Analysis of Variables in Accelerated Carbonation Environment for the Processing of Electric Arc Furnace Slag Aggregate
Abstract
1. Introduction
2. Materials and Methods
2.1. Aggregates
2.2. Methods
2.2.1. Aqueous Carbonation
2.2.2. Semi-Dry Carbonation
2.2.3. Characterisation
2.2.4. CO2 Capture
3. Results
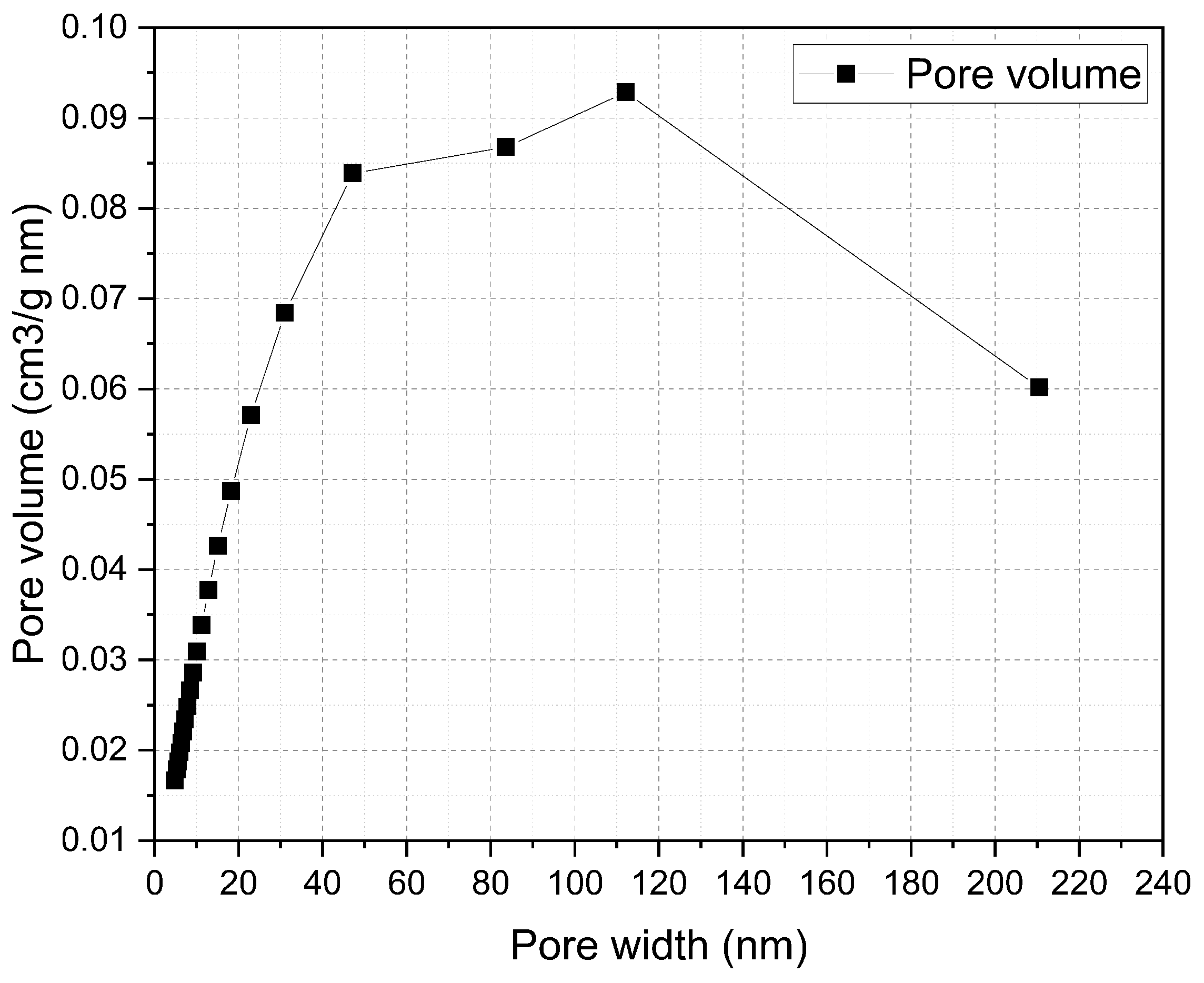
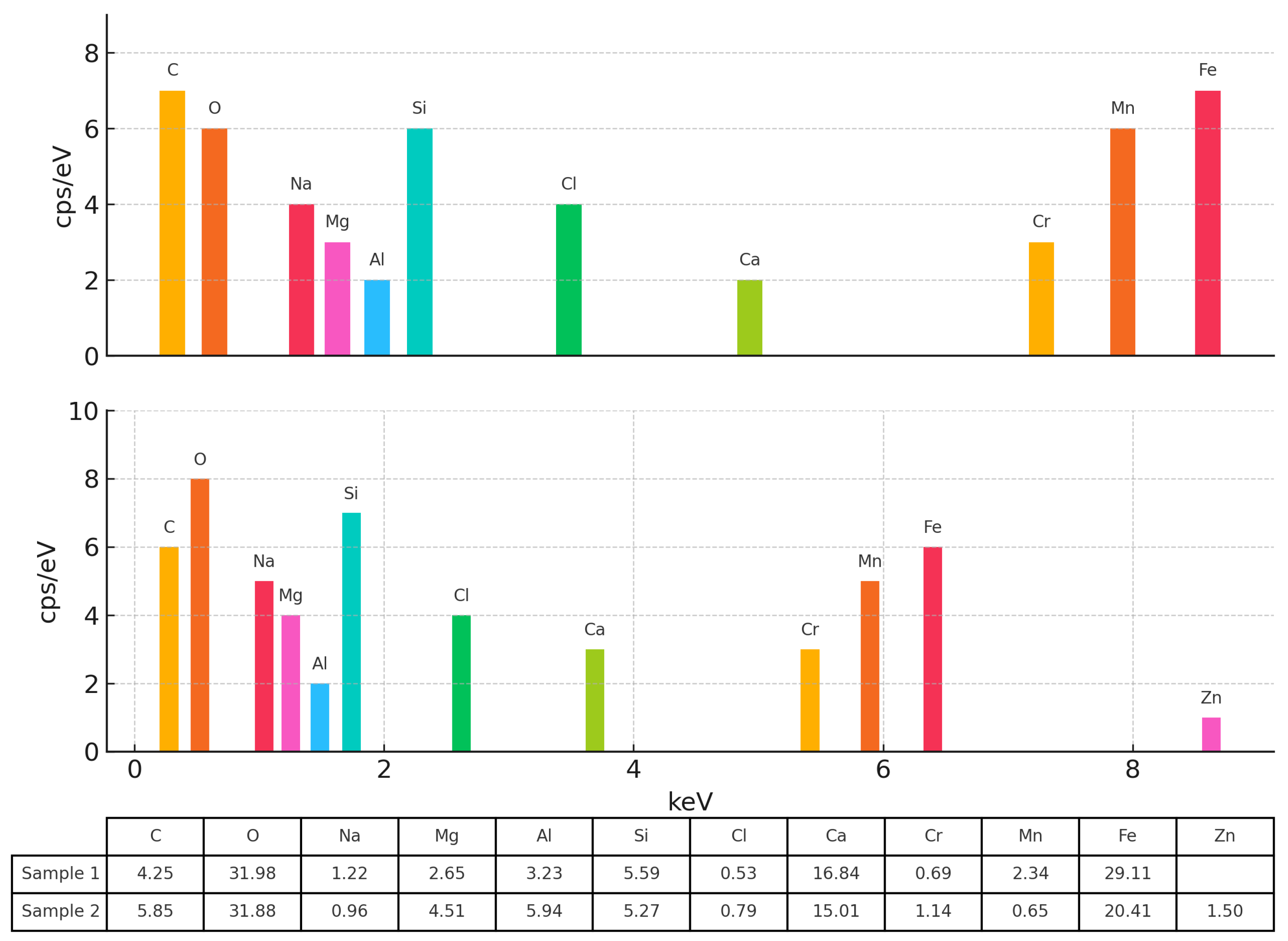
3.1. Properties of ERHA®
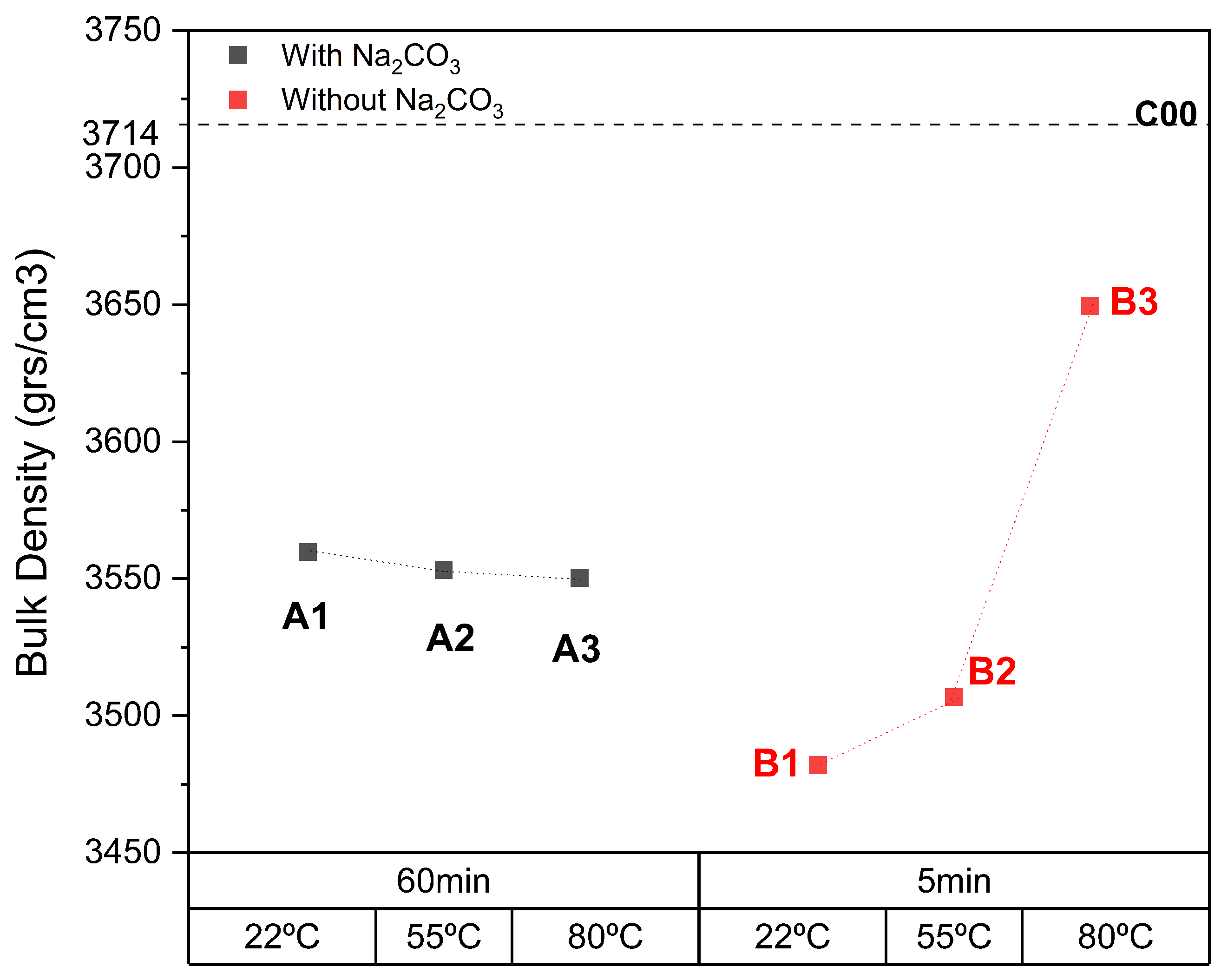
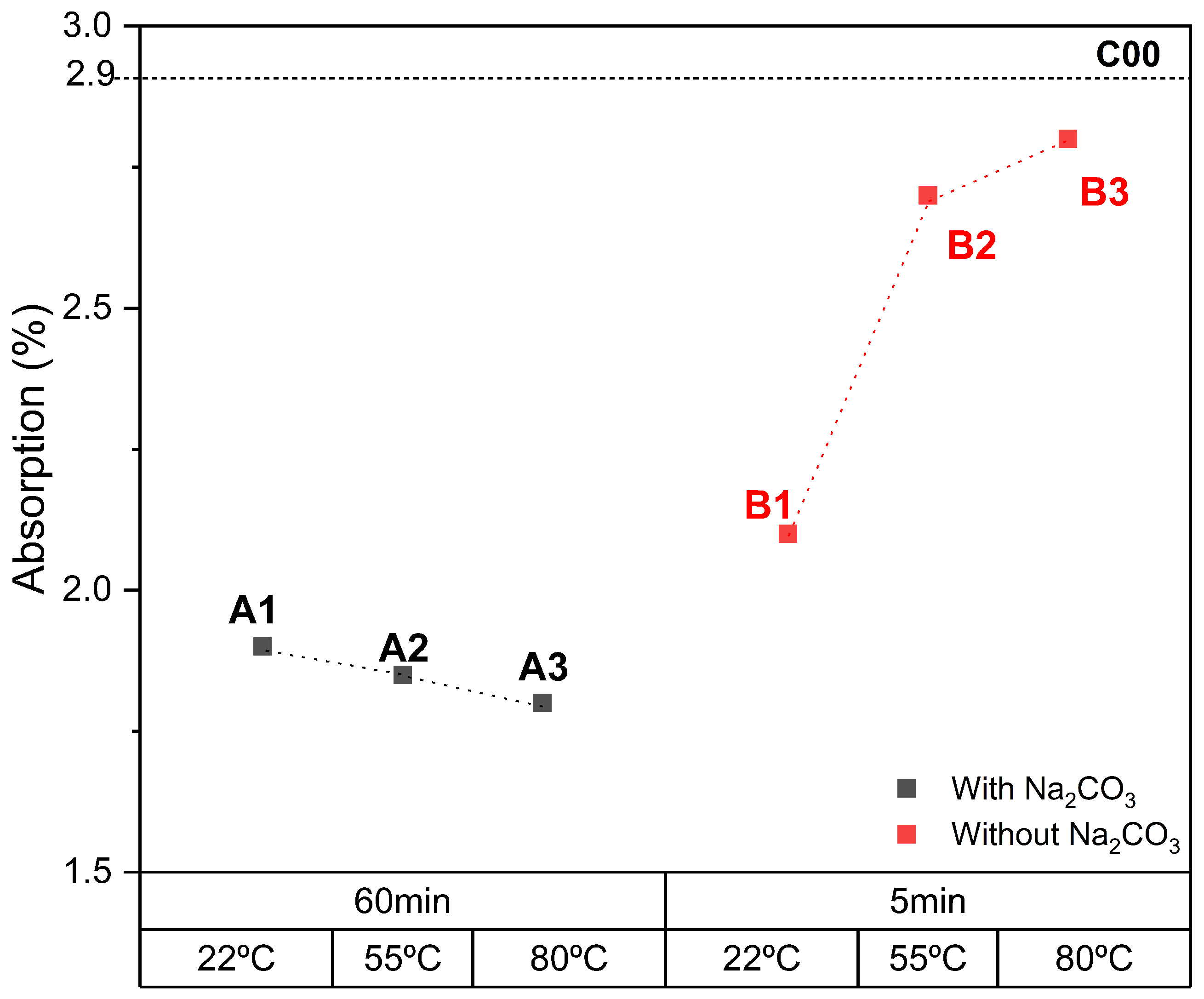
3.2. Effects of Temperature on Carbonation
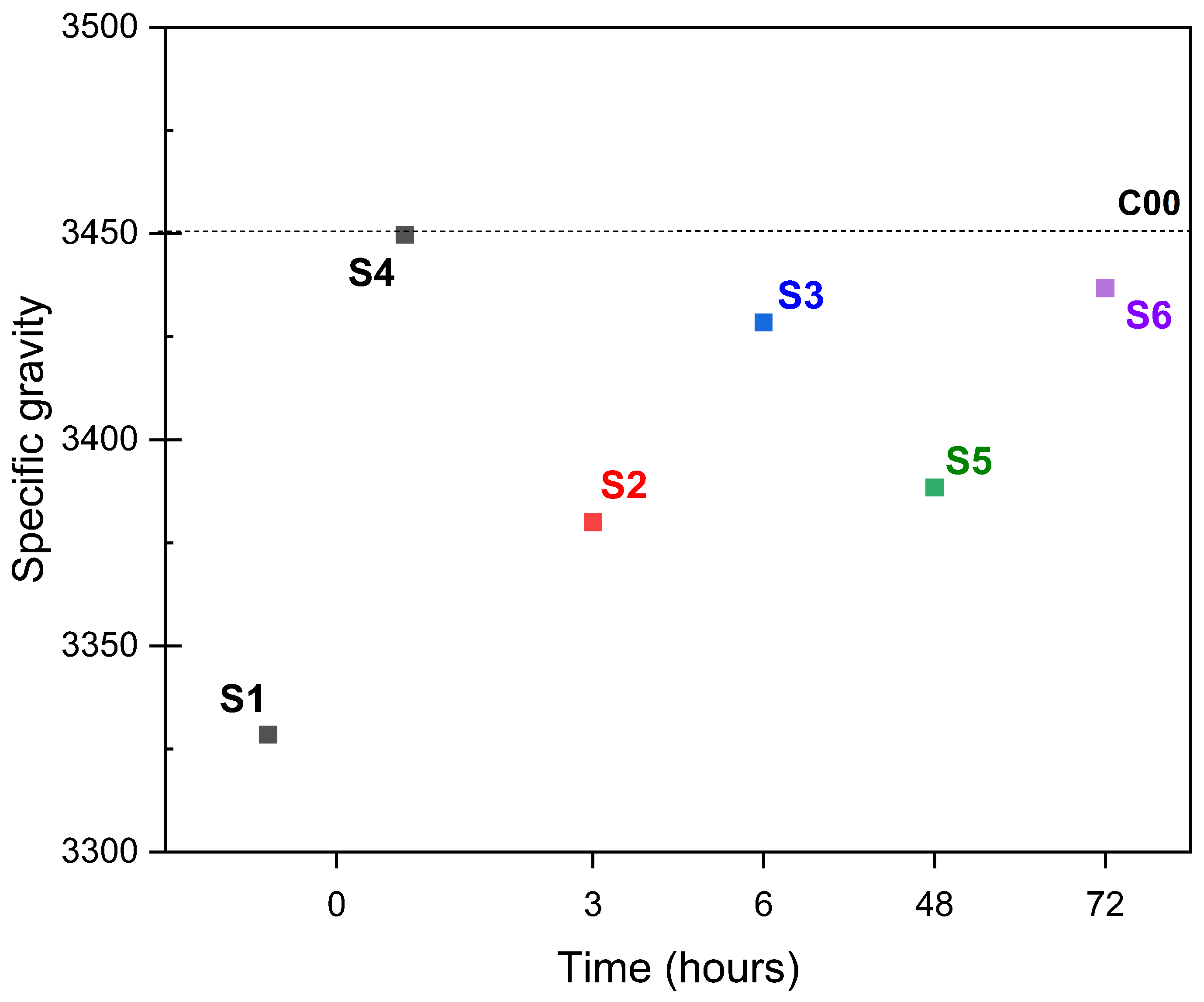
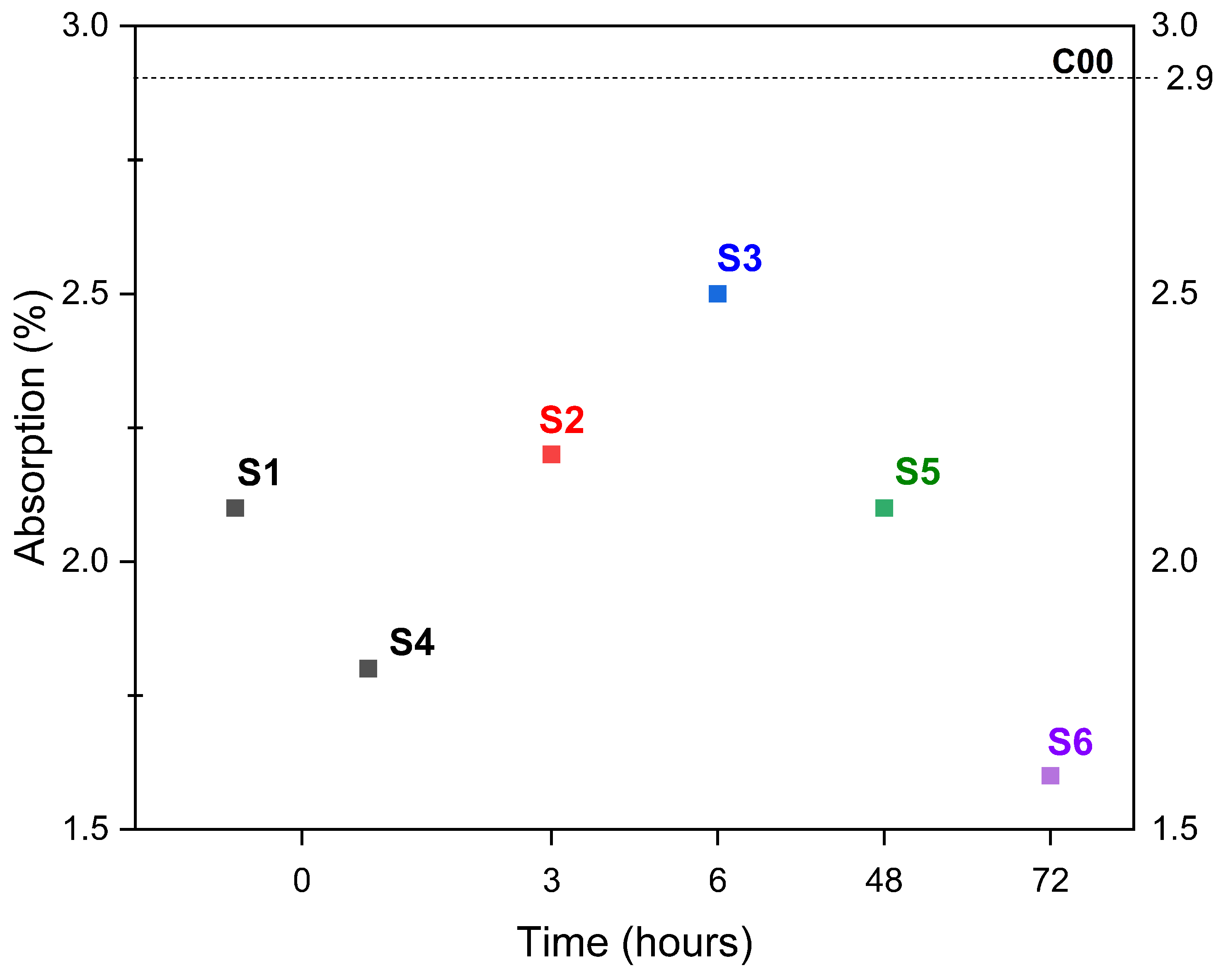
3.3. Effects of Time on Carbonation
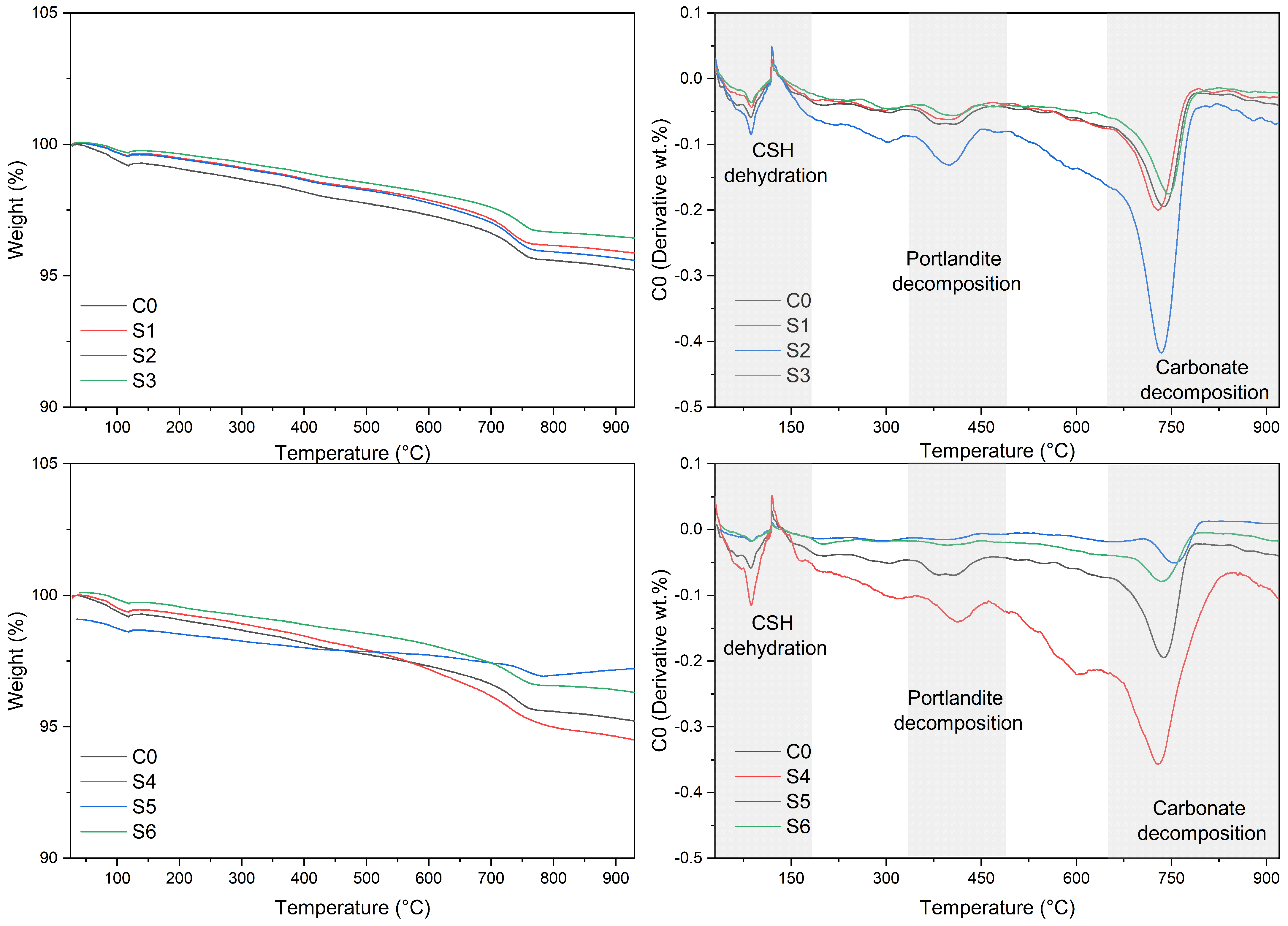
3.4. Thermogravimetric Analysis
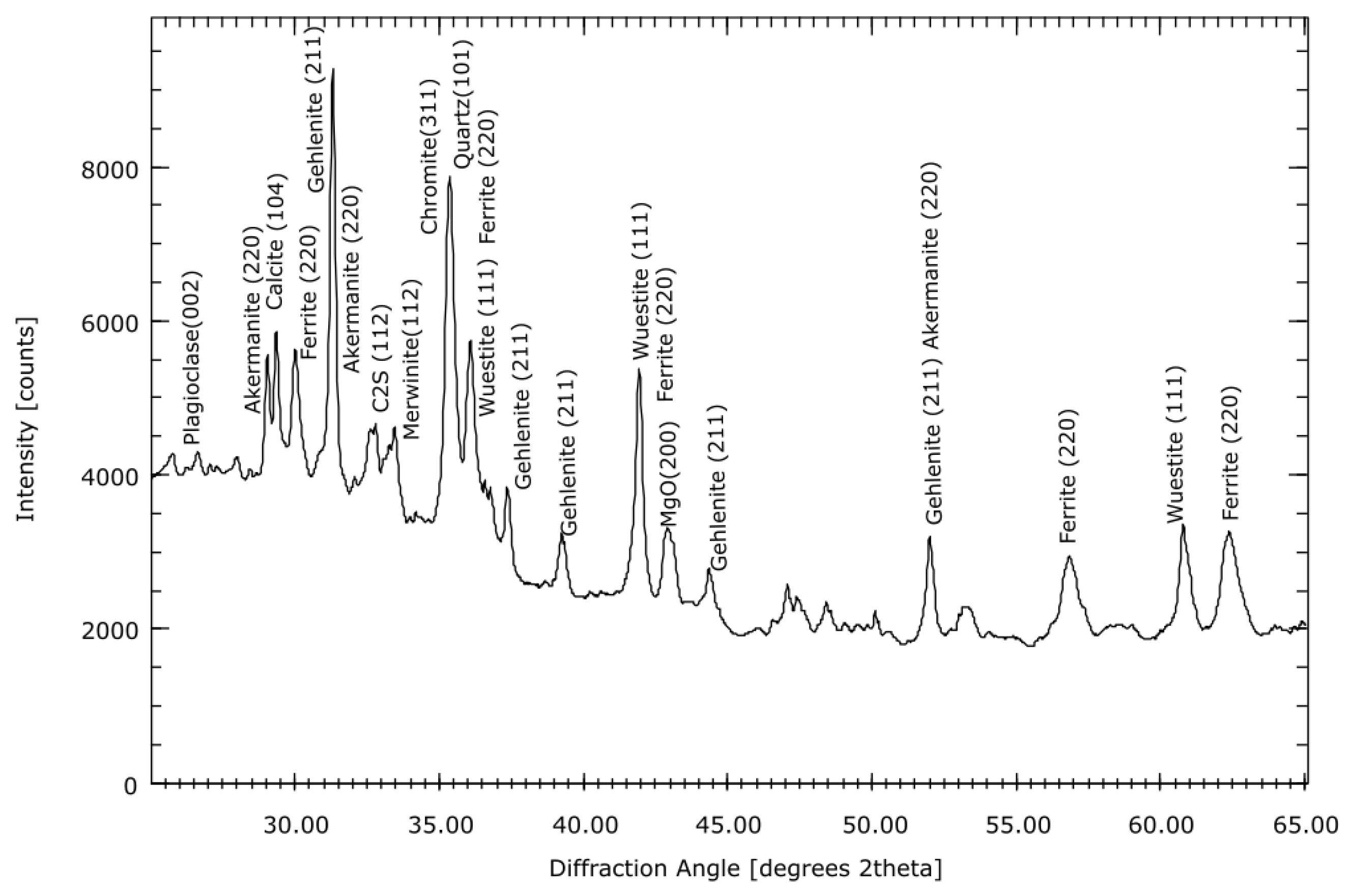
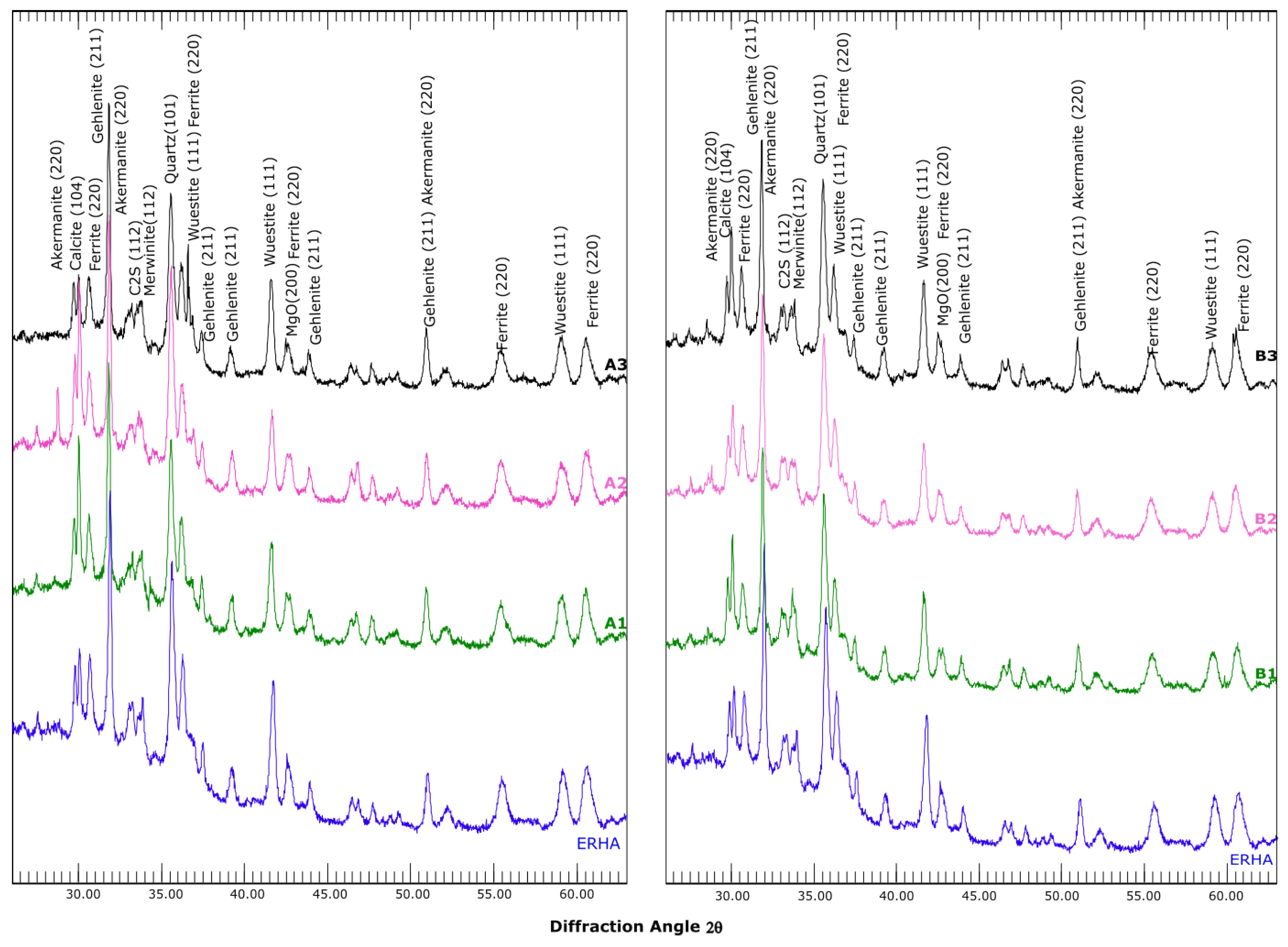
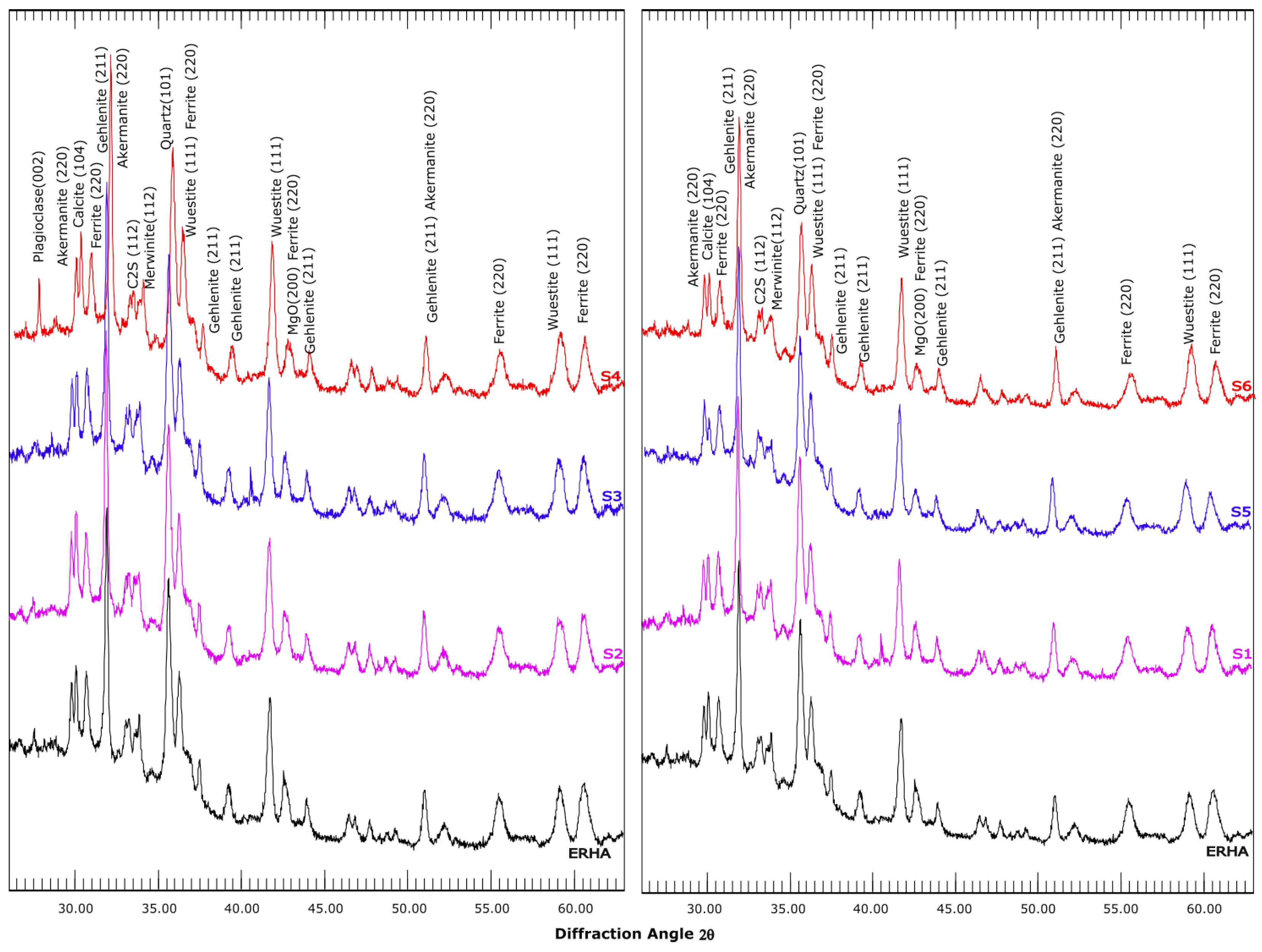
3.5. Mineralogical Analysis
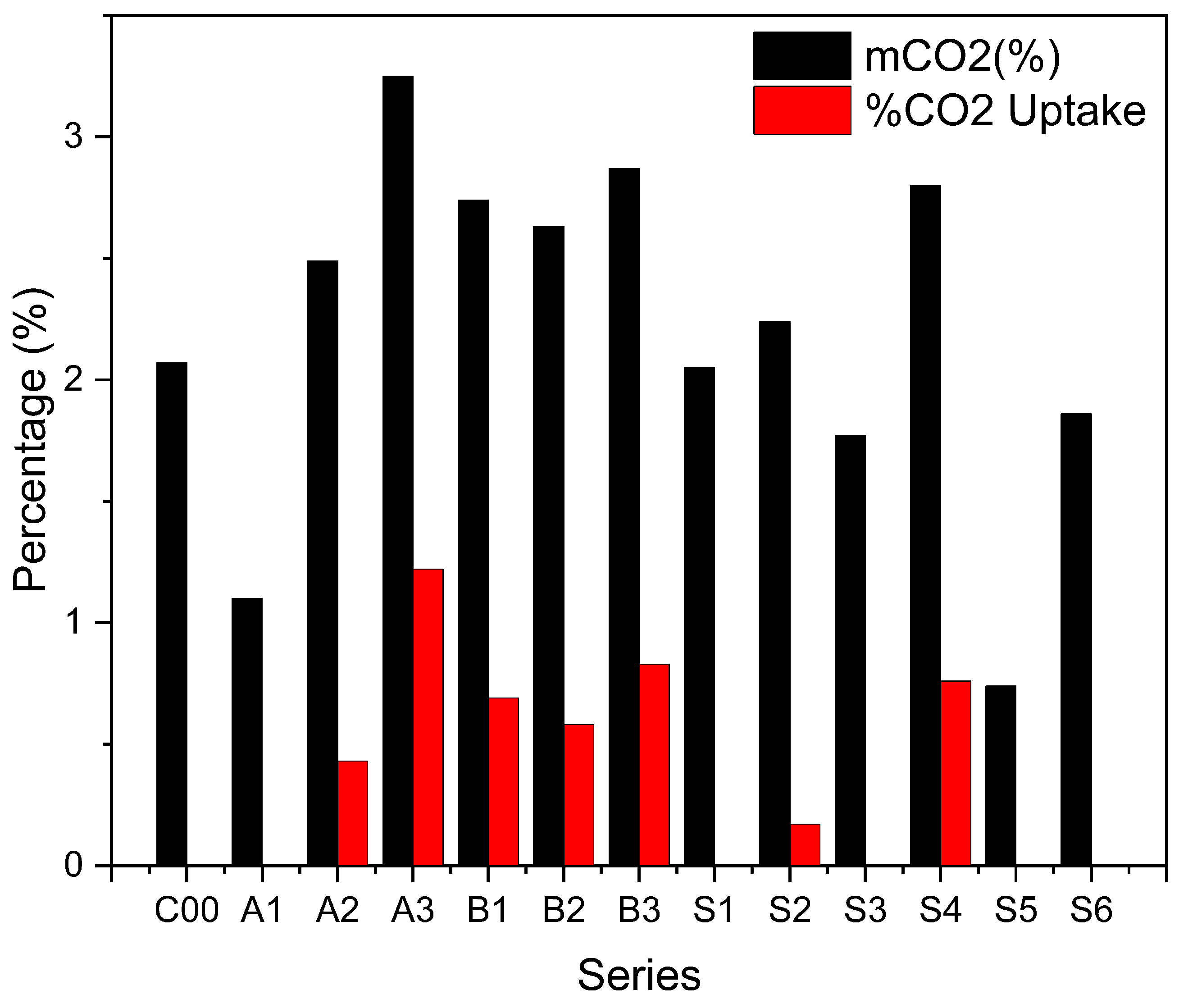
3.6. Sequestration Capacity
4. Conclusions
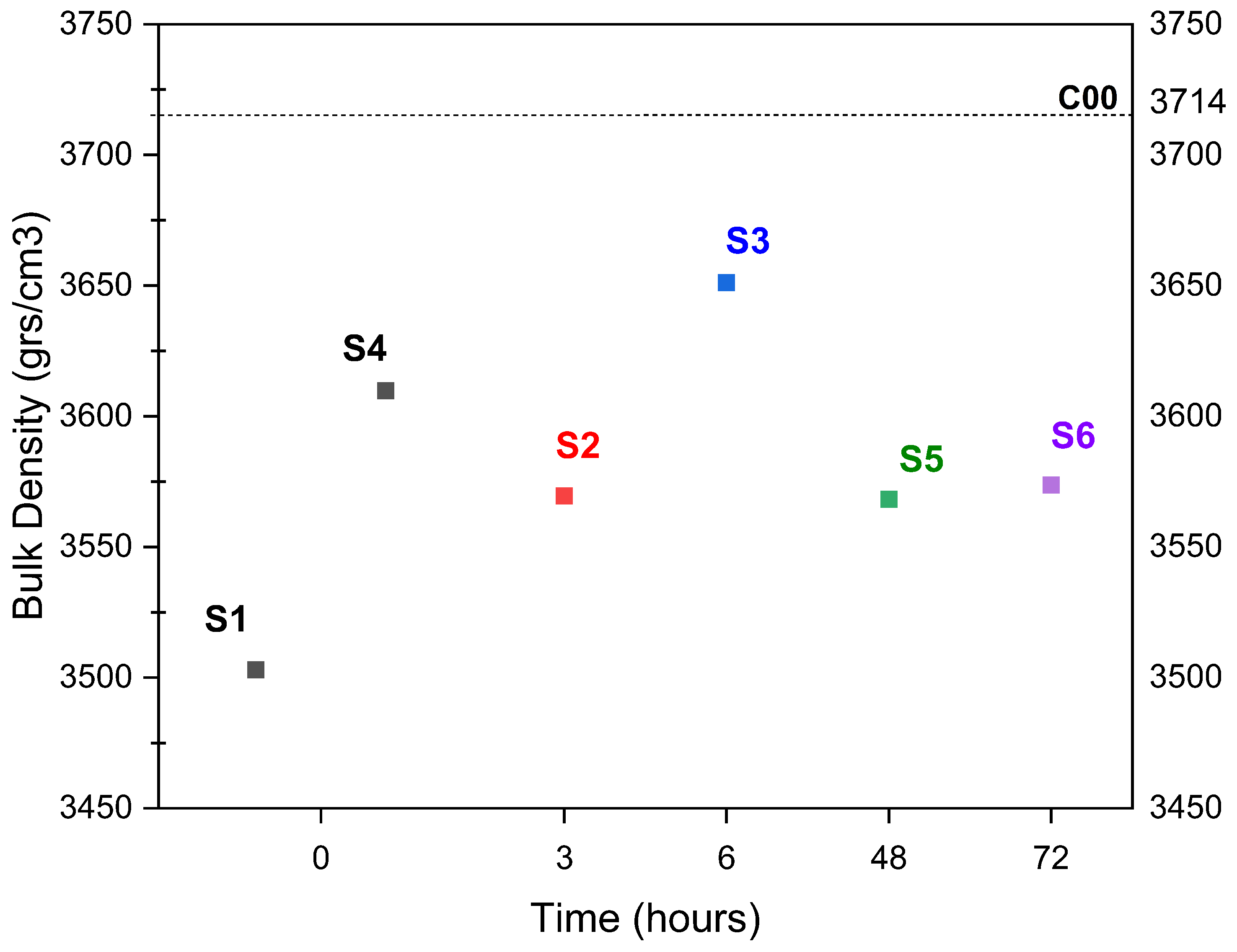
- Aqueous and semi-dry carbonation achieved a reduction in material absorption. However, increasing the temperature in the aqueous carbonation process resulted in a decrease in bulk density. In the case of semi-dry carbonation, changes in the physical properties of ERHA® were achieved as soon as a spray was applied. Based on the physical properties measured, the carbonated slag meets the requirements established with NCh163 for its use as a fine aggregate. Although mechanical testing of the aggregate itself was not performed, this material is being considered for future incorporation in cementitious mixtures to further evaluate its structural performance.
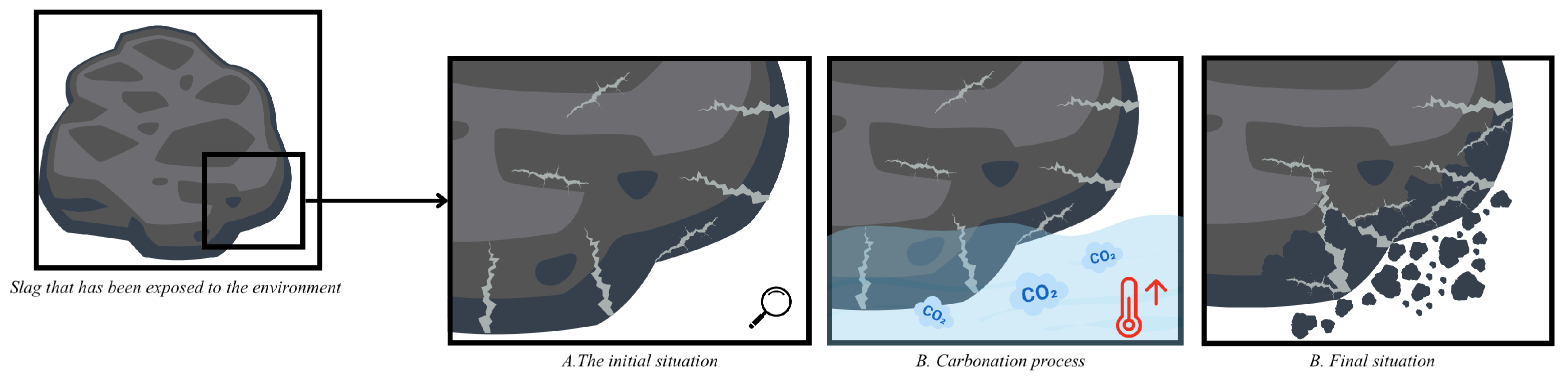
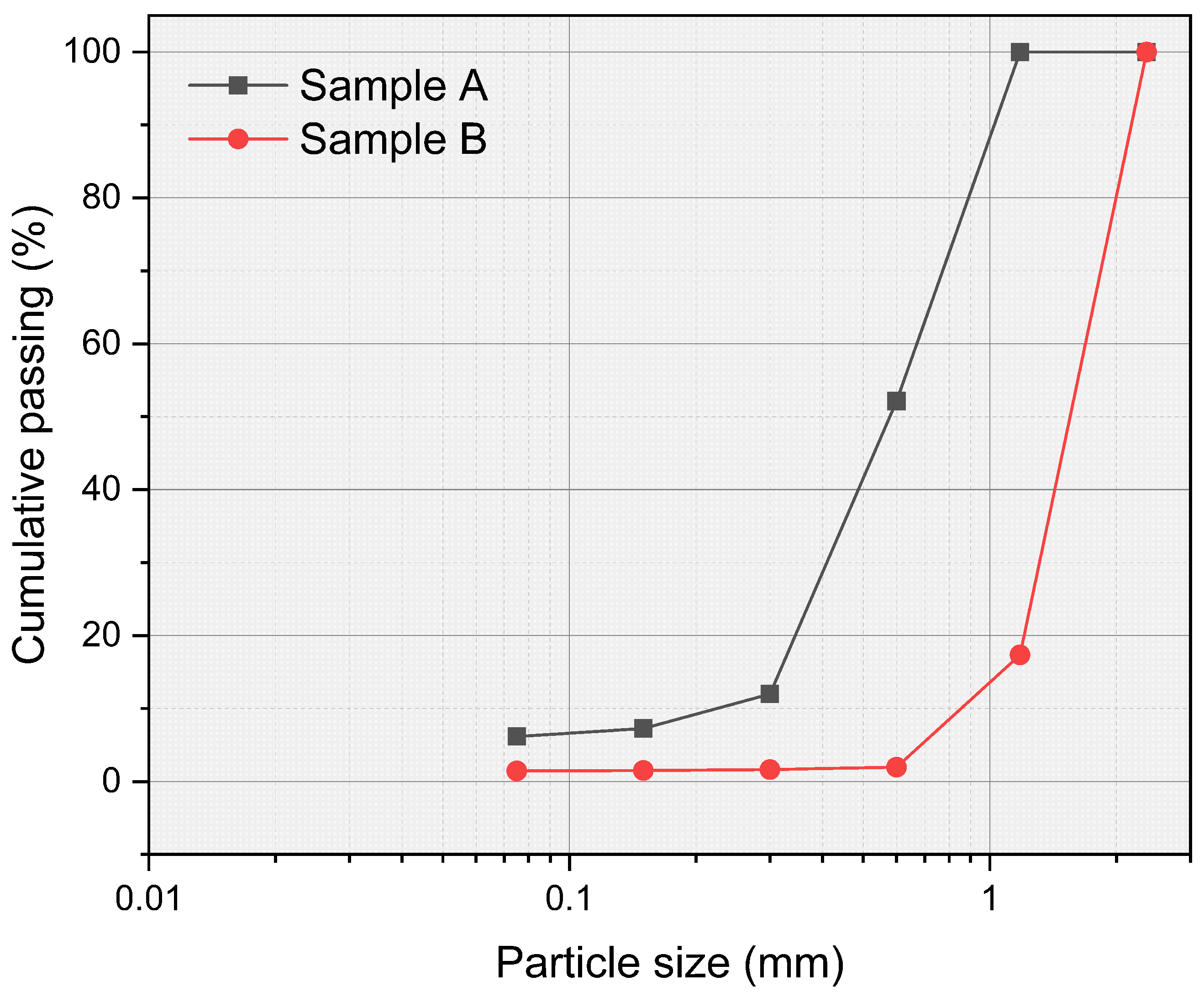
- In the aqueous carbonation series exposed for a longer period of time and at higher temperatures, a decrease in bulk density was observed. This is a very unusual effect, but after a particle size distribution was performed, it suggests an increase in microcracks in ERHA®. Notably, ERHA® already contained calcium carbonates present prior to the application of the processes.
- Thermogravimetric and X-ray diffraction analyses indicated an increase in carbonates in the carbonate samples. Aragonite was even identified in the semi-dry S3 series. An increase in vaterite was observed in most of the aqueous and semi-dry series. Furthermore, a decrease in the expansive phases, such as C2S, MgO and CaO, was observed in the samples.
- The use of sodium carbonate in aqueous carbonation implied an increase in the process time. Meanwhile, in semi-dry carbonation, it increased the formation of carbonates.
- According to the XRF, the maximum sequestration capacity of ERHA® is approximately 26%. This value is far from the carbon dioxide fixation value calculated on the basis of the thermogravimetric analysis for the series. As a result, not all series were able to exceed the value calculated for the control series.
- Based on the comparative analysis of both carbonation processes, the semi-dry method appears more attractive for industrial scalability due to its lower operational complexity, reduced water use, and absence of thermal energy requirements. Future work will focus on scaling up this method and validating its performance in full-size mortar and concrete applications.
5. Limitations and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOD | Argon oxygen decarburisation |
| ASTM | American Society for Testing and Materials |
| BET | Brunauer–Emmett–Teller (surface area method) |
| BOF | Basic oxygen furnace |
| COD | Crystallography Open Database |
| CO2 | Carbon dioxide |
| EAF | Electric arc furnace slag |
| EDS | Energy dispersive X-ray spectroscopy |
| ERHA® | Commercial electric arc furnace slag product |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| NCh | Norma Chilena (Chilean Standard) |
| SEM | Scanning electron microscopy |
| SSD | Saturated surface-dry |
| TGA | Thermogravimetric analysis |
| XRD | X-ray diffraction |
| A1, A2, A3 | Samples treated via aqueous carbonation with different variables |
| S1, S2, S3 | Samples treated via semi-dry carbonation with different variables |
References
- Baciocchi, R.; Costa, G.; Di Bartolomeo, E.; Polettini, A.; Pomi, R. Carbonation of Stainless Steel Slag as a Process for CO2 Storage and Slag Valorization. Waste Biomass Valor. 2010, 1, 467–477. [Google Scholar] [CrossRef]
- Green Steel Production: How G7 Countries Can Help Change the Global Landscape. Available online: https://www.industrytransition.org/insights/g7-green-steel-production/#:~:text=G7%20governments%20should%20accelerate%20the,funds%20dedicated%20to%20industrial%20decarbonization (accessed on 27 February 2025).
- Baena-Moreno, F.M.; Cid-Castillo, N.; Arellano-García, H.; Reina, T.R. Towards emission free steel manufacturing—Exploring the advantages of a CO2 methanation unit to minimize CO2 emissions. Sci. Total Environ. 2021, 781, 146776. [Google Scholar] [CrossRef]
- Benavides, K.; Gurgel, A.; Morris, J.; Mignone, B.; Chapman, B.; Kheshgi, H.; Herzog, H.; Paltsev, S. Mitigating emissions in the global steel industry: Representing CCS and hydrogen technologies in integrated assessment modeling. Int. J. Greenh. Gas Control 2024, 131, 103963. [Google Scholar] [CrossRef]
- European Commission. EU Climate Targets: How to Decarbonise the Steel Industry. Available online: https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/eu-climate-targets-how-decarbonise-steel-industry-2022-06-15_en (accessed on 27 February 2025).
- Rashid, M.I.; Yaqoob, Z.; Mujtaba, M.A.; Fayaz, H.; Saleel, C.A. Developments in mineral carbonation for Carbon sequestration. Heliyon 2023, 9, e21796. [Google Scholar] [CrossRef]
- Gunka, V.; Hidei, V.; Sidun, I.; Demchuk, Y.; Stadnik, V.; Shapoval, P.; Sobol, K.; Vytrykush, N.; Bratychak, M. Wastepaper Sludge Ash and Acid Tar as Activated Filler Aggregates for Stone Mastic Asphalt. Coatings 2023, 13, 1183. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.; Wang, H.; Du, Y.; Ma, J.; Zhang, X.; Wang, Z. Carbon Capture and Storage: History and the Road Ahead. Engineering 2022, 14, 33–43. [Google Scholar] [CrossRef]
- Zhang, N.; Chai, Y.E.; Santos, R.M.; Šiller, L. Advances in process development of aqueous CO2 mineralisation towards scalability. J. Environ. Chem. Eng. 2020, 8, 104453. [Google Scholar] [CrossRef]
- Song, X.; Chen, X.; Lin, Q.; Liu, Y. A Review of Mineral Carbonation from Industrial Waste. IOP Conf. Ser. Earth Environ. Sci. 2019, 401, 012008. [Google Scholar] [CrossRef]
- van Zomeren, A.; van der Laan, S.R.; Kobesen, H.B.A.; Huijgen, W.J.J.; Comans, R.N.J. Changes in mineralogical and leaching properties of converter steel slag resulting from accelerated carbonation at low CO2 pressure. Waste Manag. 2011, 31, 2236–2244. [Google Scholar] [CrossRef]
- Neeraj; Yadav, S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- Spanka, M.; Mansfeldt, T.; Bialucha, R. Influence of Natural and Accelerated Carbonation of Steel Slags on Their Leaching Behavior. Steel Res. Int. 2016, 87, 798–810. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Liu, H.; Boczkaj, G.; Cao, Y.; Wang, C. Carbon dioxide sequestration by industrial wastes through mineral carbonation: Current status and perspectives. J. Clean. Prod. 2024, 434, 140258. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Liu, Y.; Li, J. Use of steel slag as carbonation material: A review of carbonation methods and evaluation, environmental factors and carbon conversion process. J. CO2 Util. 2024, 88, 102947. [Google Scholar] [CrossRef]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef]
- Zajac, M.; Song, J.; Skocek, J.; Ben Haha, M.; Skibsted, J. Composite cements with aqueous and semi-dry carbonated recycled concrete pastes. Constr. Build. Mater. 2023, 407, 133362. [Google Scholar] [CrossRef]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Chu, H.W.; Wang, C.F.; Chiang, P.C. CO2 sequestration by carbonation of steelmaking slags in an autoclave reactor. J. Hazard. Mater. 2011, 195, 107–114. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef]
- Chang, E.E.; Chen, C.H.; Chen, Y.H.; Pan, S.Y.; Chiang, P.C. Performance evaluation for carbonation of steel-making slags in a slurry reactor. J. Hazard. Mater. 2011, 186, 558–564. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. CO2 sequestration through aqueous accelerated carbonation of BOF slag: A factorial study of parameters effects. J. Environ. Manag. 2016, 167, 185–195. [Google Scholar] [CrossRef]
- Zhu, F.; Cui, L.; Liu, Y.; Zou, L.; Hou, J.; Li, C.; Wu, G.; Xu, R.; Jiang, B.; Wang, Z. Experimental Investigation and Mechanism Analysis of Direct Aqueous Mineral Carbonation Using Steel Slag. Sustainability 2024, 16, 81. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Yang, G.; Ye, J.; Zhang, W.; Liu, Z.; Mu, Y. Effect of curing temperature on carbonation behavior of steel slag compacts. Constr. Build. Mater. 2021, 291, 123369. [Google Scholar] [CrossRef]
- Mohamed, A.M.O.; El Gamal, M.; Hameedi, S.M.; Paleologos, E.K. Chapter 9—Carbonation of steel slag. In Sustainable Utilization of Carbon Dioxide in Waste Management; Mohamed, A.M.O., El Gamal, M., Hameedi, S.M., Paleologos, E.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 327–372. [Google Scholar] [CrossRef]
- Cai, T.; Chen, X.; Johnson, J.K.; Wu, Y.; Ma, J.; Liu, D.; Liang, C. Understanding and Improving the Kinetics of Bulk Carbonation on Sodium Carbonate. J. Phys. Chem. C 2020, 124, 23106–23115. [Google Scholar] [CrossRef]
- Nielsen, P.; Boone, M.A.; Horckmans, L.; Snellings, R.; Quaghebeur, M. Accelerated carbonation of steel slag monoliths at low CO2 pressure—Microstructure and strength development. J. CO2 Util. 2020, 36, 124–134. [Google Scholar] [CrossRef]
- NCh1239:2009; Aridos para Morteros y Hormigones—Determinación de las Densidades Real y Neta y de la Absorción de Agua de las Arenas. Instituto Nacional de Normalización: Santiago, Chile, 2009.
- ASTM C128-22; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Steinour, H.H. Some Effects of Carbon Dioxide on Mortars and Concrete-Discussion. J. Am. Concr. Inst. 1959, 30, 905–907. [Google Scholar]
- Mo, L.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Moon, E.J.; Choi, Y.C. Development of carbon-capture binder using stainless steel argon oxygen decarburization slag activated by carbonation. J. Clean. Prod. 2018, 180, 642–654. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Kawatra, S.K.; Eisele, T.C.; Sutter, L.L. Carbon Dioxide Sequestration in Cement Kiln Dust through Mineral Carbonation. Environ. Sci. Technol. 2009, 43, 1986–1992. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Sutter, L.L.; Kawatra, S.K.; Eisele, T.C. Mineral carbonation for carbon sequestration in cement kiln dust from waste piles. J. Hazard. Mater. 2009, 168, 31–37. [Google Scholar] [CrossRef]
- Yang, K.H.; Chung, H.S.; Ashour, A.F. Influence of Type and Replacement Level of Recycled Aggregates on Concrete Properties. ACI Mater. J. 2008, 105, 289–296. [Google Scholar]
- NCh163:2024; Áridos para Hormigones y Morteros—Requisitos. Instituto Nacional de Normalización: Santiago, Chile, 2024.
- Chen, Z.; Cang, Z.; Yang, F.; Zhang, J.; Zhang, L. Carbonation of steelmaking slag presents an opportunity for carbon neutral: A review. J. CO2 Util. 2021, 54, 101738. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, P.; Wang, Y.; Zhao, X.; Liu, J. Effect of Fe and Mn on the hydration activity of f-CaO in steel slag. Constr. Build. Mater. 2024, 421, 135719. [Google Scholar] [CrossRef]
- Chukwuma, J.S.; Pullin, H.; Renforth, P. Assessing the carbon capture capacity of South Wales’ legacy iron and steel slag. Miner. Eng. 2021, 173, 107232. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef]
- Wang, Y.; He, F.; Wang, J.; Hu, Q. Comparison of Effects of Sodium Bicarbonate and Sodium Carbonate on the Hydration and Properties of Portland Cement Paste. Materials 2019, 12, 1033. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Dell’Aversano, R.; Gazzaniga, G.; Pastore, T. Influence of Lithium Carbonate and Sodium Carbonate on Physical and Elastic Properties and on Carbonation Resistance of Calcium Sulphoaluminate-Based Mortars. Appl. Sci. 2020, 10, 176. [Google Scholar] [CrossRef]
- Ye, J.; Liu, S.; Fang, J.; Zhang, H.; Zhu, J.; Guan, X. Effect of temperature on wet carbonation products of magnesium slag. Constr. Build. Mater. 2024, 425, 135949. [Google Scholar] [CrossRef]
- Han, J.; Sun, W.; Pan, G.; Wang, C.; Rong, H. Application of X-ray computed tomography in characterization microstructure changes of cement pastes in carbonation process. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2012, 27, 358–363. [Google Scholar] [CrossRef]
- Zhu, W.; Fusseis, F.; Lisabeth, H.; Xing, T.; Xiao, X.; De Andrade, V.; Karato, S.i. Experimental evidence of reaction-induced fracturing during olivine carbonation. Geophys. Res. Lett. 2016, 43, 9535–9543. [Google Scholar] [CrossRef]
- Ho, H.J.; Iizuka, A. Mineral carbonation using seawater for CO2 sequestration and utilization: A review. Sep. Purif. Technol. 2023, 307, 122855. [Google Scholar] [CrossRef]
- Li, Y.; Liao, H.; Guo, Y. Aqueous carbonation of steel slag and preparation of calcium carbon: A new strategy for the utilization of steel slag. J. Environ. Chem. Eng. 2025, 13, 115951. [Google Scholar] [CrossRef]
- Lin, J.Y.; Garcia, E.A.; Ballesteros, F.C.; Garcia-Segura, S.; Lu, M.C. A review on chemical precipitation in carbon capture, utilization and storage. Sustain. Environ. Res. 2022, 32, 45. [Google Scholar] [CrossRef]
- Vollprecht, D.; Wohlmuth, D. Mineral Carbonation of Basic Oxygen Furnace Slags. Recycling 2022, 7, 84. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, Z.; Gao, Y.; Shen, P.; Poon, C.S. A review on the impact of water in accelerated carbonation: Implications for producing sustainable construction materials. Cem. Concr. Compos. 2025, 157, 105902. [Google Scholar] [CrossRef]
- Ding, X.; Li, W.; Chang, J. Preparation of aragonite from calcium carbonate via wet carbonation to improve properties of steel slag building materials. Constr. Build. Mater. 2024, 451, 138763. [Google Scholar] [CrossRef]
- Zhao, D.; Williams, J.M.; Li, Z.; Park, A.H.A.; Radlińska, A.; Hou, P.; Kawashima, S. Hydration of cement pastes with calcium carbonate polymorphs. Cem. Concr. Res. 2023, 173, 107270. [Google Scholar] [CrossRef]
- Fang, Y.; Su, W.; Zhang, Y.; Zhang, M.; Ding, X.; Wang, Q. Effect of accelerated precarbonation on hydration activity and volume stability of steel slag as a supplementary cementitious material. J. Therm. Anal. Calorim. 2022, 147, 6181–6191. [Google Scholar] [CrossRef]
- Bulatbekova, D.; Vashistha, P.; Kim, H.K.; Pyo, S. Effects of basic-oxygen furnace, electric-arc furnace, and ladle furnace slags on the hydration and durability properties of construction materials: A review. J. Build. Eng. 2024, 92, 109670. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The role of Mg in the crystallization of monohydrocalcite. Geochim. Et Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Gao, J.; Huang, Y.; Wang, S.; Zhu, Z.; Song, H.; Zhang, Y.; Liu, J.; Qi, S.; Zhao, J. Mineral transformation and solidification of heavy metals during co-melting of MSWI fly ash with coal fly ash. Environ. Sci. Pollut. Res. Int. 2024, 31, 45793–45807. [Google Scholar] [CrossRef] [PubMed]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Reina, T.R.; Zhang, Z.; Vilches, L.F.; Navarrete, B. Understanding the effect of Ca and Mg ions from wastes in the solvent regeneration stage of a biogas upgrading unit. Sci. Total Environ. 2019, 691, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, F.; Ferrara, G.; Humbert, P.; Garufi, D.; Tulliani, J.M.C.; Palmero, P. CO2 Sequestration Through Aqueous Carbonation of Electric Arc Furnace Slag. J. Adv. Concr. Technol. 2024, 22, 207–218. [Google Scholar] [CrossRef]
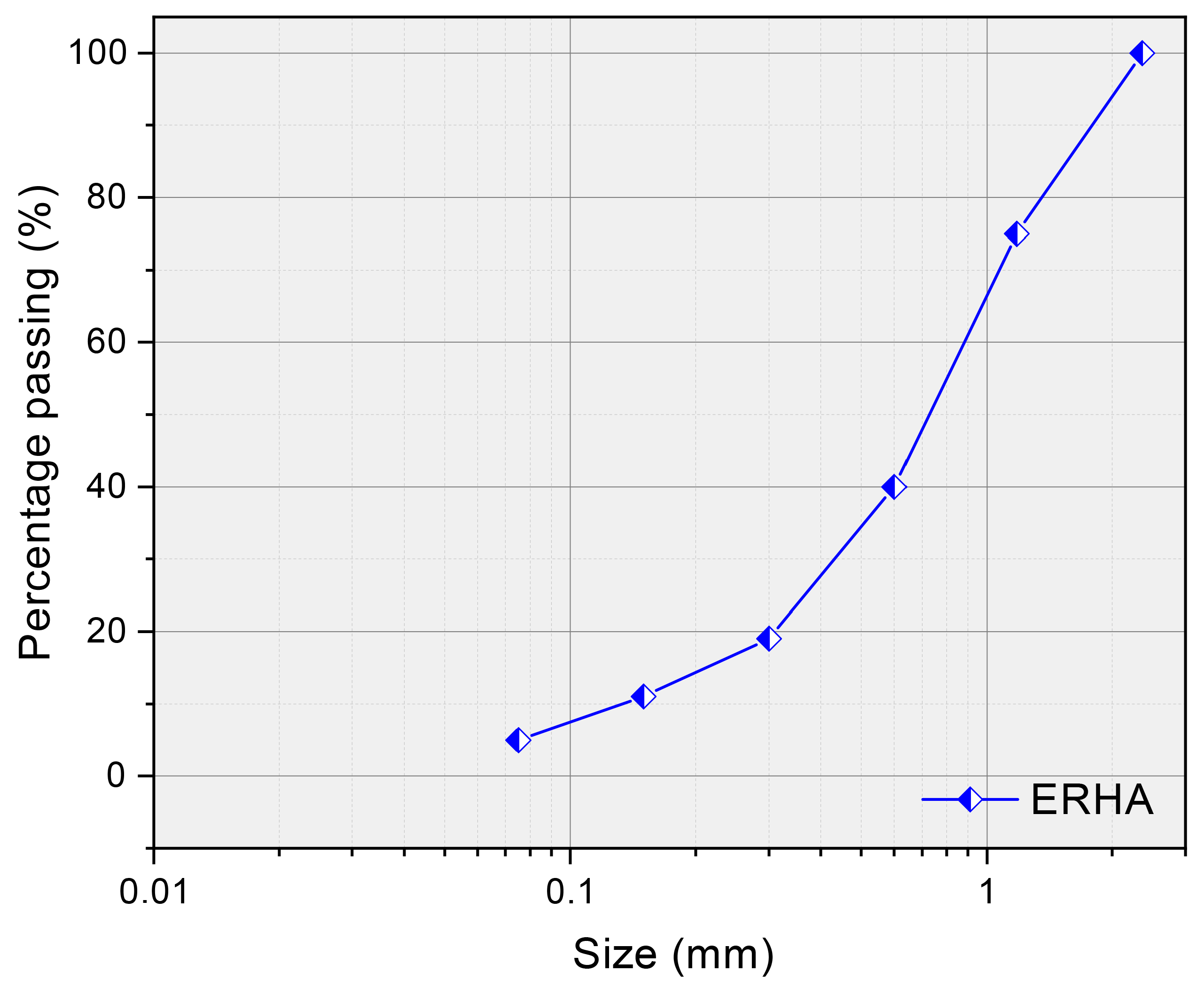
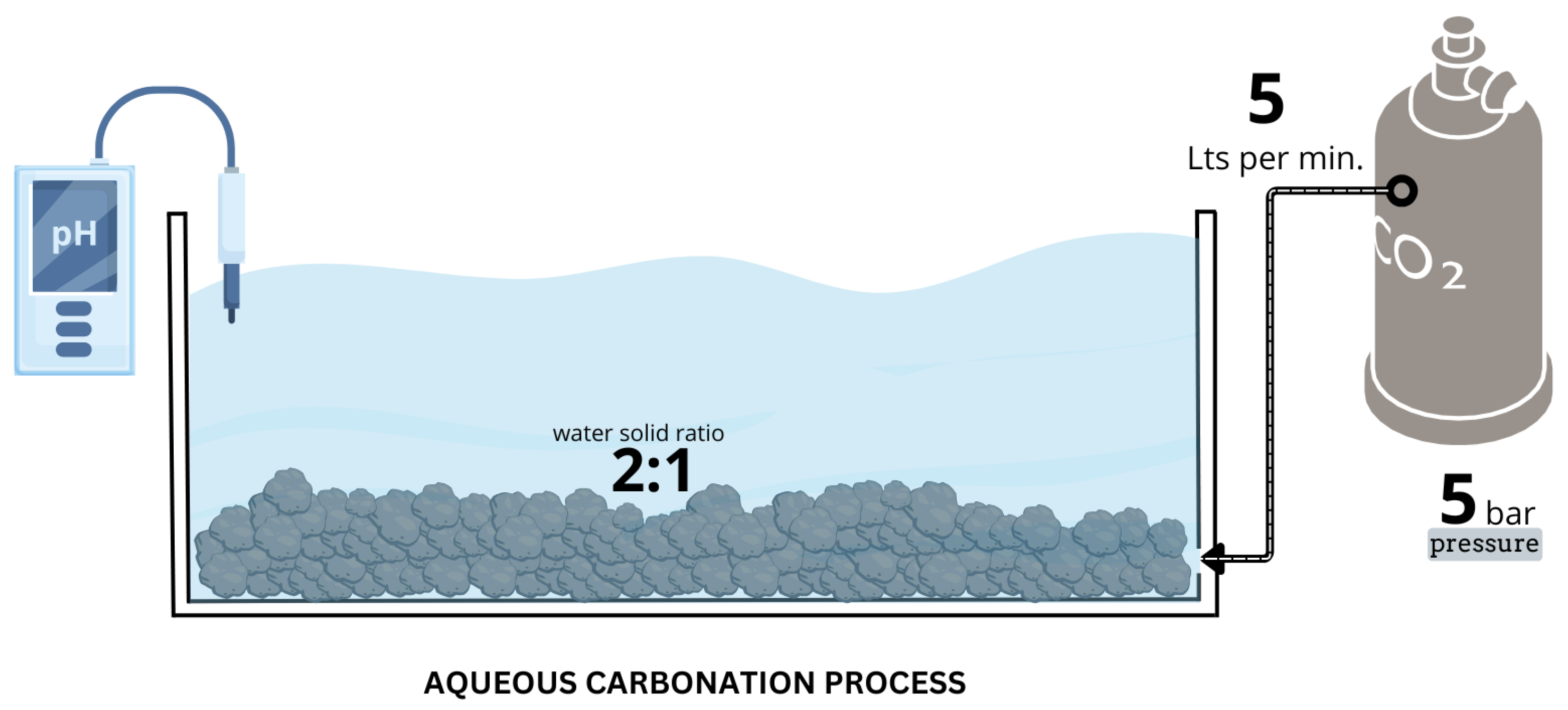
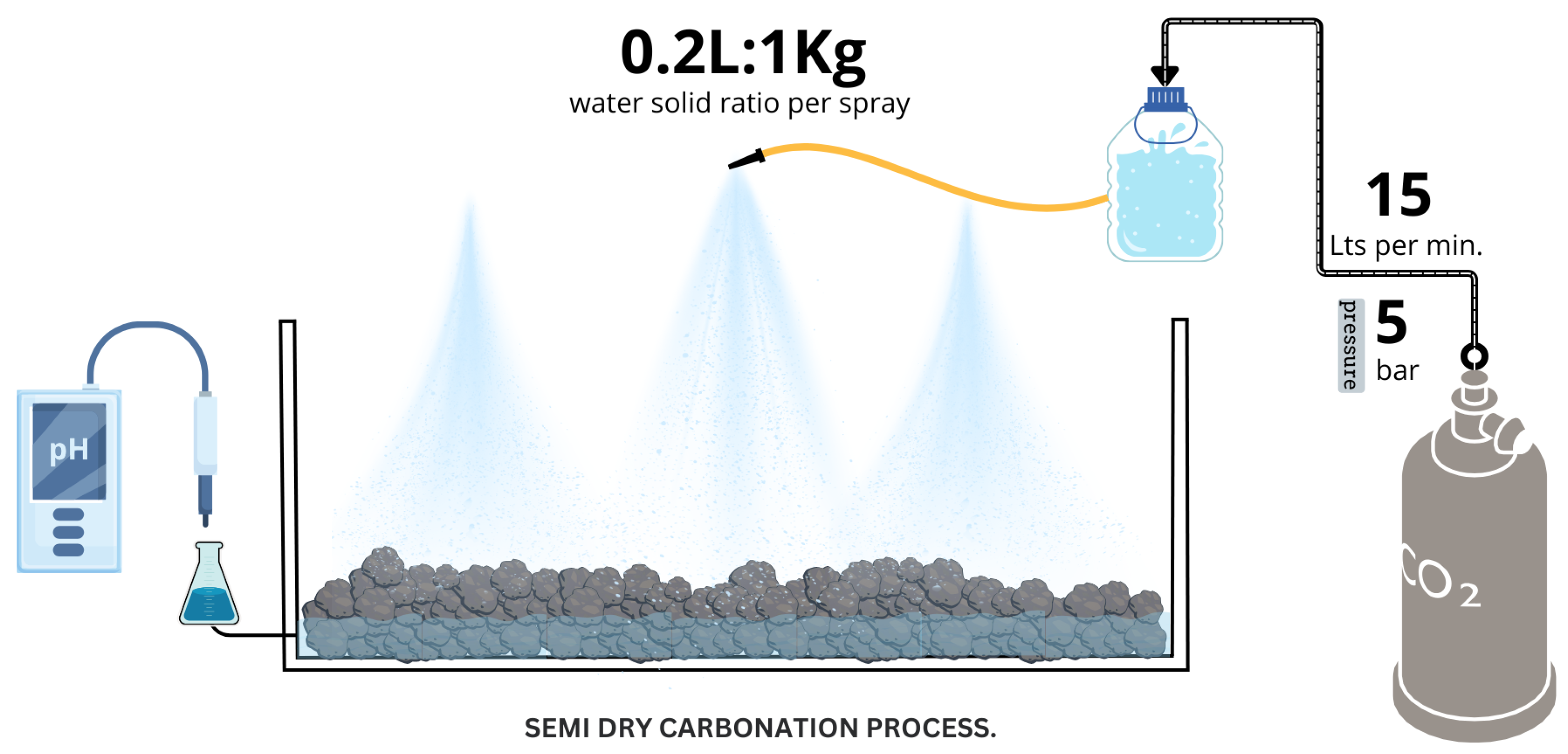

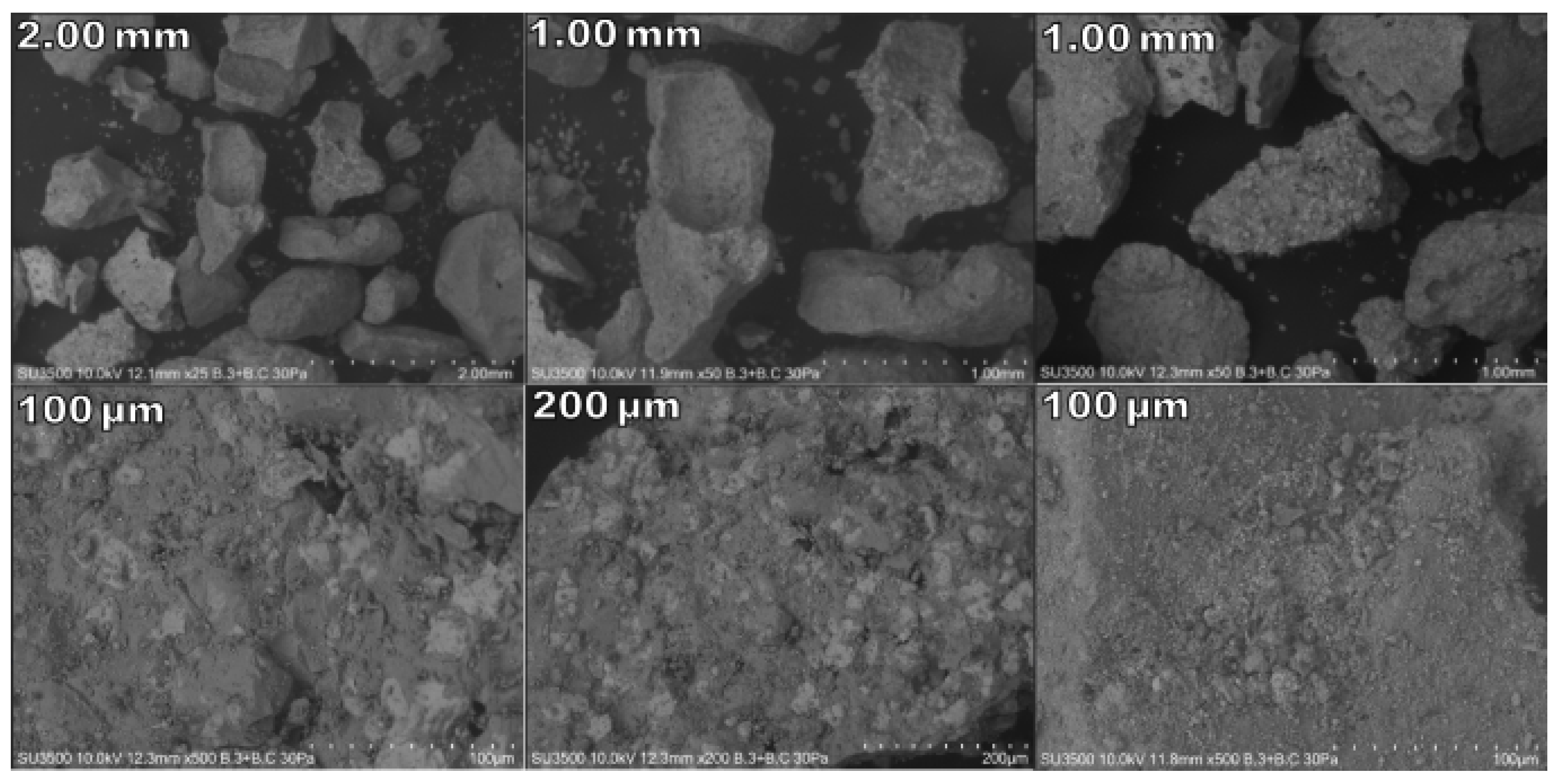




| Serie | Temperature (°C) | Time (min) | Na2CO3 |
|---|---|---|---|
| C00 | 22 | 0 | Without |
| A1 | 22 | 60 | With |
| A2 | 55 | 60 | With |
| A3 | 80 | 60 | With |
| B1 | 22 | 5 | Without |
| B2 | 55 | 5 | Without |
| B3 | 80 | 5 | Without |
| Series | Spray (Times) | Time | Na2CO3 |
|---|---|---|---|
| C00 | 0 | - | Without |
| S1 | 1 | - | Without |
| S2 | 1 every 4 h | 8 h | Without |
| S3 | 1 every 6 h | 12 h | Without |
| S4 | 1 | - | With |
| S5 | 1 per day | 2 days | Without |
| S6 | 1 per day | 3 days | Without |
| Properties | Result |
|---|---|
| Specific gravity | 3.45 |
| Bulk density (g/cm3) | 3.71 |
| Absorption (%) | 2.90 |
| Component | ERHA® [%] |
|---|---|
| Fe2O3 | 30.99 |
| CaO | 23.84 |
| SiO2 | 14.56 |
| Al2O3 | 13.42 |
| MgO | 6.73 |
| MnO | 5.14 |
| Cr2O3 | 2.43 |
| TiO2 | 0.75 |
| Na2O | 0.45 |
| P2O5 | 0.31 |
| ZnO | 0.29 |
| SO3 | 0.27 |
| BaO | 0.27 |
| Cl | 0.14 |
| V2O5 | 0.12 |
| K2O | 0.05 |
| NiO | * |
| CuO | 0.04 |
| Ga2O3 | * |
| SrO | 0.07 |
| ZrO2 | 0.02 |
| Nb2O5 | 0.03 |
| WO3 | 0.05 |
| PbO | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante, M.; Letelier, V.; Huanquilef, R.; Muñoz, P. Analysis of Variables in Accelerated Carbonation Environment for the Processing of Electric Arc Furnace Slag Aggregate. Appl. Sci. 2025, 15, 9360. https://doi.org/10.3390/app15179360
Bustamante M, Letelier V, Huanquilef R, Muñoz P. Analysis of Variables in Accelerated Carbonation Environment for the Processing of Electric Arc Furnace Slag Aggregate. Applied Sciences. 2025; 15(17):9360. https://doi.org/10.3390/app15179360
Chicago/Turabian StyleBustamante, Marion, Viviana Letelier, Ricardo Huanquilef, and Pedro Muñoz. 2025. "Analysis of Variables in Accelerated Carbonation Environment for the Processing of Electric Arc Furnace Slag Aggregate" Applied Sciences 15, no. 17: 9360. https://doi.org/10.3390/app15179360
APA StyleBustamante, M., Letelier, V., Huanquilef, R., & Muñoz, P. (2025). Analysis of Variables in Accelerated Carbonation Environment for the Processing of Electric Arc Furnace Slag Aggregate. Applied Sciences, 15(17), 9360. https://doi.org/10.3390/app15179360










