Amberlite XAD-4 Functionalized with 4-(2-Pyridylazo) Resorcinol via Aryldiazonium Chemistry for Efficient Solid-Phase Extraction of Trace Metals from Groundwater Samples
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of PAR-XAD-4 Chelating Resin
2.3. Sorption Studies and Solid-Phase Extraction Procedure
2.3.1. Sorption Experiments
2.3.2. Solid-Phase Extraction Protocol
2.4. Instrumentation and Measurements
3. Results and Discussion
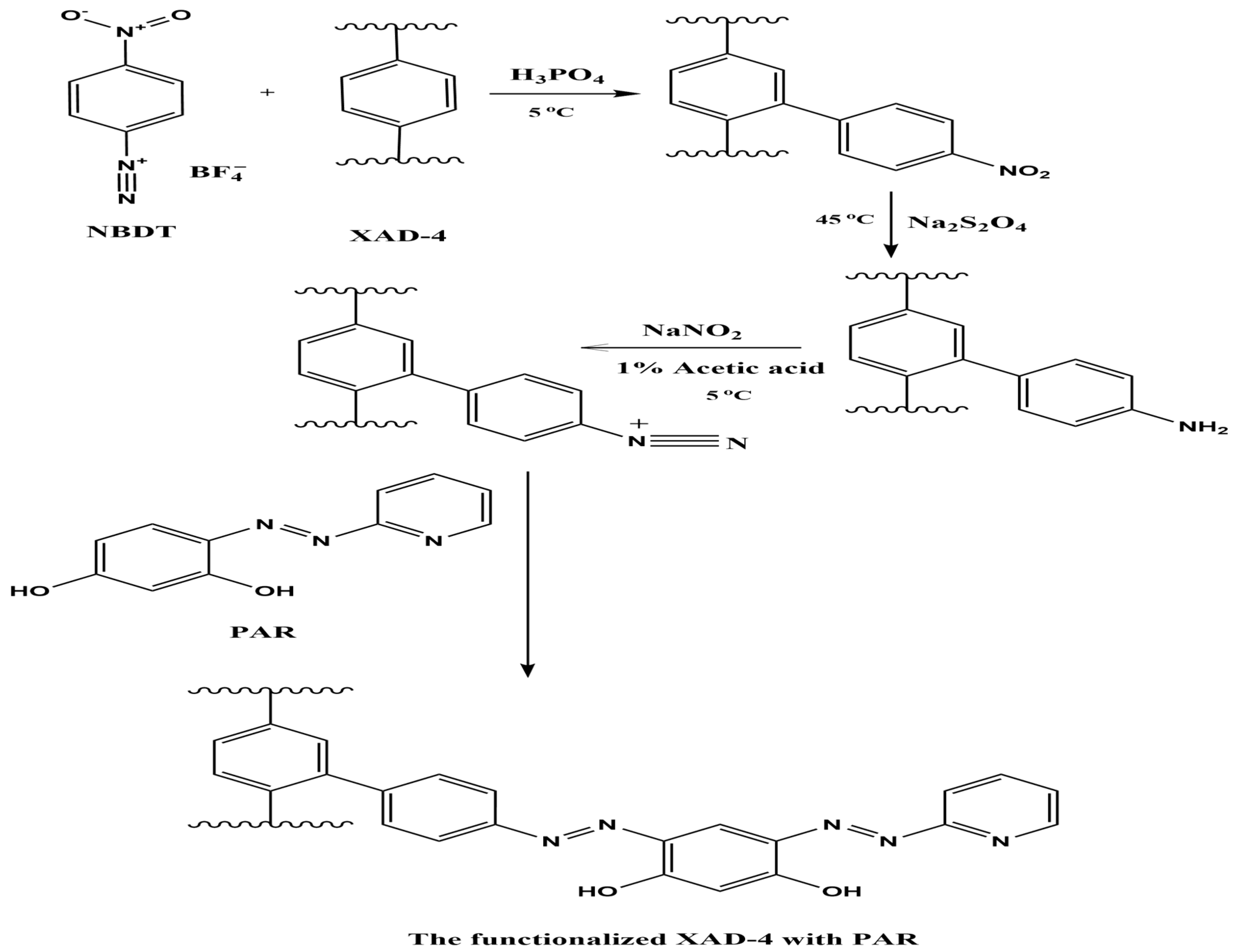
3.1. Chemical Modification Process
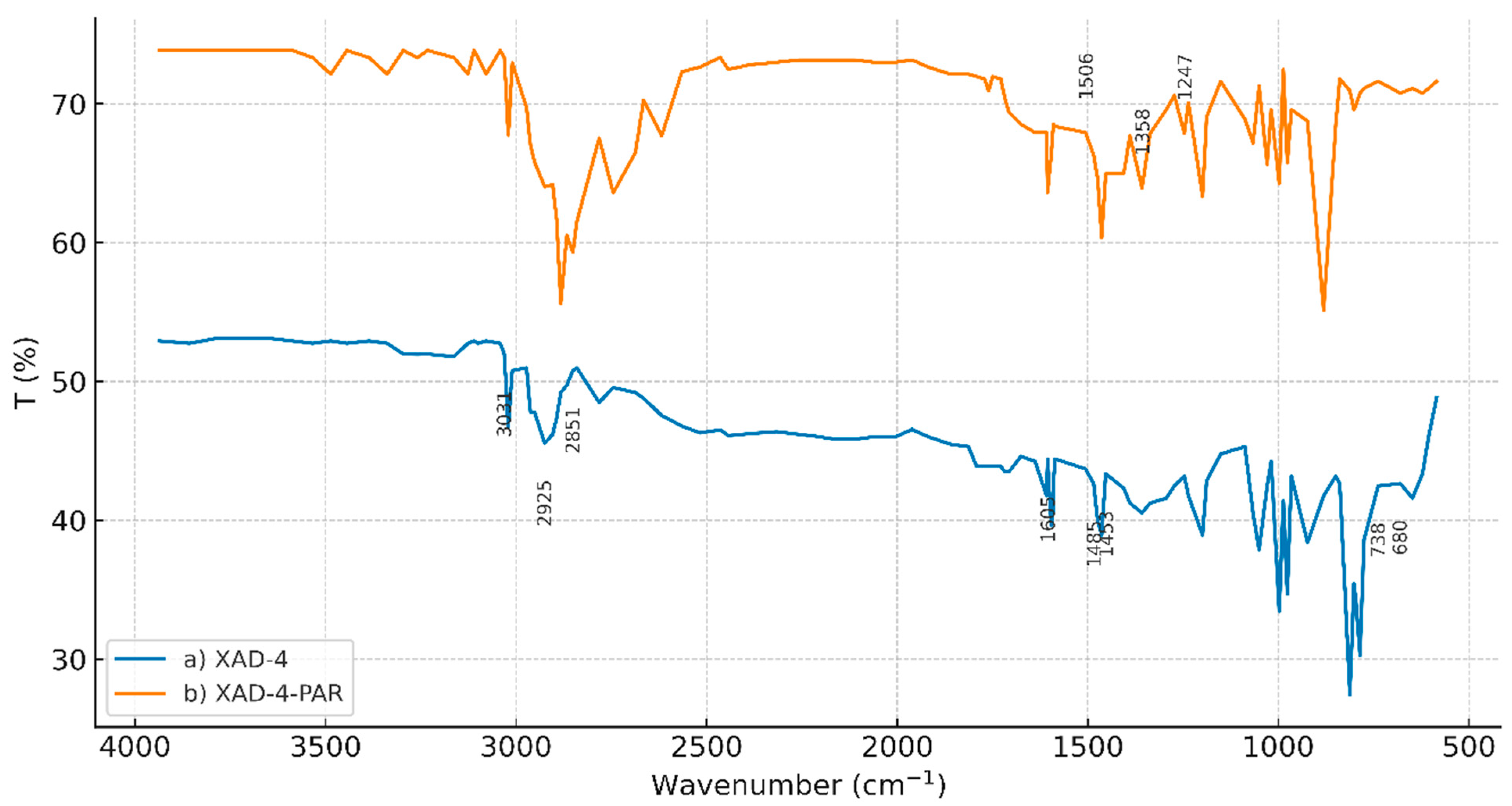
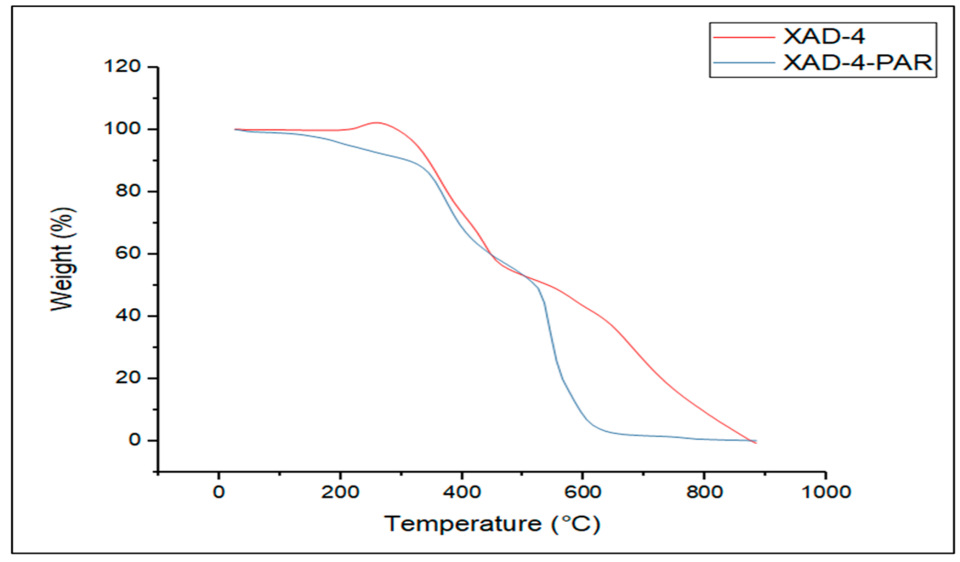
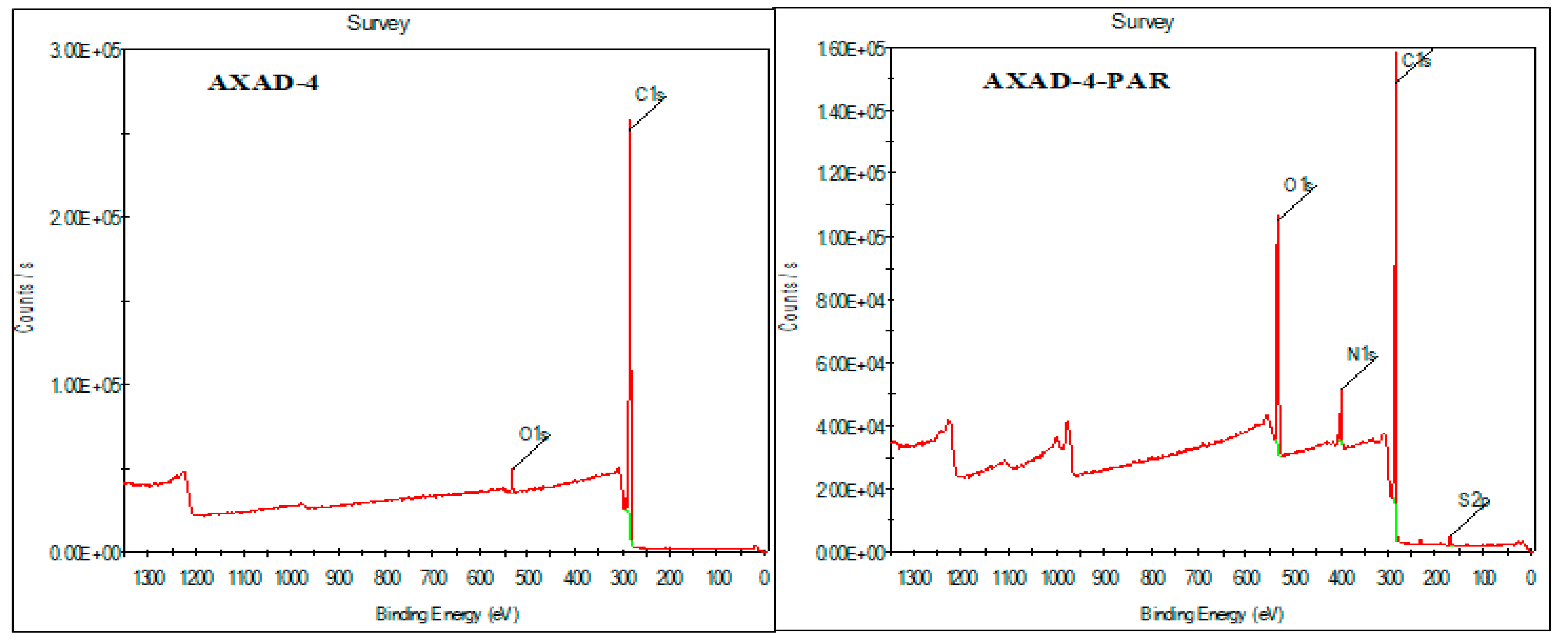
3.2. Characterization of AXAD-4-PAR Chelating Resin
3.3. Optimization of Sorption Process
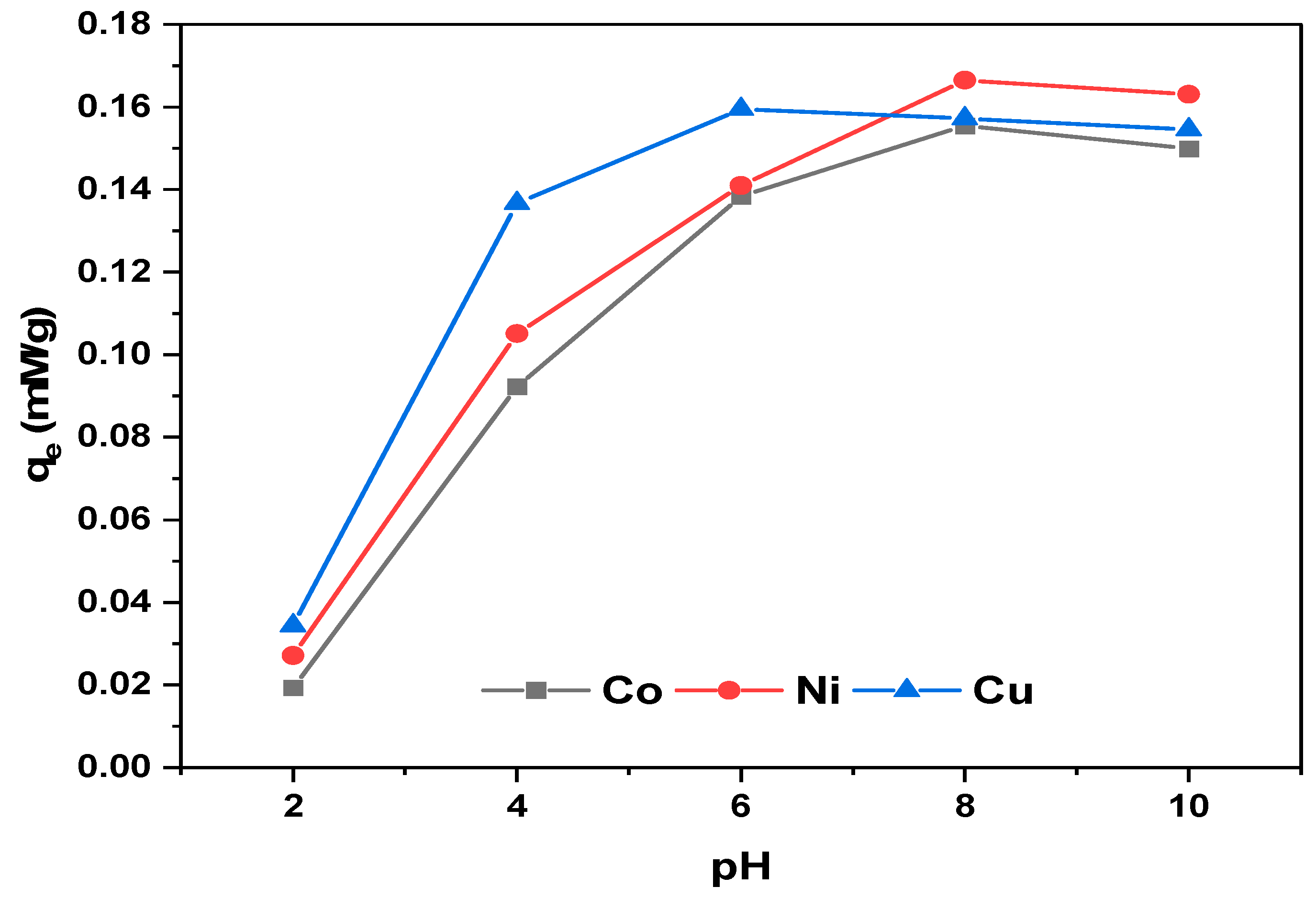
3.3.1. Effect of pH
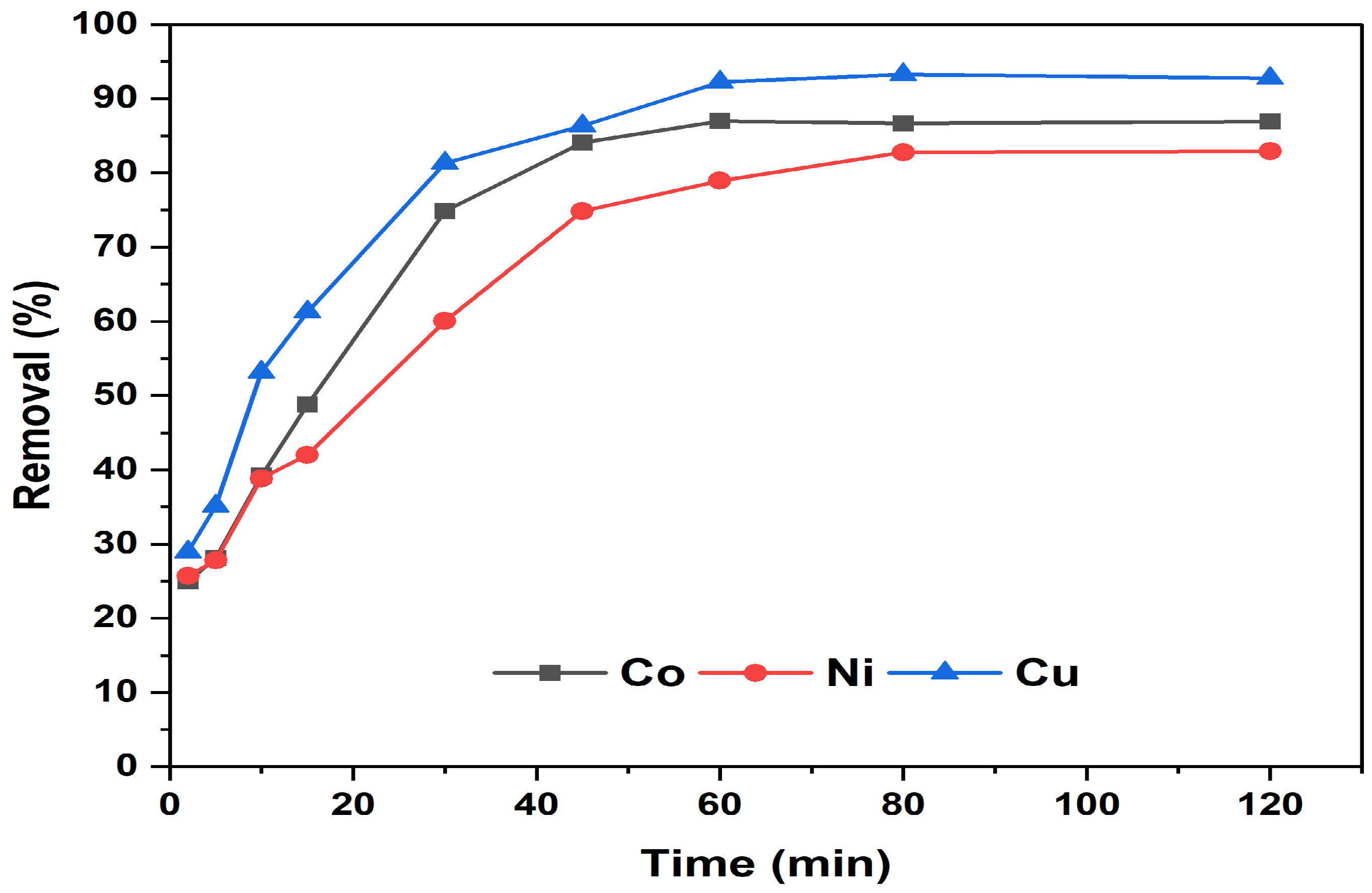
3.3.2. Effect of Contact Time
3.4. Sorption Capacity
3.5. Analytical Figure of Merits
3.6. Method Validation
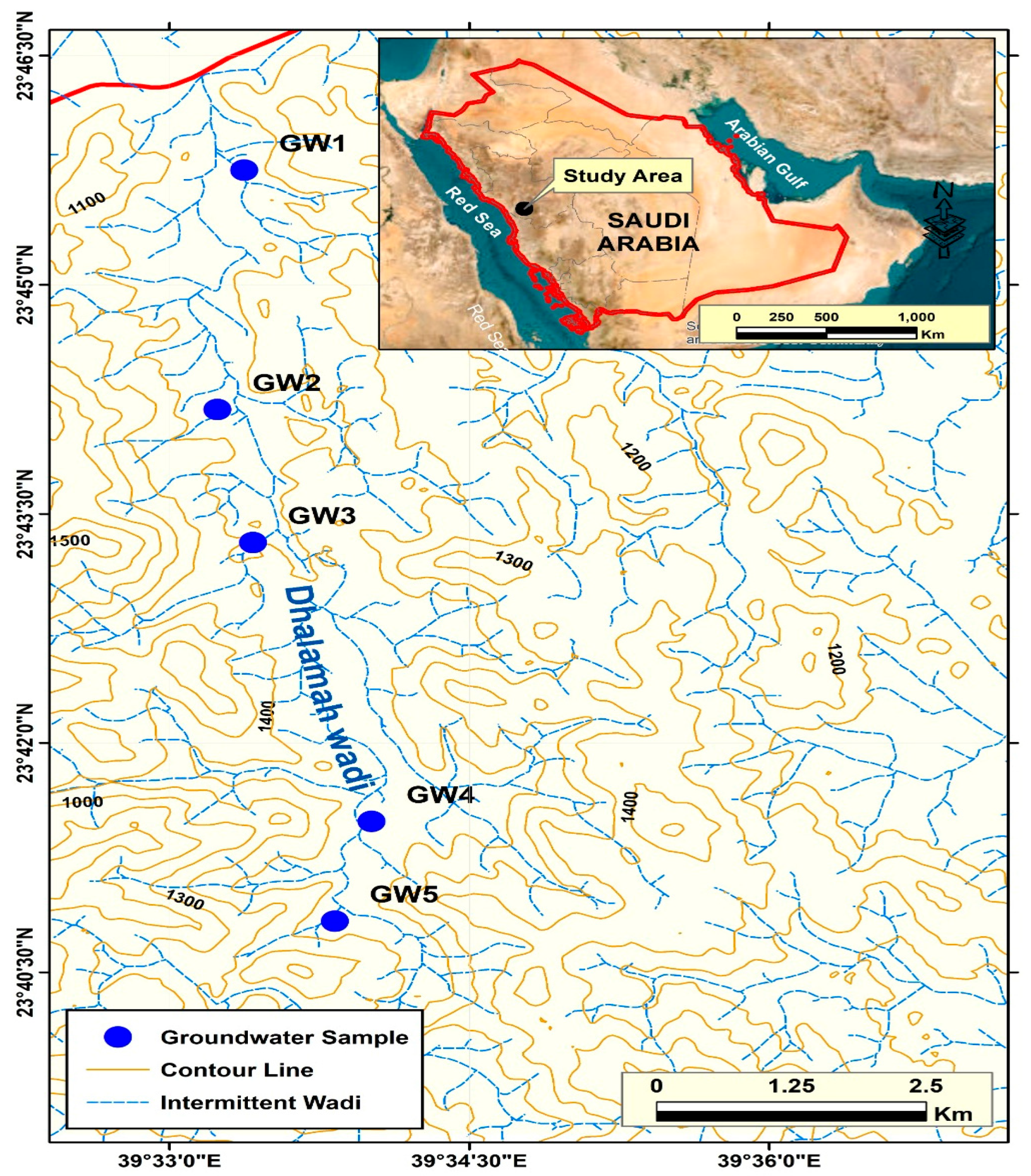
3.7. Application to Groundwater Samples
3.8. Resin Stability and Reusability
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kratochvil, B. Sampling and Sample Preservation for Trace Element Analysis. Compr. Anal. Chem. 2003, 41, 1–21. [Google Scholar] [CrossRef]
- Hu, B.; He, M. Pre-Concentration and Sample Treatment Techniques for Trace Element Analysis. Compr. Sampl. Sample Prep. Anal. Tech. Sci. 2012, 3, 365–394. [Google Scholar] [CrossRef]
- Kebbekus, B.B. Preparation of Samples for Metals Analysis. In Sample Preparation Techniques in Analytical Chemistry; Mitra, S., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Volume 162, pp. 227–270. ISBN 0471328456. [Google Scholar]
- Mendil, D.; Karatas, M.; Tuzen, M. Separation and Preconcentration of Cu(II), Pb(II), Zn(II), Fe(III) and Cr(III) Ions with Coprecipitation Method without Carrier Element and Their Determination in Food and Water Samples. Food Chem. 2015, 177, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective Removal of the Heavy Metal Ions from Waters and Industrial Wastewaters by Ion-Exchange Method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Todd, M.; Atkinson, J.W.; Scott, K. Selective Electrodeposition of Metals from Simulated Waste Solutions. J. Appl. Electrochem. 1996, 26, 379–384. [Google Scholar] [CrossRef]
- Shemirani, F.; Yousefi, S.R. Selective Extraction and Preconcentration of Cerium(IV) in Water Samples by Cloud Point Extraction and Determination by Inductively Coupled Plasma Optical Emission Spectrometry. Microchim. Acta 2007, 157, 223–227. [Google Scholar] [CrossRef]
- Soylak, M.; Divrikli, U.; Saracoglu, S.; Elci, L. Membrane Filtration—Atomic Absorption Spectrometry Combination for Copper, Cobalt, Cadmium, Lead and Chromium in Environmental Samples. Environ. Monit. Assess. 2007, 127, 169–176. [Google Scholar] [CrossRef]
- Camel, V. Solid Phase Extraction of Trace Elements. Spectrochim. Acta Part B At Spectrosc. 2003, 58, 1177–1233. [Google Scholar] [CrossRef]
- Okamoto, Y.; Nomura, Y.; Nakamura, H.; Iwamaru, K.; Fujiwara, T.; Kumamaru, T. High Preconcentration of Ultra-Trace Metal Ions by Liquid–Liquid Extraction Using Water/Oil/Water Emulsions as Liquid Surfactant Membranes. Microchem. J. 2000, 65, 341–346. [Google Scholar] [CrossRef]
- Julian Haryanto, M.; Zhang, J.; Kagaya, S.; Horikawa, K.; Shamsun Nahar, M. Preconcentration Strategies for Trace Metals Including REEs in Seawater and Porewater by Employing Commercial Chelating Resin—A Review. Microchem. J. 2024, 206, 111526. [Google Scholar] [CrossRef]
- Liška, I. Fifty Years of Solid-Phase Extraction in Water Analysis—Historical Development and Overview. J. Chromatogr. A 2000, 885, 3–16. [Google Scholar] [CrossRef]
- Garg, B.S.; Sharma, R.K.; Bhojak, N.; Mittal, S. Chelating Resins and Their Applications in the Analysis of Trace Metal Ions. Microchem. J. 1999, 61, 94–114. [Google Scholar] [CrossRef]
- Schmuckler, G. Chelating Resins—Their Analytical Properties and Applications. Talanta 1965, 12, 281–290. [Google Scholar] [CrossRef]
- Chu, S.; Feng, X.; Liu, C.; Wu, H.; Liu, X. Advances in Chelating Resins for Adsorption of Heavy Metal Ions. Ind. Eng. Chem. Res. 2022, 61, 11309–11328. [Google Scholar] [CrossRef]
- Uzun, A.; Soylak, M.; Elçi, L. Preconcentration and Separation with Amberlite XAD-4 Resin; Determination of Cu, Fe, Pb, Ni, Cd and Bi at Trace Levels in Waste Water Samples by Flame Atomic Absorption Spectrometry. Talanta 2001, 54, 197–202. [Google Scholar] [CrossRef]
- Ramesh, A.; Rama Mohan, K.; Seshaiah, K. Preconcentration of Trace Metals on Amberlite XAD-4 Resin Coated with Dithiocarbamates and Determination by Inductively Coupled Plasma-Atomic Emission Spectrometry in Saline Matrices. Talanta 2002, 57, 243–252. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Yılmaz, V.; Kartal, Ş. Solid Phase Extraction of Cu(II), Ni(II), Pb(II), Cd(II) and Mn(II) Ions with 1-(2-Thiazolylazo)-2-Naphthol Loaded Amberlite XAD-1180. Environ. Monit. Assess. 2009, 152, 369–377. [Google Scholar] [CrossRef]
- Ahmad, A.; Siddique, J.A.; Laskar, M.A.; Kumar, R.; Mohd-Setapar, S.H.; Khatoon, A.; Shiekh, R.A. New Generation Amberlite XAD Resin for the Removal of Metal Ions: A Review. J. Environ. Sci. 2015, 31, 104–123. [Google Scholar] [CrossRef]
- Cheira, M.F. Synthesis of Pyridylazo Resorcinol—Functionalized Amberlite XAD-16 and Its Characteristics for Uranium Recovery. J. Environ. Chem. Eng. 2015, 3, 642–652. [Google Scholar] [CrossRef]
- Metilda, P.; Sanghamitra, K.; Mary Gladis, J.; Naidu, G.R.K.; Prasada Rao, T. Amberlite XAD-4 Functionalized with Succinic Acid for the Solid Phase Extractive Preconcentration and Separation of Uranium(VI). Talanta 2005, 65, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rajesh, N. Augmenting the Adsorption of Palladium from Spent Catalyst Using a Thiazole Ligand Tethered on an Amine Functionalized Polymeric Resin. Chem. Eng. J. 2016, 283, 999–1008. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Abdel-Salam, A.H.; Khalil, T.E. The Impact of an Amberlite XAD-16-Based Chelating Resin for the Removal of Aqueous Cd(II) and Pb(II)Ions. Microchem. J. 2021, 165, 106097. [Google Scholar] [CrossRef]
- Meena, P.L.; Saxena, R. Applications of Amberlite XAD Based Chelating Resins in Online Pre Concentration of Metal Ions. IOSR J. Appl. Chem. 2017, 10, 44–54. [Google Scholar] [CrossRef]
- Jung, K.-H.; Byun, J.-H.; Lee, Y.-S.; Park, S.-J. Synthesis and Characterization of Chemically Modified Polystyrene as Processable Carbon Fiber Precursors. Res. Chem. Intermed. 2010, 36, 621–627. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, X.; Zhong, S.; Yi, W.-J.; Li, L.-J. A New Chloromethylation Method Based on Polystyrene–Divinylbenzene. Chem. Pap. 2019, 73, 2183–2188. [Google Scholar] [CrossRef]
- Jamil, W.; Memon, Z.; Memon, S.Q.; Samon, M.K.; Taha, M.; Khan, K.M. Environmental Friendly Synthetic Modification of Amberlite XAD-2 Resin for the Removal of Highly Toxic Hexavalent Chromium from Water. Acta Chim. Slov. 2020, 67, 260–269. [Google Scholar] [CrossRef]
- Deniau, G.; Azoulay, L.; Bougerolles, L.; Palacin, S. Surface Electroinitiated Emulsion Polymerization: Grafted Organic Coatings from Aqueous Solutions. Chem. Mater. 2006, 18, 5421–5428. [Google Scholar] [CrossRef]
- Chehimi, M.M.; Lamouri, A.; Picot, M.; Pinson, J. Surface Modification of Polymers by Reduction of Diazonium Salts: Polymethylmethacrylate as an Example. J. Mater. Chem. C Mater. 2014, 2, 356–363. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Leventis, H.C.; Davies, I.J.; Crossley, A.; Lawrence, N.S.; Jiang, L.; Jones, T.G.J.; Compton, R.G. Graphite Powder Derivatised with Poly-L-Cysteine Using “Building-Block” Chemistry—A Novel Material for the Extraction of Heavy Metal Ions. J. Mater. Chem. 2005, 15, 2375–2382. [Google Scholar] [CrossRef]
- Fioresi, F.; Vieillard, J.; Bargougui, R.; Bouazizi, N.; Fotsing, P.N.; Woumfo, E.D.; Brun, N.; Mofaddel, N.; Le Derf, F. Chemical Modification of the Cocoa Shell Surface Using Diazonium Salts. J. Colloid. Interface Sci. 2017, 494, 92–97. [Google Scholar] [CrossRef]
- Sandomierski, M.; Voelkel, A. Diazonium Modification of Inorganic and Organic Fillers for the Design of Robust Composites: A Review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1–21. [Google Scholar] [CrossRef]
- Li, D.; Luo, Y.; Onidas, D.; He, L.; Jin, M.; Gazeau, F.; Pinson, J.; Mangeney, C. Surface Functionalization of Nanomaterials by Aryl Diazonium Salts for Biomedical Sciences. Adv. Colloid Interface Sci. 2021, 294, 102479. [Google Scholar] [CrossRef]
- AlQadhi, N.F.; AlSuhaimi, A.O. Chemically Functionalized Activated Carbon with 8-Hydroxyquinoline Using Aryldiazonium Salts/Diazotization Route: Green Chemistry Synthesis for Oxins-Carbon Chelators. Arab. J. Chem. 2020, 13, 1386–1396. [Google Scholar] [CrossRef]
- Alharbi, H.N.; Alsuhaimi, A.O. Functionalization of Amberlite XAD-4 with 8-Hydroxyquinoline Chelator Using Aryldiazonium Radical Reaction and Its Application for Solid Phase Extraction of Trace Metals from Groundwater Samples. Glob. Nest J. 2020, 22, 306–314. [Google Scholar] [CrossRef]
- Ghasemi, J.; Peyman, H.; Meloun, M. Study of Complex Formation between 4-(2-Pyridylazo) Resorcinol and Al3+, Fe3+, Zn2+, and Cd2+ Ions in an Aqueous Solution at 0.1 M Ionic Strength. J. Chem. Eng. Data 2007, 52, 1171–1178. [Google Scholar] [CrossRef]
- Gaikwad, A.G.; Noguchi, H.; Yoshio, M. Solvent Extraction Studies of Metal-4-(2-Pyridyl-Azo)-Resorcinol Complexes with Potassium-Dicyclohexyl-18-Crown-6 Complex. Anal. Lett. 1991, 24, 1625–1641. [Google Scholar] [CrossRef]
- Malcik, N.; Oktar, O.; Ozser, M.E.; Caglar, P.; Bushby, L.; Vaughan, A.; Kuswandi, B.; Narayanaswamy, R. Immobilised Reagents for Optical Heavy Metal Ions Sensing. Sens. Actuators B Chem. 1998, 53, 211–221. [Google Scholar] [CrossRef]
- Asgharinezhad, A.A.; Ebrahimzadeh, H.; Rezvani, M.; Shekari, N.; Loni, M. A Novel 4-(2-Pyridylazo) Resorcinol Functionalised Magnetic Nanosorbent for Selective Extraction of Cu(II) and Pb(II) Ions from Food and Water Samples. Food Addit. Contam. Part A 2014, 31, 1196–1204. [Google Scholar] [CrossRef]
- Yanovska, E.S.; Tertykh, V.A.; Kichkiruk, O.Y.; Dadashev, A.D. Adsorption and Complexing Properties of Silicas with Analytical Reagents Grafted via the Mannich Reaction. Adsor. Sci. Tech. 2007, 25, 81–87. [Google Scholar] [CrossRef]
- Alsuhaimi, A.O. Preparation of Silica-4-(2-Pyridylazo) Resorcinol Chelator for Solid Phase Extraction of Transition Metals from Groundwater. J. Chem. Soc. Pak. 2019, 41, 151–160. [Google Scholar] [CrossRef]
- Asghari, A.; Farzinia, H.; Rajabi, M.; Ghaedi, M. Combination of Solid-Phase Extraction and Flame Atomic Absorption Spectrometry for Simultaneous Preconcentration and Determination of Some Heavy Metals in Real Samples. Desalination Water Treat 2014, 52, 5430–5441. [Google Scholar] [CrossRef]
- Kempegowda, R.G.; Malingappa, P. Diazonium Functionalized Exfoliated Graphitic Carbon as a Binderless and Covalently Modified Electrochemical Interface for Mercury Sensing. Sens. Actuators B Chem. 2013, 186, 478–485. [Google Scholar] [CrossRef]
- Scheuerman, R.A.; Tumelty, D. The Reduction of Aromatic Nitro Groups on Solid Supports Using Sodium Hydrosulfite (Na2S2O4). Tetrahedron Lett. 2000, 41, 6531–6535. [Google Scholar] [CrossRef]
- Qafoku, N.P.; Lawter, A.R.; Shao, H.; Wang, G.; Brown, C.F. Evaluating Impacts of CO2 Gas Intrusion into a Confined Sandstone Aquifer: Experimental Results. Energy Procedia 2014, 63, 3275–3284. [Google Scholar] [CrossRef]
- Wang, D.; Khan, M.K.; Moloney, M.G. Diazo and Diazonium Compounds for Surface Modification. Tetrahedron Lett. 2020, 61, 151672. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A Powerful Method for Surface Modification. Chem. Soc. Rev. 2011, 40, 3995. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectroscopy of Polymers, IX: Pendant Ester Polymers and Polycarbonates. Spectroscopy 2022, 37, 16–19,31. [Google Scholar] [CrossRef]
- Karipcin, F.; Kabalcilar, E. Spectroscopic and Thermal Studies on Solid Complexes of 4-(2-pyridylazo)resorcinol with Some Transition Metals. Acta Chim. Slov. 2007, 54, 242–247. [Google Scholar]
- Kosa, S.A.; Al-Zhrani, G.; Abdel Salam, M. Removal of Heavy Metals from Aqueous Solutions by Multi-Walled Carbon Nanotubes Modified with 8-Hydroxyquinoline. Chem. Eng. J. 2012, 181–182, 159–168. [Google Scholar] [CrossRef]
- Kocaoba, S. Determination of Some Heavy Metals from Aqueous Solutions Using Modified Amberlite XAD-4 Resin by Selective Solid-Phase Extraction. J. Anal. Sci. Technol. 2022, 13, 15. [Google Scholar] [CrossRef]
- Yamada, S.; Taki, N. Kinetic and Thermodynamic Effects on the Colour Reactions of Niobium 4-(2-Pyridylazo)Resorcinol of Auxiliary Complexing and Tantalum with Agents. Anal. Sci. 1990, 6, 567–572. [Google Scholar] [CrossRef]
- Diniz, C.V.; Doyle, F.M.; Ciminelli, V.S.T. Effect of PH on the Adsorption of Selected Heavy Metal Ions from Concentrated Chloride Solutions by the Chelating Resin Dowex M-4195. Sep. Sci. Technol. 2002, 37, 3169–3185. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Xie, F.; Lin, X.; Wu, X.; Xie, Z. Solid Phase Extraction of Lead (II), Copper (II), Cadmium (II) and Nickel (II) Using Gallic Acid-Modified Silica Gel Prior to Determination by Flame Atomic Absorption Spectrometry. Talanta 2008, 74, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F.; Gunatilleka, A.D.; Sethuraman, R. Contributions of Theory to Method Development in Solid-Phase Extraction. J. Chromatogr. A 2000, 885, 17–39. [Google Scholar] [CrossRef]
- AlSuhaimi, A.O.; AlMohaimadi, K.M.; AlAlawi, B.N.; Ali, I. A Novel Porous Silica Monolith Functionalized with 5-Amino-1,10-Phenanthroline for SPE of Metal Ions in Groundwater Samples Prior to Their Analysis Using ICP-MS. Anal. Methods 2018, 10, 2337–2346. [Google Scholar] [CrossRef]
- Labban, T.A.; AlMohaimdi, K.M.; AlAhmadi, S.; Shaikh Ishaqe, M.; AlSuhaimi, A.O. Synthesis of 8-Hydroxyquinoline-Amberlite IRC-50 Chelator for Solid Phase Extraction of Trace Metals from Groundwater Samples. J. Taibah Univ. Sci. 2020, 14, 697–708. [Google Scholar] [CrossRef]
- Bernard, J.; Branger, C.; Nguyen, T.L.A.; Denoyel, R.; Margaillan, A. Synthesis and Characterization of a Polystyrenic Resin Functionalized by Catechol: Application to Retention of Metal Ions. React. Funct. Polym. 2008, 68, 1362–1370. [Google Scholar] [CrossRef]
| Sorbent Support | Ions | Reference | ||
|---|---|---|---|---|
| Co(II) | Ni(II) | Cu(II) | ||
| AXAD-4-PAR | 0.152 | 0.167 | 0.172 | (this study) |
| PAR-Silica | nd | 0.121 | 0.132 | [41] |
| PAR-impregnated AC | 0.015 | 0.023 | 0.007 | [42] |
| PAR-Magnetic nano sorbent | nd | nd | 0.07 | [39] |
| Calibration Parameters | Metal Ions (ng/mL) | ||
|---|---|---|---|
| Co | Ni | Cu | |
| Conc. range (ng mL−1) (n = 4) | 0–15 | 0–15 | 0–20 |
| RSD at 2 ng mL−1 (n = 4) | 1.13 | 1.15 | 1.78 |
| RSD at 10 ng mL−1 (n = 4) | 1.61 | 1.07 | 2.21 |
| Correlation coefficient, R2 | 0.997 | 0.998 | 0.999 |
| Sensitivity, CPS ratio/ng mL−1 | 20038.0 | 283.95 | 3144.1 |
| LOD/ng mL−1 | 0.053 | 0.012 | 0.061 |
| CRM | Metals (Concentration in µg/L) | |||||
|---|---|---|---|---|---|---|
| Co | Cu | Ni | ||||
| Found | Certified | Found | Certified | Found | Certified | |
| SLRS-4 | 0.032 ± 0.005 | 0.033 ± 0.006 | 1.79 ± 0.08 | 1.81 ± 0.08 | 0.64 ± 0.07 | 0.67 ± 0.08 |
| Recovery (%) | 96.90% | 98.89% | 95.52% | |||
| Sample ID | Metal Concentrations (Recovery %) | ||
|---|---|---|---|
| Co(II) | Ni(II) | Cu(II) | |
| G1 | 1.07 ± 1.08 | 0.89 ± 0.03 | 2.00 ± 0.29 |
| G1 Spike | 6.48 ± 1.27 (108.2%) | 6.13 ± 1.03 (104.8%) | 7.22 ± 1.16 (104.4%) |
| G2 | 1.30 ± 0.44 | 1.05 ± 1.10 | 2.25 ± 0.53 |
| G2 Spike | 6.53 ± 1.18 (104.6%) | 6.14 ± 1.28 (101.8%) | 7.09 ± 1.73 (96.8%) |
| G3 | 1.23 ± 1.41 | 1.05 ± 1.11 | 1.96 ± 0.21 |
| G3 Spike | 6.35 ± 1.45 (102.4%) | 6.12 ± 1.19 (101.4%) | 7.62 ± 1.15(113.2%) |
| G4 | 1.15 ± 1.01 | 1.06 ± 0.02 | 1.98 ± 0.87 |
| G4 Spike | 6.33 ± 1.27 (103.6%) | 6.13 ± 1.28 (101.4%) | 7.34 ± 1.24(107.2%) |
| G5 | 1.13 ± 1.02 | 1.09 ± 1.12 | 2.00 ± 0.29 |
| G5 Spike | 6.37 ± 1.18 (104.8%) | 5.99 ± 1.29 (98%) | 7.33 ± 1.16 (106.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSuhaimi, A.O. Amberlite XAD-4 Functionalized with 4-(2-Pyridylazo) Resorcinol via Aryldiazonium Chemistry for Efficient Solid-Phase Extraction of Trace Metals from Groundwater Samples. Appl. Sci. 2025, 15, 9044. https://doi.org/10.3390/app15169044
AlSuhaimi AO. Amberlite XAD-4 Functionalized with 4-(2-Pyridylazo) Resorcinol via Aryldiazonium Chemistry for Efficient Solid-Phase Extraction of Trace Metals from Groundwater Samples. Applied Sciences. 2025; 15(16):9044. https://doi.org/10.3390/app15169044
Chicago/Turabian StyleAlSuhaimi, Awadh O. 2025. "Amberlite XAD-4 Functionalized with 4-(2-Pyridylazo) Resorcinol via Aryldiazonium Chemistry for Efficient Solid-Phase Extraction of Trace Metals from Groundwater Samples" Applied Sciences 15, no. 16: 9044. https://doi.org/10.3390/app15169044
APA StyleAlSuhaimi, A. O. (2025). Amberlite XAD-4 Functionalized with 4-(2-Pyridylazo) Resorcinol via Aryldiazonium Chemistry for Efficient Solid-Phase Extraction of Trace Metals from Groundwater Samples. Applied Sciences, 15(16), 9044. https://doi.org/10.3390/app15169044






