A Flexible Frequency-Coded Electromagnetic Sensing Array for Contactless Biological Tissues Health Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Background
2.2. Numerical Design
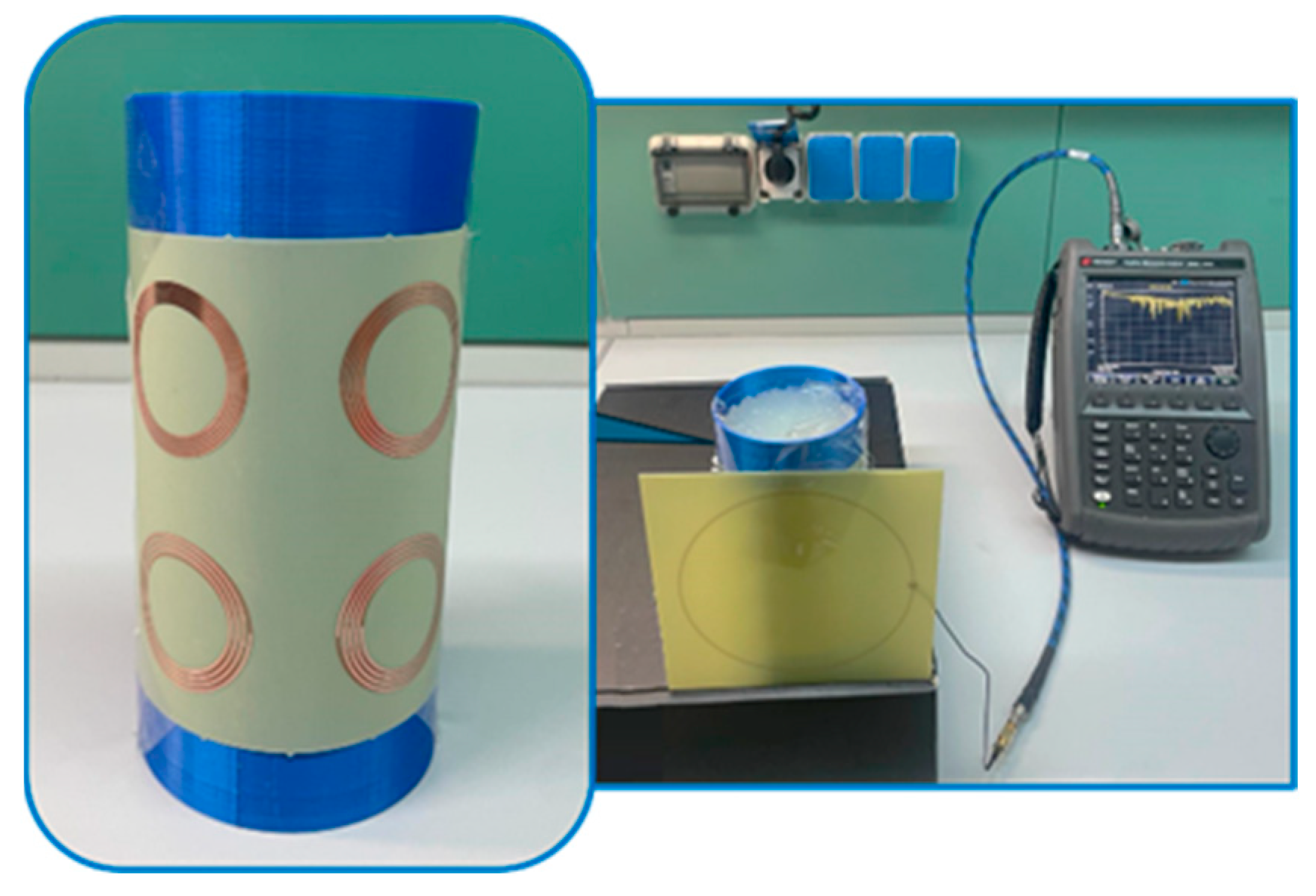
2.3. Experimental Fabrication
2.4. Homogeneous Phantom Fabrication and Dielectric Characterization
3. Numerical and Experimental Results
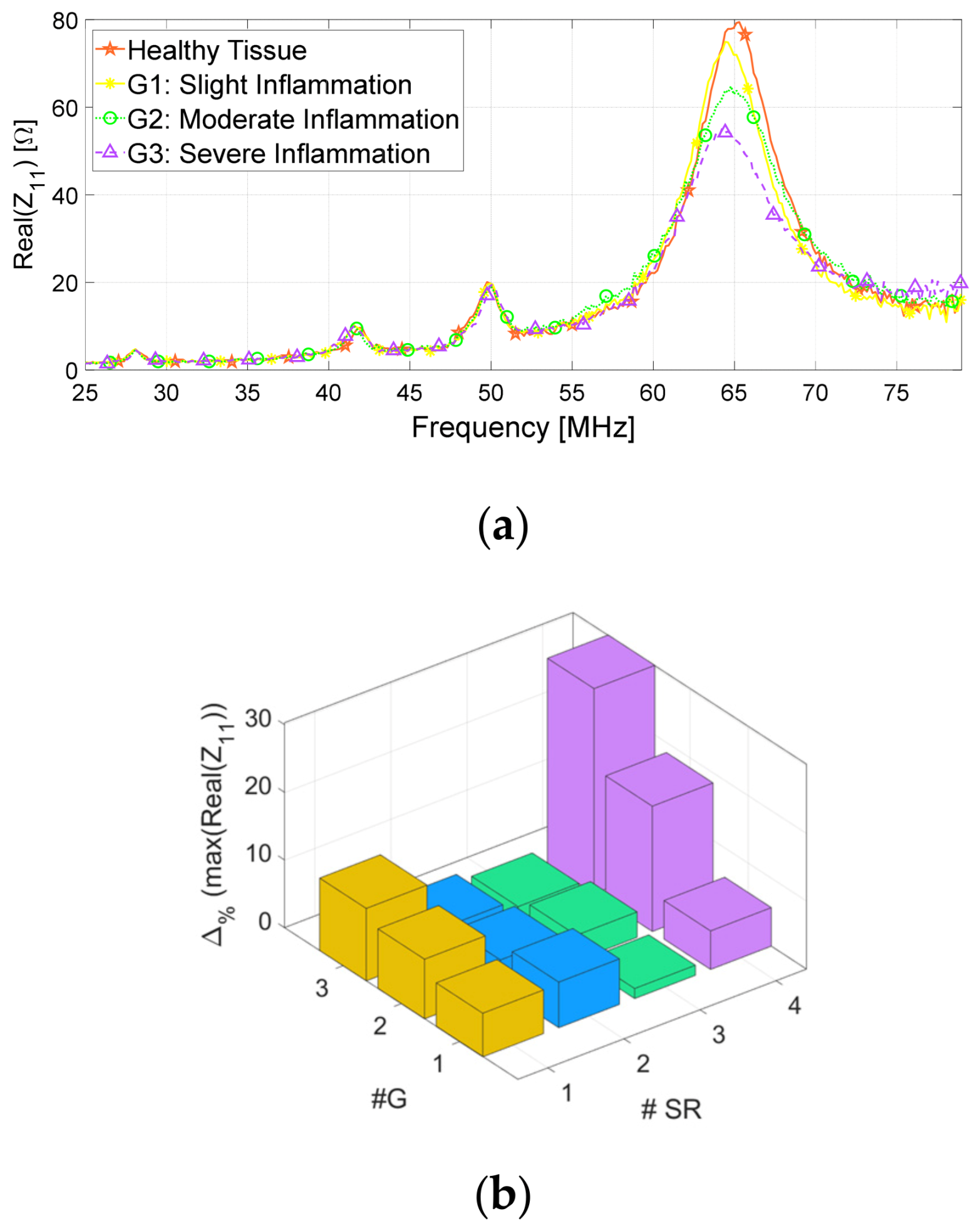
3.1. Numerical Simulations
3.2. Experimental Characterization
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD | Computer-Aided Design |
| PCB | Printed Circuit Board |
| PLA | Poly-Lactic Acid |
| RF | Radiofrequency |
| SMA | Sub-Miniature version A |
| SR | Spiral Resonator |
| VNA | Vector Network Analyzer |
References
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, S.; Khosravani, M.R. Progress and challenges in fabrication of wearable sensors for health monitoring. Sens. Actuators A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Sim, I. Mobile Devices and Health. N. Engl. J. Med. 2019, 381, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Chen, I.-M.; Luo, Z.; Yeo, S.H.; Duh, H.B.-L. A Wearable Sensing System for Tracking and Monitoring of Functional Arm Movement. IEEE/ASME Trans. Mechatron. 2010, 16, 213–220. [Google Scholar] [CrossRef]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Steinberg, I.M. Smart bandage with wireless connectivity for optical monitoring of pH. Sens. Actuators B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Mostafalu, P.; Lenk, W.; Dokmeci, M.R.; Ziaie, B.; Khademhosseini, A.; Sonkusale, S.R. Wireless Flexible Smart Bandage for Continuous Monitoring of Wound Oxygenation. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 670–677. [Google Scholar] [CrossRef]
- Ding, X.-R.; Clifton, D.; Ji, N.; Lovell, N.H.; Bonato, P.; Chen, W.; Yu, X.; Xue, Z.; Xiang, T.; Long, X.; et al. Wearable Sensing and Telehealth Technology with Potential Applications in the Coronavirus Pandemic. IEEE Rev. Biomed. Eng. 2020, 14, 48–70. [Google Scholar] [CrossRef]
- Devi, P.D.; Ilakiya, S. A Secure Employee Health Management System Using Werable Technology. In Proceedings of the 2021 International Conference on System, Computation, Automation and Networking (ICSCAN), Puducherry, India, 30–31 July 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Pal, A.; Goswami, D.; Cuellar, H.E.; Castro, B.; Kuang, S.; Martinez, R.V. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 2018, 117, 696–705. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Zehra, N.; Choudhury, A.; Iyer, P.K. Wearable Sensing Devices for Point of Care Diagnostics. ACS Appl. Bio Mater. 2020, 4, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ali, S.; Bermak, A. Recent Developments in Printing Flexible and Wearable Sensing Electronics for Healthcare Applications. Sensors 2019, 19, 1230. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 28949. [Google Scholar] [CrossRef] [PubMed]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Human-Interactive, Health-Monitoring, Wireless Devices Fabricated by Macroscale Printing Techniques. Adv. Funct. Mater. 2014, 24, 3299–3304. [Google Scholar] [CrossRef]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Rahimi, R.; Brener, U.; Chittiboyina, S.; Soleimani, T.; Detwiler, D.A.; Lelièvre, S.A.; Ziaie, B. Laser-enabled fabrication of flexible and transparent pH sensor with near-field communication for in-situ monitoring of wound infection. Sens. Actuators B Chem. 2018, 267, 198–207. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Wang, J.; Luo, X.; Xie, L.; Zhan, S.; Kim, J.; Wang, X.; Liu, X.; Ying, Y. Wearable plasmonic-metasurface sensor for noninvasive and universal molecular fingerprint detection on biointerfaces. Sci. Adv. 2021, 7, eabe4553. [Google Scholar] [CrossRef]
- Ghaffari, R.; Yang, D.S.; Kim, J.; Mansour, A.; Wright, J.A.; Model, J.B.; Wright, D.E.; Rogers, J.A.; Ray, T.R. State of Sweat: Emerging Wearable Systems for Real-Time, Noninvasive Sweat Sensing and Analytics. ACS Sens. 2021, 6, 2787–2801. [Google Scholar] [CrossRef]
- Escobedo, P.; Bhattacharjee, M.; Nikbakhtnasrabadi, F.; Dahiya, R. Smart Bandage with Wireless Strain and Temperature Sensors and Batteryless NFC Tag. IEEE Internet Things J. 2020, 8, 5093–5100. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Vardaxoglou, J.Y.; Whittow, W. A Metasurfaces Review: Definitions and Applications. Appl. Sci. 2019, 9, 2727. [Google Scholar] [CrossRef]
- Zhang, S.; Wong, C.L.; Zeng, S.; Bi, R.; Tai, K.; Dholakia, K.; Olivo, M. Metasurfaces for biomedical applications: Imaging and sensing from a nanophotonics perspective. Nanophotonics 2020, 10, 259–293. [Google Scholar] [CrossRef]
- Alsaedi, D.; El Badawe, M.; Ramahi, O.M. A Metasurface for Biomedical Imaging Applications. In Proceedings of the 2021 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting (APS/URSI), Singapore, 4–10 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 589–590. [Google Scholar]
- Jiang, S.; Liu, X.; Liu, J.; Ye, D.; Duan, Y.; Li, K.; Yin, Z.; Huang, Y. Flexible Metamaterial Electronics. Adv. Mater. 2022, 34, e2200070. [Google Scholar] [CrossRef]
- Huang, X.; Guo, W.; Liu, S.; Li, Y.; Qiu, Y.; Fang, H.; Yang, G.; Zhu, K.; Yin, Z.; Li, Z.; et al. Flexible Mechanical Metamaterials Enabled Electronic Skin for Real-Time Detection of Unstable Grasping in Robotic Manipulation. Adv. Funct. Mater. 2022, 32, 2109109. [Google Scholar] [CrossRef]
- Brizi, D.; Conte, M.; Monorchio, A. A Performance-Enhanced Antenna for Microwave Biomedical Applications by Using Metasurfaces. IEEE Trans. Antennas Propag. 2023, 71, 3314–3323. [Google Scholar] [CrossRef]
- Rotundo, S.; Brizi, D.; Flori, A.; Giovannetti, G.; Menichetti, L.; Monorchio, A. Shaping and Focusing Magnetic Field in the Human Body: State-of-the Art and Promising Technologies. Sensors 2022, 22, 5132. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Seyed, T.; Davis, J.U.; Yang, X.-D. Indutivo: Contact-Based, Object-Driven Interactions with Inductive Sensing. In Proceedings of the 31st Annual ACM Symposium on User Interface Software and Technology, Berlin, Germany, 14 October 2018; ACM: New York, NY, USA, 2018; pp. 321–333. [Google Scholar]
- Hsieh, M.-J.; Guo, J.-L.; Lu, C.-Y.; Hsieh, H.-W.; Liang, R.-H.; Chen, B.-Y. RFTouchPads: Batteryless and Wireless Modular Touch Sensor Pads Based on RFID. In Proceedings of the 32nd Annual ACM Symposium on User Interface Software and Technology, New Orleans, LA, USA, 17 October 2019; ACM: New York, NY, USA, 2019; pp. 999–1011. [Google Scholar]
- Li, Z.; Tian, X.; Qiu, C.-W.; Ho, J.S. Metasurfaces for bioelectronics and healthcare. Nat. Electron. 2021, 4, 382–391. [Google Scholar] [CrossRef]
- Brizi, D.; Stang, J.P.; Monorchio, A.; Lazzi, G. On the design of planar arrays of nonresonant coils for tunable wireless power transfer applications. IEEE Trans. Microw. Theory Tech. 2020, 68, 3814–3822. [Google Scholar] [CrossRef]
- Brizi, D.; Fontana, N.; Costa, F.; Tiberi, G.; Galante, A.; Alecci, M.; Monorchio, A. Design of Distributed Spiral Resonators for the Decoupling of MRI Double-Tuned RF Coils. IEEE Trans. Biomed. Eng. 2020, 67, 2806–2816. [Google Scholar] [CrossRef]
- Lu, D.; Yan, Y.; Avila, R.; Kandela, I.; Stepien, I.; Seo, M.; Bai, W.; Yang, Q.; Li, C.; Haney, C.R.; et al. Bioresorbable, Wireless, Passive Sensors as Temporary Implants for Monitoring Regional Body Temperature. Adv. Healthc. Mater. 2020, 9, 2000942. [Google Scholar] [CrossRef]
- Lu, D.; Yan, Y.; Deng, Y.; Yang, Q.; Zhao, J.; Seo, M.; Bai, W.; MacEwan, M.R.; Huang, Y.; Ray, W.Z.; et al. Bioresorbable Wireless Sensors as Temporary Implants for In Vivo Measurements of Pressure. Adv. Funct. Mater. 2020, 30, 202003754. [Google Scholar] [CrossRef]
- Li, S.; Lu, D.; Li, S.; Liu, J.; Xu, Y.; Yan, Y.; Rodriguez, J.Z.; Bai, H.; Avila, R.; Kang, S.; et al. Bioresorbable, wireless, passive sensors for continuous pH measurements and early detection of gastric leakage. Sci. Adv. 2024, 10, eadj0268. [Google Scholar] [CrossRef]
- Arai, T.; Hirayama, H. Folded Spiral Resonator with Double-Layered Structure for Near-Field Wireless Power Transfer. Energies 2020, 13, 1581. [Google Scholar] [CrossRef]
- Wang, X.; Pang, J.; Tan, Q.; Dong, H.; Zhao, N.; Xue, T. Design of double-layer parallel printed spiral coil for wireless power transfer applied to rotating equipment. Sens. Actuators A Phys. 2021, 331, 112761. [Google Scholar] [CrossRef]
- Masi, A.; Brizi, D.; Monorchio, A. Millimetric Inclusion Detection Through a Contactless Microwave Spiral Sensor for Biomedical Applications. IEEE Sens. J. 2023, 23, 12796–12807. [Google Scholar] [CrossRef]
- Mohammed, B.J.; Manoufali, M.; Naqvi, S.A.R.; Bialkowski, K.S.; Mills, P.C.; Abbosh, A.M. Using Dielectric Properties of Solid Fraction and Water Content to Characterize Tissues at Different Health and Age Conditions. IEEE J. Electromagn. RF Microwaves Med. Biol. 2019, 4, 69–77. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef]
- Brizi, D.; Fontana, N.; Costa, F.; Monorchio, A. Accurate Extraction of Equivalent Circuit Parameters of Spiral Resonators for the Design of Metamaterials. IEEE Trans. Microw. Theory Tech. 2018, 67, 626–633. [Google Scholar] [CrossRef]
- Gabriel, C.; Bentall, R.H.; Grant, E.H. Comparison of the dielectric properties of normal and wounded human skin material. Bioelectromagnetics 1987, 8, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.keysight.com/it/en/product/N9918A/fieldfox-a-handheld-microwave-analyzer-26-5-ghz.html (accessed on 10 June 2025).
- Ito, K.; Furuya, K.; Okano, Y.; Hamada, L. Development and characteristics of a biological tissue-equivalent phantom for microwaves. Electron. Commun. Jpn. Part I Commun. 2000, 84, 67–77. [Google Scholar] [CrossRef]
- Yu, Y.; Lowe, A.; Anand, G.; Kalra, A. Tissue phantom to mimic the dielectric properties of human muscle within 20 Hz and 100 kHz for biopotential sensing ap-plications. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 6490–6493. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Rotaru, G.-M.; Derler, S.; Spano, F.; Camenzind, M.; Annaheim, S.; Stämpfli, R.; Schmid, M.; Rossi, R.M. Materials used to simulate physical properties of human skin. Ski. Res. Technol. 2016, 22, 3–14. [Google Scholar] [CrossRef]
- Van Nunen, T.; Huismans, E.; Mestrom, R.; Bentum, M.; Visser, H. DIY Electromagnetic Phantoms for Biomedical Wireless Power Transfer Experiments. In Proceedings of the 2019 IEEE Wireless Power Transfer Conference (WPTC), London, UK, 18–21 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 399–404. [Google Scholar] [CrossRef]
- Aminzadeh, R.; Saviz, M.; Shishegar, A. Theoretical and experimental broadband tissue-equivalent phantoms at microwave and millimetre-wave frequencies. Electron. Lett. 2014, 50, 618–620. [Google Scholar] [CrossRef]
- Ianniello, C.; de Zwart, J.A.; Duan, Q.; Deniz, C.M.; Alon, L.; Lee, J.; Lattanzi, R.; Brown, R. Synthesized tissue-equivalent dielectric phantoms using salt and polyvinylpyrrolidone solutions. Magn. Reson. Med. 2017, 80, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Duyn, J.H.; Gudino, N.; de Zwart, J.A.; van Gelderen, P.; Sodickson, D.K.; Brown, R. Characterization of a dielectric phantom for high-field magnetic resonance imaging applications. Med. Phys. 2014, 41, 102303. [Google Scholar] [CrossRef] [PubMed]
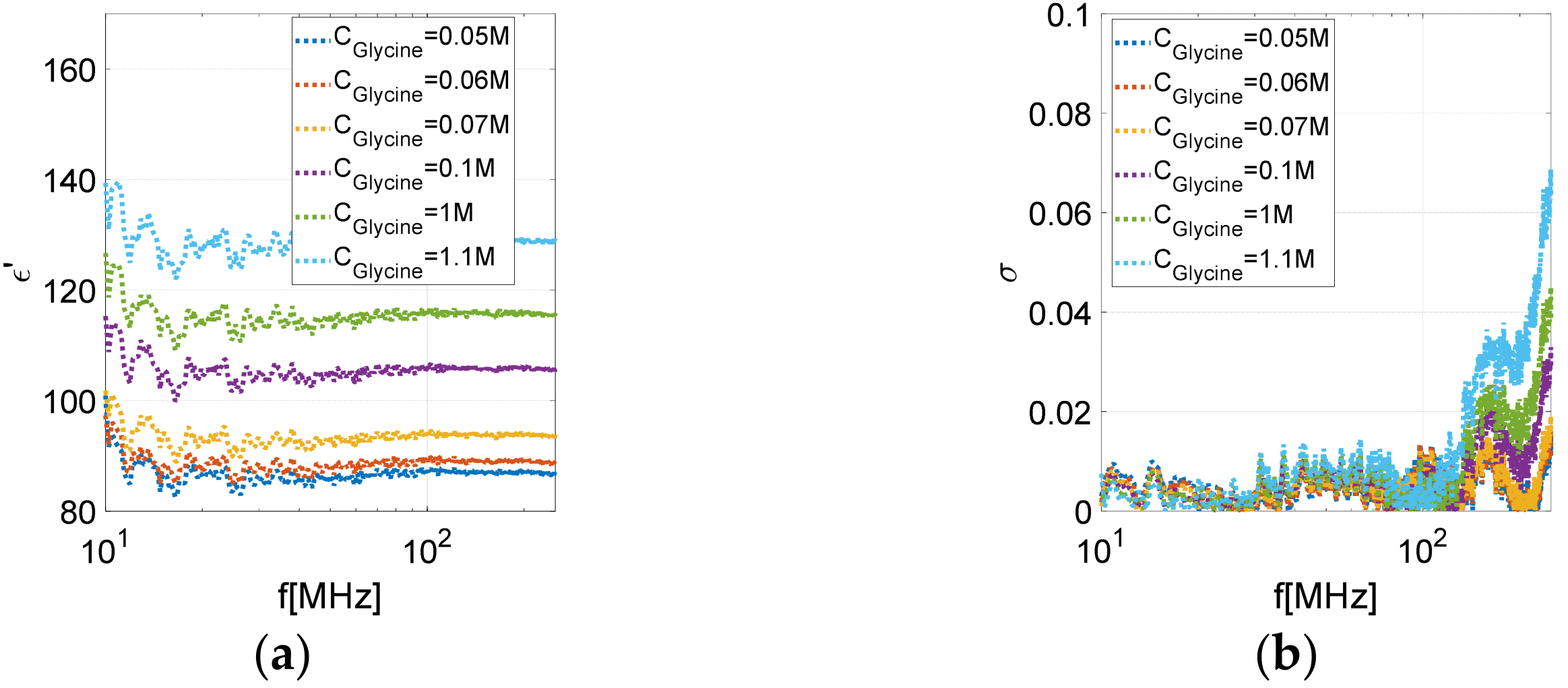
- Alwaleedy, S.; Mohemmed, S.; Karale, R.; Kumbharkhane, A.C.; Roy, B.R.; Sarode, A.V. Study of Dielectric and Thermodynamic properties of Aqueous Glycine. Review 2022. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, W.; Wang, Y.; Fan, Q.; Duan, X.; Xie, M. Spiral Resonator-based Corneal Contact Lens For Intraocular Pressure Monitoring. In Proceedings of the 2022 28th International Conference on Mechatronics and Machine Vision in Practice (M2VIP), Nanjing, China, 16–18 November 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–6. [Google Scholar] [CrossRef]
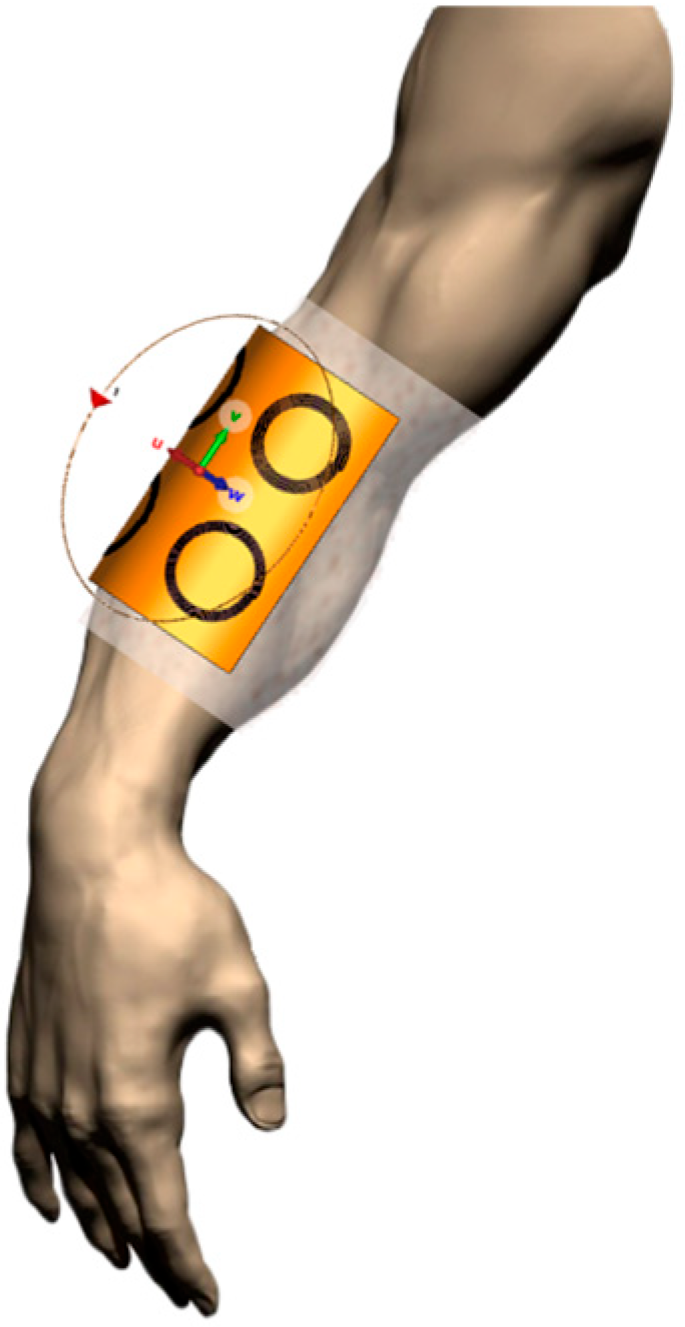
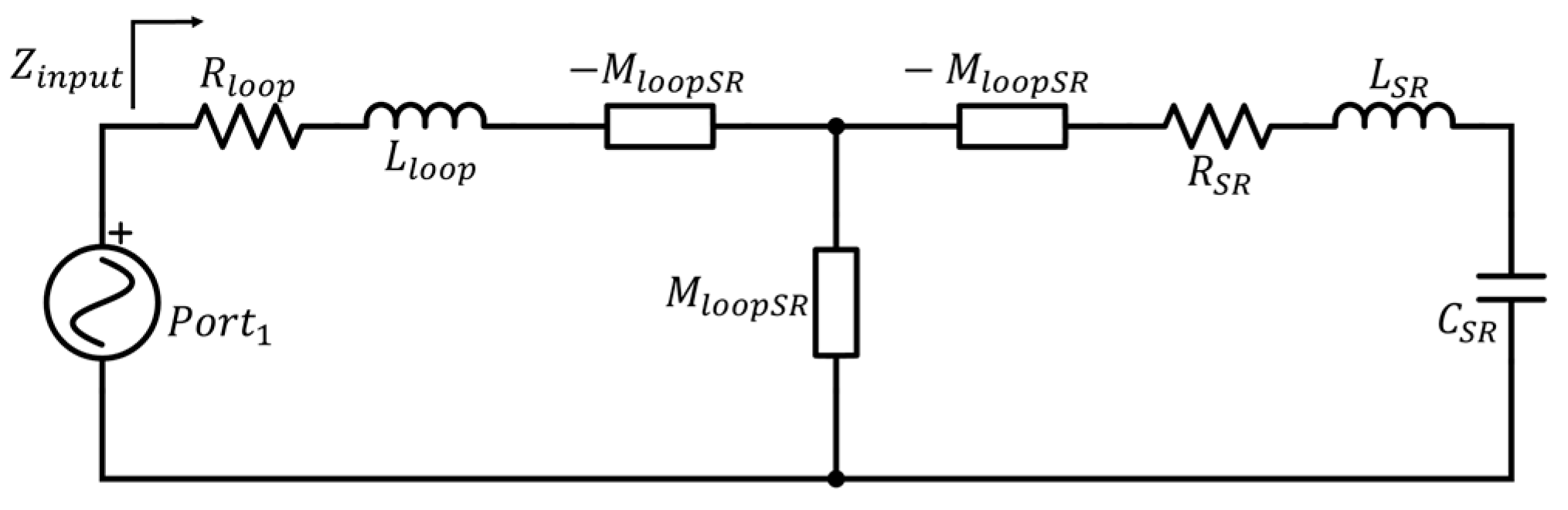
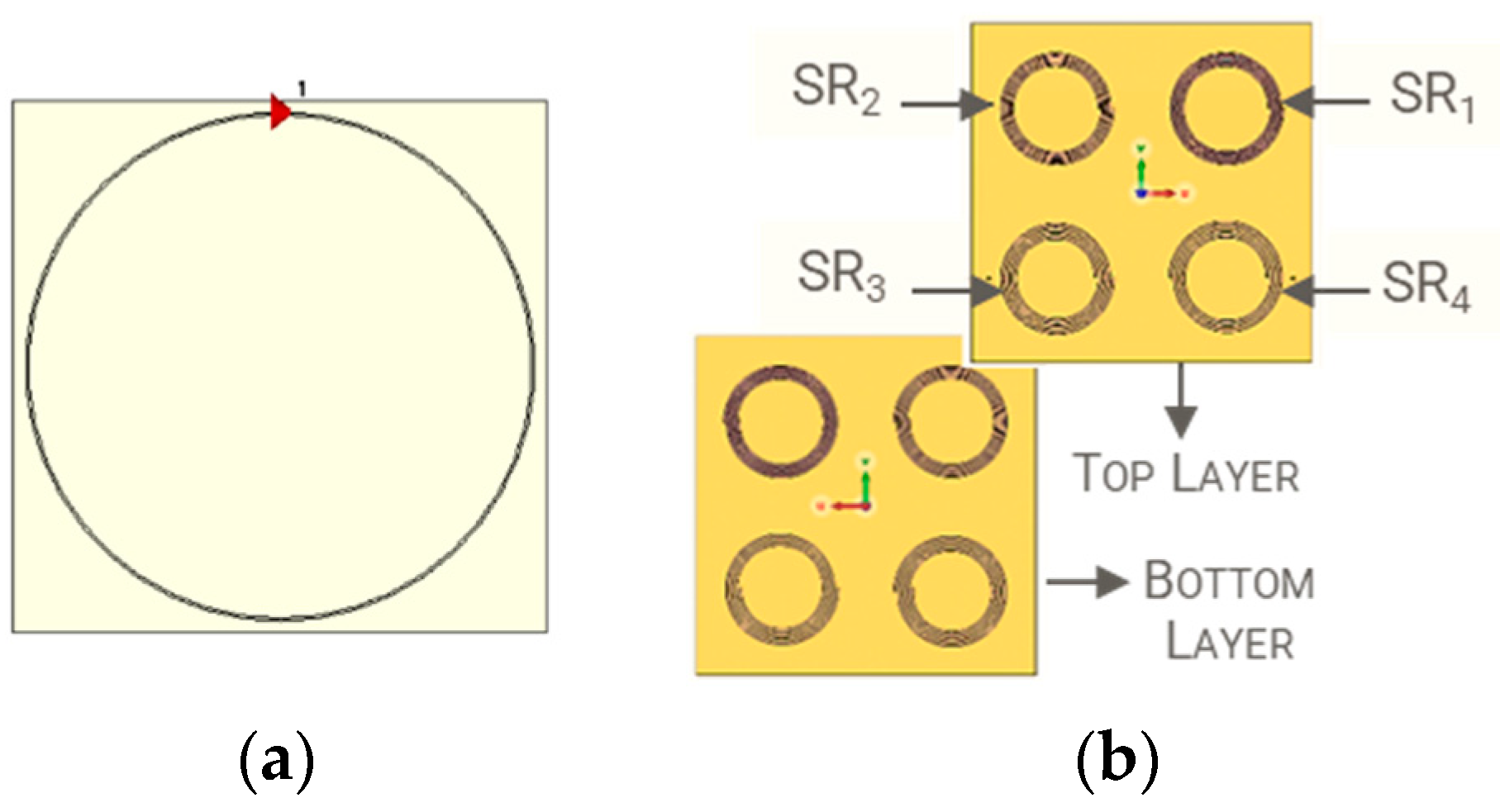



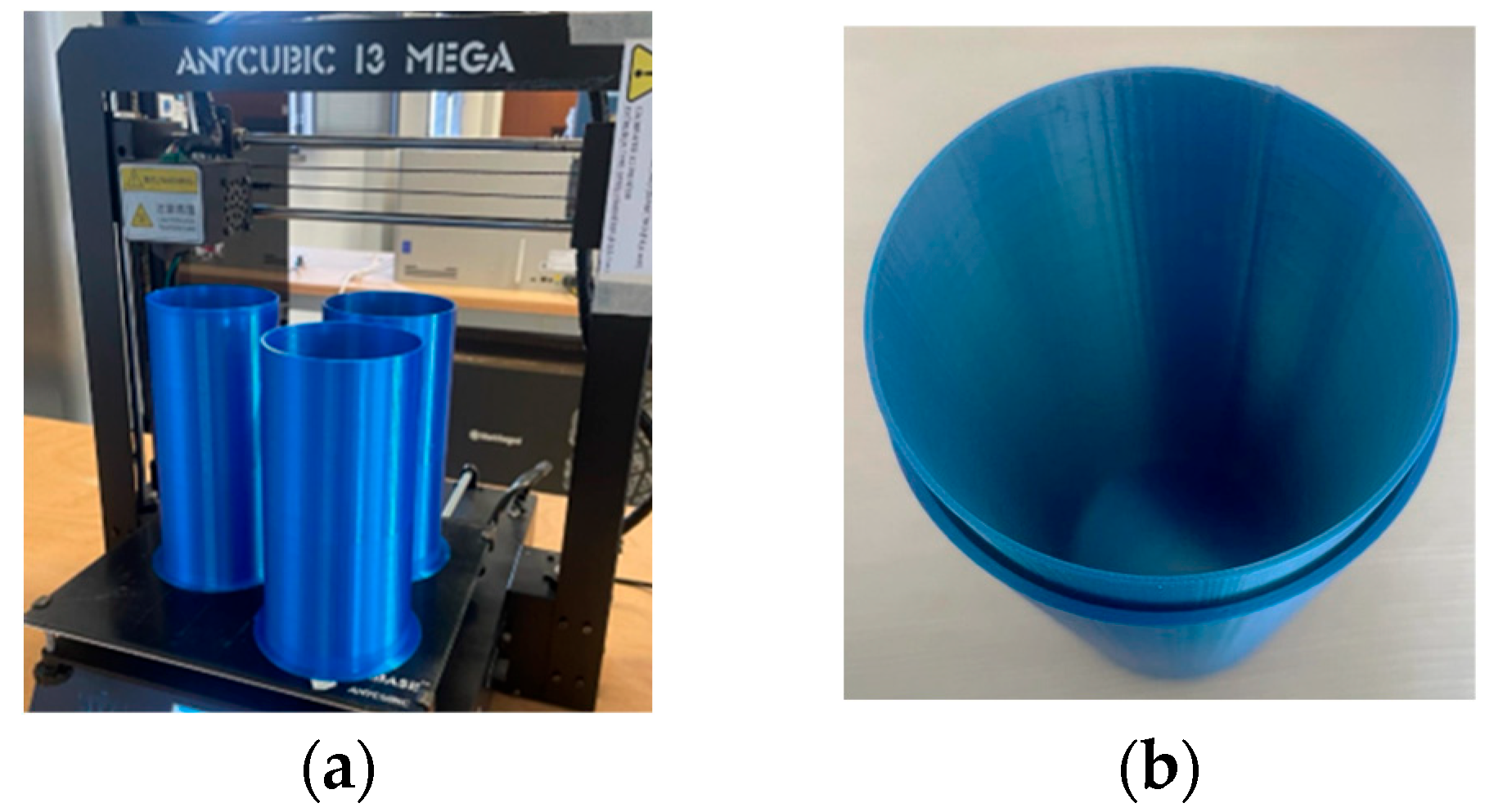

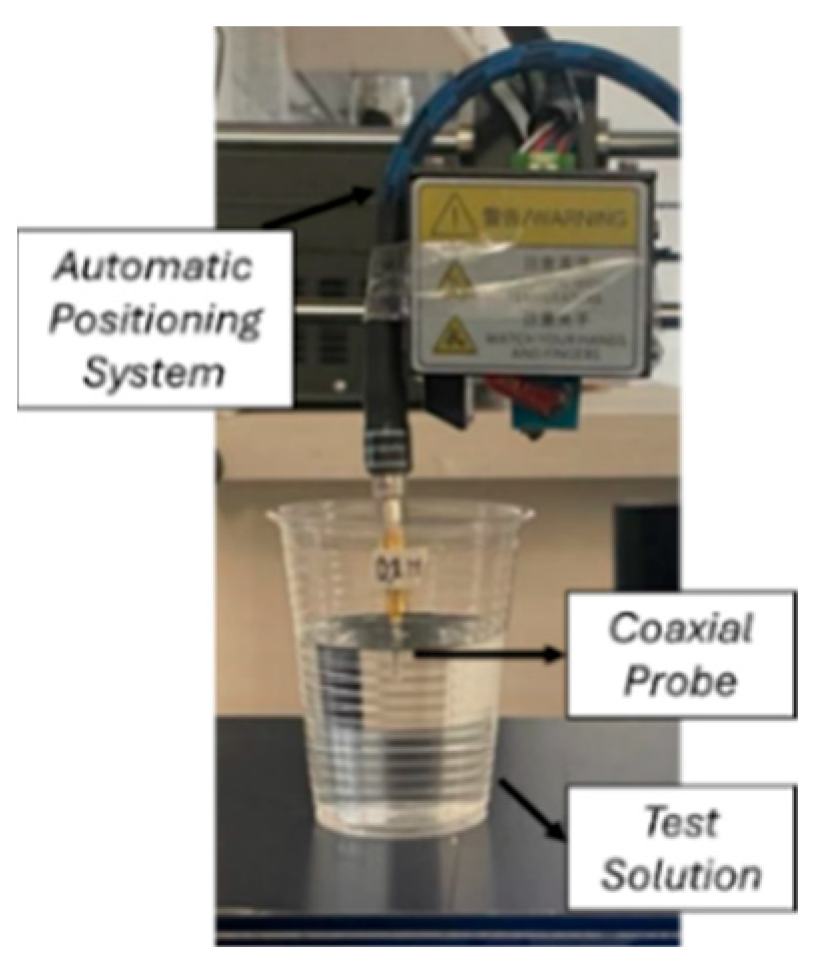


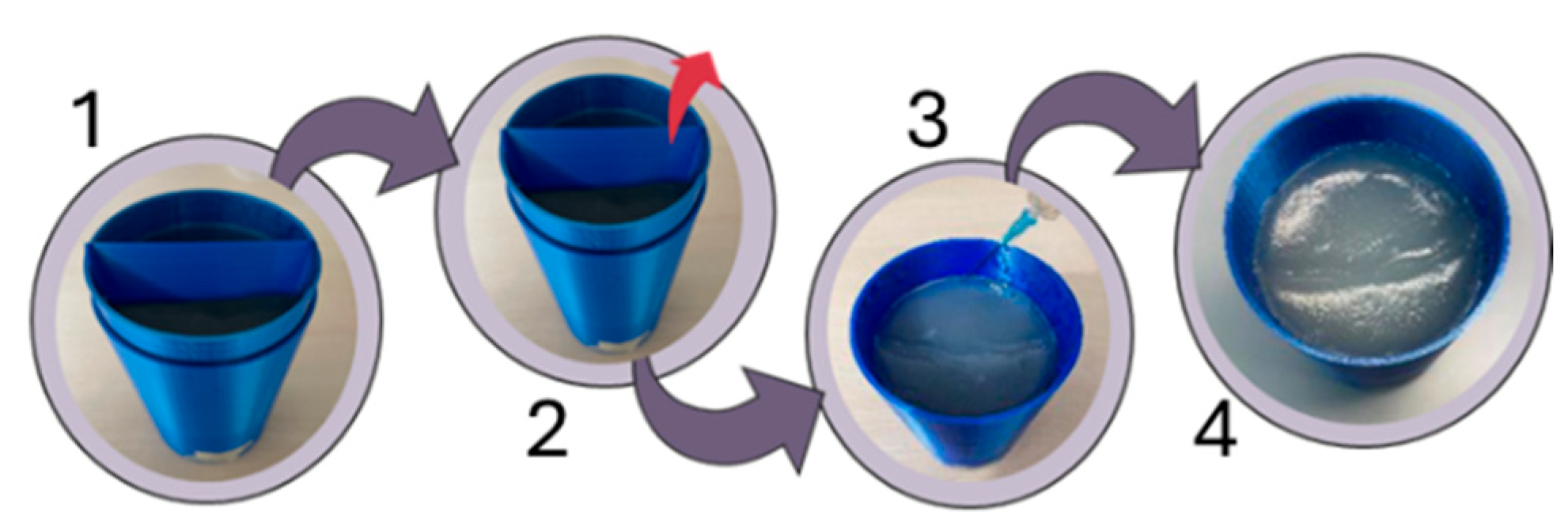


| Parameter | Probe Loop | SR1 | SR2 | SR3 | SR4 |
|---|---|---|---|---|---|
| External radius | 65 mm | 20 mm | 20 mm | 20 mm | 20 mm |
| Microstrip width wstrip | 0.700 mm | 0.700 mm | 0.700 mm | 0.700 mm | 0.700 mm |
| Microstrip thickness hstrip | 35 µm | 35 µm | 35 µm | 35 µm | 35 µm |
| Pitch | - | 1 mm | 1.25 mm | 1.54 mm | 1.61 mm |
| Number of turns | 1 | 5 | 4 | 4 | 3.5 |
| Conductive material | Copper | Copper | Copper | Copper | Copper |
| Substrate material | FR-4 (εr = 4.3, tanδ = 0.02) | ISOLA (εr = 3.45, tanδ = 0.0015) | ISOLA (εr = 3.45, tanδ = 0.0015) | ISOLA (εr = 3.45, tanδ = 0.0015) | ISOLA (εr = 3.45, tanδ = 0.0015) |
| Substrate area | 136 × 136 mm2 | 60 × 60 mm2 | 60 × 60 mm2 | 60 × 60 mm2 | 60 × 60 mm2 |
| Substrate thickness | 0.8 mm | 0.127 mm | 0.127 mm | 0.127 mm | 0.127 mm |
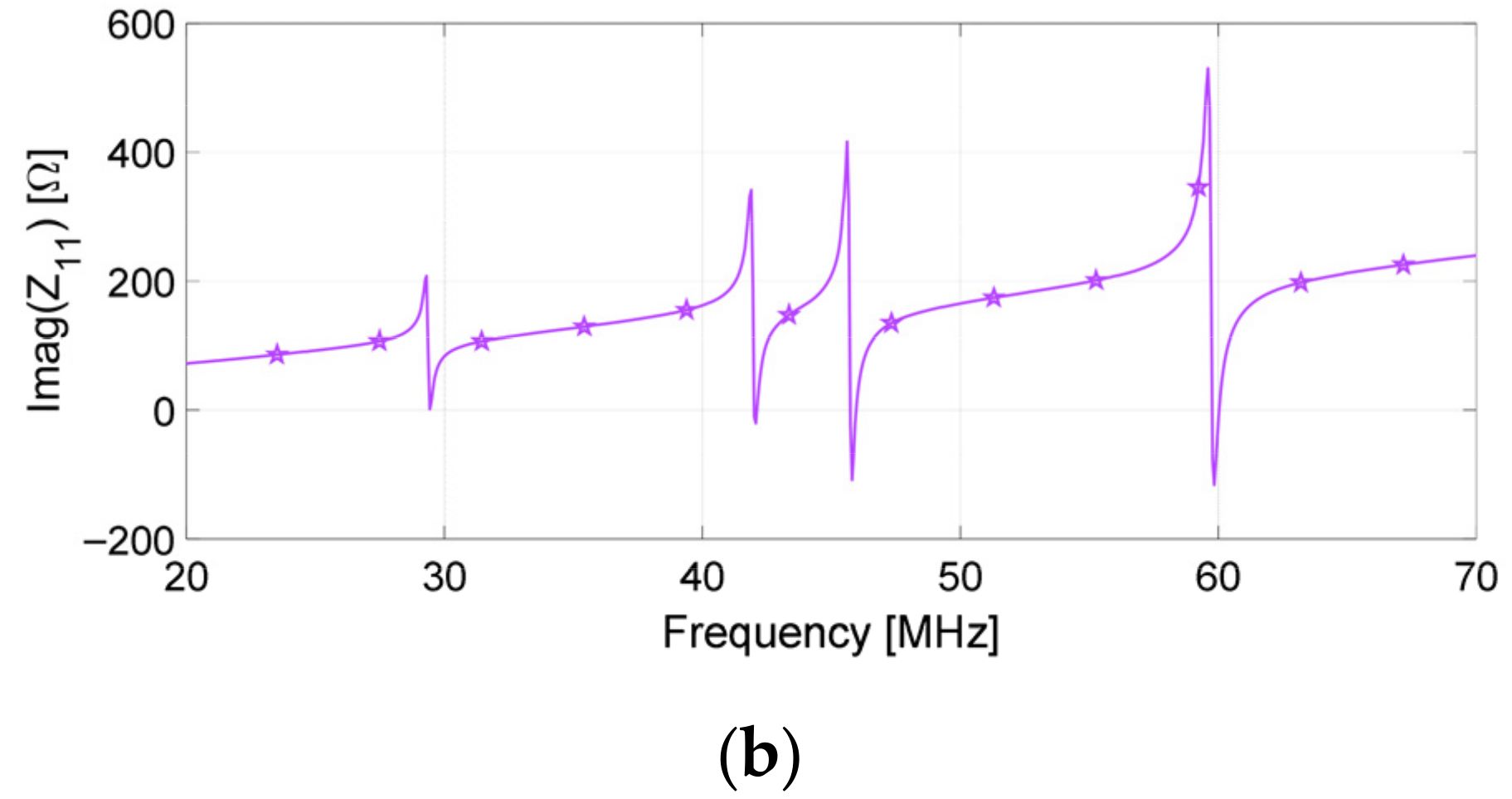
| # SR | Resonance Frequency (MHz), Planar | Resonance Frequency (MHz), Conformal |
|---|---|---|
| I | 30.1 | 49.2 |
| II | 42.6 | 70.2 |
| III | 46.3 | 75.8 |
| IV | 60.2 | 97.0 |
| Operating Frequency (MHz) | Skin (εr, σ (S/m)) | Muscle (εr, σ (S/m)) |
|---|---|---|
| 49.2 | 127.2, 0.37 | 83.5, 0.67 |
| 70.2 | 100.6, 0.42 | 74.9, 0.68 |
| 75.8 | 95.6, 0.43 | 73.3, 0.69 |
| 97.0 | 82.2, 0.46 | 69.0, 0.70 |
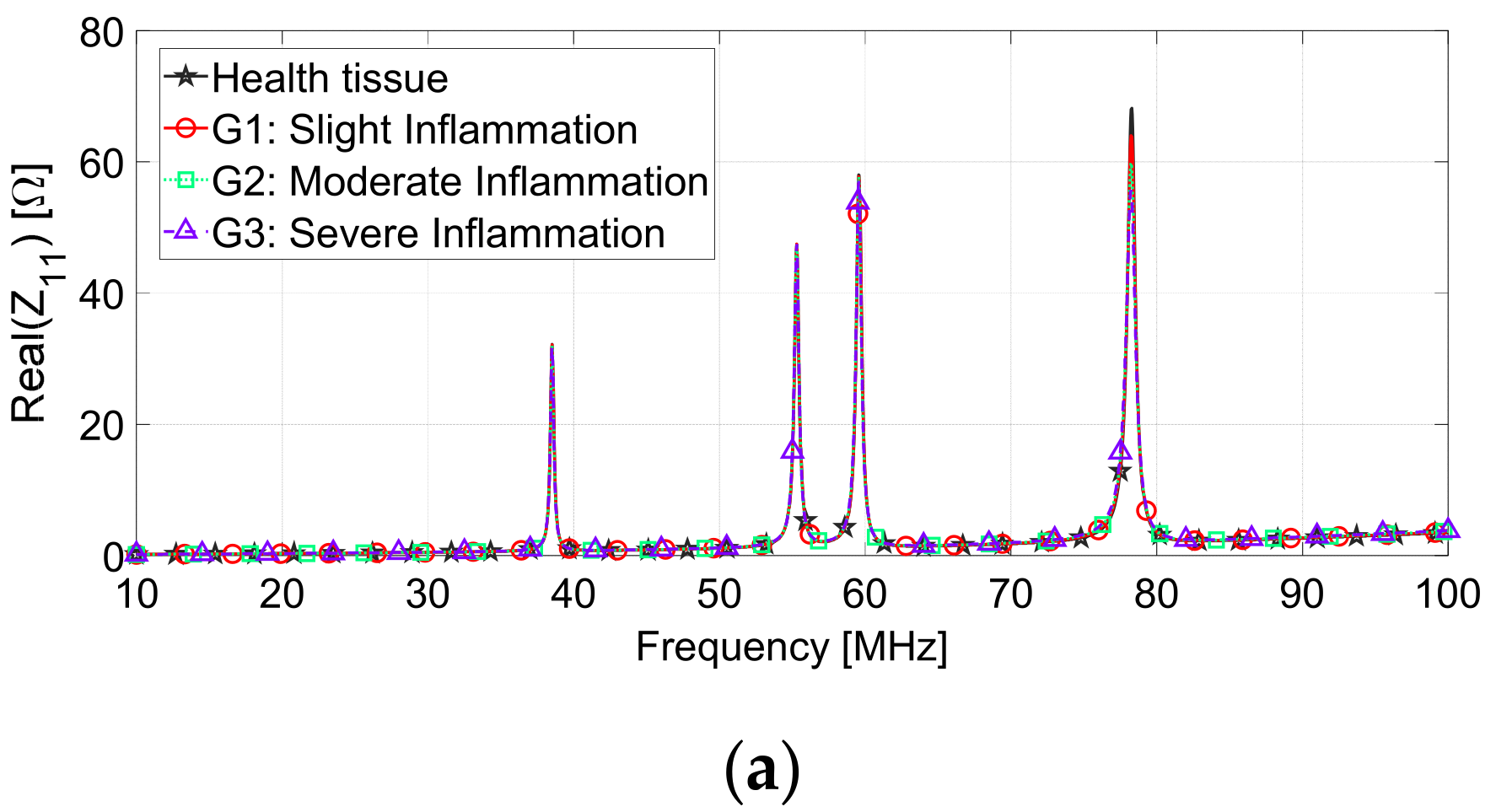
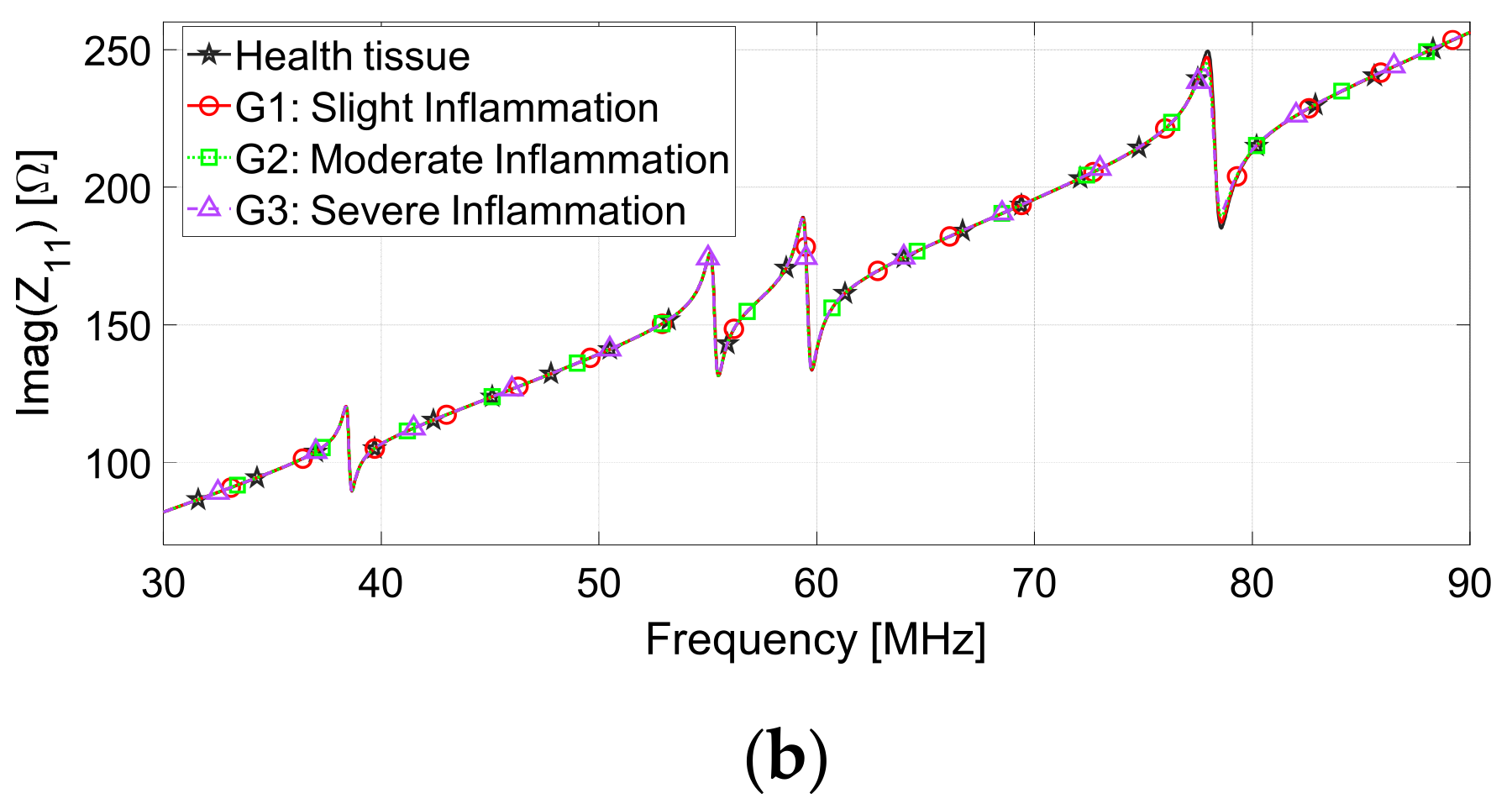
| Inflammation Severity | Skin (εr, σ (S/m)) | Muscle (εr, σ (S/m)) |
|---|---|---|
| G1 (slight) | 94.5, 0.53 | 79.3, 0.80 |
| G2 (medium) | 108.7, 0.61 | 91.2, 0.92 |
| G3 (severe) | 125.0, 0.70 | 104.9, 1.06 |
| Geometrical Features | Value (mm) |
|---|---|
| Outer Cylinder Diameter | 83.6 |
| Outer Cylinder Length | 170 |
| Outer Cylinder Thickness | 1.8 |
| Inner Cylinder Diameter | 77.8 |
| Inner Cylinder Length | 175 |
| Inner Cylinder Thickness | 1.2 |
| Base thickness | 5.0 |
| Runner Thickness | 1.8 |
| Separation Layer Thickness | 1.2 |
| Polynomial Coefficient | Glycine Permittivity Model | Salt Permittivity Model |
|---|---|---|
| a | −11.19 | −138 |
| b | 41.220 | 51.23 |
| c | 87.87 | −1.5 |
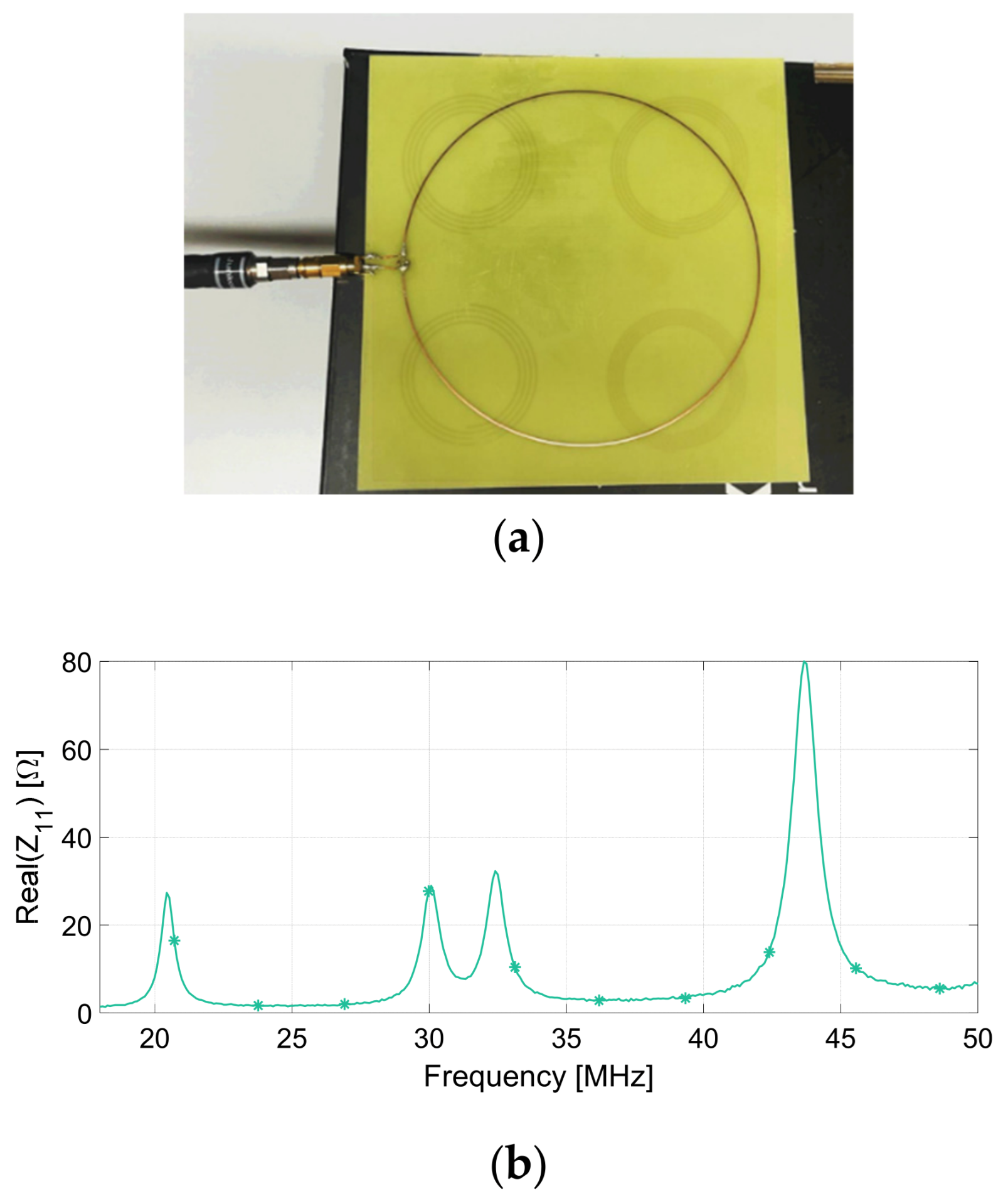
| Quadrant | Resonance Frequency (MHz), Full-Wave | Resonance Frequency (MHz), Measurement |
|---|---|---|
| I | 30 | 22 |
| II | 42 | 30 |
| III | 46 | 33 |
| IV | 60 | 44 |
| Quadrant | Resonance Frequency (MHz), Full-Wave | Resonance Frequency (MHz), Measurement |
|---|---|---|
| I | 38 | 28 |
| II | 55 | 42 |
| III | 59 | 50 |
| IV | 78 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, A.; Brizi, D.; Canicattì, E.; Rotundo, S.; Monorchio, A. A Flexible Frequency-Coded Electromagnetic Sensing Array for Contactless Biological Tissues Health Monitoring. Appl. Sci. 2025, 15, 9015. https://doi.org/10.3390/app15169015
Masi A, Brizi D, Canicattì E, Rotundo S, Monorchio A. A Flexible Frequency-Coded Electromagnetic Sensing Array for Contactless Biological Tissues Health Monitoring. Applied Sciences. 2025; 15(16):9015. https://doi.org/10.3390/app15169015
Chicago/Turabian StyleMasi, Angelica, Danilo Brizi, Eliana Canicattì, Sabrina Rotundo, and Agostino Monorchio. 2025. "A Flexible Frequency-Coded Electromagnetic Sensing Array for Contactless Biological Tissues Health Monitoring" Applied Sciences 15, no. 16: 9015. https://doi.org/10.3390/app15169015
APA StyleMasi, A., Brizi, D., Canicattì, E., Rotundo, S., & Monorchio, A. (2025). A Flexible Frequency-Coded Electromagnetic Sensing Array for Contactless Biological Tissues Health Monitoring. Applied Sciences, 15(16), 9015. https://doi.org/10.3390/app15169015






