Enhancement of Co-Production of Astaxanthin and Total Fatty Acids in Haematococcus lacustris by Combined Treatment with Exogenous Indole-3-Acetic Acid and Abscisic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Strain and Photosynthetic Cultivation
2.2. Phytohormone Treatment
2.3. Astaxanthin Quantification
2.4. Fatty Acid Quantification
2.5. Additional Analyses
2.6. Statistical Analysis
3. Results and Discussion
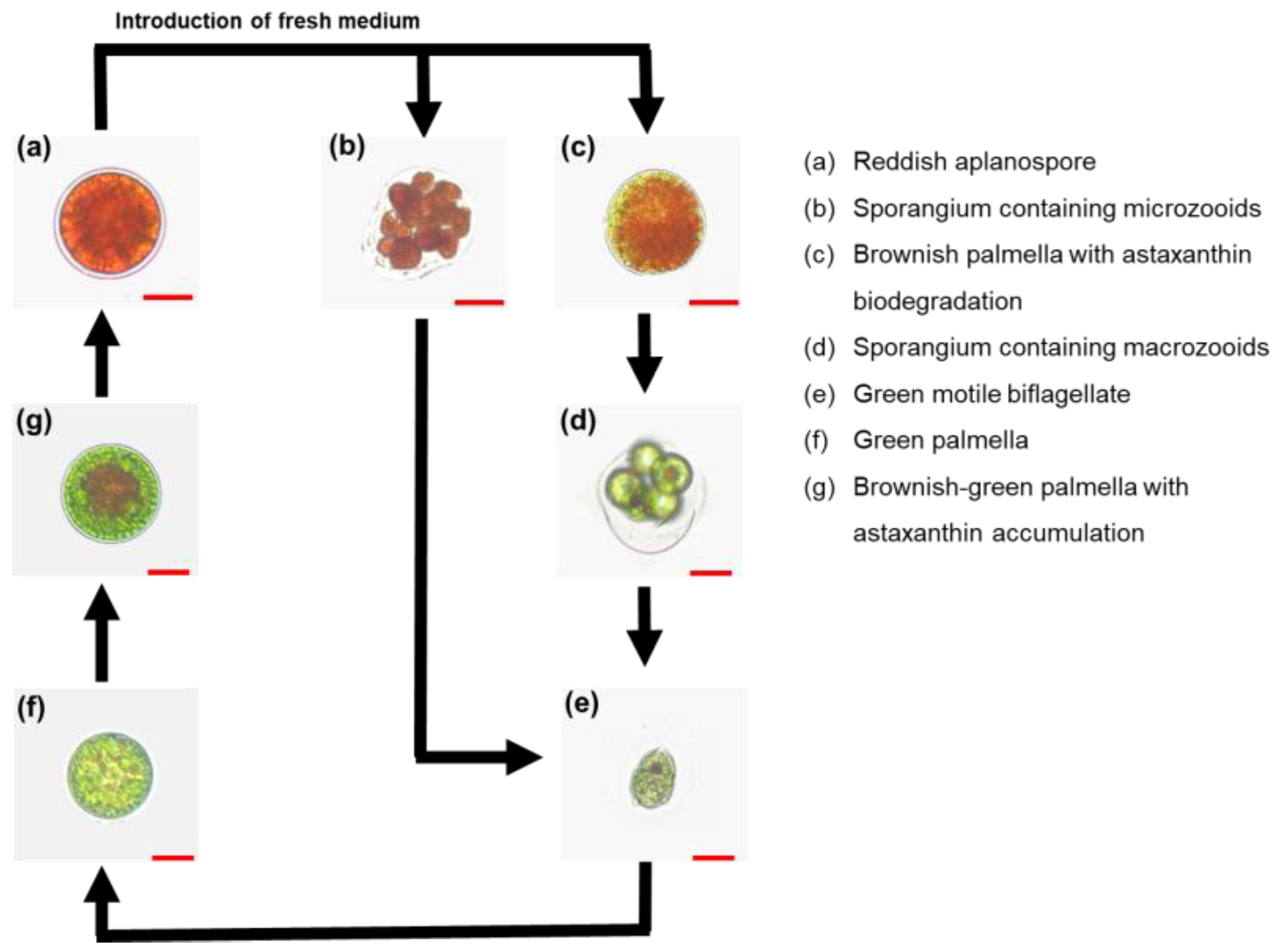
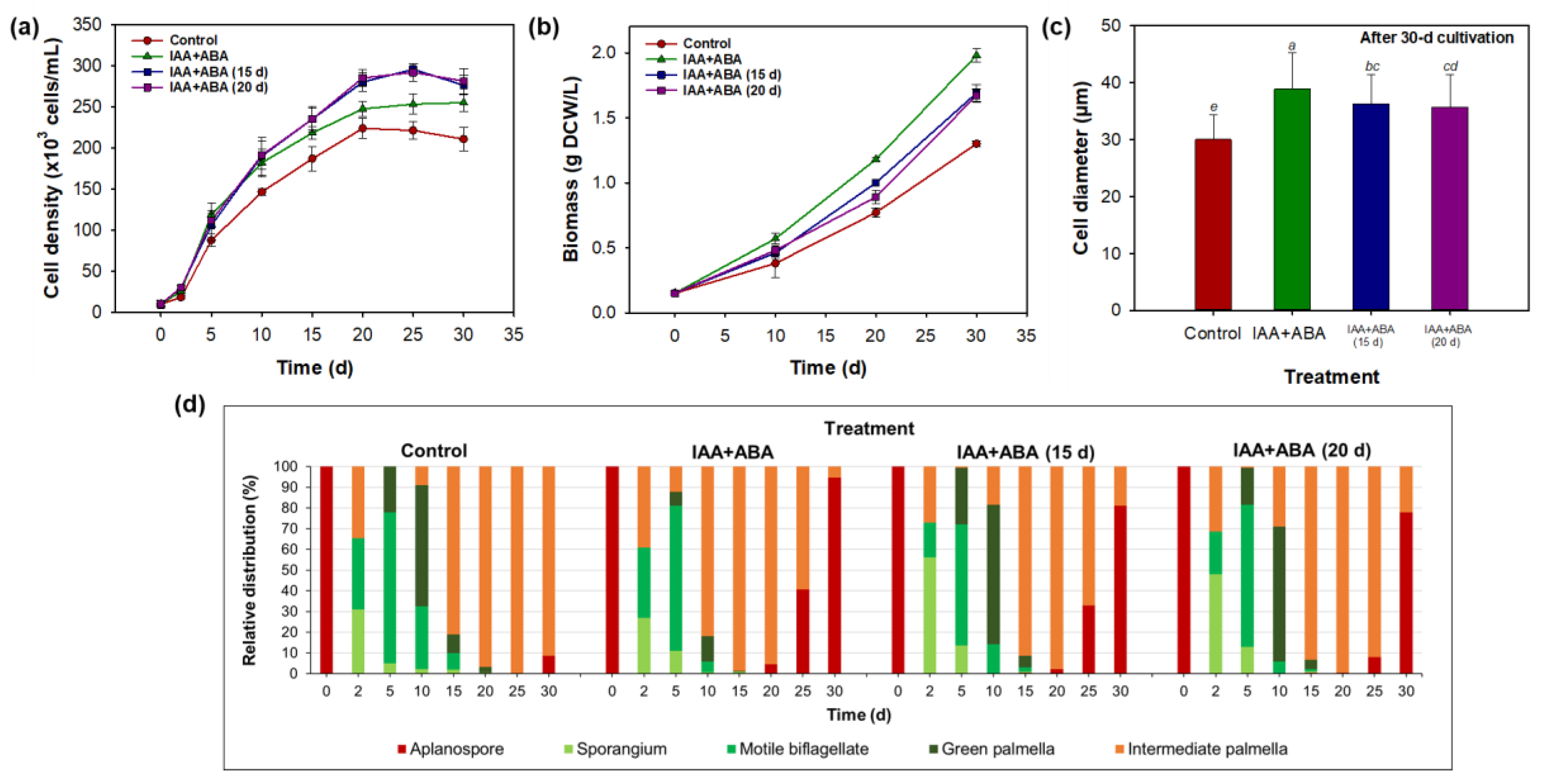
3.1. Proliferation Patterns in the Life Cycle of Haematococcus lacustris
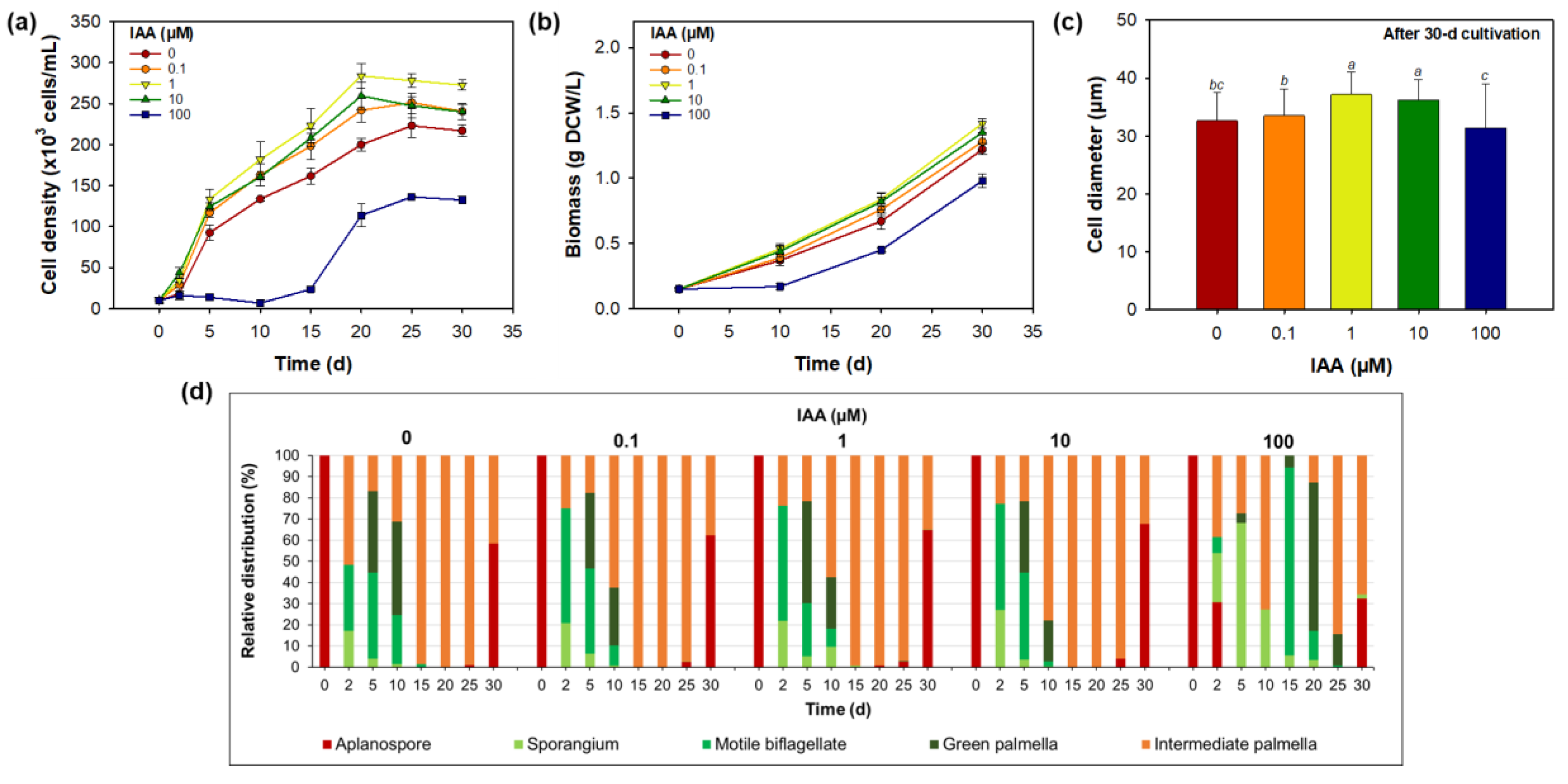
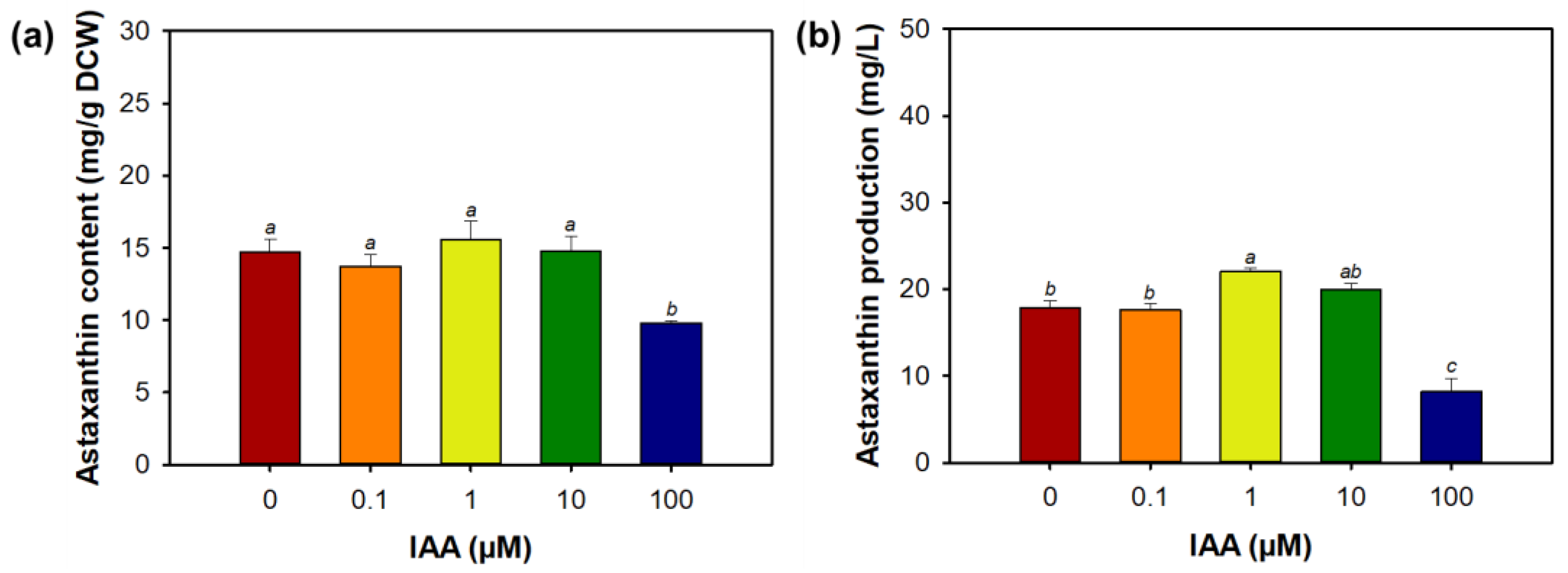
3.2. Effect of Indole-3-Acetic Acid on Morphology, Growth, and Astaxanthin Biosynthesis
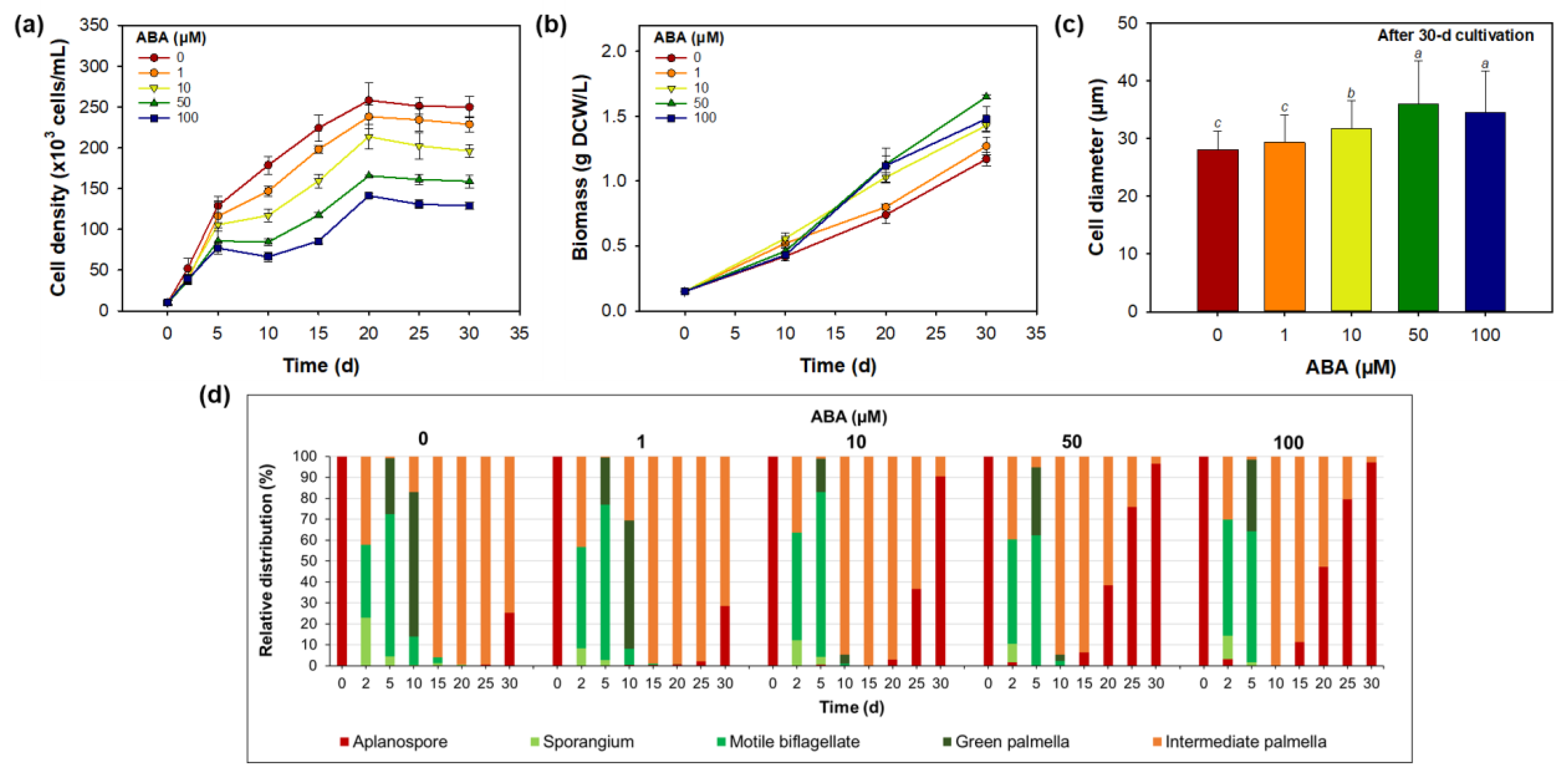
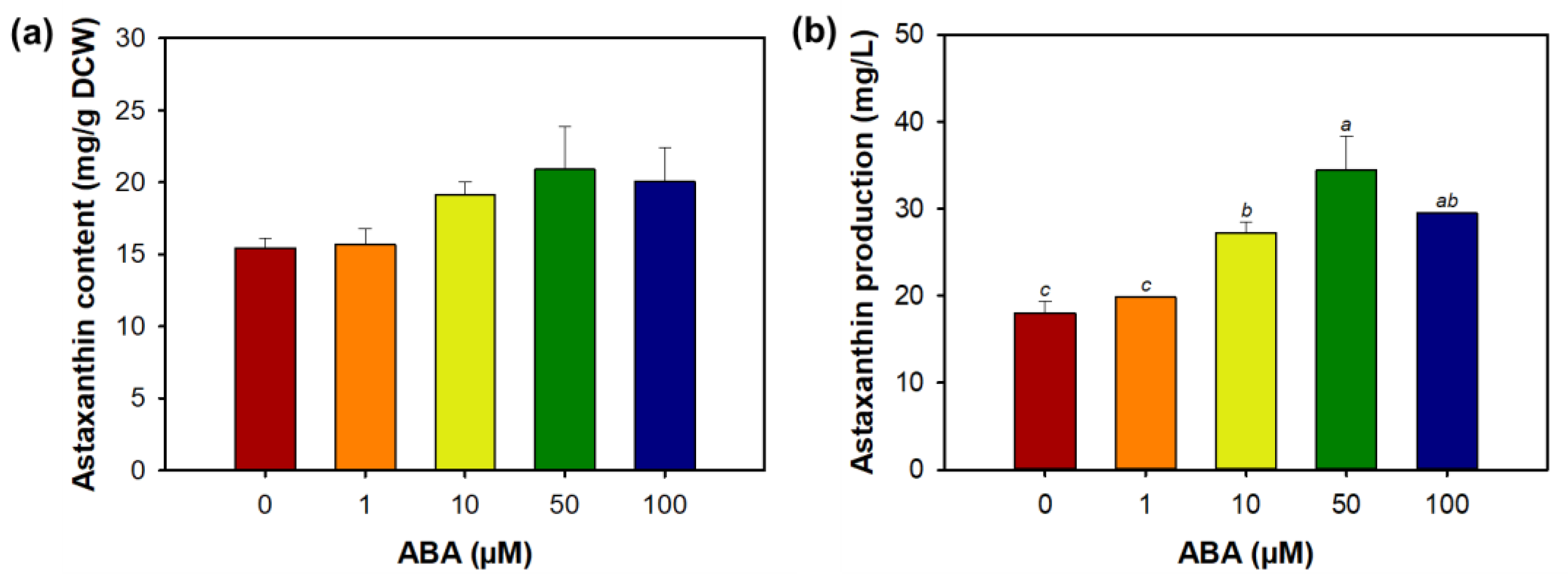
3.3. Effect of Abscisic Acid on Morphology, Growth, and Astaxanthin Biosynthesis
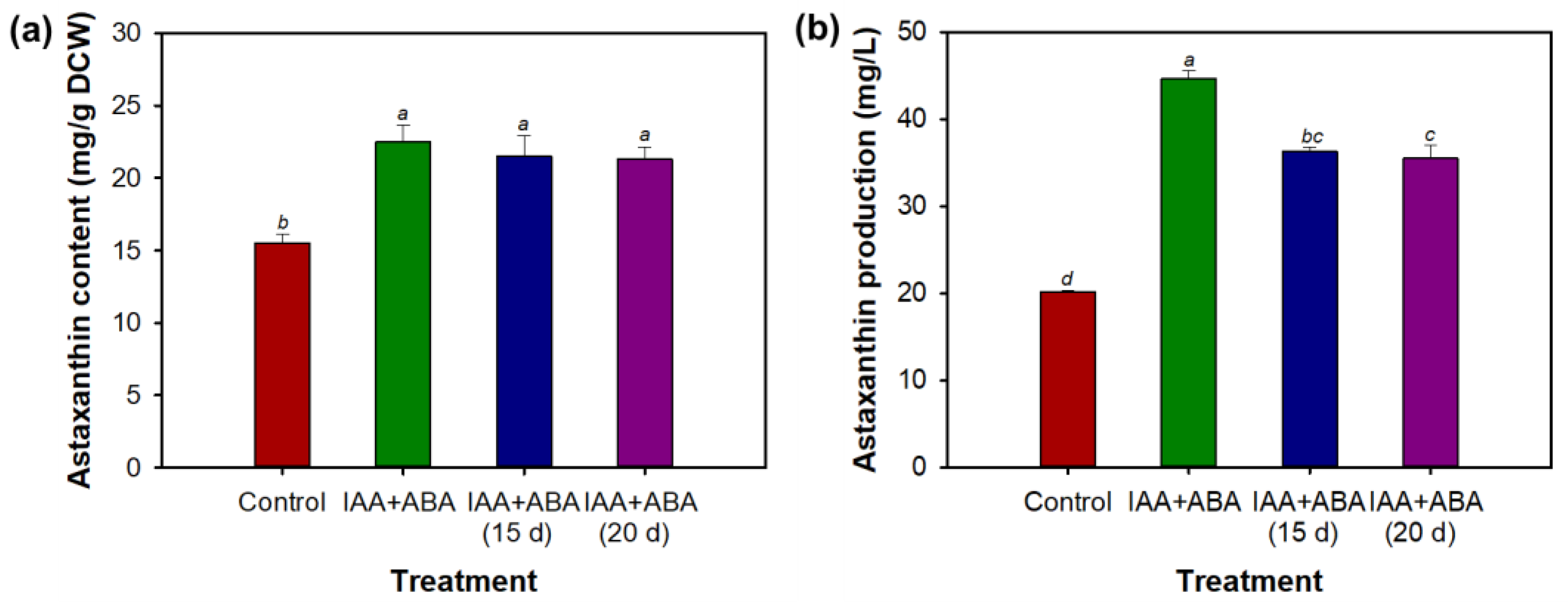
3.4. Combined Effect of Indole-3-Acetic Acid and Abscisic Acid on Morphology, Growth, and Astaxanthin Biosynthesis
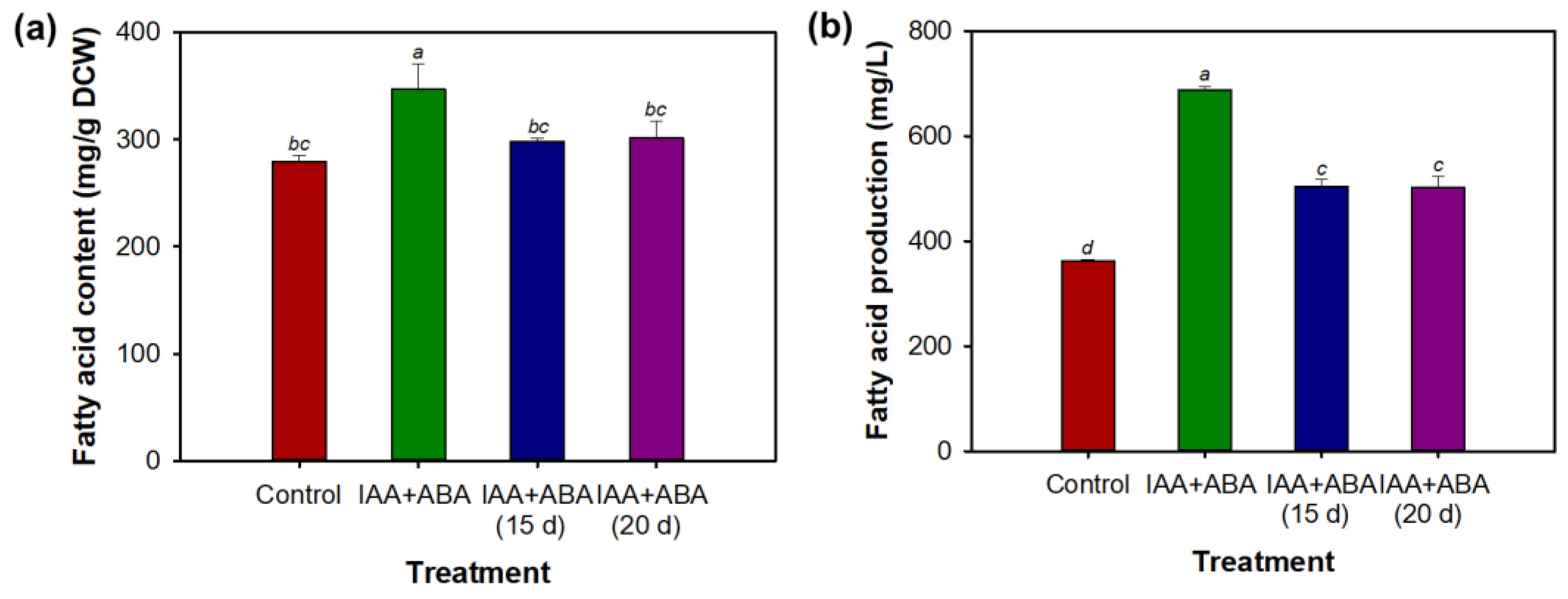
3.5. Combined Effect of Indole-3-Acetic Acid and Abscisic Acid on Fatty Acid Content and Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, H.; Kim, B.; Jeong, S.H.; Kim, T.Y.; Kim, D.; Oh, Y.; Hahn, S.K. Microalgae-Based Biohybrid Microrobot for Accelerated Diabetic Wound Healing. Small 2023, 19, 2204617. [Google Scholar] [CrossRef]
- Diankristanti, P.A.; Ho, N.H.E.; Chen, J.-H.; Nagarajan, D.; Chen, C.-Y.; Hsieh, Y.-M.; Ng, I.-S.; Chang, J.-S. Unlocking the potential of microalgae as sustainable bioresources from up to downstream processing: A critical review. Chem. Eng. J. 2024, 488, 151124. [Google Scholar] [CrossRef]
- Yoo, D.; Hong, S.-J.; Yun, S.; Kang, M.-J.; Cho, B.-K.; Lee, H.; Choi, H.-K.; Kim, D.-M.; Lee, C.-G. Metabolic engineering for redirecting carbon to enhance the fatty acid content of Synechocystis sp. Pcc6803. Biotechnol. Bioprocess Eng. 2023, 28, 274–280. [Google Scholar] [CrossRef]
- Debnath, T.; Bandyopadhyay, T.K.; Vanitha, K.; Bobby, M.N.; Nath Tiwari, O.; Bhunia, B.; Muthuraj, M. Astaxanthin from microalgae: A review on structure, biosynthesis, production strategies and application. Food Res. Int. 2024, 176, 113841. [Google Scholar] [CrossRef]
- Patel, A.K.; Tambat, V.S.; Chen, C.-W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.-Y.; Dong, C.-D.; Singhania, R.R. Recent advancements in astaxanthin production from microalgae: A review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Kim, B.; Youn Lee, S.; Lakshmi Narasimhan, A.; Kim, S.; Oh, Y.-K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124. [Google Scholar] [CrossRef]
- Hata, N.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Lakshmi Narasimhan, A.; Lee, N.; Kim, S.; Kim, Y.-E.; Christabel, C.; Yu, H.; Kim, E.-J.; Oh, Y.-K. Enhanced astaxanthin production in Haematococcus lacustris by electrochemical stimulation of cyst germination. Bioresour. Technol. 2024, 411, 131301. [Google Scholar] [CrossRef]
- Lee, C.; Choi, Y.-E.; Yun, Y.-S. A strategy for promoting astaxanthin accumulation in Haematococcus pluvialis by 1-aminocyclopropane-1-carboxylic acid application. J. Biotechnol. 2016, 236, 120–127. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Han, S.-I.; Chang, S.H.; Lee, C.; Jeon, M.S.; Heo, Y.M.; Kim, S.; Choi, Y.-E. Astaxanthin biosynthesis promotion with pH shock in the green microalga, Haematococcus lacustris. Bioresour. Technol. 2020, 314, 123725. [Google Scholar] [CrossRef]
- Samhat, K.; Kazbar, A.; Takache, H.; Gonçalves, O.; Drouin, D.; Ismail, A.; Pruvost, J. Optimization of continuous astaxanthin production by Haematococcus pluvialis in nitrogen-limited photobioreactor. Algal Res. 2024, 80, 103529. [Google Scholar] [CrossRef]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Zhang, Y.; Cheng, P.; Ma, R.; Cheng, W.; Chu, H. Enhancing astaxanthin accumulation in Haematococcus pluvialis by coupled light intensity and nitrogen starvation in column photobioreactors. J. Microbiol. Biotechnol. 2018, 28, 2019–2028. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef]
- Li, Q.; You, J.; Qiao, T.; Zhong, D.; Yu, X. Sodium chloride stimulates the biomass and astaxanthin production by Haematococcus pluvialis via a two-stage cultivation strategy. Bioresour. Technol. 2022, 344, 126214. [Google Scholar] [CrossRef]
- Yao, J.; Kim, H.S.; Kim, J.Y.; Choi, Y.-E.; Park, J. Mechanical stress induced astaxanthin accumulation of H. pluvialis on a chip. Lab Chip 2020, 20, 647–654. [Google Scholar] [CrossRef]
- Mahadi, R.; Kim, S.; Ilhamsyah, D.P.A.; Vahisan, L.P.S.; Narasimhan, A.L.; Park, G.W.; Lee, S.Y.; Oh, Y.-K. Rapid accumulation of astaxanthin in Haematococcus pluvialis induced by mild hydrostatic pressure. Biotechnol. Bioprocess Eng. 2023, 28, 345–351. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Zhang, Y.; Gao, H.; Zhao, Y.; Yu, X. Chemical inducers regulate ROS signalling to stimulate astaxanthin production in Haematococcus pluvialis under environmental stresses: A Review. Trends Food Sci. Technol. 2023, 136, 181–193. [Google Scholar] [CrossRef]
- Liu, Y.H.; Alimujiang, A.; Wang, X.; Luo, S.W.; Balamurugan, S.; Yang, W.D.; Liu, J.S.; Zhang, L.; Li, H.Y. Ethanol induced jasmonate pathway promotes astaxanthin hyperaccumulation in Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121720. [Google Scholar] [CrossRef]
- Du, F.; Hu, C.; Sun, X.; Zhang, L.; Xu, N. Transcriptome analysis reveals the promoting effect of trisodium citrate on astaxanthin accumulation in Haematococcus pluvialis under high light condition. Aquaculture 2021, 543, 736978. [Google Scholar] [CrossRef]
- Kim, Y.E.; Matter, I.A.; Lee, N.; Jung, M.; Lee, Y.C.; Choi, S.A.; Lee, S.Y.; Kim, J.R.; Oh, Y.K. Enhancement of astaxanthin production by Haematococcus pluvialis using magnesium aminoclay nanoparticles. Bioresour. Technol. 2020, 307, 123270. [Google Scholar] [CrossRef]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Madadkar Haghjou, M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef]
- Ramaih, S.; Guedira, M.; Paulsen, G.M. Relationship of indoleacetic acid and tryptophan to dormancy and preharvest sprouting of wheat. Funct. Plant Biol. 2003, 30, 939. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Hu, F.; Li, H.; Ma, L.; Xu, L. Exogenous IAA treatment enhances phytoremediation of soil contaminated with phenanthrene by promoting soil enzyme activity and increasing microbial biomass. Environ. Sci. Pollut. Res. 2016, 23, 10656–10664. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, B.; Zhang, C.; Yang, L.; Wang, Y.; Zhao, H. Effects of exogenous abscisic acid (ABA) on carotenoids and petal color in Osmanthus fragrans ‘Yanhonggui’. Plants 2020, 9, 454. [Google Scholar] [CrossRef]
- Kayani, S.-I.; Rahman, S.; Shen, Q.; Cui, Y.; Liu, W.; Hu, X.; Zhu, F.; Huo, S. Molecular approaches to enhance astaxanthin biosynthesis: Future outlook: Engineering of transcription factors in Haematococcus pluvialis. Crit. Rev. Biotechnol. 2024, 44, 514–529. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Han, B.; Yu, X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Bioresour. Technol. 2019, 274, 549–556. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A. The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul. 2014, 73, 57–66. [Google Scholar] [CrossRef]
- Mousavi, P.; Morowvat, M.; Montazeri-Najafabady, N.; Abolhassanzadeh, Z.; Mohagheghzadeh, A.; Hamidi, M.; Niazi, A.; Ghasemi, Y. Investigating the effects of phytohormones on growth and beta-carotene production in a naturally isolates stain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 6, 164–171. [Google Scholar] [CrossRef]
- Kozlova, T.A.; Kartashov, A.V.; Zadneprovskaya, E.; Krapivina, A.; Zaytsev, P.; Chivkunova, O.B.; Solovchenko, A.E. Effect of abscisic acid on growth, fatty acid profile, and pigment composition of the Chlorophyte chlorella (Chromochloris) zofingiensis and Its Co-Culture Microbiome. Life 2023, 13, 452. [Google Scholar] [CrossRef]
- Contreras-Pool, P.Y.; Peraza-Echeverria, S.; Ku-González, Á.F.; Herrera-Valencia, V.A. The phytohormone abscisic acid increases triacylglycerol content in the green microalga Chlorella saccharophila (Chlorophyta). Algae 2016, 31, 267–276. [Google Scholar] [CrossRef]
- Lin, Y.; Dai, Y.; Xu, W.; Wu, X.; Li, Y.; Zhu, H.; Zhou, H. The growth, lipid accumulation and fatty acid profile analysis by abscisic acid and indol-3-acetic acid induced in Chlorella sp. FACHB-8. Int. J. Mol. Sci. 2022, 23, 4064. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.S.; Kim, J.Y.; Lee, S.H.; Kim, D.G.; Roh, S.W.; Choi, Y.-E. Effects of an auxin-producing symbiotic bacterium on cell growth of the microalga Haematococcus pluvialis: Elevation of cell density and prolongation of exponential stage. Algal Res. 2019, 41, 101547. [Google Scholar] [CrossRef]
- Gao, Z.; Hongzheng, G.; Chunxiao, M. Effects of abscisic acid (ABA) carotenogenesis expression and astaxanthin accumulation in Haematococcus pluvialis. Res. J. Biotechnol. 2013, 8, 9–15. [Google Scholar]
- Kobayashi, M.; Hirai, N.; Kurimura, Y.; Ohigashi, H.; Tsuji, Y. Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regul. 1997, 22, 79–85. [Google Scholar] [CrossRef]
- Kobayashi, M.; Todoroki, Y.; Hirai, N.; Kurimura, Y.; Ohigashi, H.; Tsuji, Y. Biological activities of abscisic acid analogs in the morphological change of the green alga Haematococcus pluvialis. J. Ferment. Bioeng. 1998, 85, 529–531. [Google Scholar] [CrossRef]
- Mahadi, R.; Vahisan, L.P.S.; Ilhamsyah, D.P.A.; Kim, S.; Kim, B.; Lee, N.; Oh, Y.-K. Enhancement of astaxanthin and fatty acid production in Haematococcus pluvialis using strigolactone. Appl. Sci. 2022, 12, 1791. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhang, L. Cell cycles and proliferation patterns in Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2017, 35, 1205–1211. [Google Scholar] [CrossRef]
- Fábregas, J.; Domínguez, A.; Maseda, A.; Otero, A. Interactions between irradiance and nutrient availability during astaxanthin accumulation and degradation in Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2003, 61, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, B.; Li, J. The dynamic behaviors of photosynthesis during non-motile cell germination in Haematococcus pluvialis. Water 2022, 14, 1280. [Google Scholar] [CrossRef]
- Han, D.; Wang, J.; Sommerfeld, M.; Hu, Q. Susceptibility and protective mechanisms of motile and non motile cells of Haematococcus pluvialis (chlorophyceae) to photooxidative stress 1. J. Phycol. 2012, 48, 693–705. [Google Scholar] [CrossRef]
- Lee, S.-A.; Lee, N.; Oh, H.-M.; Kim, D.G.; Ahn, C.-Y. Fast-track production of astaxanthin by reduced cultivation time with the “red cell inoculation system” (RCIS) and various chemical cues in Haematococcus lacustris. J. Appl. Phycol. 2020, 32, 41–50. [Google Scholar] [CrossRef]
- Ha, N.C.; Tam, L.T.; Hien, H.T.M.; Thu, N.T.H.; Hong, D.D.; Thom, L.T. Optimization of culture conditions for high cell productivity and astaxanthin accumulation in Vietnam’s green microalgae Haematococcus pluvialis HB and a neuroprotective activity of its astaxanthin. Bioengineering 2024, 11, 1176. [Google Scholar] [CrossRef]
- Bauer, A.; Minceva, M. Examination of photo-, mixo-, and heterotrophic cultivation conditions on Haematococcus pluvialis cyst cell germination. Appl. Sci. 2021, 11, 7201. [Google Scholar] [CrossRef]
- Dao, G.-H.; Wu, G.-X.; Wang, X.-X.; Zhuang, L.-L.; Zhang, T.-Y.; Hu, H.-Y. Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. Lx1 by two types of auxin. Bioresour. Technol. 2018, 247, 561–567. [Google Scholar] [CrossRef]
- Yu, X.; Niu, X.; Zhang, X.; Pei, G.; Liu, J.; Chen, L.; Zhang, W. Identification and mechanism analysis of chemical modulators enhancing astaxanthin accumulation in Haematococcus pluvialis. Algal Res. 2015, 11, 284–293. [Google Scholar] [CrossRef]
- Chen, J.; Wei, D.; Lim, P.-E. Enhanced coproduction of astaxanthin and lipids by the green microalga Chromochloris zofingiensis: Selected phytohormones as positive stimulators. Bioresour. Technol. 2020, 295, 122242. [Google Scholar] [CrossRef]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahadi, R.; Oh, Y.-K.; Sathiyavahisan, L.P.; Lakshmi Narasimhan, A.; Kim, E.-J.; Cho, J.Y. Enhancement of Co-Production of Astaxanthin and Total Fatty Acids in Haematococcus lacustris by Combined Treatment with Exogenous Indole-3-Acetic Acid and Abscisic Acid. Appl. Sci. 2025, 15, 10136. https://doi.org/10.3390/app151810136
Mahadi R, Oh Y-K, Sathiyavahisan LP, Lakshmi Narasimhan A, Kim E-J, Cho JY. Enhancement of Co-Production of Astaxanthin and Total Fatty Acids in Haematococcus lacustris by Combined Treatment with Exogenous Indole-3-Acetic Acid and Abscisic Acid. Applied Sciences. 2025; 15(18):10136. https://doi.org/10.3390/app151810136
Chicago/Turabian StyleMahadi, Rendi, You-Kwan Oh, Laxmi Priya Sathiyavahisan, Aditya Lakshmi Narasimhan, Eui-Jin Kim, and Ja Young Cho. 2025. "Enhancement of Co-Production of Astaxanthin and Total Fatty Acids in Haematococcus lacustris by Combined Treatment with Exogenous Indole-3-Acetic Acid and Abscisic Acid" Applied Sciences 15, no. 18: 10136. https://doi.org/10.3390/app151810136
APA StyleMahadi, R., Oh, Y.-K., Sathiyavahisan, L. P., Lakshmi Narasimhan, A., Kim, E.-J., & Cho, J. Y. (2025). Enhancement of Co-Production of Astaxanthin and Total Fatty Acids in Haematococcus lacustris by Combined Treatment with Exogenous Indole-3-Acetic Acid and Abscisic Acid. Applied Sciences, 15(18), 10136. https://doi.org/10.3390/app151810136






