Abstract
This review covers the current state, challenges, and future directions of catalytic combustion technologies for mitigating fugitive methane emissions from the fossil fuel industry. Methane, a potent greenhouse gas, is released from diverse sources, including natural gas production, oil operations, coal mining, and natural gas engines. The paper details the primary emission sources, and addresses the technical difficulties associated with dilute and variable methane streams such as ventilation air methane (VAM) from underground coal mines and low-concentration leaks from oil and gas infrastructure. Catalytic combustion is a useful abatement solution due to its ability to destruct methane in lean and challenging conditions at lower temperatures than conventional combustion, thereby minimizing secondary pollutant formation such as NOX. The review surveys the key catalyst classes, including precious metals, transition metal oxides, hexa-aluminates, and perovskites, and underscores the crucial role of reactor internals, comparing packed beds, monoliths, and open-cell foams in terms of activity, mass transfer, and pressure drop. The paper discusses advanced reactor designs, including flow-reversal and other recuperative systems, modelling approaches, and the promise of advanced manufacturing for next-generation catalytic devices. The review highlights the research needs for catalyst durability, reactor integration, and real-world deployment to enable reliable methane abatement.
1. Introduction
Modern industrial society produces and uses a vast range of materials, many of which are made using chemical processes. This modern society also consumes large amounts of energy to maintain its existence. Undesired side effects from this societal model are the many concomitant environmental problems that are created. These problems often have serious effects on both human health and the natural habitat. The primary focus of this review is the emission of fugitive methane, but we also discuss a related topic, the emission of oxides of nitrogen, the so-called NOX compounds.
The majority of the energy used today is obtained through the combustion of the so-called fossil fuels, namely, coal, crude oil and its refined products, and natural gas. The products of the complete combustion of hydrocarbons are water and carbon dioxide, with some nitrogen- and sulphur-rich compounds if those elements were present in the fuel. Nitrogen oxide compounds can also be formed from the nitrogen present in the combustion air, especially when the combustion occurs at a high temperature. Emissions of oxides of sulphur and nitrogen are associated with significant health problems in humans.
One of the biggest environmental challenges that the world faces today is the rapidly changing climate, the cause of which is widely attributed to the constantly increasing levels of the so-called greenhouse gases (GHGs). Greenhouse gases contribute to global warming by absorbing and re-emitting infrared radiation, thereby trapping heat in the Earth’s atmosphere. This absorption slows the escape of heat into space, creating an energy imbalance that increases the planet’s thermal energy. Water vapour, a feedback amplifier, further intensifies warming by increasing in response to rising temperatures.
Carbon dioxide is the most abundant GHG in the atmosphere, followed by methane. Methane has a global warming potential (GWP) of between 25 and 30 times that of carbon dioxide on the 100-year time scale, or about 80 times on the 20-year time scale [1,2]. Because methane has a shorter life span in the atmosphere than carbon dioxide, its mitigation offers a much greater short-term mitigation result. Many countries have agreed to reduce methane emissions under the auspices of the Global Methane Pledge [3].
Methane is a colourless, odourless gas that is the simplest hydrocarbon molecule, comprising one atom of carbon and four of hydrogen. Methane is the principal component of natural gas. Its main direct use is as a fuel, either for space heating or electricity generation, and it is also used as a transportation fuel in the form of compressed natural gas (CNG) or liquefied natural gas (LNG). Methane is a key feedstock for the chemical process industries, especially in the manufacture of hydrogen via steam reforming or dry reforming. In addition to hydrogen, many other chemical products can be made from syngas produced by reforming. These include methanol, gasoline, methyl-tert-butyl-ether (MTBE), and formaldehyde, to mention a few [4].
This paper reviews mitigation strategies for fugitive methane emissions from the fossil fuel industry using catalytic combustion. The focus is on lower-temperature systems. In addition to the focus on fugitive methane mitigation, we also briefly discuss some other, usually high-temperature, applications, because those applications played a fundamental role in the advancement of the field of catalytic combustion. While it is not possible to provide an in-depth study of all aspects of this broad field, it is our objective to provide an overview of the key concepts, which the reader can use as a stepping-stone to achieving a greater insight of this fascinating area.
2. Sources of Fugitive Methane
2.1. Overview of Emission Sources
Recent estimates of global methane emissions from all sources give a value of approximately 610 million tonnes, or about 854 billion cubic metres per annum. This figure includes emissions from natural and anthropogenic sources. Effective mitigation strategies depend on the methane source, whether the emissions can be easily captured, and the methane concentration in the emission stream. Natural sources account for about 40% of global methane emissions. The remaining 60% are anthropogenic, of which agriculture accounts for about 50%, the energy sector 22%, and other sources such as landfills and wastewater treatment the remaining 28%. Energy sector emissions are divided amongst oil operations, including production and use, coal mining, and the natural gas supply chain [5,6].
In the following sections, we expand on emissions sources that originate in the production, transportation, and use of the three fossil fuels, coal, oil, and natural gas.
2.2. Natural Gas Production and Transportation
Global natural gas production has trended higher recently, and achieved a record high of approximately 4.19 trillion cubic metres in 2024. The United States is the world’s largest producer, accounting for about a quarter of the world’s supply. Russia is the second-largest producer, followed by Iran, China, and Canada. This continuous production growth reflects the robust global demand, particularly in the Asia-Pacific region, and is supported by the expanding infrastructure for both the pipeline and liquefied natural gas (LNG) trade. About 1% of the global natural gas production is lost to fugitive emissions. Methane is emitted at every stage, including production, storage, shipping, and use [7].
During the production stage, methane escapes for several reasons. These include some intentional venting, flaring, and loss through leakage. Leaks can occur at wellheads, piping connections, valves, and pneumatic devices that use pressurized gas for control. Completion and well flowback operations, particularly for unconventional resources like shale gas, can produce intermittent but sometimes very large emissions. After natural gas is extracted, it may be stored in underground facilities, which are also a source of emissions through leaks.
Natural gas is processed to remove impurities and separate valuable hydrocarbons, especially ethane. This stage can produce emissions from dehydrators, separation equipment, venting, pneumatic controllers, and leaks at facility components. Methane emissions at processing plants are often due to routine operations or equipment malfunction.
There can be a considerable loss of methane during shipping. This category includes the extensive pipeline networks that are used as basic transportation, as well as the large-scale movement by ship in the form of LNG.
In the pipeline network, leakage occurs throughout the chain of activities, from leaky seals, valves, pipe joints, compressors, etc. As natural gas flows through the pipelines, it requires periodic recompression to counteract the pressure loss resulting from flow, which is accomplished by periodic compressor stations. These can contribute significant emissions from several sources.
Either turbines or natural gas internal combustion (IC) engines are used to drive the compressors. While the emissions from gas turbines are fairly low, the IC engine emissions are significantly higher. These engines are operated in either lean-burn (excess oxygen) or with a stoichiometric air/fuel ratio. The IC engines in compressor stations are much larger than those used in road transportation applications, and, therefore, produce much more methane emissions. A typical 115 litre displacement natural gas lean-burn engine running at 1200 rpm produces about 61 tonnes per year of methane (1400 tonnes of CO2 equivalent), which increases as the engines age [8].
Another emission source is instrument vents. Natural gas actuates the pneumatic instruments used to control, for example, the temperature and pressure. These devices, however, bleed natural gas into the atmosphere during normal operation. This gas may be collected as a concentrated stream and vented outside of the building.
Leaking equipment is another emission source, which tends to be higher in older facilities. Methane losses through the compressor seal can range up to the tens of tonnes per year, and a single leaking valve can yield up to 1000 tonnes a year. The leaking methane is pure; however, it is rapidly diluted as it moves into the building and mixes with air [8].
Natural gas contains water vapour that must be removed to prevent pipeline corrosion and solid hydrate formation. Water is removed using triethylene glycol in a dehydration unit. The dehydration process absorbs water, methane, and other volatile organic compounds, including hazardous air pollutants such as benzene, toluene, ethyl benzene, and xylene (collectively called BTEX). When the glycol is regenerated, these emissions are released. Natural gas dehydration effluent is the third-largest methane emission source in the natural gas production industry [9].
2.3. Oil Production, Storage, and Transportation
In spite of the efforts to transition away from fossil fuels, oil production also continues at near-record rates, and is likely to do so for the foreseeable future. Methane emissions originate from numerous sources during the production and shipping of oil. They comprise intentional and unintentional releases, including operational venting, flaring, leakages, and tank/storage handling. Many of the streams are mainly methane, but can contain other light hydrocarbons. These leaks may be individually small, but there are also super-emitting cases, and, collectively, they are significant. Some of the main sources are given in the following paragraphs.
In most oil reservoirs, methane is dissolved in the oil, and is referred to as solution gas. Oil production comes from a wide variety of oil field types. Some of these fields contain a large number of highly productive wells, which, together, produce a large amount of methane. In this case, the methane might be collected and used as a fuel, or added to the pipeline. In other cases, the oil production rate is small, or there is a lack of infrastructure, so the gas is flared. On the smallest scale, flaring is not viable and the gas is simply vented. While a properly functioning flare should emit minimal methane, there are many cases of malfunctioning flares that emit significant amounts of methane. Venting and flaring have been estimated to account for about 75% of methane emissions from oil and gas operations, with venting alone responsible for 64%, and flaring for about 11% [5]. The remaining 25% of methane emissions come from a variety of sources. Methane can escape through equipment leaks at virtually every stage, from wellheads and pipelines to storage tanks and valves. Many control instruments, such as pneumatic controllers and chemical injection pumps, are powered by pressurized natural gas and intentionally release small amounts during operation. Methane is emitted from oil storage tanks (through breathing, flashing, or venting events), especially when pressure relief valves open or tanks are offloaded. Separators, used to separate oil, gas, and water, can also be major sources.
Many of these streams have a high percentage of methane, often more than 80%, especially those from leaks. Storage tank emissions can often contain significant amounts of higher hydrocarbons. Finally, a significant source of methane emissions is abandoned oil wells that have not been properly capped.
2.4. Ventilation Air Methane (VAM)
Underground coal mining presents a challenge in controlling methane emissions, a priority both for the protection of mine workers and the mitigation of environmental impacts. Methane is naturally adsorbed within coal seams and the surrounding rock strata. As coal is extracted, substantial quantities of this methane are liberated into underground mine workings. The presence of methane is a major safety hazard: in concentrations between 5 and 15% in air, it forms an explosive mixture, necessitating stringent safety protocols and continuous monitoring. To prevent hazardous accumulations, underground mines depend on powerful ventilation systems that continuously dilute and remove methane, along with other gases, by supplying large volumes of fresh air and exhausting contaminated air from the mines.
The exhaust air from these systems, known as Ventilation Air Methane (VAM), is characterized by highly dilute concentrations of methane, generally well below 1%, and frequently lower than 0.5% by volume. The enormous airflows required result in massive overall emissions. As a consequence, VAM represents the single largest source of methane emissions from most active underground coal mines. Annual methane emissions from a large coal mine ventilation shaft may reach approximately 50,000 tonnes. Considering the latest estimates of total methane emissions from mines, amounting to 41 Mt per year, and the estimated share of VAM in these emissions at 60%, the total VAM emissions are approximately 25 Mt per year [5,10,11,12].
Mitigating VAM emissions poses substantial technical challenges. The low methane concentration inhibits the use of conventional methane utilization technologies, such as flares or gas engines, which typically require higher methane contents to operate efficiently. Additionally, the variable nature of VAM, driven by factors such as the changes in coal production rates, periodic mine sealing, and atmospheric pressure fluctuations [12], complicates the implementation of consistent abatement strategies. Methane concentrations in VAM can exhibit both short-term and long-term fluctuations, and sudden changes may occur due to the operational events or geological conditions within the mine.
VAM is usually available at close to ambient temperature and may contain some water vapour. The water content may be around 2% by volume, and typically corresponds to saturated air at the local temperature. The low temperature and water content provide additional challenges for mitigation.
2.5. Natural Gas Engines
Natural gas may be used to power the engines in natural gas compressor stations. There is also an interest in the use of methane as an alternative fuel in internal combustion engines (ICEs) as a replacement for diesel or gasoline, especially in larger trucks used for the long-distance transportation of goods. Changing the fuel from diesel to natural gas results in a large reduction in the emission of greenhouse gases, provided that the methane that is not burned in the engine is successfully destroyed in a catalytic converter.
Natural gas easily forms homogeneous air–fuel mixtures which can be ignited and burned over a wide flammability range [13,14]. This pre-mixed combustion produces much lower nitrogen oxide (NOX) emissions and soot emissions compared to diesel engines. The fuel itself is chemically inert with a low photochemical reactivity and low global toxicity of the exhaust gases compared to those from diesel engines [15]. The low carbon/hydrogen ratio also permits reduced carbon dioxide emissions for a given engine efficiency [16]. Natural gas has excellent knock resistance which, when combined with the lean mixtures, permits diesel-like compression ratios for optimum engine efficiencies.
The composition and temperature of the exhaust stream from a natural gas engine vary significantly depending on whether the engine operates in a lean-burn or stoichiometric mode, each presenting distinct emissions challenges and opportunities for emission control. The excess oxygen present in lean-burn mode encourages the more complete combustion of the methane fuel, which reduces carbon monoxide emissions. The emission of nitrogen oxides (NOX) is also very low compared to traditional diesel engines. Because of the excess oxygen present in the feed, the exhaust also has excess oxygen, and, thus, a three-way converter (TWC) cannot be used. The stoichiometric natural gas engine operates with an air-to-fuel ratio close to the value required to ensure the complete combustion of the fuel, and, thus, there is little to no oxygen in the effluent. Stoichiometric engines are less fuel-efficient than lean-burn ones, and, thus, emit more carbon dioxide per unit of usable energy produced—in other words, per vehicle mile travelled.
Water is a major component of the exhaust from the engine, about 18 to 20% by volume. The value for the lean-burn operation is slightly lower than for the stoichiometric one, but is still a significant amount. Water has a strong effect on the catalyst activity for emission control. Lean-burn engines typically operate at much lower temperatures than stoichiometric ones. Stoichiometric natural gas engines typically have higher exhaust temperatures, often in the range of 450–600 °C and occasionally higher depending on the load and engine configuration. Lean-burn natural gas engines, on the other hand, produce cooler exhaust streams, with typical temperatures ranging from about 350 to 500 °C. At lower loads, the exhaust temperatures can fall below these ranges. The amount of methane emitted in the exhaust depends significantly on the engine type and operating conditions. Lean-burn engines tend toward higher emissions, in the range of 600 to 3000 ppm, with higher values at low engine loads. The methane emissions from stoichiometric engines are lower.
2.6. Summary of Emission Sources
The broad range of emission sources implies many possible compositions. Generally speaking, the composition can be divided into those containing significant quantities of water (wet) and those that do not (dry). There might also be other species present, such as sulphur compounds, BTEX, etc. The emissions of biogas might also contain a variety of species. It is often the case that the methane concentration is low, and there is an excess of oxygen (lean). As the oxygen content is reduced, the mixture becomes methane-rich. Furthermore, the temperature can be at ambient temperature or significantly higher. The composition and temperature of the emission stream, along with the ability to capture it, affects the mitigation technique that can be used.
3. Mitigation Strategies for Fugitive Methane
The mitigation strategies for methane emissions are many and varied. They can be as simple as decreasing emissions from natural gas transmission facilities by reducing leaks from valves and other equipment [17]. It has been shown that the actual leakage rate can be much higher than official inventory estimates [18]. Methane emissions from cattle are a significant agricultural source, which can be reduced by modifying their feed [19,20,21,22]. Both of these mitigation efforts rely on emissions reduction from the source, which would be the best solution; however, in many cases, anthropogenic emissions are inevitable, and, therefore, should be destroyed rather than be released into the atmosphere.
Methane is commonly burned as a fuel, and combustion technology can be used as a mitigation method for methane. Although the combustion of methane produces carbon dioxide, another greenhouse gas, it, nevertheless, results in the reduction in the greenhouse effect because of the high global warming potential of methane. Indeed, if the GWP of methane is taken as 27 times that of carbon dioxide, then the complete combustion of methane reduces the overall GWP by 90% on a mass basis, if the effects of water vapour are ignored. The combustion of fugitive methane emissions poses challenges, largely because of the conditions at which they exist, which often consist of dilute streams and relatively low temperatures. A solution, in some cases, is to use catalytic combustion, and, in the next section, we provide an overview of this methodology.
4. Combustion of Methane
Combustion is the complete oxidation of a substance (fuel) that is accompanied by a release of thermal energy. Fuels may be solids, liquids, or gases. Although many chemical species undergo combustion reactions, they are most commonly associated with fuels that are composed mainly of hydrocarbons or, for solid fuels, carbon alone. Fuels may contain other species, such as nitrogen, sulphur, or oxygen. Combustion is typically performed to provide thermal energy for heating, to generate electricity, or to produce mechanical work. Combustion may also be used to destroy unwanted material such as garbage or waste streams that should not be allowed to enter the environment.
4.1. Conventional and Catalytic Combustion
Combustion is a broad field that, because of its prevalence, has been widely researched. There are many classifications of combustion. We use the term conventional combustion to refer to the oxidation reaction that takes place between oxygen and the fuel, usually in the presence of a flame, which may or may not be visible to the naked eye. Conventional combustion is subject to a number of conditions, which constrain when it may occur. First and foremost are the flammability limits, which state that combustion can only occur over a well-defined composition. For example, for a mixture of methane in air, the lower flammability limit is about 4.4% methane and the upper limit is about 16% methane. Within this range, the mixture will burn if it is exposed to an ignition source. Conventional combustion typically requires temperatures in the range of 1300 to 2000 °C, which can lead to the formation of undesirable byproducts such as oxides of nitrogen. Finally, because of the presence of the flame, consideration must be given to the firebox design, which is often large and must be designed to accommodate the flow patterns of the fuel/air mixture. It is important to prevent flame impingement on the combustor walls.
Catalytic combustion, in contrast, is a flameless combustion process that uses a catalyst to promote the oxidation reaction. Detailed descriptions of catalytic combustion can be found in references [23,24,25]. The advantages of catalytic combustion compared to conventional combustion are many. Catalytic combustion is not constrained by flammability limits, and, thus, streams with a low fuel concentration can often be combusted without using support fuel, although very low concentrations of methane (less than 0.3% by volume) remain a challenge. Inlet reactor temperatures around 300 °C can be used for the catalytic combustion of methane, although the temperature depends on the catalyst activity and the space velocity (throughput) in the reactor. These lower reactor temperatures decrease the possibility of nitrogen oxide production.
Catalytic combustion can be divided, perhaps somewhat arbitrarily, into regions based on temperature. These temperature regions determine the dominant forces that control the reaction rate, and, critically, determine the type of catalyst and support system that is used. The temperature used in the catalytic combustion system is also a strong function of the application. There is a classical plot commonly used in the catalytic combustion literature that shows a stylized representation of the observed reaction rate expressed as a function of temperature, as shown in Figure 1. In effect, this plot can be used to illustrate and explain the roles of kinetics and heat and mass transfer in the system.
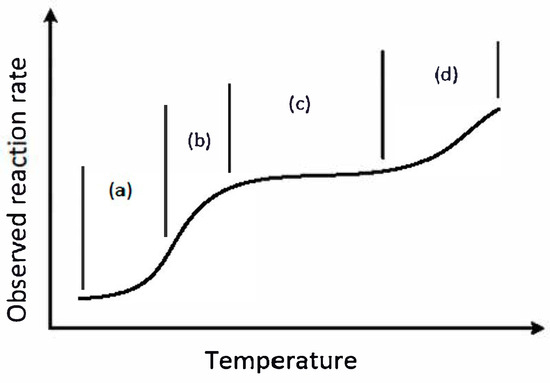
Figure 1.
Representation of the variation in observed reaction rate with temperature. The rate varies depending on the controlling factors, as the rate moves from kinetic to mass transfer control, and then as homogeneous reaction becomes important. Region (a) corresponds to kinetic control, (b) to the onset of mass transfer limitation, (c) to complete control by mass transfer and (d) the onset of catalytically supported homogeneous reaction.
In a heterogeneous catalytic system, the reaction occurs at the catalyst surface. For a porous catalyst, the bulk of the active catalyst surface lies within the bulk catalyst, that is, on the surface of the porous network. To achieve the reaction, the reactants are first transferred from the bulk gas phase to the external surface of the catalyst, through the concentration boundary layer. The reactants must then diffuse into the pores, where the reaction occurs on the surface. Both of these mass transfer steps depend on either diffusion or convection. The intrinsic reaction rate on the catalyst is controlled by the reaction kinetics. The observed rate of reaction, therefore, depends on both the rates of mass transfer and the kinetics. At relatively low temperatures, corresponding to Zone (a) in Figure 1, the reaction is intrinsically slow, and the reaction kinetics determine the rate. In this region, the rate increases exponentially with temperature, and the rate of mass transfer from the bulk becomes increasingly important. Zone (b) in Figure 1 corresponds to the region where both the reaction kinetics and the mass transfer rate control the observed rate. As the temperature increases further, and we move into Zone (c), the surface concentration becomes very small, and the mass transfer from the bulk gas phase controls the reaction. Because mass transfer coefficients are not a strong function of temperature, the observed reaction rate also only increases slowly. In this region, the kinetic expression is not important. As the temperature increases beyond this range and into Zone (d), homogeneous reactions start to occur. These homogeneous reactions are promoted by the surface catalytic reaction, and, thus, can occur outside of the standard flammability range for the fuel in question. This latter region is referred to as Catalytically Stabilized Thermal combustion, or CST [26]. It should perhaps be emphasized that, at high-temperature and -pressure operation such as that found in catalytic gas turbines, the gas phase, or homogeneous, chemistry makes a significant contribution to the overall methane conversion, and without a flame necessarily being present. We also point out that, in some turbine designs, there is a small homogeneous reaction section placed after the catalytic section [23].
The temperatures that govern the limits of the zones depicted in Figure 1 depend on the intrinsic catalyst activity, as well as the mass transfer coefficients. The latter depends on such things as the reactor geometry and the bulk gas flow regime. Although the zone limits depend on these factors, it is always important to be aware of these factors when designing a catalytic combustion system.
4.2. History and Applications of Catalytic Combustion
The development of catalytic combustion processes has been application-driven, and a brief history and the major applications are given as follows. The discovery of catalytic combustion dates to the safety lamp experiments of Sir Humphry Davy in 1818, where he observed platinum wires enabling flameless methane combustion [27]. As discussed in various publications [23,26,28], this fundamental discovery and the subsequent research into the catalysis of combustion formed the basis of the modern field of catalysis.
Catalytic combustion applications can be divided into those where the objective is to generate energy in the form of work or heat, and those in which the desire is to control fugitive emissions. While the main focus of this overview paper is on the latter application, we mention briefly some of the primary applications, because they have been important in the development of the catalytic combustion field. It is important also to realize that the two application areas often have quite different requirements, and materials that are useful in one area are not necessarily useful in others.
Some of the early work reported on catalytic combustions focuses on the applications of radiant heaters, where a catalytic active surface at high temperature supplies radiant energy. These devices ranged from large-scale process heaters to small-scale consumer products [23,26]. There was interest in the United States about using catalytic combustion for space heating during the decades of the 1950s to the 1970s.
Radiant heaters have been used with many fuels [29], and often use a metal fibre type of catalyst [21]. More recent developments in the use of porous radiant heaters based on catalytic combustion use multiple-stage designs of increasing sophistication [30,31].
There was a large increase in the interest in catalytic combustion starting in the early 1970s, with the objective of developing gas turbines that operated at relatively low temperature, compared to conventional gas turbines, as a means of reducing the production of NOX compounds in the combustion chamber. Most of these devices tended to operate in the CST region discussed earlier. There are many summary reference papers that provide details of this branch of catalytic combustion and discuss the theory of operation [23,26]. There have been numerous papers published that describe various designs at different scales, e.g., [32,33,34]. Several articles provide general discussions of the state of the technology and the remaining challenges [35,36].
A common feature of these primary combustion applications is the need for a catalyst able to withstand high temperatures for extended periods of time.
Another significant motivation for catalytic combustion came with the need to reduce atmospheric pollution from road vehicles, with catalytic converters becoming mandatory in the USA in the 1970s. This requirement led to the development of the automotive catalytic converter. Early catalytic converters destroyed carbon monoxide and hydrocarbons in vehicular exhaust, with the later appearance of the so-called three-way converter (TWC) that also reduced NOX emissions. This last technology was developed for engines operating on a stoichiometric air/fuel ratio, and purely oxidizing converters were used for lean-burn engines. An excellent review of this topic can be found in [37]. The main focus of this aspect of catalytic combustion was catalyst development, as well as the widespread adoption of monolith reactor technology, which will be discussed in more detail shortly. Although the reactions in the automotive catalytic converter for gasoline and diesel engines are quite different from the combustion of methane, there is a lot of commonality in the catalyst materials, and the associated reactor development, especially for monolith supports.
With the advent of vehicles that used natural gas as a fuel, a requirement developed for a more active catalyst that would destroy the methane present in the exhaust [38]. The remainder of the technology was similar to other automotive exhaust gas applications.
Finally, a third major area of application relates to the desire to destroy emissions from other sources. As discussed in the introduction, methane emissions from a number of sources exist, including the oil and gas sector, coal mining, agriculture, and others. In addition to methane, species such as formaldehyde, benzene, toluene, ethanol, etc. can all be readily oxidized using catalytic combustion [9]. The wide variety of properties of the emissions from these sources makes their destruction a challenge.
5. Catalysts for Methane Combustion
5.1. Overview of Catalysts Used
Catalytic combustion catalysts have been extensively studied over the past seventy years, including a large number for the combustion of methane [39]. Methane is the most stable hydrocarbon with a symmetry that makes it difficult to rupture the carbon–hydrogen bonds. The practical result is reflected in the high activation energy of the reaction, and the fact that the options for a viable catalyst are limited.
Several factors influence the catalyst performance. These include the ratio of oxygen to methane in the feed, the method of catalyst loading on the support, the nature of the support itself, the sensitivity to the catalyst structure and composition, the extent and nature of the catalyst pretreatment, the presence of other components in the feed (especially water), and the reaction conditions. The basic requirement for any combustion catalyst is that it has sufficient activity at the operating temperature desired, combined with an ignition temperature consistent with the feed properties. The operating temperature is determined by the application. For example, gas turbines and radiant heaters would normally have higher temperatures than reactors designed to destroy low-concentration emission streams. In many practical cases, there are physical constraints on the size of the reactor, and, in such cases, it is important to have high-activity catalysts. The cost can be a factor in the catalyst choice.
Although many materials have been tested in catalytic combustion applications, they can be divided into four general groups. The first group, which is considered the most active, is based on precious group metals, notably platinum and palladium. The second group consist of the metal oxide catalysts that use earth common metals. For the destruction of fugitive methane emissions, these two groups of catalysts are the most widely used.
For higher-temperature applications such as turbines and radiant heaters, research has focused on hexa-aluminate-type catalysts and those based on perovskite materials [39]. We briefly describe these four classes of catalysts. It is not the intention here to give a detailed literature review of each type, but, rather, to present an idea of the characteristics of each with some relevant references. The established literature on the catalytic combustion of methane is vast.
5.2. Catalysts Based on the Precious Group Metals (PGMs)
It is generally accepted that precious metals in either pure or oxide form are the most active catalysts for catalytic combustion [40]. These catalysts are preferred for lower-temperature applications where a highly active catalyst is required [41,42]. Palladium is the most widely used PGM catalyst for methane combustion, and research on this metal is plentiful. Two useful reviews are found in references [43,44].
Some applications of the catalytic combustion of methane, especially some low-concentration emission streams, have a feed stream that contains a large amount of water. Palladium is quite susceptible to activity loss and permanent deactivation by the presence of water. Activity loss is especially noticeable at temperatures below about 450 °C, which is attributed to the formation of Pd(OH)2 species on the surface [40,45,46]. Another suggestion is that surface hydroxyls inhibit the exchange of oxygen between the PdO, support, and the gas phase [47,48].
Platinum has also been widely used in catalytic combustion applications, especially as a key ingredient of the automotive catalytic converter. Platinum is considered to be very effective for the combustion of non-methane hydrocarbons; however, there are many references to the use of platinum for the catalytic combustion of methane. Various supports have been used [49,50,51,52,53,54].
The use of palladium or platinum alone is widespread, especially in the older literature. Combinations of different PGMs have also been presented, and typical combinations include rhodium [55,56,57,58,59,60] and gold [61,62,63]. The most recent trend today for the PGM catalysts is to use a mixture of palladium and platinum, and there is copious literature extant for this combination [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78]. The Pd:Pt combination is considered to be advantageous in the presence of water and this combination may also be more resistant to sintering. The ratio of Pd to Pt is considered to be an important factor [63,65]. It should be noted that it has been shown that, while a Pt addition enhances the catalytic activity under wet conditions, the activity can be decreased under dry feed conditions, compared to the pure Pd catalyst [76].
5.3. Deactivation of PGM-Based Catalysts
It is important that the catalyst does not suffer from excessive deactivation at the operating temperature of the system, nor from any compounds present in the feed, such as impurities or water. The inhibition of the activity of the Pd-based catalyst is discussed in Section 5.2. The presence of water can also cause long-term catalyst deactivation, especially at higher temperatures, as a result of sintering. High temperatures can also cause activity loss, even in the absence of water in the feed. Platinum catalysts are generally less susceptible to deactivation by water, but sintering and support interactions can also cause activity loss over time. The presence of even small amounts of sulphur compounds can also cause activity loss because of the irreversible adsorption on active sites.
In the case of palladium catalysts, there is an additional factor to consider. The most active surface form of palladium is PdO. At higher temperatures, even in the presence of oxygen, there is a transition to the less active Pd form. The transition temperature depends on the oxygen partial pressure [23], and is a reversible process. However, it should be considered for the case of feeds with low oxygen concentrations.
5.4. Catalysts Based on Transition Metal Oxides
Transition metal oxides represent a promising and economically attractive alternative to PGM catalysts for methane combustion, owing to the abundance and lower cost of their constituent elements. Notable oxides, such as those based on cobalt, nickel, iron, copper, and manganese, have demonstrated significant activity, selectivity, and thermal stability [79,80,81,82,83,84,85]. Among these, cobalt oxide (Co3O4) and its composites with ceria or alumina supports are particularly effective, achieving high methane conversion rates at moderate reaction temperatures and displaying an enhanced tolerance to common catalyst poisons and thermal cycling. Recent advances have focused on fine-tuning the morphological and electronic properties of these oxides via doping, surface functionalization, and nanostructuring, resulting in improved low-temperature activity, durability, and resistance to water inhibition. A recent review by [86] summarizes the current developments and emerging strategies for this family of catalysts.
5.5. Catalysts Based on Hexa-Aluminates
The third group of combustion catalysts are the hexa-aluminates and substituted hexa-aluminates, introduced for methane combustion in the 1990s [87]. Barium hexa-aluminates are especially effective [88]. As with other catalysts, the activity depends strongly on the preparation method. These materials have a very high thermal stability, although their activity is generally lower than that of the transition metal oxides.
5.6. Catalysts Based on Perovskites
For high-temperature applications, an interesting alternative to the hexa-aluminate catalysts is those made from perovskites. Perovskites are mixed oxides with a structure of the general formula ABO3. Lanthanide elements usually are at the site A position and the first-row transition metals at the site B position. Perovskites have a good thermal stability, and offer a lower-cost alternative to precious metals with acceptable high-temperature activity [89,90,91,92]. Their activity is also affected by the preparation method [93,94].
5.7. Catalyst Supports and Promotors
Catalyst systems, especially those based on PGM and metal oxide, generally consist of the active component and a support. Many permutations and combinations exist, and the situation is further complicated because components which are themselves active catalysts may be used as supports. The support can affect the catalytic mechanism, the activity, and the resulting rate equation; see references [95,96,97,98,99]. Supports include alumina [100,101,102,103,104,105,106,107], zirconia [108,109,110,111,112], silica [113,114,115], aluminosilicates [116], cobalt oxide [117,118], ceria [119,120], nickel compounds [121], and tin [122,123]. In addition to the choice of support, the preparation method [93,94,124] and the use of promoters [125,126,127] can also have a significant impact on the catalytic activity.
6. Reactor Internal Support Systems
All reactors used in catalytic combustion are multi-phase, consisting of the gaseous reacting stream and the solid catalyst system. Reactors can be divided into two categories, fixed bed and fluidized bed. In the fixed bed system, the catalyst is fixed in some manner within the reactor, and the reacting fluid flows through it. In the fluidized bed, small particles of the catalyst are placed in a bed over a distributor plate. High-speed gas is passed through the bed, causing it to move and behave like a fluid. Fluidized beds have been used for combustion applications, and a good review is available [128]. In this work, we focus exclusively on fixed bed systems.
Fixed bed reactors can be considered as a porous medium, and, in the terminology of porous media, the bed can be either consolidated or unconsolidated. In an unconsolidated porous medium, the solid phase consists of particles that are dumped into the vessel and are only constrained be the walls of the vessel. In a consolidated bed, the catalyst is typically placed on the surface of a well-defined support structure. The two most common support structures are monoliths and foams, although wires can also be used.
6.1. Unconsolidated Packed Beds
The majority of industrial chemical reactors use a fixed bed with catalyst particles in an unconsolidated bed. They have been the subject of countless publications and reviews, and all of the theory of individual catalyst particles and the packed bed is well-covered in standard chemical reaction engineering textbooks [23,129,130,131]. Their use in catalytic combustion has been reported [132]. As with all systems, there are advantages and disadvantages to using a packed bed of particles. Because of their widespread use, there is an extensive knowledge base and operational experience associated with them. Compared with the alternatives, they offer the largest bulk density possible, that is, the highest amount of catalyst per unit volume.
One of the main disadvantages is the pressure drop associated with the flow through the bed. In many commercial applications of catalytic combustion, the pressure drop is critical, and the use of packed beds is problematic. One of the consequences of the pressure drop is also the size of the catalyst particles. The pressure drop increases as the particle size decreases, and, therefore, relatively large particles, of the order of 6 mm in size, are used in industrial units. Because of this size, and considering the fast combustion reaction, the mass and heat transport effects can be significant, which significantly reduces the effectiveness of the catalyst. Finally, a packed bed of particles is prone to fouling or clogging, especially if there are particles in the feed.
6.2. Monoliths
The need for an efficient automotive catalytic converter, among other applications, led to the extensive development of the honeycomb monolith substrate [37]. This support system consists of a single block containing a series of straight, uniformly sized, and non-connected channels through which the reacting gas flows. The design maximizes the geometric surface area while maintaining a low flow resistance, making it ideal for high-throughput catalytic processes.
Monolith channels can have a variety of cross-sectional shapes, including circular, hexagonal, square, triangular, or more elaborate sinusoidal forms, chosen to balance the surface area, pressure drop, and ease of manufacture. The cross-sectional geometry, along with the channel density and wall thickness, dictates the percentage of open frontal area and the effective hydraulic diameter, which, in turn, influences the mass transfer rates and thermal response. Monolith structures are routinely manufactured with well-controlled specifications for the channel size, cell density, and wall thickness, enabling engineers to optimize for both the reaction and transport performance. Cell density is normally expressed in cells per square inch (CPSI), with commercial units ranging from around 100 to 1200 CPSI. Lower densities (100–400 CPSI) are favoured in applications requiring a very low pressure drop and high gas throughput, while high densities increase the available surface area for catalyst deposition but at the expense of a higher flow resistance. Cordierite remains the most common ceramic composition as a result of its low thermal expansion and excellent thermal shock resistance, although alternative materials such as silicon carbide (SiC) are increasingly applied in demanding, high-temperature catalytic combustion duties where a higher thermal conductivity and robustness are desirable.
The active surface of the monolith is provided by applying a high-surface-area coating, called a washcoat, onto the channel walls. This porous oxide layer, often γ-alumina or a composite oxide, hosts the catalytically active phase, typically finely dispersed precious metals or transition metal oxides. Washcoat thicknesses can range from ~10 to 150 μm, depending on the application, although deposition uniformity is challenging in non-circular geometries such as square or triangular channels [23,133]. Thicker washcoats increase catalyst loading but also risk introducing mass transfer resistance within the porous layer, which can be significant for fast, highly exothermic reactions like methane combustion. In operation, the reacting gas flows axially down each channel, with reactants transported across a diffusion boundary layer to the washcoat surface, after which molecular diffusion and the heterogeneous reaction proceed within the porous washcoat.
The heat and mass transfer characteristics of monoliths are excellent, particularly in high-pressure service where the gas flow may be in the turbulent regime within the narrow channels. At atmospheric pressure, the flow is typically laminar even at relatively high velocities; nonetheless, the high surface-to-volume ratio offsets some limitations in turbulent transport, and thermal conduction through the solid walls can help smooth temperature gradients. This is beneficial in exothermic reactions like catalytic combustion, where minimizing localized hot spots reduces catalyst sintering and structural degradation. Metal monoliths, in particular, can offer superior thermal conductivity, aiding in heat spreading and thermal cycling durability.
Mechanically, monoliths are robust, resistant to vibration, and capable of withstanding large thermal shocks, particularly when made from cordierite or suitably stabilized ceramics. Their modular form factors allow for the easy scaling from laboratory test pieces to large industrial units, and they can be assembled in stacks or arrays to treat very large flow rates. They also lend themselves well to the integration into heat exchanger/reactor hybrids, counter-diffusive configurations, or recuperative combustors, as the open-channel geometry is amenable to embedding into multi-functional reactor designs.
The properties of a low pressure drop, high geometric surface area, scalability, and good mechanical and thermal performance have seen monoliths become the dominant support system for many catalytic combustion applications, including gas turbines, automotive and stationary engine emission control, and industrial lean-methane abatement systems.
6.3. Foams
Foam materials, both regular (engineered open-cell foams) and irregular (random cellular structures), have become increasingly important as structured catalyst supports in catalytic combustion reactors, particularly for applications such as fugitive methane abatement. These porous substrates offer an innovative alternative to traditional pelletized or monolithic supports, facilitating significant improvements in reactor performance and process intensification. They have a high thermal stability and porosity. The flow patterns are typically more tortuous than monolith honeycombs, although less so than particle beds [134]. A fundamental understanding of how the geometrical and material properties of metallic and ceramic foams can optimize the catalytic reaction environments, reduce energy consumption, and enhance the overall process performance, and can be found in [135]. These properties have established foams as promising candidates for sustainable and intensified chemical engineering applications.
This new class of structured supports emerged in the 1990s [136,137,138,139,140]. These materials, available in metallic, ceramic, and composite variants, are characterized by their high porosity, large specific surface area, and interconnected open-cell structures. Metal foams, typically made from stainless steel, nickel, or FeCr alloys, offer excellent mechanical and thermal stability. Their properties can be enhanced via surface treatments and coating techniques. Ceramic foams, made from materials such as cordierite, mullite, and alumina, are noted for their superior thermal stability and resistance to mechanical stress. In addition, novel composite materials, including metal–ceramic hybrids and additively manufactured structures, further expand the range of design possibilities for catalyst supports.
A typical foam structure geometry is shown in Figure 2. Solid foams can be described as three-dimensional networks of struts and pores, forming cells (approximately spherical voids enclosed by struts) and windows (openings connecting neighbouring cells). The geometry can be expressed by overall statistical parameters, usually provided by manufacturers, such as the following: the pore density (often called grade), given as the number of pores per linear inch (PPI) and typically ranging from 5 to 100 PPI; total density; porosity; and specific internal surface area (SISA, m2/m3). Describing these parameters for irregular structures in sufficient detail for fluid-dynamic modelling is a challenge. X-ray tomographic methods have proven effective for this purpose [140].
Figure 2.
Typical appearance of a foam.
An interesting observation for metal foams (e.g., nickel- or FeCr-based ones) is that many of their mechanical and physical properties are directly influenced by the density, the porosity, the pore size, and the thickness of trigonal-structured struts. These factors collectively determine the foam performance in catalytic reactions. The SISA is, thus, closely linked to the foam grade and pore size.
The relationship between the porosity and surface area illustrates how foams differ from other reactor internals; see Figure 3. Solid foams occupy most of the available parameter space, with SISA values reaching up to 16,000 m2/m3, combined with a very high porosity. A high porosity translates into a low flow resistance when foams are used as internals in catalytic reactors. In terms of both the flow resistance and mass-transfer coefficients, foams are positioned between packed beds and monoliths with similar SISA values [141,142]. A comparison of foam carriers with standard monoliths and randomly packed beds of similar geometric surface area shows that reactors filled with solid foams can achieve the same level of methane conversion with shorter reactor lengths and a lower flow resistance [140,143]. This makes metal foam packings an attractive option for efficient reactor design by reducing the pressure drop and reactor volume requirements.
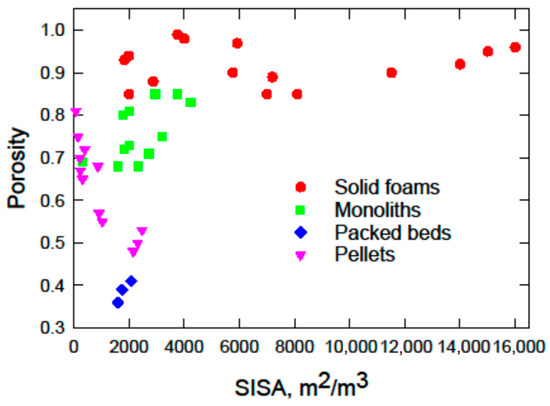
Figure 3.
Porosity and SISA for solid foams and most typical reactor packings.
Solid foams as catalytic layer carriers have been successfully tested in numerous reactions, for example, CO oxidation [141,142,144,145], Fischer–Tropsch synthesis [146,147], the abatement of vehicle exhaust emissions [137], and methane reforming [148,149].
Both open-cell ceramic and metallic foams are recognized as high-performance supports for catalytic methane combustion because of their unique structure and transport properties, particularly under conditions requiring rapid conversion and process intensification. Metal and ceramic foams (e.g., Al2O3, Fe–Ni alloys, and ZrO2) coated or impregnated with catalysts such as PdO, Co3O4, and perovskites achieve faster methane conversion rates and lower ignition temperatures than conventional supports. For example, Pd/Co3O4/Al2O3 foams achieve complete methane conversion below 400 °C at a moderate space velocity, outperforming monoliths under similar conditions [150,151,152]. Experimental studies have shown that metal foams based on FeCr or NiCr alloys exhibit Nusselt and Sherwood numbers comparable to packed beds and far exceeding those of monolithic structures [140,143,153]. Reactor modelling confirms that, for fast kinetics processes such as palladium-catalyzed methane oxidation, mass transfer in foams becomes the limiting factor that allows for shorter reactor lengths at high conversion levels [140,150,153].
For catalytic methane combustion, foams are typically coated with a thin, uniform catalyst layer such as Pd/γ-Al2O3. The effective integration of catalysts with foam carriers depends on coating methods that ensure a uniform coverage, strong adhesion, and easy access to active sites. While traditional techniques such as washcoating and sol–gel deposition are still common, electrophoretic deposition (EPD) has recently emerged as a promising and innovative alternative [154]. Unlike conventional slurry or solution-based approaches, EPD employs an electric field to drive charged catalyst particles from a stable colloidal suspension directly inside the metallic foam structure. Studies indicate that EPD offers exceptional control over the film thickness, deep penetration into the foam’s porous structure, and remarkably uniform coverage.
In summary, open-cell ceramic and metallic foams bridge the performance gap between monolithic honeycombs and randomly packed beds, combining a high specific surface area and excellent heat and mass transfer with a moderate pressure drop. Their unique 3D interconnected architecture, high porosity, and tunable pore geometry allow engineers to tailor reactor internals for both low-resistance flow and intense gas–solid contact. Compared with monoliths of an equivalent geometric surface area, foams typically deliver superior mass transfer rates, enabling shorter reactor lengths for the same conversion, while retaining mechanical robustness and good thermal conductivity, particularly in metallic variants. These traits, coupled with advances in precision coating methods such as electrophoretic deposition, make foams especially attractive for catalytic methane combustion under lean and dilute conditions, where rapid light-off, uniform temperature profiles, and compact reactor footprints are critical. While the cost, coating durability, and scale-up of fabrication remain practical considerations, the balance of the transport performance, design flexibility, and operational robustness suggests that foams will continue to gain importance as structured catalyst carriers in future catalytic combustion systems.
6.4. Comparison of Packed Beds, Monoliths, and Foams
Packed particle beds, monoliths, and foams each offer distinct performance characteristics, and their suitability depends on the balance of the pressure drop, mass and heat transfer, mechanical robustness, and fouling tolerance required by the application. Packed beds provide the highest catalyst bulk density, enabling a high active surface area per unit reactor volume. They are mechanically simple and benefit from decades of operating experience, but have a relatively high pressure drop, which limits the allowable particle size, leading to significant intraparticle diffusion and heat transfer limitations, which are particularly problematic for fast, highly exothermic reactions like methane combustion. They are also more prone to plugging or fouling when particulates are present in the feed. Monoliths overcome many of these drawbacks by providing straight, non-connected channels that combine a high geometric surface area with a low pressure drop, making them ideal for high-throughput and particulate-tolerant systems. Their geometry offers a predictable flow distribution, good heat and mass transfer (especially in turbulent, high-pressure operation), and excellent mechanical durability under vibration and thermal cycling. However, monoliths offer lower bulk catalyst loading than packed beds and can be limited by the laminar flow transport at atmospheric pressure, requiring careful washcoat optimization to avoid internal diffusion resistances. Foams occupy an intermediate position: their open, interconnected 3D structure yields a higher porosity and lower flow resistance than packed beds, with higher mass and heat transfer rates than monoliths of an equivalent surface area, especially under laminar flow. Their pore geometry can be tailored for performance, and metallic foams, in particular, offer a high thermal conductivity for temperature uniformity. Nonetheless, foams generally have a lower geometric regularity than monoliths, making precise modelling more challenging, and fabrication plus coating processes can be more costly or complex to scale. In practice, the selection between these support types hinges on the required compromise between catalyst loading, pressure loss, transport performance, fouling tolerance, thermal management, and economic factors, with packed beds favoured for the maximum catalyst density, monoliths for low-pressure-drop high-flow applications, and foams for high-intensity heat/mass transfer with a moderate flow resistance.
7. Autogenous Reactor Operation
Although there might be exceptions, it is usually desired to run the catalytic combustion reactor adiabatically, and, once the reaction has been initiated, the reactor should ideally operate in an autogenous manner. The energy provided by the combustion reaction should be sufficient to maintain the reaction, without needing an external energy supply. We distinguish autogenous operation from the broader, more general term, autothermal operation, which includes cases where the energy provided by an exothermic reaction is used to drive an endothermic one. We do not consider the latter case, although some authors use the term autothermal in a broad sense that includes autogenous operation.
7.1. Steady-State Systems with Unidirectional Flow
The first choice for reactor design is usually a single-pass unidirectional flow system, with minimum complications for construction and operation. There are many systems where design simplicity is the overwhelming criterion, and, in this case, the only option may be to select a sufficiently large reactor, with a catalyst of the appropriate activity to achieve the desired combustion efficiency. There are many cases where this method is quite feasible. For example, for the combustion of methane emissions from a natural gas vehicle operating under stoichiometric conditions, the engine exhaust gas temperature is usually sufficiently high to allow for acceptable conversion. The exhaust from a lean-burn natural gas engine has a significantly lower temperature; however, there are currently catalysts that will operate at these conditions, although it remains a challenge.
7.2. Steady-State Systems with Internal Recuperation
On the other hand, there are also many fugitive methane streams that are available at ambient temperatures, in the range of 0–30 °C. Some of these streams also have low concentrations of methane, and, thus, are difficult to combust. For these cases, some form of combined reactor and heat exchanger is needed. The obvious solution for the case of a highly exothermic reaction is to use the thermal energy generated by the combustion to heat the feed. A good overview of the broader concept of autothermal operation, including the combination of endo- and exothermic reactions, can be found in [155,156,157].
Using the hot effluent to heat the feed requires some form of heat exchanger. Conceptually, the simplest and most straightforward method of integrating heat exchange with the reactor is to add a heat exchanger as a separate unit, in which the feed and effluent exchange heat, as shown in Figure 4. If one assumes constant physical properties, and, furthermore, the use of a simple counter-current-flow single-pass heat exchanger, then the heat transferred in the exchanger is given by the following:
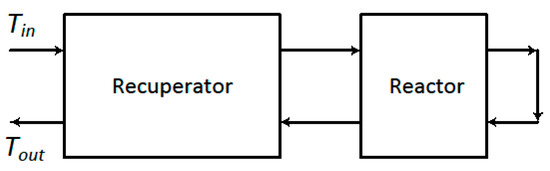
Figure 4.
Simple configuration for a combined reactor and heat exchanger. The arrows indicate the direction of flow.
The temperature driving force is the adiabatic temperature rise for the reaction, assuming 100% conversion. Thus, provided that the overall heat transfer coefficient, U, is known, then the heat exchanger area can be calculated to achieve any desired reactor inlet temperature. This design has an advantage in that one can independently control the heat transfer parameters such as the heat transfer area, and the construction may be easier.
If we move beyond the simple concept shown in Figure 4, there are more integrated systems of the reaction and heat exchange reported in the literature. Although these designs may offer an abundance of subtle variations, in a sense, they are all variations on the theme shown in Figure 5, where the heat transfer area is incorporated along the reactor surface.
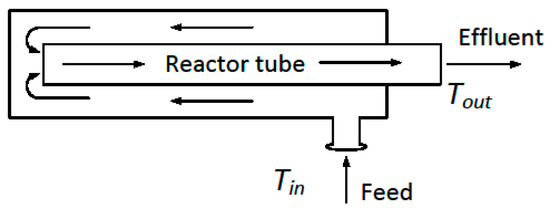
Figure 5.
Simple integrated concept of reactor and heat exchanger. The arrows indicate the direction of flow.
The design shown in Figure 5 is similar in principle to a double-pipe heat exchanger operated in counter-current flow. The cold feed that enters the system is in contact with the hot end of the reactor, and is pre-heated as it flows along the reactor wall. There have been many variations of this integrated theme. For example, there could be multiple reaction and heat transfer layers, as in the small-scale unit proposed for natural gas engines [158], as shown in Figure 6. This design was, in turn, inspired by a design from the literature [159,160]. Other examples of this arrangement have been reported, for example, [161,162]. Another variation on the theme used three parallel channels per unit, with separate reaction and recuperation sections [163].
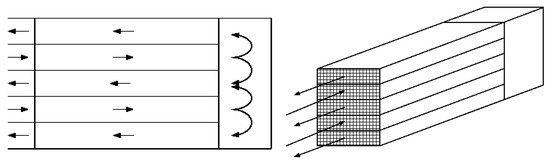
Figure 6.
Multiple channel reactor recuperator, after [158]. The arrows indicate the direction of flow.
In the application shown in Figure 6, the reactor layers were blocks of washcoated monolith, through which the reacting stream flowed. This work used highly conductive silicon carbide monoliths, and showed that efficient conversion could be achieved, with maximum temperatures larger than the adiabatic temperature rise. The concept can be used with the monoliths being replaced by any suitable reactor internal catalyst support.
Another interesting adaptation of heat exchanger technology was the incorporation of a catalytic washcoat on the walls of a double-spiral heat exchanger [164]. A spiral-design heat exchanger was also used recently as part of an integrated unit for the combustion of VAM directly in the coal mine workings, as shown in Figure 7 [165]. The spiral design for heat exchangers is well-known, and has many advantages over other heat exchanger configurations. They are generally space-efficient and offer a high heat transfer area per unit volume. The spiral design also induces turbulence which, although it increases pressure drop, also enhances the heat transfer rate. The smooth spiral shape also helps to avoid dead zones, and also sharp changes in the flow direction, thus reducing erosion–corrosion. They may, however, be more complex to construct, which can increase the cost.
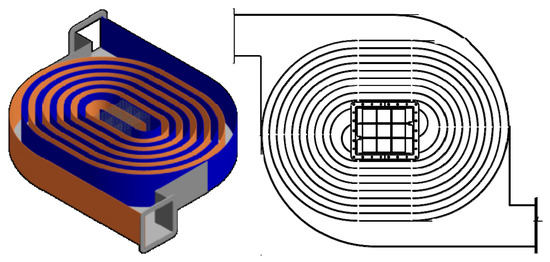
Figure 7.
An integrated reactor/recuperator system. The central catalyst section is surrounded by a spiral-type heat exchanger [165].
In the design shown in Figure 7, the catalyst was contained in a cartridge in the centre of the system. The catalyst was a series of washcoated monoliths with a state-of-the-art methane combustion catalyst. High central temperatures were achieved, which resulted in complete conversion at high values of the space velocity. Complete details are found in [165].
7.3. The Catalytic Flow-Reversal Reactor
Catalytic reactors are commonly operated at steady state, where the temperatures and concentrations are constant at any spatial position as a function of time. The advantages of running at steady state include ease of control and simplicity of operation. However, there are cases where unsteady operation is inevitable, and, over the past decades, some advantages of imposing a transient behaviour have been observed. Changes in the reaction selectivity and conversion can result from the dynamics of the adsorbed surface concentrations of reactive intermediates. There are many publications about forcing unsteady flow with a unidirectional flow; however, a reactor type of significant interest is called the catalytic flow-reversal reactor (CFRR). In this case, the feed is periodically switched between either end of the reactor using the appropriate valves, as illustrated in Figure 8.
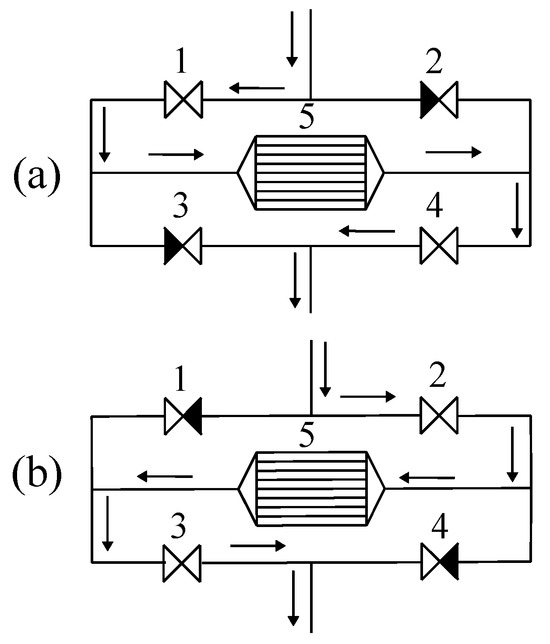
Figure 8.
Illustration of the reverse-flow reactor concept. The arrows indicate the direction of flow. Pattern (a) corresponds to forward flow and (b) corresponds to reverse flow.
We refer to Figure 8a and follow the path of the process stream. Valves 1 and 4 are open, whilst valves 2 and 3 are closed. The feed flows to the reactor from left to right (which we can call forward flow). After some time, valves 1 and 4 are closed, and valves 2 and 3 are opened, and the flow then traverses the reactor from right to left, as shown in Figure 8b. A complete cycle consists of these two operations. The elapsed time for each half-cycle is referred to as the switch time. If the forward- and reverse-flow times are the same, the operation is called symmetric reverse flow, and, if the times are different, the operating mode is called asymmetric operation. The sum of the times for forward and reverse flow is the cycle duration.
The operating principle for the CFRR can be understood by considering some temperature profiles during operation. For an exothermic reaction, the CFRR has a behaviour referred to as a heat sink [166] and a heat trap [167], which allows for an elevated temperature within the system. The principle of the heat trap effect is illustrated in Figure 9.
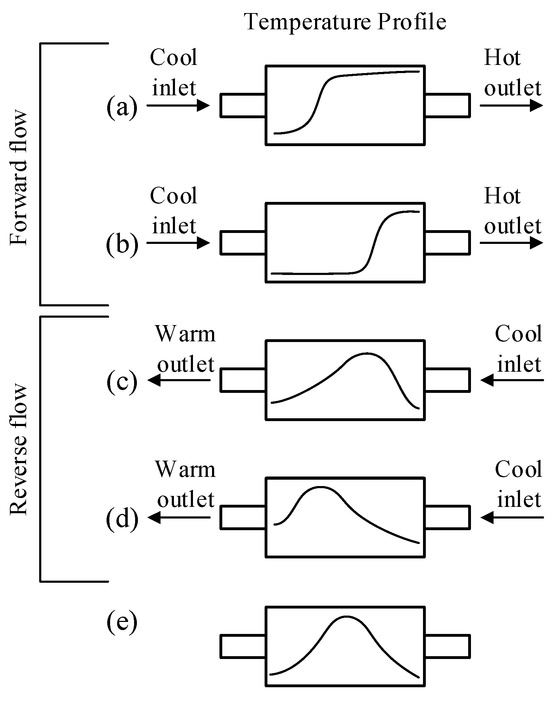
Figure 9.
Illustration of the heat trap effect for reverse-flow operation. (a) shows a possible temperature profile for unidirectional flow. (b) shows the progression of the profile towards the reactor exit. (c) shows how switching the flow prevents the hot zone from leaving the reactor, and then (d) shows the reverse progression of the temperature profile. Finally, (e) shows a possible stationary state profile that may finally be achieved.
Consider first the axial temperature profile that might be obtained in a tubular reactor with an exothermic reaction. If the reactor feed temperature is sufficient to allow for the reaction to start, then, at some point, in the reactor, the temperature will rise rapidly as the reaction rate increases exponentially, and then becomes constant as the reactants are consumed and the reaction rate decreases. Such a profile is shown in Figure 9a. Once initiated, the reactor will operate at steady state with a constant profile. However, if the feed temperature were, for example, lowered, then the reaction conditions would be insufficient to maintain autothermal operation, and the steep reaction front would progress towards the reactor exit and the reaction would extinguish. The principle behind the CFRR concept is to reverse the flow before the reaction front can leave the reactor, and the incoming cold feed now encounters a hot catalyst. The energy stored in the hot catalyst bed heats the incoming feed, and, thus, the reaction is allowed to continue, and the reaction front now moves in the opposite direction. The feed is switched back to the other end before the reaction front can exit. Provided that the reactor is initially at a sufficiently high temperature (which may require some auxiliary heat source) and the cycle duration is carefully chosen, it is possible to achieve autothermal reactor operation at feed temperatures well below those required for autothermal operation with unidirectional flow. A quasi-steady-state operation may be achieved in which the reactor temperature profile has a maximum value near the centre of the reactor, which slowly oscillates as the feed is switched between the two ends of the reactor, as shown in Figure 9c–e. Because this stored energy is added to the feed stream, it is possible to achieve temperatures higher than the adiabatic temperature rise based on the fresh feed inlet temperature. A reverse-flow operation in which the solid-phase temperature rise was as high as 13 times the adiabatic temperature rise has been reported [168].
The concept of reverse-flow operation (RFO) originated with its use in packed beds of solids [169,170]. The extension to catalytic reactors (CFRRs) was discussed by Frank-Kamenetski [171]. RFO has been applied to many processes [156]. An early commercial implementation was the oxidation of SO2 [172,173,174], and the technology has since been adapted for methanol synthesis [175,176] and NOX reduction [177,178,179,180]. Periodic flow reversal in packed bed reactors has also been shown to achieve high conversions in the catalytic decontamination of waste gases [181], with 99.5% conversion being reported. In that work, a central heat exchanger was incorporated to remove the excess heat, preventing catalyst deactivation or reactor damage, and allowing the recovered heat to be used for building heating or to drive turbines. The management of heat removal in reverse-flow reactors is further discussed in [166,181,182]. Mass extraction from the central region of the reactor as a means to withdraw energy and control temperature has also been studied [183].
The “heat trap” effect, which leads to elevated temperatures at the reactor centre, makes the process particularly suitable for the catalytic combustion of lean hydrocarbon mixtures at standard conditions. Numerous studies have explored CFRRs for catalytic combustion [181,182,184,185,186,187,188]. Many of these applications target large volumetric flow rates and employ relatively long cycle times. The technology is well-established for the combustion of VAM in underground coal mining, with large-scale units being used in, for example, China [189]. These large-scale units used for coal mines are typically located on the surface. There has been interest in small-scale units that can be used in situ, or for other applications in, for example, the oil and gas industries [183,190,191,192,193].
At a much smaller scale than these mentioned reactors, the CFRR has been used for lean methane combustion in automotive applications. The use of reverse-flow catalytic converters for natural gas and dual-fuel engines has been reported [167,194,195,196,197,198].
The CFRR often uses inert sections to allow for additional storage, and many configurations are possible [192]. Catalysts that have been reported for the catalytic combustion of methane include both fixed beds of particles [132] and monoliths [195,196,197,198]. The choice of catalyst material depends on size constraints on the unit, catalyst activity and cost, and operating variables such as the maximum allowable pressure drop.
Computational modelling can provide much needed insight into the reactor design and operation. Some basic discussion of the mathematics of the CFRR can be found in [156], and typical modelling studies can be found in [177,190,191,193,199,200]. The control of a CFRR can also be complicated, and various strategies have been proposed [201,202,203,204].
7.4. Counter-Diffusion Reactors
We have noted the challenge of achieving autothermal operation for the low-temperature feed. In the case where a fuel-rich stream is used, an interesting reactor that has seen considerable use in industry is the counter-diffusive reactor. These reactors have been used for radiant heating applications for many years [205,206]. The cross-section of a typical unit is shown in Figure 10.
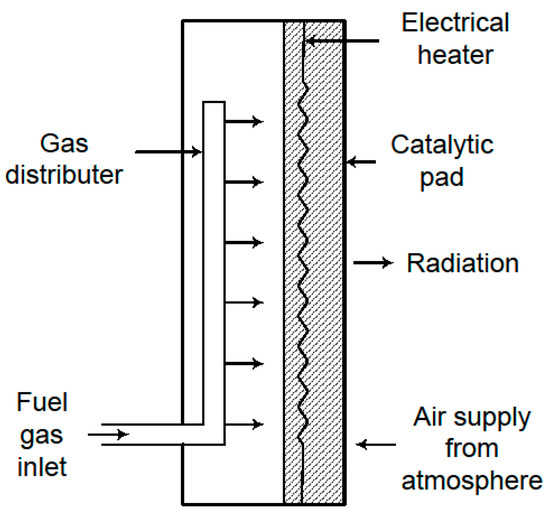
Figure 10.
Schematic of the cross-section of a counter-diffusion catalytic reactor. The arrows indicate the direction of the flow.
The reactor inlet is located at the back of the unit. Pure fuel is admitted to a plenum chamber through a fuel distributor, and is usually controlled through the use of flow and pressure regulators. The fuel then flows by convection through, first, a porous insulation blanket, and then a porous pad containing the catalyst. The porous insulation blanket reduces energy loss through the back. The catalyst pad is positioned immediately after the insulation blanket. This pad is composed of a fibrous, porous structure that incorporates the active catalytic material. Platinum is the most common catalyst used in commercial systems [207,208], though alternatives such as palladium [209] and various non-noble metals have also been documented [210]. To secure the catalyst pad, a wire mesh is typically placed at its front face. An electric heating element is generally installed between the insulation blanket and the catalyst pad; its function is to elevate the pad’s temperature to a level sufficient to trigger the combustion reaction. Once ignition occurs, the heater is turned off and the reactor operates in an auto-thermal mode.
Oxygen necessary for combustion is supplied by diffusion from the surrounding air. It must first traverse the natural convection boundary layer at the pad’s surface before diffusing into the pad itself, moving counter to the direction of the fuel flow. The principal mode of heat transfer from the reactor is via radiation emitted from the front of the catalyst pad. Additional, though lesser, heat losses occur through convection from the heater’s body and by the bulk gas flow through the system. Key experimental and modelling studies on these reactors are discussed in the following paragraphs.
The early experimental and modelling work was reported by [207,208], whose work was based on a Pt catalyst in the fibre pad. They studied the effect of the methane flow rate on the temperature distribution across and through the catalyst pad. As the methane flow rate increased, the maximum temperature moved toward the front of the pad and the combustion efficiency decreased. Their model predictions were compared to the experimental results and some agreement was obtained. The model predicted that the maximum temperature moved closer to the front of the pad as the flow rate increased. Moreover, as the flow rate increased, a higher fuel slippage was observed; that is, the combustion efficiency decreased and more methane was emitted by the system.
A counter-flow reactor model for methane combustion was proposed [211] and the modelling data were compared with the experimental results from [205] and showed good agreement. The study also reported that the combustion takes place in a thin layer near the front surface of the catalyst panel and that the oxygen diffusion into the catalyst panel is the controlling factor in the conversion efficiency.
These older observations were confirmed in a detailed experimental and modelling study of the counter-diffusive-flow reactor applied to the combustion of methane streams that contained significant amounts of impurities, such as higher hydrocarbons, water, and BTEX compounds (benzene, toluene, ethylbenzene, and xylene) [9,212]. Those investigations confirmed that the diffusion of oxygen from the surrounding air was the rate-limiting step that controlled methane conversion. Fuel slippage was dramatically reduced by enhancing the rate of mass transfer to the front surface using enhanced convection. BTEX and other hydrocarbons were preferentially converted compared to methane, and no slippage of these molecules was observed. Provided that high temperatures could be attained, no effects of large amounts of water were observed.
Radiant heaters are widely used in industry simply for their heating function, but have been increasingly used as emission control devices. They are ideally suited to situations where there are relatively low flow rate streams of the fuel-rich feed. The devices are easily portable and can be started using batteries, for example.
8. Mathematical Modelling of Catalytic Combustion Systems
8.1. Modelling Overview
The early development work in combustion systems was performed essentially on a trial-and-error basis, but the use of computational models became more prevalent as time went on. The advent and increasing availability of low-cost and powerful computational tools have allowed for more fundamental designs. In the context of the advanced computational modelling of catalytic combustion system, we discuss models that are based on the solution of the conservation equations of momentum, mass, and energy, which are represented as partial differential equations (PDEs). Today, there exist a plethora of commercial software products that can be used to solve PDE, which, in many cases, are very user-friendly.
Modelling any system is always a compromise between the computational complexity and desired model accuracy. In this section, we provide some general overview and thoughts about the future directions. We consider the variations on the fixed bed models with packed beds, monoliths, and foams as the internal structure.
8.2. Continuum Reactor Model
The models for fixed bed reactors can be divided into a number of categories. The first broad characterization is between discreet and continuum models. All of the fixed beds discussed here can be considered as a form of a porous medium, with a solid fraction and a void fraction. For the monoliths and foam varieties, the solid phase consists of the washcoat and the underlying support material. A fully discreet model for the reactor requires the solution of the governing PDE for each of the fluid and solid phases, which requires that the PDE be discretized over the entire domain. The resulting problem is computationally prohibitive, and the reactor is usually represented using a continuum model, where the domain is assumed to be a porous medium, and the conservation equations are written using a volume-averaging approach. The use of volume averaging inherent in such a model results in the loss of all spatial resolution at the pore or channel level. With such a model, it is not possible to identify processes that occur at the small-scale level.
A continuum model for a fixed bed system can be further divided into sub-categories. The first division is between the pseudo-homogeneous model and the heterogeneous model. In the former, there is no distinction made between the solid and fluid phases, and a single set of volume-averaged equations is written for the reactor. In effect, this model states that the concentration and temperature at the surface of the solid is equal to the values for the fluid. This assumption results in a simpler model, but does not account for the heat and mass transfer resistance between the fluid and the solid. On the other hand, the heterogeneous model considers each of the fluid and solid phases separately, and uses conservation equations for each phase. The two phases are coupled by heat and mass transfer coefficients.
The continuum model in either form can be modelled in one, two, or three space dimensions. A three-dimensional model includes all three space dimensions and is required for the highest level of accuracy if there is no symmetry in the system. Many reactors are cylindrical in shape, and, in this case, axial symmetry may allow for the reduction the model to two space dimensions using cylindrical coordinates. Often the radial gradients are assumed to be negligible, and the model is reduced to a single space dimension that considers only the axial direction of flow. Needless to say, the reduction to a single space dimension may result in serious inaccuracy if the radial gradients are significant. Details of these various models and the necessary conservation equations for them can be found in a variety of sources [23,129,130,131].
8.3. Discreet Reactor Models
As mentioned, a fully discreet model for the reactor requires the solution of the governing PDE for each of the fluid and solid phases, which means that the PDE be discretized over the entire domain. The resulting problem is computationally very costly, and, thus, is rarely attempted for structures such as large-scale packed particle beds or foams, except for small portions of the domain [213,214,215]. Much of this work is based on building artificial packed beds using, for example, Discreet Element Methods (DEMs). There have been some notable works reported that use fully discreet porous medium models for the real structure. These works often use a 3D map of the real geometry that can be obtained by X-ray tomography. Some notable examples for porous media combustion (PMC) can be found in [216,217]. The computational cost can be reduced by using parallel computing, which is especially suitable for computing methodologies such as the lattice Boltzmann method [217].
For a monolith, the situation is easier because of the structure built of many parallel channels. The choice can be made to model a single channel of the system, called a single-channel model (SCM). The ability to model a single channel of the reactor structure allows for the elucidation of many of the underlying phenomena in the monolith.
Monolith channels are rarely, if ever, circular in cross-section, and, thus, a three-dimensional model is required for accuracy. However, for computational cost reasons, the SCM is usually reduced to either two dimensions or one dimension. Two-dimensional and three-dimensional models use the same conservation equations for the momentum, mass, and energy. The solution domain includes the fluid-filled channel, the washcoat, and the wall. The 1D model ignores radial gradients in both the temperature and concentration and uses their mixing cup averages, giving a 1D model for the fluid. A flat velocity profile is assumed. At the washcoat interface, the discontinuity is accounted for using heat and mass transfer coefficients.
Good discussions of the many variations on the SCM can be found in, for example, [218,219,220,221,222,223,224], and a comprehensive review over the period of 1990 to 2020 provides details of the necessary modelling strategies, and the associated parameters required [225].
8.4. The Multi-Scale Problem and Model Reduction
If we consider the fixed bed reactor, we have regarded the primary internal as a packed bed of catalyst particles, a monolith block containing hundreds or thousands of parallel channels, or as a regular or random foam structure. For the monolith- and foam-type structures, the active catalyst is contained within a thin washcoat of catalytically active material. Modelling such a reactor is an exercise in multi-scale modelling. At the level of the catalyst pores, the length scale is of the order of nanometres. For particles of the porous catalyst, the typical size is around 6 mm, whilst, for monoliths and foams, the washcoat thickness is on the scale of microns. The internal void spaces in the packed bed, the monolith channels, and the void volume of the foam also have dimensions of mm. The full-scale reactors have scales of many centimetres to metres. Ideally, a model should capture accurately all of the physics and chemistry that occur at each level. Each spatial scale typically requires the solution of a set of governing PDEs for the conservation equations. If we consider the spatial scales, a model of high granularity would require the complete domain discretization down to the smallest scale, which obviously would result in a very large problem requiring vast computational resources, not to mention challenges in mesh generation. Therefore, model reduction strategies are used, with the various scales being incorporated using sub-models (also called scale bridges). Considering that all models contain error in some form, whilst, at the same time, desiring simplicity, models and scale bridges should be selected with due diligence. The use of scale-bridging in monolith reactor models has been discussed [226].
Some of the earliest efforts in model reduction involved simplifications for the reaction chemistry where detailed mechanisms were considered. Traditionally, the chemistry of each fluid element in a CFD simulation requires the direct integration of reaction rate equations. One way to save time is to compute the rate data for the given conditions and store them in a table, which can be accessed during program execution. The use of pre-computed rate data for complex reacting flows has been used for gas-phase kinetics [227,228,229]. The use of pre-computed rate data for catalytic combustion has been discussed [230]. The method was used to model the catalytic combustion of methane in a monolith reactor [226], where the use of pre-computed data was extended to scale bridges for washcoat diffusion effects in a systematic hierarchical model scale reduction exercise.
Although the use of pre-computed and tabulated data is a powerful model reduction strategy, it does have some limitations in the number of variables that can be modelled. The pre-computed data table also contains large quantities of data that are not needed during the simulation. An alternative to pre-tabulation is “on-the-fly” tabulation, known formally as In Situ Adaptive Tabulation (ISAT), which was presented in a foundational paper by Pope [231], and has been used for model reduction in monolith modelling [232]. In the ISAT algorithm, rate data are only computed and stored as required. When a chemical state is first encountered, the overall rates are computed by solving the associated differential equations directly. The compositions before and after the reaction step are recorded. When a new state arises, the ISAT table is queried. If the state exists in the table, its result is quickly retrieved. If the state is new, then the full calculation is made and the result is stored as before. By only storing the required data, the amount of storage needed is vastly reduced. ISAT has been implemented in some commercial CFD solvers.
In addition to ISAT, there have been other methods proposed for the “on-the-fly” modelling of complex reaction systems. The method of intrinsic low-dimensional manifolds (ILDMs) has been widely used as a reduction tool for detailed reaction schemes. The method has been applied to a methane and air mixture over a platinum catalyst, with both detailed heterogeneous and homogeneous kinetics, as well as a transport model [233]. Finally, we mention the computational singular perturbation (CSP) method. This method has recently been applied to a typical problem in heterogeneous catalysis [234].
9. Future Directions in Catalytic Combustion
The current state of the art in catalytic combustion is such that it is a viable option for the treatment of many fugitive methane emission streams. As with any technology, there is a potential for continuous improvement. Some extremely topical areas of research are related to the areas of artificial intelligence (AI), machine learning (ML), and advanced manufacturing (AM). In the final section of this review, we present a brief discussion of some of the potential uses for these emerging technologies for catalytic combustion.
9.1. Future Directions for Advanced Modelling
Section 8 has discussed what can be referred to as conventional reactor modelling. These models rely on the explicit formulation of physical and chemical laws expressed as conservation equations for mass, momentum, and energy. The basic PDEs are coupled with detailed kinetic models and empirical correlations for heat and mass transfer. Numerical solutions are made using finite difference, finite volume, or finite element methods. Depending on the underlying assumptions, these models provide spatially and temporally resolved predictions of reactor temperature, composition, and conversion. There are, however, still many potential problems with these conventional tools. One of the largest problems is the computational cost. High-fidelity multidimensional simulations are computationally intensive, especially for large reactors, transient processes, or detailed kinetic schemes with dozens of species and reactions. Adding to the potential computational cost is the multi-scale nature of the problem, which requires the use of scale bridges. Scale bridges are also required when the dimensionality of the problem is reduced, and the parameters and form of these scale-bridging sub-models may be not well-understood. The highly non-linear nature of many reactor simulations can also make the calculations unstable and provide difficulties in convergence.
A current trend in science and engineering is to use machine learning (ML) and artificial intelligence (AI) tools to construct models. While these techniques are not new, they are becoming increasingly popular as they become easy to use. The application of ML and AI to the modelling of catalytic reactors represents one of the most exciting frontiers in both reaction engineering and computational science. The traditional PDE-based modelling methods will continue to be foundational for understanding the reactor behaviour, ML and AI offer transformative capabilities that complement and, in some cases, transcend conventional techniques. These techniques can be used to model the complete reactor, or to build efficient sub-models for some of the small-scale heat and mass transfer phenomena, with the same purpose as the look-up tables mentioned earlier.
To do justice to this emerging field requires a review paper in itself; however, there are several recent articles that provide a good starting point. We cite, for example, four papers that focus on ML and neural networks, with reference to classical CFD modelling approaches, which give a good overview of this challenging field [235,236,237,238]. Some more specific examples of ML applied to reactors can be found in [239,240,241,242].
Accurate reactor modelling requires precise measurements of working reactor parameters that also serve to improve, test, and validate the mathematical models for a better process design. A comprehensive understanding of the spatiotemporal gradients in heterogeneous catalytic reactors by the in situ monitoring of the physical parameters and processes occurring on the catalyst surface is crucial for the rational design of advanced and more sustainable catalytic processes. Heterogeneities arise at multiple length scales, from whole reactors and catalyst bodies down to grains, active sites, and nanoparticles, leading to complex space- and time-dependent phenomena. This has been emphasized in the review [243], where in situ reactor monitoring can be leveraged by recent advances in space- and time-resolved spectroscopic methods, such as magnetic resonance, optical, and synchrotron-based techniques. These methods now allow 1D and 2D imaging, 3D depth profiling, and even time-resolved or single-molecule detection under reaction conditions. The integration of such sophisticated diagnostics will yield detailed spatial and temporal data essential for the accurate modelling of catalytic combustion reactors, supporting the development of more effective correlative approaches and, ultimately, facilitating the design of improved combustion systems. Extensive in situ data measurement and recording can result in copious amounts of data. AI and ML can both be employed to advantage to develop improved models for reactors.
Another technique of increasing interest is the measurement of the so-called thermoscalars, the local temperature and species concentrations at or near the catalyst surface. These local values can then be used as an aid in developing more accurate computational models. A recent review of this concept, in the context of catalytic combustion applications, has recently been published [244].
9.2. Advanced Manufacturing in Catalytic Combustion
Advances in the design and construction of catalytic reactors in general, and catalytic combustion systems in particular, have led to improvements in such features as the pressure drop, conversion, and reactor longevity, including prolonged structural integrity. As reviewed in this article, the development of support systems such as metal and ceramic monoliths, structured foams, and metallic gauzes has made significant contributions. These new systems, whilst they can be considered to be transformative in their own right, have occurred mostly at the macro-scale level. At the micro- or nano-scale level, progress has been slower, especially when compared to the microelectronics industry, which achieves flawless, reproducible architectures at the nanometre scale. On the other hand, catalyst and support production has struggled with variability, the incomplete control of the active site distribution, and batch-to-batch inconsistencies. This gap poses a major barrier to the next generation of clean combustion systems.
The advent of advanced manufacturing (AM) technologies, which include additive manufacturing (3D printing), atomic layer deposition (ALD), direct ink writing (DIW), digital light processing (DLP), selective laser sintering (SLS), and templated nanostructuring, marks a step-change in the design, optimization, and scalability of catalytic reactors. These techniques can play a role in the evolution of catalytic combustion systems, addressing the long-standing performance, scalability, and economic challenges while enabling new reactor designs that were previously impractical or cost-prohibitive. There are several issues to be addressed when considering the direct application of AM for catalytic converter design and manufacturing for methane combustion. We briefly discuss some of these in the following.
Conventional manufacturing methods for catalyst supports have limited flexibility and microstructural control. By contrast, AM methods can realize complex architectures unachievable by traditional means, including hierarchical porosity, optimized channel geometries, and graded structures for tailored heat and mass transfer. Recent studies have underscored the potential of 3D-printed ceramic monoliths that overcome classic limitations such as interlayer adhesion, shrinkage, and pore uniformity, paving the way for robust supports able to withstand the rigors of hydrothermal conditions and thermal cycling in catalytic combustion [245,246]. For example, the fabrication of dual-cell-density monoliths via DLP printing demonstrates how AM can deliver reactor architectures that optimize the pressure drop and active surface area simultaneously [247].
In addition to producing custom-tailored support structures, additive manufacturing enables the direct and spatially resolved incorporation of the catalytic phase. Leading-edge deposition techniques, such as ALD, inkjet printing, or electrospinning, can be used to overcome some of the limitations of traditional washcoating, which include non-uniform distribution, limited penetration, or wasted precious metals. The results include nanometre-thick catalyst layers with exceptional dispersion, finely controlled composition, and the improved accessibility of active sites. Multi-material printing can embed the catalyst, supports, and sensors/heating elements into unified structures. The prospect of multi-material printing brings closer the creation of reactors where the catalyst, support, insulation, and instrumentation are co-fabricated—this is a major step forward for both laboratory prototyping and field deployment. An example is the application of material extrusion as a 3D-printing technique to obtain highly porous silica–metal structures with a well-preserved geometry and catalytic activity [248].
A final example of note is the work reported in [249]. They used 3D metal printing to print metallic monoliths in a variety of cell shapes and sizes. They then used electrochemical deposition to place the active catalyst on the surface in a controlled way. As mentioned in the previous paragraph, this method overcomes many of the drawbacks of traditional washcoating, and is expected to play an increasing role in the future, for all types of structured supports, including foams and monoliths.
The digital-to-hardware workflow that is offered by AM allows for the much faster prototyping and development of both reactor components and complete reactor systems. Innovative geometries, multi-stage units, and integrated heat exchanger designs are feasible without costly tooling. Rapid iteration and testing help optimize the performance for different methane compositions, flow regimes, or operational temperatures, speeding up hardware innovation.
A significant advantage of AM techniques is the possibility of the straightforward manufacture of geometries that were previously impractical to make. These include microchannel or multi-zone designs, functionally graded porosity, or interleaved zones for staged catalytic and thermal operations. Reactor supports can be tuned for local variations in reactant composition or heat management, supporting a higher conversion efficiency and the native integration of sensing for real-time adaptive control, valuable for applications such as portable remediation units or unsteady emission sources.
In addition to the advances offered in novel reactor design, construction, and prototyping, AM also provides opportunities for cost savings and a reduction in the environmental impact. By optimizing the structure, catalyst placement, and scale-up procedures, there is a huge potential for waste reduction, including precious metals. Digital design and direct manufacturing simplify the move from prototype to production, while the ability to design for end-of-life disassembly and recycling contributes toward circularity and improved sustainability.
Many challenges remain in the application of AM-fabricated supports, including their use in harsh environments, integration of multi-material architectures, and long-term mechanical reliability. As these methods continue to mature, catalytic reactor technology, including catalytic combustion, stands poised to emulate the precision and reliability that microelectronics brought to information technology.
9.3. Challenges in Catalyst Development
There are a number of challenges remaining in the development of catalysts for lean methane emissions, especially for streams with a low methane concentration. Catalyst deactivation, especially under wet, low-temperature conditions, remains a challenge, as does sintering and the effect of contaminants in real emission streams. The optimization of washcoat formulations, support structures, and coating techniques will contribute to achieving a long-term, stable performance in the highly varied and sometimes harsh environments typical of methane abatement applications. This is especially true for the PGM-based catalysts, but is also a challenge for high-temperature applications.
The persistent challenge is the trade-off between cost and performance. It is expected that catalyst development will focus on minimizing the use of expensive ingredients and optimizing manufacturing techniques, incorporating advanced manufacturing techniques, such as additive manufacturing and atomic layer deposition, to permit optimized architectures and targeted placement within reactor supports. The use of advanced coating techniques should also play a role.
Over the past years, there has been a lot of interest in the use of nanotechnology to engineer very specific catalyst structures, such as core- and shell-type catalysts. It is expected that this technology will play a role in future development, although it remains to be seen how durable such structures remain at high operating temperatures over extended periods of time.
10. Summary
Catalytic combustion has demonstrated itself as a core technology for the mitigation of fugitive methane emissions produced throughout the fossil fuel supply chain. The advances reviewed in this paper reflect the substantial progress in catalyst development, reactor engineering, and the integration of catalytic combustion into practical abatement systems. Precious metal catalysts, particularly palladium- and platinum-based systems, continue to deliver the highest activity and lowest operation temperatures, making them central in applications where stringent methane destruction is required, including lean and dilute streams. Meanwhile, increased research into transition metal oxides, hexa-aluminates, and perovskite materials is opening new pathways toward cost-effective and thermally stable alternatives, which may become especially significant as large-scale deployment and long service lifetimes gain priority.
Reactor design has evolved from conventional packed beds toward structured supports such as monoliths and foams, that optimize the trade-offs between the pressure drop, catalyst accessibility, and resistance to fouling. Metallic and ceramic foams, in particular, have emerged as promising materials, combining a high specific surface area and excellent transport properties. These substrates allow for intense gas–solid interaction and shorter reactor lengths while maintaining mechanical robustness and scalability. Moreover, integrated heat exchange systems, such as recuperative and flow-reversal reactors, have improved energy recovery and enabled autogenous operation under challenging process conditions.
Some technical and economic barriers remain. In addition to the catalyst issues, mentioned earlier, the variable operating conditions also pose challenges. Methane abatement systems must be able to reliably operate across a wide range of flow rates, temperatures, and methane concentrations. As such, advances in reactor control strategies, process integration (e.g., with waste heat recovery and gas purification), and robust process modelling will be central to ensuring that catalytic solutions are both effective and cost-efficient. The incorporation of advanced manufacturing techniques, such as additive manufacturing and atomic layer deposition, promises further gains through the fabrication of supports with precisely tailored microstructures and the direct deposition of active phases.
On the computational modelling front, the continued refinement of multi-scale models and the integration of data-driven, machine-learning approaches with established reactor theories are enabling a more accurate reactor design, predictive maintenance, and operational optimization across a diverse set of operating scenarios. These tools, alongside robust experimental validation, with extensive in situ reactor monitoring, should enable technological advances.
In conclusion, catalytic combustion technologies, when coupled with the rigorous system integration and ongoing innovation in catalyst and reactor design, should play a major role in methane emission mitigation strategies. Realizing the full potential of these systems will require a sustained interdisciplinary collaboration spanning catalysis, chemical engineering, manufacturing, and systems integration. These collective efforts will aid the deployment of catalytic combustion as a robust, efficient, and scalable solution for methane mitigation.
Author Contributions
Writing—original draft preparation, R.E.H. and J.P.-P.; writing—review and editing, R.J. and J.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created in the production of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moore, S.; Freund, P.; Riemer, P.; Smith, A. Abatement of Methane Emissions; IEQ Greenhouse Gas R&D Programme: Cheltenham, NSW, Australia, 1998; ISBN 1898373167. [Google Scholar]
- Danny Harvey, L.D. A guide to global warming potentials (GWPs). Energy Policy 1993, 21, 24–34. [Google Scholar] [CrossRef]
- O’Connor, F.M. Why Methane Matters. Front. Sci. 2024, 29, 1462198. [Google Scholar] [CrossRef]
- Mokhatab, S.; Poe, W.A.; Speight, J.G. Handbook of Natural Gas Transmission and Processing, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- IEA. Global Methane Tracker 2024; IEA: Paris, France, 2024; Licence: CC BY 4.0; Available online: https://www.iea.org/reports/global-methane-tracker-2024 (accessed on 16 September 2025).
- Hayes, R.E. Catalytic solutions for fugitive methane emissions in the oil and gas sector. Chem. Eng. Sci. 2004, 59, 4073–4080. [Google Scholar] [CrossRef]
- International Energy Agency. Global Gas Security Review 2024; IEA: Paris, France, 2024. [Google Scholar]
- Litto, R.; Liu, B.; Hayes, R.E. Capturing fugitive methane emissions in natural gas compressor buildings. J. Environ. Manag. 2007, 84, 347–361. [Google Scholar] [CrossRef]
- Jodeiri, N.; Wu, L.; Mmbaga, J.P.; Hayes, R.E.; Wanke, S.E. Catalytic Combustion of VOC in a Counter-diffusive Reactor. Catal. Today 2010, 155, 147–153. [Google Scholar] [CrossRef]
- Karacan, C.Ö.; Field, R.A.; Olczak, M.; Kasprzak, M.; Ruiz, F.A.; Schwietzke, S. Mitigating climate change by abating coal mine methane: A critical review of status and opportunities. Int. J. Coal Geol. 2024, 295, 104623. [Google Scholar] [CrossRef]
- Schatzel, S.J.; Krog, R.B.; Dougherty, H. Methane emissions and airflow patterns on a longwall face: Potential influences from longwall gob permeability distributions on a bleederless longwall panel. Trans. Soc. Min. Metall. Explor. 2017, 342, 51–61. [Google Scholar] [CrossRef][Green Version]
- Swolkien, J.; Fix, A.; Gałkowski, M. Factors influencing the temporal variability of atmospheric methane emissions from Upper Silesian coal mines. Atmos. Chem. Phys. 2022, 22, 16031–16052. [Google Scholar] [CrossRef]
- Sakai, T.; Chol, B.C.; Osuga, R.; Ko, Y. Purification Characteristics of Catalytic Converters for Natural Gas Fuelled Automotive Engine 912599; SAE: Warrendale, PA, USA, 1991. [Google Scholar]
- Weaver, C.S. Natural Gas Vehicles-A Review of the State of the Art 892133; SAE: Warrendale, PA, USA, 1989. [Google Scholar]
- Gambino, M.; Cericola, R.; Corbo, P.; Iannaccone, S. Carbonyl compounds and PAH emissions from CNG heavy-duty engine. ASME J. Eng. Gas. Turbines Power 1993, 115, 747–749. [Google Scholar] [CrossRef]
- Corbo, P.; Gambino, M.; Iannaccone, S.; Unich, A. Comparison Between Lean-Burn and Stoichiometric Technologies for CNG Heavy-Duty Engines 950057; SAE: Warrendale, PA, USA, 1995. [Google Scholar]
- Edwards, M.R.; Giang, A.; Macey, G.P.; Magavi, Z.; Nicholas, D.; Ackley, R.; Schulman, A. Repair Failures Call for New Policies to Tackle Leaky Natural Gas Distribution Systems. Environ. Sci. Technol. 2021, 55, 6561–6570. [Google Scholar] [CrossRef]
- Alvarez, R.A.; Zavala-Araiza, D.; Lyon, D.R.; Allen, D.T.; Barkley, Z.R.; Brandt, A.R.; Davis, K.J.; Hendon, S.C.; Jacob, D.J.; Hamburg, S.P. Assessment of methane emissions from the U.S. oil and gas supply chain. Science 2018, 361, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Hristov, N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Montes, F.; Meinen, R.; Dell, C.; Rotz, A.; Hristov, A.N.; Oh, J.; Waghorn, G.; Gerber, P.J.; Henderson, B.; Makkar, H.P.S.; et al. SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: II. A review of manure management mitigation options. J. Anim. Sci. 2013, 91, 5070–5094. [Google Scholar] [CrossRef]
- Hristov, N.; Ott, T.; Tricarico, J.M.; Rotz, A.; Waghorn, G.; Adesogan, A.T.; Dijkstra, J.; Montes, F.; Oh, J.; Kebreab, E.; et al. SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: III. A review of animal management mitigation options. J. Anim. Sci. 2013, 91, 5095–5113. [Google Scholar] [CrossRef]
- Crowley, S.B.; Purfield, D.C.; Conroy, S.B.; Kelly, D.N.; Evans, R.D.; Ryan, C.V.; Berry, D.P. Associations between a range of enteric methane emission traits and performance traits in indoor-fed growing cattle. J. Anim. Sci. 2024, 102, skae346. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. Introduction to Catalytic Combustion; Routledge: London, UK, 1998. [Google Scholar]
- Kesselring, J.P. Catalytic Combustion. In Advanced Combustion Methods; Weinberg, J.F., Ed.; Academic Press: London, UK, 1986; pp. 237–275. [Google Scholar]
- Cottilard, S.A. (Ed.) Catalytic Combustion; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Pfefferle, L.D.; Pfefferle, W.C. Catalysis in Combustion. Catal. Rev. Sci. Eng. 1987, 29, 219–267. [Google Scholar] [CrossRef]
- Davy, H. Some new experiments and observations on the combustion of gaseous mixtures. In The Collected Works of Sir Humphrey Davy; Davy, J., Ed.; Elder and Co., Cornhill: London, UK, 1839; Volume 6. [Google Scholar]
- Kesselring, J.P.; Brown, R.A.; Schreiber, R.J.; Moyer, C.B. Catalytic Oxidation of Fuels for NOX Control from Area Sources; Environmental Protection Technology Series; EPA: Washington, DC, USA, 1976; EPA-600/2-76-037. [Google Scholar]
- Emonts, B. Catalytic radiant burner for stationary and mobile applications. Catal. Today 1999, 47, 407–414. [Google Scholar] [CrossRef]
- Specchia, S.; Toniato, G. Natural Gas Combustion Catalysts for Environmental-Friendly Domestic Burners. Catal. Today 2009, 147, S99–S106. [Google Scholar] [CrossRef]
- Ergen, T.; Tunçer, O.; Baytas, A.C. Numerical Investigation of a 5 kW Porous Medium Burner with an Integrated Heat Exchanger. J. Therm. Sci. Technol. 2016, 36, 61–68. [Google Scholar]
- Etemad, S.; Karim, H.; Smith, L.L.; Pfefferle, W.C. Advanced Technology Catalytic Combustor for High Temperature Ground Power Gas Turbine Applications. Catal. Today 1999, 47, 305–313. [Google Scholar] [CrossRef]
- Kajita, S.; Betta, R.D. Achieving Ultra Low Emissions in a Commercial 1.4 MW Gas Turbine Utilizing Catalytic Combustion. Catal. Today 2003, 83, 279–288. [Google Scholar] [CrossRef]
- Cutrone, M.B.; Beebe, K.W.; Dalla Betta, R.A.; Schlatter, J.C.; Nickolas, S.G.; Tsuchiya, T. Development of a Catalytic Combustor for a Heavy-duty Utility Gas Turbine. Catal. Today 1999, 47, 391–398. [Google Scholar] [CrossRef]
- Kolaczkowski, S.T. Catalytic Stationary Gas-turbine Combustors—A Review of the Challenges Faced to Clear the Next Set of Hurdles. Chem. Eng. Res. Design. 1995, 73, 168–190. [Google Scholar]
- Forzatti, P. Status and perspectives of catalytic combustion for gas turbines. Catal. Today 2003, 83, 3–18. [Google Scholar] [CrossRef]
- Heck, R.M.; Robert, J.; Farrauto, R.J.; Oyama, S.T. Catalytic Air Pollution Control: Commercial Technology, 4th ed.; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar]
- Huonder, A.; Olsen, D. Methane Emission Reduction Technologies for Natural Gas Engines: A Review. Energies 2023, 16, 7054. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent advances in catalysts for methane combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Cullis, C.F.; Willatt, B.M. Oxidation of methane over supported precious metal catalysts. J. Catal. 1983, 83, 267–285. [Google Scholar] [CrossRef]
- Choudhary, T.; Banerjee, S.; Choudhary, V. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Gorte, R.; Fornasiero, P. Catalytic Oxidation of Methane: Pd and Beyond. Eur. J. Inorg. Chem. 2018, 25, 2884–2893. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. App. Cat. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.; Altman, E.; Pfefferle, L.; Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev.-Sci. Eng. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Gélin, P.; Urfels, L.; Primet, M.; Tena, E. Complete oxidation of methane at low temperature over Pt and Pd catalysts for the abatement of lean-burn natural gas fuelled vehicles emissions: Influence of water and sulphur containing compounds. Catal. Today 2003, 83, 45–57. [Google Scholar] [CrossRef]
- Burch, R. Low NOx options in catalytic combustion and emission control. Catal. Today 1997, 35, 27–36. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Support interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Catalytic deactivation and role of the Support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Becker, E.; Carlsson, P.; Grönbeck, H.; Skoglundh, M. Methane oxidation over alumina supported platinum investigated by time-resolved in situ XANES spectroscopy. J. Catal. 2007, 252, 11–17. [Google Scholar] [CrossRef]
- Deutschmann, O.; Maier, L.; Riedel, U.; Stroemman, A.; Dibble, R. Hydrogen assisted catalytic combustion of methane on platinum. Catal. Today 2000, 59, 141–150. [Google Scholar] [CrossRef]
- Bui, P.; Vlachos, D.; Westmoreland, P. Catalytic ignition of methane/oxygen mixtures over platinum surfaces: Comparison of detailed simulations and experiments. Surf. Sci. 1997, 385, L1029–LI034. [Google Scholar] [CrossRef]
- Deutschmann, O.; Behrendt, F.; Warnatz, J. Modeling and simulation of heterogeneous oxidation of methane on a platinum foil. Catal. Today 1994, 21, 461–470. [Google Scholar] [CrossRef]
- Reinke, M.; Mantzaras, J.; Bombach, R.; Schenker, S.; Yylli, N. Effects of H2O and CO2 Dilution on the Catalytic and Gas-Phase Combustion of Methane, over Platinum at Elevated Pressures. Combust. Sci. Tech. 2006, 179, 553–600. [Google Scholar] [CrossRef]
- Niwa, M.; Awano, K.; Murakami, Y. Activity of supported platinum catalysts for methane oxidation. Appl. Catal. 1983, 7, 317–325. [Google Scholar] [CrossRef]
- Wang, M.; Eggenschwiler, P.D.; Tanja Franken, T.; Davide Ferri, D.; Oliver Kröcher, O. Investigation on the Role of Pd, Pt, Rh in Methane Abatement for Heavy Duty Applications. Catalysts 2022, 12, 373. [Google Scholar] [CrossRef]
- Bounechada, D.; Groppi, G.; Forzatti, P.; Kallinen, K.; Kinnunen, T. Effect of periodic lean/rich switch on methane conversion over a Ce–Zr promoted Pd-Rh/Al2O3 catalyst in the exhausts of natural gas vehicles. Appl. Catal. B Environ. 2012, 119–120, 91–99. [Google Scholar] [CrossRef]
- Ersson, A.; Kušar, H.; Carroni, R.; Griffin, T.; Järås, S. Catalytic combustion of methane over bimetallic catalysts a comparison between a novel annular reactor and a high-pressure reactor. Catal. Today 2003, 83, 265–277. [Google Scholar] [CrossRef]
- Ryu, C.K.; Ryoo, M.W.; Ryu, I.S.; Kang, S.K. Catalytic combustion of methane over supported bimetallic Pd catalysts: Effects of Ru or Rh addition. Catal. Today 1999, 47, 141–147. [Google Scholar] [CrossRef]
- Lyubovsky, M.; Smith, L.; Castaldi, M.; Karim, H.; Nentwick, B.; Etemad, S.; LaPierre, R.; Pfefferle, W. Catalytic combustion over platinum group catalysts: Fuel-lean versus fuel-rich operation. Catal. Today 2003, 83, 71–84. [Google Scholar] [CrossRef]
- Oh, S.; Mitchell, P. Effects of rhodium addition on methane oxidation behavior of alumina-supported noble metal catalysts. Appl. Catal. B Environ. 1994, 5, 165–179. [Google Scholar] [CrossRef]
- Yang, N.; Liu, J.; Sun, Y.; Zhu, Y. Au@PdOx with a PdOx-rich shell and Au-rich core embedded in Co3O4 nanorods for catalytic combustion of methane. Nanoscale 2019, 9, 4108–4109. [Google Scholar] [CrossRef]
- Venezia, A.; Murania, R.; Pantaleo, G.; Deganello, G. Pd and PdAu on mesoporous silica for methane oxidation: Effect of SO2. J. Catal. 2007, 251, 94–102. [Google Scholar] [CrossRef]
- Miao, S.; Deng, Y. Au-Pt/Co3O4 catalyst for methane combustion. Appl. Catal. B: Environ. 2001, 31, 11–14. [Google Scholar] [CrossRef]
- Lapisardi, G.; Urfels, L.; Gelin, P.; Primet, M.; Kaddouri, A.; Garbowski, E.; Toppi, S.; Tena, E. Superior catalytic behaviour of Pt-doped Pd catalysts in the complete oxidation of methane at low temperature. Catal. Today 2006, 117, 564–568. [Google Scholar] [CrossRef]
- Larpisardi, G.; Gélin, P.; Kaddouri, A.; Garbowski, E.; Da Costa, S. Pt-Pd bimetallic catalysts for methane emissions abatement. Top. Catal. 2007, 42–43, 461–464. [Google Scholar] [CrossRef]
- Kinnunen, N.; Hirvi, J.; Suvanto, M.; Pakkanen, T. Methane combustion activity of Pd–PdOx–Pt/Al2O3 catalyst: The role of platinum promoter. J. Mol. Catal. A Chem. 2012, 356, 20–28. [Google Scholar] [CrossRef]
- Bugosh, G.; Easterling, V.; Rusakova, I.; Harold, M. Anomalous steady-state and spatio-temporal features of methane oxidation on Pt/Pd/Al2O3 monolith spanning lean and rich condition. Appl. Catal. B Environ. 2015, 165, 68–78. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawashima, K.; Tagawa, Y.; Tashiro, K.; Anoda, H.; Ichioka, K.; Sumiya, S.; Zhang, G. New DOC for Light Duty Diesel DPF System; SAE Technical Paper; SAE: Warrendale, PA, USA, 2007; 2007-01-1920. [Google Scholar]
- Nomura, K.; Noro, K.; Nakamura, Y.; Yazawa, Y.; Yoshida, H.; Satsuma, A.; Hattori, T. Pd–Pt bimetallic catalyst supported on SAPO-5 for catalytic combustion of diluted methane in the presence of water vapor. Catal. Lett. 1998, 53, 167–169. [Google Scholar] [CrossRef]
- Persson, K.; Jansson, K.; Järås, S. Characterisation and microstructure of Pd and bimetallic Pd–Pt catalysts during methane oxidation. J. Catal. 2007, 245, 401–414. [Google Scholar] [CrossRef]
- Nassiri, H.; Hayes, R.E.; Semagina, N. Stability of Pd-Pt catalysts in low-temperature wet methane combustion: Metal ratio and particle reconstruction. Chem. Eng. Sci. 2018, 186, 44–51. [Google Scholar] [CrossRef]
- Goodman, E.; Dai, S.; Yang, A.; Wrasman, C.; Gallo, A.; Bare, S.; Hoffman, A.; Jaramillo, T.; Graham, G.; Pan, X.; et al. Uniform Pt/Pd Bimetallic Nanocrystals Demonstrate Platinum Effect on Palladium Methane Combustion Activity and Stability. ACS Catal. 2017, 7, 4372–4380. [Google Scholar] [CrossRef]
- Persson, K.; Ersson, A.; Jansson, K.; Fierro, J.; Jaras, S. Influence of molar ratio on Pd–Pt catalysts for methane combustion. J. Catal. 2006, 243, 14–24. [Google Scholar] [CrossRef]
- Yamamoto, H.; Uchida, H. Oxidation of methane over Pt and Pd supported on alumina in lean-burn natural-gas engine exhaust. Catal. Today 1998, 45, 147–151. [Google Scholar] [CrossRef]
- Semagina, N.; Nassiri, H.; Lee, K.; Hu, Y.; Hayes, R.E.; Scott, R.W.J. Water shifts PdO-catalyzed lean methane combustion to Pt-catalyzed rich combustion in Pd-Pt catalysts: In-situ X-ray absorption spectroscopy. J. Catal. 2017, 352, 649–656. [Google Scholar]
- Nassiri, H.; Lee, K.; Hu, Y.; Hayes, R.E.; Scott, R.W.J.; Semagina, N. Platinum inhibits low-temperature dry lean methane combustion via palladium reduction in Pd-Pt/Al2O3: An in-situ X-ray absorption study. ChemPhysChem 2017, 18, 238–244. [Google Scholar] [CrossRef]
- Chen, M.; Schmidt, L.D. Morphology and composition of Pt-Pd alloy crystallites on SiO2 in reactive atmospheres. J. Catal. 1979, 56, 198–218. [Google Scholar] [CrossRef]
- Abbasi, R.; Huang, G.; Istratescu, G.M.; Wu, L.; Hayes, R.E. Methane oxidation over Pt, Pt: Pd and Pd based catalysts: Effects of pre-treatment. Can. J. Chem. Eng. 2015, 93, 1474–1482. [Google Scholar] [CrossRef]
- Park, S.; Hwang, H.; Moon, J. Catalytic combustion of methane over rare earth stannate pyrochlore. Catal. Lett. 2003, 87, 219–223. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Z.; Yang, P. Effect of Substitution of Cobalt for Iron in Sr4Fe6O13-delta on the Catalytic Activity for Methane Combustion. Chin. J. Chem. 2011, 29, 451–454. [Google Scholar] [CrossRef]
- Zhu, W.; Jin, J.; Chen, X.; Li, C.; Wang, T.; Tsang, C.; Liang, C. Enhanced activity and stability of La-doped CeO2 monolithic catalysts for lean-oxygen methane combustion. Environ. Sci. Pollut. Res. 2018, 25, 5643–5654. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, Z.; Du, W.; Zhu, G. Morphological effect of CeO2 catalysts on their catalytic performance in lean methane combustion. Chem. Lett. 2020, 49, 461–464. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Arandiyan, H.; Peng, Y.; Chang, H.; Li, J. Low temperature complete combustion of methane over cobalt chromium oxides catalysts. Catal. Today 2013, 201, 12–18. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; González-Velasco, J.; Gutiérrez-Ortiz, J.; López-Fonseca, R. Oxidation of residual methane from VNG vehicles over Co3O4-based catalysts: Comparison among bulk, Al2O3-supported and Ce-doped catalysts. Appl. Catal. B Environ. 2018, 237, 844–854. [Google Scholar] [CrossRef]
- Liotta, L.; Di Carlo, G.; Pantaleo, G.; Deganello, G. Co3O4/CeO2 and Co3O4/CeO2–ZrO2 composite catalysts for methane combustion: Correlation between morphology reduction properties and catalytic activity. Catal. Commun. 2005, 6, 329–336. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, M.; Yang, L.; Li, Z.; Tian, F.X.; He, Y. Recent progress of catalytic methane combustion over transition metal oxide catalysts. Front. Chem. 2022, 10, 959422. [Google Scholar] [CrossRef]
- Arai, H.; Machida, M. Recent progress in high-temperature catalytic combustion. Catal. Today 1991, 10, 81–95. [Google Scholar] [CrossRef]
- Arai, H.; Machida, M. Thermal stabilization of catalyst supports and their application to high-temperature catalytic combustion. Appl. Catal. A Gen. 1996, 138, 161–176. [Google Scholar] [CrossRef]
- Prasad, R.; Kennedy, L.; Ruckenstein, E. Catalytic combustion. Catal. Rev.-Sci. Eng. 1984, 29, 219–267. [Google Scholar] [CrossRef]
- Zwinkels, M.; Jaras, S.; Menon, P.; Griffin, T. Catalytic materials for high-temperature combustion. Catal. Rev.-Sci. Eng. 1993, 35, 319–358. [Google Scholar] [CrossRef]
- Cimino, S.; Di Benedetto, A.; Pirone, R.; Russo, G. Transient behaviour of perovskite-based monolithic reactors in the catalytic combustion of methane. Catal. Today 2001, 69, 95–103. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y. Nanostructured perovskite oxides as promising substitutes of noble metals catalysts for catalytic combustion of methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar] [CrossRef]
- Lu, Y.; Michalow, K.; Matam, S.; Winkler, A.; Maeglia, A.; Yoona, S.; Heele, A.; Weidenkaffa, A.; Ferri, D. Methane abatement under stoichiometric conditions on perovskite-supported palladium catalysts prepared by flame spray synthesis. Appl. Catal. B Environ. 2014, 144, 631–643. [Google Scholar] [CrossRef]
- Lu, Y.; Eyssler, A.; Otala, E.; Matam, S.; Brunko, O.; Weidenkaff, A.; Ferri, D. Influence of the synthesis method on the structure of Pd-substituted perovskite catalysts for methane oxidation. Catal. Today 2013, 208, 42–47. [Google Scholar] [CrossRef]
- Cullis, C.; Nevell, T.; Trimm, D. Role of the catalyst support in the oxidation of methane over palladium. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1972, 68, 1406–1412. [Google Scholar] [CrossRef]
- Ribeiro, F.H.; Chow, M.; Dalla Betta, R.A. Kinetics of the complete oxidation of methane over supported palladium catalysts. J. Catal 1994, 146, 537–544. [Google Scholar]
- Barrett, W.; Shen, J.; Hu, Y.; Hayes, R.E.; Scott, R.W.J.; Semagina, N. Understanding the Role of SnO 2 Support in Water-Wolerant Methane Combustion: In situ Observation of Pd(OH) 2 and Comparison with Pd/Al2O3. ChemCatChem 2020, 12, 944–952. [Google Scholar] [CrossRef]
- Escandon, L.; Ordonez, S.; Vega, A.; Diez, F. Oxidation of methane over palladium catalysts: Effect of the support. Chemosphere 2005, 58, 9–17. [Google Scholar] [CrossRef]
- Kumar, R.; Hayes, R.; Semagina, N. Effect of support on Pd-catalyzed methane-lean combustion in the presence of water: Review. Catal. Today 2021, 382, 82–95. [Google Scholar] [CrossRef]
- Garbowski, E.; Feumi-Jantou, C.; Mouaddib, N.; Primet, M. Catalytic combustion of methane over palladium supported on alumina catalysts: Evidence for reconstruction of particles. Appl. Catal. A Gen. 1994, 109, 277–292. [Google Scholar] [CrossRef]
- Friberg, I.; Sadokhina, N.; Olsson, L. Complete methane oxidation over Ba modified Pd/Al2O3: The effect of water vapour. Appl. Catal. B. Environ. 2018, 231, 242–250. [Google Scholar] [CrossRef]
- Demoulin, O.; Navez, M.; Ruiz, P. Investigation of the behavior of a Pd/-Al2O3 catalyst during methane combustion reaction using in situ DRIFT spectroscopy. Appl. Catal. A Gen. 2005, 295, 59–70. [Google Scholar] [CrossRef]
- Demoulin, O.; Navez, M.; Gaigneaux, E.; Ruiz, P.; Mamede, A.; Granger, P.; Payen, E. Operando resonance Raman spectroscopic characterisation of the oxidation state of palladium in Pd/g-Al2O3 catalysts during the combustion of methane. Phys. Chem. Chem. Phys. 2003, 5, 4394–4401. [Google Scholar] [CrossRef]
- Datye, A.; Bravo, J.; Nelson, T.; Atanasova, P.; Lyubovsky, M.; Pfefferle, L. Catalyst microstructure and methane oxidation reactivity during the Pd-PdO transformation on alumina supports. Appl. Catal. A Gen. 2000, 198, 179–196. [Google Scholar] [CrossRef]
- Ciuparu, D.; Perkins, E.; Pfefferle, L. In situ DR-FTIR investigation of surface hydroxyls on -Al2O3 supported PdO catalysts during methane combustion. Appl. Catal. A Gen. 2004, 263, 145–153. [Google Scholar] [CrossRef]
- Castellazzi, P.; Groppi, G.; Forzatti, P.; Baylet, A.; Marecot, P.; Duprez, D. Role of Pd loading and dispersion on redox behaviour and CH4 combustion activity of Al2O3 supported catalysts. Catal. Today 2010, 155, 18–26. [Google Scholar] [CrossRef]
- Hicks, R.; Qi, H.; Young, M.; Lee, R. Effect of catalyst structure on methane oxidation over palladium on alumina. J. Catal. 1990, 122, 295–306. [Google Scholar] [CrossRef]
- Hong, E.; Kim, C.; Lim, D.; Cho, H.; Shin, C. Catalytic methane combustion over Pd/ZrO2 catalysts: Effects of crystalline structure and textural properties. Appl. Catal. B Environ. 2018, 232, 544–552. [Google Scholar] [CrossRef]
- Guerrero, S.; Araya, P.; Wolf, E. Methane oxidation on Pd supported on high area zirconia catalysts. Appl. Catal. A Gen. 2006, 298, 243–253. [Google Scholar] [CrossRef]
- Fujimoto, F.; Ribiero, R.; Avalos Borja, A.; Iglesia, E. Structure and reactivity of PdOx/ZrO2 catalysts for methane oxidation at low temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef]
- Carstens, J.; Su, S.; Bell, A. Factors Affecting the Catalytic Activity of Pd/ZrO2 for the Combustion of Methane. J. Catal. 1998, 176, 136–142. [Google Scholar] [CrossRef]
- Ibashi, W.; Groppi, G.; Forzatti, P. Kinetic measurement of CH4 combustion over a 10% PdO/ZrO2 catalyst using an annular flow micro reactor. Catal. Today 2003, 83, 115–129. [Google Scholar] [CrossRef]
- Araya, P.; Guerrero, S.; Robertson, J.; Gracia, F. Methane combustion over Pd/SiO2 catalysts with different degrees of hydrophobicity. Appl. Catal. A Gen. 2005, 283, 225–233. [Google Scholar] [CrossRef]
- Bassil, J.; Al Barazi, A.; Da Costa, P.; Boutros, M. Catalytic combustion of methane over mesoporous silica supported palladium. Catal. Today 2011, 176, 36–40. [Google Scholar] [CrossRef]
- Hoyos, L.; Praliaud, H.; Primet, M. Catalytic combustion of methane over palladium supported on alumina and silica in presence of hydrogen sulfide. Appl. Catal. A Gen. 1993, 98, 125–138. [Google Scholar] [CrossRef]
- Gannouni, A.; Albela, B.; Said Zina, M.; Bonneviot, L. Metal dispersion, accessibility and catalytic activity in methane oxidation of mesoporous templated aluminosilica supported palladium. Appl. Catal. A Gen. 2013, 464–465, 116–127. [Google Scholar] [CrossRef]
- Ercolino, G.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A.; Specchia, V. Pd/Co3O4-based catalysts prepared by solution combustion synthesis for residual methane oxidation in lean conditions. Catal. Today 2015, 257, 66–71. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Hoflund, G. In situ IR studies on the mechanism of methane oxidation over Pd/Al2O3 and Pd/Co3O4 catalysts. Fuel Process. Technol. 2003, 84, 1–11. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kreft, S.; Georgi, G.; Fulda, G.; Pohla, M.; Seeburg, D.; Berger-Karin, C.; Kondratenko, E.; Wohlrab, S. Improved catalytic methane combustion of Pd/CeO2 catalysts via porous glass integration. Appl. Catal. B Environ. 2015, 179, 313–320. [Google Scholar] [CrossRef]
- Lei, Y.; Li, W.; Liu, Q.; Lin, Q.; Zhenga, X.; Huanga, Q.; Guan, S.; Wanga, X.; Wang, C.; Li, F. Typical crystal face effects of different morphology ceria on the activity of Pd/CeO2 catalysts for lean methane combustion. Fuel 2018, 233, 10–20. [Google Scholar] [CrossRef]
- Huang, Q.; Li, W.; Lei, Y.; Guan, S.; Zheng, X.; Pan, Y.; Wen, W.; Zhu, J.; Zhang, H.; Lin, Q. Catalytic Performance of Novel Hierarchical Porous Flower-Like NiCo2O4 Supported Pd in Lean Methane Oxidation. Catal. Lett. 2018, 148, 2799–2811. [Google Scholar] [CrossRef]
- Roth, D.; Gelin, P.; Tena, E.; Primet, M. Combustion of methane at low temperature over Pd and Pt catalysts supported on Al2O3, SnO2 and Al2O3-grafted SnO2. Top. Catal. 2001, 16–17, 77–82. [Google Scholar] [CrossRef]
- Urfels, L.; Gelin, P.; Primet, M.; Tena, E. Complete oxidation of methane at low temperature over Pt catalysts supported on high surface area SnO2. Top. Catal. 2004, 30–31, 427–432. [Google Scholar] [CrossRef]
- Kinnunen, N.; Suvanto, M.; Moreno, M.; Savimaki, A.; Kallinen, K.; Kinnunen, T.; Pakkanen, T. Methane oxidation on alumina supported palladium catalysts: Effect of Pd precursor and solvent. Appl. Catal. A Gen. 2009, 370, 78–87. [Google Scholar] [CrossRef]
- Auvray, X.; Lindholm, A.; Milh, M.; Olsson, L. The addition of alkali and alkaline earth metals to Pd/Al2O3 to promote methane combustion. Effect of Pd and Ca loading. Catal. Today 2018, 299, 212–218. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Cristiani, C.; Lietti, L.; Groppi, G. The influence of ceria and other rare earth promoters on palladium-based methane combustion catalysts. Catal. Today 2012, 180, 124–130. [Google Scholar] [CrossRef]
- Kang, T.; Kim, J.; Kang, S.; Seo, G. Promotion of methane combustion activity of Pd catalyst by titania loading. Catal. Today 2000, 59, 87–93. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Kerzhentsev, M.A. Fluidized Bed Catalytic Combustion. Catal. Today 1999, 47, 339–346. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Froment, G.B.; Bischoff, K.B.; De Wilde, J. Chemical Reactor Analysis and Design, 3rd ed.; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Hayes, R.E.; Mmbaga, J.P. Introduction to Chemical Reactor Analysis, 2nd ed.; CRC Press: Baton Roca, FL, USA, 2011. [Google Scholar]
- Aubé, F.; Sapoundjiev, H. Mathematical model and numerical simulations of catalytic flow reversal reactors for industrial applications. Comput. Chem. Eng. 2000, 24, 2623–2632. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. Mass and Heat Transfer Effects in Catalytic Monolith Reactors. Chem. Eng. Sci. 1994, 49, 3587–3599. [Google Scholar] [CrossRef]
- Carty, W.M.; Lednor, P.W. Monolithic ceramics and heterogeneous catalysts: Honeycombs and foams. Curr. Opin. Solid State Mater. Sci. 1996, 1, 88–95. [Google Scholar] [CrossRef]
- Visconti, C.G.; Groppi, G.; Tronconi, E. Highly conductive “packed foams”: A new concept for the intensification of strongly endo- and exothermic catalytic processes in compact tubular reactors. Catal. Today 2016, 273, 178–186. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Fyodorov, A.A.; Gaisinovich, M.S.; Shurov, V.P.; Fyodorova, I.V.; Gubaydulina, T.A. Metal-form catalysts with supported active phase for deep oxidation of hydrocarbons. React. Kinet. Catal. Lett. 1995, 54, 167–172. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, Characterization and Application of Cellular Metals and Metal Foams. Prog. Mater. Sci. 2002, 46, 559–632. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Pushkarev, V.V.; Podyacheva, O.Y.; Koryabkina, N.A.; Veringa, H. A catalytic heat-exchanging tubular reactor for combining of high temperature exothermic and endothermic reactions. Chem. Eng. J. 2001, 82, 355–360. [Google Scholar] [CrossRef]
- Scheffler, M.; Colombo, P. Cellular Ceramics: Structure, Manufacturing, Properties, Applications; Wiley-VCH Verlag GmbH &Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Gancarczyk, A.; Sindera, K.; Iwaniszyn, M.; Piatek, M.; Macek, W.; Jodłowski, P.J.; Wroński, S.; Sitarz, M.; Łojewska, J.; Kołodziej, A. Metal Foams as Novel Catalyst Support in Environmental Processes. Catalysts 2019, 9, 587. [Google Scholar] [CrossRef]
- Patcas, F.C.; Garrido, G.I.; Kraushaar-Czarnetzki, B. CO oxidation over structured carriers:A comparison of ceramic foams, honeycombs and beads. Chem. Eng. Sci. 2007, 62, 3984–3990. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Mass-Transfer Characterization of Metallic Foams as Supports for Structured Catalysts. Ind. Eng. Chem. Res. 2005, 44, 4993–5002. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Iwaniszyn, M.; Piatek, M.; Korpyś, M.; Sindera, K.; Jodłowski, P.J.; Łojewska, J.; Kołodziej, A. Catalytic Combustion of Low-Concentration Methane on Structured Catalyst Supports. Ind. Eng. Chem. Res. 2018, 57, 10281–10291. [Google Scholar] [CrossRef]
- Sirijaruphan, A.; Goodwin, J.G.; Rice, R.W.; Wei, D.; Butcher, K.R.; Roberts, G.W.; Spivey, J.J. Metal foam supported Pt catalysts for the selective oxidation of CO in hydrogen. Appl. Catal. A Gen. 2005, 281, 1–9. [Google Scholar] [CrossRef]
- Garrido, G.I.; Patcas, F.C.; Lang, S.; Kraushaar-Czarnetzki, B. Mass transfer and pressure drop in ceramic foams: Adescription for different pore sizes and porosities. Chem. Eng. Sci. 2008, 63, 5202–5217. [Google Scholar] [CrossRef]
- Lacroix, M.; Dreibine, L.; de Tymowski, B.; Vigneron, F.; Edouard, D.; Begin, D.; Nguyen, P.; Pham, C.; Savin-Poncet, S.; Luck, F.; et al. Silicon carbide foam composite containing cobalt as a highly selective and re-usable Fischer–Tropsch synthesis catalyst. Appl. Catal. A Gen. 2011, 397, 62–72. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Visconti, C.G.; Groppi, G.; Lietti, L.; Tronconi, E. Intensifying heat transfer in Fischer-Tropsch tubular reactors through the adoption of conductive packed foams. Chem. Eng. J. 2018, 349, 829–837. [Google Scholar] [CrossRef]
- Meloni, E.; Saraceno, E.; Martino, M.; Corrado, A.; Iervolino, G.; Palma, V. SiC-based structured catalysts for a high-efficiency electrified dry reforming of methane. Renew. Energy 2023, 211, 336–346. [Google Scholar] [CrossRef]
- Balzarotti, R.; Beretta, A.; Groppi, G.; Tronconi, E. A comparison between washcoated and packed copper foams for the intensification of methane steam reforming. React. Chem. Eng. 2019, 4, 1387–1392. [Google Scholar] [CrossRef]
- Ercolino, G.; Karimi, S.; Stelmachowski, P.; Specchia, S. Catalytic combustion of residual methane on alumina monoliths and open cell foams coated with Pd/Co3O4. Chem. Eng. J. 2017, 326, 339–349. [Google Scholar] [CrossRef]
- Moncada Quintero, C.W.; Mazzei, H.G.; Servel, M.; Augier, F.; Haroun, Y.; Joly, J.F.; Specchia, S. Investigating mass transfer coefficients in lean methane combustion reaction through the morphological and geometric analysis of structured open cell foam catalysts. Chem. Eng. Sci. 2023, 281, 119138. [Google Scholar] [CrossRef]
- Choya, A.; Gudyka, S.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; Kotarba, A.; López-Fonseca, R. Novel Ce-modified cobalt catalysts supported over α-Al2O3 open cell foams for lean methane oxidation. Appl. Catal. A Gen. 2022, 632, 118511. [Google Scholar] [CrossRef]
- Korpyś, M.; Iwaniszyn, M.; Sindera, K.; Kołodziej, A.; Rotkegel, A.; Profic-Paczkowska, J.; Sitarz, M.; Gancarczyk, A. Simplified modelling of a fixed bed reactor for catalytic methane combustion. Chem. Process Eng. New Front. 2023, 44, e27. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Fiołek, A.; Sitarz, M.; Kopia, A. Effect of the Processing and Heat Treatment Route on the Microstructure of MoS2/Polyetheretherketone Coatings Obtained by Electrophoretic Deposition. J. Electrochem. Soc. 2019, 166, 151–161. [Google Scholar] [CrossRef]
- Amin, A. Review of autothermal reactors: Catalysis, reactor design, and processes. Int. J. Hydrog. Energy 2024, 65, 271–291. [Google Scholar] [CrossRef]
- Churchill, S.W. The Interaction of Reactions and Transport Part II: Combined Reactors and Exchangers. Int. J. Chem. React. Eng. 2007, 5, A55. [Google Scholar] [CrossRef]
- Kolios, G.; Frauhammer, J.; Eigenberger, G. Autothermal fixed-bed reactor concepts. Chem. Eng. Sci. 2000, 55, 5945–5967. [Google Scholar] [CrossRef]
- Litto, R.; Nien, T.; Hayes, R.E.; Mmbaga, J.P.; Votsmeier, M. Parametric Study of a Recuperative Catalytic Converter. Catalysis Today 2012, 188, 106–112. [Google Scholar] [CrossRef]
- Bernnat, J.; Rink, M.; Tuttlies, U.; Danner, T.; Nieken, U.; Eigenberger, G. Heat-integrated concepts for automotive exhaust purification. Top. Catal. 2009, 52, 2052–2057. [Google Scholar] [CrossRef]
- Kolios, G.; Gritsch, A.; Morillo, A.; Tuttlies, U.; Bernnat, J.; Opferkuch, F.; Eigenberger, G. Heat-integrated reactor concepts for catalytic reforming and automotive exhaust purification. Appl. Catal. B Environ. 2007, 70, 16–30. [Google Scholar] [CrossRef]
- Dobrego, K.V.; Gnesdilov, N.N.; Kozlov, I.M.; Bubnovich, V.I.; Gonzalez, H.A. Numerical investigation of the new regenerator–recuperator scheme of VOC oxidizer. Int. J. Heat. Mass. Transf. 2005, 48, 4695–4703. [Google Scholar] [CrossRef]
- Rebrov, E.V.; de Croon, M.H.J.M.; Schouten, J.C. Design of a microstructured reactor with integrated heat-exchanger for optimum performance of a highly exothermic reaction. Catal. Today 2001, 69, 183–192. [Google Scholar] [CrossRef]
- Salamon, E.; Cornejo, I.; Mmbaga, J.P.; Kołodziej, A.; Lojewska, J.; Hayes, R.E. Investigations of a three-channel autogenous reactor for lean methane combustion. Chem. Eng. Process. Process Intensif. 2020, 153, 107956. [Google Scholar] [CrossRef]
- Strenger, M.R.; Churchill, S.W.; Retallick, W.B. Operational Characteristics of a Double-Spiral Heat Exchanger for the Catalytic Incineration of Contaminated Air. Ind. Eng. Chem. Res. 1990, 29, 1977–1984. [Google Scholar] [CrossRef]
- Mmbaga, J.P.; Hayes, R.E.; Profic-Paczkowska, J.; Jędrzejczyk, R.; Chlebda, D.K.; Dańczak, J.; Hilderbrandt, R. Energy Recuperation in a Spiral Reactor for Lean Methane Combustion: Heat Transfer Efficiency and Design Guidelines. Processes 2025, 13, 1168. [Google Scholar] [CrossRef]
- Matros, Y.S.; Bunimovich, G.A. Reverse-flow operation in fixed bed catalytic reactors. Catal. Rev. Sci. Eng. 1996, 38, 1–68. [Google Scholar] [CrossRef]
- Strots, V.O.; Bunimovich, G.A.; Matros, Y.S.; Zheng, M.; Mirosh, E.A. Advanced catalytic converter system for natural gas-powered diesel engines. Stud. Surf. Sci. Catal. 1998, 119, 907–912. [Google Scholar]
- Hanamura, K.; Echigo, R.; Zhdanok, S.A. Superadiabatic combustion in a porous medium. Int. J. Heat Mass Transf. 1993, 36, 3201–3209. [Google Scholar] [CrossRef]
- Cottrell, F.G. Purifying Gases and Apparatus Therefor. U.S. Patent 2,121,723, 21 June 1938. [Google Scholar]
- Gilbert, N.; Daniels, K. Fixation of atmospheric nitrogen in a gas heated furnace. Ind. Eng. Chem. 1948, 40, 1719–1723. [Google Scholar] [CrossRef]
- Frank-Kamenetski, D.A. Diffusion and Heat Exchange in Chemical Kinetics; Princeton University Press: Princeton, NJ, USA, 1955. [Google Scholar]
- Boreskov, G.K.; Bunimovich, G.A.; Matros, Y.S.; Ivanov, A.A. Catalytic processes carried out under non-stationary conditions: II. Switching the direction for the feed of the reaction mixture to the catalyst bed. Experimental results. Kinet. Katal. 1982, 23, 402–406. [Google Scholar]
- Matros, Y.S. Catalytic Processes Under Unsteady State Conditions; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Bunivovich, G.A.; Strots, V.O.; Goldman, O.V. Theory and industrial application of SO2 oxidation reverse-process for sulfur acid production. In Unsteady State Processes in Catalysis; Matros, Y.S., Ed.; VPS BV: Ultrecht, The Netherlands, 1990. [Google Scholar]
- Van den Bussche, K.M.; Neophytides, S.N.; Zolotarskii, I.A.; Froment, G.F. Modelling and simulation of a reversed flow operation of a fixed-bed reactor for methanol synthesis. Chem. Eng. Sci. 1993, 48, 3335–3345. [Google Scholar] [CrossRef]
- Neophytides, S.N.; Froment, G.F. A bench scale study of reversed flow methanol synthesis. Ind. Eng. Chem. Res. 1992, 31, 1583–1589. [Google Scholar] [CrossRef]
- Guit, R.P.M. The Selective Catalytic Reduction of NOx in a Reverse Flow Reactor: A Quick Design Procedure. In Precision Process Technology; Weijnen, M.P.C., Drinkenburg, A.A.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 453–461. [Google Scholar]
- Snyder, J.D.; Subramaniam, B. Numerical simulation of a reverse-flow NOX-SCR reactor with side-stream ammonia addition. Chem. Eng. Sci. 1998, 53, 727–734. [Google Scholar] [CrossRef]
- Bobrova, L.N.; Slavinskaya, E.M.; Noskov, A.S.; Matros, Y.S. Unsteady-state performance of NOx catalytic reduction by NH3. React. Kinet. Catal. Lett. 1988, 37, 267–272. [Google Scholar] [CrossRef]
- Noskov, A.S.; Bobrova, L.M.; Matros, Y.S. Reverse-process for NOx-off gases decontamination. Catal. Today 1993, 17, 293–300. [Google Scholar] [CrossRef]
- Grozev, G.; Sapundzhiev, C.G. Modelling of the reversed flow fixed bed reactor for catalytic decontamination of waste gases. Chem. Eng. Technol. 1997, 20, 378–383. [Google Scholar] [CrossRef]
- Eigenberger, G.; Nieken, U. Catalytic combustion with periodic flow reversal. Chem. Eng. Sci. 1988, 43, 2109–2115. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hayes, R.E.; Poirier, M.; Sapoundjiev, H. Heat extraction from a flow reversal reactor in lean methane combustion. Chem. Eng. Res. Des. 2005, 83, 205–213. [Google Scholar] [CrossRef]
- Boreskov, G.K.; Matros, Y.S. Fixed catalyst bed reactors operated in steady and unsteady-state conditions. In Recent Advances in the Engineering of Chemically Reacting Systems; Doraiswamy, L.K., Ed.; Wiley: New Delhi, India, 1984; pp. 142–155. [Google Scholar]
- Matros, Y.S.; Noskov, A.S.; Chumachenko, V.A. Progress in reverse-process application to catalytic incineration problems. Chem. Eng. Process. 1993, 32, 89–98. [Google Scholar] [CrossRef]
- Sapundzhiev, C.; Chaouki, J.; Guy, C.; Klvana, D. Catalytic combustion of natural gas in a fixed bed reactor with flow reversal. Chem. Eng. Comm. 1993, 125, 171–186. [Google Scholar] [CrossRef]
- van de Beld, L.; Borman, R.A.; Derkx, O.R.; van Woezik, B.A.; Westerterp, K.R. Removal of volatile organic-compounds from polluted air in a reverse flow reactor–an experimental-study. Ind. Eng. Chem. Res. 1994, 33, 2946–2956. [Google Scholar] [CrossRef]
- Purwano, S.; Budman, H.; Silveston, R.R.; Matros, Y.S. Runaway in packed bed reactors operating with periodic flow reversal. Chem. Eng. Sci. 1994, 49, 5473–5487. [Google Scholar] [CrossRef]
- Jia, Z.; Hayes, R.E. An efficient computational scheme for building operating maps for a flow reversal reactor. Chem. Eng. Sci. 2015, 134, 423–432. [Google Scholar] [CrossRef]
- Salomons, S.; Hayes, R.E.; Poirier, M.; Sapoundjiev, H. Modelling a reverse flow reactor for the catalytic combustion of fugitive methane emissions. Comput. Chem. Eng. 2004, 28, 1599–1610. [Google Scholar] [CrossRef]
- Salomons, S.; Hayes, R.E.; Poirier, M.; Sapoundjiev, H. Flow reversal reactor for the catalytic combustion of lean methane mixtures. Catal. Today 2003, 83, 59–69. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hayes, R.E.; Poirier, M.; Sapoundjiev, H. Effect of reactor internal properties on the performance of a flow reversal catalytic reactor for methane combustion. Chem. Eng. Sci. 2004, 59, 4081–4093. [Google Scholar] [CrossRef]
- Litto, R.; Hayes, R.E.; Sapoundjiev, H.; Fuxman, A.; Forbes, F.; Liu, B.; Bertrand, F. Optimization of a flow reversal reactor for the catalytic combustion of lean methane mixtures. Catal. Today 2006, 117, 536–542. [Google Scholar] [CrossRef]
- Matros, Y.S.; Bunimovich, G.A.; Strots, V.O.; Mirosh, E.A. Reversed flow converter for emission control after automotive engines. Chem. Eng. Sci. 1999, 54, 2889–2898. [Google Scholar] [CrossRef]
- Liu, B.; Checkel, M.D.; Hayes, R.E.; Zheng, M.; Mirosh, E.A. Transient Simulation of a Catalytic Converter for a Dual Fuel Engine. Can. J. Chem. Eng. 2000, 78, 557–568. [Google Scholar] [CrossRef]
- Liu, B.; Checkel, M.D.; Hayes, R.E. Experimental study of a reverse flow catalytic converter for a dual fuel engine. Can. J. Chem. Eng. 2001, 79, 491–506. [Google Scholar] [CrossRef]
- Liu, B.; Hayes, R.E.; Checkel, M.D.; Zheng, M.; Mirosh, E.A. Reversing flow catalytic converter for a natural gas/diesel dual fuel engine. Chem. Eng. Sci. 2001, 56, 2641–2658. [Google Scholar] [CrossRef]
- Liu, B.; Checkel, M.D.; Hayes, R.E.; Zheng, M.; Mirosh, E.A. Experimental and Modelling Study of Variable Cycle Time of a Reverse Flow Catalytic Converter for Natural Gas/Diesel Dual Fuel Engines. In Proceedings of the SAE Technical Paper 2000-01-0213, Detroit, MI, USA, 6–9 March 2000. [Google Scholar] [CrossRef]
- Özgülsen, F.; Cinar, A. Forced periodic operation of tubular reactors. Chem. Eng. Sci. 1994, 49, 3409–3419. [Google Scholar] [CrossRef]
- Fabian Ramos, H.S.; Mmbaga, J.P.; Hayes, R.E. Catalyst Optimization in a Catalytic Flow Reversal Reactor for Lean Methane Combustion. Catal. Today 2023, 407, 182–193. [Google Scholar] [CrossRef]
- Fuxman, A.M.; Aksikas, I.; Forbes, J.F.; Hayes, R.E. LQ-Feedback control of a reverse flow reactor. J. Process Control 2008, 18, 654–662. [Google Scholar] [CrossRef]
- Fuxman, A.M.; Forbes, J.F.; Hayes, R.E. Characteristics-based model predictive control of a catalytic flow reversal reactor. Can. J. Chem. Eng. 2007, 84, 424–432. [Google Scholar] [CrossRef]
- Balaji, S.; Fuxman, A.; Lakshminarayanan, S.; Forbes, J.F.; Hayes, R.E. Repetitive model predictive control of a reverse flow reactor. Chem. Eng. Sci. 2007, 62, 2154–2167. [Google Scholar] [CrossRef]
- Devals, C.; Fuxman, A.M.; Bertrand, F.; Forbes, J.F.; Perrier, M.; Hayes, R.E. Enhanced model predictive control of a catalytic flow reversal reactor. Can. J. Chem. Eng. 2009, 87, 620–631. [Google Scholar] [CrossRef]
- Dongworth, M.R.; Melvin, A. Diffusive catalytic combustion. Sixt. Int. Symp. Combust. 1977, 16, 255–264. [Google Scholar] [CrossRef]
- Radcliffe, S.W.; Hickman, R.G. Diffusive catalytic combustors. J. Inst. Fuel 1975, 48, 208–214. [Google Scholar]
- Trimm, D.L.; Lam, C. The combustion of methane on platinum—Alumina fibre catalysts—I: Kinetics and mechanism. Chem. Eng. Sci. 1980, 35, 1405–1413. [Google Scholar] [CrossRef]
- Trimm, D.L.; Lam, C. The combustion of methane on platinum—Alumina fibre catalysts—II design and testing of a convective-diffusive type catalytic combustor. Chem. Eng. Sci. 1980, 35, 1731–1739. [Google Scholar] [CrossRef]
- Kiwi-Minsker, L.; Yuranov, I.; Slavinskaia, E.; Zaikovskii, V.; Renken, A. Pt and Pd supported on glass fibers as effective combustion catalysts. Catal. Today 2000, 59, 61–68. [Google Scholar] [CrossRef]
- Saracco, G.; Cerri, I.; Specchia, V.; Accornero, R. Catalytic pre-mixed fibre burners. Chem. Eng. Sci. 1999, 54, 3599–3608. [Google Scholar] [CrossRef]
- Specchia, V.; Sicardi, S.; Gianetto, A. Methane combustion with catalytic panels: Interpretation of the internal profiles of a longitudinal dispersion model. Chem. Eng. Commun. 1981, 10, 189–203. [Google Scholar] [CrossRef]
- Jodeiri, N.; Mmbaga, J.P.; Wu, L.; Wanke, S.E.; Hayes, R.E. Modelling a counter-diffusive reactor for methane combustion. Comput. Chem. Eng. 2012, 39, 47–56. [Google Scholar] [CrossRef]
- Partopour, B.; Dixon, A.G. An Integrated Workflow for Resolved-Particle Packed Bed Models with Complex Particle Shapes. Powder Technol. 2017, 322, 70–85. [Google Scholar] [CrossRef]
- Dixon, A.G. CFD as a Design Tool for Fixed-Bed Reactors. Ind. Eng. Chem. Res. 2001, 40, 5246–5254. [Google Scholar] [CrossRef]
- Reddy, K.B.V.; Dixon, A.G. Multi-scale Two-dimensional Packed Bed Reactor Model for Industrial Steam Methane Reforming. Chem. Eng. Sci. 2020, 215, 115421. [Google Scholar]
- Boigné, E.; Zirwes, T.; Parkinson, D.Y.; Vignat, G.; Muhunthan, P.; Barnard, H.S.; MacDowell, A.A.; Ihme, M.M. Integrated experimental and computational analysis of porous media combustion by combining gas-phase synchrotron CT, IR-imaging, and pore-resolved simulations. Combust. Flame 2024, 259, 113132. [Google Scholar] [CrossRef]
- Khatoonabadi, M.; Prasianakis, N.I.; Mantzaras, J. A pore-level 3D lattice Boltzmann simulation of mass transport and reaction in catalytic particles used for methane synthesis. Int. J. Heat Mass Transf. 2014, 221, 125025. [Google Scholar] [CrossRef]
- Young, L.C.; Finlayson, B.A. Mathematical Models of the Monolith Catalytic Converter: Part I. Development of Model and Application of Orthogonal Collocation. AIChE J. 1976, 22, 331–343. [Google Scholar] [CrossRef]
- Young, L.C.; Finlayson, B.A. Mathematical Models of the Monolith Catalytic Converter: Part II. Application to Automobile Exhaust. AIChE J. 1976, 22, 343–353. [Google Scholar] [CrossRef]
- Groppi, G.; Belloli, A.; Tronconi, E.; Forzatti, P. A Comparison of Lumped and Distributed Models of Monolith Catalytic Combustors. Chem. Eng. Sci. 1995, 50, 2705–2714. [Google Scholar] [CrossRef]
- Tronconi, E.; Forzatti, P. Adequacy of Lumped Parameter Models for SCR Reactors with Monolith Structure. AIChE J. 1992, 38, 201–210. [Google Scholar] [CrossRef]
- Groppi, G.; Belloli, A.; Tronconi, E.; Forzatti, P. Analysis of Multidimensional Models of Monolith Catalysts for Hybrid Combustors. AIChE J. 1995, 41, 2250–2262. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E. Theoretical analysis of mass and heat transfer in monolith catalysts with triangular channels. Chem. Eng. Sci. 1997, 52, 3521–3526. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E.; Forzatti, P. Mathematical Models of Catalytic Combustors. Catal. Rev.-Sci. Eng. 1999, 41, 227–254. [Google Scholar] [CrossRef]
- Hayes, R.E.; Cornejo, I. Multi-scale Modelling of Monolith Reactors: A Thirty-year Perspective from 1990 to 2020. Can. J. Chem. Eng. 2021, 99, 2589–2606. [Google Scholar] [CrossRef]
- Nien, T.; Mmbaga, J.P.; Hayes, R.E.; Votsmeier, M. Hierarchical multi-scale model reduction in the simulation of catalytic converters. Chem. Eng. Sci. 2013, 93, 362–375. [Google Scholar] [CrossRef]
- Meisel, W.S.; Collins, D.C. Repro-modeling: An approach to efficient model utilization and interpretation. Syst. Man Cybern. IEEE Trans. Syst. Man Cybern. SMC-3 1973, 3, 349–358. [Google Scholar] [CrossRef]
- Turányi, T. Application of repro-modeling for the reduction of combustion mechanisms. Symp. (Int.) Combust. 1994, 25, 949–955. [Google Scholar] [CrossRef]
- Turányi, T. Parameterization of reaction mechanisms using orthonormal polynomials. Comput. Chem. 1994, 18, 45–54. [Google Scholar] [CrossRef]
- Votsmeier, M. Efficient implementation of detailed surface chemistry into reactor models using mapped rate data. Chem. Eng. Sci. 2009, 64, 1384–1389. [Google Scholar] [CrossRef]
- Pope, S.B. Computationally efficient implementation of combustion chemistry using in situ adaptive tabulation. Combust. Theory Model. 1997, 1, 41–63. [Google Scholar] [CrossRef]
- Kumar, A.; Mazumder, S. Adaptation and application of the In Situ Adaptive Tabulation (ISAT) procedure to reacting flow calculations with complex surface chemistry. Comput. Chem. Eng. 2010, 35, 1317–1327. [Google Scholar] [CrossRef]
- Yan, X.; Maas, U. Intrinsic low-dimensional manifolds of heterogeneous combustion processes. Proc. Combust. Inst. 2000, 28, 1615–1621. [Google Scholar] [CrossRef]
- Diaz-Ibarra, O.; Kim, K.; Safta, C.; Zador, J.; Najm, H.N. Using computational singular perturbation as a diagnostic tool in ODE and DAE systems: A case study in heterogeneous catalysis. Combust. Theory Model. 2022, 26, 201–227. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z. Towards foundation model for chemical reactor modeling: Meta-learning with physics-informed adaptation. Chem. Eng. Res. Des. 2025, 218, 839–853. [Google Scholar] [CrossRef]
- Mowbray, M.; Vallerio, M.; Perez-Galvan, C.; Zhang, D.; Del Rio Chanona, A.; Navarro-Brull, F.J. Industrial data science—A review of machine learning applications for chemical and process industries. React. Chem. Eng. 2022, 7, 1471–1509. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, X.; Ouyang, B.; Yan, W.; Lei, H.; Chen, Z.; Luo, Z. Review of Machine Learning for Hydrodynamics, Transport, and Reactions in Multiphase Flows and Reactors. Ind. Eng. Chem. Res. 2022, 61, 9901–9949. [Google Scholar] [CrossRef]
- Babanezhad, M.; Behroyan, I.; Nakhjiri, A.T.; Marjani, A.; Rezakazemi, M.; Shirazian, S. High-performance hybrid modeling chemical reactors using differential evolution based fuzzy inference system. Sci. Rep. 2020, 10, 21304. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Ghaemi, A.; Kelishami, A.R.; Movahedirad, S. Predictive modeling of membrane reactor efficiency using advanced artificial neural networks for green hydrogen production. Sci. Rep. 2024, 14, 24211. [Google Scholar] [CrossRef]
- Shettigar, P.; Kumbhare, J.; Yadav, E.S.; Indiran, T. Wiener-Neural-Network-Based Modeling and Validation of Generalized Predictive Control on a Laboratory-Scale Batch Reactor. ACS Omega 2022, 7, 16341–16351. [Google Scholar] [CrossRef]
- Thajudeen, K.Y.; Ahmed, M.M.; Alshehri, S.A. Development of hybrid computational model for simulation of heat transfer and temperature prediction in chemical reactors. Sci. Rep. 2025, 15, 14628. [Google Scholar] [CrossRef] [PubMed]
- Tom Savage, T.; Basha, N.; McDonough, J.; Krassowski, J.; Matar, O.; del Rio Chanona, E.A. Machine learning-assisted discovery of flow reactor designs. Nat. Chem. Eng. 2024, 1, 522–531. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. Chemical Imaging of Spatial Heterogeneities in Catalytic Solids at Different Length and Time Scales. Angew. Chem. Int. Ed. 2009, 48, 4910–4943. [Google Scholar] [CrossRef]
- Mantzaras, J. Progress in non-intrusive laser-based measurements of gas-phase thermoscalars and supporting modeling near catalytic interfaces. Prog. Energy Combust. Sci. 2019, 70, 169–211. [Google Scholar] [CrossRef]
- Kanitkar, S.R.; Dutta, B.; Md Abedin, A.; Bai, X.; Haynes, D.J. Advanced Manufacturing in Heterogeneous Catalysis; RCS SPR–Catalysis; SPR: Putrajaya, Malaysia, 2024; pp. 1–41. [Google Scholar]
- Rosseau, L.R.S.; Middelkoop, V.; Willemsen, H.A.M.; Roghai, I.; van Sint Annaland, M. Review on Additive Manufacturing of Catalysts and Sorbents and the Potential for Process Intensification. Front. Chem. Eng. 2022, 4, 8–26. [Google Scholar] [CrossRef]
- Reinao, C.; Diaz, P.; Cornejo, I. A new model for the pressure drop in dual-cell density monoliths: Experimental validation and CFD. Chem. Eng. J. 2025, 516, 163599. [Google Scholar] [CrossRef]
- Mackiewicz, E.; Wejrzanowski, T.; Nowacki, R.; Jaroszewicz, J.; Marchewka, J.; Wilk, L.; Bezkosty, P.; Sitarz, M. 3D hierarchical porous structures printed from a silica-nickel composite paste. Appl. Mater. Today 2023, 33, 101859. [Google Scholar] [CrossRef]
- Bazte, O.; Botana, F.J.; Calvino, J.J.; Cauqui, M.A.; Gatica, J.M.; Vidal, H.; Gonzalez-Rovira, L.; Lopez-Castro, J.; Yeste, M.P.; Blanco, G.; et al. Novel combination of 3D printing and electrochemical deposition to design and prepare metallic honeycomb supported catalysts for dry reforming of methane. Chem. Eng. J. 2025, 506, 159939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).