1. Introduction
The advancement of the economy and individuals’ quest for a pleasant lifestyle have facilitated the extensive utilization of air conditioning. The utilization of eco-friendly refrigerants, including R32, R410A, and R290, has markedly risen in air conditioning circulation systems [
1]. The novel refrigerants are set to gradually replace Freon-based refrigerants. Nonetheless, environmentally friendly refrigerants regularly demonstrate flammability. In recent years, the incidence of fires and explosions resulting from the leakage of flammable refrigerants has increased [
2]. The cracking of copper tubes may be the primary cause of refrigerant leakage, ultimately leading to air conditioning fires. Ruptures in copper tubes frequently occur in the high-pressure pipelines of air conditioning systems, particularly in air conditioning condensers.
The extensive usage of flammable refrigerants in air conditioning has received significant attention due to the fire safety concerns arising from their leakage [
3,
4,
5,
6]. He et al. discovered that the ignition probability of refrigerants correlates with the duration of leaks [
7]. Their results indicated that the ignition probability increased when flammable refrigerant gas clouds persisted for extended periods. Li et al. investigated the combustion properties and safety hazards of novel refrigerants (R1234yf, R32, and R600a) under varying ambient conditions [
8]. R600a exhibited the maximum adiabatic combustion temperature, while R1234yf demonstrated the lowest. R32 possessed the largest explosion pressure, whereas R600a showed the lowest explosion pressure.
The existing research discussed copper tube corrosion failure in finned heat exchangers [
9], apartment fire sprinkler systems [
10], air conditioning systems [
11,
12], air source heat pump water heaters [
13], and in a refrigerating plant [
14]. Faes reviewed the corrosion types and preventive measures on heat exchangers made of different materials, including copper, yellow brass, aluminum, and stainless steel [
15]. Corrosion forms including ant nest corrosion, pitting corrosion, and stress corrosion cracking are the main causes of copper tube failure [
11]. Corrosion impact factors in air conditioning include copper tube processing oils, fin stamping oils, and compressor oils circulating within the tubes, all of which can hydrolyze to generate organic acid vapor. Impact factors such as refrigerant decomposition products, impurities in aqueous media, and oxygen concentration can accelerate the corrosion process. Sulfur and chlorine constituents in air pollution can contribute to pitting corrosion of copper tubes [
16]. Nelson et al. found that the presence of copper oxide during manufacture caused the failure of copper alloy tubes [
17]. Tambang et al. explored the effects of phosphorus content inside phosphorus-deoxidized copper tubes on ant nest corrosion. It indicated that phosphorus content in copper tubes had no effect on the acceleration of ant nest corrosion [
18]. Marzena studied formicary corrosion on copper tubes. A corrosion attack can be initiated in the area of non-metallic inclusions rich in the tube material [
19].
Researchers performed experimental studies employing morphological analysis techniques, such as stereo-microscopy, metallographic microscopy, scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS) to characterize the macroscopic and microscopic morphology of the rupture sites in copper tubes, thereby deducing the cause of failure. Ou et al. examined the morphological features of the crack in the copper tube of the air conditioning condenser in a fire [
20]. Ant nest corrosion was discovered in the fire case. Li et al. investigated the corrosion characteristics of copper tubes in a formic acid environment by performing vapor corrosion experiments and SEM-EDS analysis [
21]. The findings indicated that corrosion of an ant nest initiates with the dissolving of the surface oxide layer by formic acid, leading to the exposure of the copper substrate. The copper substrate serves as the anode, and the undissolved oxide layer functions as the cathode due to the potential difference. The formation of corrosion products including Cu(COOH)
2 and Cu
2O creates a wedging action, resulting in the enlargement of microcracks and penetration of the tube wall. Nano X-ray CT was used to examine the ant nest corrosion on copper tube. The results showed that corrosion begins on the copper surface and progresses to localized branching tunnels that penetrate the wall [
22]. Elragei et al. investigated the corrosion failure of 90-10 copper–nickel alloy heat exchanger tubes in a seawater desalination facility [
23]. The results indicated that the failure was attributed to an elevated iron content (up to 6%), significantly exceeding the international standard limit of 2%. The existence of iron weakened the alloy’s corrosion resistance and ultimately caused pitting corrosion and fracture.
Current studies have contributed to understanding the fire hazards associated with air conditioning refrigerant leaks and the corrosion properties of copper tubes in air conditioning systems. In the actual manufacture process, volatile lubricating oil residuals used in the drawing of air conditioning condenser tubes can be found on copper tubes if not cleaned thoroughly. If the refrigerant filling process of an air conditioning unit is not performed properly, allowing errors such as introducing air containing water vapor, volatile lubricating oil will hydrolyze to produce carboxylic acids, leading to ant nest corrosion of copper pipes [
24]. The main components produced by the hydrolysis of lubricating oil include acetic acid [
13]. Therefore, acetic acid vapor emitted by acetic acid aqueous solution was utilized to corrode copper tubes in this study. In addition, the piston and slide of the rolling rotor compressor inside the air conditioner are in linear contact [
25]. Friction marks can be found on the outer surface of the piston. In this process, material exchange between the piston and the slide can result in scratches. The iron filings generated by friction will enter the air conditioning pipeline with the refrigerant. Research into the effects of iron fillings on the corrosion behavior of copper tubes in air conditioners has seldom been conducted. In order to simulate the environmental conditions inside an air conditioning system before the occurrence of copper tube rupture and refrigerant leakage accidents, this study utilized acetic acid vapor to corrode copper tubes and explored the effects of lubricating oil and iron powder on copper tube corrosion. The research findings can assist air conditioning failure analyzers, fire investigators, and manufactures in comprehending the corrosion and fracture characteristics of copper tubes in air conditioning systems influenced by factors involving acetic acid, lubricating oil, and iron powder.
The rest of this paper is organized as follows.
Section 2 introduces the corrosion experiment method.
Section 3 describes the experiment’s results regarding the morphology and composition of the corrosion products.
Section 4 discusses the corrosion mechanism.
Section 5 summarizes the main findings of this study.
2. Materials and Methods
The study utilized the gas atmosphere technique to replicate the corrosion of copper tubes subjected to acetic acid vapor. The corrosion features, including surface and internal surfaces, of copper tubes in corrosive environments distinguished by different concentrations of acetic acid, POE lubricating oil, and iron powder were examined. Five sets of operational conditions were established. The stereo microscope and metallographic microscope were employed to examine the macroscopic morphology of the corroded surface and cross-section, respectively. Scanning electron microscopy, energy dispersive spectroscopy, and X-ray diffraction were used to examine the microstructure and composition of corrosion products. This work presents a comprehensive introduction to the corrosion environment simulation method and the characterization approach for corrosion products.
2.1. Corrosion Method
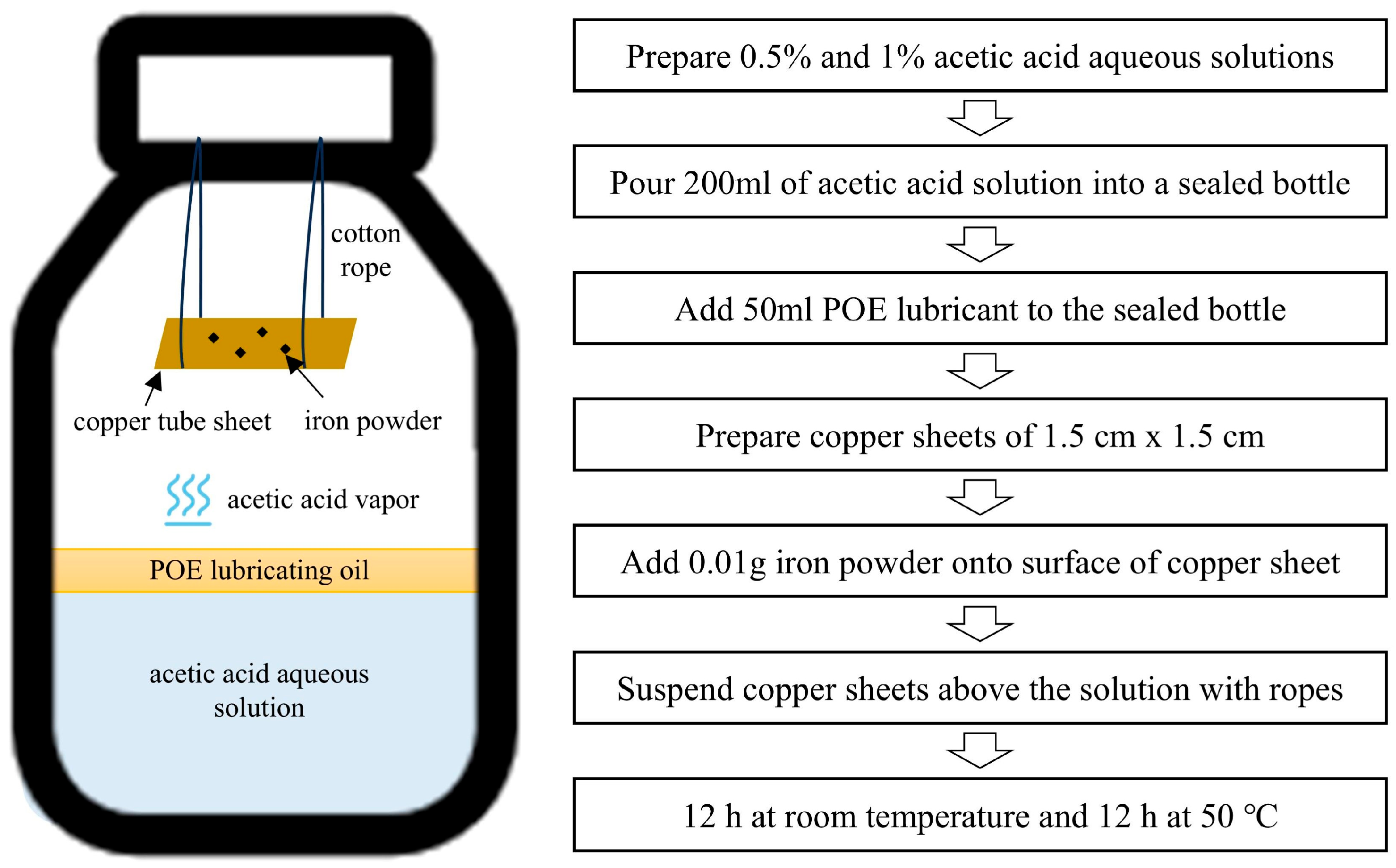
In current research, the experimental methods for studying copper tube corrosion mainly include the immersion method and atmosphere method. The immersion method accelerates the corrosion process by completely immersing the sample in an organic acid solution. Directly immersing in an organic acid solution environment does not conform to the actual situation in which air conditioning condenser copper pipes are corroded by acetic acid. The operating principle of air conditioning indicates that the superheated steam compressed by the compressor to high pressure and high temperature will enter the condenser, and the heat released by the refrigerant will be carried away by the outdoor unit condensing fan, causing the high pressure and high temperature refrigerant to condense from gas to liquid. During this process, acetic acid will exist in the form of steam in the condenser copper tube. Therefore, in this experiment, we used the atmosphere method, which means that the acetic acid vapor emitted from the acetic acid aqueous solution corrodes the copper tube. The atmosphere method is more in line with the corrosive environment in which the air conditioning condenser operates.
Figure 1 shows that the organic acid solution is contained in a sealed bottle, with the test sample situated away from the liquid surface, enabling the vapor released by the organic acid to corrode the sample.
POE lubricating oil experiences hydrolysis, resulting in the formation of minor carboxylic acids, including acetic acid and propionic acid, with an increased concentration of acetic acid under elevated temperature and humidity conditions. The content of the acids varies from 0.1% to 1% [
26]. Consequently, a 36% acetic acid standard solution was dissolved into purified water to formulate acetic acid aqueous solutions with concentrations of 0.5% and 1% in the experiments. 200 mL of the acetic acid solution was poured into a 500 mL bottle.
The predominant refrigerants utilized in air conditioning systems are R32 and R410A. POE lubricant is the predominant compatible lubricant. Consequently, 50 mL of POE lubricant was added into the sealed container. A TP2 internally threaded copper tube, typically utilized in air conditioning condenser tubes, is cut into copper sheets of 1.5 cm × 1.5 cm. The TP2 internally threaded copper tube (ZHUHAI GANGLONG METAL Co., Ltd., Zhuhai, China) used in the experiment was a phosphorus de-oxidized copper with a copper content of 99.5%. Copper sheets were suspended above the solution using a cotton rope. The container was tightly closed to keep out air. Moreover, the operation of the air conditioner will result in compressor wear, producing iron particles that will travel to the copper tube with the refrigerant. 0.01 g of iron powder was applied to the surface of a copper sheet to examine the influence of iron powder on the corrosion of copper tubes. The composition of the iron powder (Nangong Yingtai Metal Materials Co., Ltd., Xingtai, China) had an iron content of 99%. Iron powder can pass through a sieve with a pore size of 0.25 mm.
The condensation temperature of air conditioning frequently exceeds 10 °C above the ambient temperature. Summer ambient temperatures can reach 40 °C. Consequently, the container was positioned in a drying oven to sustain a temperature of 50 °C during the experiment. The experiment was performed in a diurnal alternating mode, indicating that after 12 h, the samples were removed from the oven and placed at ambient temperature for an additional 12 h.
The corrosive factors and temperature parameters were considered when creating the corrosive environment close to the actual working conditions of air conditioning. Copper sheets were extracted on days 7, 14, 21, and 28 for observation and deep analysis.
2.2. Material Characterization Method
After the corrosion test, the samples were cleaned with ultrasonic waves, soaked in anhydrous ethanol, and dried with a hair dryer to obtain the dried samples. The samples were cut into small pieces for the convenience of observation. Samples were placed under a stereo microscope and a scanning electron microscope for observation. The macroscopic morphological features of the copper tube’s surface were examined using a stereo microscope (DSX110, OLYMPUS, Tokyo, Japan). The corrosion morphology of the copper tube surface was examined using an SEM-EDS (SU3800-ELEMENT, Hitachi, Hitachi City, Japan) and the elemental content of the corrosion products was evaluated qualitatively.
The copper specimen was put into an embedding machine. Epoxy resin embedding powder was added into the machine. The heating temperature was set to 145 °C, the cooling temperature was set to 70 °C, and the holding time was set to 100 s. An automatic grinding and polishing machine was used to polish the embedded samples with 400 #, 800 #, 1200 #, and 2000 # abrasive paper. A diamond spray polishing agent was used to obtain smooth and flat sample surfaces. The surface of the sample attached with polishing agent was then rinsed with clean water. The sample was soaked in anhydrous ethanol for 2 min, then dried with a hair dryer. Finally, the corrosion path and depth of the copper tube cross-section were examined using a metallurgical microscope (DSX510i, OLYMPUS).
To analyze the phase of copper corrosion products, we gently scraped off the surface products. We then ground the surface corrosion products of the copper tube into powder. The products were examined by XRD apparatus. XRD (Rigaku SmartLab SE, Rigaku, Tokyo, Japan) elucidates crystal structures by producing distinct diffraction patterns. The Cu target was chosen for the analysis of copper corrosion product phases, utilizing a diffraction angle between 10° and 80° and a scanning rate of 10°/min. XRD (Aeris, Malvern Panalytical, Malvern, UK), with a cobalt target, operates at a diffraction angle of 10° to 80° and a scanning speed of 5°/min to examine the phase of corrosion products associated with iron powder.
2.3. Corrosion Test Conditions
Table 1 enumerates five distinct sets of corrosion situations. Groups 1 and 2 each add 200 mL of acetic acid at concentrations of 0.5% and 1% without POE lubricants and iron powder, respectively. Group 3 adds 50 mL of POE lubricating oil on the basis of Group 2. Group 4 incorporates 0.01 g of iron powder on the basis of Group 2. Group 5 incorporates both iron powder and POE lubricating oil on the basis of Group 2.
3. Results
3.1. The Morphology Characteristics of the Corrosion Products
(1) Surface morphology
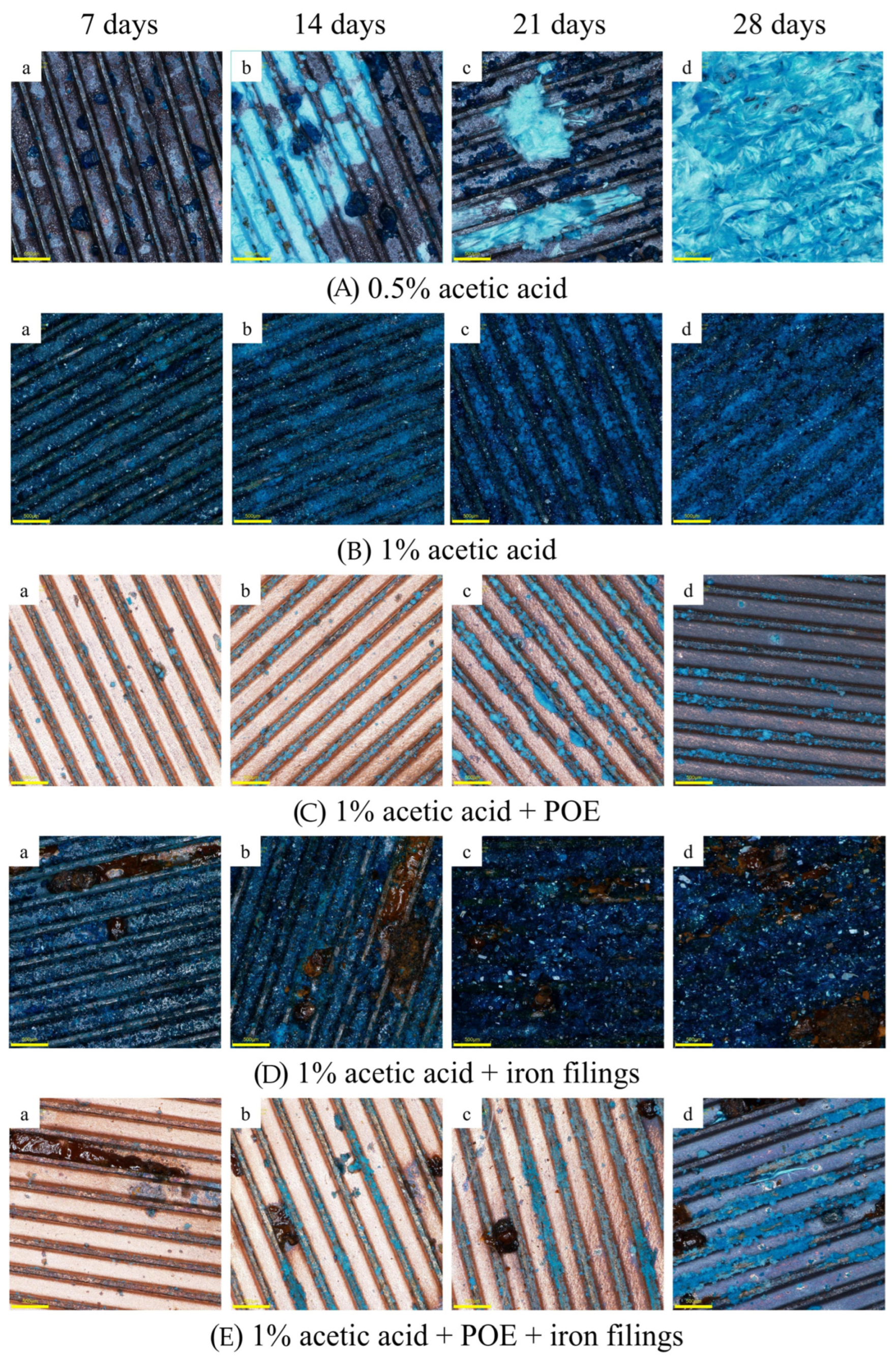
Figure 2 illustrates the macroscopic morphology of copper tubes subjected to corrosion for 7, 14, 21, and 28 days across five distinct corrosion regimes, magnified 96 times. The color and morphology of the corrosion products on the copper sheet evolved over time.
Following seven days of corrosion in 0.5% acetic acid vapor, dark blue crystalline deposits are dispersed at the base of the copper tube groove, surrounded by brick-red substances. Cuprous oxides Cu2O have been suggested to have formed. On 14th day, a layer of turquoise-blue corrosion products developed at the base of the groove. At 21 days, the yield of turquoise-blue crystalline materials markedly escalated, evolving into a loose flocculent structure.
The products are susceptible to separation from the copper substrate owing to insufficient adhesive strength. By day 28, a substantial accumulation of flocculent corrosion products had developed, entirely covering the inner surface of the copper tube. Consequently, when the duration of corrosion exposure increases, the deterioration of copper in a 0.5% acetic acid vapor environment intensifies, and the shape of the corrosion products experiences substantial alterations. The corrosion products evolve from the initial dark blue and brick-red substances to turquoise-blue flocculent materials. As the concentration of acetic acid rises to 1%, the quantity of corrosion products increases with the same period of corrosion. After 7 days, the thread grooves on the inner surface of the copper tube are saturated with a substantial accumulation of agglomerated blue corrosion products. The products entirely envelop the groove bottom by day 21. At 28 days, the accumulated height of corrosion products surpasses the height of the internal thread teeth. No flocculent corrosion products were detected as generated in 0.5% acetic acid vapor.
In the presence of POE lubricating oil, copper tubes demonstrate a reduction in corrosion products, which appear as a turquoise-blue color. After seven days, the surface of the copper tube maintains a metallic sheen, with blue corrosion products dispersed on the internal thread teeth. The turquoise-blue products had markedly increased, gathering near the base of the grooves on day 21. By day 28, the surface of the copper tube was entirely oxidized and corroded. The aforementioned facts demonstrate that POE lubricant mitigates the corrosion rate of copper tubes caused by acetic acid. Upon the addition of iron powder, it undergoes corrosion by acetic acid, resulting in a brownish-yellow colloidal deposit. In contrast to
Figure 2b, the copper corrosion products in
Figure 2d, with the addition of iron powder, exhibit increased brightness and enhanced light reflectivity, signifying a greater degree of crystallization of the copper corrosion products. It suggests that iron powder facilitates the crystallization of copper corrosion products throughout the corrosion process. In the presence of iron powder, POE lubricating oil, and acetic acid vapor, the iron powder undergoes corrosion first, whereas no significant copper corrosion products are observed by day 7. Distinct blue copper corrosion products emerge on the surface of the copper tube on day 14. By day 21, the blue copper corrosion products have increased, predominantly accumulating on the upper surfaces of the internal thread teeth of the copper tube. By day 28, flocculent blue corrosion products are being locally produced, indicating a greater extent of corrosion relative to
Figure 2c.
(2) Corrosion in cross-section
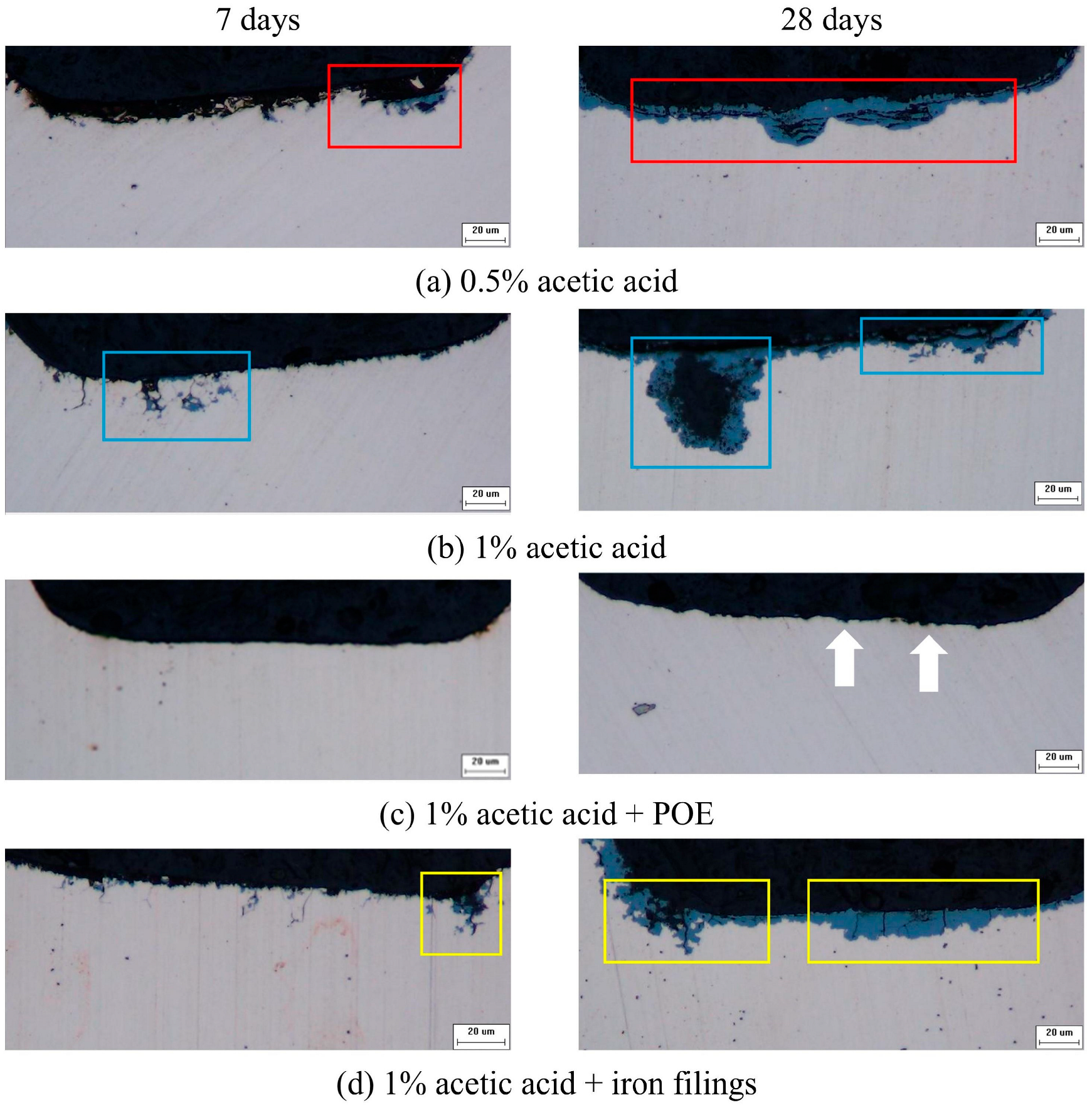
Figure 3 illustrates the cross-section of copper tubes subjected to corrosion for 7 and 28 days across four distinct environments. Corrosion markings manifested on the cross-sections of the copper tube after 7 days in 0.5% and 1% acetic acid vapor. The cross-section displayed a corrosion pattern like an ant nest, defined by a network of interconnected channels filled with blue corrosion products in 1% acetic acid vapor. On the 28th day, the copper tube exposed to 0.5% acetic acid exhibited localized pitting and consistent surface corrosion, with a maximum corrosion depth of 21 μm. The copper tube associated with 1% acetic acid displayed greater degrees of pitting, with the pitting edges filled with corrosion products. Regions free of pitting exhibit signs of ant nest corrosion, with a maximum corrosion depth of 51 μm. In a 1% acetic acid vapor corrosion environment with the addition of POE oil, little pitting was seen on the surface of the copper tube after 28 days. The corrosion features of ant nests were investigated in a 1% acetic acid vapor environment supplemented with iron powder. The cross-sectional image on the 28th day exhibited pronounced tree branch-like corrosion pits on the left side and extensive pits resulting from a merger of corrosion pits on the right side, with a maximum corrosion depth of 13 μm.
During the early stage of acetic acid corrosion, localized ant nest corrosion manifests on the copper tube, displaying a dendritic or reticulate corrosion pattern. In the later stages, uniform corrosion dominates, as little corrosion pits merge into larger, deeper pits, yet tree-branch-like corrosion pathways continue to exist in localized areas. The corrosion products of copper progressively infiltrate the interior of the copper tube, creating dendritic corrosion pathways that may lead to the fracturing of the tube.
(3) Surface microtopography
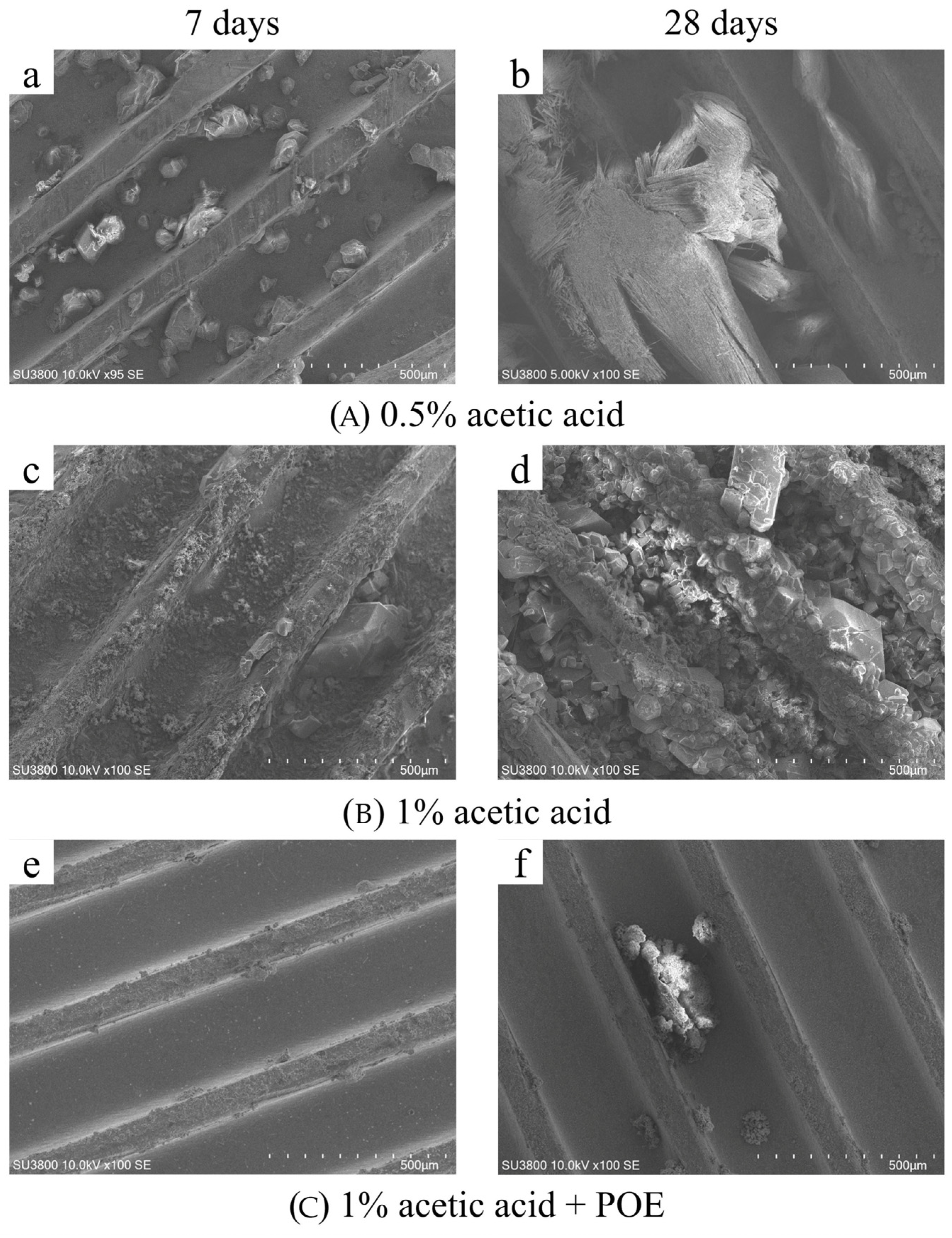
Figure 4 displays scanning electron microscopy pictures of the surface morphology of copper tubes subjected to the initial three corrosion conditions on the 7th and 28th days. In 0.5% acetic acid, the copper corrosion products after seven days were predominantly crystalline and deposited at the base of the copper tube grooves. On the 28th day, the surface of the copper tube displayed flocculent, needle-shaped crystals with enlarged grain size. On the seventh day in 1% acetic acid, the surface of the copper tube was predominantly characterized by small granular crystals, with corrosion products uniformly coating the interior surface of the tube. On the 28th day, the corrosion products intensified, the crystallization degree elevated, and the grains expanded. Following the application of POE lubricating oil, the corrosion byproducts on the copper tube surface on the seventh day were predominantly evenly distributed at the top of the thread teeth. On the 28th day, crystals similar to those first formed in 0.5% acetic acid were deposited near the base of the grooves. The residual components predominantly displayed surface corrosion.
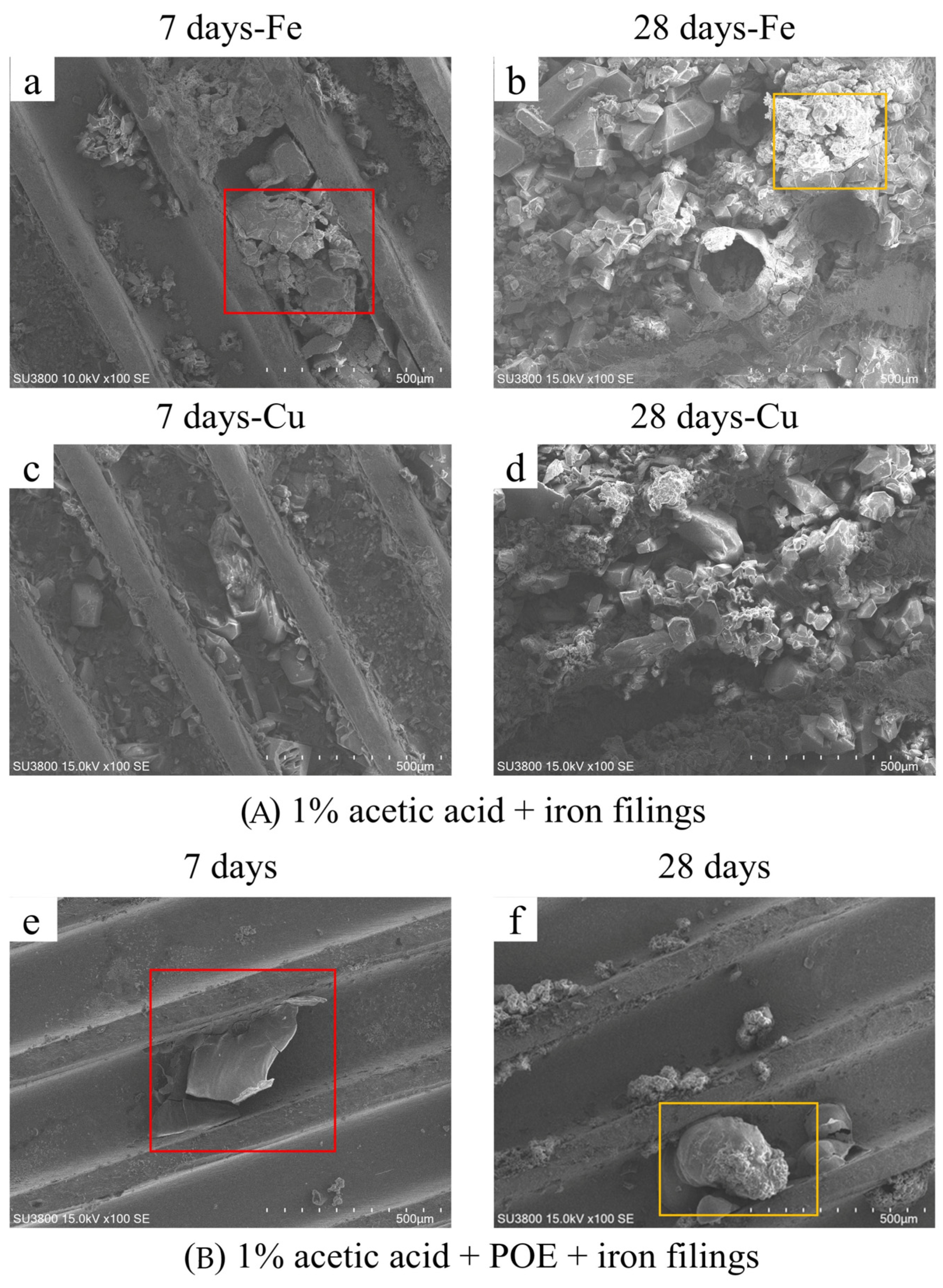
Figure 5 illustrates the corrosion morphology on the surfaces of copper tubes subsequent to the incorporation of iron powder. After seven days, the red-framed region in
Figure 5a exhibits lamellar or blocky iron corrosion products characterized by a rough surface. After 28 days, the iron corrosion products evolve into a layered cluster structure, exhibiting considerable crystal growth.
Figure 5c,d illustrates the corrosion products of copper. The copper corrosion products progressively evolve from a dispersed form to a continuous layer over bigger grains, aligning with prior macroscopic morphological data. The influence of acetic acid vapor corrosion on copper tubes exacerbates over time, leading to increased crystallization of the resultant corrosion products.
Figure 5e,f illustrates a condition in which POE lubricant and iron particles are present simultaneously. After seven days, the iron corrosion products within the red frame exhibit a lamellar structure and stick to the groove’s bottom, while copper shows minimal corrosion. After 28 days, the morphology of the iron-containing corrosion products exhibits substantial alterations, with the corrosion products at the base of the framed area appearing granular. A substantial aggregation of deposits develops on the surface of the corrosion products. Subsequently, EDS and XRD were employed to examine the elemental composition and phase of the products.
3.2. Composition of Corrosion Products
(1) Element composition based on EDS analysis
Table 2 presents the findings on the surface EDS composition study conducted after 7 and 28 days in five distinct corrosive conditions. Analysis of the corrosion data reveals that the copper content in the 1% acetic acid vapor concentration was lower than that in the 0.5% acetic acid vapor concentration on the seventh day. The ratio of carbon to oxygen components rose, signifying that the rise in acetic acid concentration during the initial phase of corrosion accelerated the corrosion process. In the presence of POE lubricating oil, the copper concentration exceeds that observed in 0.5% acetic acid but is lower than that in 1% acetic acid. It means that the corrosion rate of copper in a POE lubricating oil environment is lower than in 1% acetic acid but higher than in 0.5% acetic acid.
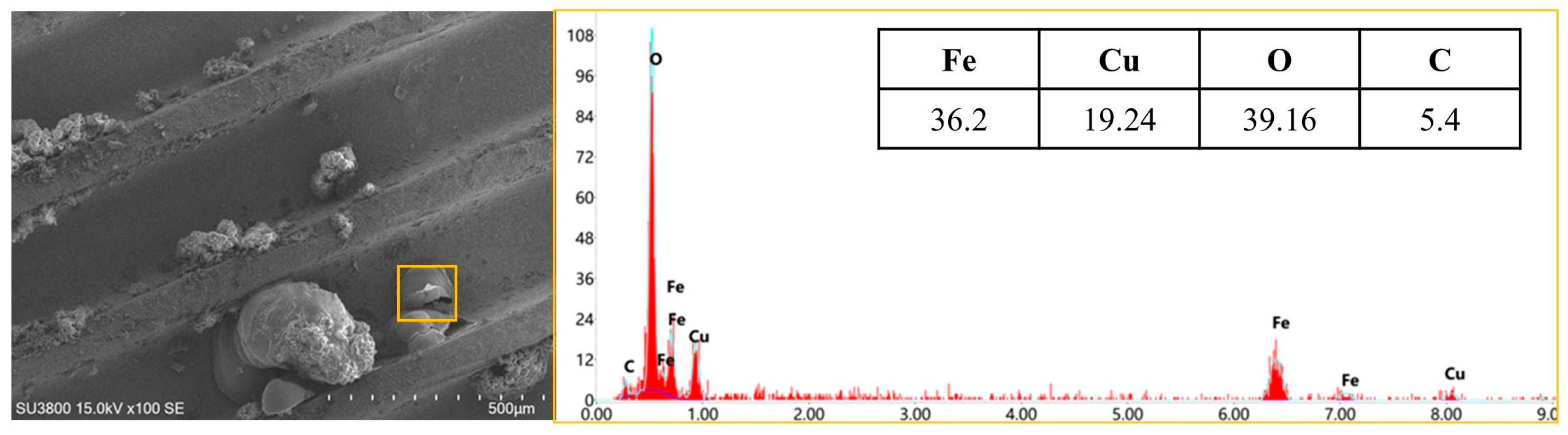
Following the addition of iron powder, the mass percentage of the Fe element in the initial corrosion product of lamellar iron was 74.86%. On the 28th day, the mass percentage of Fe in the collected iron corrosion products diminished to 61.15%. The ratio of oxygen and carbon components has risen, suggesting that the initially formed iron corrosion products will persist in reacting with acetic acid, yielding corrosion products. The copper concentration in copper corrosion products diminishes to below 40%. On the seventh day, the primary constituents of corrosion products, in the presence of both iron powder and POE, are Fe, O, and C. A composite material comprising both Cu and Fe elements was produced on the 28th day, as illustrated in
Figure 6.
(2) Phase analysis by XRD
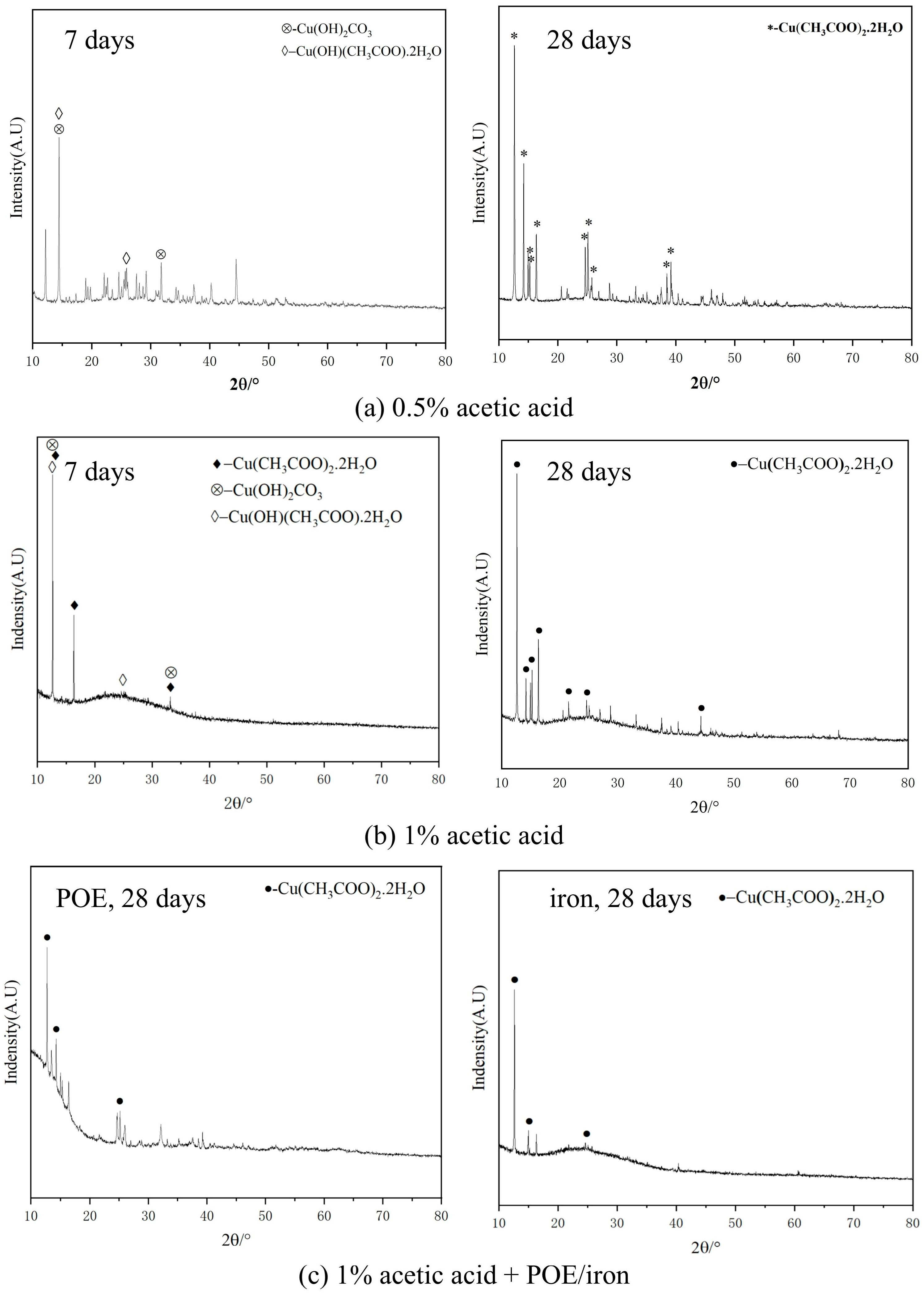
Figure 7 shows the XRD patterns of corrosion products on copper pipes under different corrosion conditions. Based on the position and intensity of its diffraction peaks, we compared the PDF cards of different substances to determine the type of product. In addition, the substance was marked by comparing the XRD spectrum with the experimental results in [
27]. The corrosion products corresponding to 0.5% acetic acid were mainly cupric carbonate basic, Cu
2(OH)
2CO
3, and basic acetic acid copper hydrate Cu(OH)(CH
3COO)·2H
2O on the 7th day. It is difficult to identify some crystal phases among them. The unidentified crystal phase is Cu
4(OH)(CH
3COO)7·2H
2O owing to the isomorphism with cobalt hydroxide acetate Co
4(OH)(CH
3COO)
7·2H
2O [
27]. On the 28th day, no crystalline phase other than copper acetate hydrate was observed, signifying that all corrosion products had been transformed into Cu(CH
3COO)
2·2H
2O. On the 7th day, the corrosion products of copper pipes in 1% acetic acid vapor concentration were Cu
2(OH)
2CO
3, Cu(OH)(CH
3COO)·2H
2O, and Cu(CH
3COO)
2·2H
2O. By the 28th day, all the corrosion products had converted into Cu(CH
3COO)
2·2H
2O.
In the experiment with additional POE, there were fewer initial corrosion products; therefore, XRD analysis was conducted on the corrosion products from day 28. The results indicated that the primary product was Cu(CH3COO)2·2H2O, aligning with earlier macroscopic and microscopic findings. The inclusion of POE lubricating oil or iron powder did not change the final copper corrosion products.
Figure 8 displays the spectrum of corrosion products from iron powder, as determined by XRD using a cobalt target. It was found that the corrosion products of iron were Fe
2O
3 and FeOOH. Fe
2O
3 is the ultimate product of iron powder oxidation, remaining stable in acidic environments and serving as a protective barrier. Consequently, the incorporation of iron powder retards the corrosion of copper. The synthesis of FeOOH necessitates the involvement of O
2 and H
2O. Dissolved oxygen in acetic acid vapor facilitates the oxidation of iron, resulting in the formation of Fe
3+. Fe
3+ further oxidizes copper, elevating the concentration of local copper ions and facilitating the crystallization of copper corrosion products.
4. Discussions
The following discusses the corrosion mechanism of copper pipes in acetic acid vapor atmosphere, as well as the influence of POE lubricating oil and iron powder in the corrosion process.
(1) Corrosion mechanism of copper pipes in acetic acid vapor environment
Figure 9 illustrates the schematic diagram of the copper tube corrosion mechanism. Copper reacts with oxygen under acidic circumstances as follows. The surface observation results in
Figure 2 show that a thin layer of brick-red substances has formed on the surface of the copper tube. The cross-section observations in
Figure 3 shows that the corrosion products show gray with a blue color. It is consistent with the color of cuprous oxide, as seen in [
28]. The above findings indicate that cuprous oxide is one of the main corrosion products [
10]. There are two paths to form cuprous oxide. The first path is that copper reacts with water and oxygen on the surface.
Copper undergoes oxidation at the anode:
The cathodic reaction is:
In weakly alkaline environments, copper ions combine with hydroxide ions to form copper hydroxide, Cu(OH)2.
In atmospheres with a low vapor content of the acid, a basic copper acetate forms preferentially, according to the reaction [
29,
30]:
The formula of the intermediate products is Cu4(OH)(CH3COO)7·2H2O or Cu(OH)(CH3COO)·2H2O, which is determined by the relative humidity and potential of hydrogen.
As the concentration of acetic acid increases, the copper hydroxide acetate evolves to form copper acetate via the reaction:
In addition, carbon dioxide within the container will interact with copper hydroxide to produce basic copper carbonate:
Similarly, acetic acid continues to react with 2Cu
2(OH)
2CO
3 to produce copper acetate, with the content of acetic acid content increasing.
The second path to produce cuprous oxide is that cuprous acetate can be oxidized by oxygen and transform into cupric carboxylate and cuprous oxide. Cupric carboxylate will continue to react with copper to produce cuprous acetate.
Reactions (9) and (10) are repeated over and over again to promote corrosion [
29]. Cuprous oxide is fragile and prone to internal cracks, allowing substances such as acetic acid, acetate salts, and oxygen to invade and continuously corrode the copper inside. Consequently, basic copper acetate will be produced during the corrosion process in environments with varying amounts of acetic acid, while the ultimate corrosion product will manifest as copper acetate hydrate. The produced corrosion products will aggregate on the surface of the copper tube and progressively infiltrate its interior, establishing a branched corrosion pathway and precipitating the rupture of the copper tube.
(2) Shielding effect of POE lubricating oil
The primary mechanism of POE oil in the corrosion process is exhibited as a shielding effect. The phase analysis results demonstrate that the ultimate corrosion product of copper pipes is Cu(CH3COO)2·2H2O, irrespective of the presence of POE lubricating oil in the environment. The presence of POE oil substantially influences the corrosion rate. POE oil creates a thick oil layer on the surface of an acetic acid aqueous solution, impeding the volatilization of acetic acid and water, minimizing direct contact between organic acids and copper tubes and thus decelerating the corrosion reaction. The shielding effect can significantly impede the oxidation and corrosion of copper, demonstrating the protective role of POE lubricating oil in corrosive situations.
POE lubricating oil can hydrolyze to generate carboxylic acids, hence elevating the concentration of these acids in the solution and facilitating corrosion. Nevertheless, the ambient temperature of 50 °C during trials is detrimental to the hydrolysis of POE lubricating oil. The quantity of carboxylic acid produced by hydrolysis is minimal, exerting negligible influence on the enhancement of the corrosion rate. Chandra et al. also found that the measured acidity numbers of the POE oils were negligible and showed no significant change in the acidity [
14]. Furthermore, POE oil is immiscible with acetic acid aqueous solution, instead creating an oil film on the solution’s surface, which obstructs the evaporation of acetic acid and water vapor, leading to a reduction in copper corrosion products. In the air conditioning refrigeration cycle system, POE oil and refrigerant are compatible and engage in the full refrigeration cycle. The entirely immiscible lubricating oil will create a dense lubricating layer on the inner wall surface of the copper tube, thereby safeguarding the copper tube.
(3) Mechanism of Iron Powder on Corrosion Process
The effect of iron powder on copper tube corrosion is mainly manifested in two aspects. Firstly, iron powder reacts with oxygen and water, with Fe functioning as an anode, oxidizing into Fe
2+.
The cathodic reaction is the same as reaction (2). Iron can precipitate out of solution to form hydrated ferric oxide or rust by the series of reactions presented below [
31].
Therefore, the corrosion of iron occurs first, which provides protection to the copper tube. As the concentration of acetic acid increases with time, FeOOH will react with acetic acid so that Fe
3+ are present on the surface of the copper.
Fe3+ functions as a potent oxidizing agent, converting Cu to Cu2+. This reaction indirectly facilitates the dissolution of copper in acetic acid vapor, leading to a substantial elevation in the concentration of Cu2+ in the aqueous environment around the copper tube’s surface. When the concentration surpasses the solubility of copper acetate and the solution attains supersaturation, the crystallization process can be markedly enhanced, expediting the deposition of copper acetate. Therefore, it is necessary to clean the iron filings formed in the manufacturing of air conditioning compressors. In addition, adding appropriate lubricating oil can reduce the iron particles generated during operation of the compressor.
5. Conclusions
This article simulates the effects of acetic acid vapor corrosion on air conditioner’s condenser copper pipes. Due to the mixture of refrigerant and lubricating oil circulating in the air conditioning system, the lubricating oil will adhere to the inner surface of the condenser copper tube. In addition, the iron filings generated by the wear of the air conditioning compressor will flow to the condenser pipe with the refrigerant. Therefore, the influences of acetic acid vapor concentration, POE oil, and iron powder were taken into account in this study. The macroscopic and microscopic morphological properties and phase transformations of copper pipes caused by carboxylic acid corrosion have been elucidated. The subsequent conclusions can be stated as follows.
(1) In the initial stage, copper hydroxy acetate can be produced at low concentrations. As the acetic acid concentration increases with time, copper hydroxy acetate turns into copper acetate hydrate. The corrosion products predominantly exhibit as blue-turquoise crystals. The ultimate products are flocculent substances in 0.5% acetic acid and dark blue crystals in 1% acetic acid. Ant nest corrosion pattern is observed. Little corrosion pits merge into larger, deeper pits, exhibiting tree-branch-like corrosion pathways as corrosion proceeds.
(2) POE lubricating oil has a shielding effect on copper pipes corroded by acetic acid. POE lubricating oil hinders the evaporation of acetic acid from contact with copper pipes, resulting in only trace amounts of blue corrosion products. The final corrosion product is mainly copper acetate hydrate.
(3) When iron powder is present in the system, the iron powder is initially oxidized into FeOOH and Fe2O3. Thus, iron powders can protect copper from corrosion in the early stage. As the content of acetic acid increases, iron oxide can react with acetic acid and produce Fe3+. Fe3+ will oxidize copper into Cu2+, facilitating its reaction with acetic acid and exacerbating copper corrosion. The crystallinity of copper corrosion products is enhanced when iron powder is added. The inclusion of iron powder in 1% acetic acid does not alter the final corrosion products of copper inside the acetic acid environment.
Further research can be conducted on the coupling influence of factors such as acetic acid concentration, ambient temperature, iron powder content, and POE lubricant content on the corrosion characteristics of copper tubes.