Abstract
In this work, eco-friendly ceric ammonium nitrate (CAN) and ferric chloride hexahydrate catalysts in ethanol/acetonitrile systems were used to efficiently synthesize novel dihydropyrimidinone (-thione) derivatives via the Biginelli reaction. The obtained compounds with phenolic fragments at the C4 position demonstrated enhanced antioxidant properties. Significant structure–activity relationships were indicated by three complementary assays (PFRAP, ABTS, and AAPH-induced DNA oxidation): oxo-derivatives demonstrated superior ferric ion reduction (PFRAP), while thio-substituted analogs consistently outperformed their carbonyl counterparts in radical scavenging. Remarkably, all compounds surpassed the reference antioxidant BHT, demonstrating the potential of synthesized dihydropyrimidine structures as multifunctional antioxidants for therapeutic applications. The study also shows the relationship between the catalyst–solvent system and its effect on product yields, using ceric ammonium nitrate and ferric chloride hexahydrate.
1. Introduction
Oxidation is a fundamental radical chain chemical process that affects both industrial materials and biological systems [1]. As regards petroleum chemistry, oxidation leads to the degradation of fuels and lubricants, reducing their quality and lifespan [2,3]. Similarly, uncontrolled oxidation in living organisms causes oxidative stress—an imbalance between reactive oxygen species (ROS) and antioxidant defenses [4,5]. Thus, the search for new antioxidants remains a critical task in different spheres, including medicinal chemistry, pharmacology, and the oil industry.
Nitrogen-containing heterocycles are one of the most significant and pharmacologically attractive classes of organic compounds. Within this group, pyrimidines hold particular significance, being a part of the structure of nucleic acids [6], vitamins (e.g., thiamine) [7,8], and numerous synthetic drugs [9,10]. A notable subclass of pyrimidine derivatives is dihydropyrimidinones (DHPMs) and their thione analogs [11]. These scaffolds have attracted considerable attention due to their wide-ranging therapeutic applications. Over the past century, DHPMs have been extensively investigated as calcium channel blockers (e.g., nifedipine analogs—compound (I) in Figure 1) [12,13], anticancer agents [14] (e.g., monastrol (II) [15], a selective mitotic kinesin inhibitor), and antimicrobial (III) [16,17,18] and anti-inflammatory [19] compounds.
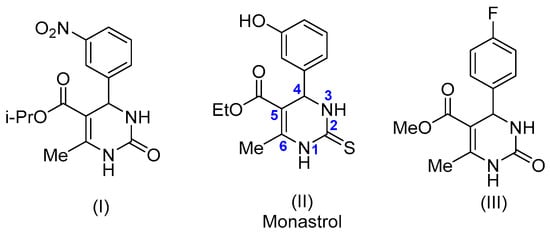
Figure 1.
Examples of DHPMs that exhibit biological activity.
The easiest way to obtain DHPMs and their thione analogs is the Biginelli reaction [20], a three-component condensation of an aldehyde, β-ketoester, and urea, which is particularly valued for its synthetic flexibility, as it tolerates a broad array of catalysts and solvents. Under classical conditions (typically using ethanol and hydrochloric acid), moderate yields of 40–60% are commonly observed [21]. Different advancements have demonstrated that Brønsted acids like trifluoroacetic acid [22] or para-toluenesulfonic acid [23] can enhance reaction rates by activating the aldehyde carbonyl toward imine formation [24,25]. Lewis acid catalysts, including metal triflates [26,27] (Yb (OTf)3, Zn (OTf)2) and transition metal salts (FeCl3, CuCl2) [28], have proven particularly effective, often improving yields to 80–95% [29]. In recent years, heterogeneous catalytic systems [30] have gained popularity due to their compatibility with green chemistry principles [31]. Materials such as zeolites, montmorillonite, and silica-supported nanoparticles offer advantages in terms of recyclability and a reduced environmental footprint [32,33]. At the same time, the solvent plays an equally critical role: polar protic solvents like methanol favor intermediate solubility but may promote side reactions, while aprotic systems such as acetonitrile or DMF enhance carbonyl activation at the expense of requiring anhydrous conditions [34,35]. Nevertheless, a lot of researchers have proved that solvent-free approaches are also effective, including those relying on mechanochemical methods or microwave irradiation, often delivering yields in the 85–92% range while minimizing organic solvent usage [36,37]. This remarkable flexibility in catalysts and solvents allows precise control over the electronic and steric properties of the obtained compounds, making the Biginelli reaction an effective tool for pharmaceutical applications in which structural fine-tuning is crucial for bioactivity optimization.
Recent studies highlight that functionalization at the C4 [38], C5 [39], and C2 positions of DHPMs can further enhance their pharmacological properties [40,41,42]. At the same time, phenolic compounds are well known for their antioxidant activity, primarily due to the ability to neutralize free radicals [43,44]. By incorporating phenolic fragments into dihydropyrimidinone (-thione) scaffolds at C4, enhanced antioxidant properties compounds can be synthesized. A synergistic effect between the heterocycle and the redox-active phenolic fragment can increase their radical-scavenging capabilities. Such dual functionality makes phenol-substituted DHPMs promising candidates for combating oxidative stress, which leads to aging, neurodegeneration, and chronic inflammation [45]. Moreover, the DHPM structure provides a versatile platform for further structural modifications.
Radical-scavenging mechanisms have a complex nature; due to this, there are numerous assays for antioxidant property evaluation, and no universal standard has been established [46]. This methodological variability requires a complementary approach, utilizing different assays to understand various facets of antioxidant behavior.
In this study, we used three well-validated spectrophotometric methods to comprehensively evaluate the antioxidant potential of synthesized dihydropyrimidinone (-thione) derivatives—the ABTS and PFRAP (Ferric Reducing Antioxidant Power) assays [47] and DNA protective capacity [48]. These methods were selected to probe different antioxidant mechanisms: while ABTS measures the compounds’ ability to neutralize stable radical cations through single-electron transfer (SET), PFRAP evaluates their capacity to reduce ferric ions (Fe3+ to Fe2+), reflecting hydrogen atom transfer (HAT) activity [49,50]. Meanwhile, the AAPH-induced DNA oxidation assay measures the compounds’ ability to protect salmon testes DNA from oxidative damage.
2. Materials and Methods
The initial reagents and solvents were obtained from commercial suppliers (Acros (Geel, Belgium) and Sigma-Aldrich (St. Louis, MI, USA)). The compounds 3-oxo-N-phenylbutanamide, 3-oxo-N-(p-tolyl)butanamide, and N-(4-iodophenyl)-3-oxobutanamide were synthesized using a well-established standard procedure. Melting points were determined using a Stuart SMP30 apparatus (Bibby Scientific Ltd., Staffordshire, UK). FTIR spectra was recorded on an Agilent Cary 660 spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an attenuated total reflectance (ATR) accessory (ZnSe crystal), averaging 32 scans per measurement. NMR spectra (1H at 300 MHz, 13C at 75 MHz) were acquired on a Bruker Avance II 300 spectrometer (Bruker Corporation, Billerica, MA, USA) using DMSO-d6 as the solvent and tetramethylsilane (TMS) as the internal standard. The spectral data is provided in the Supplementary Materials (Figures S1–S27, Tables S1–S3). Elemental analysis was performed on a Vario MicroCube analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany).
3. Experimental Section
3.1. General Procedure for the Preparation of Dihydropyrimidinones (-thiones) 1a and 2–5a,b (Method A)
A mixture of 4-hydroxy-3,5-dimethoxybenzaldehyde (5 mmol, 0.91 g), corresponding amide (10 mmol), urea or thiourea (15 mmol), and ceric ammonium nitrate (CAN) as the catalyst (0.5 mmol) was refluxed in 20 mL of ethanol for 8 h under stirring. During the reaction, precipitation was observed for compounds. For compounds 2a,b and 4–5a,b, a precipitate formed during the reaction, which was subsequently isolated by filtration. In contrast, compounds 1a and 3a,b were obtained by concentrating the reaction mixture under reduced pressure using a rotary evaporator, followed by dilution with water and filtration of the resulting solids. All isolated compounds were further purified by recrystallization from individually selected solvents.
5-Acetyl-4-(4-hydroxy-3,5-dimethoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (1a). Yield 38.3% (recrystallized from MeCN), m.p. 157–158 °C (lit. 153–155 °C [51]).
1H NMR (300 MHz, DMSO-d6, δ): 9.09 (s, 1H, OH), 8.31 (s, 1H, NH), 7.68 (s, 1H, NH), 6.51 (d, J = 1.8 Hz, 2H, Har), 5.19 (d, J = 3.3 Hz, 1H, CH), 3.71 (s, 6H, OCH3), 2.29 (s, 3H, COCH3), 2.08 (d, J = 1.1 Hz, 3H, CH3). 13C NMR (75 MHz, DMSO- d6, δ): 195.1, 152.5, 148.4, 148.2, 135.7, 134.7, 109.6, 104.7, 56.5, 54.5, 31.1, 30.5, 19.2. IR, cm−1: 3341 (ν O-H), 3212 (ν N-H), 3088 (ν N-H), 1713 (ν C=O). Elemental analysis found C, 58.64; H, 6.07; N, 9.22. Calculated for C15H18N2O5: C, 58.82; H, 5.92; N, 9.15.
Ethyl 4-(4-hydroxy-3,5-dimethoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (2a). Yield 48.7% (recrystallized from MeCN:H2O = 1:5), m.p. 173–175 °C (lit. 169.8–171.7 °C [52]).
1H NMR (300 MHz, DMSO-d6, δ): 9.10 (s, 1H, OH), 8.28 (s, 1H, NH), 7.62 (s, 1H, NH), 6.49 (s, 2H, Har), 5.08 (s, 1H, CH), 4.01 (q, J = 7.5 Hz, 2H, CH2), 3.71 (s, 6H, OCH3), 2.25 (s, 3H, CH3), 1.12 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 165.9, 152.8, 148.5, 148.2, 135.4, 104.2, 99.9, 59.6, 56.4, 54.2, 18.2, 14.6. IR, cm−1: 3520 (ν O-H), 3227 (ν N-H), 3095 (ν N-H), 1723 (ν C=O). Elemental analysis found C, 57.01; H, 6.12; N, 8.41. Calculated for C16H20N2O6: C, 57.14; H, 5.99; N, 8.33.
Ethyl 4-(4-hydroxy-3,5-dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (2b). Yield 75.2% (recrystallized from EtOH:H2O = 1:1), m.p. 193–195 °C (lit. 189–191 °C [53]).
1H NMR (300 MHz, DMSO-d6, δ): 10.28 (s, J = 1.8 Hz, 1H, OH), 9.57 (s, J = 3.9, 1.8 Hz, 1H, NH), 8.41 (s, 1H, NH), 6.47 (s, 2H, Har), 5.11 (d, J = 3.7 Hz, 1H, CH), 4.04 (q, J = 7.1 Hz, 2H, CH2), 3.71 (s, 6H, OCH3), 2.28 (s, 3H, CH3), 1.14 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 174.7, 165.7, 148.3, 145.2, 135.8, 134.1, 104.4, 101.4, 60.0, 56.4, 54.3, 17.6, 14.6. IR, cm−1: 3472 (ν O-H), 3325 (ν N-H), 3193 (ν N-H), 1661 (ν C=O). Elemental analysis found C, 54.70; H, 5.87; N, 7.92; S, 9.16. Calculated for C16H20N2O5S: C, 54.53; H, 5.72; N, 7.95; S, 9.10.
3.2. General Procedure for the Preparation of Dihydropyrimidinones (-thiones) 3–5a,b (Method B)
A mixture of 4-hydroxy-3,5-dimethoxybenzaldehyde (5 mmol, 0.91 g), corresponding amide (10 mmol), urea or thiourea (15 mmol), and ferric chloride hexahydrate as the catalyst (0,5 mmol) was refluxed in 20 mL of ethanol for 8 h under stirring. During the reaction, precipitation was observed for compounds 3–4a,b and 5b, whereas no precipitate formed for compound 5a. Upon completion of the reaction, the mixture was poured into cold water, and the resulting solid was collected by filtration. All obtained compounds were subsequently purified by recrystallization from individually selected solvents.
4-(4-Hydroxy-3,5-dimethoxyphenyl)-6-methyl-2-oxo-N-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxamide (3a). Yield 81.5% (recrystallized from MeCN:H2O = 1:2), m.p. 227–229 °C.
1H NMR (300 MHz, DMSO-d6) 9.44 (s, 1H, OH), 8.57 (s, 1H, CONH), 8.29 (s, 1H, NH), 7.41 (d, J = 1.9 Hz, 2H, Har), 7.40 (s, 1H, NH), 7.15–7.00 (m, 2H, Har), 6.52 (s, 2H, Har), 5.32 (d, J = 2.8 Hz, 1H, CH), 3.66 (d, J = 1.5 Hz, 6H, OCH3), 2.21 (s, 3H, CH3), 2.07 (d, J = 1.7 Hz, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 165.9, 153.0, 148.3, 137.9, 137.0, 135.5, 134.7, 132.6, 129.4, 120.2, 106.1, 104.5, 56.5, 55.6, 31.1, 20.9, 17.4. IR, cm−1: 3352 (ν O-H), 3276 (ν N-H), 1671 (ν C=O). Elemental analysis found C, 63.61; H, 5.98; N, 10.42. Calculated for C21H23N3O5: C, 63.47; H, 5.83; N, 10.57.
4-(4-Hydroxy-3,5-dimethoxyphenyl)-6-methyl-2-thioxo-N-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxamide (3b). Yield 72.1% (recrystallized from MeCN:H2O = 3:1), m.p. 267–269 °C.
1H NMR (300 MHz, DMSO-d6, δ): 9.87 (s, 1H, OH), 9.61 (s, 1H, CONH), 9.27 (s, 1H, NH), 8.35 (s, 1H, NH), 7.42 (d, J = 8.0 Hz, 2H, Ar H), 7.07 (d, J = 8.0 Hz, 2H, Ar H), 6.53 (s, 2H, Ar H), 5.33 (d, J = 3.1 Hz, 1H), 3.68 (s, 6H, OCH3), 2.22 (s, 3H, CH3), 2.05 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 174.5, 165.5, 148.4, 136.9, 135.9, 135.2, 133.5, 132.8, 129.4, 120.3, 108.0, 104.6, 56.5, 55.6, 31.1, 20.9, 16.9. IR, cm−1: 3497 (ν O-H), 3356 (ν N-H), 3268 (ν N-H), 3176 (ν N-H), 1665 (ν C=O). Elemental analysis found C, 61.05; H, 5.87; N, 10.32; S, 7.83. Calculated for C21H23N3O4S: C, 61.00; H, 5.61; N, 10.16; S, 7.75.
4-(4-Hydroxy-3,5-dimethoxyphenyl)-N-(4-iodophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide (4a). Yield 20.9% (recrystallized from MeCN:H2O = 2:1), m.p. 247–249 °C.
1H NMR (300 MHz, DMSO-d6, δ): 9.48 (s, 1H, OH), 8.52 (s, 1H, CONH), 8.07 (s, 1H, NH), 7.58 (d, J = 8.4 Hz, 2H, Har), 7.39 (d, J = 8.3 Hz, 2H, Har), 7.33 (s, 1H, NH), 6.55 (s, 2H, Har), 5.34 (s, 1H, CH), 3.68 (s, 6H, OCH3), 2.05 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 166.1, 152.9, 148.3, 139.5, 137.6, 135.4, 134.7, 122.2, 105.7, 104.3, 86.9, 56.4, 55.5, 17.5. IR, cm−1: 3330 (ν O-H), 1665 (ν C=O). Elemental analysis found C, 47.11; H, 4.12; N, 8.38. Calculated for C20H20IN3O5: C, 47.17; H, 3.96; N, 8.25.
4-(4-Hydroxy-3,5-dimethoxyphenyl)-N-(4-iodophenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide (4b). Yield 27.2% (recrystallized from MeCN:H2O = 2:1), m.p. 249–251 °C.
1H NMR (300 MHz, DMSO-d6, δ): 9.94 (s, 1H, OH), 9.80 (s, 1H, CONH), 9.34 (s, 1H, NH), 8.37 (s, 1H, NH), 7.61 (d, J = 8.3 Hz, 2H, Har), 7.40 (d, J = 8.6 Hz, 2H, Har), 6.51 (s, 2H, Har), 5.36–5.30 (m, 1H, CH), 3.67 (s, 6H, OCH3), 2.05 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 174.4, 165.8, 148.4, 139.2, 137.8, 135.9, 135.8, 133.4, 122.3, 121.7, 107.5, 104.4, 87.4, 56.5, 55.4, 16.9. IR, cm−1: 3485 (ν O-H), 3354 (ν N-H), 3288 (ν N-H), 3218 (ν N-H), 1673 (ν C=O). Elemental analysis found C, 45.85; H, 4.02; N, 7.91; S, 6.01. Calculated for C20H20IN3O4S: C, 45.72; H, 3.84; N, 8.00; S, 6.10.
4-(4-Hydroxy-3,5-dimethoxyphenyl)-6-methyl-2-oxo-N-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxamide (5a). Yield 23.0% (recrystallized from MeCN:H2O = 1:8), m.p. 146–148 °C.
1H NMR (300 MHz, DMSO-d6, δ): 9.53 (s, 1H, OH), 8.64 (s, 1H, CONH), 8.27 (s, 1H, NH), 7.55 (d, J = 8.5, 1.3 Hz, 2H, Har), 7.46 (s, 1H, NH), 7.25 (t, J = 7.8 Hz, 2H, Har), 7.06–6.94 (t, 1H, Har), 6.54 (d, J = 1.2 Hz, 2H, Har), 5.35 (d, J = 2.4 Hz, 1H, CH), 3.67 (d, J = 1.2 Hz, 6H, OCH3), 2.04 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 166.0, 153.0, 148.3, 139.6, 138.2, 135.4, 134.7, 129.0, 123.6, 120.1, 106.0, 104.3, 56.4, 55.6, 17.4. IR, cm−1: 3534 (ν O-H), 3272 (ν N-H), 3110 (ν N-H), 1701 (ν C=O), 1672 (ν C=O). Elemental analysis found C, 62.48; H, 5.73; N, 10.84. Calculated for C20H21N3O5: C, 62.65; H, 5.52; N, 10.96.
4-(4-Hydroxy-3,5-dimethoxyphenyl)-6-methyl-N-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide (5b). Yield 12.0% (recrystallized from MeCN), m.p. 261–263 °C.
1H NMR (300 MHz, DMSO-d6, δ): 9.93 (s, 1H, OH), 9.73 (s, 1H, CONH), 9.33 (s, 1H, NH), 8.39 (s, 1H, NH), 7.60–7.51 (d, 2H, Har), 7.27 (t, J = 7.7 Hz, 2H, Har), 7.02 (t, J = 7.3 Hz, 1H, Har), 6.53 (s, 2H, CH2), 5.35 (d, J = 3.1 Hz, 1H, CH), 3.68 (s, 6H, OCH3), 2.08 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6, δ): 174.4, 165.6, 148.4, 139.4, 135.8, 135.4, 133.5, 129.1, 123.8, 120.2, 107.9, 104.5, 56.5, 55.6, 31.2, 16.9. IR, cm−1: 3489 (ν O-H), 3362 (ν N-H), 3287 (ν N-H), 3208 (ν N-H), 1675 (ν C=O), 1632 (ν C=O). Elemental analysis found C, 60.30; H, 5.45; N, 10.44; S, 8.07. Calculated for C20H21N3O4S: C, 60.14; H, 5.30; N, 10.52; S, 8.03.
3.3. Antioxidant Activity
Antioxidant activity was evaluated using three complementary spectrophotometric assays (ABTS, PFRAP, AAPH-DNA) to assess different mechanistic pathways: radical scavenging, ferric ion reduction, and biomacromolecule protection. All experiments were performed in triplicate on freshly prepared solutions using a SOLAR UV–Vis spectrophotometer PB 2201 (SOLAR Laser Systems, Minsk, Belarus) with temperature-controlled cuvette holders (±0.5 °C). The measurements were carried out in a 1 cm quartz cuvette at a temperature of 25 °C; the spectral scanning step was 0.5 nm.
3.3.1. ABTS Assay
The ABTS radical cation (ABTS+) was generated by reacting 7 mM ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) in distilled water with 2.45 mM potassium persulfate (K2S2O8) in a 1:1 (v/v) ratio [54]. The obtained reaction mixture was incubated in the dark at ambient temperature (20–25 °C) for 12–16 h to yield a stable dark-blue solution. Prior to analysis, the stock solution was diluted with absolute ethanol to achieve an absorbance of 0.70 ± 0.02 at 734 nm.
For antioxidant activity assessment, 300 µL of test compounds (50 µM in DMSO) were introduced into 2.7 mL of the diluted ABTS+. solution. Following a 10 min incubation period under subdued light, the reduction in absorbance at 734 nm was recorded. The radical-scavenging capacity (%) was evaluated using the following formula:
where Acontrol and Asample represent the absorbances of the ABTS+. solution without and with the test compound, respectively.
Scavenging activity (%) = (Acontrol − Asample)/Acontrol,
3.3.2. Ferric Ion-Reducing Capacity (PFRAP) Assay
A 1% potassium ferricyanide solution was prepared by dissolving 1.0 g of K3[Fe(CN)6] in 1 mL of 1 M hydrochloric acid followed by dilution to a final volume of 100 mL with water. Similarly, a 0.2% ferric chloride solution was obtained by dissolving 0.2 g of FeCl3·6H2O in 1 mL of 1 M HCl with a small volume of water, adjusting to 100 mL with additional water. For the sodium dodecyl sulfate (SDS) solution, 1.0 g of SDS was dissolved in 100 mL of deionized water to achieve a 1% concentration.
The antioxidant assay was performed by sequentially mixing 100 µL of the test compound solution (500 µM), 400 µL of 96% ethanol, 2.5 mL of deionized water, 750 µL of 1 M HCl, 750 µL of the 1% potassium ferricyanide solution, 250 µL of 1% SDS solution, and 250 µL of 0.2% ferric chloride solution. The resulting mixture was incubated at 50 °C in a water bath for precisely 20 min; the samples were then cooled to room temperature (20–25 °C), and the absorbance was measured at 750 nm using a spectrophotometer, with a reagent blank (prepared identically but without the test substance) serving as the reference [55].
3.3.3. AAPH-Induced Oxidation of the DNA Assay
The protective effects of the obtained compounds against oxidative DNA damage were evaluated using an AAPH (2,2′-azobis(2-amidinopropane) dihydrochloride) system with modifications to established protocols [56].
In this assay, 20 μL of each compound dissolved in DMSO (500 μM) was introduced to phosphate-buffered saline (PBS, pH 7.4) containing salmon testes DNA (2.5 mg·mL−1) and AAPH (40 mM). The reaction mixture was aliquoted into test tubes (2.0 mL per tube) and incubated at 37 °C for 2.5 h to induce oxidative damage.
Following incubation, samples were rapidly cooled and treated with 1.0 mL of thiobarbituric acid (TBA) reagent (prepared by dissolving 1.00 g TBA and 0.40 g NaOH in 100 mL PBS, pH 7.4) along with 1.0 mL of 3.0% trichloroacetic acid solution. The mixtures were then heated in a boiling water bath for 15 min to develop chromogenic reaction products. After cooling, 2.0 mL of n-butanol was added to each tube, followed by vigorous mixing to extract thiobarbituric acid reactive substances (TBARSs). The organic phase was separated, and its absorbance was measured spectrophotometrically at 535 nm compared with distilled water as a blank.
Antioxidant efficacy was evaluated by comparing sample absorbance (Asample) to the blank control (Acontrol) using the following formula:
Antioxidant activity (%) = (1 − (Asample/Acontrol))
This formula represents the compound’s ability to prevent TBARS formation during AAPH-induced DNA oxidation, with BHT serving as a reference antioxidant standard.
4. Results and Discussion
In this study, we successfully synthesized a series of dihydropyrimidinones and dihydropyrimidinthiones via the Biginelli reaction in acetonitrile using ceric ammonium nitrate (CAN) as catalysts (method A) and ferric chloride hexahydrate and in ethanol (method B) under reflux. The reaction was carried out using 4-hydroxy-3,5-dimethoxybenzaldehyde as the limiting reagent, with two equivalents of the appropriate dicarbonyl compound and three equivalents of either urea or thiourea, and 10 mol% of the catalyst. Acetylacetone, acetoacetic ester, and amides of acetoacetic ester (N-phenyl, N-para-tolyl, and N-para-iodophenyl) were used as dicarbonyl compounds (Scheme 1).
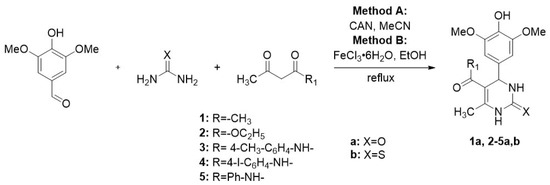
Scheme 1.
Synthesis of dihydropyrimidinones (-thiones) 1,5a,b.
Our study reveals a dependence of product yields on catalyst–solvent combinations (Table 1), particularly for compounds 3–5a,b. It was demonstrated that for oxo-derivatives 3a, 4a, 5a that the FeCl3·6H2O/EtOH system proved markedly superior, yielding 81.5% for 3a compared with only 21.9% with CAN/MeCN. This trend held for 5a (47.9% with CAN vs. 23.0% with ferric chloride hexahydrate), though it was less pronounced for 4a. Thio-derivatives showed more complex behavior, while FeCl3·6H2O/EtOH gave optimal results for 3b (72.1% vs. 48.9%), CAN/MeCN proved better for 4b and 5b. These patterns suggest that ethanol enhances Fe3+-catalyzed cyclizations for most oxo-compounds, while thiourea derivatives require case-specific optimization.

Table 1.
Reaction yields.
In the 1H NMR spectra of the prepared compounds, there are no peaks in the region of 10.4 ppm corresponding to the proton of the aldehyde group. Peaks at 9.10–10.28 ppm corresponding to the proton of the phenolic group are present in all spectra. Peaks of NH protons in the spectrum of derivatives with C=S and shifted to a higher field (9.61–9,80 ppm), in comparison with derivatives with the C=O group (8.28–8.64 ppm).
The IR spectra of the synthesized compounds contain absorption bands characteristic of the stretching vibrations of O-H bonds (3330–3534 cm−1), N-H bonds in the dihydropyrimidine ring (3300–3088 cm−1), as well as stretching vibrations of C=O bonds at around 1700 for products 1,2 and at around 1660 for amide derivatives 3–5 [57].
The obtained dihydropyrimidinone (-thione) derivatives exhibited notable antioxidant properties, as evidenced by AAPH-induced oxidation of the DNA (Figure 2), PFRAP (Ferric Reducing Antioxidant Power) (Figure 3), and ABTS radical-scavenging (Figure 4) assays. A comparative analysis reveals distinct trends correlating structural features with antioxidant efficacy.
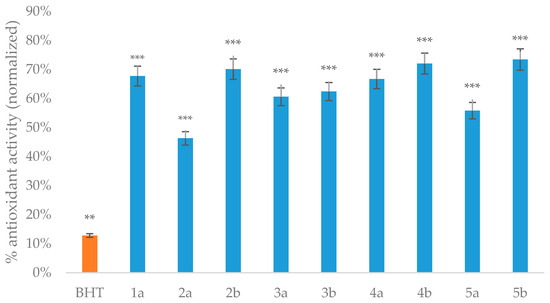
Figure 2.
AAPH-induced oxidation of the DNA assay results of the obtained compounds. Concentration of compounds was 500 µM. The results are expressed as mean ± standard deviation (** p < 0.01, *** p < 0.001).
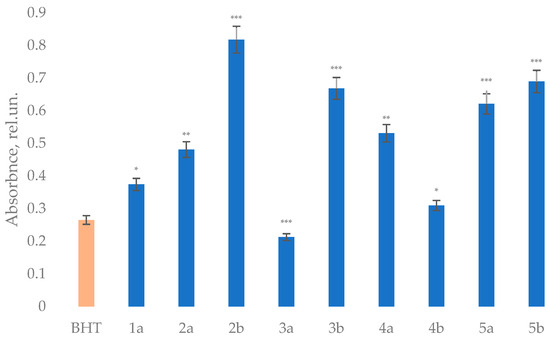
Figure 3.
Ferric ion-reducing capacity of the obtained compounds. Concentration of compounds was 250 µM. The results are expressed as mean ± standard deviation compared to BHT (* p < 0.05, ** p < 0.01, *** p < 0.001).
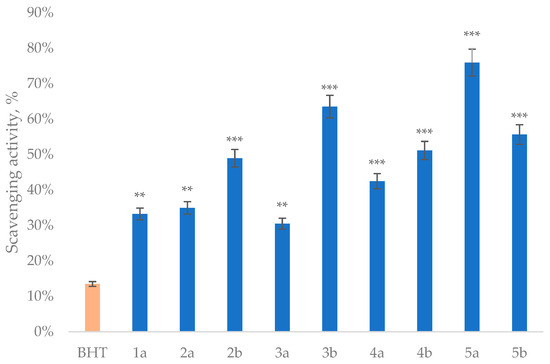
Figure 4.
ABTS scavenging activity of the obtained compounds. Concentration of compounds was 50 µM (** p < 0.01, *** p < 0.001).
DNA oxidation is considered a major factor in carcinogenesis [58]. Since excessive oxidative stress damages double-stranded DNA, it alters gene expression, leading to possible mutagenesis [59]. 2,2′-Azobis (2-amidopropane) dihydrochloride (AAPH) has the ability to undergo spontaneous decomposition in an aqueous solution, resulting in the formation of peroxyl radicals (ROO•) [60,61]. ROO• can abstract hydrogen atoms from DNA, leading to chain oxidation reactions. Although TBARS are most commonly used to detect malondialdehyde generated during lipid peroxidation, it can also detect aldehydic fragments produced during the oxidative degradation of the DNA [62,63]. AAPH is able to convert the supercoiled DNA strand to open circular and linear forms and more than 20 carbonyl species eventually. The reaction of these aldehydic products with thiobarbituric acid yields a chromophoric adduct with maximum absorbance at 535 nm, which serves as an indirect marker of DNA damage [64].
All the synthesized compounds demonstrated superior protective effects compared with the reference antioxidant BHT (26%) in the AAPH-induced DNA oxidation assay. The thione derivatives consistently outperformed their oxo analogs, with compound 4b (86%) with a 4-iodophenylcarboxamide fragment and compound 5b (87%) with a simple phenylcarboxamide showing nearly complete DNA protection. The presence of the thione group appears crucial for this protective effect, as evidenced by the 20–30% activity increase in thione versus oxo pairs. The carboxamide derivatives generally showed better protection than ester- or acetyl-substituted compounds, indicating the importance of the hydrogen-bonding capable amide group in DNA protection mechanisms.
The ferric ion reduction capacity measured by the FRAP assay showed more varied structure–activity relationships. While most compounds exceeded BHT’s reducing power (0.265), the ethyl ester thione derivative 2b exhibited exceptional activity (0.818), suggesting its optimal electronic configuration for electron transfer processes. At the same time, iodo-substituted thione 4b showed relatively modest FRAP activity (0.310) despite its excellent AAPH results, highlighting how different antioxidant mechanisms respond to structural features. The phenylcarboxamide derivatives displayed intermediate reducing power, while the p-tolyl analog 3b showed surprisingly strong activity (0.669) given its modest performance in other assays. These variations suggest that ferric reduction depends on subtle electronic effects of the C5 substituents that differ from radical-scavenging requirements.
The ABTS assay results showed yet another distinct structure–activity profile, with phenylcarboxamide derivatives 5a (76%) and p-tolyl analog 3b (64%) showing the most potent radical-scavenging capacity, exceeding that of BHT (13%). Unlike the AAPH results, the thione effect was less pronounced in ABTS, with some oxo derivatives like 5a performing exceptionally well. This suggests that the thione group enhances peroxyl radical interception (AAPH) and electron transfer (FRAP); the parent oxo compounds can be equally effective at donating hydrogen atoms to stabilize the ABTS radical cation. The 4-hydroxy-3,5-dimethoxyphenyl fragment appears particularly well suited for ABTS scavenging, as all derivatives significantly outperformed BHT despite its tert-butyl protection groups.
There are three key structural features that emerge as critical for optimal antioxidant performance: the thione group at C2 position consistently enhances activity across all assays, particularly for DNA protection; carboxamide derivatives at C5 generally outperform ester and acetyl analogs, with phenyl and p-tolyl substitutions being most favorable; and the conserved 4-hydroxy-3,5-dimethoxyphenyl fragment at C4 provides essential radical stabilization. The most balanced antioxidant profile was shown by compound 5b, which combined all these favorable features to deliver top-tier performance in both radical-scavenging ABTS and AAPH-induced oxidation of the DNA assays, along with strong reducing power (FRAP). These results demonstrate that careful structural modulation of dihydropyrimidinone structures can yield tunable antioxidants surpassing industrial standards, with different substituents favoring specific antioxidant mechanisms. The consistent superiority over BHT across all assay systems highlights the potential of these phenolic-dihydropyrimidinone hybrids as multifunctional antioxidants for pharmaceutical applications in which comprehensive oxidative protection is required.
5. Conclusions
In this study the efficient synthesis of novel dihydropyrimidinone (-thione) derivatives via the Biginelli reaction using eco-friendly catalytic systems was carried out. This offers a more sustainable alternative to traditional acid-catalyzed Biginelli reactions, reducing environmental impact while maintaining high yields. The influence of catalyst–solvent systems on reaction yields was also shown, with ferric chloride hexahydrate in ethanol proving optimal for oxo-derivatives and CAN in acetonitrile favoring certain thio-compounds. The incorporation of a 4-hydroxy-3,5-dimethoxyphenyl fragment at the C4 position of the DHPM ring allowed to obtain new effective antioxidants. Compound 5b, containing a thione group at position 2 and an N-phenylamide fragment at position 5, showed better radical-scavenging ability, reduced Fe+3 ions, and better DNA protective properties.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15169152/s1: Figure S1: 1H NMR spectra of 1a; Figure S2: 13C NMR spectra of 1a; Figure S3: IR spectra of 1a; Figure S4: 1H NMR spectra of 2a; Figure S5: 13C NMR spectra of 2a; Figure S6: IR spectra of 2a; Figure S7: 1H NMR spectra of 2b; Figure S8: 13C NMR spectra of 2b; Figure S9: IR spectra of 2b; Figure S10: 1H NMR spectra of 3a; Figure S11: 13C NMR spectra of 3a; Figure S12: IR spectra of 3a; Figure S13: 1H NMR spectra of 3b; Figure S14: 13C NMR spectra of 3b; Figure S15: IR spectra of 3b; Figure S16: 1H NMR spectra of 4a; Figure S17: 13C NMR spectra of 4a; Figure S18: IR spectra of 4a; Figure S19: 1H NMR spectra of 4b; Figure S20: 13C NMR spectra of 4b; Figure S21: IR spectra of 4b; Figure S22: 1H NMR spectra of 5a; Figure S23: 13C NMR spectra of 5a; Figure S24: IR spectra of 5a; Figure S25: 1H NMR spectra of 5b; Figure S26: 13C NMR spectra of 5b; Figure S27: IR spectra of 5b; Table S1. ABTS+∙ scavenging activity of the obtained compounds; Table S2. Ferric ion-reducing capacity of the obtained compounds; Table S3. AAPH-Induced Oxidation of the DNA assay results of the obtained compounds.
Author Contributions
Conceptualization, O.V.S. and V.N.K.; methodology, O.V.S.; validation, E.R.S. and V.N.K.; formal analysis, V.N.K.; investigation, O.V.S. and V.A.K.; resources, E.R.S.; writing—review and editing, O.V.S. and E.R.S.; visualization, E.R.S. and V.A.K.; supervision, V.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Kovalsky, B.; Shram, V.; Bezborodov, Y.N.; Petrov, O.; Lysyannikova, N.; Kravtsova, E. The results of the study of the mechanism of oxidation of motor oil. J. Phys. Conf. Ser. 2019, 1399, 055014. [Google Scholar] [CrossRef]
- Aguilar, G.; Mazzamaro, G.; Rasberger, M. Oxidative degradation and stabilisation of mineral oil-based lubricants. In Chemistry and Technology of Lubricants; Springer: Berlin/Heidelberg, Germany, 2009; pp. 107–152. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Velena, A.; Zarkovic, N.; Gall Troselj, K.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1, 4-Dihydropyridine Derivatives: Dihydronicotinamide Analogues—Model Compounds Targeting Oxidative Stress. Oxidative Med. Cell. Longev. 2016, 2016, 1892412. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Anthoni Samy, H.N.; Sivaperuman, A.; Subramani, A. Structure-activity relationships of pyrimidine derivatives and their biological activity—A review. Med. Chem. 2023, 19, 10–30. [Google Scholar] [CrossRef]
- Nair, N.; Majeed, J.; Pandey, P.; Sweety, R.; Thakur, R. Antioxidant potential of pyrimidine derivatives against oxidative stress. Indian J. Pharm. Sci. 2022, 84, 14–26. [Google Scholar] [CrossRef]
- Parkhomenko, Y.M.; Vovk, A.; Protasova, Z.; Chornyy, S.; Kobzar, O.; Stepanenko, S.; Chekhivska, L. Molecular Structural Features that Determine the Neurotropic Activity of Thiamine Derivatives. Neurophysiology 2022, 54, 82–93. [Google Scholar] [CrossRef]
- Manzoor, S.; Prajapati, S.K.; Majumdar, S.; Raza, M.K.; Gabr, M.T.; Kumar, S.; Pal, K.; Rashid, H.; Kumar, S.; Krishnamurthy, S. Discovery of new phenyl sulfonyl-pyrimidine carboxylate derivatives as the potential multi-target drugs with effective anti-Alzheimer’s action: Design, synthesis, crystal structure and in-vitro biological evaluation. Eur. J. Med. Chem. 2021, 215, 113224. [Google Scholar] [CrossRef]
- Nerkar, A.U. Use of pyrimidine and its derivative in pharmaceuticals: A review. J. Adv. Chem. Sci. 2021, 7, 729–732. [Google Scholar] [CrossRef]
- Kappe, C.O. 100 years of the Biginelli dihydropyrimidine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Zohny, Y.M.; Awad, S.M.; Rabie, M.A.; Al-Saidan, O.A. Synthesis of dihydropyrimidines: Isosteres of Nifedipine and evaluation of their calcium channel blocking efficiency. Molecules 2023, 28, 784. [Google Scholar] [CrossRef]
- Kappe, C.O. 4-Aryldihydropyrimidines via the Biginelli condensation: Aza-analogs of nifedipine-type calcium channel modulators. Molecules 1998, 3, 1–9. [Google Scholar] [CrossRef]
- Dowarah, J.; Patel, D.; Marak, B.N.; Yadav, U.C.S.; Shah, P.K.; Shukla, P.K.; Singh, V.P. Green synthesis, structural analysis and anticancer activity of dihydropyrimidinone derivatives. RSC Adv. 2021, 11, 35737–35753. [Google Scholar] [CrossRef] [PubMed]
- Ragab, F.A.; Abou-Seri, S.M.; Abdel-Aziz, S.A.; Alfayomy, A.M.; Aboelmagd, M. Design, synthesis and anticancer activity of new monastrol analogues bearing 1, 3, 4-oxadiazole moiety. Eur. J. Med. Chem. 2017, 138, 140–151. [Google Scholar] [CrossRef]
- Castro Jara, M.; Silva, A.C.A.; Ritter, M.; da Silva, A.F.; Gonçalves, C.L.; Dos Santos, P.R.; Borja, L.S.; de Pereira, C.M.P.; da Silva Nascente, P. Dihydropyrimidinones against multiresistant bacteria. Front. Microbiol. 2022, 13, 743213. [Google Scholar] [CrossRef]
- Sarvaiya, B.H.; Vaja, P.I.; Paghdar, N.A.; Ghelani, S.M. Medicinal perspective of a promising scaffold–dihydropyrimidinones: A review. J. Heterocycl. Chem. 2024, 61, 1325–1348. [Google Scholar] [CrossRef]
- Gawdzik, B.; Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Masternak, J.; Kramkowski, K.; Wypych, A.; Ostaszewski, R. The Evaluation of DHPMs as Biotoxic Agents on Pathogen Bacterial Membranes. Membranes 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef]
- Panda, S.S.; Khanna, P.; Khanna, L. Biginelli reaction: A green perspective. Curr. Org. Chem. 2012, 16, 507–520. [Google Scholar] [CrossRef]
- Kappe, C.O. The generation of dihydropyrimidine libraries utilizing Biginelli multicomponent chemistry. QSAR Comb. Sci. 2003, 22, 630–645. [Google Scholar] [CrossRef]
- Mohammadizadeh, M.R.; Firoozi, N. Trifluoroacetic Acid as an Effective Catalyst for Biginelli Reaction: One-Pot, Three-Component Synthesis of 3, 4-Dihydropyrimidin-2 (1H)-ones (and-Thiones). J. Chem. 2011, 8, S266–S270. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, S.; Li, T. p-toluenesulfonic acid-catalyzed efficient synthesis of dihydropyrimidines: Improved high yielding protocol for the Biginelli reaction. Synth. Commun. 2002, 32, 1847–1851. [Google Scholar] [CrossRef]
- Xin, J.; Chang, L.; Hou, Z.; Shang, D.; Liu, X.; Feng, X. An enantioselective Biginelli reaction catalyzed by a simple chiral secondary amine and achiral Brønsted acid by a dual-activation route. Chem. A Eur. J. 2008, 14, 3177–3181. [Google Scholar] [CrossRef]
- Wan, J.-P.; Lin, Y.; Liu, Y. Catalytic asymmetric Biginelli reaction for the enantioselective synthesis of 3, 4-dihydropyrimidinones (DHPMs). Curr. Org. Chem. 2014, 18, 687–699. [Google Scholar] [CrossRef]
- Wang, L.; Qian, C.; Tian, H.; Ma, Y. Lanthanide triflate catalyzed one-pot synthesis of dihydropyrimidin-2 (1 H)-thiones by a three-component of 1, 3-dicarbonyl compounds, aldehydes, and thiourea using a solvent-free Biginelli condensation. Synth. Commun. 2003, 33, 1459–1468. [Google Scholar] [CrossRef]
- Heravi, M.M.; Moradi, R.; Mohammadkhani, L.; Moradi, B. Current progress in asymmetric Biginelli reaction: An update. Mol. Divers. 2018, 22, 751–767. [Google Scholar] [CrossRef]
- Pathak, V.N.; Gupta, R.; Varshney, B. An efficient, inexpensive’Green Chemistry’route to multicomponent Biginelli condensation catalyzed by CuCl2.2H2O-HCl. Indian J. Chem. Sect. B Org. Incl. Med. 2008, 47, 434. [Google Scholar]
- Liu, Z.-L.; Zhang, R.-M.; Liu, Y.; Guo, Y.; Meng, Q.-G. The effects of different catalysts, substituted aromatic aldehydes on one-pot three-component Biginelli reaction. Curr. Org. Synth. 2019, 16, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chopda, L.V.; Dave, P.N. Recent advances in homogeneous and heterogeneous catalyst in Biginelli reaction from 2015-19: A concise review. ChemistrySelect 2020, 5, 5552–5572. [Google Scholar] [CrossRef]
- Shumaila, A.M.; Al-Thulaia, A.A. Mini-review on the synthesis of Biginelli analogs using greener heterogeneous catalysis: Recent strategies with the support or direct catalyzing of inorganic catalysts. Synth. Commun. 2019, 49, 1613–1632. [Google Scholar] [CrossRef]
- Bhatt, B.R.; Dixit, B.C.; Kataria, V.B.; Dixit, R.B.; Saiyad, S. Recent Advances in Biginelli Reaction using Nanoparticles, Zeolites and Metal Compounds as Catalyst: A Concise Review. Lett. Org. Chem. 2024, 21, 821–846. [Google Scholar] [CrossRef]
- Patil, R.V.; Chavan, J.U.; Dalal, D.S.; Shinde, V.S.; Beldar, A.G. Biginelli reaction: Polymer supported catalytic approaches. ACS Comb. Sci. 2019, 21, 105–148. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J.; Sherwood, J. The combined role of catalysis and solvent effects on the Biginelli reaction: Improving efficiency and sustainability. Chem. A Eur. J. 2013, 19, 5174–5182. [Google Scholar] [CrossRef]
- Beck, P.S.; Leitão, A.G.; Santana, Y.B.; Correa, J.R.; Rodrigues, C.V.; Machado, D.F.; Matos, G.D.; Ramos, L.M.; Gatto, C.C.; Oliveira, S.C. Revisiting Biginelli-like reactions: Solvent effects, mechanisms, biological applications and correction of several literature reports. Org. Biomol. Chem. 2024, 22, 3630–3651. [Google Scholar] [CrossRef]
- Costa dos Santos, P.H.; Guimar Souza, V.L.; Carvalho Santos, A.C.; Esteves, H.; Modolo, L.V.; de Fma, N. Synthesis of Biginelli Compounds using Microwave-Assisted Methods. Curr. Microw. Chem. 2023, 10, 70–87. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Bose, A.; Mal, P. Solvent-Free Ball-Milling Biginelli Reaction by Subcomponent Synthesis. Eur. J. Org. Chem. 2015, 2015, 6994–6998. [Google Scholar] [CrossRef]
- Matache, M.; Dobrota, C.; Bogdan, N.D.; Funeriu, D.P. Recent developments in the reactivity of the Biginelli compounds. Curr. Org. Synth. 2011, 8, 356–373. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Creating chemical diversity space by scaffold decoration of dihydropyrimidines. Pure Appl. Chem. 2005, 77, 155–161. [Google Scholar] [CrossRef]
- Wan, J.-P.; Pan, Y. Recent advance in the pharmacology of dihydropyrimidinone. Mini Rev. Med. Chem. 2012, 12, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Ghalehbin, B.; Najafi, S.; Razzaghi-Asl, N. Synthesis and antileishmanial effect of a few cyclic and non-cyclic n-aryl enamino amide derivatives. Res. Pharm. Sci. 2020, 15, 340–349. [Google Scholar] [CrossRef]
- Singh, K.; Singh, S. A mild and practical method for the regioselective synthesis of N-acylated 3, 4-dihydropyrimidin-2-ones. New acyl transfer reagents. Tetrahedron Lett. 2006, 47, 8143–8146. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y.; Yokoe, I. Radical-scavenging activity of butylated hydroxytoluene (BHT) and its metabolites. Chem. Phys. Lipids 2004, 130, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy, M. Antioxidant properties of BHT estimated by ABTS assay in systems differing in pH or metal ion or water concentration. Eur. Food Res. Technol. 2011, 232, 837–842. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.-Q. Ferrocene as a functional group enhances the inhibitive effect of dihydropyrimidine on radical-induced oxidation of DNA. Org. Chem. Front. 2014, 1, 792–797. [Google Scholar] [CrossRef]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546. [Google Scholar] [CrossRef]
- Koshelev, V.N.; Primerova, O.V.; Vorobyev, S.V.; Stupnikova, A.S.; Ivanova, L.V. Synthesis and Antioxidant Activity of Novel Thiazole and Thiazolidinone Derivatives with Phenolic Fragments. Appl. Sci. 2023, 13, 13112. [Google Scholar] [CrossRef]
- Qin, B.; Yang, K.; Cao, R. Synthesis, radical-scavenging activities, and protective effects against AAPH-induced oxidative damage in DNA and erythrocytes of piperine derivatives. J. Chem. 2020, 2020, 9026286. [Google Scholar] [CrossRef]
- Mittal, A.; Vashistha, V.K.; Das, D.K. Recent advances in the antioxidant activity and mechanisms of chalcone derivatives: A computational review. Free. Radic. Res. 2022, 56, 378–397. [Google Scholar] [CrossRef]
- Bhuyan, U.; Handique, J.G. Plant polyphenols as potent antioxidants: Highlighting the mechanism of antioxidant activity and synthesis/development of some polyphenol conjugates. Stud. Nat. Prod. Chem. 2022, 75, 243–266. [Google Scholar] [CrossRef]
- Shamim, S.; Khan, K.M.; Salar, U.; Ali, F.; Lodhi, M.A.; Taha, M.; Khan, F.A.; Ashraf, S.; Ul-Haq, Z.; Ali, M. 5-Acetyl-6-methyl-4-aryl-3, 4-dihydropyrimidin-2 (1H)-ones: As potent urease inhibitors; synthesis, in vitro screening, and molecular modeling study. Bioorganic Chem. 2018, 76, 37–52. [Google Scholar] [CrossRef]
- Liberto, N.A.; de Paiva Silva, S.; de Fatima, A.; Fernandes, S.A. β-Cyclodextrin-assisted synthesis of Biginelli adducts under solvent-free conditions. Tetrahedron 2013, 69, 8245–8249. [Google Scholar] [CrossRef]
- da Silva, D.L.; Reis, F.S.; Muniz, D.R.; Ruiz, A.L.T.; de Carvalho, J.E.; Sabino, A.A.; Modolo, L.V.; de Fátima, Â. Free radical scavenging and antiproliferative properties of Biginelli adducts. Bioorganic Med. Chem. 2012, 20, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy, M. The importance of solvent type in estimating antioxidant properties of phenolic compounds by ABTS assay. Eur. Food Res. Technol. 2013, 236, 1099–1105. [Google Scholar] [CrossRef]
- Işıl Berker, K.; Güçlü, K.; Tor, İ.; Demirata, B.; Apak, R. Total antioxidant capacity assay using optimized ferricyanide/prussian blue method. Food Anal. Methods 2010, 3, 154–168. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Burmistrova, D.A.; Arsenyev, M.V.; Polovinkina, M.A.; Pomortseva, N.P.; Fukin, G.K.; Poddel’sky, A.I.; Berberova, N.T. Synthesis and antioxidant activity of new catechol thioethers with the methylene linker. Molecules 2022, 27, 3169. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Behbahani, F.K.; Marandi, G.B.; Mirza, B. One-pot synthesis of 3, 4-dihydropyrimidin-2 (1 H)-ones, thiones and 2-selenoxo DHPMs using 1-butyl-3-methylimidazolium hydrogen sulfate as non-halogenated ionic liquid. Phosphorus Sulfur Silicon Relat. Elem. 2020, 196, 54–60. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Das, N.; Parvin, M.S.; Hasan, M.; Akter, M.; Hossain, M.S.; Parvez, G.M.; Sarker, A.K.; Rahman, M.A.A.; Mamun, A.; Islam, M.E. A flavone from the ethyl acetate extract of Leea rubra leaves with DNA damage protection and antineoplastic activity. Biochem. Biophys. Rep. 2022, 30, 101244. [Google Scholar] [CrossRef]
- Fuentes-Lemus, E.; Dorta, E.; Escobar, E.; Aspee, A.; Pino, E.; Abasq, M.; Speisky, H.; Silva, E.; Lissi, E.; Davies, M. Oxidation of free, peptide and protein tryptophan residues mediated by AAPH-derived free radicals: Role of alkoxyl and peroxyl radicals. Rsc Adv. 2016, 6, 57948–57955. [Google Scholar] [CrossRef]
- Chen, J.-F.; Liu, Z.-Q. Ferrocenyl-appended aurone and flavone: Which possesses higher inhibitory effects on DNA oxidation and radicals? Chem. Res. Toxicol. 2015, 28, 451–459. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Tang, Y.Z.; Wu, D. Antioxidant effects of phenothiazine, phenoxazine, and iminostilbene on free-radical-induced oxidation of linoleic acid and DNA. J. Phys. Org. Chem. 2009, 22, 1009–1014. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Z.Q. The protective effect of hydroxyl-substituted Schiff bases on the radical-induced oxidation of DNA. J. Phys. Org. Chem. 2009, 22, 791–798. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free radical-induced damage to DNA: Mechanisms and measurement. Free. Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).