Abstract
Background: Natural products have been used worldwide as alternatives to treat or prevent different chronic diseases. Euterpe oleracea Mart. (açaí) has bioactive molecules in its chemical matrix, such as epicatechin, apigenin, and cyanidin-3-O-rutinoside. These molecules guarantee açaí’s antioxidant, anti-inflammatory, and antitumor potential. Açaí’s chemical matrix is susceptible to degradation. Nanocarriers are appropriate to use with NP. The aim of this study was to produce, characterize, and analyze the in vitro safety profile of a nanoemulsion (NE) containing açaí extract. Methods: Different NEs were prepared with açaí extract (0.83–20 mg/mL). A characterization was performed considering physical–chemical parameters and a morphological analysis. The most stable NE was evaluated for in vitro safety in fibroblasts. Fibroblasts were exposed to a concentration curve of NEs for 24 h. Cellular viability and proliferation, the levels of nitric oxide, reactive oxygen species (ROS), and the release of dsDNA were measured. Possible DNA damage was also measured. Results: It was possible to determine that the NE with 4 mg/mL of açaí extract was the most stable under refrigeration, presenting a favorable in vitro safety profile since fibroblasts kept their homeostasis aspects under most of the concentrations tested as well as their DNA integrity. Conclusion: The obtained results show that a stable NE was produced, maintaining the NP antioxidant capacity and non-toxic effects in fibroblasts.
1. Introduction
Natural health products have been investigated by many research groups worldwide due to their biological properties, such as their antioxidant, anti-inflammatory, and antitumor behavior, and others [1,2,3]. The Euterpe oleracea Mart., popularly known as açaí, is a great example. Açaí is a Brazilian native fruit from the Amazon rainforest that has been used by the indigenous and rural population based on folk knowledge for many years [4].
Spada et al. (2009) [5] were some of the first researchers to investigate the biological properties of açaí. The authors developed an experimental protocol using an in vivo model where animals were exposed to hydrogen peroxide (H2O2) and treated with açaí extract. The study demonstrated the antioxidant capacity of açaí, mainly acting as a cytoprotective agent for the brain. Later, in 2016, Barbosa et al. [6] also demonstrated the antioxidant effects of açaí in humans who regularly consumed açaí juice. These findings drew increased attention to the berry, with the authors attributing its effects to its unique chemical composition.
Our research group has also been studying açaí extract for years. Machado et al. (2016) [7] developed an experimental model using neuron-like cells exposed to rotenone, a mitochondrial complex I inhibitor. After causing mitochondrial impairment, the cells were treated with a concentration–response curve of açaí hydroalcoholic extract. It was possible to observe that, mainly at 5 ug/mL, the extract could recover mitochondrial function. This effect occurred due to the modulation of some important genes related to the mitochondrial complex I protein composition, such as NDUFS7 and NDUFS8. In 2019, Machado et al. [8] showed the anti-inflammatory potential of açaí extract in phytohemagglutinin-activated macrophages. This effect looked to be related to NLRP3 inflammasome modulation. Souza et al. (2020) [9] observed a similar anti-neuroinflammatory profile in microglia cells activated by lipopolysaccharide exposure. Finally, Cadoná et al. (2021) [10] proved the mechanism of how açaí extract could perform its anti-neuroinflammatory activity. The authors activated microglia cells with LPS and nigericin to specifically activate the inflammation via the NLRP3 inflammasome recruitment. Then, the cells were treated with freeze-dried açaí extract. The extract could reduce the inflammation by mitigating the NLRP3 priming and assembly signals.
Interestingly, most of the studies with açaí correlate its properties to its chemical matrix composition. Açaí extract has a very heterogeneous composition, being formed by several bioactive molecules, such as orientin, apigenin, catechin, epicatechin, and others. However, despite numerous evidence regarding the biological activities of açaí extract, this product of natural origin may present some limitations of use/applicability, including low bioavailability, photosensitivity, and the possibility of the degradation of the chemical matrix due to oxidation, among others. Also, nanotechnology has been considered a promising tool against this challenge [11]. There are many different nanocarriers that can be used to protect the chemical matrix of natural products, such as nanocapsules, liposomes, and nanoemulsions, for example [12].
Nanoemulsions are heterogeneous structures formed by two immiscible liquid phases, one of which is oily (O) and the other water (W) [13]. The O/W formulation is composed of oil droplets in an aqueous medium and stabilized by surfactants, which are responsible for reducing surface tension and preventing droplet aggregation [14]. Among the advantages of using this carrier, the stability can be highlighted [15], as well as the fact that nanoemulsions are non-toxic, biocompatible, and able to increase the bioavailability and absorption rate of the substances carried [16,17]. Furthermore, nanoemulsions can protect unstable molecules, such as anthocyanins, from photodegradation, oxidation, and the variations in pH and temperature that can occur during both storage and future administration in the human body [18,19]. There are already some studies where nanoemulsions have been developed to carry natural products [20,21,22]. However, there is still a lack of scientific studies to develop, characterize, and investigate the safety profile of nanoemulsions containing açaí extract.
In this regard, the objective of this study was to develop, characterize, and evaluate the in vitro safety profile of a nanoemulsion containing a freeze-dried hydroalcoholic açaí extract.
2. Materials and Methods
2.1. Experimental Design
This is an experimental study where initially a freeze-dried hydroalcoholic açaí extract was produced and characterized. Then, a nanoemulsion containing this extract (NE) was also produced and characterized with the aim of having a nanocarrier capable of protecting the açaí extract chemical matrix. Later, an in vitro experimental model was conducted to evaluate the safety profile of both the free extract and the NE in fibroblasts. Genotoxicity was also evaluated.
2.2. Production and Characterization of Açaí Freeze-Dried Hydroalcoholic Extract
The methods used to produce and characterize a freeze-dried hydroalcoholic açaí extract are described below.
2.2.1. Freeze-Dried Hydroalcoholic Açaí Extract Production
Fresh açaí fruits were obtained from a harvesting area located in the Amazon rainforest (protocol SisGen: AF1630A). These fruits were transported to the Laboratory of Cellular Culture and Bioactive Effects of the Franciscan University under refrigeration (4 °C). Freeze-dried açaí hydroalcoholic extract was prepared following the methods described by Bittencourt et al. (2013) [23] and adapted by Machado et al. (2016) [7]. Whole fruits were macerated and transferred to amber flasks at a final concentration of 300 mg/mL. A 70% ethanol solution was used. The entire process of extraction was performed for 21 days. Every 7 days, the material was filtered and the solvent was replaced. At the end of the extraction, the obtained material was rotaevaporated and lyophilized.
2.2.2. Freeze-Dried Hydroalcoholic Açaí Extract Characterization
Powdered açaí extract was utilized for characterization via high-performance liquid chromatography (HPLC). This process was performed following conditions previously published by Boligon et al. (2015) [24]. The açaí extract concentration used was 10 mg/mL. The equipment used was the Shimadzu™ Autosampler machine (model SIL-20A, São Paulo, Brazil). The extract was purified in solid phase using the C-18 cartridge (SPE-C18, Strata C18-E, Phenomenex, Torrance, CA, USA). Non-anthocyanin phenolic compounds were detected using a reverse phase C-18 Hyoersil Gold column. The volume of injection was 20 µL and the mobile phase was composed of 5% (v/v) of formic acid to acetonitrile. Chromatograms for the purpose of quantifying non-anthocyanin phenolics were obtained at 280 nm (hydroxybenzoate derivatives), 320 nm (hydroxycinnamate derivatives), and 360 nm (flavonol derivatives). The phenolic compounds of the samples were identified by comparison with the retention time of authentic standards and with the spectral data obtained from UV–visible absorption.
With the aim of quantifying anthocyanin molecules, purified samples were injected onto a C-18 Core–Shell Kinetex reversed phase column at 38 °C. The injection volume was 20 µL and the mobile phase was a solution of 3% formic acid in water (v/v) and 100% acetonitrile at a flow rate of 0.9 mL/min. Chromatograms were obtained at 520 nm for quantification purposes. Cyanidin and peonidin derivatives were identified based on the order of the elution and absorption spectrum and quantified as the equivalent to cyanidin-3-O-glucoside. All of the results were expressed as mg/100 g of lyophilized extract.
2.3. Production and Characterization of Nanoemulsion Containing Açaí Extract
The methods used to produce and characterize the nanoemulsion containing açaí extract are described below.
2.3.1. Nanoemulsion Containing Açaí Extract Production
The nanoemulsion preparation protocol carried out was conducted based on Bazana et al. (2019) [25]. To identify the ideal nanoemulsion formulation, six different concentrations of açaí extract were used. Table 1 presents the composition of the six NE initially prepared and labelled as follows: NE1 (0.83 mg/mL of extract); NE2 (2 mg/mL of extract); NE3 (4 mg/mL of extract); NE4 (6 mg/mL of extract); NE5 (8 mg/mL of extract); and NE6 (20 mg/mL of extract).

Table 1.
Composition of each phase of the six nanoemulsion formulations containing açaí extract prepared. Final volume of 25 mL.
The aqueous phase (AP) was composed of polysorbate 80 (Tween 80®) and ultrapure water. The oily phase (OP) consisted of medium chain triglycerides (MCTs), sorbitan monooleate (Span 80®), a filtered solution of açaí extract (a dilution of 1.0 g of freeze-dried açaí extract was used and 100 mL of 70% ethanol was added and filtered through a 0.45 µm filter), and 70% ethanol. Both phases were placed on magnetic stirrers (without heating) until the complete solubilization of their constituents. Then, the oil phase was injected into the aqueous phase under magnetic stirring (without heating). Magnetic stirring was maintained for 10 min and then the formulation was taken to the rotary evaporator at 40 °C and 60 rpm to remove the organic solvent and obtain the final volume of 25 mL.
2.3.2. Average Droplet Size and Polydispersity Index
To determine the average droplet diameter and polydispersity index (PDI) of the nanoemulsion, the dynamic light scattering method was used on the Zetasizer ® equipment (Zetasizer® nano-ZS model ZEN 3600, Malvern Instruments®, Malvern, UK) with a laser source in the length waveform of 532 nm and a measurement angle of 173°. To achieve this, the formulation was diluted 500 times (v/v) in an ultrapure water solution (previously filtered using a syringe and a 0.45 µm diameter membrane). The results were expressed in nanometers (nm) for diameter and analyzed using the average reading of three repetitions.
2.3.3. Zeta Potential
The evaluation of the zeta potential was carried out using the Zetasizer equipment (Zetasizer® nano-Zs model ZEN 3600, Malvern, UK) which uses the laser Doppler microelectrophoresis technique, which expresses the results in millivolts (mV). The analyses were carried out after diluting the formulations in a 10 mM NaCl solution previously filtered through a 0.45 μm membrane.
2.3.4. pH Measurement
The NE pH was obtained directly from the formulations using a calibrated potentiometer (DM-22, Digimed®, São Paulo, SP, Brazil).
2.3.5. Microscopic Analysis
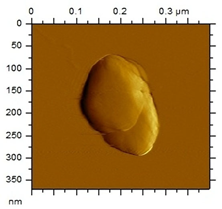
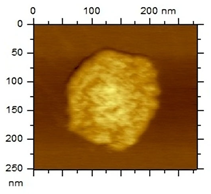
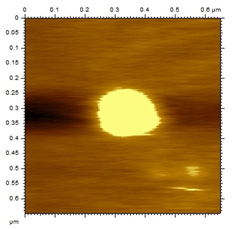
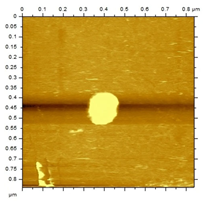
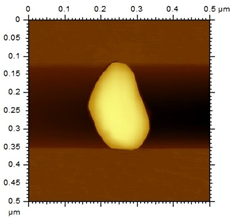
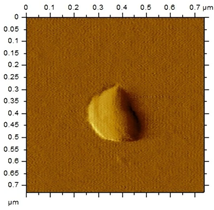
The morphological analysis of the NEs was carried out using the atomic force microscopy (MFA) technique, using the Agilent Technologies 5500 equipment (Agilent, Santa Clara, CA, USA), according to an adaptation by Gündel and collaborators (2018) [26]. To this end, the samples were diluted in ultrapure water (1:10), and then a drop was added to cleaved mica until dry. The micrographs were obtained at room temperature using a non-contact mode with high-resolution SSS-NCL tips (Nanosensors, force constant 48 N/m and resonance frequency 154 kHz, Neuchatel, Switzerland). Images were captured using PicoView 1.14.4 software (Molecular Imaging Corporation, San Diego, CA, USA) and analyzed using PicoImage 5.
2.3.6. Stability Evaluation
The evaluation of the stability of the NEs was carried out following the Cosmetic Product Stability Guide/ANVISA (2004) and Godoi et al. (2017) [27], where the NEs (n = 3) were evaluated according to their physicochemical parameters. The samples were stored in different conditions, room temperature (25 °C)—RT, refrigeration (4 °C)—RE, and a climatic chamber (40 °C and 65% humidity)—CC, and monitored for 0, 7, 15, and 30 days after the day of preparation. We assessed the average droplet diameter, polydispersity index, zeta potential, and pH.
2.3.7. Evaluation of the Antioxidant Capacity
Based on the characterization and stability assessments, we continued with the assessment of antioxidant capacity, using only with the most stable formulations from the different times and storage conditions tested (NE1, NE2, and NE3). To verify the antioxidant capacity of the nanoemulsions, they were divided and stored under different climatic conditions, RT (25 °C), RE (4 °C), CC (40 °C and 65% humidity), and monitored on days 0, 15, and 30 after preparation. The antioxidant capacity was assessed by evaluating the activity of ABTS+· [28] and DPPH· radical scavenging [29]. For the ABTS+· assay, we prepared a Trolox curve including 8 concentrations (10–150 µg/mL). The blank was composed of only ultrapure water. The absorbance reduction was registered for each sample, as well as for the ABTS+·. The antioxidant activity was expressed as µM of Trolox equivalent/μL of the sample. Additionally, for the DPPH· the Trolox curve was used with concentration from 10 to 175 µg/mL. The blank was made of methanol and the DPPH· antioxidant activity was expressed as µM of Trolox equivalent/μL of the sample.
2.3.8. Quantification of Phenolic Compounds
To evaluate the stability profile of the phenolic components present, the free and nanostructured (NE3) açaí extract (4 mg/mL) were quantified immediately after preparation and 30 days after conditioning at RT (25 °C), RE (4 °C), and in CC (40 °C and 65% humidity). Initially, the nanoemulsions containing açaí extract were degraded according to Hara and Radin (1978) [30] and Vassar et al. (2006) [31] using hexane:isopropanol.
2.3.9. Thermal Analysis
Based on the results obtained in the stability assessment and subsequently by analyzing the antioxidant capacity, it was possible to define the formulation that presented the best performance and evaluate its thermal behavior and quantification of phenolic compounds, as well as the safety profile tests against human cells. The thermal evaluation of the free and nanoemulsified extract (4 mg/mL) was carried out according to Song et al. (2021) [32], through thermogravimetric analysis (TGA) using a thermoanalyzer system (TGA—51H from Shimadzu, São Paulo, SP, Brazil) at temperatures that varied until reaching 300 °C, a heating rate of 5 °C/min, and a nitrogen flow rate of 50 mL/min. The TGA data were plotted as temperature versus percentage by mass, from which the start and end temperatures of decomposition were obtained.
2.4. In Vitro Safety Profile
With the aim of evaluating the in vitro safety profile of the açaí-free extract as well as of the NEs, an experimental protocol using human fibroblasts was performed as described below.
2.4.1. Cellular Culture and Treatments
Human fibroblasts (HFF-1 cell line, ATCC SCRC-1041) were purchased from the Rio de Janeiro Cell Bank. These cells were cultured using Dulbecco’s Modified Eagle Medium (DMEM) with 10% of fetal bovine serum (FBS) and 1% of antibiotics (penicillin and streptomycin). The cells were kept in a CO2 incubator at 5% CO2 and 37 °C. Then, the fibroblasts were exposed to a concentration–response curve (0.001–1000 μg/mL) of the açaí-free extract and of the NEs during 24 h. After the incubations, colorimetric and fluorimetric assays were performed to evaluate cellular viability and proliferation as well as parameters related to the oxidative metabolism. The negative control group was composed of cells with cell culture medium only. The following were used for the positive controls: (i) 25 μM of hydrogen peroxide (H2O2) for the viability analysis, the semi-quantification of ROS, and the determination of dsDNA release assays; (ii) 10 μM of sodium nitroprusside for the NO quantification test.
2.4.2. Cellular Viability and Genotoxicity Analysis
After treatments, the HFF-1 cells were evaluated regarding cellular viability and proliferation indexes through MTT assay. The MTT reagent is metabolized by mitochondrial enzymes of alive cells, forming intracellular purple formazan crystals. By adding dimethylsulfoxide (DMSO), formazan crystals are solubilized in the extracellular medium. Absorbance was measured at 570 nm wavelength in a plate reader [2].
To complement the results obtained via MTT assay, we performed the Clonogenic Cell Viability Assay [33]. Cells were plated in a 12-well plate at a final concentration of 1 × 105 cells/mL. After stabilization (overnight), the cells were exposed to the free and nanostructured açaí extract for 24 h at the same concentrations used for the other assays. After the incubation, each well was stained with 0.2% gentian violet dye for 30 min at 37 °C. Then, excess dye was removed using distilled water. The plates were dried, and high-quality images were collected. The number of viable cells was determined using Image J software version 13.0.6.
To measure possible DNA damage, we performed the comet assay [34]. After treating the fibroblasts, the cells were lysed. After the electrophoresis of the slides (prepared with conventional agarose and low-melting agarose), silver nitrate staining was performed. One hundred nuclei per slide (treatment groups) were analyzed in triplicate. The damage index was calculated using the following equation: (n° of nucleusX0+ n° of nucleusX1+ n° of nucleusX2+ n° of nucleusX3+ n° of nucleusX4)+apoptosis. In the equation, 0 = no DNA damage, 4 = maximum DNA damage, and apoptosis = complete DNA degradation and cell death.
Additionally, we performed the GEMO assay [35]. This assay allows the analysis of the treatments’ effect directly on DNA. A free standard DNA was exposed to the concentration of açaí-free extract or nanoemulsion containing the extract for 30 min. Then, PicoGreenTM (Thermo Fisher-P11495; São Paulo, SP, Brazil) reagent was added to check the fluorescence intensity.
2.4.3. Total Levels of Reactive Oxygen Species Measurement
The total levels of ROS were measured via 2′7′ dichlorofluorescein diacetate (DCFH-DA) assay. The DCFH-DA reagent is deacetylated by the cells, forming dichlorofluorescein (DCFH). DCFH can react with ROS molecules, mainly hydrogen peroxide, being transformed into dichlorodihydrofluorescein (DCF) which emits fluorescence. The fluorescence intensity was measured at 525 nm emission and 488 nm excitation in a plate reader (Molecular Devices, San Jose, CA, USA).
2.4.4. Indirect Measurement of Nitric Oxide Production
Nitric oxide (NO) levels were determined through an indirect method based on the use of the Greiss reagent. Absorbance was quantified at 540 nm using an Anthos 2010 plate reader (Biochrom® Anthos 2010, London, UK). This colorimetric assay detects organic nitrate which is a NO metabolite [36].
2.4.5. Analysis of Extracellular dsDNA Release
To complement the MTT assay, the cellular mortality index was determined via extracellular double-strand DNA (dsDNA) quantification using a fluorescent probe called Quant-iTTM PicoGreenTM (Thermo Fisher-P11495; São Paulo, SP, Brazil). PicoGreen has a high affinity to dsDNA. In this sense, an increased fluorescence measured in the extracellular supernatant indicates the release of dsDNA from dead cells [37].
2.5. Statistical Analysis
The obtained results were initially plotted in a Microsoft Excel table (version 2010) and transformed to a percentage of the negative control. Then, the statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test using the GraphPad Prism software (version 5.0). Results with p < 0.05 were considered significant.
3. Results
3.1. Freeze-Dried Hydroalcoholic Açaí Extract Production and Characterization
A purple and homogeneous açaí extract powder was obtained. This extract was characterized via HPLC. Different bioactive molecules were found as part of the extract’s chemical matrix. Considering the non-anthocyanin phenolic compounds, epicatechin was the most present (76.2 ± 1.5 mg/100 g of extract), followed by luteolin and apigenin (42.8 ± 0.6 and 49.9 ± 0.6 mg/100 g of extract, respectively). Regarding the anthocyanin compounds, cyanidin-3-O-rutinoside was the most present (25.9 ± 0.2 mg/100 g of extract).
3.2. Nanoemulsion Containing Açaí Extract Production and Characterization
Six different nanoemulsions were produced using six different concentrations of açaí extract in the formulations NE1-NE6 (0.83; 2; 4; 6; 8; and 20 mg/mL). Table 2 presents the characterization of each nanoformulation after their preparation. It was observed that all the nanoemulsions presented ideal characteristics considering materials in nanoscale. In general, the particles’ average sizes varied between 170 and 200 nm once the açaí extract was increased in the formulations. The PDI was kept below 0.3 for all conditions, except for formulation 6 (20 mg/mL of extract). The ZP was negative for all of the nanoformulations, especially for the nanoemulsion 6, and the pH varied between the nanoemulsions and did not follow a progression pattern.

Table 2.
Characterization of nanoemulsions containing açaí extract immediately after preparation (time zero) at different concentrations NE1-6 (0.83; 2; 4; 6; 8; and 20 mg/mL).
3.2.1. Stability Evaluation
All of the nanoemulsions (NE1–NE6) and the free extract (for the pH) were evaluated regarding their stability over different time points and storage conditions (Table 3). At time zero (after the preparation), NE1 presented a size of 172 ± 1.68 nm, a PDI of 0.207 ± 0.13, a ZP of −12.12 ± 0.39 mV, and a pH of 5.31 ± 0.32. No significant changes were observed in NE1 after 7 days, except for the pH under refrigeration. After 15 days, the ZP decreased to −9.91 ± 1.18 mV at room temperature, while after 30 days, its characteristics remained comparable to the initial values.

Table 3.
Microscopic analysis and stability of nanoemulsions containing açaí extract at different concentrations NE1-NE6 (0.83; 2; 4; 6; 8; and 20 mg/mL).
NE2 presented a size of 194 ± 26.93 nm, a PDI of 0.200 ± 0.05, a ZP of −10.65 ± 0.55 mV, and a pH of 4.97 ± 0.70. After 7 days of analysis, only the ZP significantly decreased to −12.96 ± 1.32 mV under refrigeration. After 15 days, the PDI increased to 0.303 ± 0.05 nm at room temperature, while after 30 days, NE2 presented a significant decrease in the ZP under all storage conditions.
NE3 initially had a size of 194 ± 34.92 nm, a PDI of 0.226 ± 0.10, a ZP of −10.15 ± 3.34 mV, and a pH of 6.59 ± 0.32. After 7 days the pH decreased to 5.32 ± 0.37 at room temperature and 5.00 ± 0.13 at the climatic chamber. After 15 and 30 days, the pH significantly declined under all conditions, accompanied by notable changes in the ZP under refrigeration and the climatic chamber.
NE4 had a size of 187 ± 18.73 nm, a PDI was 0.243 ± 0.06, a zeta potential −14.47 ± 1.16 mV, and a pH of 5.56 ± 0.01. After 7 and 15 days of analysis, the pH significantly changed under room temperature and the climatic chamber, while after 30 days the samples in the climatic chamber did not present any conserved characteristics and it was not possible to continue the measurements.
NE5 at time zero presented a size of 202 ± 13.33 nm, a PDI of 0.240 ± 0.06, a ZP of −13.22 ± 0.83 mV, and a pH of 4.61 ± 0.60. After 7 days, the ZP significantly changed to −16.72 ± 2.01 mV. Interestingly, after 15 and 30 days, only the nanoemulsions kept under refrigeration presented the ideal characteristics. This same profile was found for NE6.
For the free extract, most concentrations showed significant pH variations under all temperature conditions. The morphological analysis via MFA confirmed the expected spherical (though not fully homogeneous) structures of the nanoemulsions, with sizes consistent with dynamic light scattering measurements.
3.2.2. Evaluation of the Antioxidant Capacity
The antioxidant capacity of the NEs and the free extract (Figure 1A–F) was measured using the ABTS+· and DPPH· methods. The evaluations were performed at time zero and after 30 days of maintenance under different temperature conditions. For the ABTS+· assay, it was found that, under refrigeration, NE1 presented 0.25 μmol/μL of Trolox, while after 30 days this formulation presented 0.42 μmol/μL of Trolox. The free extract interestingly showed a higher antioxidant capacity, since at the initial point a value of 1.38 μmol/μL of Trolox was observed for the ABTS+· and 1.35 μmol/μL of Trolox. Under room temperature and the climatic chamber, the obtained values were very similar to the refrigeration, except for the nanoemulsion after 30 days, in which the result was 1.18 μmol/μL of Trolox. Considering NE2, a higher antioxidant activity was observed on the first day of evaluation for all of the temperature conditions when compared with the analysis after 30 days. A similar profile was found for the free extract, and the difference between the nanoemulsions and the free extract was not too evident. NE3 presented an antioxidant capacity similar between nanoemulsions evaluated at time zero and 30 days after production. However, at time zero, the free extract showed a very high antioxidant potential compared with the values found after 30 days.
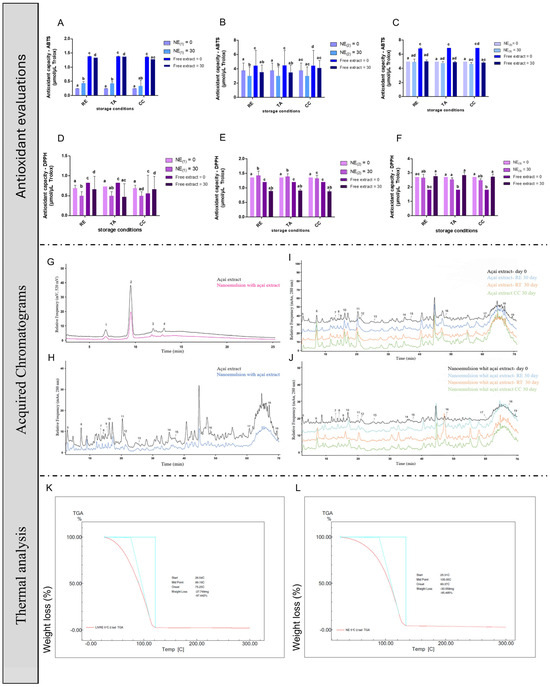
Figure 1.
Antioxidant capacity of açaí-free extract and NEs and stability. The antioxidant capacity of açaí-free extract and NE storage in different conditions using DPPH· and ABTS+· methods. (A) ABTS+· for NE1. (B) ABTS+· for NE2. (C) ABTS+· for NE3. (D) DPPH· for NE1. (E) DPPH· for NE2. (F) DPPH· for NE3. (G,H) HPLC curves for açaí-free extract and NEs. (I) HPLC for açaí-free extract in different temperature conditions. (J) HPLC for NEs in different temperature conditions. (K) Thermal analysis for açaí-free extract. Peak 1: Cyanidin-3-O-glucoside; Peak 2: Cyanidin-3-O-rutinoside; Peak 3: Peonidin-3-O-glucoside; Peak 4: Peonidin-3-O-rutinoside; Peak 5: Gallic acid; Peak 6: Protocatechuic acid; Peak 7: Epigallocatechin; Peak 8: Catechin; Peak 9: Caffeic acid; Peak 10: Vanilic acid; Peak 11:Epicatechin; Peak 12: Syringic acid; Peak 13: p-Coumaric acid; Peak 14: t-Ferulic acid; Peak 15: Dihydroquercetin; Peak 16: Orienthin; Peak 17: t-Cinnamic acid; Peak 18: Luteolin; Peak 19: Apigenin and (L) Thermal analysis of NEs. Statistical analysis was performed via one-way ANOVA, followed by Tukey’s post hoc test. Results with p < 0.05 were considered significant. Different letters indicate statistical differences between groups.
Additionally, regarding the DPPH· assay, the most notable difference compared with the ABTS+· results was that NE2 and NE3 exhibited a higher antioxidant activity in the nanoformulated forms than the free extract across all tested temperatures. In contrast, NE1 did not show the same profile of results, where the free extract had a bigger antioxidant capacity than the nanoemulsion.
The following results were expressed in equivalence to Trolox: evaluation against the radical ABTS+· in refrigeration conditions, ambient temperature, and climatic chamber; availability against the DPPH· radical in refrigeration conditions, ambient temperature, and climatic chamber. The data were expressed as the median and standard deviation (S.D.). The results were analyzed with the aim of comparing the NEs and free extract in their respective concentrations by two-way ANOVA followed by Bonferroni’s post hoc test. Significant results were considered those with p < 0.05 (# p < 0.05; ## p < 0.01; ### p < 0.001). The results were also analyzed with the aim of comparing time zero and 30-day point of each group in their respective concentrations by means of two-way ANOVA followed by Tukey’s post hoc test. Significant results were considered those with p < 0.05 (* p < 0.05; ** p < 0.01; *** p < 0.001).
3.2.3. Quantification of Phenolic Compounds
Figure 1G–J show the quantification of phenolic compounds in the açaí-free extract and in the NEs (at its best formulation—NE3). Figure 1G shows the obtained peaks 1–4, which indicate the presence of Cyanidin-3-O-glucoside, Cyanidin-3-O-rutinoside, Peonidin-3-O-glucoside, and Peonidin-3-O-rutinoside, respectively. Cyanidin-3-O-rutinoside was the major compound found in the açaí chemical matrix. Figure 1H shows the peaks from 5 to 19, representing Gallic acid, Protocatechuic acid, Epigallocatechin, Catechin, Caffeic acid, Vanilic acid, Epicatechin, Syringic acid, p-Coumaric acid, t-Ferulic acid, Dihydroquercetin, Orienthin, t-Cinnamic acid, Luteolin, and Apigenin, respectively.
The major phenolic acid in NE3 and the free extract (4 mg/mL) was vanillic acid. After 30 days, the condition that provided the lowest loss of the majority compound for the açaí-free extract was the climatic chamber and for NE3 it was refrigeration. The major flavonoid in the free extract was epicatechin and the condition that best favored its quantification in 30 days was refrigeration; while the major flavonoid of NE3 was luteolin and its potential shelf life for 30 days was also under refrigeration conditions. The major anthocyanin of NE3 and the free extract was cyanidin-3-O-rutinoside. After 30 days, the free extract in refrigerated conditions was the one that best maintained the proportion of this compound. Meanwhile, NE3 had all its anthocyanins degraded after 30 days, making it impossible to quantify them.
3.2.4. Thermal Analysis
Figure 1K,L represent the mass loss of the groups during the increase in the system temperature. When exposing the free extract to the heating ramp, the first and only stage detected was in the range of 75–100 °C in relation to the loss of free water, resulting in a mass loss of 97% and it being completely degraded after 100 °C. Regarding NE3, we can see a late profile for the beginning of the water loss process, starting its loss process at 89 °C and ending the process at 105 °C, with loss 95% quantified at the end.
3.3. In Vitro Safety Profile
The in vitro safety profile analysis was performed using human fibroblasts (HFF-1 cell line, ATCC RCSC-1041) (Figure 2). The cells were exposed to a concentration curve of NE3 (Figure 2E–H) or to a curve of the açaí-free extract (Figure 2A–D) for 24 h. After 24 h of exposure to the free extract, the fibroblasts maintained a cellular viability index at similar levels to the negative control. Only the highest concentration of the extract increased the levels of NO and none of the concentrations increased the ROS production and dsDNA release compared with the untreated cells.
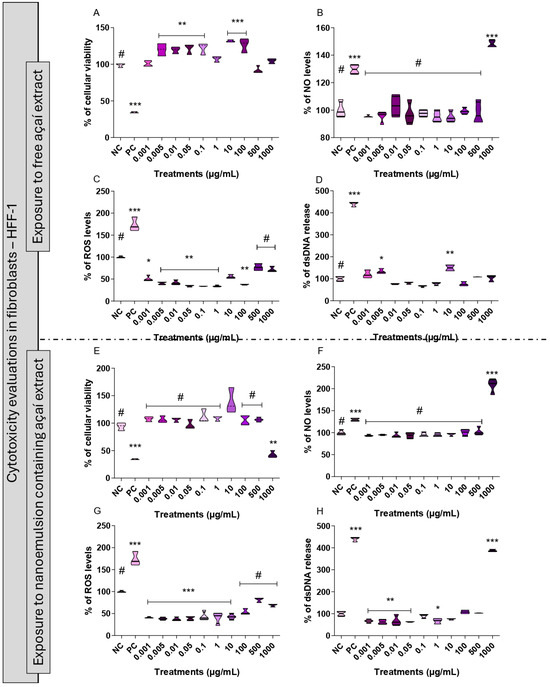
Figure 2.
Safety profile of açaí-free extract and NE3. HFF-1 cell exposure to açaí-free extract for 24 h (A) cell viability, (B) indirect measurement of nitric oxide production, (C) indirect measurement of the production of reactive oxygen species, (D) quantification of extracellular dsDNA. HFF-1 exposure to NE3 for 24 h (E) cell viability, (F) indirect measurement of nitric oxide production, (G) indirect measurement of the production of reactive oxygen species, (H) quantification of extracellular dsDNA. Statistical analysis was performed via one-way ANOVA, followed by Tukey’s post hoc test. Results with p < 0.05 were considered significant. * indicates statistical difference compared with NC. NC = negative control (untreated cells). PC = positive control (cells exposed to hydrogen peroxide or sodium nitroprusside). * p < 0.05 in comparison to NC; ** p < 0.01 in comparison to NC; *** p < 0.001 in comparison to NC; # p < 0.05 in comparison to PC.
A very similar profile was found in the treatments with NE after 24 h of exposure; the fibroblasts maintained a cell viability index similar to the negative control except under the highest concentration tested. In relation to the production of NO and dsDNA, only the highest concentration showed a significant increase in these parameters, but to produce ROS there was no modification when compared with the untreated cells.
Additionally, through the clonogenic assay (Figure 3A–D), it was possible to confirm the cellular response observed via MTT assay. All concentrations of açaí-free extract kept the cellular viability at similar levels to the negative control. The cells exposed to the nanoemulsion containing açaí extract also maintained cellular viability levels close to those found for untreated cells, except for the treatments of 500 and 1000 μg/mL.
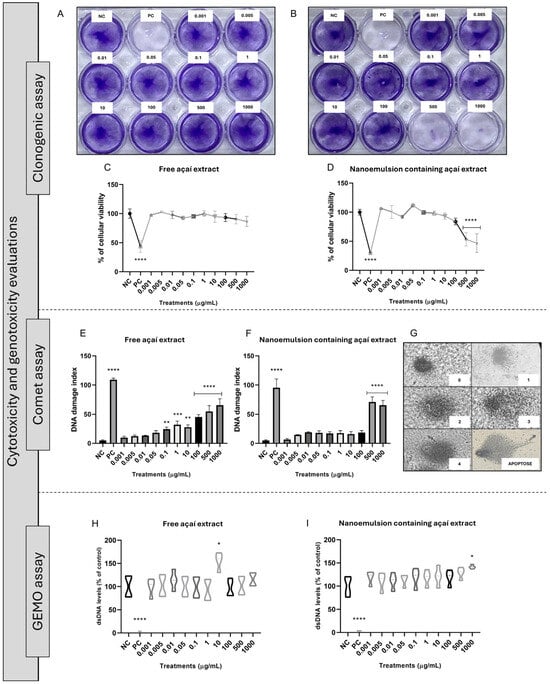
Figure 3.
Reproductive survival of cells and DNA damage assays of açaí-free extract and NE3. (A,C) Clonogenic assay of the HFF-1 cells exposed to açaí-free extracts. (B,D) Clonogenic assay of the HFF-1 cells exposed to NE3. (E) Comet assay of açaí-free extract, (F) comet assay of NE3, (G) GEMO assay of açaí-free extract, (H) GEMO assay of free açaí extract, (I) GEMO assay of NE3. Statistical analysis was performed via one-way ANOVA, followed by Tukey’s post hoc test. Results with p < 0.05 were considered significant. * indicates statistical difference compared with NC. NC = negative control (untreated cells). PC = positive control (cells exposed to hydrogen peroxide or sodium nitroprusside). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Figure 3E,F show the comet assay results. Interestingly, from 0.1 to 1000 μg/mL of the açaí-free extract the cells presented an increased DNA damage index in comparison to the untreated cells. On the other hand, considering the nanoemulsion containing açaí extract, only the concentrations of 500 and 1000 μg/mL caused potential DNA damage compared with the negative control. Figure 3H,I demonstrate the obtained results for the GEMO assay. None of the tested concentrations of free or nanostructured açaí extract caused DNA breaks directly in comparison to the negative control.
4. Discussion
This experimental study aimed to develop and characterize a nanoemulsion containing açaí extract (NE) and assess its in vitro safety profile using a cell culture of human cells. The findings revealed promising results, highlighting the potential of nanotechnology for natural health products, such as açaí extract.
After the preparation of the hydroalcoholic açaí extract, it was lyophilized, yielding a purple-colored powder. An HPLC analysis revealed the presence of various bioactive molecules, confirming the complex chemical composition of the açaí extract. Considering the non-anthocyanin compounds, epicatechin was the most present, followed by luteolin and apigenin, while considering the anthocyanin molecules, cyanidin-3-O-rutinoside (82.6% of anthocyanins) was the most concentrated one, followed by cyanidin-3-O-glucoside (11.3% of anthocyanins). These results corroborate the chemical matrix found by Machado et al. (2016) [7], as well as the analysis performed by Neri-Numa et al. (2018) [38] and Yamaguchi et al. (2015) [4]. Additionally, Schauss et al. (2006) [39] and Matta et al. (2020) [40] also performed experimental studies with açaí extract and they were able to detect the presence of cyanidin-3-O-rutinoside and cyanidin-3-O-glucoside as major compounds.
Later, different NEs were developed. In general, these nanoformulations presented a size of approximately 170–200 nm. According to Komaiko & Mcclements (2015) [41], this size standard is characteristic of nanoemulsions produced via the spontaneous emulsification method. It is important to mention that similar sizes were found for nanodroplets containing açaí oil [42]. These nanoformulations showed a PDI under 0.3, which indicates size uniformity [43]. ZP was also verified, and it is used to determine the nanodroplet surface electric potential that is dependent on the degree of ionization of the molecules present at the O/W interface. A ZP of ±30 mV would be enough to guarantee system stability [44] for systems stabilized by electrostatic mechanisms. The NEs presented a ZP that varied between −10 and −23 mV. The negative charge could be due to the presence of Tween 80® in the formulation, because this reagent provides to the system a characteristic that the nanodroplets end up being stable against aggregation by steric repulsion, not electrostatic, because of the presence of large polymeric groups. Finally, in terms of physicochemical characterization, the obtained pH was acidic.
Microscopic analysis techniques are well recognized as being fundamentally important for validating the results obtained through dynamic light scattering [45]. Atomic force microscopy uses a microscope that allows the development of analytical techniques used for ultra-high resolution surface analysis, generating micrographs of surface topography and being able to measure three-dimensional profiles with nanometric resolution [46]. Through the microscopic analysis of each nanoformulation (NE1-NE6) it was possible to observe spherical droplets characteristic of nanoemulsions. Additionally, the microscopy corroborates the results found using the dynamic light scattering technique, since the NE1-NE6 presented sizes of 183, 174, 175, 169, 171, and 180 nm, respectively.
Another important aspect to be considered when working with nanoformulations is the analysis of stability. NE1 maintained most of its physical chemical characteristics for 30 days; however, there was a significant alteration in its condition of assembly in the CC, since in this condition there was an increase in its diameter after 30 days compared with time zero. NE4 from the 15th day of evaluation showed alterations in the CC condition with its average diameter above 900 nm and the PDI of 0.6. In addition to presenting a macroscopically viscous appearance, the analysis of stability and subsequent antioxidant capacity is therefore ruled out for this NE. NE5 from the 15th day of testing showed alterations under the RT condition, with its average diameter being above 900 nm and PDI > 1, demonstrating the total destabilization of the formulation. Subsequently, on the 30th day of evaluation, alterations were observed in the CC condition for NE5 with its average diameter exceeding 700 nm and PDI 0.3, thus both formulations, NE4 and NE5, were discarded in the analysis of stability and antioxidant capacity. After 7 days of preparation, NE6 presented alterations in the condition of RT with its average diameter passing 600 nm and the PDI was 0.5. Based on the results obtained, NE2 and NE3 consistently maintained satisfactory nanometric characteristics across all preparation conditions and evaluation time points.
To evaluate the antioxidant activity, the free extracts and NEs (NE1, NE2, and NE3) were evaluated in periods of 0 and 30 days under different storage conditions, employing ABTS+· and DPPH· radical sequestration techniques. It was observed that in all the concentrations of the NE1-3 they presented free radical scavenging activity against the ABTS+· radical in a crescent manner. Under refrigeration it was observed that NE2-3 exhibited behavior like the free extract. This same profile is repeated with greater intensity against the DPPH· radical. Thus, this data demonstrates that nanosystems can protect natural extracts from possible degradation, enabling the greater detection of antioxidant activity when nanoemulsified. It is important to mention that, when there is interest in determining the antioxidant capacity of a natural product, it is necessary to use different methods to better show their effect [47]. This is the reason why it was decided to perform two different assays to achieve this objective. Del Pozo-Insfran, Brenes and Talcott, (2004) [48] worked with açaí pulp and showed a relatively high antioxidant capacity (48.6 μmol Trolox equivalents/mL) in relation to other well-known antioxidant fruits (rich in anthocyanins), such as bilberries (4.6–31.1 μmol TE/g), strawberries (18.3–22.9), raspberries (19.2–22.6), and blackberries (13.7–25.1), among others. Yamaguchi et al. (2015) [4] performed a study to identify the antioxidant activity of açaí pulp. The authors demonstrated that this natural product presents significant antioxidant activity, corroborating with the data found in this study. Martinez et al. (2018) [47] evaluated the hydroalcoholic extract of açaí seeds against DPPH· and ABTS+· and confirmed the antioxidant profile of this fruit. Additionally, the study carried out by Melo et al. (2021) [49] evaluated the antioxidant potential of the açaí seeds’ extract prepared at RT (25 °C), and the extract showed antioxidant activity against DPPH· (622.81 μmol TEAC/g) and ABTS+· (763.09 μmol TEAC/g) radicals. In this regard, based on the stability tests as well as on the antioxidant determination, it was decided to continue all the additional assay using only NE3.
The thermal analysis showed that the degradation temperature of the NE was higher than that of the açaí-free extract. This result confirms the protective effect of the nanocarrier produced in this study. Izadiyan et al. (2021) [50] performed a study using 5-fluorouracil, a chemotherapy drug, and a nanoemulsion containing this drug. Similarly to the findings here, the authors found that the nanoemulsion could protect the drug against the increase in temperature.
The stability profile of the phenolic compounds that are part of the açaí chemical matrix was tested. The composition of the NE and of the extract in its free form were determined immediately after preparation and 30 days after storage under different temperature conditions. After 30 days, the condition that provided a minor loss of the major compound for the free extract was CC, while for NE3 was under refrigeration. The major flavonoid in the free extract was epicatechin and the condition that best favored its quantification after 30 days was refrigeration; the major flavonoid of NE3 was luteolin and its storage potential for 30 days was also best under refrigeration. The most present anthocyanin for both the free extract and NE3 was cyanidin-3-O-rutinoside. After 30 days, the free extract under refrigeration was the one which better maintained the proportion of this compound. Meanwhile, NE3 had all of its anthocyanins degraded after the 30 days of preparation, making it impossible to quantify. The compounds quantified for the free extract and for NE3 corroborate the results presented by Melo et al. (2016) [49]. These authors describe the quantification via UHPLC-LTQ-Orbitrap MS/MS of a crude açaí seed extract, identifying vanillic acid, coumaric acid, ferulic acid, catechin, caffeic acid, and apigenin, which were reported for the first time in açaí seeds. In the study carried out by Del Pozo-Insfran, Brenes and Talcott (2004) [48], the authors state that the predominant polyphenols found in açaí pulp were ferulic acid, epicatechin, p-hydroxybenzoic acid, gallic acid, protocatechuic acid, catechin, ellagic acid, vanillic acid, and p-coumaric acid.
After establishing the production and characterization of the NE, the determination of its in vitro safety profile using a human cell line was conducted. According to Bondarenko et al. (2021) [51], in vitro experimental models are a very common way to scientifically evaluate the nanotoxicity of nanomaterials, using different assays to elucidate the biological mechanisms generated by nanocarriers. A comparison between the free and nanostructured açaí extract was performed. Initially, human fibroblasts were exposed to the açaí-free extract for 24 h. After 24 h of incubation, none of the concentrations reduced cell viability compared with the control, although 1000 μg/mL increased the NO levels. A similar profile was observed with the nanoemulsion: at 1000 μg/mL, it reduced cell viability and elevated NO, ROS (also at 500 μg/mL), and dsDNA release. Overall, both the free and nanoformulated açaí extract demonstrated a satisfactory in vitro safety profile, as most tested concentrations did not adversely affect cellular homeostasis. This pattern was also confirmed via clonogenic assay, where only the 500 and 1000 μg/mL concentrations of the nanoemulsion caused cellular death and none of the tested concentrations of free extract induced any impact to the cellular viability. It is important to mention that these fibroblasts are not colony-forming; however, most of the cells were concentrated in the center of the wells. Additionally, the comet and GEMO assays demonstrated that mainly the nanoemulsion containing açaí extract does not cause expressive DNA damage. As expected, only the 500 and 1000 μg/mL concentrations caused significant DNA damage compared with the negative control. In the study carried out by Zain et al., (2021) [52], they evaluated the cell viability of 3T3 fibroblast cells against a palm leaf extract enriched with flavonoids (Elaeis guineensis Jacq.) that were free and nanoemulsified (7.81 to 1000 µg/mL) using an MTT assay. The results showed that the free extract did not demonstrate cytotoxicity effects against 3T3 cells, while the NE was considered non-toxic at concentrations lower than 500 µg/mL. When we relate the study by Zain et al. (2021) [52] with the data presented in this study, we can verify similar profiles of apparent non-toxicity in both groups, both the free and nanoemulsified extracts against HFF-1 cells at the lowest concentrations. Furthermore, they carried out the evaluation after only 48 h of exposure, while in this work an evaluation was conducted up to 72 h of exposure, demonstrating an increased action profile of the açaí-free extract, as well as its nanoemulsified version. Our research group has been working with açaí extract for 10 years. Many of our published studies have already investigated the in vitro safety profile of açaí-free extract in different cells lines [7,9,53]. However, this is the first study which demonstrates the in vitro safety profile of a NE, reinforcing the innovative aspect of this experimental investigation.
There are some limitations of this study that must be declared. To develop the nanoemulsions, surfactants that are not considered green materials were used. The safety evaluations were carried out using cell culture and only one cell line; further experiments with other cell lines could help confirm the obtained results described here. The use of more complex systems to analyze nanotoxicity, such as in vivo experiments, for example, would be of great interest.
5. Conclusions
In this study, NEs were developed through the spontaneous emulsification method. According to the data found, we can state that it was possible to formulate stable and homogeneous NEs. According to the morphological evaluation of the six NEs, their average droplet diameters corroborate the values obtained using the dynamic light scattering technique. Furthermore, the physicochemical stability profile was also analyzed for 30 days under three different storage conditions: climatic chamber, room temperature, and refrigeration. According to the study of antioxidant capacity using the ABTS+· and DPPH· radical methods, it was found that the best storage condition was refrigeration, and the formulations that maintained its physicochemical stability and antioxidant capacity was 4 mg/mL. It was also found that the 4 mg/mL NE had a better thermal profile compared with the free extract. In vitro safety profile tests were also carried out; through these tests it was possible to observe that both the free extract and the NE were not cytotoxic or genotoxic and a cytoprotective effect was also detected in relation to a possible oxidative stress process, thus suggesting antioxidant action. Based on the results found, it is concluded that it was possible to develop, characterize, and evaluate the safety profile of NE containing a hydroalcoholic extract of Euterpe oleracea Mart. as a promising nanosystem for diverse applicability.
Author Contributions
Conceptualization, S.N.d.G., D.V.d.S., L.P. and F.R.F.; methodology, S.N.d.G., D.V.d.S., T.F., L.P., F.R.F., G.K.C.B., C.B.D. and S.S.; validation, S.N.d.G., D.V.d.S., L.P., F.R.F. and S.S.; formal analysis, S.N.d.G., D.V.d.S., L.P., F.R.F. and S.S.; investigation, S.N.d.G., D.V.d.S., T.F., L.P. and F.R.F.; resources, T.E., F.D.P.M., A.G., D.A.P., A.K.M. and A.F.O.; data curation, S.N.d.G., D.V.d.S., L.P., G.K.C.B., C.B.D. and F.R.F.; writing—original draft preparation, S.N.d.G., D.V.d.S., T.F., L.P., G.K.C.B., C.B.D., A.K.M. and A.F.O.; writing—review and editing, S.N.d.G., D.V.d.S., T.F., L.P., F.R.F., S.S., T.E., F.D.P.M., A.G., D.A.P., A.K.M. and A.F.O.; visualization, T.E., F.D.P.M., A.G., D.A.P., A.K.M. and A.F.O.; supervision, A.K.M. and A.F.O.; project administration, A.K.M. and A.F.O.; funding acquisition, A.K.M. and A.F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES)-Brazil, grant number 001 and by “Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul” (FAPERGS), grant number 24/2551-0001295-8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the financial support of CAPES and Franciscan University in Brazil. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) is also acknowledged for the fellowship awarded to A.F.O. The authors extend their appreciation to Linear Scientific Visual Communication (linear.ilustra@gmail.com) for the professional design of the Graphical Abstract presented in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AP | Aqueous phase |
| CC | Climatic chamber |
| MCTs | Medium chain triglycerides |
| NE | Nanoemulsion containing açaí extract |
| NE1 | Nanoemulsion 1 with 0.83 mg/mL of extract |
| NE2 | Nanoemulsion 2 with 2 mg/mL of extract |
| NE3 | Nanoemulsion 3 with 4 mg/mL of extract |
| NE4 | Nanoemulsion 4 with 6 mg/mL of extract |
| NE5 | Nanoemulsion 5 with 8 mg/mL of extract |
| NE6 | Nanoemulsion 6 with 20 mg/mL of extract |
| NO | Nitric oxide |
| O | Oily |
| OP | Oily phase |
| PDI | Polydispersity index |
| RE | Refrigeration |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| ZP | Zeta potential |
| W | Water |
References
- Silva, D.F.; Vidal, F.C.; Santos, D.; Costa, M.C.; Morgado-Díaz, J.A.; do Desterro Soares Brandão Nascimento, M.; de Moura, R.S. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement. Altern. Med. 2014, 14, 175. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Wu, T.; Jensen, G.S.; Shauss, A.G.; Wu, X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem. 2010, 122, 610–617. [Google Scholar] [CrossRef]
- Dias, M.M.; Martino, H.S.; Noratto, G.; Roque-Andrade, A.; Stringheta, P.C.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Anti-inflammatory activity of polyphenolics from açai (Euterpe oleracea Martius) in intestinal myofibroblasts CCD-18Co cells. Food Funct. 2015, 6, 3249–3256. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.; Pereira, L.F.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Spada, P.D.; Dani, C.; Bortolini, G.V.; Funchal, C.; Henriques, J.A.; Salvador, M. Frozen fruit pulp of Euterpe oleraceae Mart. (Acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J. Med. Food 2009, 12, 1084–1088. [Google Scholar] [CrossRef]
- Barbosa, P.O.; Pala, D.; Silva, C.T.; de Souza, M.O.; do Amaral, J.F.; Vieira, R.A.; Folly, G.A.; Volp, A.C.; de Freitas, R.N. Açai (Euterpe oleracea Mart.) pulp dietary intake improves cellular antioxidant enzymes and biomarkers of serum in healthy women. Nutrition 2016, 32, 674–680. [Google Scholar] [CrossRef]
- Machado, A.K.; Andreazza, A.C.; da Silva, T.M.; Boligon, A.A.; do Nascimento, V.; Scola, G.; Duong, A.; Cadoná, F.C.; Ribeiro, E.E.; da Cruz, I.B. Neuroprotective Effects of Açaí (Euterpe oleracea Mart.) against Rotenone In Vitro Exposure. Oxid. Med. Cell Longev. 2016, 2016, 8940850. [Google Scholar] [CrossRef]
- Machado, A.K.; Cadona, F.C.; Assmann, C.E.; Andreazza, A.C.; Duarte, M.M.M.F.; dos Santos Branco, C.; Zhou, X.; de Souza, D.V.; Ribeiro, E.E.; da Cruz, I.B.M. Açaí (Euterpe oleracea Mart.) has anti-inflammatory potential through NLRP3-inflammasome modulation. J. Funct. Foods 2019, 56, 364–371. [Google Scholar] [CrossRef]
- de Souza, D.V.; Pappis, L.; Bandeira, T.T.; Sangoi, G.G.; Fontana, T.; Rissi, V.B.; Sagrillo, M.R.; Duarte, M.M.; Duarte, T.; Bodenstein, D.F.; et al. Açaí (Euterpe oleracea Mart.) presents anti-neuroinflammatory capacity in LPS-activated microglia cells. Nutr. Neurosci. 2022, 25, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Cadoná, F.C.; de Souza, D.V.; Fontana, T.; Bodenstein, D.F.; Ramos, A.P.; Sagrillo, M.R.; Salvador, M.; Mota, K.; Davidson, C.B.; Ribeiro, E.E.; et al. Açaí (Euterpe oleracea Mart.) as a Potential Anti-neuroinflammatory Agent: NLRP3 Priming and Activating Signal Pathway Modulation. Mol. Neurobiol. 2021, 58, 4460–4476. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2007, 23, 1361–1394. [Google Scholar] [CrossRef]
- Montagner, G.E.; Ribeiro, M.F.; Cadoná, F.C.; Franco, C.; Gomes, P. Liposomes loading grape seed extract: A nanotechnological solution to reduce wine-making waste and obtain health-promoting products. Future Foods 2022, 5, 100144. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mandal, A. Formation, characteristics and oil industry applications of nanoemulsions: A review. J. Pet. Sci. Eng. 2021, 206, 109402. [Google Scholar] [CrossRef]
- Montes de Oca-Ávalos, J.M.; Candal, R.J.; Herrera, M.L. Colloidal properties of sodium caseinate-stabilized nanoemulsions prepared by a combination of a high-energy homogenization and evaporative ripening methods. Food Res. Int. 2017, 100, 143–150. [Google Scholar] [CrossRef]
- Ismail, A.; Nasr, M.; Sammour, O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: Improved pharmacokinetic/pharmacodynamic properties. Int. J. Pharm. 2020, 583, 119402. [Google Scholar] [CrossRef]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef]
- Chen, B.H.; Stephen Inbaraj, B. Nanoemulsion and Nanoliposome Based Strategies for Improving Anthocyanin Stability and Bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, C.A.S.; Taarji, N.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A. Formulation and characterization of water-in-oil nanoemulsions loaded with açaí berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Res. Int. 2018, 106, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chavhan, S.; Sawant, K.K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; Dos Santos Almeida, J.C.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef]
- Copetti, P.M.; Gündel, S.D.S.; de Oliveira, P.S.B.; Favarin, F.R.; Ramos, A.P.; Pintos, F.G.; Pappis, L.; Gündel, A.; Machado, A.K.; Ourique, A.F.; et al. Development, characterisation, stability study and antileukemic evaluation of nanoemulsions containing. Nat. Prod. Res. 2022, 36, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.S.; Machado, D.C.; Machado, M.M.; Dos Santos, G.F.; Algarve, T.D.; Marinowic, D.R.; Ribeiro, E.E.; Soares, F.A.; Barbisan, F.; Athayde, M.L.; et al. The protective effects of guaraná extract (Paullinia cupana) on fibroblast NIH-3T3 cells exposed to sodium nitroprusside. Food Chem. Toxicol. 2013, 53, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; Piana, M.; Kubiça, T.F.; Mario, D.N.; Dalmolin, T.V.; Bonez, P.C.; Weiblen, R.; Lovato, L.; Alvez, S.H.; Campos, M.M.A.; et al. HPLC analysis and antimicrobial, antimycobacterial and antiviral activities of Tabernaemontana catharinensis. J. Appl. Biomed. 2015, 13, 7–18. [Google Scholar] [CrossRef]
- Bazana, M.T.; da Silva, S.S.; Codevilla, C.F.; de Deus, C.; Lucas, B.N.; Ugalde, G.A.; Mazutti, M.A.; Flores, E.M.M.; Barin, J.S.; da Silva, C.B.; et al. Development of nanoemulsions containing Physalis peruviana calyx extract: A study on stability and antioxidant capacity. Food Res. Int. 2019, 125, 108645. [Google Scholar] [CrossRef]
- da Silva Gündel, S.; de Souza, M.E.; Quatrin, P.M.; Klein, B.; Wagner, R.; Gündel, A.; Vaucher, R.A.; Santos, R.C.V.; Ourique, A.F. Nanoemulsions containing Cymbopogon flexuosus essential oil: Development, characterization, stability study and evaluation of antimicrobial and antibiofilm activities. Microb. Pathog. 2018, 118, 268–276. [Google Scholar] [CrossRef] [PubMed]
- de Godoi, S.N.; Quatrin, P.M.; Sagrillo, M.R.; Nascimento, K.; Wagner, R.; Klein, B.; Santos, R.C.V.; Ourique, A.F. Evaluation of Stability and In Vitro Security of Nanoemulsions Containing. Biomed Res. Int. 2017, 2017, 2723418. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Vassar, V.; Hagen, C.; Ludwig, J.; Thomas, R.; Zhou, J. One-step method of phosphatidylcholine extraction and separation. BioTechniques 2006, 42, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef]
- Cubillos-Rojas, M.; Amair-Pinedo, F.; Peiró-Jordán, R.; Bartrons, R.; Ventura, F.; Rosa, J.L. The E3 ubiquitin protein ligase HERC2 modulates the activity of tumor protein p53 by regulating its oligomerization. J. Biol. Chem. 2014, 289, 14782–14795. [Google Scholar] [CrossRef]
- Nadin, S.B.; Vargas-Roig, L.M.; Ciocca, D.R. A silver staining method for single-cell gel assay. J. Histochem. Cytochem. 2001, 49, 1183–1186. [Google Scholar] [CrossRef]
- Cadoná, F.; Mania-Cattani, M.; Machado, A.; Oliveira, R.; Flôres, E.; Assmann, C.; Algarve, T.; da Cruz, I. Genomodifier capacity assay: A non-cell test using dsDNA molecules to evaluate the genotoxic/genoprotective properties of chemical compounds. Anal. Methods 2014, 21, 8559–8568. [Google Scholar] [CrossRef]
- Choi, W.S.; Shin, P.G.; Lee, J.H.; Kim, G.D. The regulatory effect of veratric acid on NO production in LPS-stimulated RAW264.7 macrophage cells. Cell Immunol. 2012, 280, 164–170. [Google Scholar] [CrossRef]
- Susan, J.A.; José, C.; Janet, R.E. Picogreen quantitation of DNA: Effective evaluation of samples pre-or post-PCR. Nucleic Acids Res. 1996, 13, 2623–2625. [Google Scholar]
- Neri-Numa, I.A.; Soriano Sancho, R.A.; Pereira, A.P.A.; Pastore, G.M. Small Brazilian wild fruits: Nutrients, bioactive compounds, health-promotion properties and commercial interest. Food Res. Int. 2018, 103, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef] [PubMed]
- Komaiko, J.; Sastrosubroto, A.; McClements, D.J. Formation of oil-in-water emulsions from natural emulsifiers using spontaneous emulsification: Sunflower phospholipids. J. Agric. Food Chem. 2015, 63, 10078–10088. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P.; et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B 2017, 166, 301–310. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J. Control Release 2020, 328, 895–916. [Google Scholar] [CrossRef]
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal nanomedicines: Recent advancements, challenges, opportunities and regulatory overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef]
- Martinez, R.M.; Guimarães, D.A.B.; Berniz, C.R.; Abreu, J.P.; Rocha, A.P.M.D.; Moura, R.S.; Resende, A.C.; Teodoro, A.J. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods 2018, 7, 178. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Talcott, S.T. Phytochemical composition and pigment stability of Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef]
- Melo, P.S.; Arrivetti, L.O.R.; Alencar, S.M.; Skibsted, L.H. Antioxidative and prooxidative effects in food lipids and synergism with α-tocopherol of açaí seed extracts and grape rachis extracts. Food Chem. 2016, 213, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Shameli, K.; Teow, S.-Y.; Yusefi, M.; Kia, P.; Rasouli, E.; Tareq, M.A. Anticancer Activity of 5-Fluorouracil-Loaded Nanoemulsions Containing Fe3O4/Au Core-Shell Nanoparticles. J. Mol. Struct. 2021, 1245, 131075. [Google Scholar] [CrossRef]
- Bondarenko, O.; Mortimer, M.; Kahru, A.; Feliu, N.; Javed, I.; Kakinen, A.; Lin, S.; Xia, T.; Song, Y.; Davis, T.P.; et al. Nanotoxicology and Nanomedicine: The Yin and Yang of Nano-Bio Interactions for the New Decade. Nano Today 2021, 39, 101184. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.S.C.; Ediriisinghe, S.L.; Kim, C.-H.; de Zoysa, M.; Shaari, K. Nanoemulsion of flavonoid-enriched oil palm (Elaeis guineensis Jacq.) leaf extract enhances wound healing in zebrafish. Phytomedicine Plus 2021, 1, 100124. [Google Scholar] [CrossRef]
- Davidson, C.B.; Sangoi, G.; de Souza, D.; Fontana, T.; Bonazza, G.K.C.; Schultz, J.V.; Martins, M.O.; Fagan, S.B.; Machado, A.K. Potencial anti-inflamatório do extrato de Euterpe oleracea Mart. em células pulmonares ativadas. Discip. Sci. 2022, 23, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).