Microbial and Sensory Evaluation of Halophytes Cultivated in a Soilless System Under Different Salinities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Halophyte Species
2.2. Growth Conditions
2.3. Microbial Quality Evaluation
2.4. Sensory Analytical Method
2.5. Statistical Analysis
3. Results and Discussion
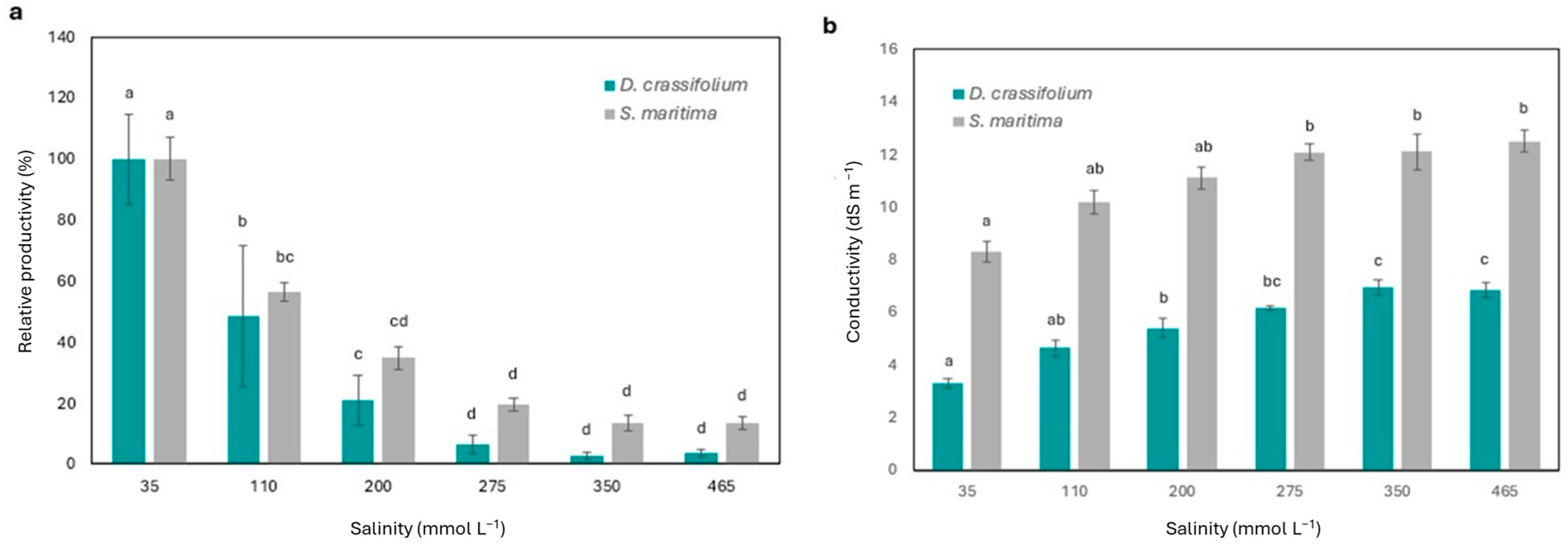
3.1. Effects of Salinity on Productivity and Conductivity
3.2. Microbial Quality
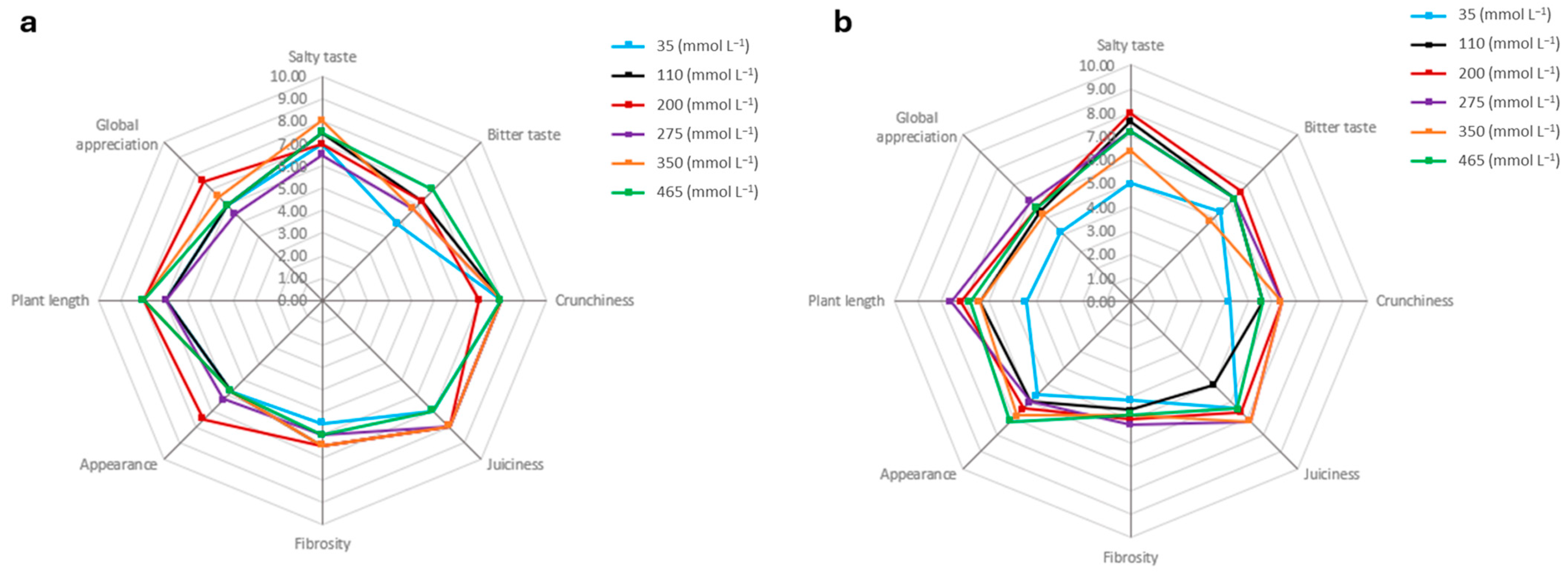
3.3. Sensory Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as New Model Plant Species for Salt Tolerance Strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Vineeth, V.T.; Kumar, S.; Shukla, M.; Chinchmalatpure, A.; Sharma, P.C. Ecological and Economic Potential of Major Halophytes and Salt Tolerant Vegetation in India. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83881-063-4. [Google Scholar]
- Fatnani, D.; Patel, M.; Parida, A.K. Regulation of Chromium Translocation to Shoot and Physiological, Metabolomic, and Ionomic Adjustments Confer Chromium Stress Tolerance in the Halophyte Suaeda maritima. Environ. Pollut. 2023, 320, 121046. [Google Scholar] [CrossRef]
- Grigore, M. Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer: New York, NY, USA, 2020; ISBN 9783030576349. [Google Scholar]
- Farrell, C.; Mitchell, R.E.; Szota, C.; Rayner, J.P.; Williams, N.S.G. Green Roofs for Hot and Dry Climates: Interacting Effects of Plant Water Use, Succulence and Substrate. Ecol. Eng. 2012, 49, 270–276. [Google Scholar] [CrossRef]
- Zurayk, R.A.; Baalbaki, R. Inula crithmoides: A Candidate Plant for Saline Agriculture. Arid Soil Res. Rehabil. 1996, 10, 213–223. [Google Scholar] [CrossRef]
- Castroviejo, S.; Aedo, C.; Laínz, M.; Muñoz Garmedndia, F.; Nieto Feliner, G.P.J.; Benedí, C. Flora Ibérica: Plantas Vasculares de La Península Ibérica e Islas Baleares. In Flora Iberica: Real Jardín Botánico; CSIC: Madrid, Spain, 1997. [Google Scholar]
- Mohamed, N.A.F.; Al-Touby, S.S.; Hossain, M.A. Evaluation of Cytotoxic and Antioxidant Activities of Different Polarities Extracts of Suaeda maritima. Biocatal. Agric. Biotechnol. 2022, 42, 102370. [Google Scholar] [CrossRef]
- Lima, A.R.; Gama, F.; Castañeda-Loaiza, V.; Costa, C.; Schüler, L.M.; Santos, T.; Salazar, M.; Nunes, C.; Cruz, R.M.S.; Varela, J.; et al. Nutritional and Functional Evaluation of Inula crithmoides and Mesembryanthemum nodiflorum Grown in Different Salinities for Human Consumption. Molecules 2021, 26, 4543. [Google Scholar] [CrossRef]
- Duarte, B.; Caçador, I. Iberian Halophytes as Agroecological Solutions for Degraded Lands and Biosaline Agriculture. Sustainability 2021, 13, 1005. [Google Scholar] [CrossRef]
- Wungrampha, S.; Rawat, N.; Pareek, A. Survival Strategies in Halophytes: Adaptation and Regulation. In Handbook of Halophytes; Grigore, M., Ed.; Springer: Cham, Switzerland, 2021; pp. 1591–1612. ISBN 978-3-030-57635-6. [Google Scholar]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible Halophytes of the Mediterranean Basin: Potential Candidates for Novel Food Products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Linekha, R.; Jeno, J.G.A.; Abirami, K.; Yamunadevi, B.; Nakkeeran, E. Halophiles and Their Adaptations: A Comprehensive Review on Recent Progress and Prospects in Biodesalination Applications. Clean 2024, 52, 2300260. [Google Scholar] [CrossRef]
- Correia, I.; Antunes, M.; Tecelão, C.; Neves, M.; Pires, C.L.; Cruz, P.F.; Rodrigues, M.; Peralta, C.; Pereira, D.; Reboredo, F.; et al. Nutritive Value and Bioactivities of a Halophyte Edible Plant. Plants 2024, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs Cultivated Halophytes: Nutritional and Functional Differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Peddi, P.; Ptsrk, P.R.; Rani, N.U.; Tulasi, S.L. Green Synthesis, Characterization, Antioxidant, Antibacterial, and Photocatalytic Activity of Suaeda maritima (L.) Dumort Aqueous Extract-Mediated Copper Oxide Nanoparticles. J. Genet. Eng. Biotechnol. 2021, 19, 131. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Sousa, J.; Bento-Silva, A.; Duarte, B.; Caçador, I.; Salazar, M.; Mecha, E.; Serra, A.T.; Bronze, M.R. Soilless Cultivated Halophyte Plants: Volatile, Nutritional, Phytochemical, and Biological Differences. Antioxidants 2023, 12, 1161. [Google Scholar] [CrossRef]
- Giamperi, L.; Bucchini, A.; Fraternale, D.; Genovese, S.; Curini, M.; Ricci, D. Composition and Antioxidant Activity of Inula crithmoides Essential Oil Grown in Central Italy (Marche Region). Nat. Prod. Commun. 2010, 5, 315–318. [Google Scholar] [CrossRef]
- Qneibi, M.; Hanania, M.; Jaradat, N.; Emwas, N.; Radwan, S. Inula Viscosa (L.) Greuter, Phytochemical Composition, Antioxidant, Total Phenolic Content, Total Flavonoids Content and Neuroprotective Effects. Eur. J. Integr. Med. 2021, 42, 101291. [Google Scholar] [CrossRef]
- Hoather, R.C.; Rackham, R.F. Oxidised Nitrogen in Waters and Sewage Effluents Observed by Ultra-Violet Spectrophotometry. Analyst 1959, 84, 548–551. [Google Scholar] [CrossRef]
- ISO 4833-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 17410; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. ISO: Geneva, Switzerland, 2019.
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0,95. ISO: Geneva, Switzerland, 2008.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli. ISO: Geneva, Switzerland, 2001.
- ISO 6888-1_2021; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species)—Part 1_ Method Using Baird-Parker Agar Medium. ISO: Geneva, Switzerland, 2021.
- Stone, H.; Sidel, J.; Oliver, S.; Woolsey, A.; Singleton, R.C. Sensory Evaluation by Quantitative Descriptive Analysis. In Descriptive Sensory Analysis in Practice; Gacula, M.C., Jr., Ed.; Food & Nutrition Press, Inc.: Scottsdale, AZ, USA, 2004; pp. 24–32. ISBN 9780470385036. [Google Scholar]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Salt Stress Effects on Growth, Pigments, Proteins and Lipid Peroxidation in Salicornia persica and S. europaea. Biol. Plant. 2009, 53, 243–248. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Matabane, R. Tolerance of Salt Marsh Ecotone Species to Salinity and Inundation. Master’s Thesis, Nelson Mandela University, Gqeberha, South Africa, 2018. [Google Scholar]
- Hassan, M.A.; Chaura, J.; López-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-Invasive Plants vs. Halophytes in Mediterranean Salt Marshes: Stress Tolerance Mechanisms in Two Related Species. Front. Plant Sci. 2016, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Agarie, S.; Shimoda, T.; Shimizu, Y.; Baumann, K.; Sunagawa, H.; Kondo, A.; Ueno, O.; Nakahara, T.; Nose, A.; Cushman, J.C. Salt Tolerance, Salt Accumulation, and Ionic Homeostasis in an Epidermal Bladder-Cell-Less Mutant of the Common Ice Plant Mesembryanthemum crystallinum. J. Exp. Bot. 2007, 58, 1957–1967. [Google Scholar] [CrossRef]
- Choi, S.-C.; Lim, S.-H.; Kim, S.-H.; Choi, D.-G.; Kim, J.-G.; Choo, Y.-S. Growth and Solute Pattern of Suaeda maritima and Suaeda asparagoides in an Abandoned Salt Field. J. Ecol. Environ. 2012, 35, 351–358. [Google Scholar] [CrossRef]
- Alhdad, G.M. Responses of Suaeda maritima to Flooding and Salinity. PhD Thesis, University of Sussex, Brighton, UK, 2012. [Google Scholar]
- Lopes, T.F.; Ortigueira, J.; Matos, C.T.; Reis, A.; Francisco, G. Conceptual Design of an Autotrophic Multi-Strain Microalgae-Based Biorefinery: Preliminary Techno-Economic and Life Cycle Assessments. Fermentation 2023, 9, 255. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official Journal of the European Union, L 338, 1–26, 22 December 2005. Available online: https://eur-lex.europa.eu/eli/reg/2005/2073/oj/eng (accessed on 9 April 2025).
- European Commission. Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Official Journal of the European Union, L 322, 12–29, 7 December 2007. Available online: https://eur-lex.europa.eu/eli/reg/2007/1441/oj/eng (accessed on 9 April 2025).
- Nikalje, G.C.; Nikam, T.D.; Suprasanna, P. Looking at Halophytic Adaptation to High Salinity Through Genomics Landscape. Curr. Genom. 2017, 18, 542–552. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Gaudette, N.J. The Impact of Salt Replacers and Fl Avor Enhancer on the Processing Characteristics and Consumer Acceptance of Restructured Cooked Hams. Meat Sci. 2014, 96, 1165–1170. [Google Scholar] [CrossRef]
- Martins-Noguerol, R.; Cambrollé, J.; Machilla-Leytón, J.M.; Puerto-Marchena, A.; Muñoz-Vallés, S.; Millian-Linares, M.C.; Millán, F.; Martínez-Force, E.; Figueroa, M.E.; Pedroche, J.; et al. Influence of Soil Salinity on the Protein and Fatty Acid Composition of the Edible Halophyte Halimione portulacoides. Food Chem. 2021, 352, 129370. [Google Scholar] [CrossRef] [PubMed]

| Halophyte Species | Microbial Groups (Log CFU g/plant −1) | Salinity (mmol L−1) | ||
|---|---|---|---|---|
| 35 | 200 | 465 | ||
| D. crassifolium | Aerobic microorganisms at 30 °C | 2.00 ± 0.06 aA | 3.08 ± 0.05 bB | 3.61 ± 0.05 cC |
| M. nodiflorum | 2.98 ± 0.03 aB | 3.26 ± 0.12 aB | 3.06 ± 0.08 aB | |
| I. crithmoides | 4.54 ± 0.20 aC | 5.18 ± 0.14 bC | 5.38 ± 0.05 bD | |
| S. maritima | 2.65 ± 0.03 bB | 2.48 ± 0.01 aA | 2.60 ± 0.06 abA | |
| D. crassifolium | Aerobic microorganisms at 6.5 °C (psychrotrophics) | 2.00 ±0.01 aA | 2.35 ± 0.49 aA | 3.07 ± 0.16 aB |
| M. nodiflorum | 2.35 ± 0.49 aA | 2.15 ± 0.21 aA | 2.97 ± 0.10 aB | |
| I. crithmoides | 2.35 ± 0.49 aA | 2.50 ± 0.28 aA | 2.15 ± 0.21 aA | |
| S. maritima | 2.48 ± 0.01 aA | 2.80 ± 0.14 abA | 3.14 ± 0.20 cB | |
| D. crassifolium | Filamentous fungi | 2.45 ± 0.21 aAB | 3.80 ± 0.03 bA | 4.38 ± 0.05 cC |
| M. nodiflorum | 2.15 ± 0.21 aA | 1.24 ± 1.75 aA | 2.30 ± 0.43 aA | |
| I. crithmoides | 2.63 ± 0.21 aAB | 3.00 ± 0.06 aA | 3.11 ± 0.01 aAB | |
| S. maritima | 3.03 ± 0.11 aB | 3.02 ± 0.09 aA | 3.29 ± 0.02 aB | |
| D. crassifolium | Yeasts | ≤2 | ≤2 | ≤2 |
| M. nodiflorum | ≤2 | ≤2 | ≤2 | |
| I. crithmoides | ≤2 | ≤2 | ≤2 | |
| S. maritima | ≤2 | ≤2 | ≤2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintas, C.; Lima, A.R.; Gama, F.; Nunes, C.; Salazar, M.; Barreira, L. Microbial and Sensory Evaluation of Halophytes Cultivated in a Soilless System Under Different Salinities. Appl. Sci. 2025, 15, 8799. https://doi.org/10.3390/app15168799
Quintas C, Lima AR, Gama F, Nunes C, Salazar M, Barreira L. Microbial and Sensory Evaluation of Halophytes Cultivated in a Soilless System Under Different Salinities. Applied Sciences. 2025; 15(16):8799. https://doi.org/10.3390/app15168799
Chicago/Turabian StyleQuintas, Célia, Alexandre R. Lima, Florinda Gama, Carla Nunes, Miguel Salazar, and Luísa Barreira. 2025. "Microbial and Sensory Evaluation of Halophytes Cultivated in a Soilless System Under Different Salinities" Applied Sciences 15, no. 16: 8799. https://doi.org/10.3390/app15168799
APA StyleQuintas, C., Lima, A. R., Gama, F., Nunes, C., Salazar, M., & Barreira, L. (2025). Microbial and Sensory Evaluation of Halophytes Cultivated in a Soilless System Under Different Salinities. Applied Sciences, 15(16), 8799. https://doi.org/10.3390/app15168799






