Flow Velocity Distribution Downstream of Nanofibrous Filter in Minichannel Determined by Particle Image Velocimetry Method
Abstract
1. Introduction
2. Materials and Methods
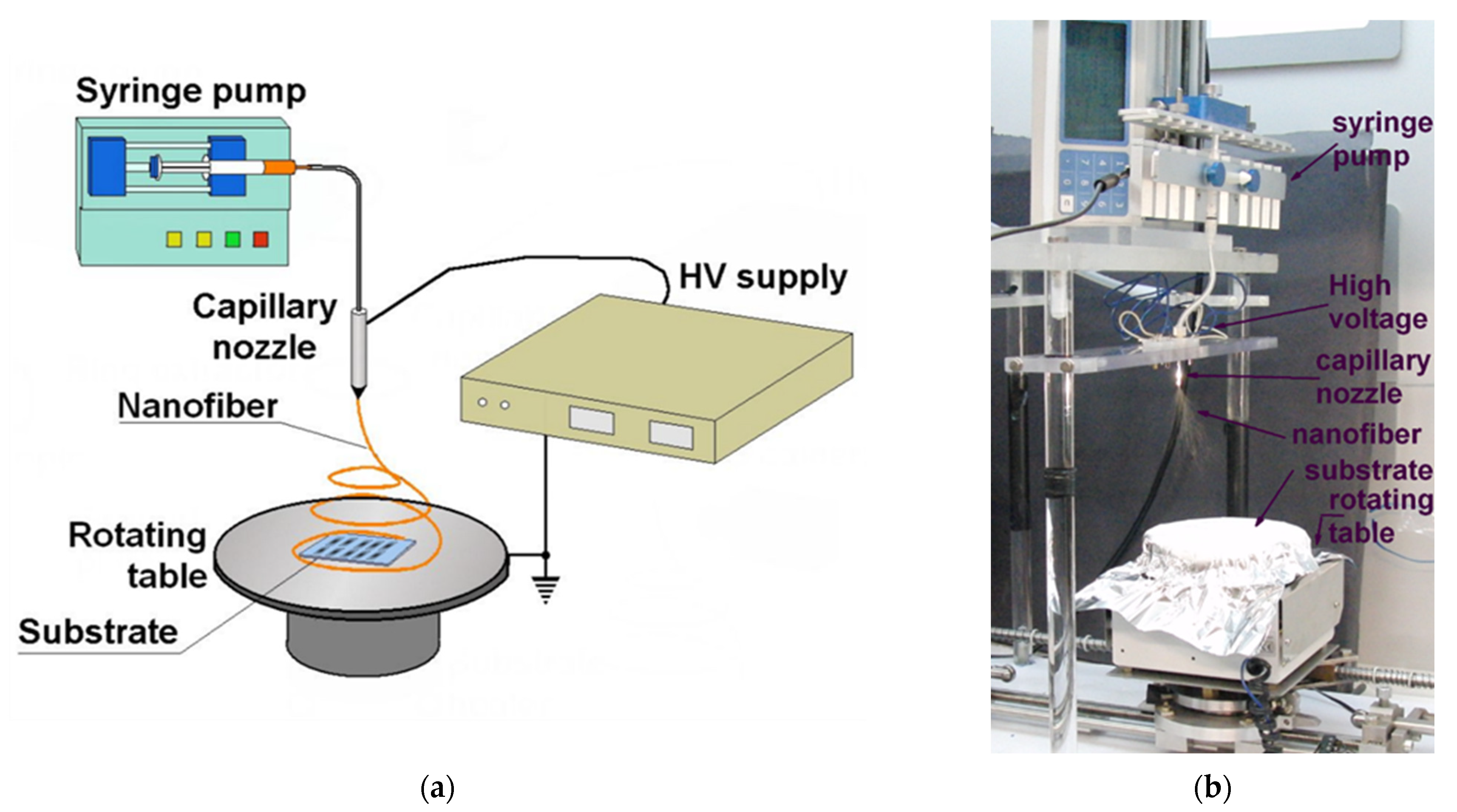
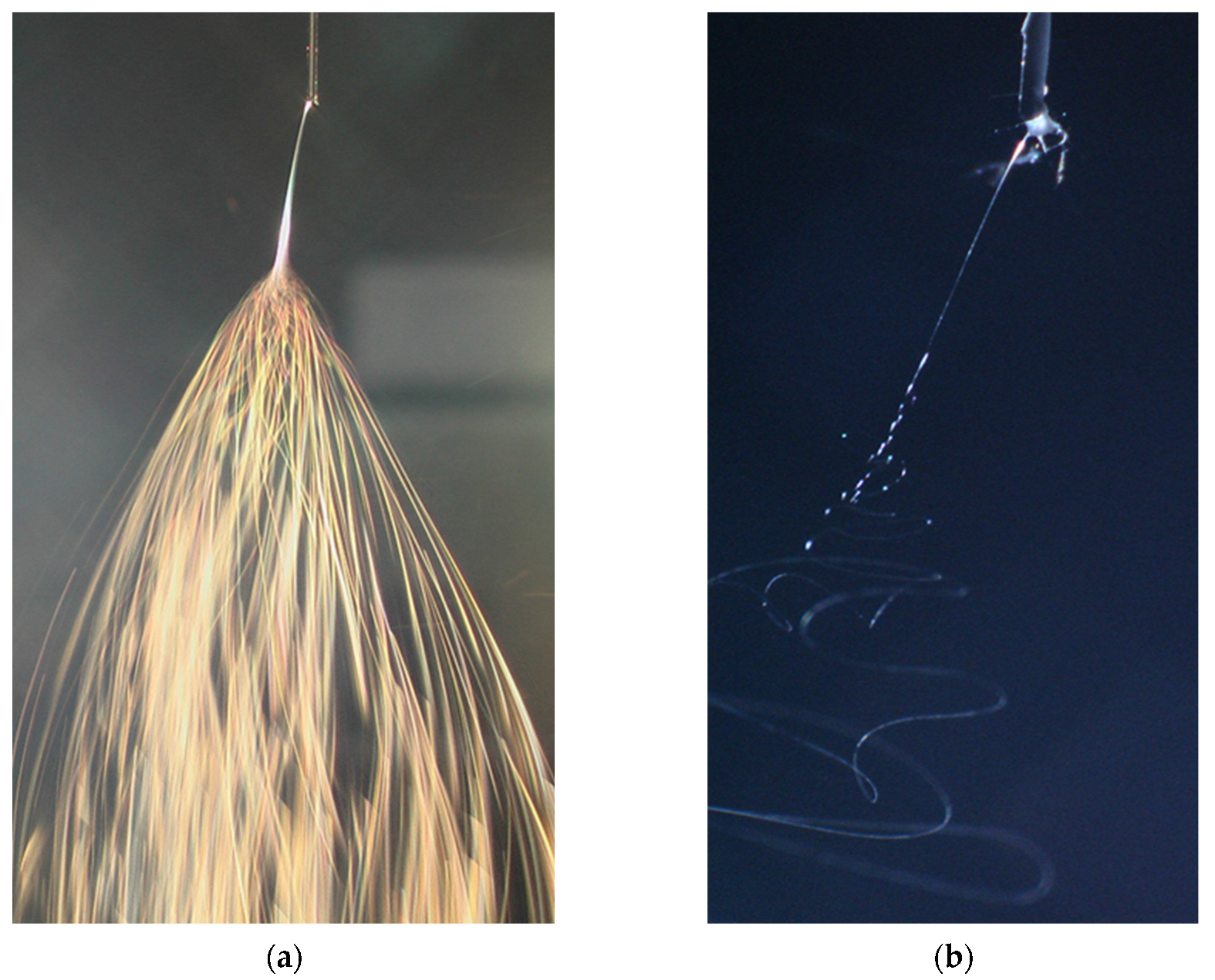
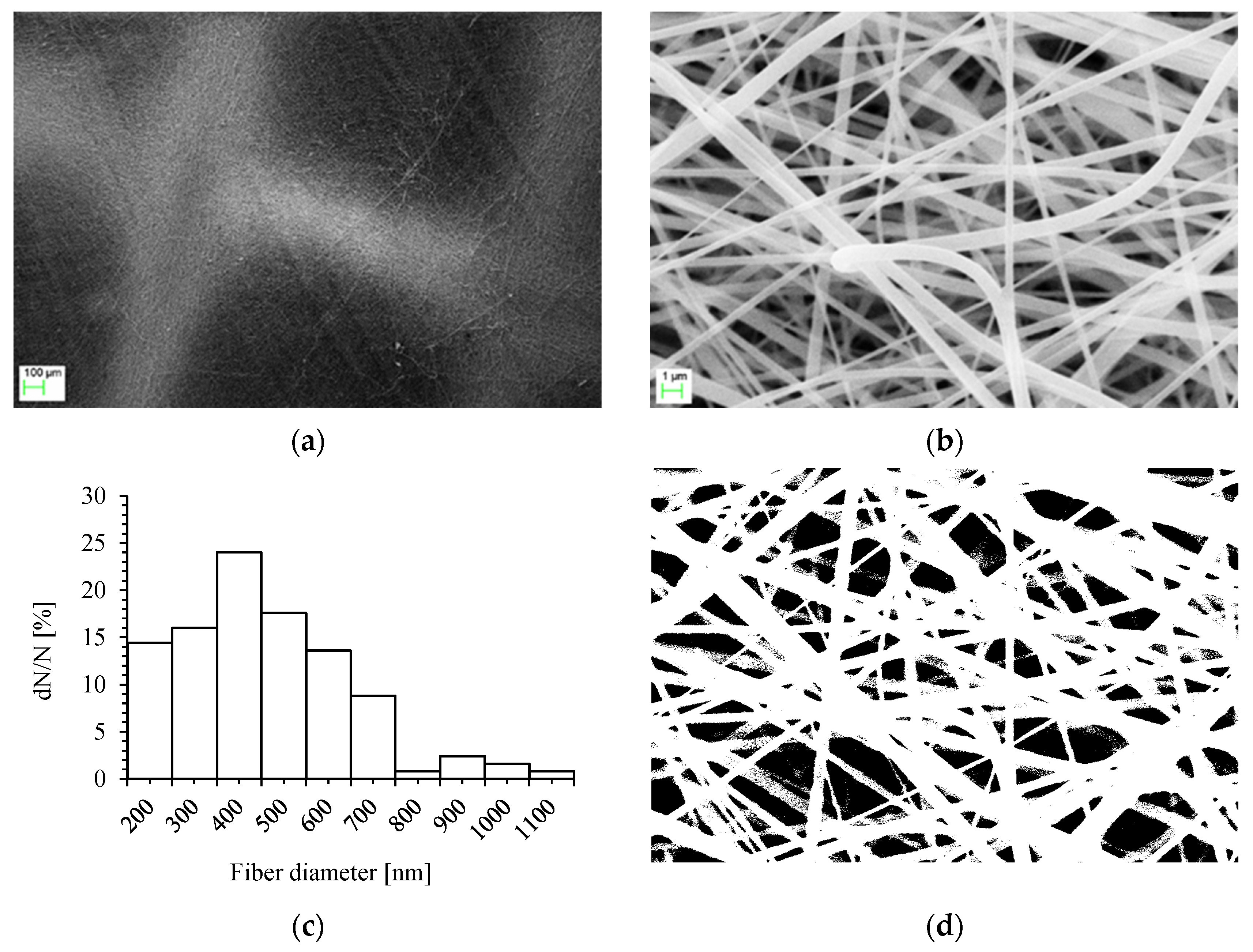
2.1. Nonwoven Nanofibrous Filter Production by Electrospinning
2.2. Experimental Stand
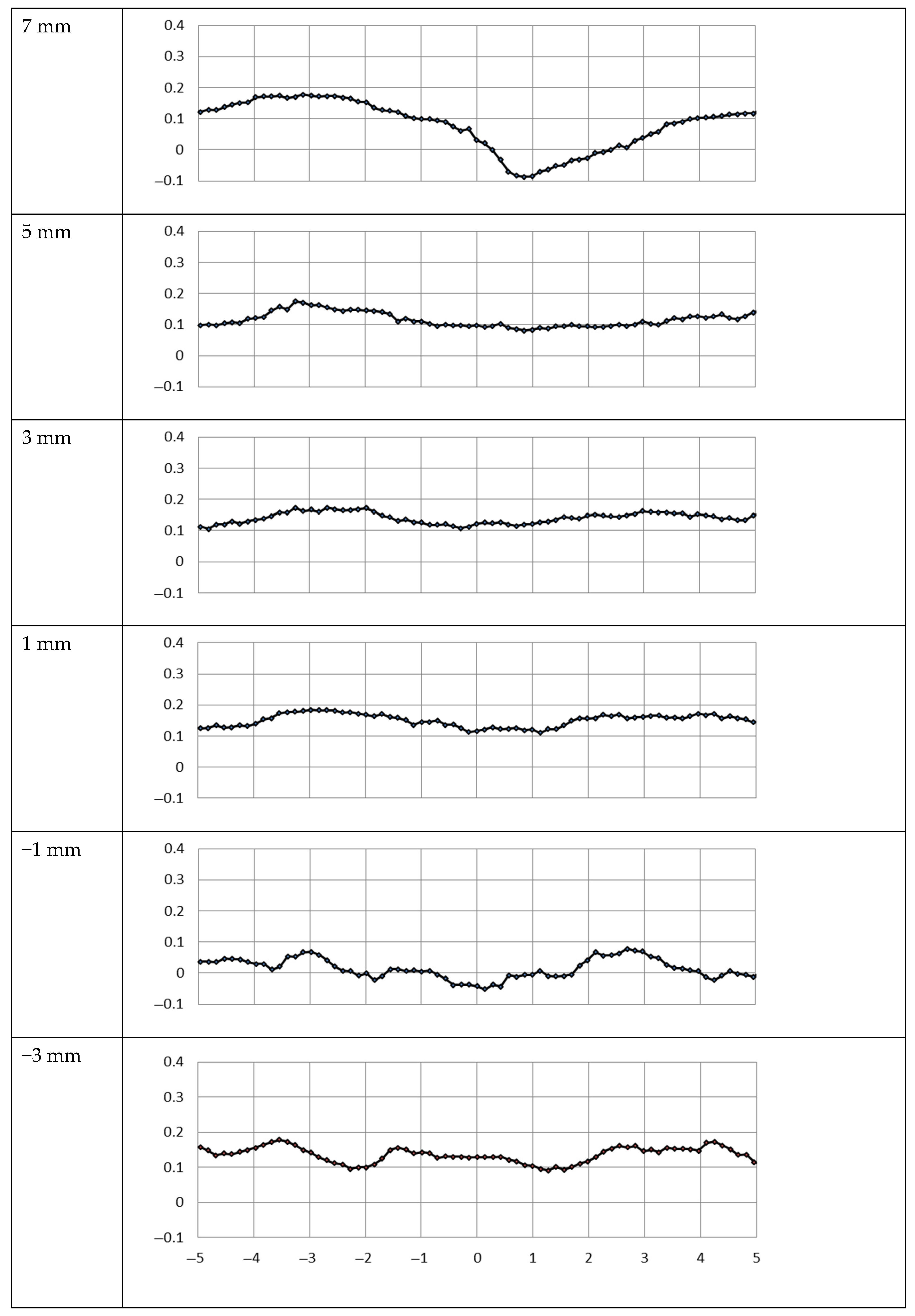
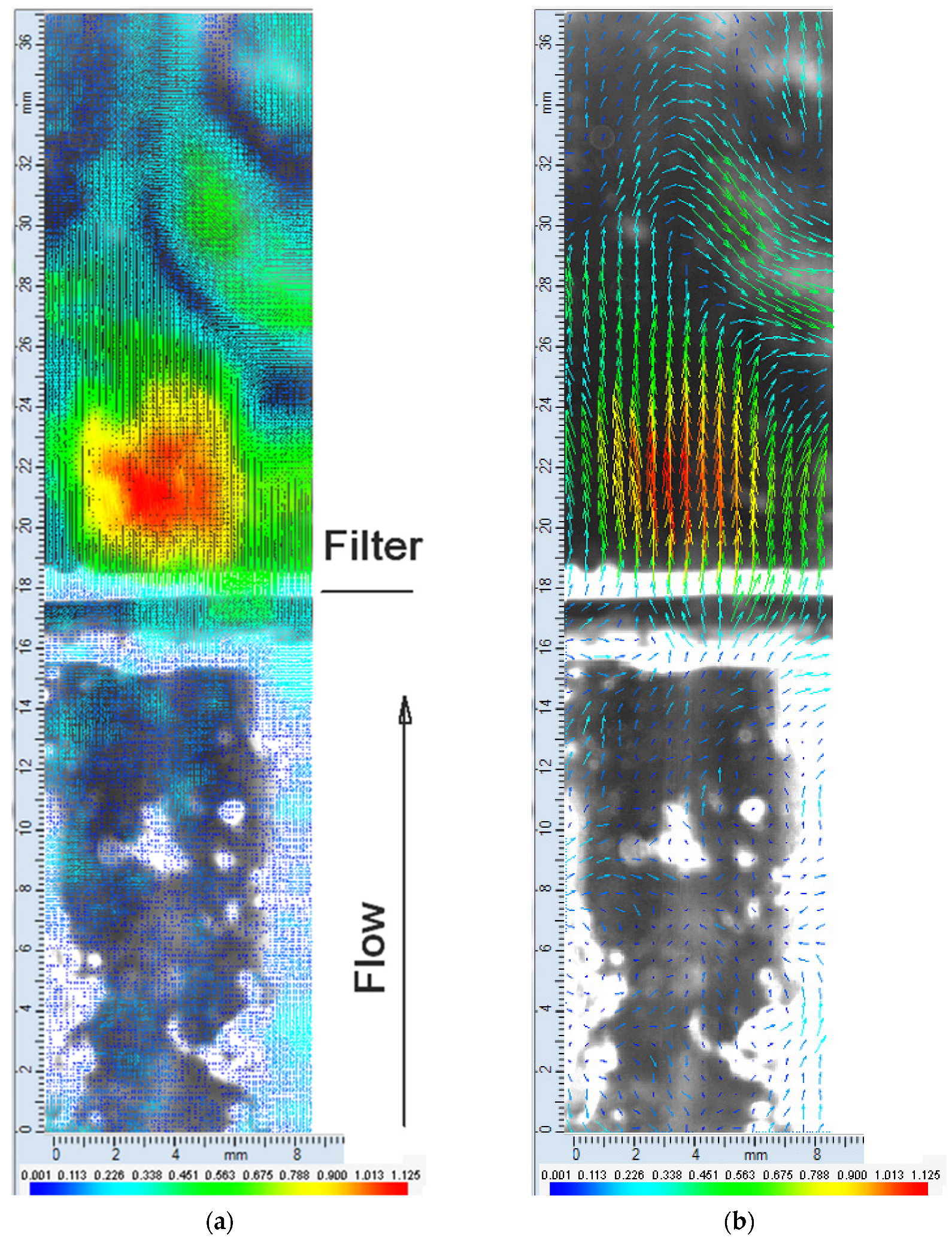
3. Results
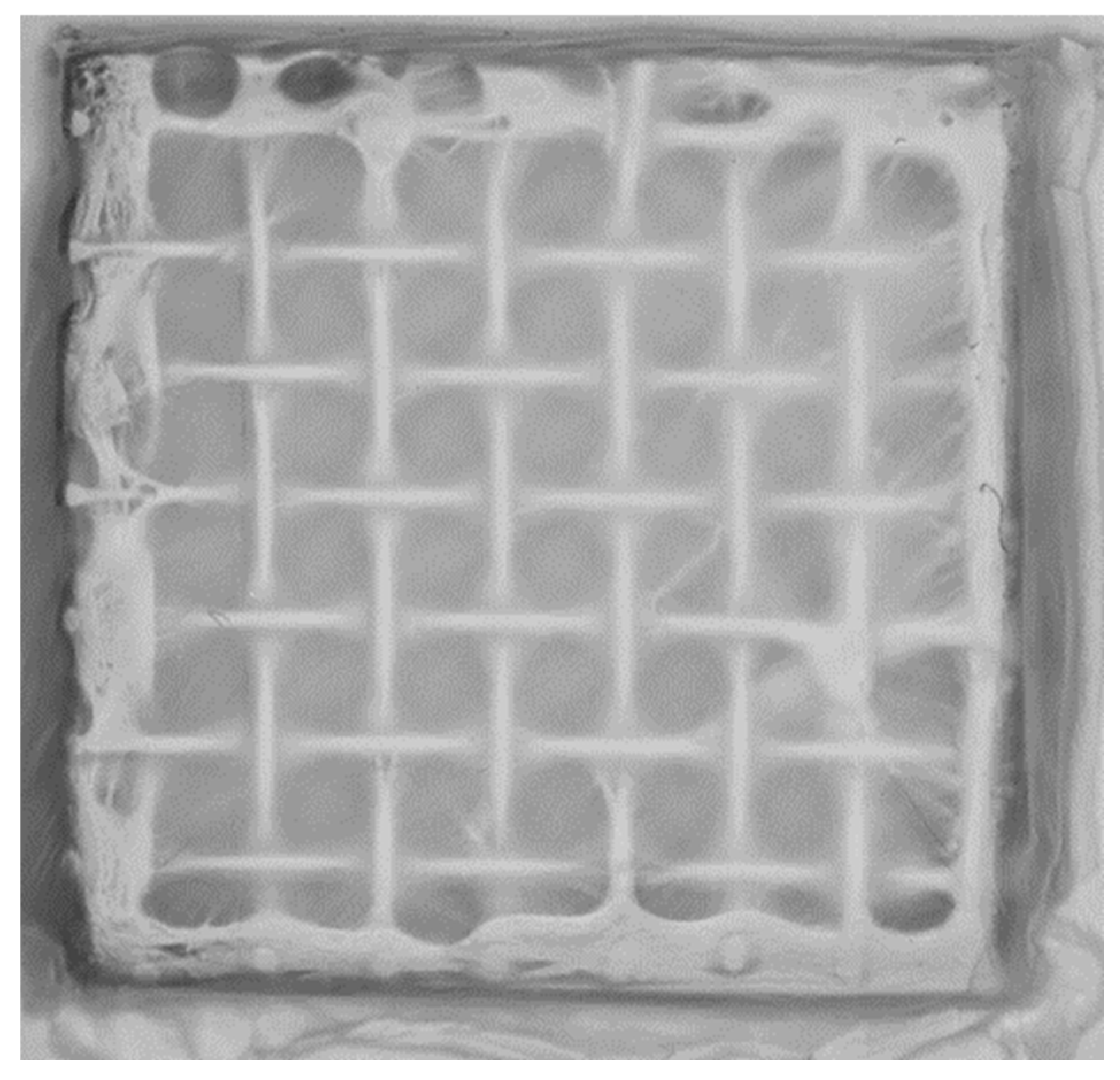
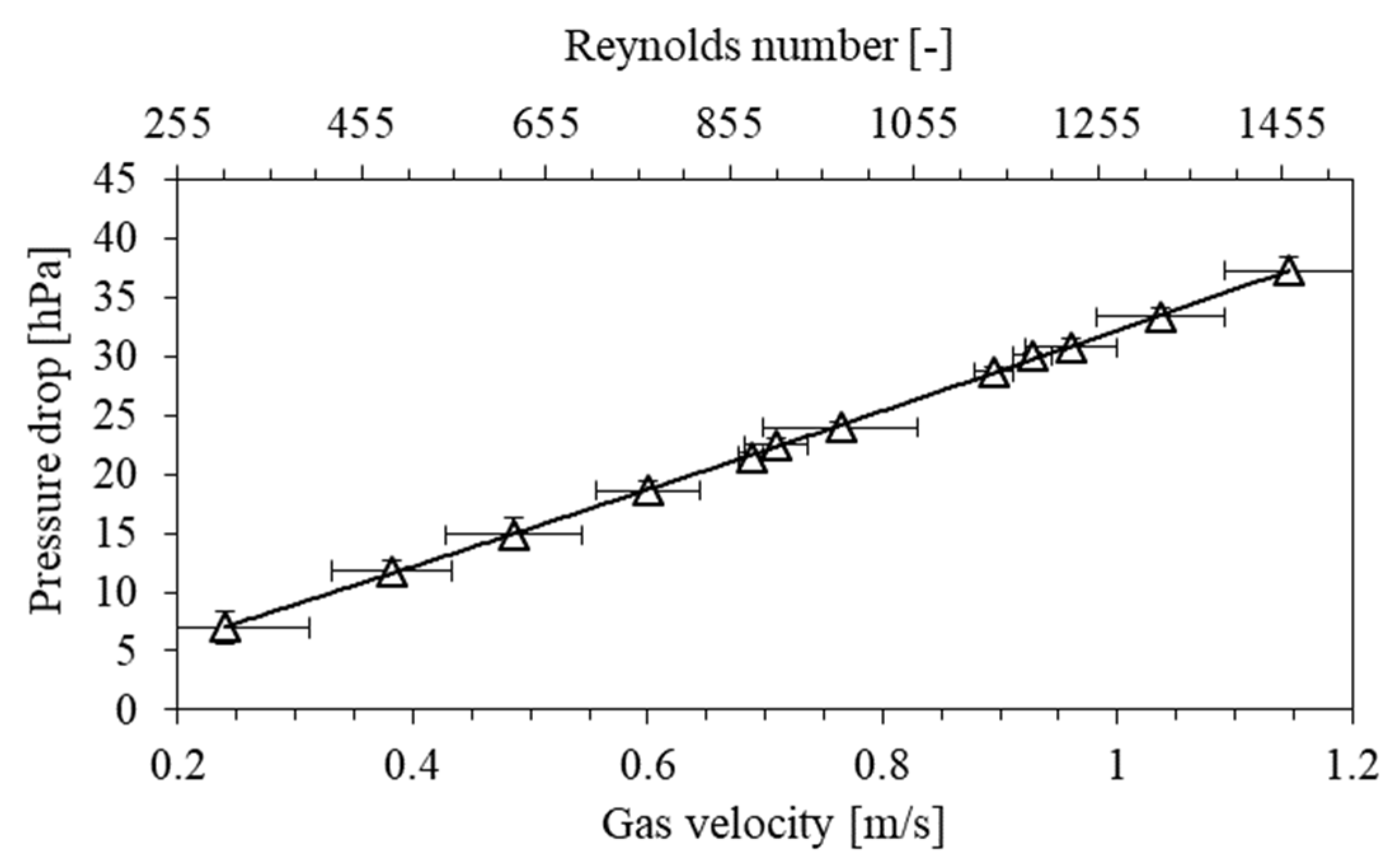
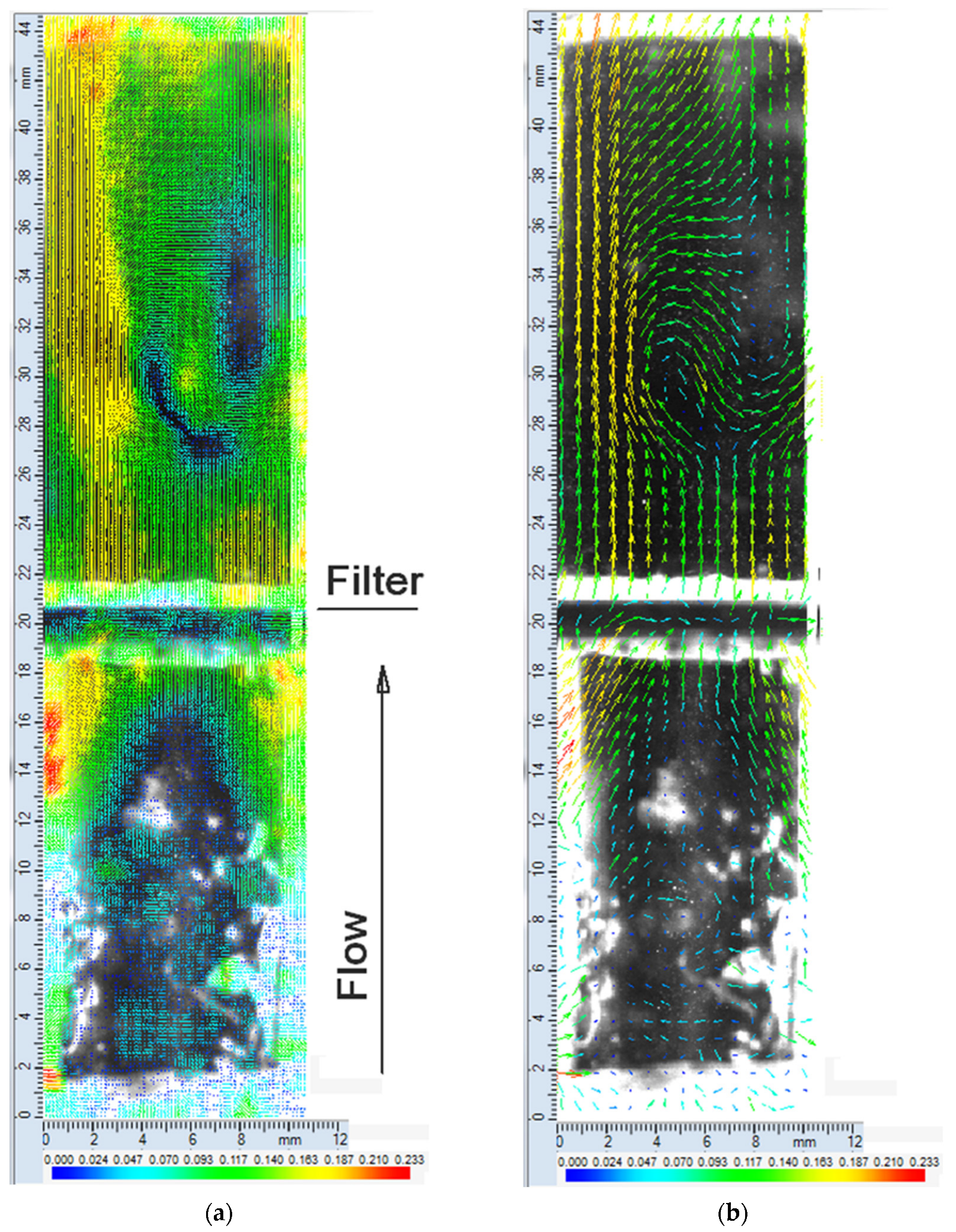
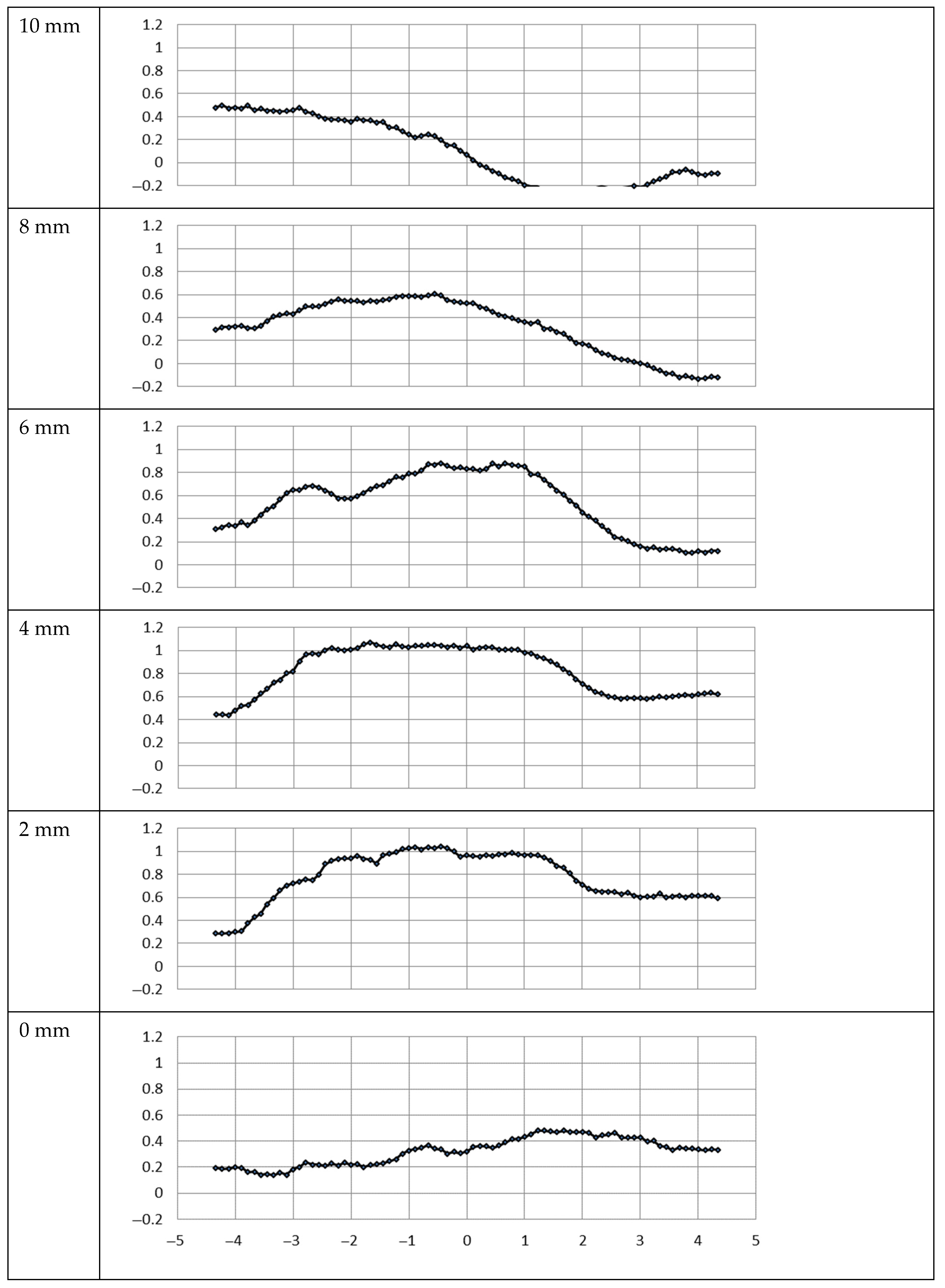
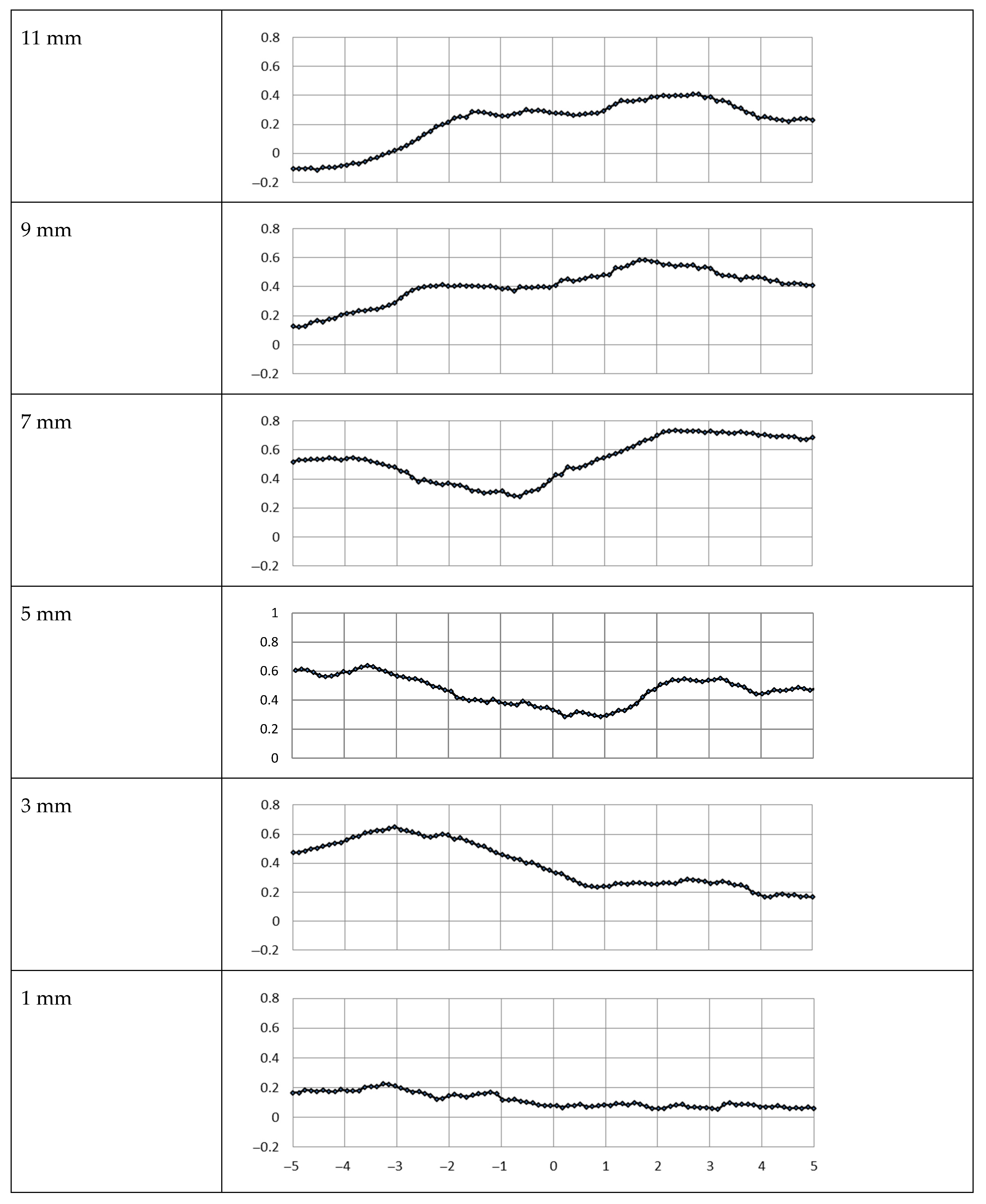
3.1. Metal Mesh
3.2. PVDF Filter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshari, A.; Matson, U.; Ekberg, L.E. Characterization of indoor sources of fine and ultrafine particles: A study conducted in a full-scale chamber. Indoor Air 2005, 15, 141–150. [Google Scholar] [CrossRef]
- Wallace, L.; Ott, W. Personal exposure to ultrafine particles. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 20–30. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Personal exposure to ultrafine particles: The influence of time-activity patterns. Sci. Total Environ. 2014, 468–469, 903–907. [Google Scholar] [CrossRef]
- Lunden, M.M.; Delp, W.W.; Singer, B.C. Capture efficiency of cooking-related fine and ultrafine particles by residential exhaust hoods. Indoor Air 2015, 25, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Lee, S.-G.; Hwang, J. Application of corona discharge-generated air ions for filtration of aerosolized virus and inactivation of filtered virus. J. Aerosol Sci. 2017, 107, 31–40. [Google Scholar] [CrossRef]
- Abdillah, S.F.I.; Wang, Y.-F. Ambient ultrafine particle (PM0.1): Sources, characteristics, measurements and exposure implications on human health. Environ. Res. 2023, 218, 115061. [Google Scholar] [CrossRef]
- Pertegal, V.; Riquelme, E.; Lozano-Serra, J.; Cañizares, P.; Rodrigo, M.A.; Saez, C.; Lacasa, E. Cleaning technologies integrated in duct flows for the inactivation of pathogenic microorganisms in indoor environments: A critical review of recent innovations and future challenges. J. Environ. Manag. 2023, 345, 118798. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.R.; Dudhwadkar, S.; Raju, N.P.; Tandon, S. Public health risk assessment and speciation of air-borne microorganisms in an office building. J. Aerosol Sci. 2024, 179, 106362. [Google Scholar] [CrossRef]
- Moreno-Martín, V.; Lopez, M.; Roldan, C.; Bou, D.; Fraga, S.; Teixeira, J.P.; Lopez-Lilao, A.; Sanfelix, V.; Moliner, R.; Monfort, E.; et al. Potential human exposure and risks of incidental nanoparticles released during rotary dry cutting of ceramic tiles. J. Aerosol Sci. 2025, 183, 106485. [Google Scholar] [CrossRef]
- McAuley, T.; Fisher, R.; Zhou, X.; Jaques, P.; Ferro, A. Relationships of outdoor and indoor ultrafine particles at residences downwind of a major international border crossing in Buffalo, NY. Indoor Air 2010, 20, 298–308. [Google Scholar] [CrossRef]
- Rim, D.; Wallace, L.; Persily, A. Infiltration of outdoor ultrafine particles into a test house. Environ. Sci. Technol. 2010, 4, 5908–5913. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.; Siegel, J.A. Comparison of test methods for determining the particle removal efficiency of filters in residential and light-commercial central HVAC systems. Aerosol Sci. Technol. 2012, 46, 504–513. [Google Scholar] [CrossRef]
- Stephens, B.; Siegel, J.A. Ultrafine particle removal by residential heating, ventilating, and air-conditioning filters. Indoor Air 2013, 23, 488–497. [Google Scholar] [CrossRef]
- Azimi, P.; Zhao, D.; Stephens, B. Estimates of HVAC filtration efficiency for fine and ultrafine particles of outdoor origin. Atmos. Environ. 2014, 98, 337–346. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, T.; Liu, Y.; Li, L.; Liu, J.; Yang, B.; Ramakrishna, S.; Long, Y.-Z. Efficient air filtration through advanced electrospinning techniques in nanofibrous Materials: A review. Sep. Purif. Technol. 2024, 349, 127773. [Google Scholar] [CrossRef]
- Fang, X.; Chang, R.; Zuo, J.; Zhang, W.E.; Zou, Y.; Li, K. How do environmental and operational factors impact particulate matter dynamics in building construction?-Insights from real-time sensing. J. Environ. Manag. 2025, 380, 125098. [Google Scholar] [CrossRef]
- Ma, C.J.; Lee, K.B.; Kim, S.D.; Sera, K. Thermal and hygroscopic properties of indoor particulate matter collected on an underground subway platform. Asian J. Atmos. Environ. 2015, 9, 165–172. [Google Scholar] [CrossRef]
- Font, O.; Moreno, T.; Querol, X.; Martins, V.; Sánchez Rodas, D.; de Miguel, E.; Capdevila, M. Origin and speciation of major and trace PM elements in the Barcelona subway system. Transp. Res. Part D Transp. Environ. 2019, 72, 17–35. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Lee, Y.Y.C.; Chun, H.S.; Moon, J.Y.; Choi, J.S.; Park, D.; Lee, Y.Y.C. Correlation of α/γ-Fe2O3 nanoparticles with the toxicity of particulate matter originating from subway tunnels in Seoul stations, Korea. J. Hazard. Mater. 2020, 382, 121175. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Oh, M.; Yoon, Y.; Park, E.; Lee, K. Source identification of PM10 pollution in subway passenger cabins using positive matrix factorization. Atmos. Environ. 2012, 49, 180–185. [Google Scholar] [CrossRef]
- Moreno, T.; Reche, C.; Minguillón, M.C.; Capdevila, M.; de Miguel, E.; Querol, X. The effect of ventilation protocols on airborne particulate matter in subway systems. Sci. Total Environ. 2017, 584–585, 1317–1323. [Google Scholar] [CrossRef]
- Moreno, T.; de Miguel, E. Improving air quality in subway systems: An overview. Environ. Pollut. 2018, 239, 829–831. [Google Scholar] [CrossRef]
- Lee, H. Generation characteristics of the airborne wear particles emitted from the wheel–rail contact for various train velocities and their generation relation with the train velocity. Atmos. Environ. X 2020, 5, 100068. [Google Scholar] [CrossRef]
- Passi, A.; Nagendra, S.M.S.; Maiya, M.P. Assessment of exposure to airborne aerosol and bio-aerosol particles and their deposition in the respiratory tract of subway metro passengers and workers. Atmos. Pollut. Res. 2021, 12, 101218. [Google Scholar] [CrossRef]
- Passi, A.; Nagendra, S.; Maiya, M.P. Assessment of indoor and outdoor exposure to airborne particles in subway metro stations and their associated health risks. WIT Trans. Ecol. Environ. 2021, 252, 85–96. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Podgórski, A.; Maisser, A.; Szymański, W.W.; Jackiewicz, A.; Gradoń, L. Penetration of monodisperse, singly charged nanoparticles through polydisperse fibrous filters. Aerosol Sci. Technol. 2011, 45, 215–233. [Google Scholar] [CrossRef]
- Chang, D.-Q.; Chen, S.-C.; Fox, A.R.; Viner, A.S.; Pui, D.Y.H. Penetration of sub-50 nm nanoparticles through electret HVAC filters used in residence. Aerosol Sci. Technol. 2015, 49, 966–976. [Google Scholar] [CrossRef]
- Woo, S.-H.; Cheon, T.-W.; Lee, G.; Kim, J.B.; Bae, G.-N.; Kwon, S.-B.; Jang, H.R.; Yook, S.-J. Performance evaluation of a hybrid dust collector for removing particles during subway train operation. Aerosol Sci. Technol. 2019, 53, 562–574. [Google Scholar] [CrossRef]
- Afshari, A.; Ekberg, L.; Forejt, L.; Mo, J.; Rahimi, S.; Siegel, J.; Chen, W.; Wargocki, P.; Zurami, S.; Zhang, J. Electrostatic precipitators as an indoor air cleaner—A Literature Review. Sustainability 2020, 12, 8774. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.-S.; Lee, H.; Park, Y.; Lee, G.; An, S.-H.; Han, B.; Kim, Y.-J.; Kim, H.-J. Retrofitting of an air handling unit by a two-stage electrostatic precipitator. IEEE Trans. Ind. Appl. 2024, 60, 1656–1664. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Muzamil, K. Recent progress on electrospun nanofibrous polymer membranes for water and air purification: A review. Chemosphere 2023, 310, 136886. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Zhang, C.; Zhou, Y.; Yu, D.-G. Electrospun nanofiber as building blocks for high-performance air filter: A review. Nano Today 2024, 55, 102161. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Lim, T.C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.; Krupa, A.; Sobczyk, A.T.; Lackowski, M.; Czech, T.; Ramakrishna SSundarrajan, S.; Pliszka, D. Electrospray nanocoating of microfibres. Solid State Phenom. 2008, 140, 127–132. [Google Scholar] [CrossRef]
- Lackowski, M.; Krupa, A.; Jaworek, A. Nonwoven filtration mat production by electrospinning method. J. Phys. Conf. Ser. 2011, 301, 012013. [Google Scholar] [CrossRef]
- Lackowski, M.; Krupa, A.; Jaworek, A. Nanofabric nonwoven mat for filtration smoke and nanoparticles. Pol. J. Chem. Technol. 2013, 15, 48–52. [Google Scholar] [CrossRef]
- Baghali, M.; Jayathilaka, W.A.D.M.; Ramakrishna, S. The Role of Electrospun Nanomaterials in the Future of Energy and Environment. Materials 2021, 14, 558. [Google Scholar] [CrossRef]
- Yao, S.; Ramakrishna, S.; Chen, G. Recent Advances in Metal–Organic Frameworks Based on Electrospinning for Energy Storage. Adv. Fiber Mater. 2023, 5, 1592–1617. [Google Scholar] [CrossRef]
- Yun, K.M.; Hogan, C.J., Jr.; Matsubayashi, Y.; Kawabe, M.; Iskandar, F.; Okuyama, K. Nanoparticle filtration by electrospun polymer fibers. Chem. Eng. Sci. 2007, 62, 4751–4759. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yin, X.; Li, Z.; Yu, J.; Ding, B. Tailoring mechanically robust poly (m-phenylene isophthalamide) nanofiber/nets for ultrathin high-efficiency air filter. Sci. Rep. 2017, 7, 40550. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Qi, H.; Dai, Y.; Jiang, S.; Ding, B.; Wang, X.; Li, C.; Zeng, J.; Wu, T.; et al. Electrospinning and electrospun nanofibers: From academic research to industrial production. Prog. Mater. Sci. 2025, 154, 101494. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, Y.; Wei, B.; Chen, J.; Zhang, Y.; Hu, S.; Li, J.; Wang, Y. Sustainable self-powered PVDF fibrous filter medias based on triboelectric nanogenerator with efficient dust collection for personal occupational protection. J. Colloid Interface Sci. 2025, 696, 137871. [Google Scholar] [CrossRef] [PubMed]
- Przekop, R.; Gradoń, L. Deposition and filtration of nanoparticles in the composites of nano-and microsized fibers. Aerosol Sci. Technol. 2008, 42, 483–493. [Google Scholar] [CrossRef]
- Jackiewicz, A.; Bałazy, A.; Podgórski, A. Investigation of aerosol dispersion in fibrous filters. Pol. J. Chem. Technol. 2008, 10, 66–72. [Google Scholar] [CrossRef]
- Podgórski, A. Estimation of the upper limit of aerosol nanoparticles penetration through inhomogeneous fibrous filters. J. Nanoparticles Res. 2009, 11, 197–207. [Google Scholar] [CrossRef]
- Podgórski, A.; Bałazy, A. Novel formulae for deposition efficiency of electrically neutral, submicron aerosol particles in bipolarly charged fibrous filters derived using Brownian dynamics approach. Aerosol Sci. Technol. 2008, 42, 123–133. [Google Scholar] [CrossRef]
- Khandaker, M.; Progri, H.; Arasu, D.T.; Nikfarjam, S.; Shamim, N. Use of polycaprolactone electrospun nanofiber mesh in a face mask. Materials 2021, 14, 4272. [Google Scholar] [CrossRef]
- Liang, L.; Wu, L.; Xu, W.; Liu, C.; Liu, X.; Cheng, H.; Liu, Y.; Zhang, G.; Che, H.; Sun, J.; et al. The characterization and quantification of viable and dead airborne biological particles using flow cytometry and double fluorescent staining. J. Aerosol Sci. 2022, 165, 106019. [Google Scholar] [CrossRef]
- Peimbert, M.; Alcaraz, L.D. Where environmental microbiome meets its host: Subway and passenger microbiome relationships. Mol. Ecol. 2022, 32, 2602–2618. [Google Scholar] [CrossRef]
- Su, Q.; Huang, Y.; Wei, Z.; Zhu, C.; Zeng, W.; Wang, S.; Long, S.; Zhang, G.; Yang, J.; Wang, X. A novel multi-gradient PASS nanofibrous membranes with outstanding particulate matter removal efficiency and excellent antimicrobial property. Sep. Purif. Technol. 2023, 307, 122652. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhong, Q.; Wang, F.; Yang, B. Preparation and filtration performance of antibacterial PVDF/SiO2/Ag composite nanofiber membrane. J. Build. Eng. 2023, 74, 106864. [Google Scholar] [CrossRef]
- Yu, K.-P.; Lee, G.W.-M.; Lin, S.-Y.; Huang, C.P. Removal of bioaerosols by the combination of a photocatalytic filter and negative air ions. J. Aerosol Sci. 2008, 39, 377–392. [Google Scholar] [CrossRef]
- Richter, W.R.; Wood, J.P.; Wendling, M.Q.S.; Rogers, J.V. Inactivation of Bacillus anthracis spores to decontaminate subway railcar and related materials via the fogging of peracetic acid and hydrogen peroxide sporicidal liquids. J. Environ. Manag. 2018, 206, 800–806. [Google Scholar] [CrossRef]
- Dehghan, S.F.; Golbabaei, F.; Mousavi, T.; Mohammadi, H.; Kohneshahri, M.H.; Bakhtiari, R. Production of nanofibers containing magnesium oxide nanoparticles for the purpose of bioaerosol removal. Pollution 2020, 6, 185–196. [Google Scholar] [CrossRef]
- Sohrabi, M.; Abbasi, M.; Sadighzadeh, A. Fabrication and evaluation of electrospun polyacrylonitrile/silver nanofiber membranes for air filtration and antibacterial activity. Polym. Bull. 2023, 80, 5481–5499. [Google Scholar] [CrossRef]
- Jaworek, A.; Krupa, A.; Lackowski, M.; Sobczyk, A.T.; Czech, T.; Ramakrishna, S.; Sundarrajan, S.; Pliszka, D. Nanocomposite fabric formation by electrospinning and electrospraying technology. J. Electrost. 2009, 67, 435–438. [Google Scholar] [CrossRef]
- Jaworek, A.; Krupa, A.; Lackowski, M.; Sobczyk, A.T.; Czech, T.; Ramakrishna, S.; Sundarrajan, S.; Pliszka, D. Electrostatic method for the production of polymer nanofibers blended with metal-oxide nanoparticles. J. Phys. Conf. Ser. 2009, 146, 012006. [Google Scholar] [CrossRef]
- Blosi, M.; Costa, A.L.; Ortelli, S.; Belosi, F.; Ravegnani, F.; Varesano, A.; Tonetti, C.; Zanoni, I.; Vineis, C. Polyvinyl alcohol/silver electrospun nanofibers: Biocidal filter media capturing virus-size particles. J. Appl. Polym. Sci. 2021, 138, 51380. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kang, S.; Park, J.-W.; Hwang, J. Fabrication of silver nanowire coated fibrous air filter medium via a two-step process of electrospinning and electrospray for anti-bioaerosol treatment. J. Hazard. Mater. 2021, 411, 125043. [Google Scholar] [CrossRef]
- De Almeida, D.S.; Martins, L.D.; Aguiar, M.L. Air pollution control for indoor environments using nanofiber filters: A brief review and post-pandemic perspectives. Chem. Eng. J. Adv. 2022, 11, 100330. [Google Scholar] [CrossRef]
- Salkovskiy, Y.; Fadeev, A. High-efficiency retention of ultrafine aerosols by electrospun nanofibers. Sci. Rep. 2022, 12, 20850. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Penicot, P.; Contal, P.; Leclerc, D.; Vendel, J. Clogging of fibrous filters by solid aerosol particles Experimental and modelling study. Chem. Eng. Sci. 2001, 56, 3549–3561. [Google Scholar] [CrossRef]
- Leung, W.W.-F.; Hung, C.-H. Investigation on pressure drop evolution of fibrous filter operating in aerodynamic slip regime under continuous loading of sub-micron aerosols. Sep. Purif. Technol. 2008, 63, 691–700. [Google Scholar] [CrossRef]
- Wang, J.; Kim, S.C.; Pui, D.Y.H. Investigation of the figure of merit for filters with a single nanofiber layer on a substrate. J. Aerosol Sci. 2008, 39, 323–334. [Google Scholar] [CrossRef]
- Bao, B.; He, W.; Zhao, H.; Xu, B.; Lin, Z. Modeling penetration through fibrous filter during dynamic filtration. Aerosol Air Qual. Res. 2015, 15, 648–656. [Google Scholar] [CrossRef]
- Zheng, C.-H.; Kanaoka, C. Recent advances in dust collection technology and ISO standardization in bag filtration. J. Zhejiang Univ.-Sci A (Appl. Phys. Eng.) 2018, 19, 21–33. [Google Scholar] [CrossRef]
- Pei, C.; Ou, Q.; Pui, D.Y.H. Effects of temperature and relative humidity on laboratory air filter loading test by hygroscopic salts. Sep. Purif. Technol. 2021, 255, 117679. [Google Scholar] [CrossRef]
- Berry, G.; Beckman, I.; Cho, H. A comprehensive review of particle loading models of fibrous air filters. J. Aerosol Sci. 2023, 167, 106078. [Google Scholar] [CrossRef]
- Aghdasi, M.; Nazari, M.; Yonesi Holari, S.; Hashemi, N.N. Designing a new microchannel to collect microparticles using dielectrophoretic forces: Numerical and experimental investigation. J. Electrost. 2024, 127, 103879. [Google Scholar] [CrossRef]
- Kirsh, A.A.; Stechkina, I.B.; Fuchs, N.A. Effect of the gas slip on the pressure drop in a system of parallel cylinders at small Reynolds numbers. J. Colloid Interface Sci. 1971, 37, 458–461. [Google Scholar] [CrossRef]
- Kirsh, V.A.; Budyka, A.K.; Kirsh, A.A. Simulation of nanofibrous filters produced by the electrospinning method: 2. The effect of gas slip on the pressure drop. Colloid J. 2008, 70, 584–588. [Google Scholar] [CrossRef]
- Kirsch, A.A.; Stechkina, I.B.; Fuchs, N.A. Effect of gas slip on the pressure drop in fibrous filters. J. Aerosol Sci. 1973, 4, 287–293. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kumita, M.; Seto, T.; Inui, Y.; Bao, L.; Fujimoto, T.; Otani, Y. Effect of slip flow on pressure drop of nanofiber filters. J. Aerosol Sci. 2017, 114, 244–249. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Yin, X.; Yu, J.; Ding, B. Slip-effect functional air filter for efficient purification of PM2.5. Sci. Rep. 2016, 6, 35472. [Google Scholar] [CrossRef]
- Xia, T.; Bian, Y.; Zhang, L.; Chen, C. Relationship between pressure drop and face velocity for electrospun nanofiber filters. Energy Build. 2018, 158, 987–999. [Google Scholar] [CrossRef]
- Azimian, M.; Naderi, M.; Soltani, P.; Cheng, L.; Naderi, K.; Linden, S.; Wiegmann, A. Experimental and CFD analysis of fluid flow through nanofiber filter media. Sci. Rep. 2024, 14, 16128. [Google Scholar] [CrossRef] [PubMed]
- Mizeraczyk, J.; Dekowski, J.; Podliński, J.; Kocik, M.; Ohkubo, T.; Kanazawa, S. Laser flow visualization and velocity fields by particle image velocimetry in an electrostatic precipitator model. J. Visualiz. 2003, 6, 125–133. [Google Scholar] [CrossRef]
- Niewulis, A.; Podliński, J.; Kocik, M.; Barbucha, R.; Mizeraczyk, J.; Mizuno, A. EHD flow measured by 3D PIV in a narrow electrostatic precipitator with longitudinal-to-flow wire electrode and smooth or flocking grounded plane electrode. J. Electrost. 2007, 65, 728–734. [Google Scholar] [CrossRef]
- Podliński, J.; Berendt, A.; Mizeraczyk, J. Particle Image Velocimetry of airflow in electrohydrodynamic device for dust particle collection. Int. J. Plasma Environ. Sci. Technol. 2013, 7, 82–88. [Google Scholar]
- Krupa, A.; Podliński, J.; Mizeraczyk, J.; Jaworek, A. Velocity field of EHD flow during back corona discharge in electrostatic precipitator. Powder Technol. 2019, 344, 475–486. [Google Scholar] [CrossRef]
- Yuan, Z.; Yanagawa, K.; Ehara, Y. Visualization and analysis of a re-entrained particle with an electrostatic precipitator using PIV method. J. Electrost. 2022, 119, 103751. [Google Scholar] [CrossRef]
- Tański, M.; Reza, A.; Przytuła, D.; Garasz, K.; Tomaszewski, A. Electrostatic precipitator with Surface Dielectric Barrier Discharge ionizer. J. Clean. Prod. 2023, 404, 136990. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, X.; Li Abbas, G.; Yu, Y.; Zhang, Z. EHD flow auxiliary particle collection in HVDC electrostatic precipitator based on PIV flow visualization technology. Phys. Scr. 2023, 98, 045002. [Google Scholar] [CrossRef]
- Kang, S.; Bock, N.; Swanson, J.; Pui, D.Y.H. Characterization of pleated filter media using particle image velocimetry. Sep. Purif. Technol. 2020, 237, 116333. [Google Scholar] [CrossRef]
- Pliszka, D.; Sundarrajan, S.; Jaworek, A.; Krupa, A.; Lackowski, M.; Ramakrishna, S. Optimization of electrospray process by PIV in nanostructured membrane preparation. Adv. Sci. Technol. 2008, 60, 117–122. [Google Scholar] [CrossRef]
- Kähler, C.J.; Hain, R. Fundamental protective mechanisms of face masks against droplet infections. J. Aerosol Sci. 2020, 148, 105617. [Google Scholar] [CrossRef]
- Migliorini, M.; Zachos, P.K.; MacManus, D.G.; Giannouloudis, A. Experimental investigation of unsteady fan-intake interactions using time-resolved stereoscopic particle image velocimetry. Exp. Therm. Fluid Sci. 2025, 166, 111482. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Pliszka, D.; Jaworek, A.; Krupa, A.; Lackowski, M.; Ramakrishna, S. A novel process for the fabrication of nanocomposites membranes. J. Nanosci. Nanotechnol. 2009, 9, 4442–4447. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Semnani, D.; Morshed, M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J. Appl. Polym. Sci. 2007, 106, 2536–2542. [Google Scholar] [CrossRef]
- VDI Germany 2016. Available online: www.vdi.de/fileadmin/pages/vdi_de/redakteure/richtlinien/inhaltsverzeichnisse/2417322.pdf (accessed on 10 March 2025).
- Podgórski, A.; Bałazy, A.; Gradoń, L. Application of nanofibers to improve the filtration efficiency of the most penetrating aerosol particles in fibrous filters. Chem. Eng. Sci. 2006, 61, 6804–6815. [Google Scholar] [CrossRef]
- Bilek, P.; Sidlof, P. Optical measuring and vizualization of efficiency and homogenity of nanofiber filtration materials. In Proceedings of the Nanocon 2011 Conference Proceedings 3rd International Conference, Brno, Czech Republic, 21–23 September 2011; pp. 503–508. [Google Scholar]
- Bilek, P.; Hruza, J. Influence of structure uniformity of nanofibrous filters on their homogeneity of filtration efficiency. In Proceedings of the Nanocon 2014 Conference Proceedings 6th International Conference, Brno, Czech Republic, 5–7 November 2014; pp. 427–436. [Google Scholar]
- Agrawal, V. Development of Image Filtration Algorithms for PIV and PTV Technique in Suspension Flow of Bifurcating Micro-Channels. Bachelor’s Thesis, Indian Institute of Technology Guwahati, Guwahati, India, 2015. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, A.; Wardach-Święcicka, I.; Ronewicz, K.; Jaworek, A. Flow Velocity Distribution Downstream of Nanofibrous Filter in Minichannel Determined by Particle Image Velocimetry Method. Appl. Sci. 2025, 15, 8728. https://doi.org/10.3390/app15158728
Krupa A, Wardach-Święcicka I, Ronewicz K, Jaworek A. Flow Velocity Distribution Downstream of Nanofibrous Filter in Minichannel Determined by Particle Image Velocimetry Method. Applied Sciences. 2025; 15(15):8728. https://doi.org/10.3390/app15158728
Chicago/Turabian StyleKrupa, Andrzej, Izabela Wardach-Święcicka, Karol Ronewicz, and Anatol Jaworek. 2025. "Flow Velocity Distribution Downstream of Nanofibrous Filter in Minichannel Determined by Particle Image Velocimetry Method" Applied Sciences 15, no. 15: 8728. https://doi.org/10.3390/app15158728
APA StyleKrupa, A., Wardach-Święcicka, I., Ronewicz, K., & Jaworek, A. (2025). Flow Velocity Distribution Downstream of Nanofibrous Filter in Minichannel Determined by Particle Image Velocimetry Method. Applied Sciences, 15(15), 8728. https://doi.org/10.3390/app15158728






