Magnetic Properties of Commercial Cornflakes
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Iron
2.2. Experimental Characterizations
3. Results
3.1. The XRF Spectra
3.2. XRD Spectrum
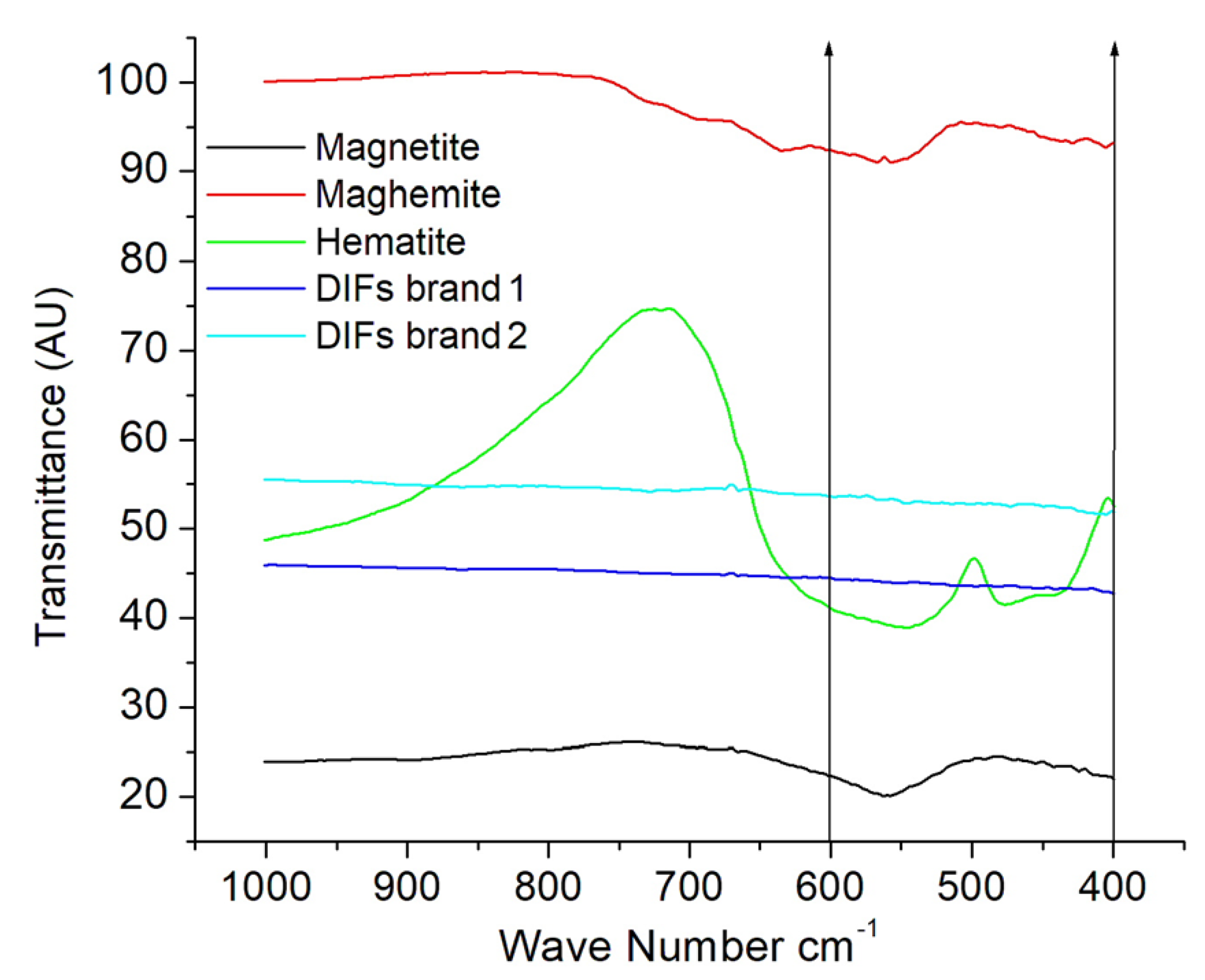
3.3. The FTIR Spectra
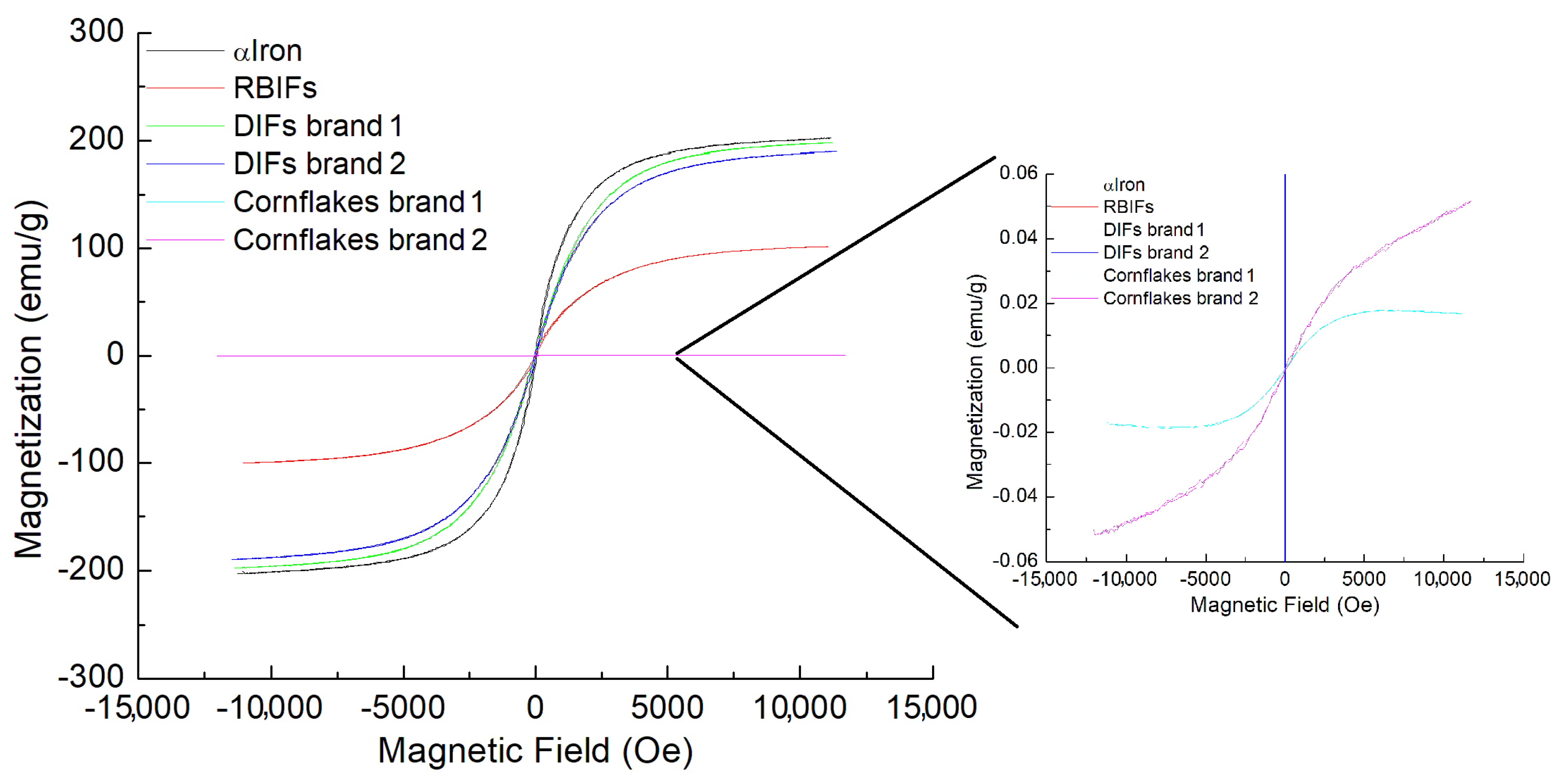
3.4. Magnetization Loops
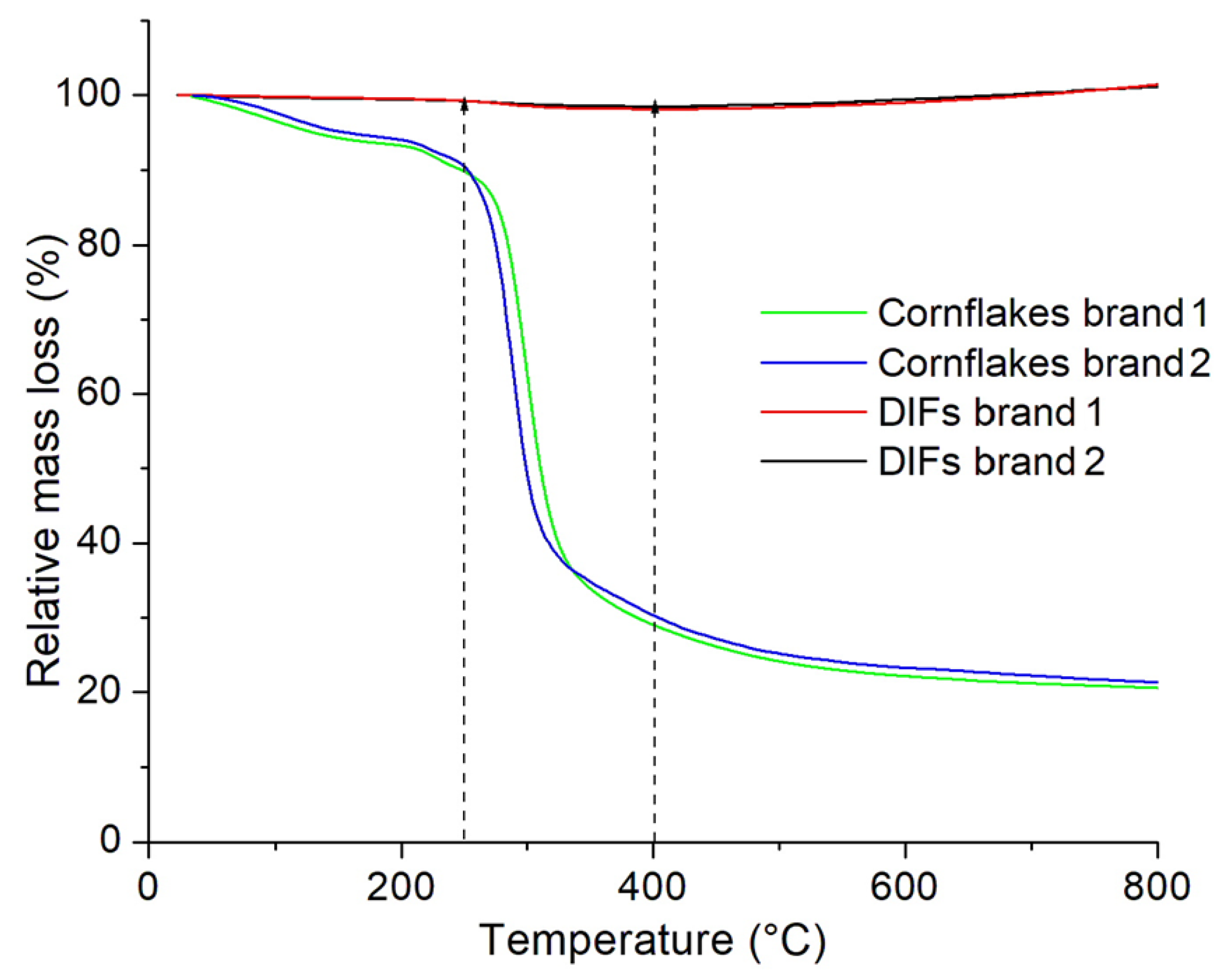
3.5. Thermograms
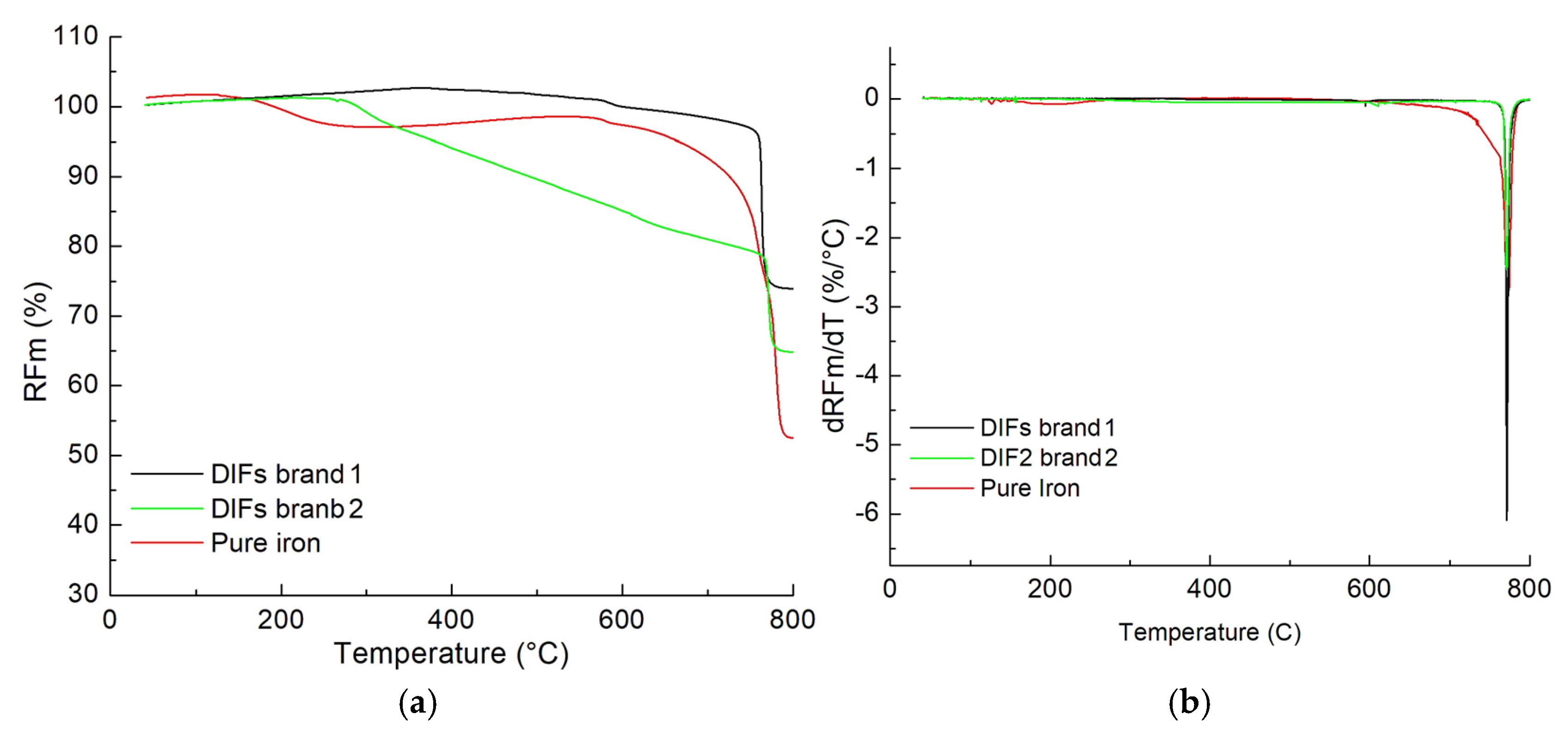
3.6. Magneto-Thermograms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EFSA | The European Food Safety Authority |
| NOAEL | No observable adverse effect level |
| DIFs | Dark iron filings |
| BCC | Body-centered cubic |
| RBIFs | Red/brown iron filings |
References
- Latunde-Dada, G.O. Iron Intake and Human Health. Nutrients 2024, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- McMillen, S.A.; Dean, R.; Dihardja, E.; Ji, P.; Lönnerdal, B. Benefits and risks of early life iron supplementation. Nutrients 2022, 14, 4380. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F. Ensuring the efficacious iron fortification of foods: A tale of two barriers. Nutrients 2022, 14, 1609. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; De Benoist, B.; Dairy, O.; Hurrel, R. Guidelines on Food Fortification with Micronutrients. World Health Organization, 2006. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=bbb90acf54fdd30ed9ebfc018b298e3515aefdca (accessed on 5 October 2024).
- Lee, K.; Clydesdale, F.M.; Tannenbaum, S.R. Iron sources used in food fortification and their changes due to food processing. Crit. Rev. Food Sci. Nutr. 1979, 11, 117–153. [Google Scholar] [CrossRef]
- Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417–24450. [Google Scholar] [CrossRef]
- Sakhare, D.T. Green Synthesis, Characterization And Antimicrobial Activity of Iron Nanoparticles Using Hibiscus Leaf Extract. SN Appl. Sci. 2020, 2, 898. [Google Scholar] [CrossRef]
- Osman, A.G.; El-Desouky, A.I.; Morsy, M.K.; Aboud, A.A.; Mohamed, M.H. Production and evaluation of fortified biscuit with iron nanoparticles for anemia as functional food. Egypt. J. Chem. 2022, 65, 181–194. [Google Scholar] [CrossRef]
- Salaheldin, T.A.; Regheb, E.M. In-Vivo Nutritional and Toxicological Evaluation of Nano Iron Fortified Biscuits as Food Supplement for Iron Deficient Anemia. J. Nanomed. Res. 2016, 3, 00049. [Google Scholar] [CrossRef]
- Garcés, V.; González, A.; Sabio, L.; Sánchez-Arévalo, C.M.; Gálvez, N.; Dominguez-Vera, J.M. Magnetic and golden yogurts. Foods a potential nanomedicine carrier. Materials 2020, 13, 481. [Google Scholar] [CrossRef]
- Góral, D.; Marczuk, A.; Góral-Kowalczyk, M.; Koval, I.; Andrejko, D. Application of iron nanoparticle-based materials in the food industry. Materials 2023, 16, 780. [Google Scholar] [CrossRef]
- Sieg, H.; Schaar, C.; Fouquet, N.; Böhmert, L.; Thünemann, A.F.; Braeuning, A. Particulate iron oxide food colourants (E 172) during artificial digestion and their uptake and impact on intestinal cells. Toxicol. Vitr. 2024, 96, 105772. [Google Scholar] [CrossRef]
- Voss, L.; Hsiao, I.L.; Ebisch, M.; Vidmar, J.; Dreiack, N.; Böhmert, L.; Stock, V.; Braeuning, A.; Loeschner, K.; Laux, P.; et al. The presence of iron oxide nanoparticles in the food pigment E172. Food Chem. 2020, 327, 127000. [Google Scholar] [CrossRef]
- Askri, D.; Ouni, S.; Galai, S.; Chovelon, B.; Arnaud, J.; Sturm, N.; Lehmann, S.G.; Sakly, M.; Amara, S.; Sève, M. Nanoparticles in foods? A multiscale physiopathological investigation of iron oxide nanoparticle effects on rats after an acute oral exposure: Trace element biodistribution and cognitive capacities. Food Chem. Toxicol. 2019, 127, 173–181. [Google Scholar] [CrossRef]
- Kumari, A.; Chauhan, A.K. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: A review. J. Food Sci. Technol. 2022, 59, 3319–3335. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the re-evaluation of iron oxides and hydroxides (E 172) as food additives. EFSA J. 2015, 13, 4317. [Google Scholar] [CrossRef]
- Lermyte, F.; Zhang, W.Y.; Brooks, J.; Huband, S.; Collingwood, J.F.; Lees, M.R.; Rayman, M.P.; Sadler, P.J. Metallic iron in cornflakes. Food Funct. 2020, 11, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.; Parcell, L.M. Beauty and the Bran: Kellogg’s Campaign to “Correct Faulty Elimination” and Conquer the Cereal Industry. J. Hist. 2022, 48, 324–348. [Google Scholar] [CrossRef]
- Lysenko, E.; Surzhikov, A.; Nikolaev, E.; Starý, O. Curie temperature control of magnetic materials using thermogravimetric measurements in a magnetic field. In Recent Developments in the Field of Non-Destructive Testing, Safety and Materials Science, Proceedings of the ICMTNT 2021, Tomsk, Russia, 9–11 November 2021; Studies in Systems, Decision and Control; Springer: Cham, Switzerland, 2021; Volume 433, pp. 195–203. [Google Scholar] [CrossRef]
- Hull, A.W. A New Method of X-Ray Crystal Analysis. Phys. Rev. 1917, 10, 661–696. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. Part A 2007, 80, 333–341. [Google Scholar] [CrossRef]
- Graham, C.D. Iron and nickel as magnetization standards. J. Appl. Phys. 1982, 53, 2032–2034. [Google Scholar] [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of oxidation on the magnetization of nanoparticulate magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef]
- Millan, A.; Urtizberea, A.; Silva, N.J.; Palacio, F.; Amaral, V.S.; Snoeck, E.; Serin, V. Surface effects in maghemite nanoparticles. J. Magn. Magn. Mater. 2007, 312, L5–L9. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Liu, H.; Chen, L.; Li, L. Thermal decomposition of corn starch with different amylose/amylopectin ratios in open and sealed systems. Cereal Chem. 2009, 86, 383–385. [Google Scholar] [CrossRef]
- Chakraborty, A. Kinetics of the reduction of hematite to magnetite near its Curie transition. J. Magn. Magn. Mater. 1999, 204, 57–60. [Google Scholar] [CrossRef]
- Laughlin, D.E. The β iron controversy revisited. J. Phase Equilibria Diffus. 2018, 39, 274–279. [Google Scholar] [CrossRef]
- Copeland, C.S.; Rock, K.L.; Pinhal, A.; Chapman, R.C.; Chilcott, R.P. A fatal case report of barium chloride toxicity. J. Anal. Toxicol. 2023, 47, e33–e41. [Google Scholar] [CrossRef]
- Johnson, C.H.; Van Tassell, V.J. Acute barium poisoning with respiratory failure and rhabdomyolysis. Ann. Emerg. Med. 1991, 20, 1138–1142. [Google Scholar] [CrossRef]
- Boyd, E.M.; Abel, M. The acute toxicity of barium sulfate administered intragastrically. Can. Med. Assoc. J. 1966, 94, 849–853. [Google Scholar]
- Aziz, H.A.; Ghazali, M.F.; Hung, Y.T.; Wang, L.K. Toxicity, source, and control of barium in the environment. In Handbook of Advanced Industrial and Hazardous Wastes Management; CRC Press: Boca Raton, FL, USA, 2017; pp. 463–482. [Google Scholar] [CrossRef]
- Leeuw, N.H.; Cooper, T.G. Surface simulation studies of the hydration of white rust Fe (OH) 2, goethite α-FeO (OH) and hematite α-Fe2O3. Geochim. Cosmochim. Acta 2007, 71, 1655–1673. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Raman, A. The application of infrared spectroscopy to the study of rust systems—II. Study of cation deficiency in magnetite (Fe3O4) produced during its transformation to maghemite (γ-Fe2O3) and hematite (α-Fe2O3). Corros. Sci. 1993, 34, 1355–1365. [Google Scholar] [CrossRef]
- Voss, L.; Hoché, E.; Stock, V.; Böhmert, L.; Braeuning, A.; Thünemann, A.F.; Sieg, H. Intestinal and hepatic effects of iron oxide nanoparticles. Arch. Toxicol. 2021, 95, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Ranjbary, A.G.; Saleh, G.K.; Azimi, M.; Karimian, F.; Mehrzad, J.; Zohdi, J. Superparamagnetic Iron Oxide Nanoparticles Induce Apoptosis in HT-29 Cells by Stimulating Oxidative Stress and Damaging DNA. Biol. Trace Elem. Res. 2023, 201, 1163–1173. [Google Scholar] [CrossRef] [PubMed]

| Element | Concentration (mg/100 g) |
|---|---|
| Cl | 271.0 |
| K | 117.8 |
| P | 92.4 |
| Ca | 44.2 |
| S | 41.3 |
| Fe | 10.9 |
| Ba | 3.28 |
| Zn | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholico, F.A.; Orozco, A.A.; Quintero, L.H.; Knauth, P.; López, Z.; Paz, J.A.; Velásquez, C.A.; Bernal, J.d.J.; Cano, M.E. Magnetic Properties of Commercial Cornflakes. Appl. Sci. 2025, 15, 8652. https://doi.org/10.3390/app15158652
Cholico FA, Orozco AA, Quintero LH, Knauth P, López Z, Paz JA, Velásquez CA, Bernal JdJ, Cano ME. Magnetic Properties of Commercial Cornflakes. Applied Sciences. 2025; 15(15):8652. https://doi.org/10.3390/app15158652
Chicago/Turabian StyleCholico, Francisco A., Aldo A. Orozco, Luis H. Quintero, Peter Knauth, Zaira López, José A. Paz, Celso A. Velásquez, Jose de Jesús Bernal, and Mario E. Cano. 2025. "Magnetic Properties of Commercial Cornflakes" Applied Sciences 15, no. 15: 8652. https://doi.org/10.3390/app15158652
APA StyleCholico, F. A., Orozco, A. A., Quintero, L. H., Knauth, P., López, Z., Paz, J. A., Velásquez, C. A., Bernal, J. d. J., & Cano, M. E. (2025). Magnetic Properties of Commercial Cornflakes. Applied Sciences, 15(15), 8652. https://doi.org/10.3390/app15158652








