Design of Forward Osmosis Desalination Configurations: Exergy and Energy Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Forward Osmosis Desalination System Design
2.2. Exergy Analysis Methodology
- The system operates under steady-state conditions;
- Kinetic and potential contributions are neglected [46];
- Pump work is assumed to be supplied by electrical energy.
3. Results and Discussion
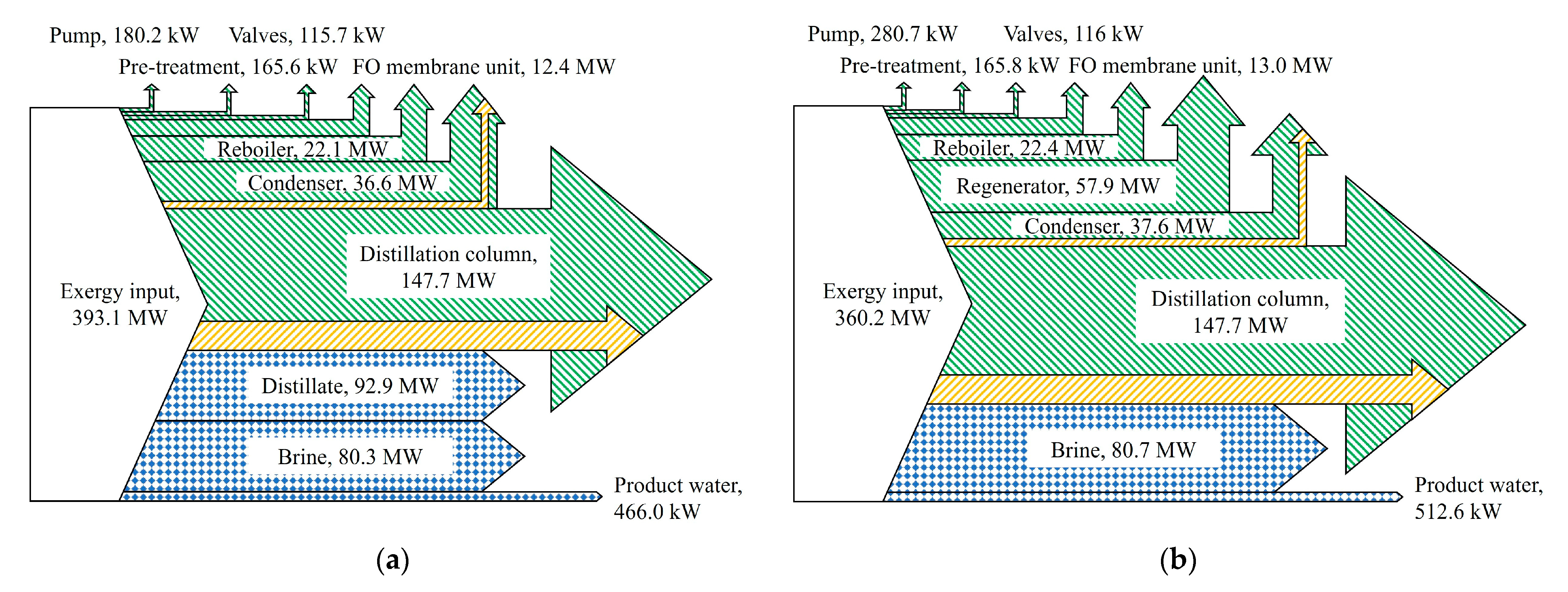
3.1. Energy Analysis of FO Desalination Systems
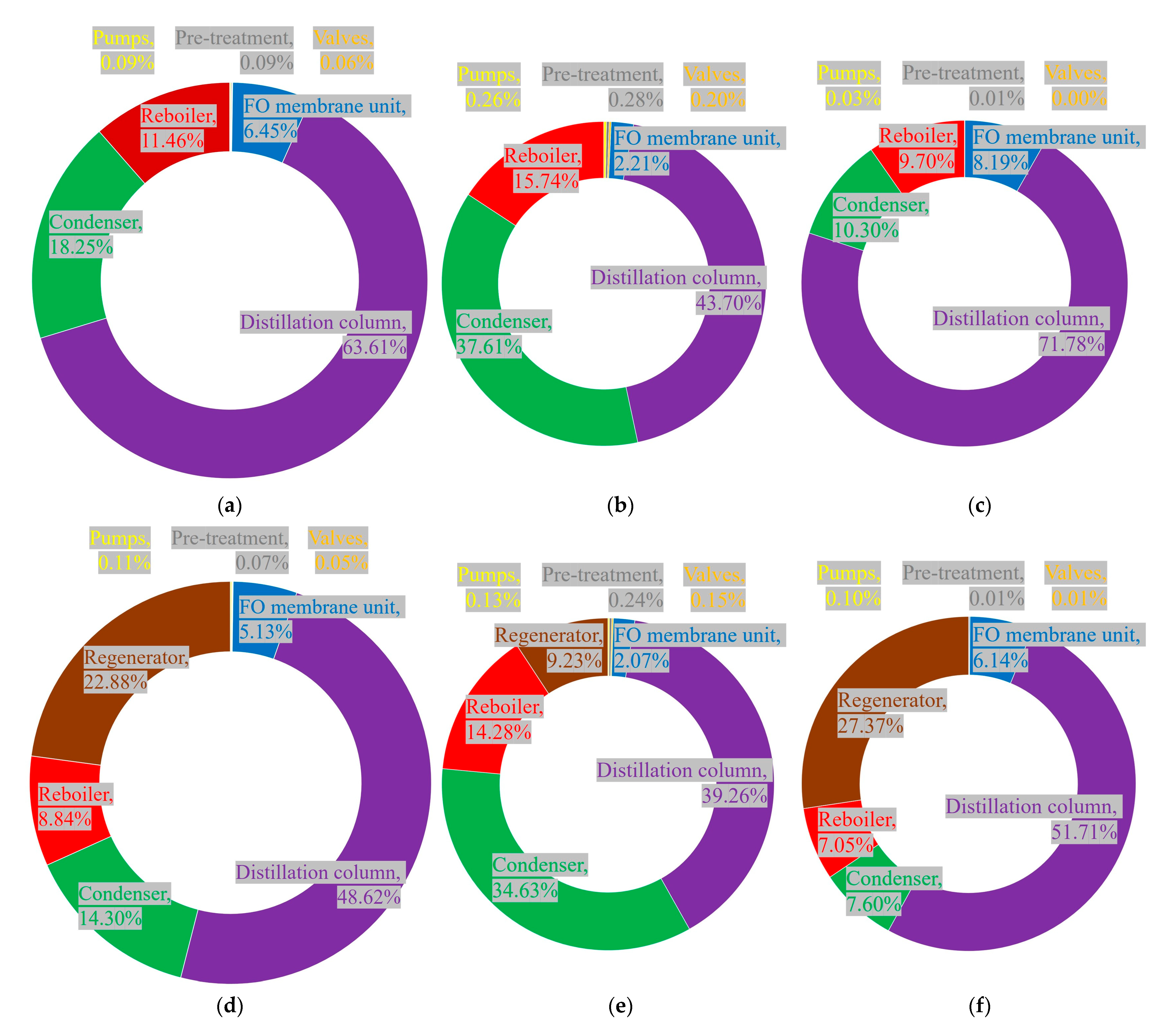
3.2. Component-Wise Exergy Analysis of FO Desalination Systems by Components
- Previous studies have also shown that connecting FO membrane units in series can reduce chemical exergy destruction by stabilizing concentration gradients across the membrane [45].
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Altmann, T.; Robert, J.; Bouma, A.; Swaminathan, J. Primary energy and exergy of desalination technologies in a power-water cogeneration scheme. Appl. Energy 2019, 252, 113319. [Google Scholar] [CrossRef]
- Water, U.N. The United Nations World Water Development Report 2021: Valuing Water; UN Water: Geneva, Switzerland, 2021. [Google Scholar]
- Koncagül, E.; Tran, M.; Connor, R.; Uhlenbrook, S. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; Facts and Figures. 2018. Available online: https://coilink.org/20.500.12592/jsv90z (accessed on 18 August 2025).
- Bank, F. World Energy Outlook 2018; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Rosen, M.; Farsi, A. CHAPTER EIGHT—Seawater desalination systems using sustainable energy technologies. In Sustainable Energy Technologies for Seawater Desalination; Rosen, M., Farsi, A., Eds.; Academic Press: Cambridge, UK, 2022; pp. 277–360. [Google Scholar]
- Khan, M.A.; Rehman, S.; Al-Sulaiman, F.A. A hybrid renewable energy system as a potential energy source for water desalination using reverse osmosis: A review. Renew. Sustain. Energy Rev. 2018, 97, 456–477. [Google Scholar] [CrossRef]
- Dubreuil, A.; Assoumou, E.; Bouckaert, S.; Selosse, S.; Maı, N. Water modeling in an energy optimization framework—The water-scarce middle east context. Appl. Energy 2013, 101, 268–279. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, Z.Y.; Han, M.Y.; Wang, G.D.; Hayat, T.; Chen, G.Q. Energy-water nexus in seawater desalination project: A typical water production system in China. J. Clean. Prod. 2021, 279, 123412. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K. Environmental impacts of desalination and brine treatment-Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [CrossRef]
- Panagopoulos, A. A comparative study on minimum and actual energy consumption for the treatment of desalination brine. Energy 2020, 212, 118733. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Darwish, M.A.; Al-Najem, N. The water problem in Kuwait. Desalination 2005, 177, 167–177. [Google Scholar] [CrossRef]
- Drioli, E.; Criscuoli, A.; Curcio, E. Integrated membrane operations for seawater desalination. Desalination 2002, 147, 77–81. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Rosen, M.; Farsi, A. CHAPTER TEN—Optimization of seawater desalination systems. In Sustainable Energy Technologies for Seawater Desalination; Rosen, M., Farsi, A., Eds.; Academic Press: Cambridge, UK, 2022; pp. 439–496. [Google Scholar]
- Okampo, E.J.; Nwulu, N. Optimisation of renewable energy powered reverse osmosis desalination systems: A state-of-the-art review. Renew. Sustain. Energy Rev. 2021, 140, 110712. [Google Scholar] [CrossRef]
- Rosen, M.; Farsi, A. CHAPTER SIX—Application of energy and exergy methods for assessing seawater desalination systems. In Sustainable Energy Technologies for Seawater Desalination; Rosen, M., Farsi, A., Eds.; Academic Press: Cambridge, UK, 2022; pp. 201–230. [Google Scholar]
- Farsi, A.; Dincer, I. Development and evaluation of an integrated MED/membrane desalination system. Desalination 2019, 463, 55–68. [Google Scholar] [CrossRef]
- Ali, E.; Orfi, J.; AlAnsary, H.; Soukane, S.; Elcik, H.; Alpatova, A.; Ghaffour, N. Cost analysis of multiple effect evaporation and membrane distillation hybrid desalination system. Desalination 2021, 517, 115258. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.W.; Burhan, M.; Ang, L.; Ng, K.C. Energy-water-environment nexus underpinning future desalination sustainability. Desalination 2017, 413, 52–64. [Google Scholar] [CrossRef]
- Zarzo, D.; Prats, D. Desalination and energy consumption—What can we expect in the near future? Desalination 2018, 427, 1–9. [Google Scholar] [CrossRef]
- Zhu, A.; Christofides, P.D.; Cohen, Y. On RO membrane and energy costs and associated incentives for future enhancements of membrane permeability. J. Membr. Sci. 2009, 344, 1–5. [Google Scholar] [CrossRef]
- Castel, C.; Favre, E. Membrane separations and energy efficiency. J. Membr. Sci. 2018, 548, 345–357. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Roesink, H.D.; Kemperman, A.J. Forward osmosis: A critical review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Francis, L.; Ogunbiyi, O.; Saththasivam, J.; Lawler, J.; Liu, Z. A comprehensive review of forward osmosis and niche applications. Environ. Sci. Water Res. Technol. 2020, 6, 1986–2015. [Google Scholar] [CrossRef]
- Kim, T.; Kim, Y.; Yun, C.; Jang, H.; Kim, W.; Park, S. Systematic approach for draw solute selection and optimal system design for forward osmosis desalination. Desalination 2012, 284, 253–260. [Google Scholar] [CrossRef]
- Ge, Q.; Ling, M.; Chung, T. Draw solutions for forward osmosis processes: Developments, challenges, and prospects for the future. J. Membr. Sci. 2013, 442, 225–237. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.H.; Kim, Y.C.; Lee, K.H.; Park, I.S.; Park, S. Operation and simulation of pilot-scale forward osmosis desalination with ammonium bicarbonate. Chem. Eng. Res. Des. 2015, 94, 390–395. [Google Scholar] [CrossRef]
- Tsatsaronis, G.; Cziesla, F. Basic exergy concepts, exergy balance and exergetic efficiency, exergy analysis of simple processes, energetic and exergetic analysis of complex systems, strength and limitations of exergy analysis. In Encyclopedia of Life Support Systems (EOLSS), Topic Energy, Developed under the Auspices of the UNESCO; EOLSS Publishers: Oxford, UK, 2004. [Google Scholar]
- Aljundi, I.H. Energy and exergy analysis of a steam power plant in Jordan. Appl. Therm. Eng. 2009, 29, 324–328. [Google Scholar] [CrossRef]
- Ehsana, A.; Yilmazoglu, Z. Design and exergy analysis of a thermal power plant using different types of Turkish lignite. Int. J. Thermodyn. 2011, 14, 125–133. [Google Scholar] [CrossRef]
- Suresh, M.; Reddy, K.S.; Kolar, A.K. 3-E analysis of advanced power plants based on high ash coal. Int. J. Energy Res. 2010, 34, 716–735. [Google Scholar] [CrossRef]
- Elsner, W.; Kowalczyk, Ł.; Marek, M. Numerical thermodynamic optimization of supercritical coal fired power plant with support of IPSEpro software. Arch. Thermodyn. 2012, 33, 96–105. [Google Scholar] [CrossRef]
- Singh, O.K.; Kaushik, S.C. Variables influencing the exergy based performance of a steam power plant. Int. J. Green Energy 2013, 10, 257–284. [Google Scholar] [CrossRef]
- Siva Reddy, V.; Kaushik, S.C.; Tyagi, S.K. Exergetic analysis and evaluation of coal-fired supercritical thermal power plant and natural gas-fired combined cycle power plant. Clean Technol. Environ. Policy 2014, 16, 489–499. [Google Scholar] [CrossRef]
- Cavalcanti, E.J.; Santos, E.B.; Carvalho, M. Energetic and exergetic performances of a multi-effect desalination unit driven by a gas turbine. Environ. Prog. Sustain. Energy 2024, 43, e14324. [Google Scholar] [CrossRef]
- Arakcheeva El Kori, N.; Blanco-Marigorta, A.M.; Melián Martel, N. Definition of Exergetic Efficiency in the Main and Emerging Thermal Desalination Technologies: A Proposal. Water 2024, 16, 1254. [Google Scholar] [CrossRef]
- Patel, D.; Mudgal, A.; Patel, V.; Patel, J.; Park, K.; Davies, P.; Dhakal, N. Exergy analysis for enhanced performance of integrated batch reverse osmosis—Forward osmosis system for brackish water treatment. Desalination 2024, 580, 117548. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Energy requirements of ammonia–carbon dioxide forward osmosis desalination. Desalination 2007, 207, 370–382. [Google Scholar] [CrossRef]
- Kolliopoulos, G.; Martin, J.T.; Papangelakis, V.G. Energy requirements in the separation-regeneration step in forward osmosis using TMA–CO2–H2O as the draw solution. Chem. Eng. Res. Des. 2018, 140, 166–174. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Zubair, S.M. On exergy calculations of seawater with applications in desalination systems. Int. J. Therm. Sci. 2011, 50, 187–196. [Google Scholar] [CrossRef]
- Al-Weshahi, M.A.; Anderson, A.; Tian, G. Exergy efficiency enhancement of MSF desalination by heat recovery from hot distillate water stages. Appl. Therm. Eng. 2013, 53, 226–233. [Google Scholar] [CrossRef]
- Patel, D.; Mudgal, A.; Patel, V.; Patel, J.; Park, K.; Davies, P.; Alegre, R.R. Energy, exergy, economic and environment analysis of standalone forward osmosis (FO) system for domestic wastewater treatment. Desalination 2023, 567, 116995. [Google Scholar] [CrossRef]
- Querol, E.; Gonzalez-Regueral, B.; Ramos, A.; Perez-Benedito, J.L. Novel application for exergy and thermoeconomic analysis of processes simulated with Aspen Plus®. Energy 2011, 36, 964–974. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Lienhard, J.H.; Zubair, S.M. Thermophysical properties of seawater: A review of existing correlations and data. Desalination Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Huang, S.; Li, N.; Low, E.; Xiao, L.; Liang, C. Entropy and exergy analysis of a liquid-liquid air-gap hollow fiber membrane contactor. Int. J. Therm. Sci. 2020, 158, 106543. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Li, Z.; Valladares, R.; Li, Q.; Amy, G. Indirect desalination of red sea water with forward osmosis and low pressure reverse osmosis for water reuse. Desalination 2011, 280, 160–166. [Google Scholar] [CrossRef]
- Choi, B.G.; Zhan, M.; Shin, K.; Lee, S.; Hong, S. Pilot-scale evaluation of FO-RO osmotic dilution process for treating wastewater from coal-fired power plant integrated with seawater desalination. J. Membr. Sci. 2017, 540, 78–87. [Google Scholar] [CrossRef]
- Zaviska, F.; Chun, Y.; Heran, M.; Zou, L. Using FO as pre-treatment of RO for high scaling potential brackish water: Energy and performance optimisation. J. Membr. Sci. 2015, 492, 430–438. [Google Scholar] [CrossRef]
- Nassrullah, H.; Anis, S.F.; Hashaikeh, R.; Hilal, N. Energy for desalination: A state-of- the-art review. Desalination 2020, 491, 114569. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Chia, W.Y.; Chia, S.R.; Khoo, K.S.; Chew, K.W.; Show, P.L. Sustainable membrane technology for resource recovery from wastewater: Forward osmosis and pressure retarded osmosis. J. Water Process Eng. 2021, 39, 101758. [Google Scholar] [CrossRef]
- Hafiz Al Hariri, A.; Khalifa, A.E. Heat recovery of multistage circulated permeate gap membrane distillation for energy-efficient water production. Sep. Purif. Technol. 2025, 352, 128284. [Google Scholar] [CrossRef]
- Toth, A.J. Modelling and optimisation of multi-stage flash distillation and reverse osmosis for desalination of saline process wastewater sources. Membranes 2020, 10, 265. [Google Scholar] [CrossRef]
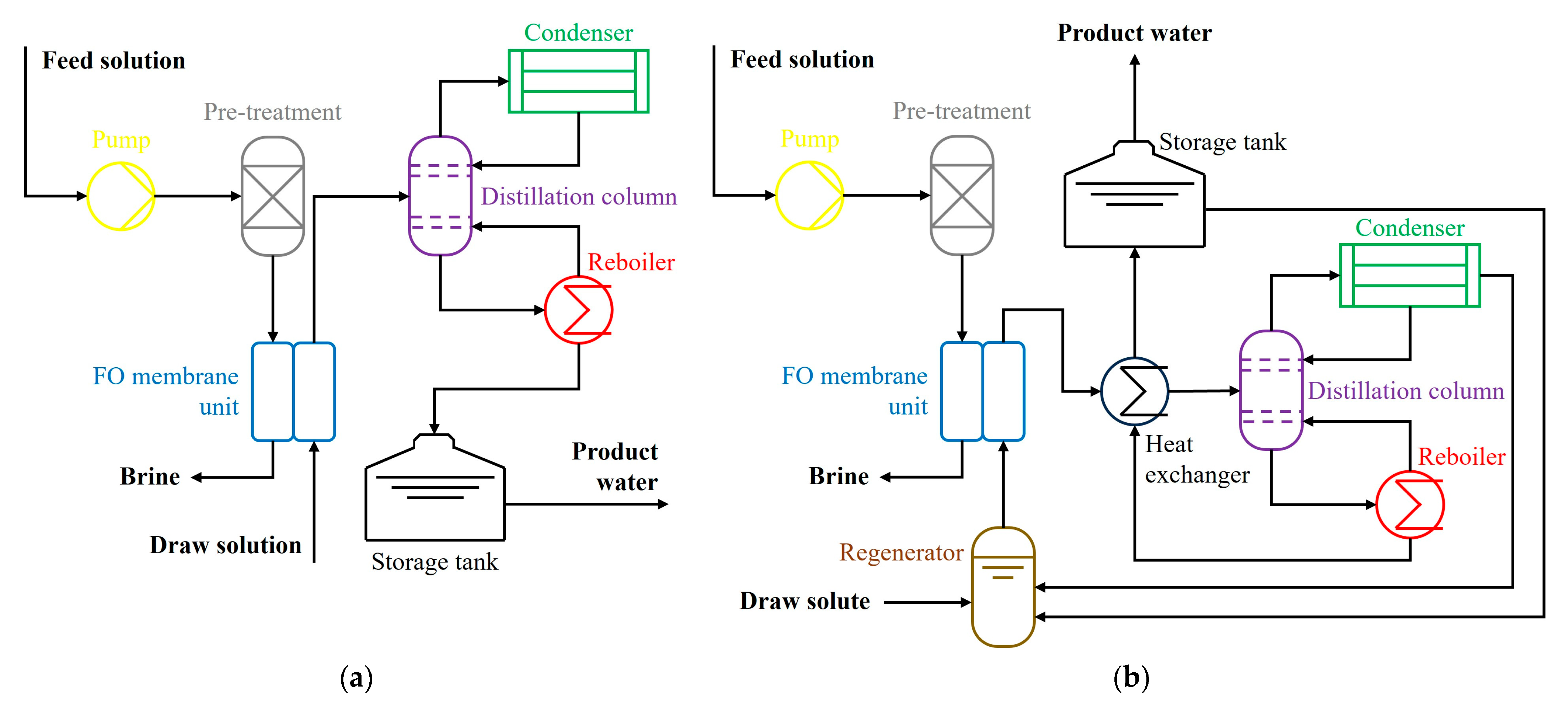
| Single-Pass FO Desalination System | Regenerative FO Desalination System | |
|---|---|---|
| Thermal energy consumption | 114.5 MW | 114.5 MW |
| Electrical energy consumption | 98.1 kW | 188.9 kW |
| Salt rejection rate | 98% | 98% |
| Water recovery rate | 60% | 54% |
| Equivalent work | 1.4 kWh/m3 | 1.8 kWh/m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Kim, Y.; Kim, D. Design of Forward Osmosis Desalination Configurations: Exergy and Energy Perspectives. Appl. Sci. 2025, 15, 9168. https://doi.org/10.3390/app15169168
Park C, Kim Y, Kim D. Design of Forward Osmosis Desalination Configurations: Exergy and Energy Perspectives. Applied Sciences. 2025; 15(16):9168. https://doi.org/10.3390/app15169168
Chicago/Turabian StylePark, Chulwoo, Yonghyuk Kim, and Daejoong Kim. 2025. "Design of Forward Osmosis Desalination Configurations: Exergy and Energy Perspectives" Applied Sciences 15, no. 16: 9168. https://doi.org/10.3390/app15169168
APA StylePark, C., Kim, Y., & Kim, D. (2025). Design of Forward Osmosis Desalination Configurations: Exergy and Energy Perspectives. Applied Sciences, 15(16), 9168. https://doi.org/10.3390/app15169168






