Evaluation of the Microstructure and Corrosion Resistance of the 800HT Alloy After Long-Term Operation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- -
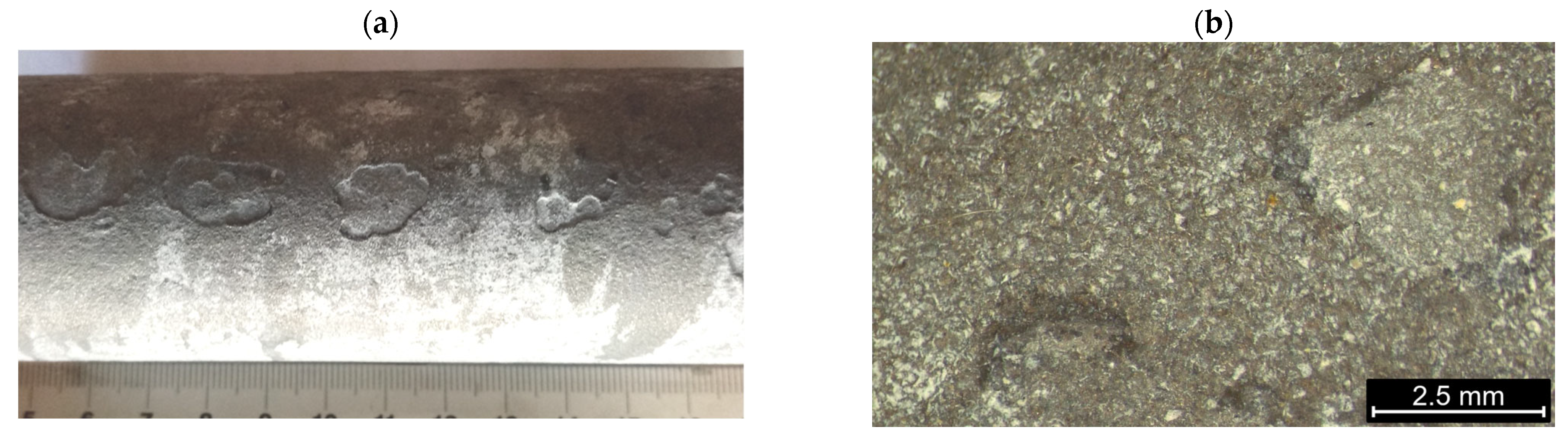
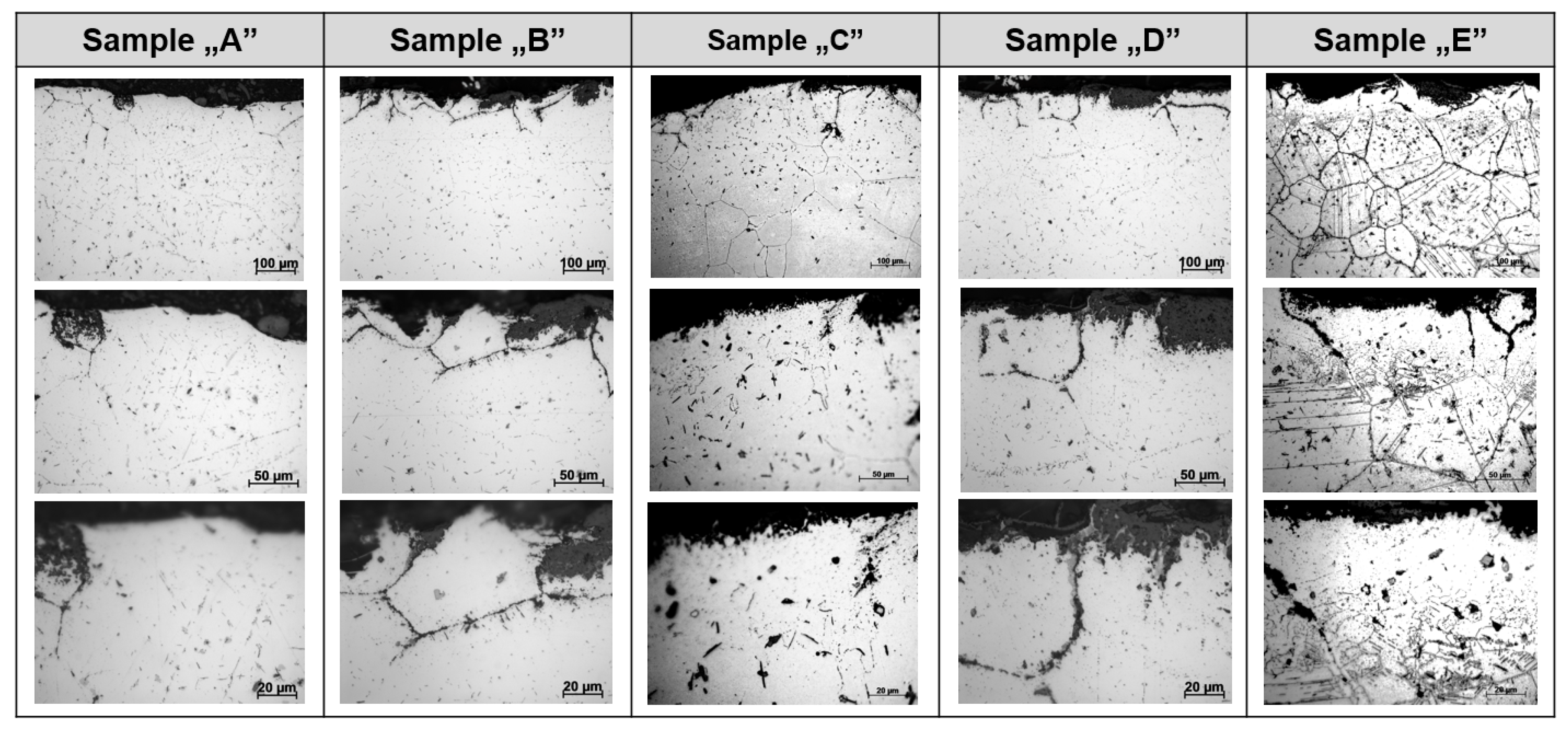
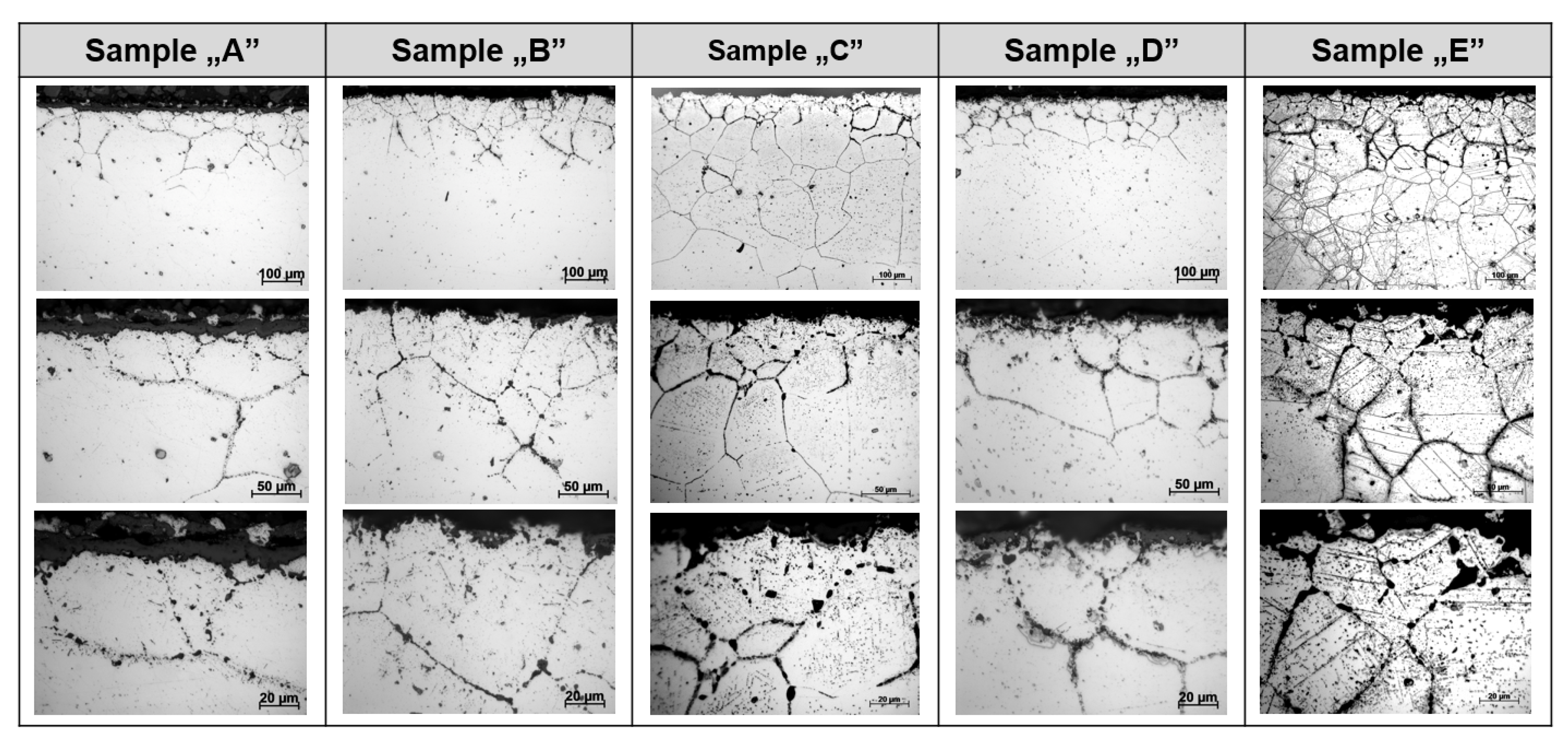
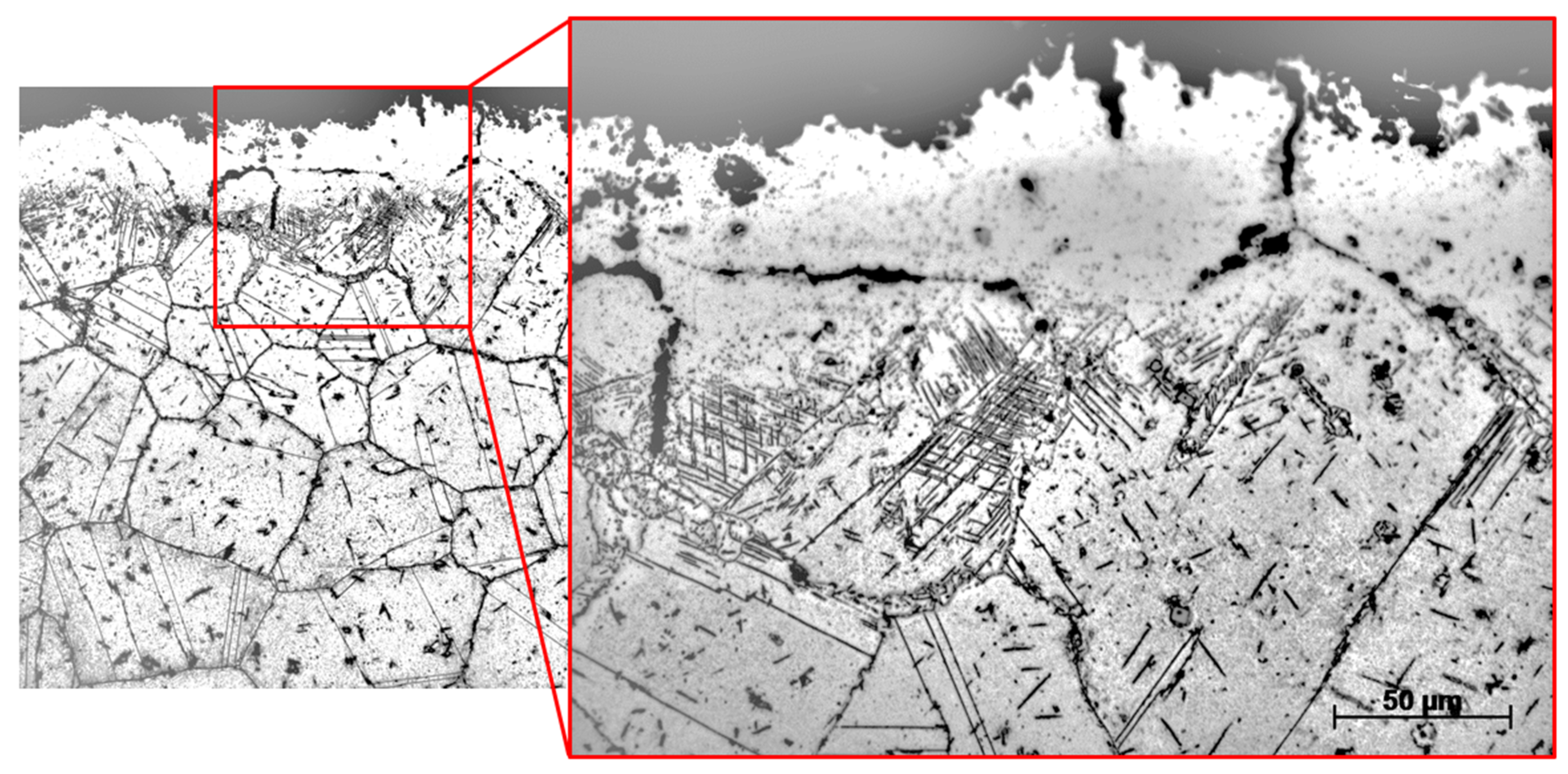
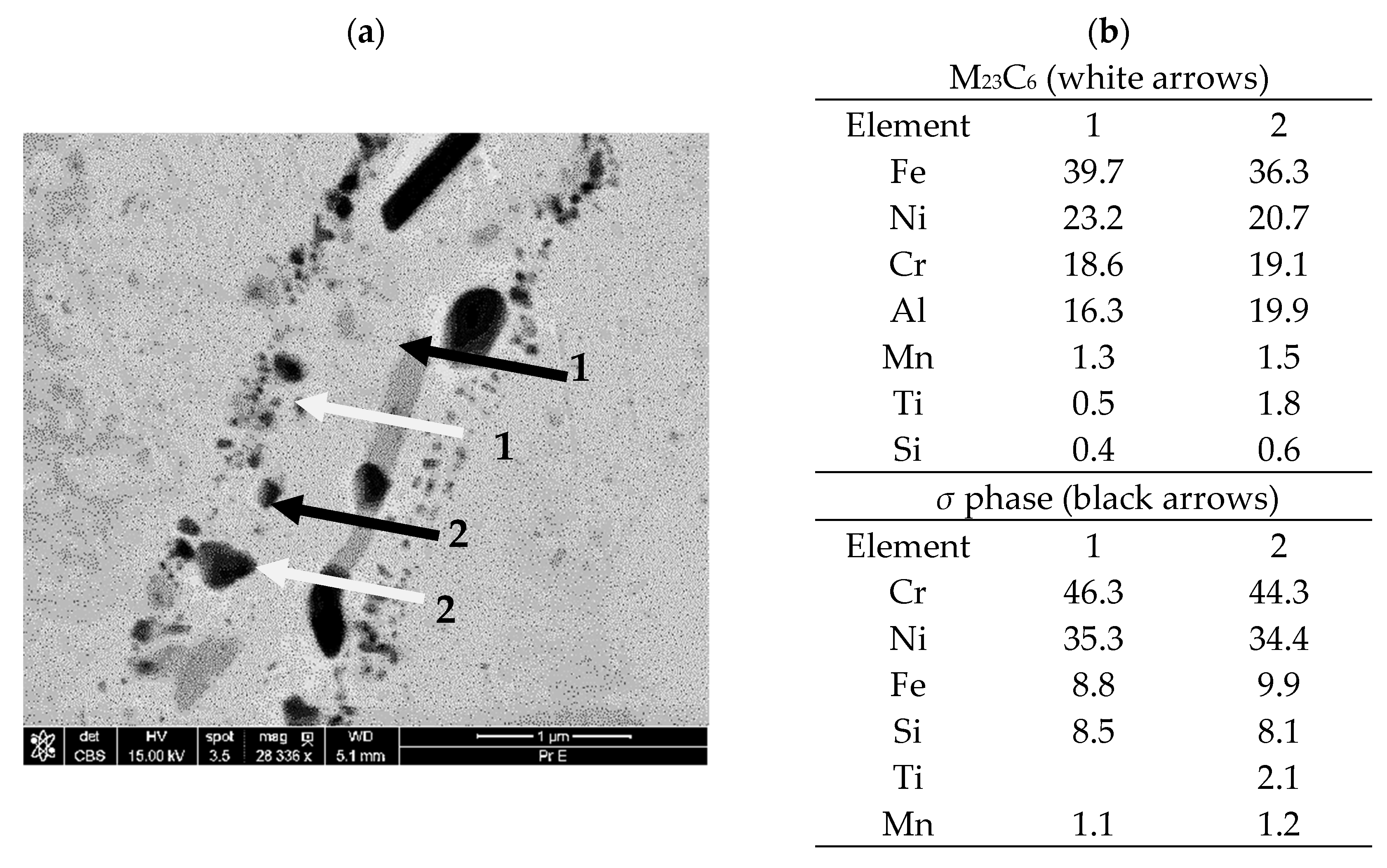
- Observations of the microstructure of the tested post-operational samples showed that, as a result of degradation in high temperature conditions, intensive precipitation of σ and γ’ phases and M23C6-type carbides and dispersion phases is observed.
- -
- The favorable areas for the formation of precipitates are grain boundaries, but their precipitation also occurs inside the grains. The precipitates are initially lamellar in nature, and then, as a result of long-term operation, they grow and take on a spherical shape.
- -
- Long-term operation did not cause significant changes in hardness that could indicate a decrease in the plasticity of the tested sample.
- -
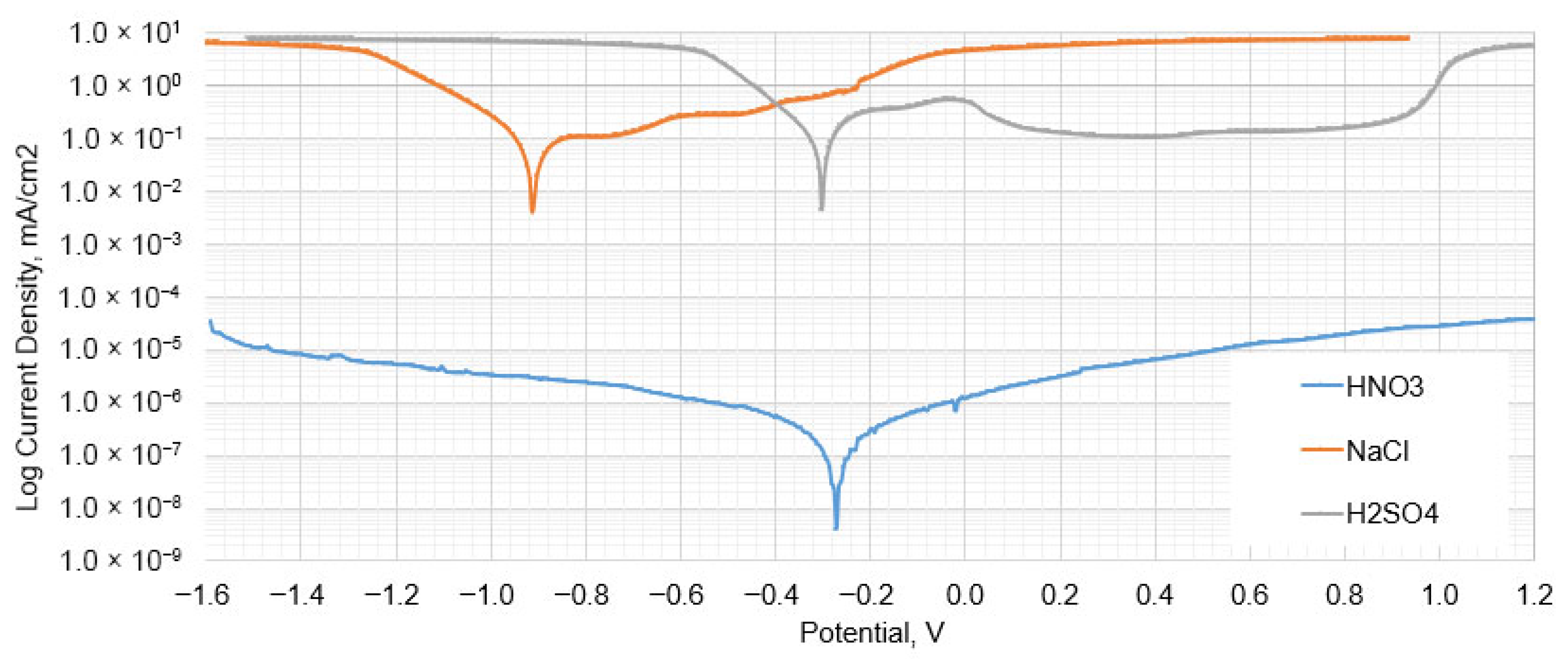
- Operating conditions in the high temperature range inhibit electrochemical corrosion processes, but also allow condensates to form on the surface and promote pitting corrosion. The most unfavorable environment is chloride, where the corrosion rate can reach over 9 mm/year at a potential of −0.9 V, and the most favorable is nitric acid, where the loss of thickness is the smallest. The corrosion potential of sulfuric acid and nitric acid is similar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tapping, R.L.; Lu, Y.C.; Pandey, M.D. Alloy 800 SG tubing: Current Status and future challenges. In Proceedings of the 13th International Conference on Environmental Degradation of Materials in Nuclear Power Systems–Water Reactors, Canadian Nuclear Society (CNS), Whistler, BC, Canada; 2007; pp. 2033–2051. [Google Scholar]
- Page, R.A. Small-Angle Neutron Scattering Investigation of Creep Damage in Type 304 Stainless Steel and Alloy 800. Met. Trans. A 1985, 16, 2283–2289. [Google Scholar] [CrossRef]
- Tabasov, A.; Krop, J.; Kermes, V.; Nemet, A.; Stehlík, P. Waste-to-energy technologies: Impact on environment. Energy 2012, 44, 146–155. [Google Scholar] [CrossRef]
- Mahajan, S.; Chibber, R. Hot corrosion studies of boiler steels exposed to different molten salt mixtures at 950 °C. Eng. Fail. Anal. 2019, 99, 210–224. [Google Scholar] [CrossRef]
- Li, Y.S.; Niu, Y.; Wu, W.T. Accelerated corrosion of pure Fe, Ni, Cr and several Fe-based alloys induced by ZnCl2–KCl at 450 °C in oxidizing environment. Mater. Sci. Eng. A 2003, 345, 64–71. [Google Scholar] [CrossRef]
- Zhang, K.; Niu, Y.; Zeng, C.; Wu, W. Corrosion of iron and four commercial steels in a Cl-containing oxidizing atmosphere at 500–600 °C. J. Mater. Sci. Technol. 2004, 20, 213–216. [Google Scholar]
- Anand, K.; Kumar, S.A.; Tamilmannan, K.; Sathiya, P.; Arivazhagan, B. Metallurgical characterizations and mechanical properties on friction welding of Incoloy 800H joints. J. Mater. Res. 2016, 31, 2173–2185. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Liu, C.; Lian, X. Damage analysis of 800 series materials from reformer tube outlet components. Eng. Fail. Anal. 2023, 146, 107134. [Google Scholar] [CrossRef]
- Tan, L.; Rakotojaona, L.; Allen, T.R.; Nanstad, R.K.; Busby, J.T. Microstructure optimization of austenitic Alloy 800H (Fe–21Cr–32Ni). Mater. Sci. Eng. A 2011, 528, 2755–2761. [Google Scholar] [CrossRef]
- Mirzadeh, H. Constitutive modeling and prediction of hot deformation flow stress under dynamic recrystallization conditions. Mech. Mater. 2015, 85, 66–79. [Google Scholar] [CrossRef]
- Xiao, L.; Li, W.; Cota-Sanchez, G. Mechanical property and microstructure characterization of incoloy 800H alloy and its welds after corrosion testing in high temperature steam. Nucl. Eng. Des. 2022, 398, 111970. [Google Scholar] [CrossRef]
- Cao, Y.; Di, H.; Zhang, J.; Yang, Y. Dynamic behavior and microstructural evolution during moderate to high strain rate hot deformation of a Fe–Ni–Cr alloy (alloy 800H). J. Nucl. Mater. 2015, 456, 133–141. [Google Scholar] [CrossRef]
- Natesan, K.; Shankar, P.S. Uniaxial creep response of Alloy 800H in impure helium and in low oxygen potential environments for nuclear reactor applications. J. Nucl. Mater. 2009, 394, 46–51. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J. Hot corrosion of Fe and Ni-based alloys in Wase-to-energy environment at 850 °C. Eng. Fail. Anal. 2022, 133, 105964. [Google Scholar] [CrossRef]
- Uusitalo, M.A.; Vuoristo, P.M.J.; Mäntylä, T.A. High temperature corrosion of coatings and boiler steels in reducing chlorine-containing atmosphere. Surf. Coat. Technol. 2002, 161, 275–285. [Google Scholar] [CrossRef]
- Goyal, K.; Bhatia, R. Effect of nano yttria stabilized zirconia on corrosion behaviour of chromium oxide coatings on boiler steel. J. Electrochem. Sci. Eng. 2023, 13, 995–1004. [Google Scholar] [CrossRef]
- Bankiewicz, D.; Yrjas, P.; Hupa, M. High-Temperature Corrosion of Superheater Tube Materials Exposed to Zinc Salts. Energy Fuels 2009, 23, 3469–3474. [Google Scholar] [CrossRef]
- Tuz, L.; Kąc, S.; Sierakowski, D. Technology of electron beam welding of 10CrMo9-10 steel with the specific quality requirements. Manuf. Lett. 2023, 35, 53–57. [Google Scholar] [CrossRef]
- Zaghloul, M.B.; Sadek, A.A.; El-Batahgy, A.M.; Hanafy, M. Effect of Welding Parameters on Hot Cracking Susceptibility of Alloy 800. Q. J. Jpn. Weld. Soc. 1994, 12, 335–341. [Google Scholar] [CrossRef]
- Bisht, H.; Singh, R.P.; Sharma, V. Study on Welding Parameters in TIG Welding of Incoloy-800: An Investigation with Designed Experiments and ARAS Method; Optimization Methods in Engineering; Springer Nature: New York, NY, USA, 2021; pp. 209–233. [Google Scholar] [CrossRef]
- Srirangan, A.K.; Paulraj, S. Multi-response optimization of process parameters for TIG welding of Incoloy 800HT by Taguchi grey relational analysis. Eng. Sci. Technol. Int. J. 2016, 19, 811–817. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, D.L.; Na, Y.; Zhou, Y.; Han, X.L.; Wang, J. Effects of TIG welding parameters on morphology and mechanical properties of welded joint of Ni-base superalloy. Procedia Eng. 2011, 10, 37–41. [Google Scholar] [CrossRef]
- Gudenko, A.S.; Skorobogatykh, V.N.; Korneev, A.A.; Bardin, I.V.; Parshikova, N.V.; Bukharin, I.I.; Makarova, E.A. Effect of the Operating Temperature of a Steam Methane Reforming Furnace on Structural Changes and Crack Susceptibility in Welding 800HT Nickel Alloy. Metallurgist 2023, 67, 17–24. [Google Scholar] [CrossRef]
- Dehmolaei, R.; Shamanian, M.; Kermanpur, A. Microstructural changes and mechanical properties of Incoloy 800 after 15 years service. Mater. Charact. 2009, 60, 246–250. [Google Scholar] [CrossRef]
- El-Magd, E.; Nicolini, G.; Farag, M. Effect of Carbide Precipitation in the Creep Behaviour of Alloy 800HT in the Temperature Range 700 °C to 900 °C. Metall. Mater. Trans. A 1996, 27, 747–756. [Google Scholar] [CrossRef]

| Fe | C | Si | Mn | P | S | Cr | Ni | N | Al | Ti | Co | Cu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Required | min. 39.5 | 0.05 ÷ 0.10 | max 0.700 | max 1.500 | max 0.015 | max 0.010 | 19.0 ÷ 22.0 | 30.0 ÷ 34.0 | max 0.030 | 0.25 ÷ 0.65 | 0.25 ÷ 0.65 | max 0.50 | max 0.50 |
| Test result | 45.7 | 0.121 | 0.469 | 0.773 | 0.019 | 0.071 | 19.9 | 31.5 | - | 0.258 | 0.397 | 0.078 | 0.203 |
| Mo ∼ 0.251; Nb ∼ 0.060; V ∼ 0.0704; W ∼ 0.020 | |||||||||||||
| Sample No. | Solutioning | Annealing | ||
|---|---|---|---|---|
| Temperature | Time | Temperature | Time | |
| Sample A | - | - | - | - |
| Sample B | 1100 °C | 1 h | - | - |
| Sample C | 1100 °C | 10 h | - | - |
| Sample D | 1100 °C | 10 h | 750 °C | 24 h |
| Sample E | 1100 °C | 10 h | 750 °C | 72 h |
| Parameter | Unit | H2SO4 | HNO3 | NaCl | |
|---|---|---|---|---|---|
| Corrosion potential | ECORR | mV | −302.81 | −272.48 | −910.57 |
| Corrosion current | jCORR | μA/cm2 | 111.79 | 8.50 × 10−4 | 395.75 |
| Corrosion rate | CR | mm/year | 2.576 | 1.96 × 10−5 | 9.1192 |
| Tafel anodic constant | βa | V/dec | 0.1374 | 1.1549 | −0.98375 |
| Tafel cathodic constant | βc | V/dec | 0.15078 | 0.85665 | 0.28637 |
| Polarization resistance | Rp | Ω | 279.29 | 2.51 × 108 | 443.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierakowski, D.; Tuz, L.; Kąc, S. Evaluation of the Microstructure and Corrosion Resistance of the 800HT Alloy After Long-Term Operation. Appl. Sci. 2025, 15, 9188. https://doi.org/10.3390/app15169188
Sierakowski D, Tuz L, Kąc S. Evaluation of the Microstructure and Corrosion Resistance of the 800HT Alloy After Long-Term Operation. Applied Sciences. 2025; 15(16):9188. https://doi.org/10.3390/app15169188
Chicago/Turabian StyleSierakowski, Damian, Lechosław Tuz, and Sławomir Kąc. 2025. "Evaluation of the Microstructure and Corrosion Resistance of the 800HT Alloy After Long-Term Operation" Applied Sciences 15, no. 16: 9188. https://doi.org/10.3390/app15169188
APA StyleSierakowski, D., Tuz, L., & Kąc, S. (2025). Evaluation of the Microstructure and Corrosion Resistance of the 800HT Alloy After Long-Term Operation. Applied Sciences, 15(16), 9188. https://doi.org/10.3390/app15169188







