Physicochemical Stability of the Pigment Produced by Pseudofusicoccum adansoniae: Influence of pH, Temperature, Additives, and Light Exposure
Abstract
1. Introduction
2. Methods
2.1. Submerged Culture and Extract Production
2.2. Stability of the Crude Pigment Extract
2.3. Temperature Stability
2.4. pH Stability
2.4.1. Effect of Additives and Ions
2.4.2. Effect of the Storage
2.5. Statistical Analysis
3. Results
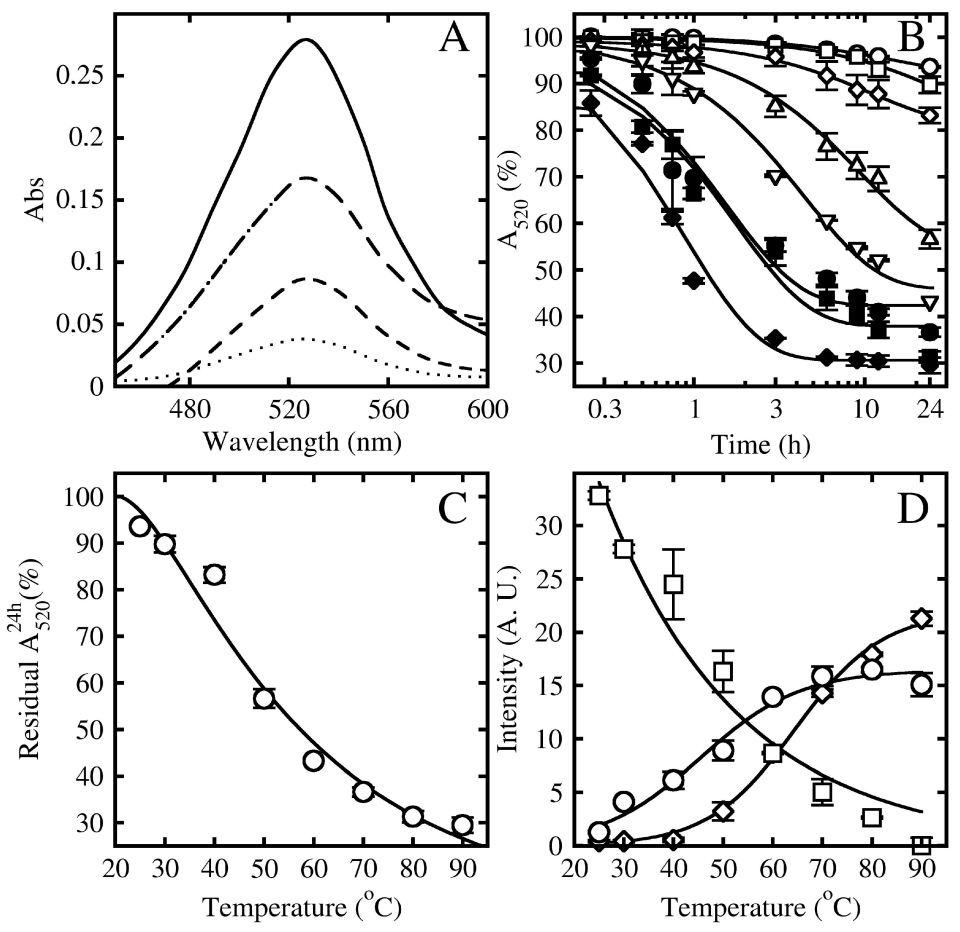
3.1. Effect of Temperature
3.2. Effect of pH
3.3. Effect of Additives and Ions on Color Stability
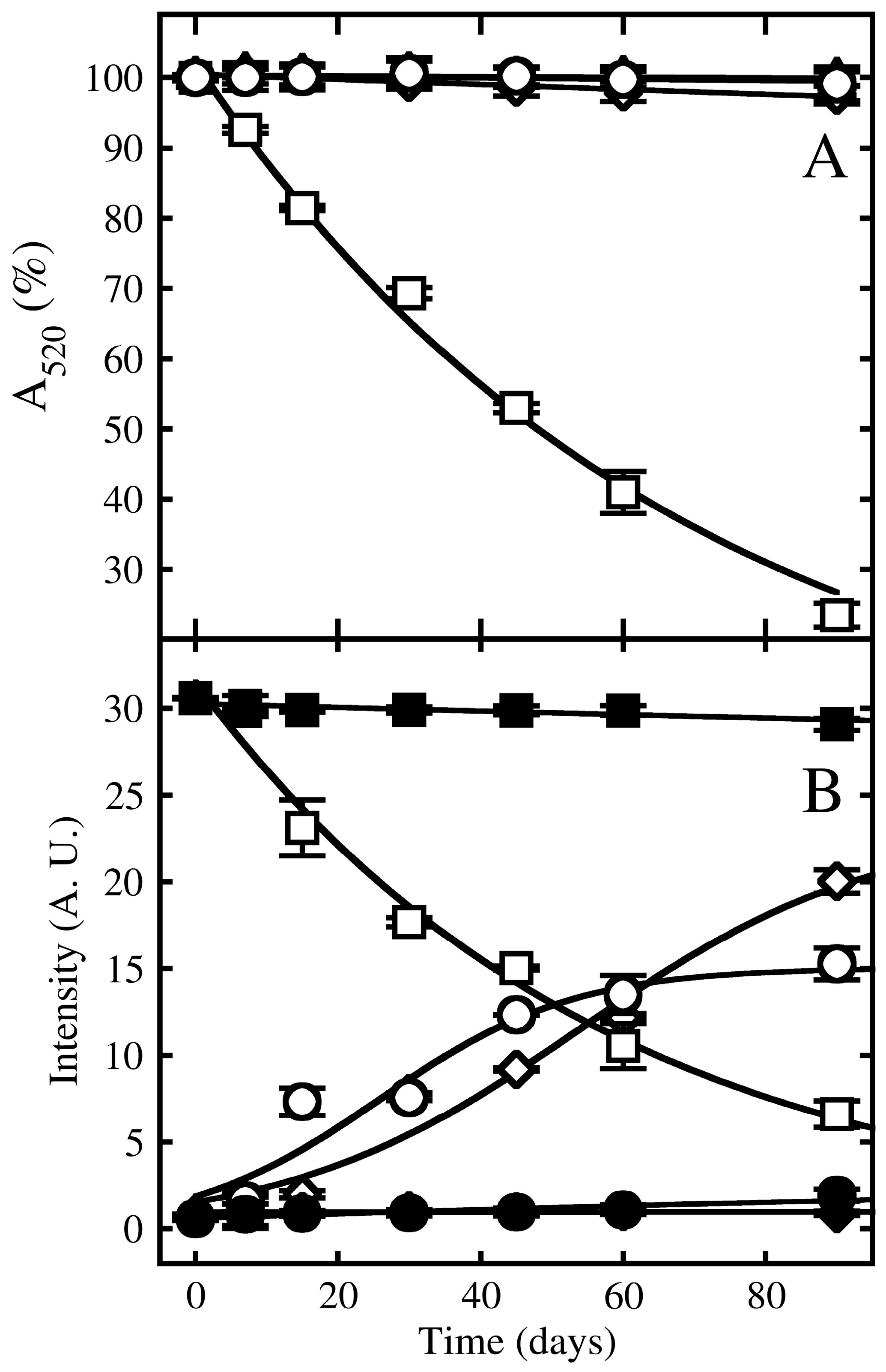
3.4. Effect of Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413. [Google Scholar] [CrossRef]
- Dufossé, L. Biotechnological approaches in the production of fungal pigments. In Fungal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2025; pp. 449–466. [Google Scholar] [CrossRef]
- Sivakumar, B.; Rao, N.R.; Poornamath, B.P.; Jayaram, S.; Sarojini, S. Multifarious pigment producing fungi of Western Ghats and their potential. Plant Sci. Today 2022, 9, 733–747. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current perspective of yellowish-orange pigments from microorganisms—A review. J. Clean. Prod. 2018, 180, 168–182. [Google Scholar] [CrossRef]
- Aguilar, D.; Morales-Oyervides, L.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Raso, J.; Montañez, J. Effect of ozone processing conditions on stability of fungal pigments. Innov. Food Sci. Emerg. Technol. 2018, 45, 255–263. [Google Scholar] [CrossRef]
- Kour, D.; Yadav, N.; Yadav, A.N. Endophytic Fungi as Emerging Bioresources for Bioactive Compounds for Sustainable Development. J. Appl. Biol. Biotechnol. 2023, 11, i–iii. [Google Scholar] [CrossRef]
- Umar, A.; Darwish, D.B.E.; Alenezi, M.A. Fungal pigments: Secondary metabolites and their application. In Fungal Secondary Metabolites: Synthesis and Applications in Agroecosystem; Elsevier: Amsterdam, The Netherlands, 2024; pp. 173–195. [Google Scholar] [CrossRef]
- Chatragadda, R.; Dufossé, L. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Global Natural Colorants Market Research Report 2022 (Status and Outlook), 2022. Available online: https://www.databridgemarketresearch.com/reports/global-colorants-market (accessed on 12 December 2023).
- Anshi; Kapil, S.; Goswami, L.; Sharma, V. Unveiling the Intricacies of Microbial Pigments as Sustainable Alternatives to Synthetic Colorants: Recent Trends and Advancements. Micro 2024, 4, 621–640. [Google Scholar] [CrossRef]
- Abel, G.; Amobonye, A.; Bhagwat, P.; Pillai, S. Diversity, stability and applications of mycopigments. Process Biochem. 2023, 133, 270–284. [Google Scholar] [CrossRef]
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Lee, S.Y.; Coutinho, J.A.P.; Weingarten, M. Sustainable recovery of microbial-derived natural pigments using deep eutectic solvents: Advances, potential, and challenges. Sep. Purif. Technol. 2025, 361, 131413. [Google Scholar] [CrossRef]
- Gonçalves, B.R.P.; Machado, B.A.S.; Hanna, S.A.; Umsza-Guez, M.A. Prospective Study of Microbial Colorants under the Focus of Patent Documents. Recent Pat. Biotechnol. 2020, 14, 184–193. [Google Scholar] [CrossRef]
- de Souza, T.L.S.; de Souza, C.O.; Umsza-Guez, M.A. Technological and Scientific Prospection on Pigments Produced by Microorganisms. Recent Pat. Biotechnol. 2022, 17, 364–375. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, P.; Singh, M.; Joshi, D.; Soni, R.; Suyal, D.C. Microbial pigments: Overview and industrial perspective. In Microbial Bioactive Compounds: Industrial and Agricultural Applications; Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 291–297. [Google Scholar] [CrossRef]
- Malik, K.; Tokas, J.; Tokkas, J.; Goyal, S.; Pigments, M. 2012. Available online: http://ijmrt.inpressco.com (accessed on 12 December 2023).
- Lin, L.; Xu, J. Production of Fungal Pigments: Molecular Processes and Their Applications. J. Fungi 2023, 9, 44. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Torres, F.A.E.; Zaccarim, B.R.; de Lencastre Novaes, L.C.; Jozala, A.F.; Santos, C.A.D.; Teixeira, M.F.S.; Santos-Ebinuma, V.C. Natural colorants from filamentous fungi. Appl. Microbiol. Biotechnol. 2016, 100, 2511–2521. [Google Scholar] [CrossRef]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Molelekoa, T.B.J.; Augustyn, W.; Regnier, T.; da Silva, L.S. Chemical characterization and toxicity evaluation of fungal pigments for potential application in food, phamarceutical and agricultural industries. Saudi J. Biol. Sci. 2023, 30, 103630. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, D.Í.; Gurgel, R.S.; de Souza, A.T.F.; Matias, R.R.; de Souza Falcão, L.; Chaves, F.C.M.; da Silva, G.F.; Martínez, J.G.; de Lima Procópio, R.E.; Fantin, C.; et al. Isolation and Identification of Pigment-Producing Endophytic Fungi from the Amazonian Species Fridericia chica. J. Fungi 2024, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, Y.; Fang, X.; Wu, L.; Zhang, Y. Isolation and identification of pigment-producing filamentous fungus DBFL05 and its pigment characteristics and chemical structure. CyTA-J. Food 2023, 21, 374–385. [Google Scholar] [CrossRef]
- Venil, C.K.; Lakshmanaperumalsamy, P.; Venil, C.K.; Lakshmanaperumalsamy, P. An Insightful Overview on Microbial Pigment, Prodigiosin, An Insightful Overview on Microbial Pigment, Prodigiosin. Electron. J. Biol. 2013, 5, 49–61. [Google Scholar]
- Venil, C.K.; Lakshmanaperumalsamy, P. Application of response surface methodology in medium optimization for protease production by the new strain of Serratia marcescens SB08. Pol. J. Microbiol. 2009, 58, 117–124. [Google Scholar]
- Parmar, R.S.; Singh, C. A comprehensive study of eco-friendly natural pigment and its applications. Biochem. Biophys. Rep. 2018, 13, 22–26. [Google Scholar] [CrossRef]
- Maeda, R.N.; Pantoja, L.; Kiyoko, L.; Yuyama, O.; Chaar, J.M. Stability of ascorbic acid and anthocyanin on camu-camu (Myrciaria dubia McVaugh) nectar. Ciênc. Tecnol. Aliment. 2007, 27, 313–316. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U. Colorimetric Characterization for Comparative Analysis of Fungal Pigments and Natural Food Colorants. J. Agric. Food Chem. 2006, 54, 7027–7035. [Google Scholar] [CrossRef] [PubMed]
- De Faria Silva, L.A.; Alves, M.F.; Filho, D.F.; Takahashi, J.A.; Santos, L.S.; De Carvalho, S.A. Pigment produced from Arcopilus aureus isolated from grapevines: Promising natural yellow colorants for the food industry. Food Chem. 2022, 389, 132967. [Google Scholar] [CrossRef] [PubMed]
- Gudepu, R.; Thirunahari, U. Exploring the Therapeutic Potential of Microbial Pigments: A Comprehensive Review. Int. J. Sci. Res. (IJSR) 2024, 13, 1233–1240. [Google Scholar] [CrossRef]
- Pavlic, D.; Wingfield, M.J.; Barber, P.; Slippers, B.; Hardy, G.E.S.J.; Burgess, T.I. Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 2008, 100, 851–866. [Google Scholar] [CrossRef]
- Alves, B.V.B.; Borges, L.J.; Hanna, S.A.; Soares, M.B.P.; Bezerra, D.P.; Moreira, L.L.P.F.; Borges, W.d.S.; Portela, R.W.D.; Fernandez, C.C.; Umsza-Guez, M.A. Pigment Production by Pseudofusicoccum sp.: Extract Production, Cytotoxicity Activity, and Diketopiperazines Identified. Microorganisms 2025, 13, 277. [Google Scholar] [CrossRef]
- Ministério da Saúde-MS Agência Nacional de Vigilância Sanitária-ANVISA. Available online: https://www.gov.br/anvisa/pt-br (accessed on 12 December 2023).
- Reynoso, R.; Garcia, F.A.; Morales, D.; de Mejia, E.G. Stability of Betalain Pigments from a Cactacea Fruit. J. Agric. Food Chem. 1997, 45, 2884–2889. [Google Scholar] [CrossRef]
- Luo, W.; Xue, H.; Xiong, C.; Li, J.; Tu, Y.; Zhao, Y. Effects of temperature on quality of preserved eggs during storage. Poult. Sci. 2020, 99, 3144–3157. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, S.; Zavanella, L. Chilled or frozen? Decision strategies for sustainable food supply chains. Int. J. Prod. Econ. 2012, 140, 731–736. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Oishi, B.O.; Pandey, A.; Soccol, C.R. Biopigments from Monascus: Strains Selection, Citrinin Production and Color Stability. Braz. Arch. Biol. Technol. 2005, 48, 885–894. [Google Scholar] [CrossRef]
- Fabre, C.E.; Santerre, A.L.; Loret, M.O.; Baberian, R.; Pareilleux, A.; Goma, G.; Blanc, P.J. Production and Food Applications of the Red Pigments of Monascus ruber. J. Food Sci. 1993, 58, 1099–1102. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Müller, B.L.; Moritz, D.E.; De Oliveira, D.; Schmidell, W.; Ninow, J.L. Thermal stability of natural pigments produced by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2013, 2, 278–284. [Google Scholar] [CrossRef]
- Santos-Ebinuma, V.C.; Roberto, I.C.; Teixeira, M.F.S.; Pessoa, A. Improving of red colorants production by a new Penicillium purpurogenum strain in submerged culture and the effect of different parameters in their stability. Biotechnol. Prog. 2013, 29, 778–785. [Google Scholar] [CrossRef]
- Amr, A.; Al-Tamimi, E. Stability of the crude extracts of Ranunculus asiaticus anthocyanins and their use as food colourants. Int. J. Food Sci. Technol. 2007, 42, 985–991. [Google Scholar] [CrossRef]
- Righetto, A.M.; Beleia, A.; Helena, S.; Ferreira, P. Physicochemical Stability of Natural or Pre-Sweetened Frozen Passion Fruit Juice. Braz. Arch. Biol. Technol. 1999, 42, 1–4. [Google Scholar] [CrossRef][Green Version]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U. Photostability of Natural Orange−Red and Yellow Fungal Pigments in Liquid Food Model Systems. J. Agric. Food Chem. 2009, 57, 6253–6261. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation—Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Santos, D.T.; Angela, M.; Meireles, A. Carotenoid Pigments Encapsulation: Fundamentals. Tech. Recent Trends 2010, 4, TOCENGJ-4-42. [Google Scholar] [CrossRef]
- Priatni, S. Encapsulation and Stability Study of Monascus Fermented Rice Extract. Procedia Chem. 2015, 17, 189–193. [Google Scholar] [CrossRef]


| Additives (BCR (%)/Standard Deviation) | Time (h) | ||
|---|---|---|---|
| 0 | 12 | 24 | |
| Control | 100 ± 0.06 | 95.86 ± 0.06 | 93.59 ± 0.15 |
| Aspartame | 100 ± 0.46 | 94.86 ± 0.99 | 91.72 ± 1.36 |
| Sodium cyclamate | 100 ± 1.56 | 95.69 ± 0.82 | 92.38 ± 1.46 |
| Calcium propionate | 100 ± 1.52 | 95.59 ± 0.97 | 94.40 ± 1.23 |
| Potassium sorbate | 100 ± 2.02 | 96.05 ± 1.67 | 94.12 ± 1.46 |
| Acesulfame | 100 ± 1.95 | 87.74 ± 0.97 | 84.50 ± 0.83 |
| Maltitol | 100 ± 2.29 | 95.81 ± 1.49 | 94.68 ± 1.53 |
| Inulin | 100 ± 1.24 | 96.19 ± 1.30 | 94.05 ± 1.42 |
| Potassium benzoate | 100 ± 1.36 | 93.62 ± 2.06 | 91.01 ± 1.78 |
| Ions (BCR (%)/Standard Deviation) | |||
| Control | 100 ± 0.06 | 95.86 ± 0.06 | 93.59 ± 0.15 |
| Calcium chloride 0.1 M | 100 ± 1.39 | 94.90 ± 1.52 | 92.42 ± 1.35 |
| Calcium chloride 0.5 M | 100 ± 1.41 | 97.53 ± 1.30 | 96.17 ± 1.28 |
| Sodium nitrate 0.1 M | 100 ± 1.74 | 94.33 ± 1.53 | 92.89 ± 1.46 |
| Sodium nitrate 0.5 M | 100 ± 1.78 | 98.16 ± 0.41 | 96.86 ± 0.50 |
| Magnesium sulfate 0.1 M | 100 ± 1.18 | 94.81 ± 1.34 | 93.00 ± 1.49 |
| Magnesium sulfate 0.5 M | 100 ± 0.74 | 94.16 ± 1.72 | 92.85 ± 1.64 |
| Zinc sulfate 0.1 M | 100 ± 4.17 | 91.92 ± 3.13 | 89.81 ± 2.90 |
| Zinc sulfate 0.5 M | 100 ± 1.20 | 94.01 ± 0.97 | 93.03 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, B.V.B.; Borges, L.J.; Moreau, V.H.; Hanna, S.A.; Umsza-Guez, M.A. Physicochemical Stability of the Pigment Produced by Pseudofusicoccum adansoniae: Influence of pH, Temperature, Additives, and Light Exposure. Appl. Sci. 2025, 15, 8800. https://doi.org/10.3390/app15168800
Alves BVB, Borges LJ, Moreau VH, Hanna SA, Umsza-Guez MA. Physicochemical Stability of the Pigment Produced by Pseudofusicoccum adansoniae: Influence of pH, Temperature, Additives, and Light Exposure. Applied Sciences. 2025; 15(16):8800. https://doi.org/10.3390/app15168800
Chicago/Turabian StyleAlves, Bianca Vilas Boas, Letícia Jambeiro Borges, Vitor Hugo Moreau, Samira Abdallah Hanna, and Marcelo Andrés Umsza-Guez. 2025. "Physicochemical Stability of the Pigment Produced by Pseudofusicoccum adansoniae: Influence of pH, Temperature, Additives, and Light Exposure" Applied Sciences 15, no. 16: 8800. https://doi.org/10.3390/app15168800
APA StyleAlves, B. V. B., Borges, L. J., Moreau, V. H., Hanna, S. A., & Umsza-Guez, M. A. (2025). Physicochemical Stability of the Pigment Produced by Pseudofusicoccum adansoniae: Influence of pH, Temperature, Additives, and Light Exposure. Applied Sciences, 15(16), 8800. https://doi.org/10.3390/app15168800







