expands the potential for on-board functionalization [
35,
37]. For example, conductive or semiconducting filaments and resins can be deposited side by side with structural materials, enabling localized heater traces or sensor arrays to be seamlessly embedded within the microfluidic chip [
38,
42]. This monolithic approach reduces the risk of interface leakage and eliminates additional assembly steps, improving device reliability while lowering total fabrication time. From an application standpoint, such readily customizable platforms are particularly compelling in point-of-care or personalized diagnostic devices, where integrated sensing and controlled heating or cooling are essential for processing patient samples reliably and on demand [
44,
45].
Finally, the inherent design freedom of AM facilitates rapid iteration, so developers can easily fine-tune heater layouts or sensor placements, as well as channel geometries for highly localized cooling, without retooling or relaying photomasks [
46,
47]. These benefits have led to a growing body of work on fully 3D-printed microfluidic pumps, valves, mixers, and reaction chambers, many of which now incorporate real-time sensing, thermal regulation, or fluid actuation elements in a single device [
38,
40,
48]. As these technologies mature, the potential for creating highly integrated, multifunctional microfluidic chips becomes ever more tangible, establishing a clear path toward practical, cost-effective research and clinical applications solutions.
Table 4 below summarizes key technical information on how additive manufacturing (AM) has enabled new possibilities for integrating heaters, sensors, or cooling elements into microfluidic chips. Citations are indicated in brackets.
Now, an example performance from AM platforms, Vat-photopolymerization has produced optically clear channels with sub-100 µm features, allowing heater/sensor proximity that reduces thermal lag compared with post-bonded inserts [
38,
40,
48]. Co-located resistive traces adjacent to flow paths yield thermal time constants on the order of 10–200 ms (device-dependent) and lower control effort by limiting parasitic heat spreading. AM also enhances power efficiency by directing heat to targeted regions and supports iterative optimization of layouts (e.g., trace geometry, channel–heater offset) across print cycles [
38,
40,
48].
- B.
Nanomaterials in Temperature Regulation:
The emerging use of carbon nanotubes (CNTs) filled with low-melting-point metals, such as gallium, introduces a micro- to nano-scale approach for temperature measurement that is reminiscent of traditional mercury thermometers, yet adapted to tiny volumes [
51,
52]. In this concept, gallium is confined within the hollow core of the nanotubes, and any change in temperature results in volumetric expansion or contraction of the gallium column. One can infer the fluid or environment’s temperature by monitoring the degree of this expansion.
Like other micro-nano sensing methods, this approach addresses accuracy challenges often encountered at microscopic scales. The gallium-filled CNT thermometer can be integrated into microfluidic systems or small-scale reaction setups where precise temperature monitoring is crucial. Although it does not necessarily achieve the ultra-high precision of certain dedicated MEMS or nanophotonic sensors, the gallium-in-CNT strategy benefits from a straightforward readout mechanism that is purely volumetric. Additionally, it is robust in settings where electromagnetic or optical interference might hamper more conventional sensors. Moreover, this method exhibits several advantages for different applications. First, it provides direct, contact-based thermometric feedback within confined or miniaturized regions, which is highly attractive for lab-on-chip devices requiring local temperature measurements. Second, because the nanotube geometry can be customized, there is potential for tailoring the sensitivity range by adjusting the inner diameter or wall thickness of the CNT. Finally, its operational principle, a “micro/nano-scale mercury thermometer”, offers intuitive visualization and could allow more straightforward instrumentation than complex electronic or optical-based systems in specific scenarios.
Here, carbon nanotubes filled with gallium deliver an effective micro/nano temperature monitoring technique, especially valuable in microfluidics, biomedical applications, and any domain where localized, real-time thermal mapping is needed. Future work may focus on improving their measurement resolution, stability, and integration with broader sensing arrays to enhance further the versatility and fidelity of these novel nanothermometers [
51,
52].
Compared with other nanoscale thermometry methods, gallium-filled carbon nanotubes provide contact, volumetric readouts with sub-degree accuracy after calibration and are resistant to electromagnetic and optical interference, though they require mechanical anchoring and may drift under repeated thermal cycling. Optical quantum-dot thermometry enables non-contact spatial mapping with sub-degree resolution within a calibrated range but demands optical access and careful management of photophysical effects such as bleaching and environmental sensitivity. Thin-film RTDs and micro-thermocouples typically deliver ~0.1–0.2 °C accuracy with rapid electrical response, yet as contact sensors they risk perturbing delicate flows or chemistries. A practical approach is hybridization: RTDs or TECs can provide fast inner-loop control, while Quantum dots (QDs) or Gallium-filled carbon nanotube (Ga-CNTs) are used intermittently for spatial or localized corrections in contexts where direct contact is not feasible. The (Ga-CNTs) thermometers typically achieve errors of ≤0.5 °C across their operating window. For comparison, thin-film RTDs positioned near the channel routinely provide ~0.1–0.5 °C accuracy, while quantum-dot luminescence thermometry offers non-contact spatial mapping with sub-degree resolution (≈0.1–1 °C) within a calibrated range. QDs, however, require optical access and careful management of photophysical effects, whereas Ga-CNTs offer simple volumetric readout but demand secure mechanical anchoring.
- C.
Nanomaterial-based Sensing:
Quantum dots (QDs) are semiconductor nanoparticles characterized by their nanoscale size, typically ranging from 1 to 10 nm, which leads to unique optical and electronic properties due to quantum confinement effects. This confinement occurs when the size of the QDs is smaller than the Bohr radius of the electron-hole pair, resulting in discrete energy levels and size-dependent emission wavelengths [
53,
54,
55]. These properties make QDs act like artificial atoms, with controllable discrete energy levels, and they can be optically excited to produce photoluminescence, where absorbed photons create excitons that recombine to emit light. The emission spectra of QDs are narrower and more symmetric than conventional organic dyes, enhancing their sensitivity for detection applications. QDs are used in a variety of applications, including biological imaging, solar cells, and as fluorescence resonance energy transfer donors due to their high brightness, stability, and quantum efficiency [
53]. They can be synthesized using various methods, such as pulsed laser ablation in liquids, which allows for the production of highly pure QDs with tunable shapes and sizes [
54]. The composition of QDs can vary, including materials like CdSe, ZnS, and carbon, each offering different advantages such as reduced toxicity or enhanced biocompatibility [
56,
57]. The ability to modify their surface with functional groups further extends their applicability in biological and medical sciences, enabling uses in drug delivery and cellular imaging [
56]. Despite their potential, the toxicity of certain QDs, particularly those containing heavy metals like cadmium, remains a concern, prompting the development of alternative materials like carbon quantum dots, which offer lower toxicity and better biocompatibility [
56,
57].
Now, in the context of temperature regulation and monitoring in microfluidic devices, as explored by Meng et al. [
29], quantum dots serve a dual role: as sensitive thermal reporters and as embedded fluorescent markers for temperature calibration in highly localized microscale regions. Their integration into microfluidic systems enhances spatial resolution and enables indirect, non-contact thermal sensing, particularly valuable where direct sensor insertion is impractical or disruptive to flow dynamics [
58]. Microfluidic systems are known for their high surface-to-volume ratios, enabling efficient heat transfer, but they are equally susceptible to rapid temperature fluctuations and gradients due to scale limitations and non-uniform thermal fields [
23,
59,
60,
61,
62]. Here, QDs offer a distinct advantage over traditional thermocouples or resistive sensors. As indicated by the optical thermometry discussions in Meng et al. [
29]. The temperature-dependent fluorescence intensity or lifetime of QDs can be harnessed to map thermal variations across microchannels optically. This optical technique avoids the heat dissipation problems inherent to contact-based sensors, such as thermocouples [
63,
64,
65] or platinum films [
66,
67], which can locally perturb the temperature field due to their self-heating effects [
66]. The advantages of QDs are particularly emphasized in cases where precise thermal regulation is essential, such as nucleic acid amplification reactions (e.g., isothermal PCR). Meng et al. [
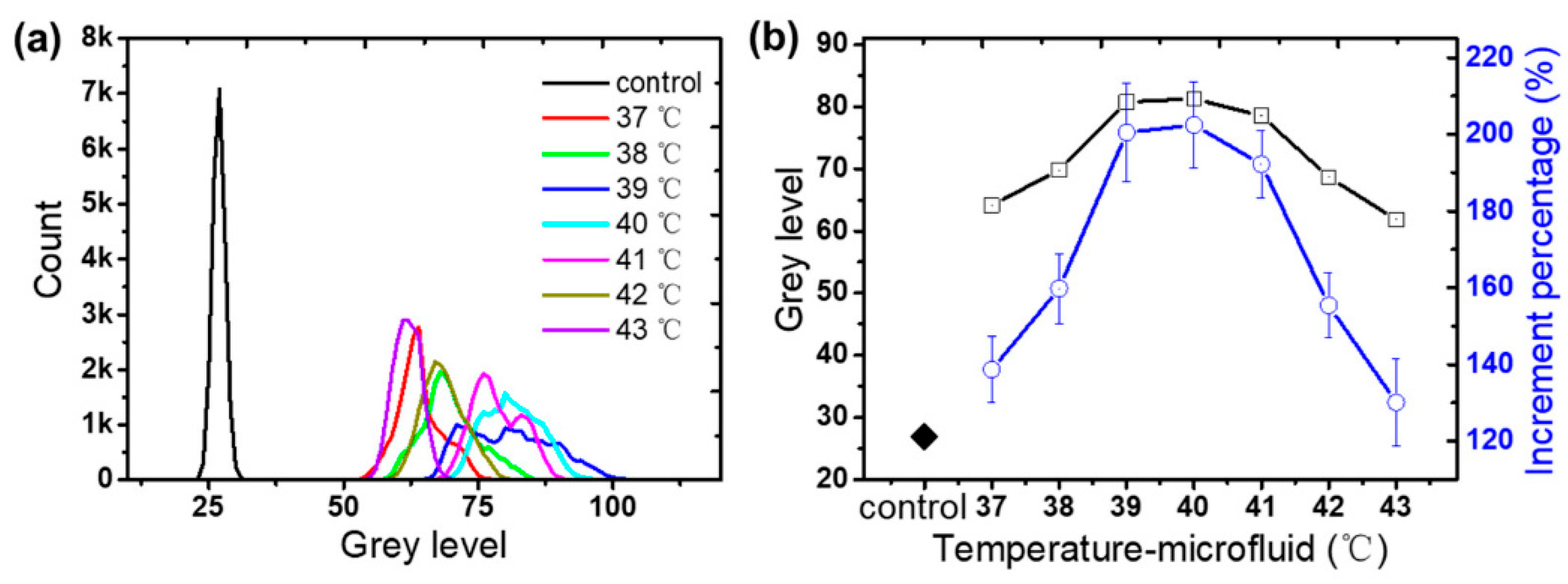
29]. demonstrated that a 1 °C difference in amplification temperature leads to distinguishable changes in fluorescence intensity during PCR assays (
Figure 7b). The grayscale temperature response is non-monotonic over the full range, so calibration is limited to a monotonic sub-range or implemented as a two-branch fit, with branch selection guided by an auxiliary cue (e.g., RTD estimate, continuity across frames, or dual-wavelength ratiometry). Alternatively, lifetime-based readout can be employed to obtain a monotonic temperature dependence within the operating window.
Such acceptable discrimination underscores the potential of QDs in thermal feedback loops, where even minute shifts must be detected and compensated. Although the primary method proposed in the work utilized liquid metal-coupled platinum resistance sensors (PT100), the reference to QDs in optical sensing recognizes their complementary role in expanding microfluidic thermometry approaches [
58]. Despite their benefits, QDs are not without limitations. The authors note that the emission behavior of QDs can be influenced by their size and shape distribution, which is temperature-dependent, potentially introducing emission non-uniformity [
58]. This variance complicates calibration and may reduce measurement fidelity unless uniform QD batches and rigorous characterization are employed. Furthermore, their use often requires additional optical components (excitation sources, filters), and their integration must be carefully aligned with existing microchannel geometries to prevent signal overlap or crosstalk [
68]. Nonetheless, quantum dots remain a valuable tool for thermal analysis within microfluidic architectures, mainly when applied with compensation mechanisms like those devised by Meng et al. [
29]. Here, the liquid metal-mediated interface between macro-scale industrial sensors and microfluidic channels yielded a reliable and cost-effective alternative to direct thermal sensing, as shown in
Figure 8. At the same time, QDs offer complementary, spatially resolved, non-invasive monitoring. Integrating both strategies could offer a hybridized system with macro-level control and microscale feedback precision.
The sensing approach described in
Section 2B effectively bridges macro-scale sensing with microfluidic temperature monitoring through a well-calibrated indirect method. The authors achieve high precision without intruding into the microchannel by measuring the glass substrate temperature using a PT100 sensor and enhancing thermal contact with liquid metal. The inclusion of gallium significantly improves thermal conductivity and reduces measurement error (~0.5 °C), as shown in
Figure 8a. The derived compensation function (
Tmicro = 0.96
Tset + 1.37) enables reliable real-time estimation across a broad temperature range (30–100 °C). This strategy balances simplicity, repeatability, and low cost, offering a scalable solution for integrated thermal sensing in lab-on-chip systems. However, substrate uniformity and bonding quality remain potential sources of error, suggesting future opportunities for combining with optical thermometry [
58,
69].
Gallium-filled carbon nanotube (Ga-CNT) thermometers provide localized contact measurements through the volumetric expansion of confined Ga columns, while quantum-dot (QD) optical thermometers enable non-contact mapping via changes in fluorescence intensity or lifetime [
58,
69]. For Ga-CNTs, reliable operation requires mechanical anchoring and passivation at the insertion site, controlled Ga wetting, and consideration of nearby electrical environments. Calibration is typically performed against traceable setpoints (e.g., stage or bath) with hysteresis checks during heating–cooling ramps to capture volume-lag artifacts, the response is generally monotonic but device-specific. For QDs, calibration must account for batch-to-batch spectral variance, photobleaching, local pH/ionic strength, and optical path changes. Lifetime-based detection is generally more robust than intensity-only metrics [
58,
69], with demonstrating biological applications. Ga-CNTs may drift due to creep or interfacial slip, which can be mitigated by surface treatments and periodic two-point recalibration. QDs are susceptible to photobleaching, reabsorption, scattering, and temperature-insensitive background, mitigation strategies include ratiometric probes, lifetime detection, and reference dyes [
58,
69]. Established contact sensors (e.g., RTDs, thermocouples) and TEC-based platforms are already deployed in microfluidic PCR and diagnostics [
58,
69]. In contrast, Ga-CNT thermometry remains at an early translational stage, while QD thermometry is at a mid-readiness level, primarily in research tools and some chip integrations [
58,
69] Current use cases include co-validation (e.g., QD mapping to guide placement of conventional sensors) and hotspot diagnostics rather than serving as primary metrology in regulated assays. Ga-CNTs are best suited for sub-100 µm confined regions where embedded contact sensing is acceptable. QDs are preferred for rapid spatial mapping and non-contact feedback in development platforms. Material choice must also consider safety and biocompatibility, with cadmium-free or carbon-based QDs increasingly favored [
58,
69].
- D.
Real-time Temperature Sensing and AI-driven Feedback:
Real-time temperature sensing and AI-driven feedback control have substantially reshaped microfluidic temperature management, delivering unprecedented precision, responsiveness, and adaptability essential for advanced diagnostic applications. Temperature control is especially critical in microfluidic systems employed for nucleic acid amplification (e.g., PCR, LAMP), enzymatic reactions, and cell-based assays, where thermal fluctuations can severely impact assay reproducibility and sensitivity [
70,
71]. Traditional heating strategies, often relying on resistive elements or bulk thermal cyclers, struggled with spatial resolution and feedback latency, resulting in heterogeneous thermal zones within the chip and poor reproducibility across runs [
72].
Concretely, adaptive fuzzy-PID control on TEC-based chips has demonstrated tight tracking under programmed gradients with sub-degree dispersion (σ ≈ 0.19–0.31 °C, steady-state error |Ess| ≤ 0.1 °C), even as thermal loads varied—performance that is difficult to achieve with fixed-gain PID on the same hardware. Vision-in-the-loop controllers on digital microfluidic platforms detect droplet states such as splitting, merging, or evaporation in real time and adjust drive patterns to stabilize thermally sensitive workflows, thereby improving run-to-run reproducibility. At the design stage, machine-learning approaches further reduce empirical tuning by predicting operating windows that minimize thermal lag and hot-spot formation before fabrication.
Despite these advances, two limitations remain significant. The first is data dependence and domain shift: models trained on one chip, assay, or ambient condition may degrade when applied to another, leading to drift or unsafe control actions. Mitigation strategies include transfer learning, periodic recalibration, and conservative fallback controllers. The second is latency and interpretability: camera- or network-based loops may miss fast transients, and opaque policies complicate failure analysis. Practical implementations therefore combine a fast inner loop (RTD-based PID or MPC at 10–100 ms updates) with slower AI corrections operating at camera rates, while enforcing hard constraints on actuator power, ramp rates (ΔT/Δt), and hot-side TEC limits. To support fair assessment, robust reporting should include update period, σ, Ess, overshoot, and disturbance-rejection metrics under representative thermal loads.r
The implementation of real-time temperature sensors, such as thin-film RTDs, thermocouples, and infrared (IR) microthermal detectors, has enabled localized, high-resolution thermal monitoring in complex microfluidic environments [
73,
74]. These sensors capture temperature variations across micrometer-scale reaction zones, generating time-resolved thermal profiles that form the basis for dynamic control. However, the mere acquisition of temperature data does not inherently lead to optimal performance without a feedback mechanism capable of adapting control in response to these measurements. This is where AI-driven control algorithms offer transformative value. Microfluidic systems can autonomously adjust thermal inputs in real time by leveraging machine learning (ML), deep learning, and reinforcement learning frameworks. AI models are trained on extensive datasets consisting of prior thermal performance, fluid dynamics, droplet behavior, and biochemical outcomes, enabling them to predict the most efficient heating or cooling sequence for a given condition [
75,
76]. As demonstrated in the AI-assisted digital microfluidic system named μDropAI, semantic segmentation models are used to identify various droplet states, such as splitting, merging, or evaporation-induced deformation, based on visual feedback, which correlates with thermal shifts [
73]. These insights guide electrode activation patterns in digital microfluidics (DMF), maintaining precise thermal conditions dynamically.
Furthermore, AI-driven thermal feedback systems provide a robust response to non-idealities such as air bubbles, variable sample viscosities, and non-uniform reagent mixing, challenges that are notoriously difficult for conventional PID controllers to manage [
77,
78]. In complex diagnostic workflows like enzyme-linked immunosorbent assays (ELISA) or multiplexed nucleic acid amplification, AI has been used to detect anomalies such as incomplete filling or thermal lag and initiate corrective protocols in situ, thereby preserving analytical accuracy. In addition to real-time applications, AI facilitates predictive temperature control through simulation-driven design tools. For instance, DAFD 3.0 (Design Automation for Fluid Dynamics) uses consensus ML models, combining boosted decision trees and neural networks, to automatically recommend optimal device geometries and flow rates that result in targeted thermal behavior for single and double emulsions [
75]. These predictive models account for variations in capillary numbers, flow rate ratios, and geometrical constraints to design thermally stable microenvironments in silico, drastically reducing the time and cost associated with empirical optimization. Notably, such systems are also being integrated into robotic microfluidic interfaces (e.g., RoMI) that carry out autonomous ELISA workflows, combining pneumatically actuated microchannels and machine vision-guided AI to control thermal-dependent incubation steps [
79,
80]. This combination of AI-enabled fluidics and temperature control creates a
human-free, sample-to-answer diagnostic pipeline, which is especially relevant for remote or point-of-care (POC) diagnostics where precision and repeatability are paramount [
81,
82].
Thus, the convergence of real-time temperature sensing and AI-driven control mechanisms establishes a new paradigm in microfluidic temperature regulation. These technologies address long-standing challenges in assay precision, reliability, and automation by providing high-resolution spatiotemporal feedback, enabling adaptive and predictive thermal control, and facilitating self-correcting protocols. Such advancements are central to fulfilling the vision of next-generation laboratory medicine that is not only efficient but also intelligent, scalable, and globally deployable [
83,
84].
Taken together, the comparison in
Table 5 highlights that no single approach is universally optimal. TEC combined with adaptive or fuzzy PID achieves the most precise tracking among reported systems (σ ≈ 0.19–0.31 °C, Ess ≤ 0.1 °C), provided the hot side is adequately heat-sunk. Additive manufacturing (AM) reduces thermal lag by positioning heaters and sensors closer to the channel, although the magnitude of improvement remains device specific. Non-contact optical thermometry (e.g., QD- or luminescence-based) delivers sub-degree spatial maps within calibrated ranges, functioning best as a complement rather than a substitute for contact sensors. Integrated endo/exothermic schemes enable compact, localized actuation without compressors but introduce added complexity in reagent handling.
According to the comparison, TEC with adaptive or fuzzy PID provides the tightest tracking among reported systems (σ ≈ 0.19–0.31 °C, Ess ≤ 0.1 °C) when hot-side heat removal is sufficient. Additive manufacturing (AM) shortens thermal lag by positioning heaters and sensors closer to the channel, although the performance gain remains device specific. Quantum-dot (QD) thermometry enables sub-degree, non-contact spatial mapping within calibrated ranges and serves best as a complement, rather than a substitute, for contact sensors. Integrated endo/exothermic schemes deliver compact, localized actuation without compressors but introduce reagent-handling requirements. Overall, method selection should be guided by temperature range, spatial uniformity, sensor-placement constraints, and power budget, with the reported values drawn directly from the cited studies.
Here also an example performance from AI-assisted control. Adaptive fuzzy control on TEC-cooled chips maintains |Ess| ≤ 0.1 °C with σ ≈ 0.19–0.31 °C across multi-step setpoints, even under nonlinear load changes [
15]. In digital microfluidic platforms, computer-vision models stabilize droplet operations against thermally induced variability (e.g., evaporation, wetting shifts), improving run-to-run assay reproducibility [
73]. Design-automation frameworks further reduce empirical tuning by recommending geometries that pre-condition thermal behavior prior to fabrication [
75].
4. Discussion: Future Directions and Challenges in Microfluidic Devices’ Temperature Control
Embedding thermally isolated polymer tubing within PDMS enables convective heating/cooling with low power (≈12–17% of supplied heat) and minimal electrical load. These tubes, positioned in parallel or cross configurations, use convective heat transfer to regulate temperature without needing energy-intensive resistive or Joule heating elements [
13,
85,
88,
89]. By modulating the inlet flow rate and temperature of water, uniform heating or controlled gradients can be achieved with minimal power, only 12–17% of the supplied heat is needed to maintain target temperatures [
13,
85,
86,
87]. Thermal losses are minimized by optimizing tube placement and using multilayer PDMS to insulate against free convection [
85,
90,
91,
92]. The simple fabrication process, relying on casting and bonding rather than cleanroom techniques, reduces environmental impact and enables scalable, low-cost production [
93,
94,
95,
96]. Temperature cycling is achieved by alternately flowing hot and cold fluids, reducing electrical load while maintaining dynamic thermal control [
85,
97,
98]. Further improvements involve integrating miniaturized pumps and sensors for closed-loop control [
99,
100], and using liquid metals for enhanced thermal conductivity with lower flow requirements [
101,
102]. These strategies establish a foundation for sustainable, portable, and energy-conscious microfluidic platforms.
On the other hand, Artificial intelligence (AI), automation, and machine learning (ML) present new pathways for achieving high-precision, real-time thermal regulation in microfluidic systems. Unlike conventional control strategies, AI and ML can adapt to nonlinear, time-varying behaviors inherent to microfluidics without extensive physical modeling. Through learning from experimental data, ML models can predict temperature deviations and dynamically adjust control inputs to maintain stability, reducing overshoot and steady-state error under varying thermal loads. Reinforcement learning, in particular, can optimize temperature control policies through continuous feedback, allowing the system to adapt in real time. Moreover, integrating AI with automated feedback loops enables self-calibration and fault detection, significantly improving system robustness and reproducibility. These intelligent methods are especially suited for applications requiring precise gradient control, such as droplet formation and biochemical reactions, where temperature fluctuations can lead to experimental failure [
17,
103,
104,
105]. By incorporating data-driven intelligence, AI and ML provide a flexible and scalable framework for microfluidic thermal management, offering enhanced control accuracy and system responsiveness beyond the limits of traditional PID-based methods [
106,
107,
108].
The scale-up of temperature-controlled microfluidic technologies for clinical and industrial applications introduces several critical challenges that must be addressed to ensure reliable operation and manufacturability. A primary concern lies in developing effective active control mechanisms capable of managing the complex thermal and hydrodynamic behavior of microfluidic systems. These systems often exhibit nonlinear and time-varying dynamics, mainly due to hydrodynamic interactions, which complicate the realization of stable temperature profiles and precise functional performance [
109,
110]. Therefore, the implementation of advanced control strategies such as Model Predictive Control (MPC) or adaptive fuzzy PID control becomes necessary to achieve accurate and robust temperature regulation, as demonstrated in recent studies employing thermoelectric coolers (TECs) [
111,
112]. Another substantial challenge involves the miniaturization of microfluidic devices. While reducing device dimensions facilitates integration and lowers operational costs, it simultaneously introduces experimental uncertainties and constraints on multifunctional integration, thereby increasing design complexity [
110,
113]. Moreover, the lack of standardized manufacturing and testing protocols impedes reproducibility and hinders regulatory approval, presenting a bottleneck for the transition from laboratory prototypes to commercial products [
113,
114]. These limitations are compounded by discrepancies in expectations and methodologies between academic research and industrial implementation, often leading to misalignment during technology transfer [
115]. Additionally, maintaining consistent performance during scale-up remains technically challenging, especially when transitioning from small-batch laboratory fabrication to large-scale production lines [
104,
116]. Reliable thermal management is also critical, as thermal instability can significantly affect the performance of temperature-sensitive microfluidic applications such as droplet generation and cell analysis [
105,
108].
In this context, the development of adaptive, intelligent control frameworks for thermoelectric-based systems is essential to overcoming performance degradation due to overshoot, steady-state error, and external disturbances [
111,
112].
To improve reproducibility and accelerate technology transfer, we recommend a compact standardization package embedded in Methods or Supporting Information and enforced during review. This should include the use of ITS-90–traceable references with both setpoint and ramp calibrations, incorporating hysteresis checks and ≥8 h drift, and reporting explicit sampling rates alongside stability metrics such as σ (RMS deviation) and Ess (steady-state error). Authors should provide a concise reporting checklist covering sensor type and placement relative to the channel, the thermal stack of the substrate (conductivity and thickness of each layer), controller architecture with tuned parameters, ambient conditions, and thermal load, including flow rates and reaction enthalpy. To enable cross-laboratory comparisons, open benchmark test chips with canonical heater-plus-channel layouts and documented boundary conditions should be made available. Benchmarking of control strategies such as PID, fuzzy logic, MPC, and AI should be performed on shared datasets, with results reported in terms of rise and settling times, overshoot, disturbance rejection, σ, Ess, and the control or update period. Materials guidance tables should also be included, summarizing properties such as thermal conductivity, surface roughness, permeability (e.g., PDMS versus glass), and biocompatibility constraints relevant to thermometry, for example the use of cadmium-free or carbon-based QDs. Finally, validation should follow a tiered structure: Tier-1 benchtop (open chip, sensors visible), Tier-2 semi-integrated (packaged module), and Tier-3 application-grade (enclosed, assay-ready), each with acceptance thresholds for σ and Ess. Collectively, these elements align academic prototypes with manufacturing and regulatory expectations and shorten the path to point-of-care and industrial deployment.
5. Conclusions
Temperature regulation within microfluidic systems is critical to their functional accuracy, reproducibility, and operational stability, particularly for thermally sensitive processes such as polymerase chain reaction (PCR), single-cell assays, and high-throughput biochemical reactions. This review highlights that no singular strategy universally addresses the diverse thermal demands of microfluidic applications; instead, a spectrum of methods has evolved, each tailored to specific operational constraints and functional objectives. Traditional proportional–integral–derivative (PID) control algorithms, particularly when enhanced with fuzzy logic, remain foundational due to their real-time error correction capabilities, minimal steady-state error, and applicability across a wide temperature range [
15,
26]. However, their performance hinges on precise parameter tuning, which can be both computationally intensive and application specific.
Segmental and localized temperature control strategies, such as those using liquid metal interfaces, offer significant advantages in micro–macro thermal interfacing by enabling accurate temperature estimation without direct sensor contact with the microfluid [
29]. These approaches leverage the thermal conductivity of intermediary materials like gallium to bridge macro-scale sensing systems with micro-scale environments, providing high spatial fidelity and minimizing contamination risks. Thermoelectric cooling methods, particularly when combined with adaptive fuzzy PID algorithms, represent a versatile class of bidirectional temperature control systems capable of maintaining small and large gradient thermal profiles with exceptional precision and low deviation [
15]. However, their efficiency decreases with increasing temperature differentials, and they require robust heat dissipation infrastructure to maintain performance.
Recent advancements in fabrication techniques, mainly additive manufacturing (AM), have further transformed the thermal landscape of microfluidic systems. By enabling the co-fabrication of functional components such as resistive heaters, thermistors, and cooling channels within a monolithic architecture, AM bypasses the need for multi-step assembly or post-bonding insertions [
34]. This integration improves thermal efficiency, reduces dead volume, and enhances device reproducibility. At the same time, the use of novel nanomaterials, such as gallium-filled carbon nanotubes and temperature-sensitive quantum dots, provides new paradigms for localized, non-invasive, and real-time thermal sensing with microscale precision [
29,
58]. These optical thermometric approaches eliminate self-heating effects and allow spatial temperature mapping without physical intrusion into fluidic channels.
Integrating artificial intelligence (AI) and machine learning (ML) represents a transformative direction in microfluidic temperature control. Real-time thermal feedback systems augmented by AI can dynamically compensate for thermal lag, fluidic variability, and system non-linearities that conventional PID controllers struggle to address. By learning from experimental datasets, AI algorithms can optimize heating and cooling sequences, enabling predictive control and fault detection in complex thermal environments [
58]. Such adaptive frameworks are particularly relevant for droplet microfluidics, multiplexed nucleic acid amplification, and on-chip diagnostic workflows where thermal uniformity is paramount.
For future improvements, several strategies are recommended. First, developing energy-efficient thermal control systems, such as convective microchannel heating or embedded thermally isolated tubing, can minimize power consumption while maintaining tight temperature regulation. Second, hybrid approaches that integrate macro-level sensing (e.g., platinum RTDs with liquid metal interfaces) and microscale optical thermometry (e.g., quantum dot fluorescence) offer a pathway to enhanced resolution and calibration-free monitoring. Third, standardization in design, calibration protocols, and material integration is essential for bridging the gap between research-grade prototypes and clinically or industrially viable microfluidic platforms. Lastly, adopting AI-driven, closed-loop thermal control systems will be crucial to overcoming the dynamic and non-linear nature of microscale thermal environments, ensuring reproducibility, robustness, and scalability in next-generation lab-on-chip systems.
Looking ahead, a practical path forward is to integrate a digital twin with edge-level predictive control and an event-driven fallback within a unified stack. The twin can be implemented as a reduced-order thermal model derived from CFD and system identification, continuously updated by live sensor data through estimators such as extended Kalman or moving-horizon filters that fuse macro-scale RTD/thermocouple readings with periodic microscale optical maps (e.g., QD/luminescence) to correct spatial bias. With this calibrated state, a predictive controller, whether MPC or adaptive PID with look-ahead, can schedule ramps, preheat cold zones, and enforce actuator limits (TEC current, heater power, ΔT/Δt) to suppress overshoot and minimize σ and Ess under setpoint changes or flow disturbances. Executing this framework on the edge (MCU, FPGA, or SoC adjacent to the chip) enables update periods below 100 ms without network latency while supporting energy-aware strategies such as duty-cycling heaters, shaping TEC setpoints to respect hot-side constraints, and auto-tuning PID gains under load shifts. For ultra-low-power or portable formats, an event-driven (neuromorphic) reflex can monitor sparse cues, threshold crossings in sentinel sensors, or rapid intensity shifts in minimal ROIs and trigger micro-corrections when the main controller is idle, maintaining stability at milliwatt budgets. The most robust near-term architecture is hybrid: a fast closed loop around the TEC/RTD provides coarse regulation, intermittent optical thermometry supplies spatial corrections, both streams are fused in the estimator, and multi-zone heaters or the TEC are commanded through the predictive layer. Additive manufacturing closes the loop physically by co-locating heaters and RTDs within tens to hundreds of micrometers of the channel, thereby shortening thermal lag and improving model fidelity. This combination, twin-guided prediction, edge execution, event-driven safety net, and AM-enabled co-location, offers stable sub-degree control with graceful disturbance rejection while remaining compatible with the power and latency constraints of field-deployable microfluidic platforms.