Synergistic Valorization of Refuse-Derived Fuel and Animal Fat Waste Through Dry and Hydrothermal Co-Carbonization
Abstract
1. Introduction
2. Materials and Methods Introduction
2.1. Feedstock
2.2. Carbonization Tests
2.3. Chars, Hydrochars, and Effluent Characterization
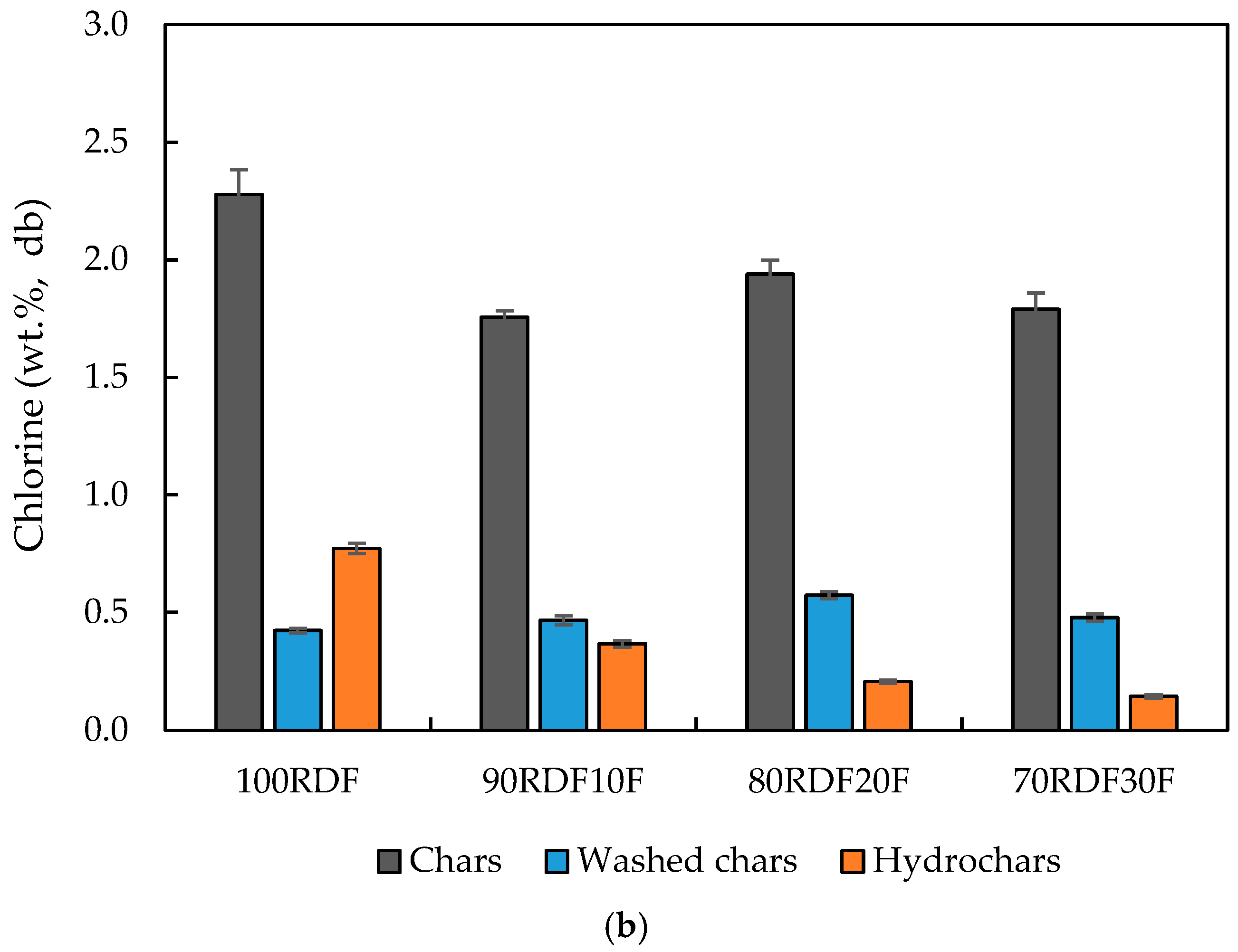
2.4. Char Washing for Chlorine Removal
3. Results
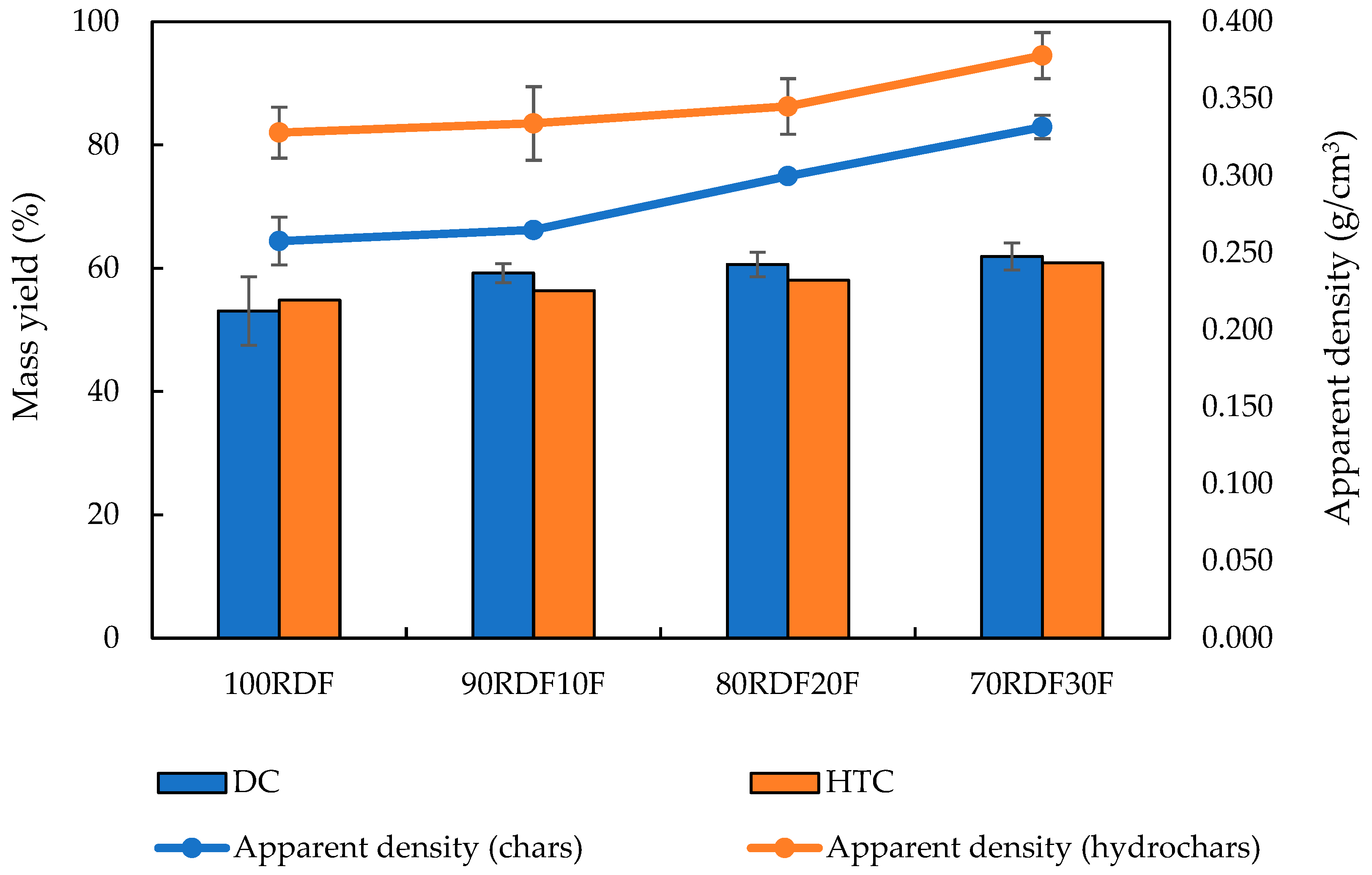
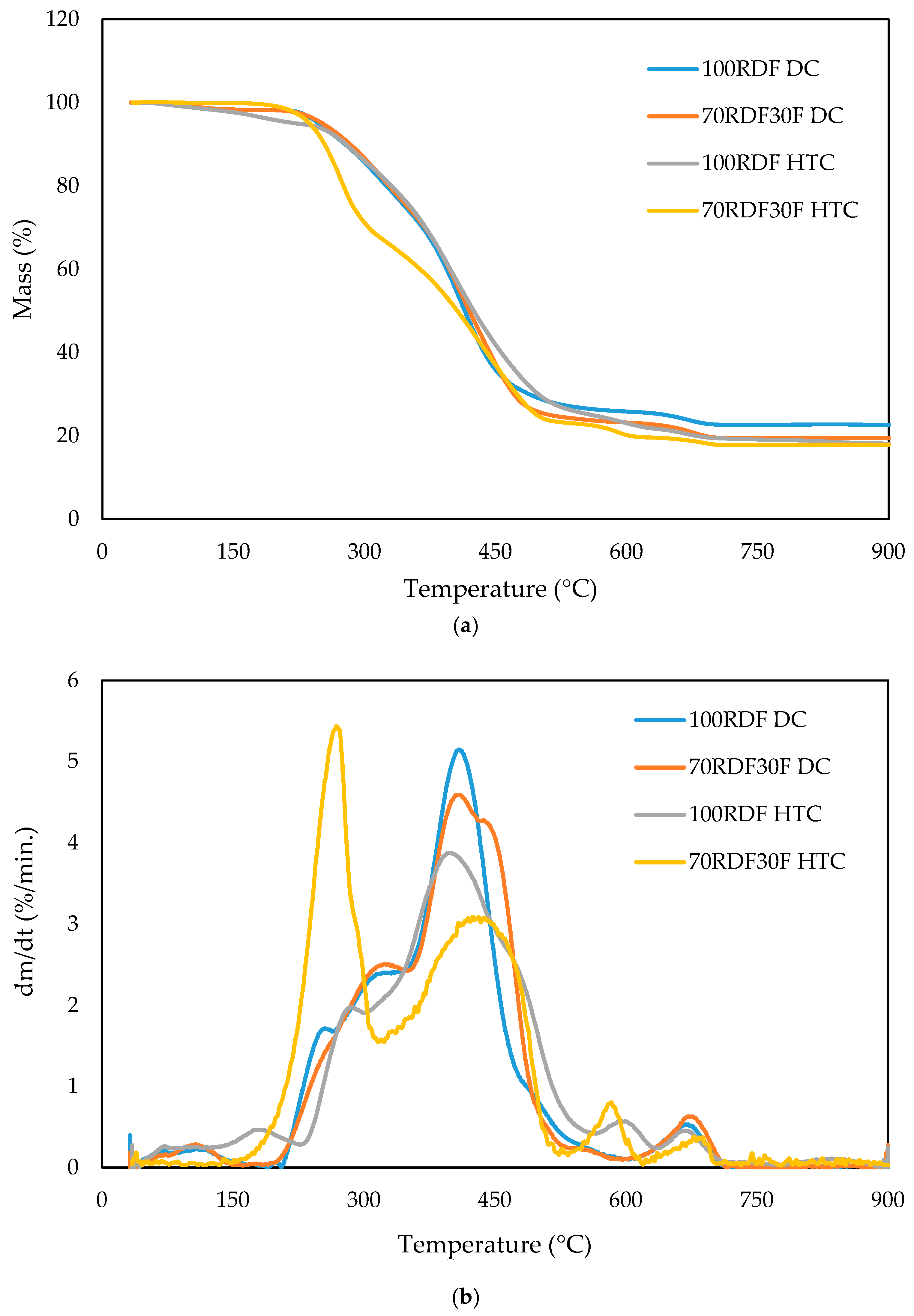
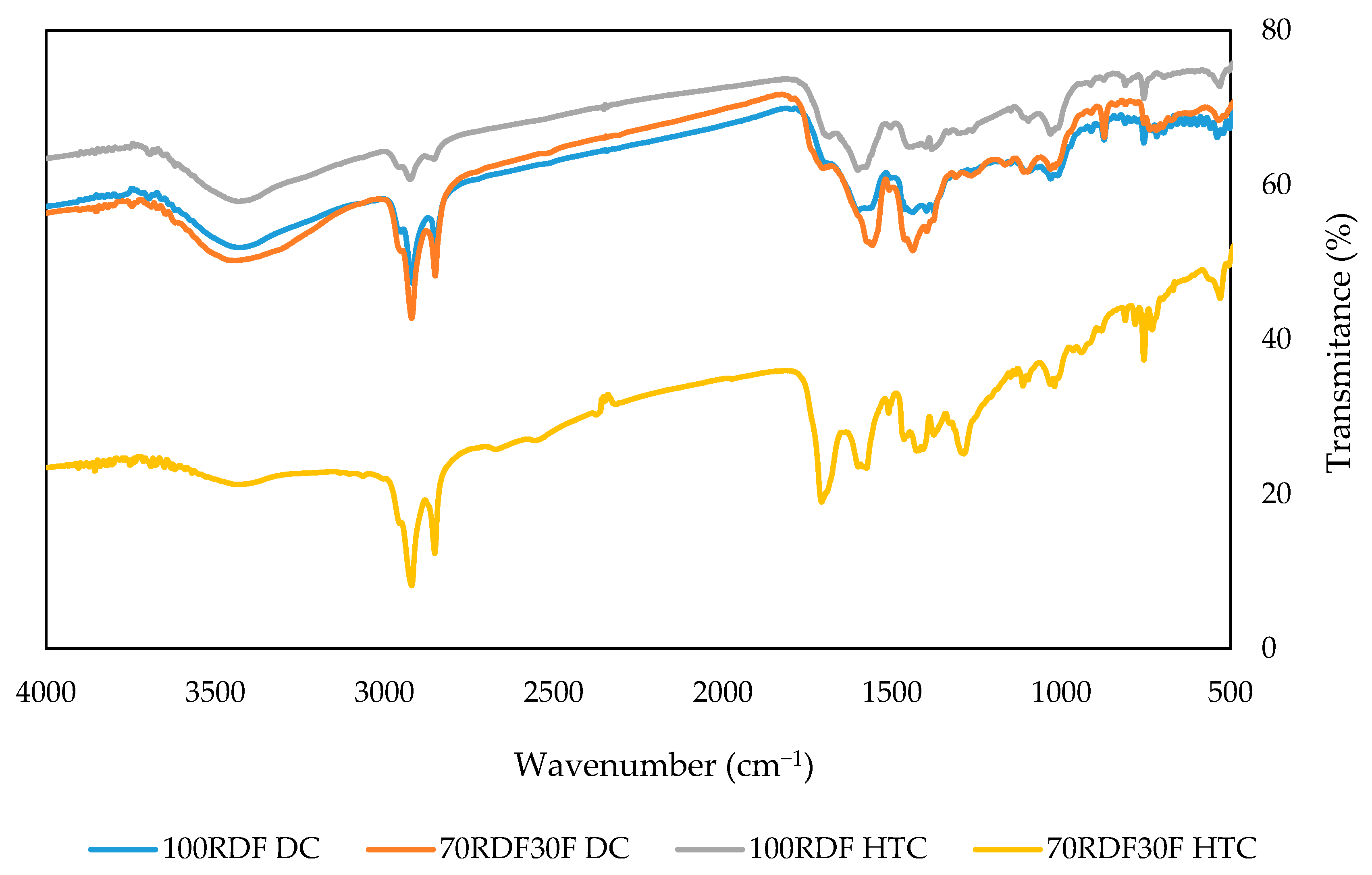
3.1. Dry and Hydrothermal Carbonization Tests
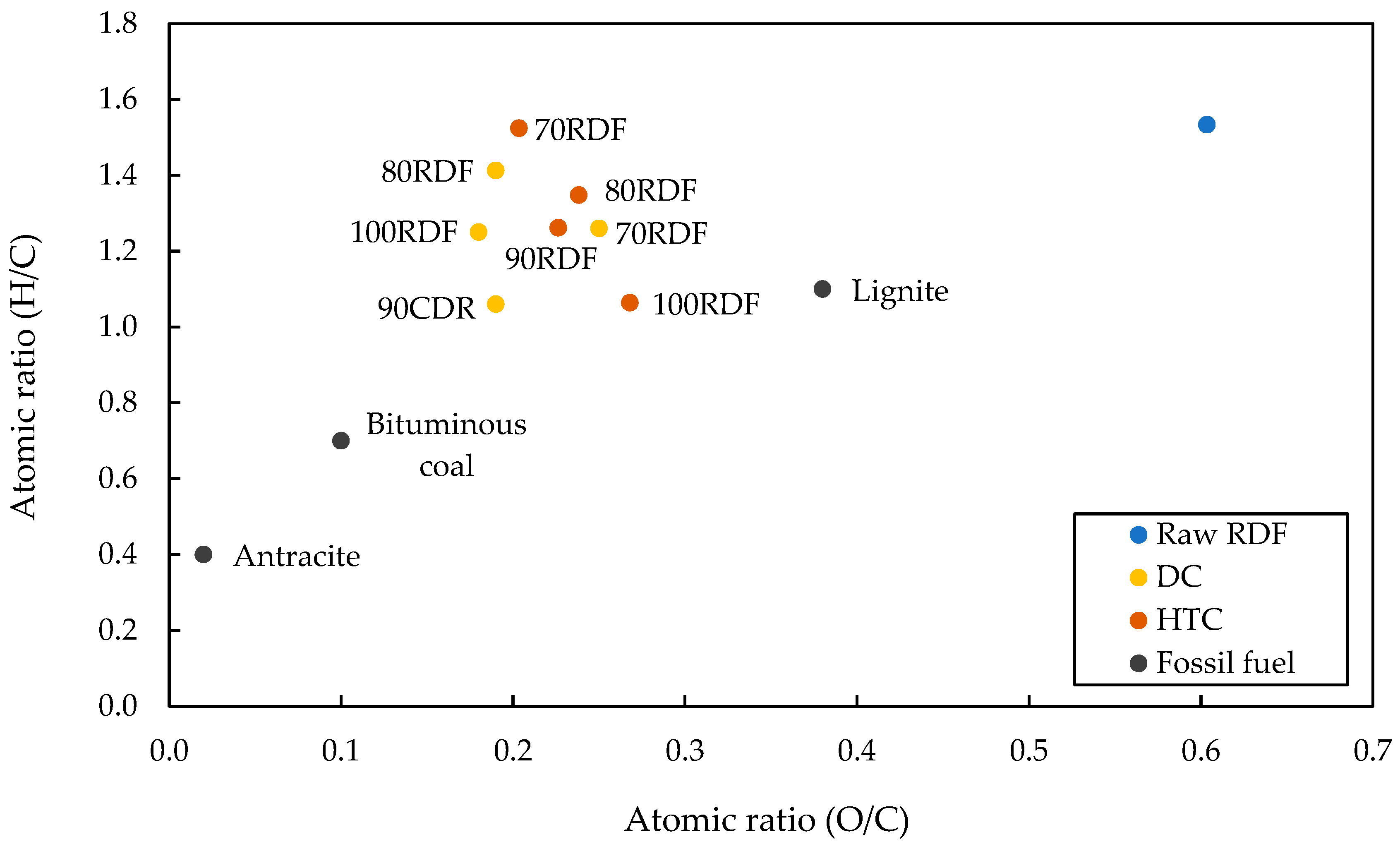
3.2. Feedstock and Char Characterization
3.3. Effluent Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, C.; Zuo, W.; Yuan, S.; Zhao, P.; Zhou, H. EEffect of Moisture on Gasification of Hydrochar Derived from Real-MSW. Biomass Bioenergy 2023, 178, 106976. [Google Scholar] [CrossRef]
- Bardhan, M.; Novera, T.M.; Tabassum, M.; Islam, M.A.; Islam, M.A.; Hameed, B.H. Co-Hydrothermal Carbonization of Different Feedstocks to Hydrochar as Potential Energy for the Future World: A Review. J. Clean. Prod. 2021, 298, 126734. [Google Scholar] [CrossRef]
- Niu, M.; Sun, R.; Ding, K.; Gu, H.; Cui, X.; Wang, L.; Hu, J. Synergistic Effect on Thermal Behavior and Product Characteristics during Co-Pyrolysis of Biomass and Waste Tire: Influence of Biomass Species and Waste Blending Ratios. Energy 2021, 240, 122808. [Google Scholar] [CrossRef]
- Tauseef, M.; Ansari, A.; Khoja, A.; Naqvi, S.R.; Liaquat, R.; Nimmo, W.; Daood, S. Thermokinetics Synergistic Effects on Co-Pyrolysis of Coal and Rice Husk Blends for Bioenergy Production. Fuel 2022, 318, 123685. [Google Scholar] [CrossRef]
- Mishra, R.; Sahoo, A.; Mohanty, K. Pyrolysis Kinetics and Synergistic Effect in Co-Pyrolysis of Samanea Saman Seeds and Polyethylene Terephthalate Using Thermogravimetric Analyser. Bioresour. Technol. 2019, 289, 121608. [Google Scholar] [CrossRef]
- Infiesta, L.R.; Ferreira, C.R.N.; Trovó, A.G.; Borges, V.L.; Carvalho, S.R. Design of an Industrial Solid Waste Processing Line to Produce Refuse-Derived Fuel. J. Environ. Manag. 2019, 236, 715–719. [Google Scholar] [CrossRef]
- Nobre, C.; Vilarinho, C.; Alves, O.; Mendes, B.; Gonçalves, M. Upgrading of Refuse Derived Fuel through Torrefaction and Carbonization: Evaluation of RDF Char Fuel Properties. Energy 2019, 181, 66–76. [Google Scholar] [CrossRef]
- Verhoeff, F.; Adell, A.; Boersma, A.A.R.; Pels, J.R.; Lensselink, J.; Kiel, J.H.A.; Schukken, H. TorTech Torrefaction Technology for the Production of Solid Bioenergy Carriers from Biomass and Waste; ECN: Petten, The Neherlands, 2011. [Google Scholar]
- Barskov, S.; Zappi, M.; Buchireddy, P.; Dufreche, S.; Guillory, J.; Gang, D.; Hernandez, R.; Bajpai, R.; Baudier, J.; Cooper, R.; et al. Torrefaction of Biomass: A Review of Production Methods for Biocoal from Cultured and Waste Lignocellulosic Feedstocks. Renew. Energy 2019, 142, 624–642. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, P.; Liu, R. Synergistic Combination of Biomass Torrefaction and Co-Gasification: 1. Reactivity Studies. Bioresour. Technol. 2017, 245, 225–233. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass Torrefaction: An Overview on Process Parameters, Economic and Environmental Aspects and Recent Advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Huang, Z.; Qin, L.; Chen, W.; Zhao, B.; Xing, F. Refused Derived Fuel from Municipal Solid Waste Used as an Alternative Fuel during the Iron Ore Sinter Process. J. Clean. Prod. 2021, 278, 123594. [Google Scholar] [CrossRef]
- Manyà, J.J.; García-Ceballos, F.; Azuara, M.; Latorre, N.; Royo, C. Pyrolysis and Char Reactivity of a Poor-Quality Refuse-Derived Fuel (RDF) from Municipal Solid Waste. Fuel Process. Technol. 2015, 140, 276–284. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Longo, A.; Vilarinho, C.; Gonçalves, M. Torrefaction and Carbonization of Refuse Derived Fuel: Char Characterization and Evaluation of Gaseous and Liquid Emissions. Bioresour. Technol. 2019, 285, 121325. [Google Scholar] [CrossRef]
- Rago, Y.P.; Collard, F.X.; Görgens, J.F.; Surroop, D.; Mohee, R. Torrefaction of Biomass and Plastic from Municipal Solid Waste Streams and Their Blends: Evaluation of Interactive Effects. Fuel 2020, 277, 118089. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Z.; Zhang, J.; Zhan, W.; Gao, L.; He, Z. Synthesis and Characteristics of Carbon-Based Synfuel from Biomass and Coal Powder by Synergistic Co-Carbonization Technology. Renew. Energy 2024, 227, 120458. [Google Scholar] [CrossRef]
- Wei, Y.; Fakudze, S.; Zhang, Y.; Ma, R.; Shang, Q.; Chen, J.; Liu, C.; Chu, Q. Co-Hydrothermal Carbonization of Pomelo Peel and PVC for Production of Hydrochar Pellets with Enhanced Fuel Properties and Dechlorination. Energy 2022, 239, 122350. [Google Scholar] [CrossRef]
- Mahata, S.; Periyavaram, S.R.; Akkupalli, N.K.; Srivastava, S.; Matli, C. A Review on Co-Hydrothermal Carbonization of Sludge: Effect of Process Parameters, Reaction Pathway, and Pollutant Transport. J. Energy Inst. 2023, 110, 101340. [Google Scholar] [CrossRef]
- Piboonudomkarn, S.; Khemthong, P.; Youngjan, S.; Wantala, K. Co-Hydrothermally Carbonized Sewage Sludge and Lignocellulosic Biomass: An Efficiently Renewable Solid Fuel. Arab. J. Chem. 2023, 16, 105315. [Google Scholar] [CrossRef]
- Sharma, H.B.; Dubey, B.K. Co-Hydrothermal Carbonization of Food Waste with Yard Waste for Solid Biofuel Production: Hydrochar Characterization and Its Pelletization. Waste Manag. 2020, 118, 521–533. [Google Scholar] [CrossRef]
- Wang, T.; Si, B.; Gong, Z.; Zhai, Y.; Cao, M.; Peng, C. Co-Hydrothermal Carbonization of Food Waste-Woody Sawdust Blend: Interaction Effects on the Hydrochar Properties and Nutrients Characteristics. Bioresour. Technol. 2020, 316, 123900. [Google Scholar] [CrossRef]
- Ul Saqib, N.; Sarmah, A.K.; Baroutian, S. Effect of Temperature on the Fuel Properties of Food Waste and Coal Blend Treated under Co-Hydrothermal Carbonization. Waste Manag. 2019, 89, 236–246. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Ge, C.; Liu, W.; Tang, Y.; Zhuang, X.; Qiu, R. Synergistic Effect of Hydrothermal Co-Carbonization of Sewage Sludge with Fruit and Agricultural Wastes on Hydrochar Fuel Quality and Combustion Behavior. Waste Manag. 2019, 100, 171–181. [Google Scholar] [CrossRef]
- González-Arias, J.; Carnicero, A.; Sánchez, M.E.; Martínez, E.J.; López, R.; Cara-Jiménez, J. Management of Off-Specification Compost by Using Co-Hydrothermal Carbonization with Olive Tree Pruning. Assessing Energy Potential of Hydrochar. Waste Manag. 2021, 124, 224–234. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, C.; Ma, X.; Zhou, Y.; Wu, J. Co-Hydrothermal Carbonization of Sewage Sludge and Banana Stalk: Fuel Properties of Hydrochar and Environmental Risks of Heavy Metals. J. Environ. Chem. Eng. 2021, 9, 106051. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Gholizadeh, M.; Hu, X.; Yuan, X.; Sarkar, B.; Vithanage, M.; Mašek, O.; Ok, Y.S. Co-Hydrothermal Carbonization of Swine and Chicken Manure: Influence of Cross-Interaction on Hydrochar and Liquid Characteristics. Sci. Total Environ. 2021, 786, 147381. [Google Scholar] [CrossRef]
- Li, Q.; Lin, H.; Zhang, S.; Yuan, X.; Gholizadeh, M.; Wang, Y.; Xiang, J.; Hu, S.; Hu, X. Co-Hydrothermal Carbonization of Swine Manure and Cellulose: Influence of Mutual Interaction of Intermediates on Properties of the Products. Sci. Total Environ. 2021, 791, 148134. [Google Scholar] [CrossRef]
- Belete, Y.Z.; Mau, V.; Yahav Spitzer, R.; Posmanik, R.; Jassby, D.; Iddya, A.; Kassem, N.; Tester, J.W.; Gross, A. Hydrothermal Carbonization of Anaerobic Digestate and Manure from a Dairy Farm on Energy Recovery and the Fate of Nutrients. Bioresour. Technol. 2021, 333, 125164. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, R.; Zhang, D.; Gao, Y.; Xiong, M.; Chen, W. Co-Hydrothermal Carbonization of Cotton Textile Waste and Polyvinyl Chloride Waste for the Production of Solid Fuel: Interaction Mechanisms and Combustion Behaviors. J. Clean. Prod. 2021, 316, 128306. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Li, A. Co-Hydrothermal Carbonization of Lignocellulosic Biomass and Waste Polyvinyl Chloride for High-Quality Solid Fuel Production: Hydrochar Properties and Its Combustion and Pyrolysis Behaviors. Bioresour. Technol. 2019, 294, 122113. [Google Scholar] [CrossRef]
- Lin, C.; Zhao, P.; Ding, Y.; Cui, X.; Liu, F.; Wang, C.; Guo, Q. Hydrogen-Rich Gas Production from Hydrochar Derived from Hydrothermal Carbonization of PVC and Alkali Coal. Fuel Process. Technol. 2021, 222, 106959. [Google Scholar] [CrossRef]
- Huang, N.; Zhao, P.; Ghosh, S.; Fedyukhin, A. Co-Hydrothermal Carbonization of Polyvinyl Chloride and Moist Biomass to Remove Chlorine and Inorganics for Clean Fuel Production. Appl. Energy 2019, 240, 882–892. [Google Scholar] [CrossRef]
- Shen, Y. A Review on Hydrothermal Carbonization of Biomass and Plastic Wastes to Energy Products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Kossińska, N.; Grosser, A.; Kwapińska, M.; Kwapiński, W.; Ghazal, H.; Jouhara, H.; Krzyżyńska, R. Co-Hydrothermal Carbonization as a Potential Method of Utilising Digested Sludge and Screenings from Wastewater Treatment Plants towards Energy Application. Energy 2024, 299, 131456. [Google Scholar] [CrossRef]
- Petrovič, A.; Predikaka, T.C.; Škodič, L.; Vohl, S.; Čuček, L. Hydrothermal Co-Carbonization of Sewage Sludge and Whey: Enhancement of Product Properties and Potential Application in Agriculture. Fuel 2023, 350, 128807. [Google Scholar] [CrossRef]
- Li, C.S.; Cai, R.R.; Hasan, A.; Lu, X.L.; Yang, X.X.; Zhang, Y.G. Fertility Assessment and Nutrient Conversion of Hydrochars Derived from Co-Hydrothermal Carbonization between Livestock Manure and Corn Cob. J. Environ. Chem. Eng. 2023, 11, 109166. [Google Scholar] [CrossRef]
- Ebrahim Malool, M.; Keshavarz Moraveji, M.; Shayegan, J. Co-Hydrothermal Carbonization of Digested Sewage Sludge and Sugarcane Bagasse: Integrated Approach for Waste Management, Optimized Production, Characterization and Pb(II) Adsorption. Alex. Eng. J. 2023, 74, 79–105. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, T.; Wang, S.; Andrea, K.; Peng, H.; Yuan, H.; Zhu, Z. Multivariate and Multi-Interface Insights into Carbon and Energy Recovery and Conversion Characteristics of Hydrothermal Carbonization of Biomass Waste from Duck Farm. Waste Manag. 2023, 170, 154–165. [Google Scholar] [CrossRef]
- Yatish, K.V.; Ningaraju, C.; Lalithamba, H.S.; Sakar, M.; Geetha Balakrishna, R. Demonstrating Photocatalytic Esterification as a Potential Strategy to Improve the Properties of Feedstock Oil Derived from Dairy Waste Scum for Biodiesel Production. Energy Convers. Manag. 2024, 309, 118463. [Google Scholar] [CrossRef]
- Valizadeh, S.; Valizadeh, B.; Khani, Y.; Jae, J.; Hyun Ko, C.; Park, Y.K. Production of Biodiesel via Esterification of Coffee Waste-Derived Bio-Oil Using Sulfonated Catalysts. Bioresour. Technol. 2024, 404, 130908. [Google Scholar] [CrossRef]
- Paula Soares Dias, A.; Saraiva, N.; Rijo, B.; Francisco Costa Pereira, M.; Santos, L.F.; Galhano, R.; Paulo, I. Sugar Derived Hydrochar Catalysts for Enhanced Biodiesel Production via Esterification. Fuel 2024, 374, 132459. [Google Scholar] [CrossRef]
- Shah, M.; Poudel, J.; Kwak, H.; Oh, S.C. Kinetic Analysis of Transesterification of Waste Pig Fat in Supercritical Alcohols. Process Saf. Environ. Prot. 2015, 98, 239–244. [Google Scholar] [CrossRef]
- Hariprasath, P.; Vijayakumar, V.; Selvamani, S.T.; Vigneshwar, M.; Palanikumar, K. Some Studies on Waste Animal Tallow Biodiesel Produced by Modified Transesterification Method Using Heterogeneous Catalyst. Mater. Today Proc. 2019, 16, 1271–1278. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Veiga-del-Baño, J.M.; Chica, A.; Quesada-Medina, J. Waste Animal Fats as Feedstock for Biodiesel Production Using Non-Catalytic Supercritical Alcohol Transesterification: A Perspective by the PRISMA Methodology. Energy Sustain. Dev. 2022, 69, 150–163. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N. Pre-Esterification of High Acidity Animal Fats to Produce Biodiesel: A Kinetic Study. Arab. J. Chem. 2021, 14, 103048. [Google Scholar] [CrossRef]
- Magdziarz, A.; Jerzak, W.; Wądrzyk, M.; Sieradzka, M. Benefits from Co-Pyrolysis of Biomass and Refuse Derived Fuel for Biofuels Production: Experimental Investigations. Renew. Energy 2024, 230, 120808. [Google Scholar] [CrossRef]
- Zaini, I.; Wen, Y.; Mousa, E.; Jönsson, P.; Yang, W. Primary Fragmentation Behavior of Refuse Derived Fuel Pellets during Rapid Pyrolysis. Fuel Process. Technol. 2021, 216, 106796. [Google Scholar] [CrossRef]
- Bhatt, M.; Wagh, S.; Chakinala, A.; Pant, K.; Sharma, T.; Joshi, J.; Shah, K.; Sharma, A. Conversion of Refuse Derived Fuel from Municipal Solid Waste into Valuable Chemicals Using Advanced Thermo-Chemical Process. J. Clean. Prod. 2021, 329, 129653. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Durão, L.; Şen, A.; Vilarinho, C.; Gonçalves, M. Characterization of Hydrochar and Process Water from the Hydrothermal Carbonization of Refuse Derived Fuel. Waste Manag. 2020, 120, 303–313. [Google Scholar] [CrossRef]
- Longo, A.; Alves, O.; Șen, A.U.; Nobre, C.; Brito, P.; Gonçalves, M. Dry and Hydrothermal Co-Carbonization of Mixed Refuse-Derived Fuel (RDF) for Solid Fuel Production. Reactions 2024, 5, 77–97. [Google Scholar] [CrossRef]
- Longo, A.; Pacheco, N.; Panizio, R.; Vilarinho, C.; Brito, P.; Gonçalves, M. Carbonization of Refuse-Derived Fuel Pellets with Biomass Incorporation to Solid Fuel Production. Fuels 2024, 5, 746–761. [Google Scholar] [CrossRef]
- CEN/TS 15414-1; Solid Recoverd Fuels–Determination of Moisture Content Usign the Oven Dry Method–Part 1: Determinations of Total Moisture by a Reference Method. European Committee for Standarization (CEN): Brussels, Belgium, 2010; Volume 1.
- EN 15402:2011; Solid Recovered Fuels–Determination of the Content of Volatile Matter. European Committee for Standarization (CEN): Brussels, Belgium, 2010.
- EN 15403; Solid Recoverd Fuels–Determination of Ash Content. European Committee for Standarization (CEN): Brussels, Belgium, 2011.
- Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B., Eaton, A.D., Rice, E., Eds.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- EPA-SW-9253; Clhoride (Titrimetric, Silver Nitrate). United States Environmental Protection Agency (EPA): Washington, DC, USA, 1994; Volume 16.
- Liu, X.; Tan, H.; Wang, X.; Wang, Z.; Xiong, X. Oxidation Reactivity and Kinetic Analysis of Bituminous Coal Char from High-Temperature Pyrolysis: Effect of Heating Rate and Pyrolysis Temperature. Thermochim. Acta 2020, 690, 178660. [Google Scholar] [CrossRef]
- Ovčačíková, H.; Velička, M.; Vlček, J.; Topinková, M.; Klárová, M.; Burda, J. Corrosive Effect of Wood Ash Produced by Biomass Combustion on Refractory Materials in a Binary Al–Si System. Materials 2022, 15, 5796. [Google Scholar] [CrossRef]
- Benavente, V.; Lage, S.; Gentili, F.; Jansson, S. Influence of Lipid Extraction and Processing Conditions on Hydrothermal Conversion of Microalgae Feedstocks – Effect on Hydrochar Composition, Secondary Char Formation and Phytotoxicity. Chem. Eng. J. 2021, 428, 129559. [Google Scholar] [CrossRef]
- Adeniyi, A.; Iwuozor, K.; Emenike, E.; Ajala, O.; Ogunniyi, S.; Muritala, K. Thermochemical Co-Conversion of Biomass-Plastic Waste to Biochar: A Review. Green Chem. Eng. 2023, 5, 31–49. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Experimental Analysis of the Effects of Feedstock Composition on the Plastic and Biomass Co-Gasification Process. Renew. Energy 2024, 231, 120960. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Z.; Chen, G.; Zhang, M.; Sun, T.; Wang, Q.; Du, Z.; Chen, Y.; Wu, M.; Li, Z.; et al. Co-Pyrolysis Characteristics of Forestry and Agricultural Residues and Waste Plastics: Thermal Decomposition and Products Distribution. Process Saf. Environ. Prot. 2023, 177, 380–390. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M.; Goldfarb, J.; Maag, A. Effect of Solvent and Feedstock Selection on Primary and Secondary Chars Produced via Hydrothermal Carbonization of Food Wastes. Bioresour. Technol. 2022, 348, 126799. [Google Scholar] [CrossRef]
- Broch, A.; Jena, U.; Hoekman, S.; Langford, J. Analysis of Solid and Aqueous Phase Products from Hydrothermal Carbonization of Whole and Lipid-Extracted Algae. Energies 2013, 7, 62–79. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xiao, K.; Liu, X.; Hu, H.; Li, X.; Yao, H. Correlations between the Physicochemical Properties of Hydrochar and Specific Components of Waste Lettuce: Influence of Moisture, Carbohydrates, Proteins and Lipids. Bioresour. Technol. 2019, 272, 482–488. [Google Scholar] [CrossRef]
- Aliyu, M.; Iwabuchi, K.; Itoh, T. Upgrading the Fuel Properties of Hydrochar by Co-Hydrothermal Carbonisation of Dairy Manure and Japanese Larch (Larix Kaempferi): Product Characterisation, Thermal Behaviour, Kinetics and Thermodynamic Properties. Biomass Convers. Biorefinery 2023, 13, 11917–11932. [Google Scholar] [CrossRef]
- Figueiró, C.G.; Vital, B.R.; de Carneiro, A.C.O.; da Silva, C.M.S.; Magalhães, M.A.; de Fialho, L.F. Energy Valorization of Woody Biomass by Torrefaction Treatment: A Brazilian Experimental Study. Maderas Cienc. Tecnol. 2019, 21, 297–304. [Google Scholar] [CrossRef]
- Liu, X.; Peng, L.; Deng, P.; Xu, Y.; Wang, P.; Tan, Q.; Zhang, C.; Dai, X. Co-Hydrothermal Carbonization of Sewage Sludge and Rice Straw to Improve Hydrochar Quality: Effects of Mixing Ratio and Hydrothermal Temperature. Bioresour. Technol. 2024, 415, 131665. [Google Scholar] [CrossRef]
- Petrovič, A.; Predikaka, T.C.; Vuković, J.P.; Jednačak, T.; Hribernik, S.; Vohl, S.; Urbancl, D.; Tišma, M.; Čuček, L. Sustainable Hydrothermal Co-Carbonization of Residues from the Vegetable Oil Industry and Sewage Sludge: Hydrochar Production and Liquid Fraction Valorisation. Energy 2024, 307, 132760. [Google Scholar] [CrossRef]
- Shen, Q.; Zhu, X.; Peng, Y.; Xu, M.-M.; Huang, Y.; Xia, A.; Zhu, X.; Liao, Q. Structure Evolution Characteristic of Hydrochar and Nitrogen Transformation Mechanism during Co-Hydrothermal Carbonization Process of Microalgae and Biomass. Energy 2024, 295, 131028. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Ghiasi, B.; Soelberg, N.R.; Sokhansanj, S. Biomass Torrefaction Process, Product Properties, Reactor Types, and Moving Bed Reactor Design Concepts. Front. Energy Res. 2021, 9, 1–20. [Google Scholar] [CrossRef]
- ÖzyuǧUran, A.; Yaman, S. Prediction of Calorific Value of Biomass from Proximate Analysis. Energy Procedia 2017, 107, 130–136. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, Y.; Niu, J.; Zeng, X.; Zheng, X.; Wu, C. Effect of Low-Nitrogen Combustion System with Flue Gas Circulation Technology on the Performance of NOx Emission in Waste-to-Energy Power Plant. Chem. Eng. Process. - Process Intensif. 2022, 175, 108910. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kourtelesis, M.; Moschovi, A.M.; Sakkas, K.M.; Yakoumis, I. Selected Techniques for Cutting SOx Emissions in Maritime Industry. Technologies 2022, 10, 99. [Google Scholar] [CrossRef]
- Chen, W.H.; Lu, K.M.; Tsai, C.M. An Experimental Analysis on Property and Structure Variations of Agricultural Wastes Undergoing Torrefaction. Appl. Energy 2012, 100, 318–325. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Afzal, M.T. HHV Predicting Correlations for Torrefied Biomass Using Proximate and Ultimate Analyses. Bioengineering 2017, 4, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S.; Li, W.; Gu, J.; Yuan, H.; Ling, X.; Chen, Y. Chlorine Migration Mechanisms during Torrefaction of Fermentation Residue from Food Waste. Bioresour. Technol. 2019, 271, 9–15. [Google Scholar] [CrossRef]
- Mota-Panizio, R.; Hermoso-Orzáez, M.J.; Carmo-Calado, L.; Calado, H.; Goncalves, M.M.; Brito, P. Co-Carbonization of a Mixture of Waste Insulation Electric Cables (WIEC) and Lignocellulosic Waste, for the Removal of Chlorine: Biochar Properties and Their Behaviors. Fuel 2022, 320, 123932. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N.; Abelha, P. Evaluation of Slagging and Fouling Tendency during Biomass Co-Firing with Coal in a Fluidized Bed. Biomass Bioenergy 2012, 39, 192–203. [Google Scholar] [CrossRef]
- Porshnov, D.; Ozols, V.; Ansone-Bertina, L.; Burlakovs, J.; Klavins, M. Thermal Decomposition Study of Major Refuse Derived Fuel Components. Energy Procedia 2018, 147, 48–53. [Google Scholar] [CrossRef]
- Lu, J.J.; Chen, W.H. Investigation on the Ignition and Burnout Temperatures of Bamboo and Sugarcane Bagasse by Thermogravimetric Analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]
- Çepelioğullar; Haykiri-Açma, H.; Yaman, S. Kinetic Modelling of RDF Pyrolysis: Model-Fitting and Model-Free Approaches. Waste Manag. 2016, 48, 275–284. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X. Characteristics of Co-Hydrothermal Carbonization on Polyvinyl Chloride Wastes with Bamboo. Bioresour. Technol. 2018, 247, 302–309. [Google Scholar] [CrossRef] [PubMed]
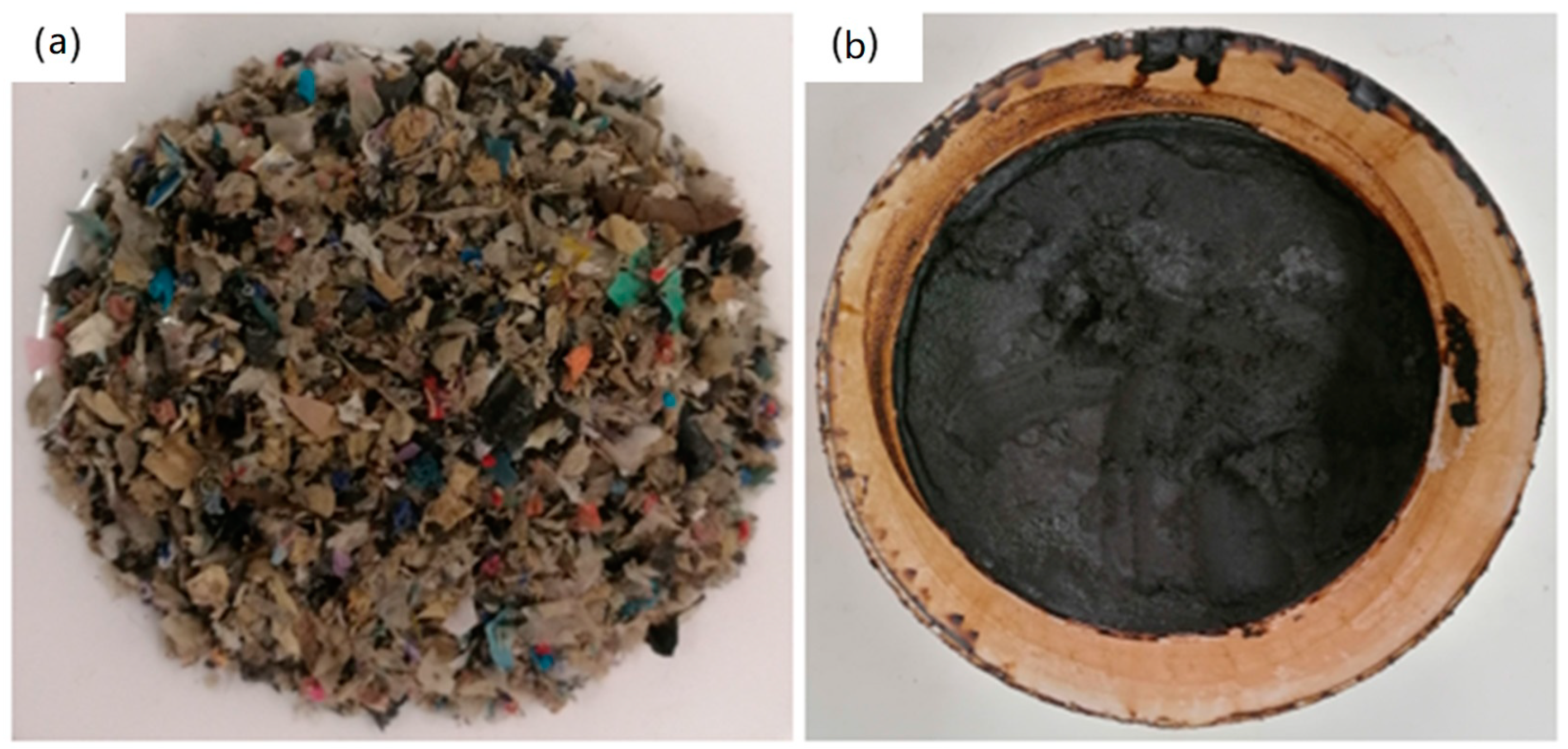
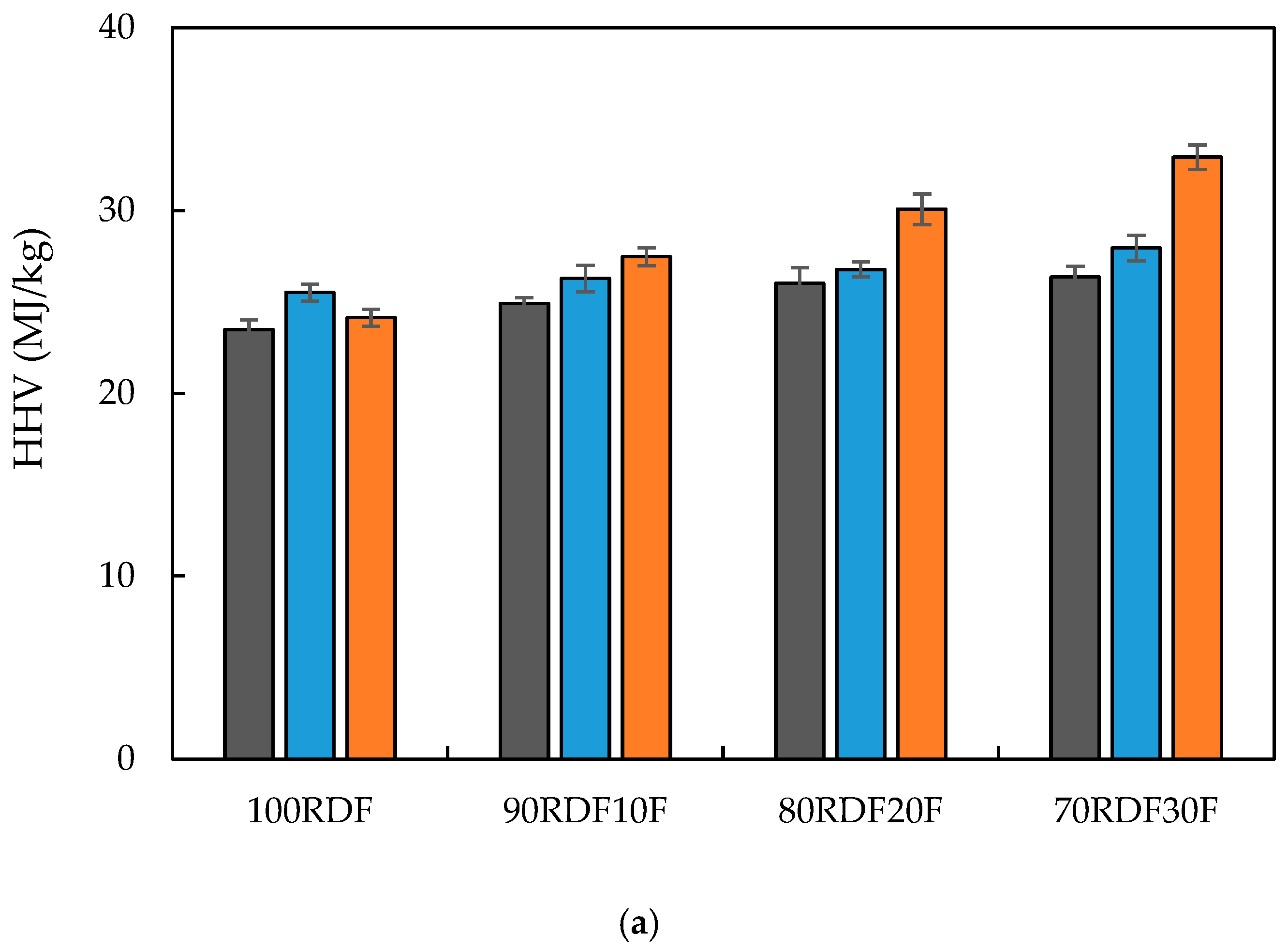
| Thermochemical Method | Sample | Composition (wt.%) | T (°C) | t (min) | |
|---|---|---|---|---|---|
| RDF | Fat | ||||
| Dry carbonization (DC) | 100RDF | 100 | 0 | 425 | 30 |
| 90RDF10F | 90 | 10 | |||
| 80RDF20F | 80 | 20 | |||
| 70RDF30F | 70 | 30 | |||
| Hydrothermal carbonization (HTC) | 100RDF | 100 | 0 | 300 | |
| 90RDF10F | 90 | 10 | |||
| 80RDF20F | 80 | 20 | |||
| 70RDF30F | 70 | 30 | |||
| Analysis | Equipment/Methodology | Conditions |
|---|---|---|
| Mass yield | Equation (1) | Calculated as the mass of the product divided by the initial biomass feedstock mass, expressed as a percentage. |
| Apparent density | Equation (2) | Determined by dividing the dry mass of the sample by its geometric volume. |
| Proximate composition | CEN/TS 15414-3:2010 [52], EN 15402:2011 [53], EN 15403:2011 [54] | Moisture by drying at 105 °C. Volatile matter by heating at ~900 °C under inert atmosphere. Ash by combustion at 550 °C. Fixed carbon was determined by difference. |
| Elemental composition | Thermo Finnigan Flash EA 112 CHNS analyzer (Waltham, MA, USA) | Sample combusted at high temperature (~1000 °C) in oxygen-rich environment. Combustion gases (CO2, H2O, N2, SO2) measured via thermal conductivity and infrared detectors. Oxygen content calculated by difference from total. |
| Ash mineral composition | Horiba Jobin-Yvon, Ultima Model ICP (Inductively Coupled Plasma) (Lyon, France) | Ash sample digested in acid mixture (e.g., HNO3/HCl). Solution analyzed by ICP to quantify mineral elements (Ca, Mg, K, Na, Fe, etc.) |
| Chlorine content | ThermoFisher Scientific Niton XL3t XRF Analyzer (Waltham, MA, USA) | Non-destructive X-ray fluorescence on solid samples, quantifying chlorine via characteristic X-ray emission. |
| HHV (Higher Heating Value) | IKA® C200 calorimeter (Staufen, Germany) | Bomb calorimeter combusts sample in oxygen at constant volume. Temperature rise used to calculate energy content (MJ/kg) |
| FT-IR | ThermoFisher Scientific Nicolet 174 iS10 FT-IR Spectrophotometer (Waltham, MA, USA) | Samples analyzed in mid-infrared range (4000–400 cm−1) to identify functional groups. Measurements performed using ATR. |
| Thermogravimetric analysis | Waters Company, TA Instruments Q50 TG analyzer (New Castle, DE, USA) | Samples heated from room temperature to 900 °C at 20 °C/min in air. Weight loss monitored to assess thermal stability and composition changes |
| pH | Crison MicropH 2001 pH meter (Barcelona, Spain) | Measured at ambient temperature in aqueous suspensions or extracts, using calibrated glass electrode. |
| COD (Chemical Oxygen Demand) | Standard Methods 5220B [55] | Organic matter oxidized by potassium dichromate (K2Cr2O7) in acidic solution under reflux. Remaining dichromate titrated with ferrous ammonium sulfate to quantify oxygen demand. |
| Chlorides | Titration EPA-SW-948 (Test method 9253) [56] | Volumetric titration of chloride ions with silver nitrate (AgNO3) using chromate indicator (Mohr method). |
| Total solids | Standard Methods 2540B [55] | Drying sample at 105 °C until constant weight to quantify total solids content. |
| Ash composition (aqueous samples) | Horiba Jobin-Yvon, Ultima Model ICP (Inductively Coupled Plasma) (Lyon, France) | Ash sample digested in acid mixture (HNO3). Solution analyzed by ICP to quantify mineral elements (Ca, Mg, K, Na, Fe, etc.) |
| Analysis | 100RDF | 90RDF10F | 80RDF20F | 70RDF30F | ||||
|---|---|---|---|---|---|---|---|---|
| DC | HTC | DC | HTC | DC | HTC | DC | HTC | |
| Proximate Analysis (wt.%, db) | ||||||||
| Moisture * | 4.1 ± 0.1 BC | 2.6 ± 0.5 DE | 4.7 ± 0.3 AB | 2.4 ± 0.3 E | 4.8 ± 0.6 A | 1.8 ± 0.1 F | 2.8 ± 0.6 D | 1.1 ± 0.0 G |
| Volatile matter | 66.9 ± 1.3 F | 77.2 ± 4.5 B | 68.3 ± 1.0 E | 72.7 ± 1.2 D | 71.7 ± 0.4 CD | 80.1 ± 1.4 A | 74.6 ± 2.7 C | 81.1 ± 2.9 A |
| Ash | 20.0 ± 0.7 A | 14.5 ± 0.6 D | 17.1 ± 1.7 B | 15.3 ± 0.7 D | 14.8 ± 1.7 C | 10.5 ± 0.6 E | 11.4 ± 2.3 F | 9.5 ± 0.5 E |
| Fixed carbon | 13.1 ± 0.6 C | 8.3 ± 3.4 E | 14.6 ± 1.3 B | 12.0 ± 0.7 D | 13.4 ± 2.0 BC | 9.3 ± 1.0 F | 14.0 ± 4.8 A | 9.3 ± 1.1 F |
| Ultimate analysis (wt.%, daf) | ||||||||
| C | 60.7 ± 3.0 A | 57.6 ± 2.2 B | 60.6 ± 2.0 A | 58.8 ± 3.0 B | 61.7 ± 2.8 A | 61.3 ± 2.7 A | 60.6 ± 2.6 A | 63.0 ± 2.6 A |
| H | 5.4 ± 0.5 D | 5.1 ± 0.3 E | 5.4 ± 0.3 D | 6.2 ± 0.5 C | 6.4 ± 0.5 C | 6.9 ± 0.4 B | 6.4 ± 0.5 C | 8.0 ± 0.5 A |
| N | 1.2 ± 0.3 E | 2.2 ± 0.3 A | 1.6 ± 0.2 D | 1.9 ± 0.4 C | 1.2 ± 0.2 E | 1.7 ± 0.3 C | 1.2 ± 0.2 E | 1.3 ± 0.2 D |
| S | 0.0 ± 0.0 B | 0.0 ± 0.0 B | 0.2 ± 0.0 A | 0.2 ± 0.1 A | 0.1 ± 0.0 A | 0.2 ± 0.1 A | 0.1 ± 0.0 A | 1.1 ± 0.4 A |
| O | 12.6 ± 2.5 C | 20.6 ± 2.7 A | 15.1 ± 1.8 B | 17.7 ± 3.2 B | 15.7 ± 3.3 B | 19.5 ± 3.1 B | 20.3 ± 2.1 A | 17.1 ± 2.5 B |
| O/C | 0.18 | 0.27 | 0.19 | 0.22 | 0.19 | 0.24 | 0.25 | 0.20 |
| H/C | 1.25 | 1.06 | 1.06 | 1.26 | 1.41 | 1.35 | 1.26 | 1.52 |
| Ash Composition | Char Sample | ||||
|---|---|---|---|---|---|
| DC | HTC | ||||
| 100RDF | 70RDF30F | 100RDF | 70RDF30F | ||
| Oxides (wt.%, db) | Al2O3 | 13.4 | 9.1 | 7.5 | 10.1 |
| CaO | 51.5 | 42.5 | 23.6 | 29.4 | |
| Fe2O3 | 12.1 | 3.2 | 2.1 | 3.3 | |
| K2O | 2.1 | 2.0 | 1.1 | 0.9 | |
| MgO | 4.4 | 4.3 | 4.8 | 3.7 | |
| Na2O | 0.2 | 1.0 | 0.5 | 0.3 | |
| SiO2 | 3.0 | 5.4 | 2.1 | 0.6 | |
| TiO2 | 0.9 | 0.2 | 0.3 | 0.1 | |
| Fouling and slagging index | B/A | 4.1 high | 3.6 high | 3.2 high | 3.4 high |
| BAI | 5.4 low | 1.0 low | 1.3 low | 2.7 low | |
| Fu | 9.1 high | 10.8 high | 5.3 high | 4.2 high | |
| Sr | 0.2 low | 0.6 low | 0.3 low | 0.1 low | |
| TA | 2.2 high | 3.0 high | 1.6 high | 1.2 high | |
| Chlorine (wt.%, db) | 0.4 | 0.4 | 0.5 | 0.8 | |
| Ash content (wt.%, db) | 20.1 | 17.3 | 11.4 | 14.5 | |
| Sample | Ti (°C) | Tb (°C) | T1 (°C) | T2 (°C) | DTG1 (%/s) | DTG2 (%/s) |
|---|---|---|---|---|---|---|
| 100RDF DC | 297 | 678 | 407 | 671 | 5.1 | 0.5 |
| 70RDF30F DC | 285 | 684 | 408 | 676 | 4.6 | 0.6 |
| 100RDF HTC | 289 | 786 | 401 | 670 | 3.9 | 0.6 |
| 70RDF30F HTC | 235 | 678 | 275 | 589 | 5.4 | 0.8 |
| Analysis | Samples | ||||
|---|---|---|---|---|---|
| DC | HTC | ||||
| 100RDF | 70RDF30F | 100RDF | 70RDF30F | ||
| pH | 6.1 | 7.6 | 4.6 | 4.6 | |
| COD (g/L) | 4.5 | 7.0 | 47.9 | 49.6 | |
| Chlorides (g/L) | 3.3 | 2.0 | 1.7 | 1.3 | |
| Total solids (g/L) | 7.9 | 5.4 | 25.8 | 23.0 | |
| Volatile solids (g/L) | 3.7 | 3.4 | 19.5 | 19.4 | |
| Fixed solids (g/L) | 4.2 | 1.9 | 6.4 | 3.6 | |
| Ash mineral composition (wt.%, db) | Al2O3 | 0.4 | 3.4 | 0.5 | 2.5 |
| CaO | 7.9 | 27.6 | 29.4 | 58.9 | |
| Fe2O3 | 0.1 | 0.3 | 0.2 | 0.9 | |
| K2O | 1.7 | 16.4 | 6.7 | 7.2 | |
| MgO | 0.7 | 7.8 | 5.8 | 14.9 | |
| Na2O | 0.4 | 13.7 | 4.4 | 6.7 | |
| SiO2 | 1.3 | 5.0 | 0.4 | 7.6 | |
| TiO2 | 0.0 | 0.0 | 0.0 | 0.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, A.; Brito, P.; Gonçalves, M.; Nobre, C. Synergistic Valorization of Refuse-Derived Fuel and Animal Fat Waste Through Dry and Hydrothermal Co-Carbonization. Appl. Sci. 2025, 15, 9315. https://doi.org/10.3390/app15179315
Longo A, Brito P, Gonçalves M, Nobre C. Synergistic Valorization of Refuse-Derived Fuel and Animal Fat Waste Through Dry and Hydrothermal Co-Carbonization. Applied Sciences. 2025; 15(17):9315. https://doi.org/10.3390/app15179315
Chicago/Turabian StyleLongo, Andrei, Paulo Brito, Margarida Gonçalves, and Catarina Nobre. 2025. "Synergistic Valorization of Refuse-Derived Fuel and Animal Fat Waste Through Dry and Hydrothermal Co-Carbonization" Applied Sciences 15, no. 17: 9315. https://doi.org/10.3390/app15179315
APA StyleLongo, A., Brito, P., Gonçalves, M., & Nobre, C. (2025). Synergistic Valorization of Refuse-Derived Fuel and Animal Fat Waste Through Dry and Hydrothermal Co-Carbonization. Applied Sciences, 15(17), 9315. https://doi.org/10.3390/app15179315










