Fabrication of Mechanically Robust Water-Soluble Core Molds and Experimental Validation to Manufacture a Composite Part
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
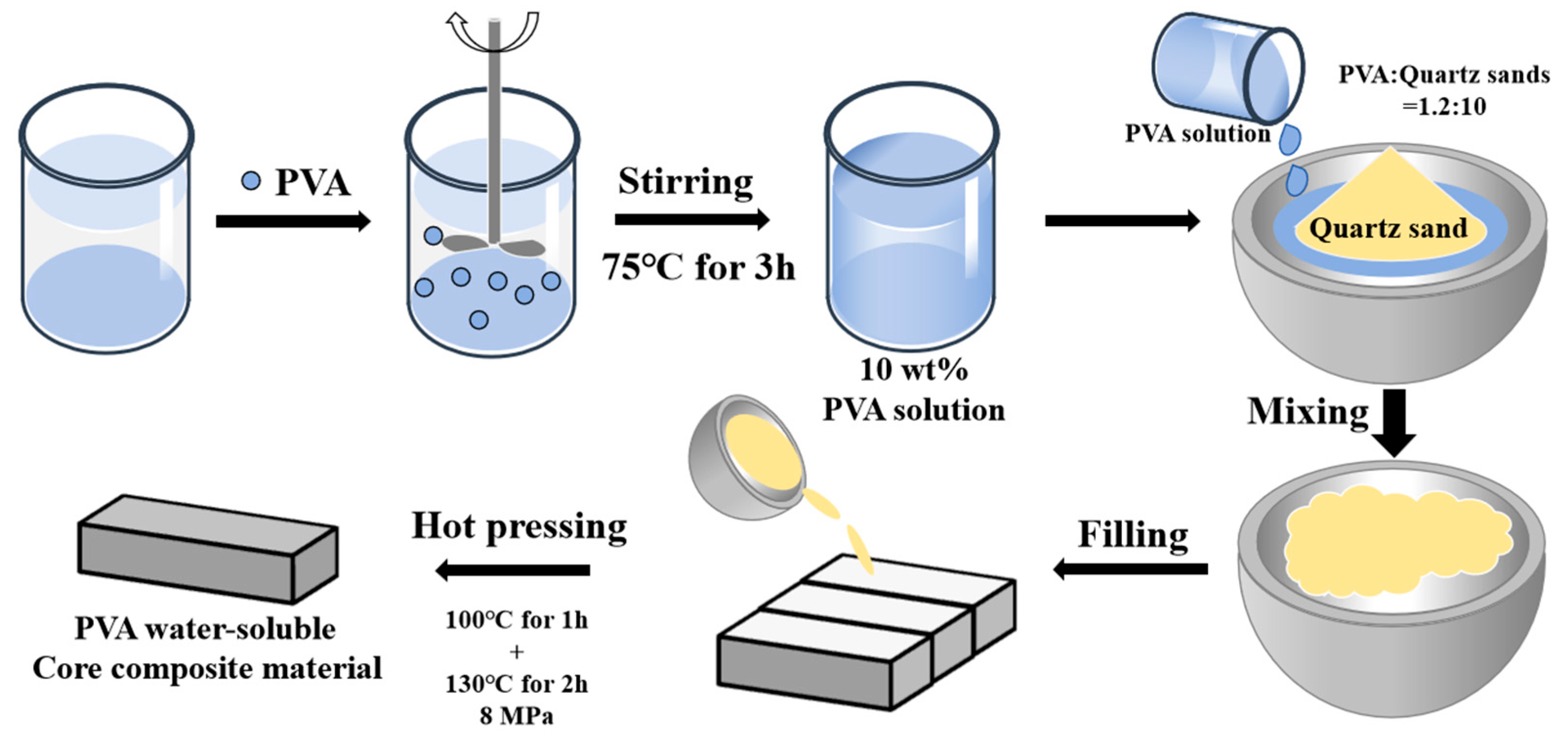
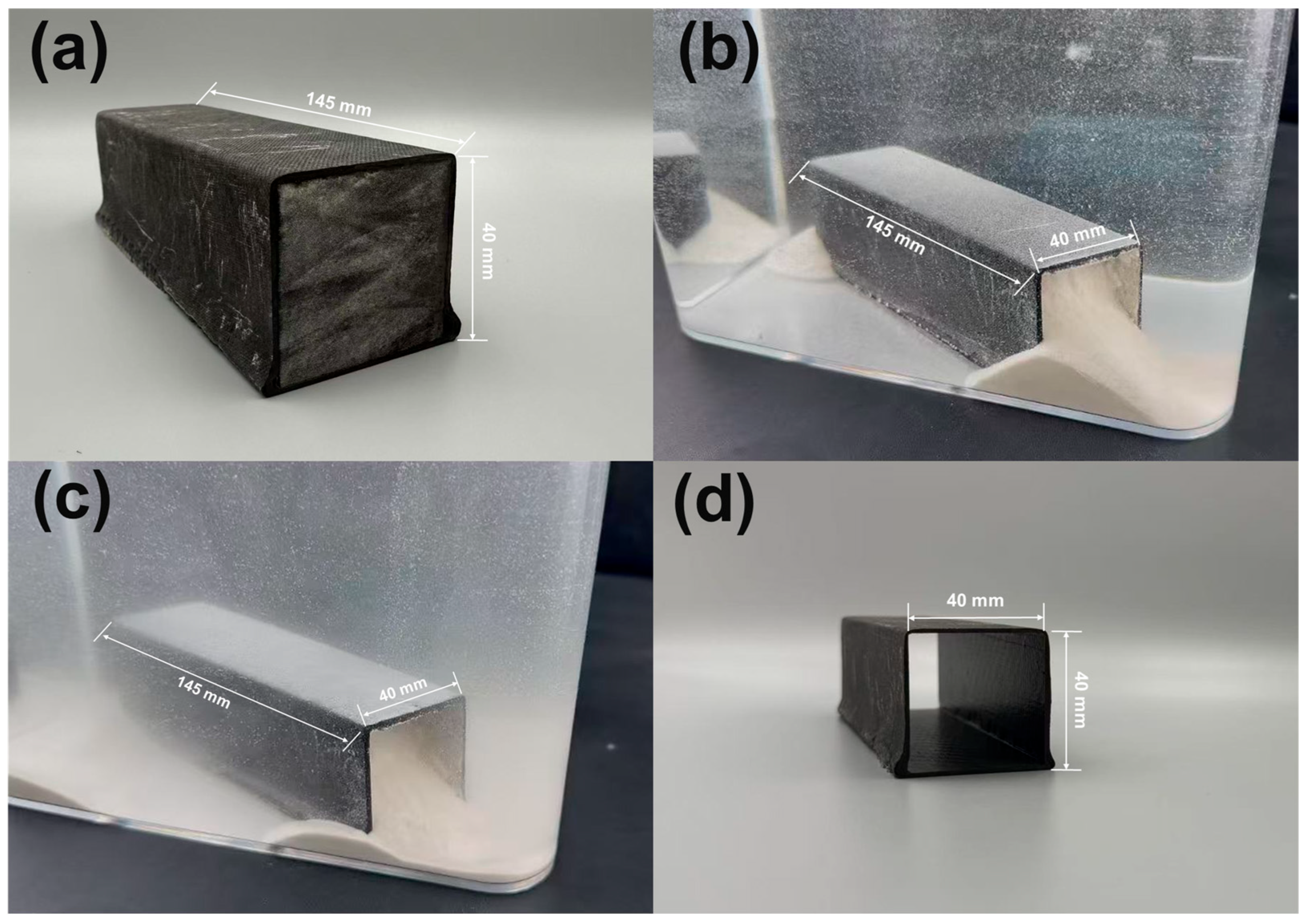
2.2. Preparation of Water-Soluble Core Mold and Its Application
2.3. Characterization
3. Results and Discussion
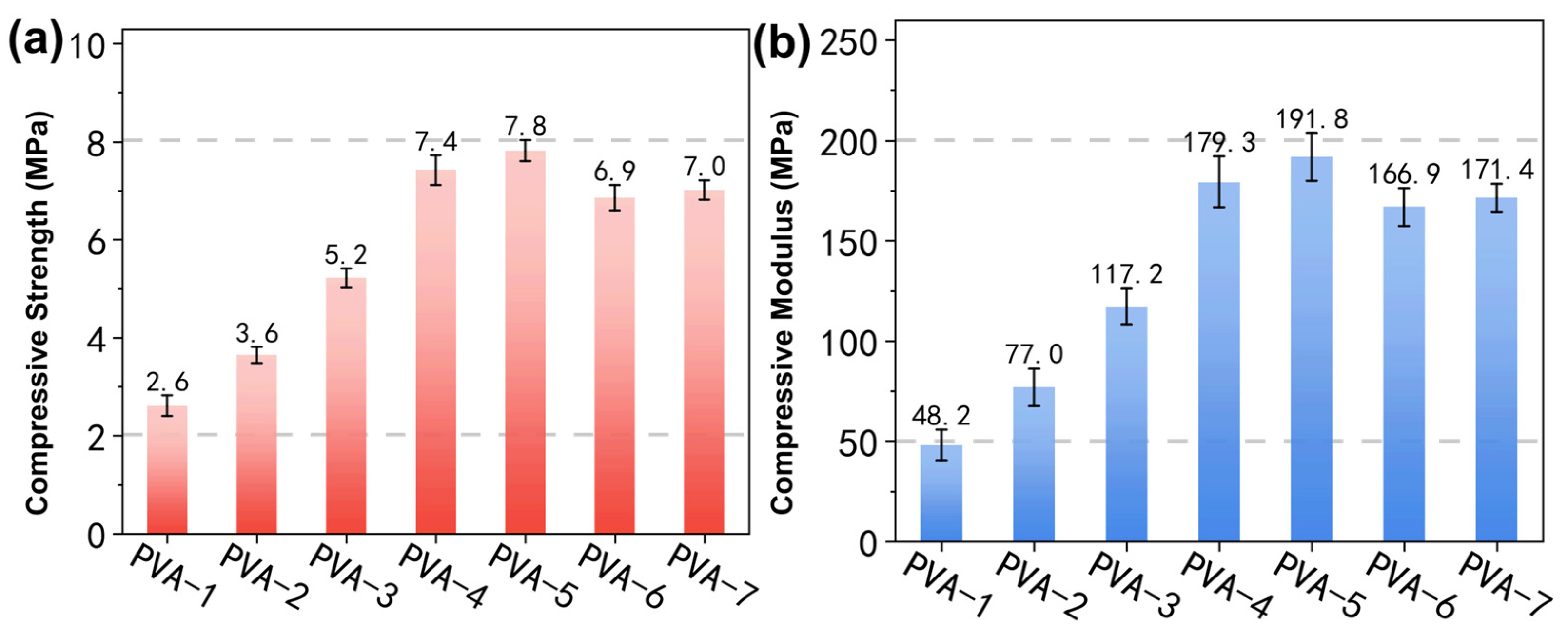
3.1. Compressive Properties of Core Molds
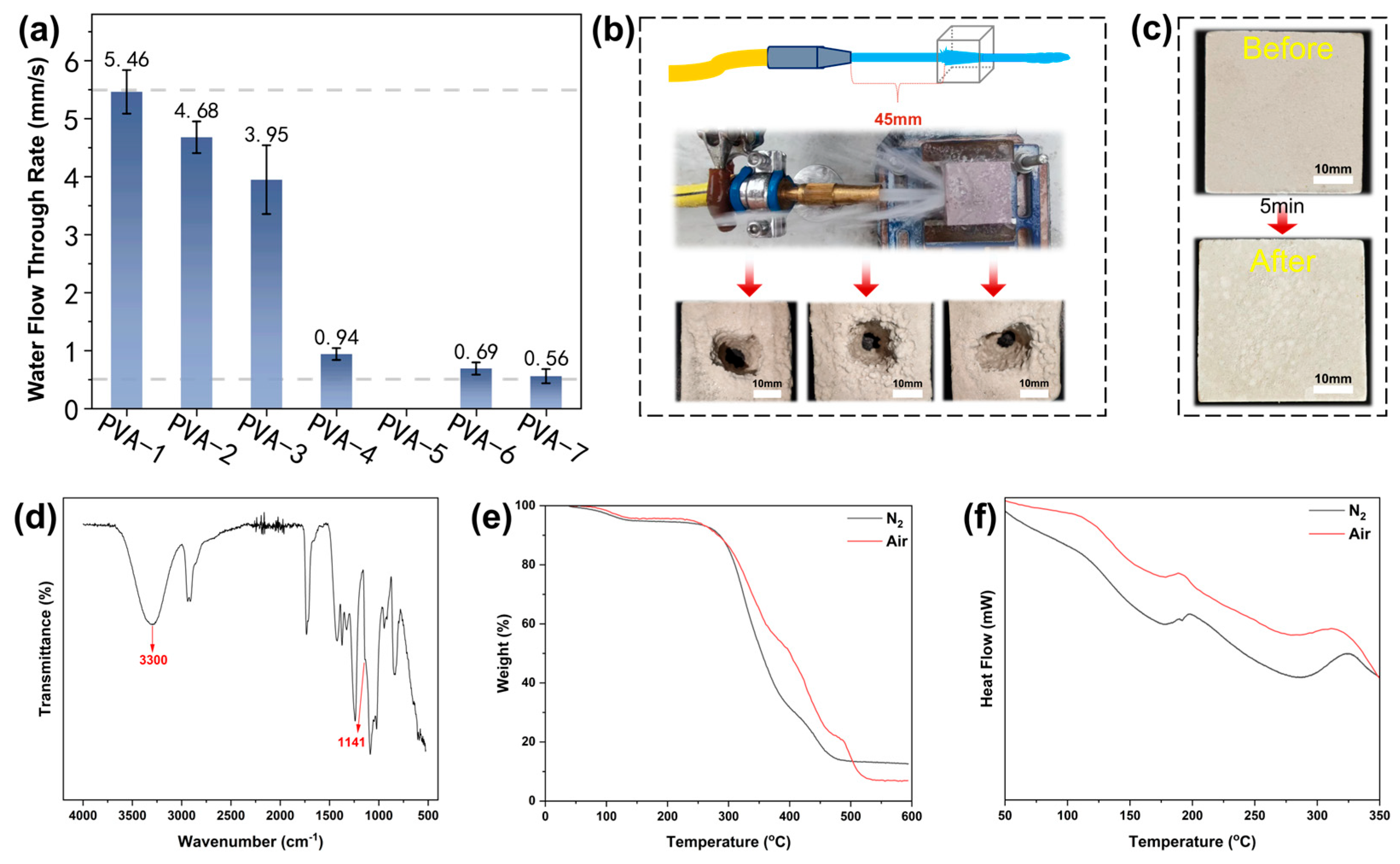
3.2. Water Penetration Rate of Core Molds
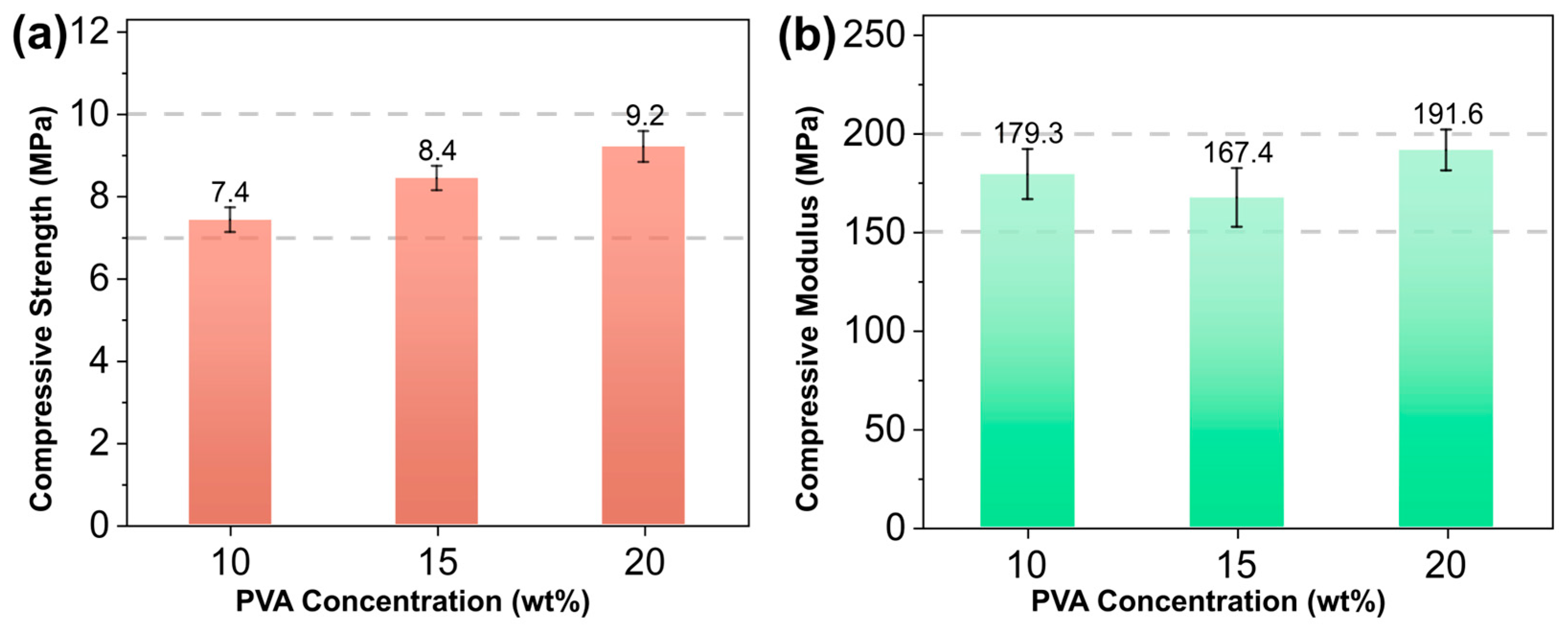
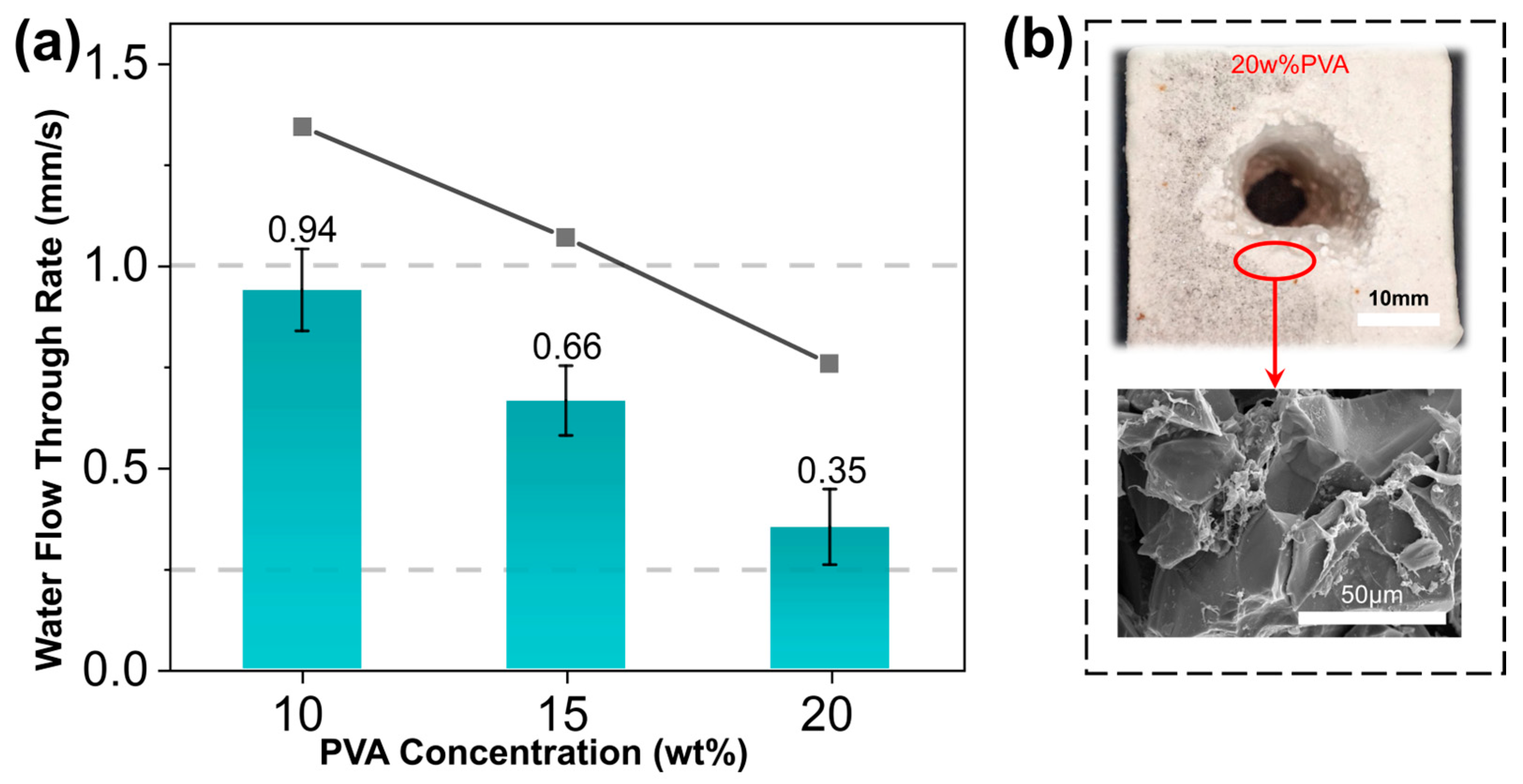
3.3. Effect of PVA Concentration on the Performance of Core Molds
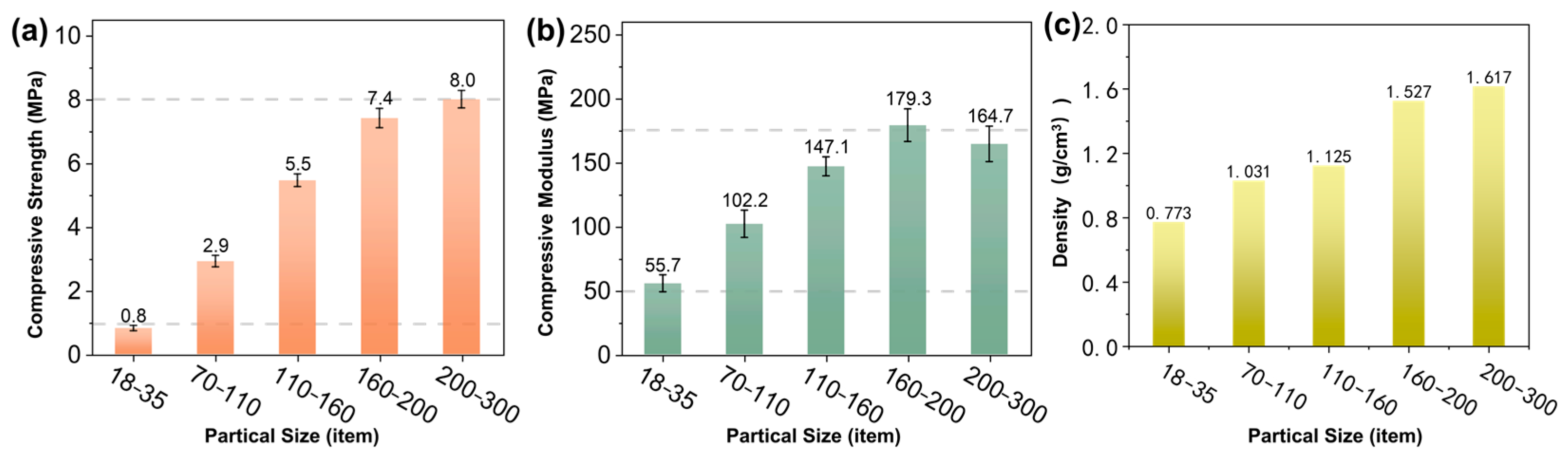
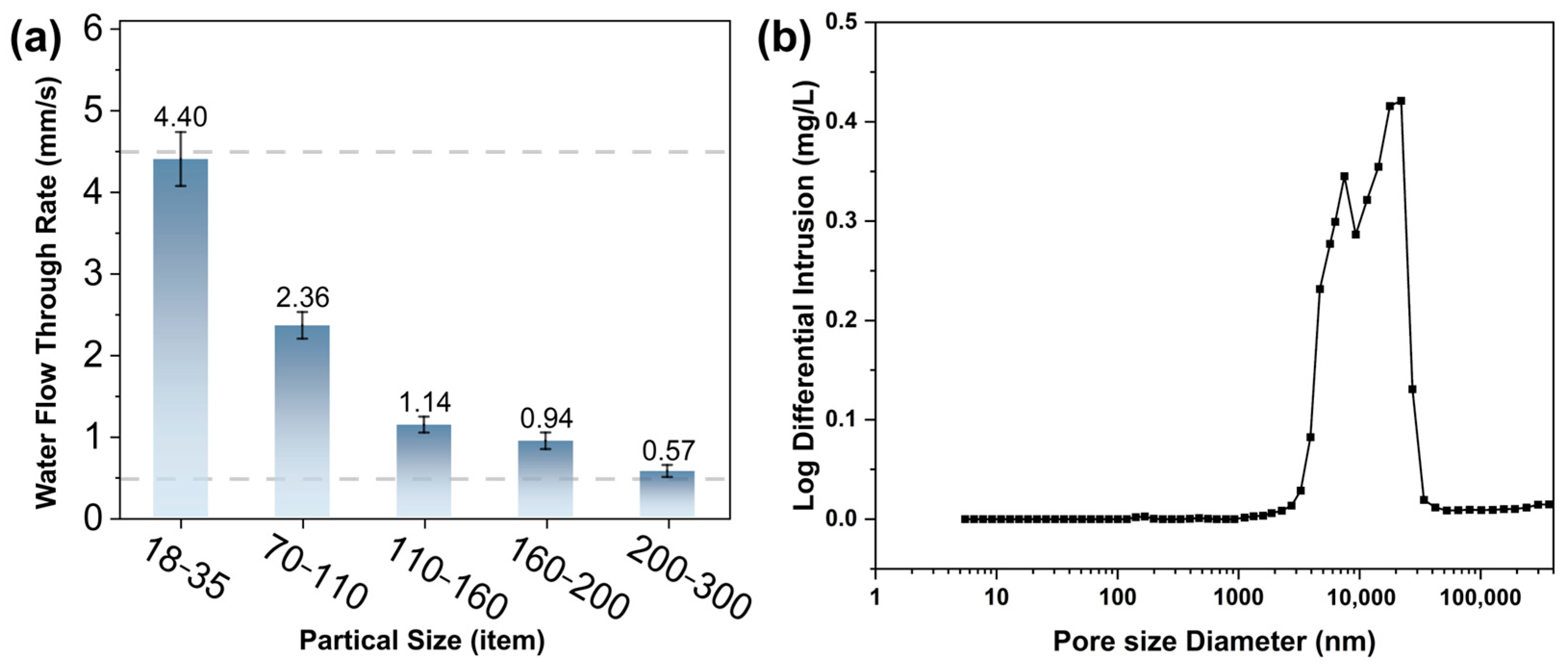
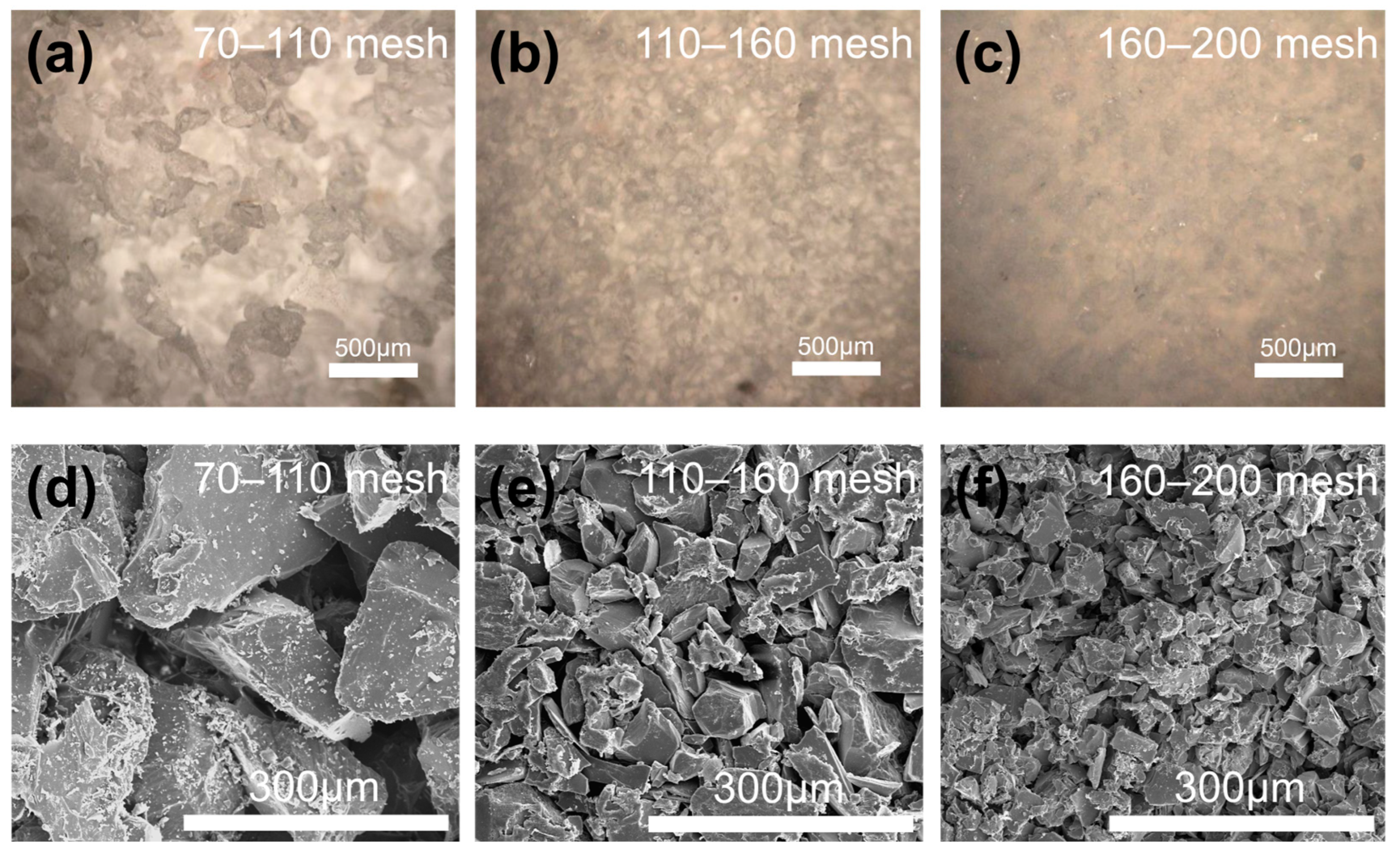
3.4. Effect of Quartz Sand Size on the Performance of Core Molds
3.5. Experimental Validation of Core Molds
4. Conclusions
- (1)
- The density of the core molds was increased by reducing the particle size of quartz sands, and it reached 1.617 g/cm3 for samples containing 200–300 mesh quartz sands. The 160 °C compressive strength of the core molds reached as high as 8.0 MPa, representing a seven-fold increase when compared with those with 18–35 mesh quartz sands.
- (2)
- The water penetration rate decreased with the increasing molecular weight and alcoholysis degree of PVA, the concentration of PVA and the packing density of quartz sands. Samples which were prepared using 10 wt% PVA-4 and 160–200 mesh quartz sands yielded optimal mechanical performance and water solubility that met the criteria for practical application in industrial sectors.
- (3)
- A validation experiment was conducted to verify the efficacy of using the as-prepared core molds to prepare carbon-fiber-reinforced composite parts, and this provided a satisfactory outcome.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Du, B.; He, J.; Long, W.; Su, G.; Liu, J.; Fan, Z.; Chen, L. A review of thermoplastic composites on wind turbine blades. Compos. Part B Eng. 2025, 299, 112411. [Google Scholar] [CrossRef]
- Ayyagari, S.; Al-Haik, M.; Ren, Y.; Abbott, A.; Trigg, E.; Zheng, B.; Koerner, H. Metal organic frameworks modification of carbon fiber composite interface. Compos. Part B Eng. 2021, 224, 109197. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, G.; Vaidya, U.; Wang, H. Past, present and future prospective of global carbon fibre composite developments and applications. Compos. Part B Eng. 2023, 250, 110463. [Google Scholar] [CrossRef]
- Nitai, A.S.; Chowdhury, T.; Inam, M.N.; Rahman, S.; Mondal, I.H.; Johir, M.A.H.; Hessel, V.; Fattah, I.M.R.; Kalam, A.; Suwaileh, W.A.; et al. Carbon fiber and carbon fiber composites—creating defects for superior material properties. Adv. Compos. Hybrid Mater. 2024, 7, 169. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, P.; Huang, Y.; Jiang, W.; Liu, X.; Fan, Z. A water-soluble magnesium sulfate bonded sand core material for manufacturing hollow composite castings. Compos. Struct. 2018, 201, 553–560. [Google Scholar] [CrossRef]
- Xiao, X.; Gong, X.; Zhao, J.; Fan, Z. Preparation of alumina powder-reinforced high-strength salt cores produced via 3D direct printing for aluminum alloy casting. Int. J. Met. 2024, 18, 2210–2224. [Google Scholar] [CrossRef]
- Kluck, S.; Hambitzer, L.; Luitz, M.; Mader, M.; Sanjaya, M.; Balster, A.; Milich, M.; Greiner, C.; Kotz-Helmer, F.; Rapp, B.E. Replicative manufacturing of metal moulds for low surface roughness polymer replication. Nat. Commun. 2022, 13, 5048. [Google Scholar] [CrossRef]
- Schillfahrt, C.; Fauster, E.; Schledjewski, R. A methodology for determining preform compaction in bladder-assisted resin transfer molding with elastomeric bladders for tubular composite parts. Int. J. Mater. Form. 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Xiao, Z.; Harper, L.T.; Kennedy, A.R.; Warrior, N. A water-soluble core material for manufacturing hollow composite sections. Compos. Struct. 2017, 182, 380–390. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Ran, S.; Zhang, L.; Hu, C.; Wang, H. Water-soluble sand core made by binder jetting printing with the binder of potassium carbonate solution. Int. J. Met. 2023, 17, 2286–2297. [Google Scholar] [CrossRef]
- Lombardi, J.L.; Vaidyanathan, K.; Artz, G.; Gillespie, J.; Yarlagadda, S. A water soluble mandrel material for fabricating complex polymer composite components. In Proceedings of the SAMPE 2001, Long Beach, CA, USA, 6–10 May 2001; Volume 46, pp. 1316–1319. [Google Scholar]
- Li, Q.Z.; Wu, X.Q. Preparation and Properties of a Novel Water Soluble Core Material for the Resin Transfer Molding Process. In Proceedings of the 1st International Congress on Advanced Materials, Jinan, China, 13–16 May 2011; University of Jinan: Jinan, China, 2011; pp. 844–847. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, F.; Zhang, C.; Lin, Y.; Wu, M.; Cai, J.; Han, G.; Fan, Z. Fabrication of high-strength and anti-hydration water-soluble calcia-based ceramic core modified with nano-ZrO2 via direct ink writing method. Ceram. Int. 2023, 49, 38623–38634. [Google Scholar] [CrossRef]
- Heinzmann, C.; Weder, C.; De Espinosa, L.M. Supramolecular polymer adhesives: Advanced materials inspired by nature. Chem. Soc. Rev. 2016, 45, 342–358. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.; Kim, Y.J.; Kim, S.; Kim, H.J.; Ahn, S.; An, S.Y.; et al. Recent application developments of water-soluble synthetic polymers. J. Ind. Eng. Chem. 2014, 20, 3913–3918. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Li, W.; Sun, J. Superstrong Water-Based Supramolecular Adhesives Derived from Poly(vinyl alcohol)/Poly(acrylic acid) Complexes. ACS Mater. Lett. 2021, 3, 875–882. [Google Scholar] [CrossRef]
- Bjørsvik, M.; Høiland, H.; Skauge, A. Formation of colloidal dispersion gels from aqueous polyacrylamide solutions. Colloids Surf. Physicochem. Eng. Asp. 2008, 317, 504–511. [Google Scholar] [CrossRef]
- Shah, N.; Hussain, M.; Rehan, T.; Khan, A.; Khan, Z.U. Overview of Polyethylene Glycol-based Materials with a Special Focus on Core-Shell Particles for Drug Delivery Application. Curr. Pharm. Des. 2022, 28, 352–367. [Google Scholar] [CrossRef]
- Thompson, C.S.; Zou, M. Silica nanoparticle antireflective coating with PVP adhesion layer. In Proceedings of the 2013 IEEE 39th Photovoltaic Specialists Conference (PVSC), Tampa, FL, USA, 16–21 June 2013; pp. 1489–1491. [Google Scholar] [CrossRef]
- Al-Nasassrah, M.A.; Podczeck, F.; Newton, J.M. The effect of an increase in chain length on the mechanical properties of polyethylene glycols. Eur. J. Pharm. Biopharm. 1998, 46, 31–38. [Google Scholar] [CrossRef]
- McNeill, I.C.; Sadeghi, S.M.T. Thermal stability and degradation mechanisms of poly(acrylic acid) and its salts: Part 1—Poly(acrylic acid). Polym. Degrad. Stab. 1990, 29, 233–246. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’ANtone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J.; et al. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Klimenko, N.N.; Mikhailenko, N.Y.; Delitsin, L.M.; Sigaev, V.N. Effect of Particle Size of Quartz Sand Filler on Microstructure and Strength of Alkali-Activated Slag-Based Materials. Arab. J. Sci. Eng. 2021, 46, 4337–4352. [Google Scholar] [CrossRef]
- Kumar, S. Strength, abrasion and permeability studies on cement concrete containing quartz sandstone coarse aggregates. Constr. Build. Mater. 2016, 125, 884–891. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Y.; Xu, Z.; Xiao, X.; Li, P.; Wang, X.; Guo, H. Solubilization of fully hydrolyzed poly (vinyl alcohol) at room temperature for fabricating recyclable hydrogels. ACS Macro Lett. 2023, 12, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhao, J.; Wang, Y.; Li, Z.; Zheng, J. A highly stretchable, self-healing, recyclable and interfacial adhesion gel: Preparation, characterization and applications. Chem. Eng. J. 2019, 360, 334–341. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, T.; Yue, B.; Gu, D.; Cui, L.; Qiu, Y.; Dong, Y. Performance of a Water-Soluble Ceramic Core and Its Application in Aluminum Casting. Int. J. Met. 2025, 19, 977–988. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, W.; Sun, X.; Zhou, S.; Zhang, K.; Li, S.; Liu, B.; Wang, G. Effect of particle grading on water-soluble salt-based ceramic core based on vat photopolymerization. Ceram. Int. 2025, 51, 9893–9903. [Google Scholar] [CrossRef]
- ISO 679:1989; Cement-Test Methods. International Organization for Standardization: Geneva, Switzerland, 1989.
- Chu, W.-B.; Yang, J.-W.; Liu, T.-J.; Tiu, C.; Guo, J. The effects of pH, molecular weight and degree of hydrolysis of poly (vinyl alcohol) on slot die coating of PVA suspensions of TiO2 and SiO2. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 1–10. [Google Scholar] [CrossRef]
- Labidi, N.S.; Djebaili, A. Studies of the mechanism of polyvinyl alcohol adsorption on the calcite/water interface in the presence of sodium oleate. J. Miner. Mater. Charact. Eng. 2008, 7, 147–161. [Google Scholar] [CrossRef]
- Zor, M.; Şen, F.; Candan, Z.; Ivanov, E.; Batakliev, T.; Georgiev, V.; Menseidov, D. Preparation and characterization of polyvinyl alcohol (PVA)/carbonized waste rubber biocomposite films. Polymers 2024, 16, 1050. [Google Scholar] [CrossRef]
- Cai, J.; Jin, T.; Kou, J.; Zou, S.; Xiao, J.; Meng, Q. Lucas–Washburn equation-based modeling of capillary-driven flow in porous systems. Langmuir 2021, 37, 1623–1636. [Google Scholar] [CrossRef]
- Lebeau, M.; Konrad, J.M. A new capillary and thin film flow model for predicting the hydraulic conductivity of unsaturated porous media. Water Resour. Res. 2010, 46, W12554. [Google Scholar] [CrossRef]


| Sample ID (Tradename) | Degree of Polymerization | Degree of Alcoholysis (%) |
|---|---|---|
| PVA-1 (PVA 5088) | 500 | 88 |
| PVA-2 (PVA 0872) | 800 | 72 |
| PVA-3 (PVA 1780) | 1700 | 80 |
| PVA-4 (PVA 2488) | 2400 | 88 |
| PVA-5 (PVA 2499) | 2400 | 99 |
| PVA-6 (PVA 2688) | 2600 | 88 |
| PVA-7 (PVA 3688) | 3600 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Tan, L.; Fu, Y.; Sun, Z.; Chen, Y.; Luo, W.; Zhou, S.; Liang, M.; Zou, H. Fabrication of Mechanically Robust Water-Soluble Core Molds and Experimental Validation to Manufacture a Composite Part. Appl. Sci. 2025, 15, 10039. https://doi.org/10.3390/app151810039
Yang T, Tan L, Fu Y, Sun Z, Chen Y, Luo W, Zhou S, Liang M, Zou H. Fabrication of Mechanically Robust Water-Soluble Core Molds and Experimental Validation to Manufacture a Composite Part. Applied Sciences. 2025; 15(18):10039. https://doi.org/10.3390/app151810039
Chicago/Turabian StyleYang, Tianbo, Lei Tan, Yuntao Fu, Ziwen Sun, Yang Chen, Wei Luo, Shengtai Zhou, Mei Liang, and Huawei Zou. 2025. "Fabrication of Mechanically Robust Water-Soluble Core Molds and Experimental Validation to Manufacture a Composite Part" Applied Sciences 15, no. 18: 10039. https://doi.org/10.3390/app151810039
APA StyleYang, T., Tan, L., Fu, Y., Sun, Z., Chen, Y., Luo, W., Zhou, S., Liang, M., & Zou, H. (2025). Fabrication of Mechanically Robust Water-Soluble Core Molds and Experimental Validation to Manufacture a Composite Part. Applied Sciences, 15(18), 10039. https://doi.org/10.3390/app151810039









