A Novel Effective Arsenic Removal Technique for High-Arsenic Copper Minerals: Two-Stage Filtration Technology Based on Fe-25Al Porous Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Arsenic-Bearing Copper Mineral
2.2. Fe-25Al Porous Material Preparation
2.3. Thermogravimetric Differential Thermal Analysis
2.4. Experimental Conditions
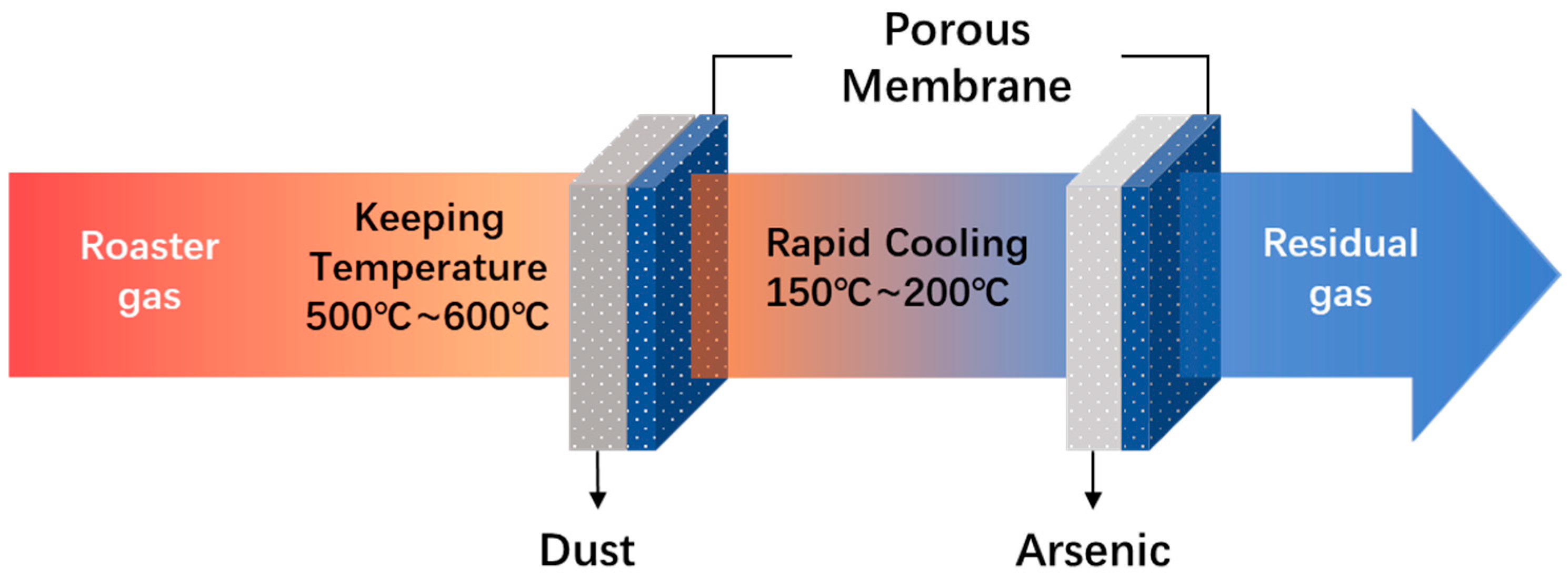
2.5. Two-Stage Filtration with Gradient Temperature Control
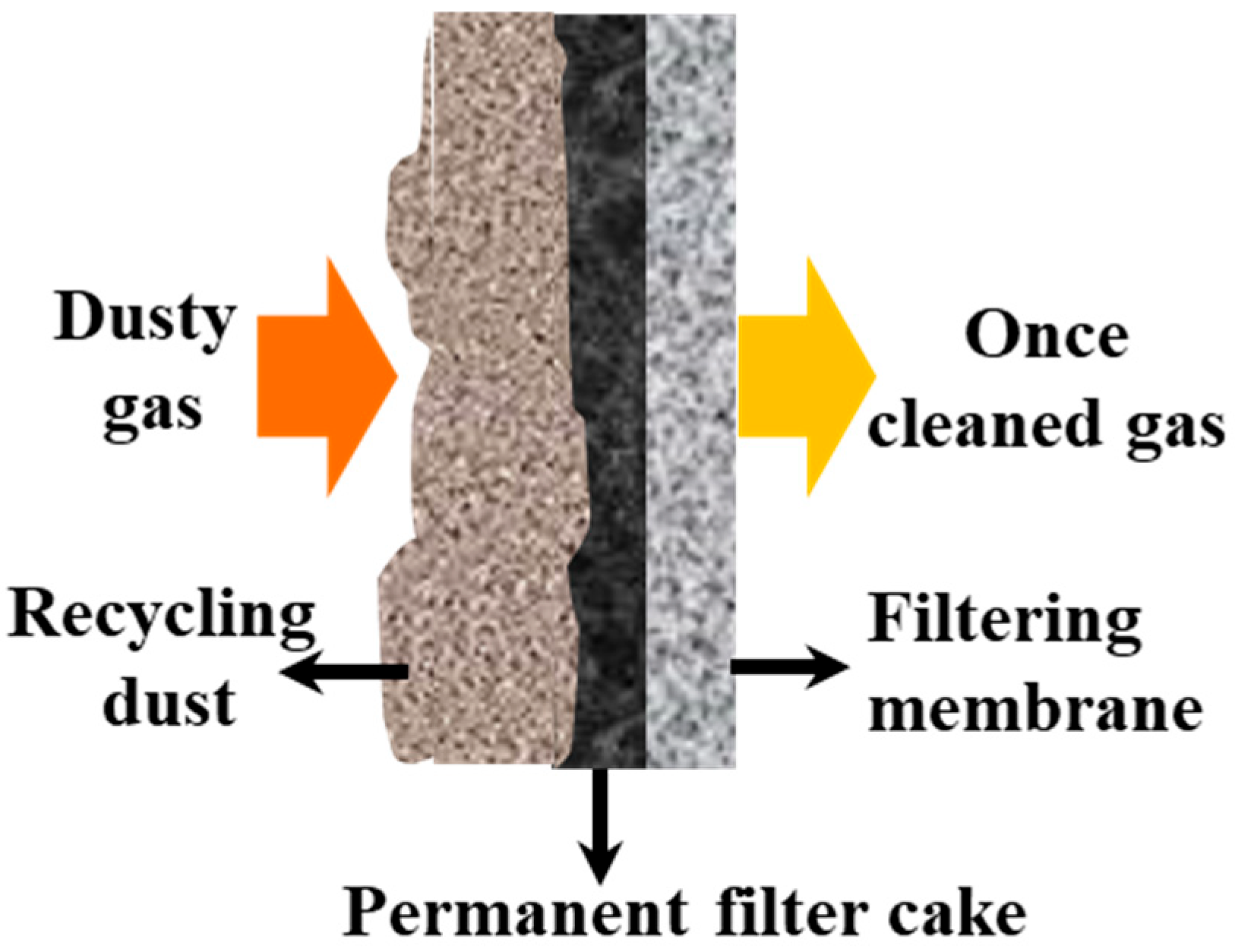
2.5.1. First Filtration System
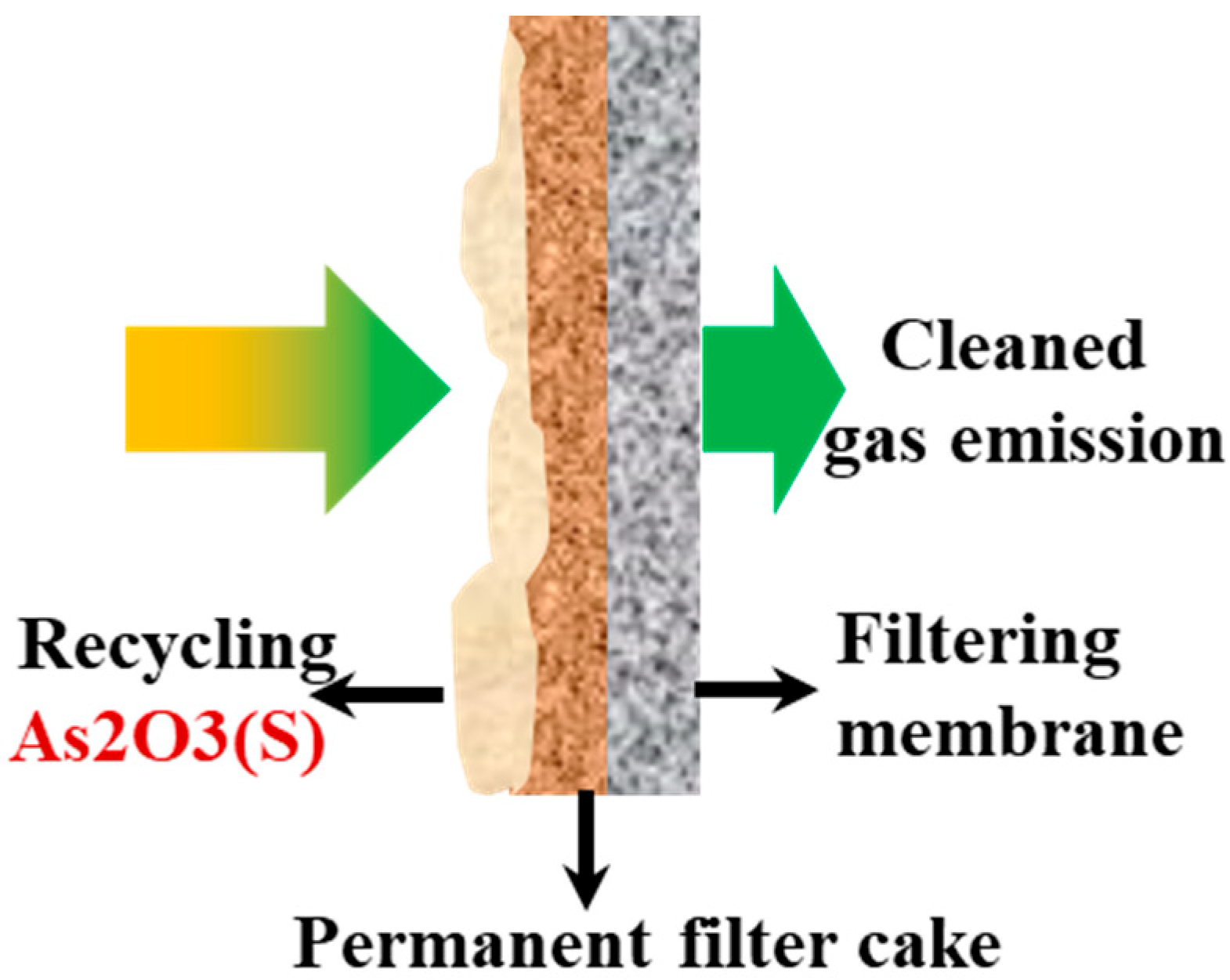
2.5.2. Secondary Filtration System
2.5.3. Blowback Regeneration System
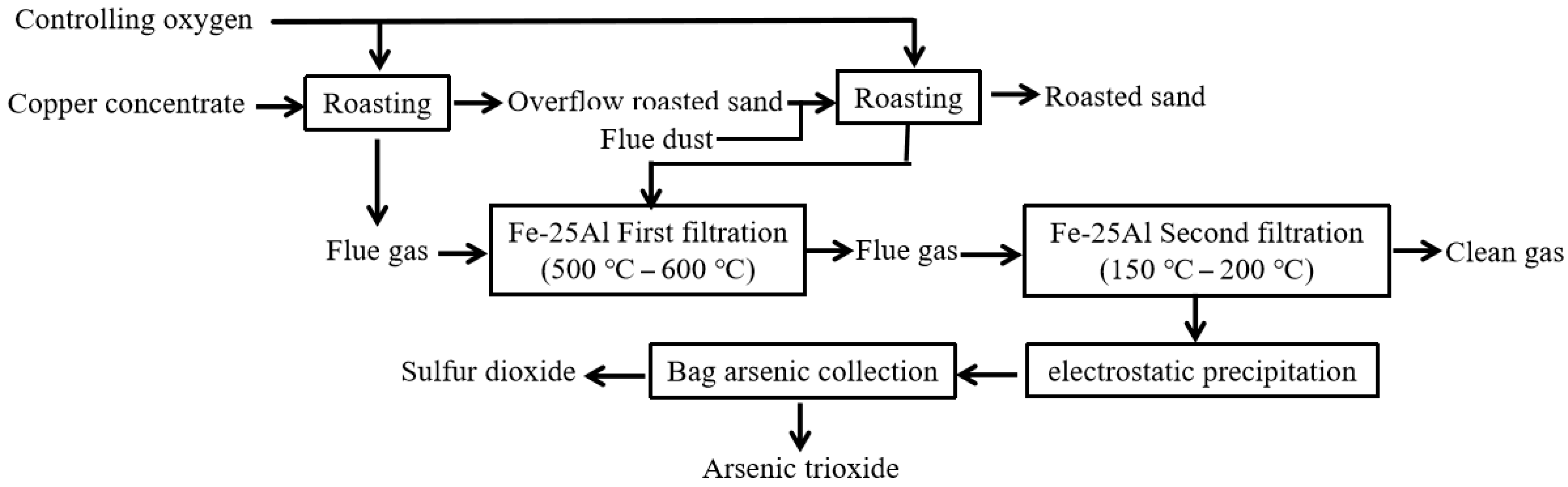
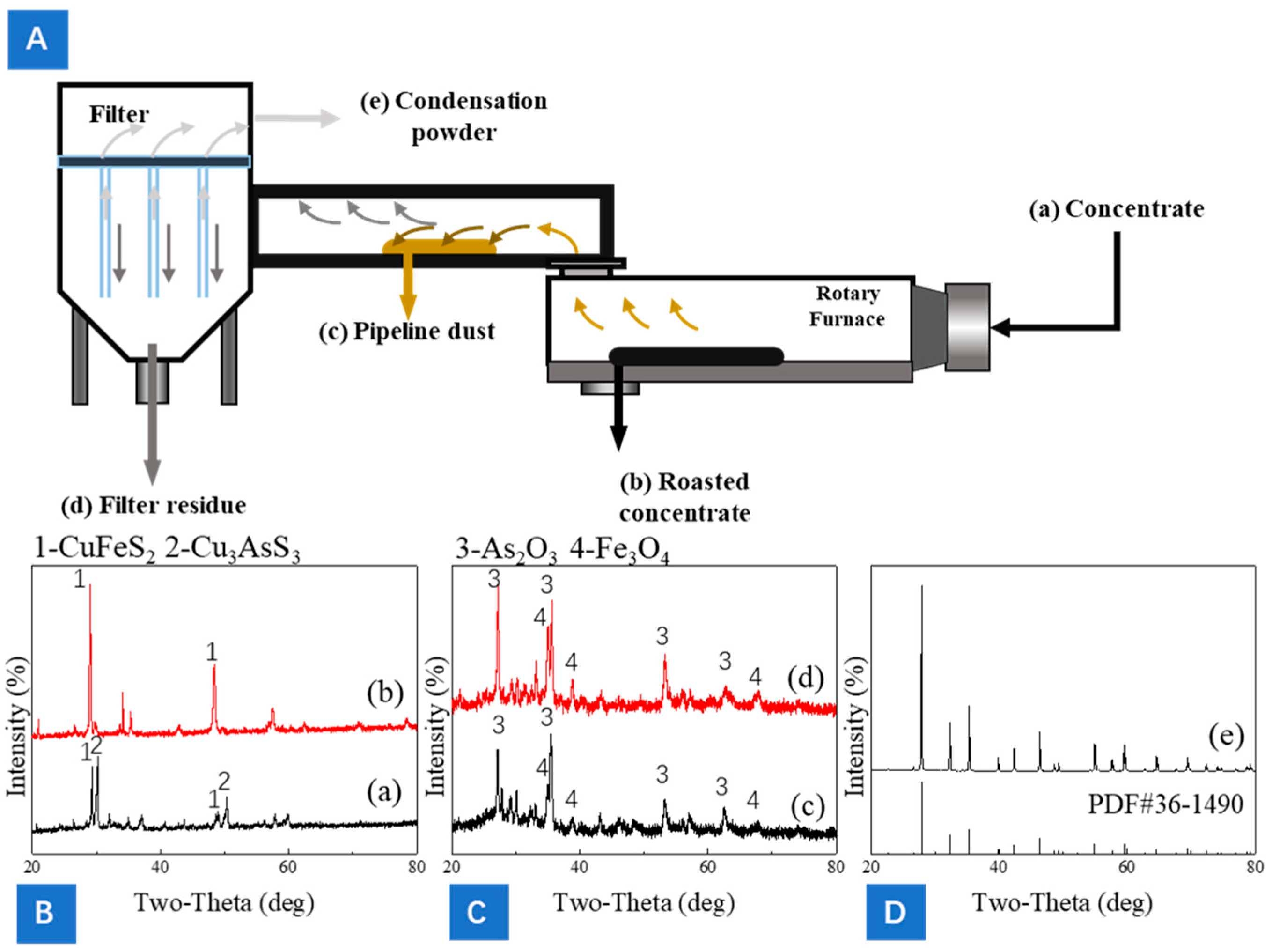
2.6. Process Flow and Equipment Structure
2.7. Preliminary Experiments
2.8. Optimized Experiments
2.9. Continuous Operation Experiment
3. Results and Discussion
3.1. TG-DTA Analysis of Arsenic-Bearing Copper Mineral Roasting
3.2. Preliminary Experiment Analysis
3.3. Optimization Experiment Analysis
3.4. Continuous Operation Experiment Analysis
3.5. Arsenic Removal Effect of Fe-25Al Porous Material on HACM Smelting
3.6. Roasted Mineral Balance and Energy Consumption Analysis
3.7. Equipment and Running Costs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villena, M.; Greve, F. On resource depletion and productivity: The case of the Chilean copper industry. Resour. Policy 2018, 59, 553–562. [Google Scholar] [CrossRef]
- Luo, Z.Q.; Liu, G.Q.; Guo, S.X.; Mine, C.C.; Corporation, J.C. The research on the index regulation of copper concentrate in Chengmenshan Copper Mine. Copp. Eng. 2017, 6, 74–76. [Google Scholar]
- Hu, Q.J. Mining loss and depletion management practice in Fenghuangshan Copper Mine. World Nonferrous Met. 2019, 7, 201–202. [Google Scholar]
- Chen, J.P.; Zhang, Y.; Wang, J.X.; Liao, K.Y.; Lou, B.; Ding, J.H.; Yin, J.N.; Xiang, J. On present situation and potential analysis of copper resources in China. J. Geol. 2013, 37, 358–365. [Google Scholar]
- Liu, G.; Zhu, R. Study of China’s current copper slag on the use of resources. Min. Metall. 2008, 3, 59–63. [Google Scholar]
- Wang, Z.; Qin, K. Types, metallogenic environments and characteristics of temporal and spatial distribution of copper deposits in China. Acta Geol. Sin.-Engl. Ed. 1989, 2, 79–92. [Google Scholar]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2016, 174, 258–281. [Google Scholar] [CrossRef]
- Deng, H.; Xiao, K. Experiments on mineral processing of high arsenic-copper ore. Nonferrous Met. 2010, 6, 17–19. [Google Scholar]
- Taheri, M.; Mehrzad, J.; Mahmudy Gharaie, M.H.; Afshari, R.; Dadsetan, A.; Hami, S. High soil and groundwater arsenic levels induce high body arsenic loads, health risk and potential anemia for inhabitants of northeastern Iran. Environ. Geochem. Health 2016, 38, 469–482. [Google Scholar] [CrossRef]
- Lin, L.; Liu, X.; Liang, Y.; Xu, W.; Li, Y.; Lin, Z. Analysis of mineral phases in heavy-metal hazardous waste under the interdisciplinary scope of data science and chemistry. Prog. Chem. 2021, 33, 2163–2172. [Google Scholar]
- Taylor, P.R.; Putra, T.A.R. Pyrometallurgical processing technologies for treating high arsenic copper concentrates. In Celebrating the Megascale: Proceedings of the Extraction and Processing Division Symposium on Pyrometallurgy in Honor of David G.C. Robertson; Springer: Cham, Switzerland, 2014; pp. 197–211. [Google Scholar]
- Smith, J.G.; Johnson, R.L. Arsenic speciation in nonferrous metallurgy: Environmental impacts and mitigation strategies. Environ. Sci. Technol. 2020, 12, 7123–7134. [Google Scholar]
- Brooks, G.A.; Rankin, W.J.; Gray, N.B. Thermal separation of arsenic and antimony oxides. Metall. Mater. Trans. B 1994, 25, 873–884. [Google Scholar] [CrossRef]
- Padilla, R.; Aracena, A.; Ruiz, M.C. Reaction mechanism and kinetics of enargite oxidation at roasting temperatures. Metall. Mater. Trans. B 2012, 43, 1119–1126. [Google Scholar] [CrossRef]
- Chambers, B.; Pickles, C.A.; Peacey, J.G. Thermodynamic analysis of the sulphation roasting of enargite concentrates. High Temp. Mater. Process. 2012, 31, 613–625. [Google Scholar] [CrossRef]
- Conner, K.; Anderson, C. Enargite treatments and pressure oxidation of concentrates. J. Metall. Eng. 2013, 2, 9. [Google Scholar]
- Zhou, S.; Li, T.; Wang, C.; Tan, Z.; Guan, X.; Li, H.; Zhang, W. Tennantite and enargite rejection in the copper flotation—A mini-review. J. Miner. Mater. Charact. Eng. 2023, 11, 63–68. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Abashina, T.; Vainshtein, M. Review on arsenic removal from sulfide minerals: An emphasis on enargite and arsenopyrite. Miner. Eng. 2021, 172, 172. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection; General Administration of Quality Supervision; Inspection and Quarantine of P.R.C. Announcement No. 106. 2017. Available online: http://www.mofcom.gov.cn/article/b/g/201803/20180302716892.shtml (accessed on 9 July 2025).
- Liu, R.; Zhang, N. Analysis of the operation situation of copper industry. Resour. Recycl. 2024, 2, 36–37. [Google Scholar]
- Bruckard, W.J.; Davey, K.J.; Jorgensen, F.R.A.; Wright, S.; Brew, D.R.M.; Haque, N.; Vance, E.R. Development and evaluation of an early removal process for the beneficiation of arsenic-bearing copper ores. Miner. Eng. 2010, 23, 1167–1173. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Han, H.; Sun, W.; Hu, Y.; Wang, T.; Yue, L.; Yang, Y.; Cao, X.; Tang, H. Arsenic removal from arsenic-containing copper and cobalt slag using alkaline leaching technology and MgNH4AsO4 precipitation. Sep. Purif. Technol. 2020, 238, 116422. [Google Scholar] [CrossRef]
- Yi, Y.; Shi, J.; Tian, Q.H.; Guo, X.Y. Arsenic removal from high-arsenic dust by NaOH-Na2S alkaline leaching. Chin. J. Nonferrous Met. 2015, 25, 806–814. [Google Scholar]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Arsenic removal from copper ores and concentrates through alkaline leaching in NaHS media. Hydrometallurgy 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Rao, M.; Xia, H.; Xu, Y.; Jiang, G.; Zhang, Q.; Yuan, Y.; Zhang, L. Study on ultrasonic assisted intensive leaching of germanium from germanium concentrate using HCl/NaOCl. Hydrometallurgy 2024, 230, 106385. [Google Scholar] [CrossRef]
- Nakazawa, S.; Takeda, Y.; Uumetsu, Y. Thermodynamic Reassessment of As-S System with Emphasis on Liquid Phase Properties. Calphad 2019, 67, 101675. [Google Scholar]
- Henao, H.; Paredes, I.; Diaz, R.; Ortiz, J. Characterization and pyrometallurgical removal of arsenic from copper concentrate roasting dust. J. Miner. Mater. Charact. Eng. 2021, 9, 609–620. [Google Scholar] [CrossRef]
- Henao, H.; Paredes, I.; Diaz, R.; Ortiz, J. Pyrometallurgical Removal of Arsenic from Electrostatic Precipitators Dusts of Copper Smelting. J. Miner. Mater. Charact. Eng. 2021, 9, 545–565. [Google Scholar] [CrossRef]
- Chi, X.P.; Guo, Y.S.; Lv, X.L.; Zhong, S.P.; Liu, C.; Chen, H.; Wang, J.E. The research status and progress of arsenic removal from copper slag. World Nonferrous Met. 2018, 23, 12–16. [Google Scholar]
- Dalewski, F. Removing arsenic from copper smelter gases. JOM 1999, 51, 24–26. [Google Scholar] [CrossRef]
- Zhang, W.; Che, J.; Wen, P.; Xia, L.; Ma, B.; Chen, J.; Wang, C. Co-treatment of copper smelting flue dust and arsenic sulfide residue by a pyrometallurgical approach for simultaneous removal and recovery of arsenic. J. Hazard. Mater. 2021, 416, 126149. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Qi, Y.; Li, F.; Shu, J.; Sun, Z.; Sun, S.; Chen, M.; Pu, S. Effect of electrolyte reuse on metal recovery from waste CPU slots by slurry electrolysis. Waste Manag. 2019, 95, 370–376. [Google Scholar] [CrossRef]
- Sahu, U.K.; Ji, W.; Liang, Y.; Ma, H.; Pu, S. Mechanism enhanced active biochar support magnetic nano zero-valent iron for efficient removal of Cr(VI) from simulated polluted water. J. Environ. Chem. Eng. 2022, 10, 107077. [Google Scholar] [CrossRef]
- Fu, X.; Niu, Z.; Peng, C.; Han, H.; Sun, W.; Yue, T. Quantitative synergistic adsorption affinity of Ca(II) and sodium oleate to predict the surface reactivity of hematite and quartz. Sep. Purif. Technol. 2025, 360, 131196. [Google Scholar] [CrossRef]
- Peng, C.; Fu, X.; Niu, Z.; Sun, W.; Yue, T. Protonation behavior study of the active sites on typical sulfide minerals surface using surface complexation model. Colloids Surf. A Physicochem. Eng. Asp. 2025, 710, 136307. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Li, N.; Yang, B.; Xia, H.; Yu, K.; Yang, H.; Zhang, L. Ultrasound-assisted activation of zinc powder by antimony salts for the removal of Co and Cd from zinc sulfate solution. Sep. Purif. Technol. 2025, 375, 133781. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, X.; Sun, Z.; Zhang, H.; Akhtar, F.; Czujko, T.; Feng, P. Fabrication and characterization of highly porous FeAl based intermetallics by thermal explosion reaction. Adv. Eng. Mater. 2019, 21, 1801110. [Google Scholar] [CrossRef]
- Tang, X.; He, Y. Fe-25Al intermetallic material filtrating to remove arsenic at hightemperature: A novel effective proposal of removal arsenic fromarsenic-bearing copper ores. Mater. Today Commun. 2025, 43, 111712. [Google Scholar] [CrossRef]
- Tang, X.; He, Y. Arsenic extraction from copper concentrate usingcontrolled oxidative roasting and filtration process. Trans. Nonferrous Met. Soc. China 2023, 33, 3198–3209. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.; Liu, C.T. Review of porous intermetalliccompounds by reactive synthesis of elemental powders. Intermetallics 2018, 93, 217–226. [Google Scholar] [CrossRef]
- Karczewski, K.; Stępniowski, W.J.; Salerno, M. Fabrication of FeAl Intermetallic Foams by Tartaric Acid-Assisted Self-Propagating High-Temperature Synthesis. Materials 2018, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Liang, Y.; Dong, D.; Lin, J. FeAl/Al2O3 porous composite microfiltration membrane for highly efficiency high-temperature particulate matter capturing. Journal of Porous Materials. 2021, 28, 955–961. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Jiang, Y.; Gao, L.; Yu, L.; Lin, N.; Liu, C.T. Direct separation of arsenic and antimony oxides by high-temperature filtration with porous FeAl intermetallic. J. Hazard. Mater. 2017, 338, 364–371. [Google Scholar] [CrossRef]
- Yuan, Y.Z.; Hao, Y.; Wang, W. Differential thermal/thermogravimetric analysis (dta-tg) and thermal expansion anomaly properties of Tm2Fe17 compound. In Proceedings of the 2010 International Conference on Mechanic Automation and Control Engineering, Wuhan, China, 26–28 June 2010; IEEE publisher: New York, NY, USA, 2010. [Google Scholar]
- Nakazaw, S.; Yazawa, A.; Jorgensen, F.R.A. Simulation of the removal of arsenic during the roasting of copper concentrate. Metall. Mater. Trans. B 1999, 30, 393–401. [Google Scholar] [CrossRef]
- Tang, X.W.; He, Y.H. An arsenic removal technology and its application in arsenic-containing copper. ChemEngineering 2024, 8, 56. [Google Scholar] [CrossRef]
- Li, T.T.; Peng, C.Q.; Wang, R.C.; Wang, X.F.; Wang, Z.Y. Research progress in porous Fe-Al, Ti-Al and Ni-Al intermetallic compound porous materials. Chin. J. Nonferrous Met. 2011, 21, 784–795. [Google Scholar]
- Amaya, M.; Espinosa-Medina, M.A.; Porcayo-Calderon, J.; Martinez, L.; Gonzalez-Rodriguez, J.G. High temperature corrosion performance of FeAl intermetallic alloys in molten salts. Mater. Sci. Eng. A 2003, 349, 12–19. [Google Scholar] [CrossRef]



| Element | Cu | Fe | S | As | Bi | Sb | Zn |
|---|---|---|---|---|---|---|---|
| wt% | 23.2 | 23.4 | 34.8 | 11.8 | 0.22 | 0.89 | 1.27 |
| Mineral | Enargite (Cu3AsS4) | Tennantite (Cu12As4S13) | Chalcopyrite (CuFeS2) | Pyrite (FeS2) | Arsenopyrite (FeAsS) |
|---|---|---|---|---|---|
| wt% | 19.5 | 23.1 | 7.64 | 27.4 | 15.6 |
| No | Sand (%) | Charcoal (%) | Feedrate (kg/h) | Total Amount (kg) | Temperature (℃) | Time (min) | Furnace Pressure (Pa) | Air Blow | Nitrogen (MP) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | / | 100 | 360 | 660~710 | 90 | −200 | Y | / | |
| 2 | 10 | 2 | 200 | 550 | 690~740 | 130 | −100 | N | 0.2 |
| 3 | 10 | 2 | 100 | 330 | 670~710 | 150 | −200 | N | 0.2 |
| 4 | 10 | 2 | 100 | 300 | 700~740 | 120 | −100 | N | 0.2 |
| 5 | 10 | 3 | 50 | - | 750~760 | 0 | −200 | Y | 0.25 |
| 6 | 10 | 3 | 50 | - | 780~800 | 0 | −100 | Y | 0.25 |
| 7 | 10 | 3 | 50 | - | 760~810 | 0 | −200 | Y | 0.25 |
| 8 | 10 | 3 | 50 | - | 730~760 | 0 | −100 | Y | 0.25 |
| 9 | 10 | 3 | 50 | 150 | 700~750 | 210 | −160 | Y | 0.2 |
| 10 | 10 | 3 | 100 | 300 | 700~740 | 120 | −160 | Y | 0.2 |
| 11 | 10 | 3 | 120 | 360 | 700~740 | 120 | −100 | Y | 0.2 |
| 12 | 10 | 3 | 80 | 280 | 700~740 | 120 | −60 | Y | 0.2 |
| No | Sand (%) | Charcoal (%) | Feedrate (kg/h) | Total Amount (kg) | Temperature (°C) | Furnace Pressure (Pa) |
|---|---|---|---|---|---|---|
| A | 10 | 3 | 100 | 300 | 650~730 | −200 |
| B | A returned to the furnace | 100 | 130 | 700~730 | −100 | |
| C | 10 | 3 | 90 | 315 | 700~730 | −200 |
| D | C returned to the furnace | 100 | 170 | 700~710 | −100 | |
| E | 10 | 3 | 80 | 320 | 700~730 | −200 |
| F | E returned to the furnace | 80 | 170 | 660~700 | −100 | |
| G | 10 | 3 | 90 | 315 | 730~760 | −200 |
| H | G returned to the furnace | 90 | 135 | 740~760 | −100 | |
| I | 10 | 3 | 80 | 200 | 730~760 | −160 |
| J | 15 | 0 | 80 | 200 | 730~740 | −160 |
| K | 5 | 3 | 80 | 260 | 730~740 | −100 |
| L | 5 | 0 | 80 | 200 | 700~730 | −60 |
| Elements | Raw Copper Mineral | Arsenic Removal From Copper Mineral | Arsenic Removal Efficiency | Sulfur Fixation Efficiency |
|---|---|---|---|---|
| As | 10.94% | 1.07% | 90.31% | |
| S | 27.40% | 23.17% | 84.56% |
| No | Arsenic Content (wt%) | Sulfur Content (wt%) | Arsenic Removal Efficiency (%) | Sulfur Fixation Efficiency (%) |
|---|---|---|---|---|
| 1 | 0.93 | 16.55 | 12.27 | 71.42 |
| 2 | 0.83 | 21.75 | 92.5 | 79.38 |
| 3 | 0.78 | 16.76 | 92.87 | 61.68 |
| 4 | 0.74 | 15.39 | 93.24 | 56.19 |
| 5 | 5.73 | 2.57 | 47.62 | 9.38 |
| 6 | 4.14 | 2.03 | 62.16 | 7.40 |
| 7 | 3.26 | 1.81 | 70.20 | 6.61 |
| 8 | 2.97 | 1.48 | 73.13 | 5.40 |
| 9 | 3.06 | 3.16 | 72.03 | 11.53 |
| 10 | 0.60 | 24.74 | 94.51 | 90.29 |
| 11 | 0.66 | 24.62 | 93.98 | 89.85 |
| 12 | 0.48 | 19.24 | 95.61 | 70.22 |
| No | Arsenic Content (wt%) | Sulfur Content (wt%) | Arsenic Removal Efficiency (%) | Sulfur Fixation Efficiency (%) |
|---|---|---|---|---|
| A | 1.65 | 24.39 | 84.92 | 89.01 |
| B | 0.88 | 14.82 | 91.96 | 54.09 |
| C | 1.43 | 23.06 | 86.93 | 84.20 |
| D | 0.61 | 15.38 | 94.41 | 56.13 |
| E | 0.94 | 11.97 | 91.41 | 43.78 |
| F | 0.86 | 21.36 | 92.14 | 77.96 |
| G | 1.29 | 22.49 | 88.21 | 41.93 |
| H | 0.56 | 20.68 | 94.88 | 76.13 |
| I | 0.47 | 17.86 | 95.70 | 65.26 |
| J | 0.88 | 21.94 | 91.96 | 80.11 |
| K | 1.23 | 22.22 | 88.76 | 81.09 |
| L | 1.04 | 24.84 | 90.49 | 90.66 |
| No | Sand (%) | Charcoal (%) | Feedrate (kg/h) | Total Amount (kg) | Diesel Consumption (L) | Electricity Consumption (kWh) |
|---|---|---|---|---|---|---|
| A | 10 | 3 | 100 | 300 | 75 | 190 |
| B | A returned to the furnace | 100 | 130 | |||
| C | 10 | 3 | 90 | 315 | 60 | 210 |
| D | C returned to the furnace | 100 | 170 | |||
| E | 10 | 3 | 80 | 320 | 70 | 210 |
| F | E returned to the furnace | 80 | 170 | |||
| G | 10 | 3 | 90 | 315 | 60 | 205 |
| H | G returned to the furnace | 90 | 135 | |||
| I | 10 | 3 | 80 | 200 | 60 | 215 |
| J | 15 | 0 | 80 | 200 | ||
| K | 5 | 3 | 80 | 260 | 75 | 305 |
| L | 5 | 0 | 80 | 200 | ||
| No. | Items | Cost (Million CNY) | Notes |
|---|---|---|---|
| 1 | Mixing and feeding/discharging device | 1.50 | |
| 2 | Preheating furnace | 3.00 | |
| 3 | Oxygen controlled roasting furnace | 5.60 | |
| 4 | High temperature filter | 3.20 | |
| 5 | Combustion and heating system | 1.80 | |
| 6 | Reduction furnace | 2.00 | Alternative |
| 7 | Condensation arsenic collection device | 1.80 | |
| 8 | Tail gas purification device | 2.00 | |
| 9 | Auxiliary equipment | 2.00 | Air compressor and nitrogen generator |
| 10 | Electrical and Instrumentation System | 1.80 | |
| 11 | Installation and debugging fees | 3.70 | 15% of all equipment costs in the front |
| 12 | Simple factory building | 2.25 | |
| Total project investment | 30.65 |
| No. | Items | Cost (Million CNY) | Notes |
|---|---|---|---|
| 1 | Gas | 12.00 | Gas consumption 40 m3/t, price 3.0 CNY/m3 |
| 2 | Electricity | 6.22 | Total power 1800 kw, price 0.6 CNY/kw·h |
| 3 | Water | 0.90 | Water fee, 6 CNY/t |
| 4 | Texturizer | 3.00 | 10 k tons per year, 300 CNY/ t |
| 5 | Carbon | 9.50 | 25 kg carbon per year, price 3.8 CNY/kg |
| 6 | Pharmaceutical | 2.00 | Chemicals consumed per ton of material 20 CNY |
| 7 | Labor | 6.00 | 60 people, 100 k CNY/person/year |
| 8 | Equipment maintenance fee | 0.86 | Calculated at 3.5% of the total equipment cost |
| 9 | Management expense | 2.02 | 5% of the sum of 1–8 expenses |
| Annual total operating cost | 42.50 | ||
| Unit operating cost per tons | 425.10 | Annual processing of 100 k tons | |
| Depreciation and amortization per tons | 61.31 | 5-year comprehensive depreciation | |
| Tax per tons | 42.00 | 6% of income | |
| Total cost per tons | 528.41 | Processing cost of arsenic containing metal |
| Process | Principle | Advantage | Disadvantage |
|---|---|---|---|
| Pyrometallurgical | Utilizing the volatile nature of arsenic to separate arsenic from metal compounds through roasting. | Efficient, short process, and wide applicability | Serious pollution of arsenic-bearing smoke and dust emissions |
| Hydrometallurgical | Utilizing the different solubility characteristics of metals and arsenic to separate arsenic. | Well arsenic removal effect, mild conditions, and low environmental risk | High demands for pharmaceuticals and equipment, difficult treatment of waste liquids |
| Biological | Using arsenic-loving microorganisms, such as the genus Thiobacillus, to separate arsenic. | Environmentally friendly, low-cost, suitable for low-grade minerals | Long cycle, poor adaptability of bacterial strains, and difficulty in industrialization |
| Two-stage filtration | Using Fe-25Al porous material filtration based on pyrometallurgical method to separate arsenic | Efficient, short process, wide applicability, environmentally friendly | Process complex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; He, Y. A Novel Effective Arsenic Removal Technique for High-Arsenic Copper Minerals: Two-Stage Filtration Technology Based on Fe-25Al Porous Material. Appl. Sci. 2025, 15, 8899. https://doi.org/10.3390/app15168899
Tang X, He Y. A Novel Effective Arsenic Removal Technique for High-Arsenic Copper Minerals: Two-Stage Filtration Technology Based on Fe-25Al Porous Material. Applied Sciences. 2025; 15(16):8899. https://doi.org/10.3390/app15168899
Chicago/Turabian StyleTang, Xiaowei, and Yuehui He. 2025. "A Novel Effective Arsenic Removal Technique for High-Arsenic Copper Minerals: Two-Stage Filtration Technology Based on Fe-25Al Porous Material" Applied Sciences 15, no. 16: 8899. https://doi.org/10.3390/app15168899
APA StyleTang, X., & He, Y. (2025). A Novel Effective Arsenic Removal Technique for High-Arsenic Copper Minerals: Two-Stage Filtration Technology Based on Fe-25Al Porous Material. Applied Sciences, 15(16), 8899. https://doi.org/10.3390/app15168899






