The Influence of Ultraviolet-C Light Pretreatment on Blackcurrant (Ribes nigrum) Quality During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Light Treatment
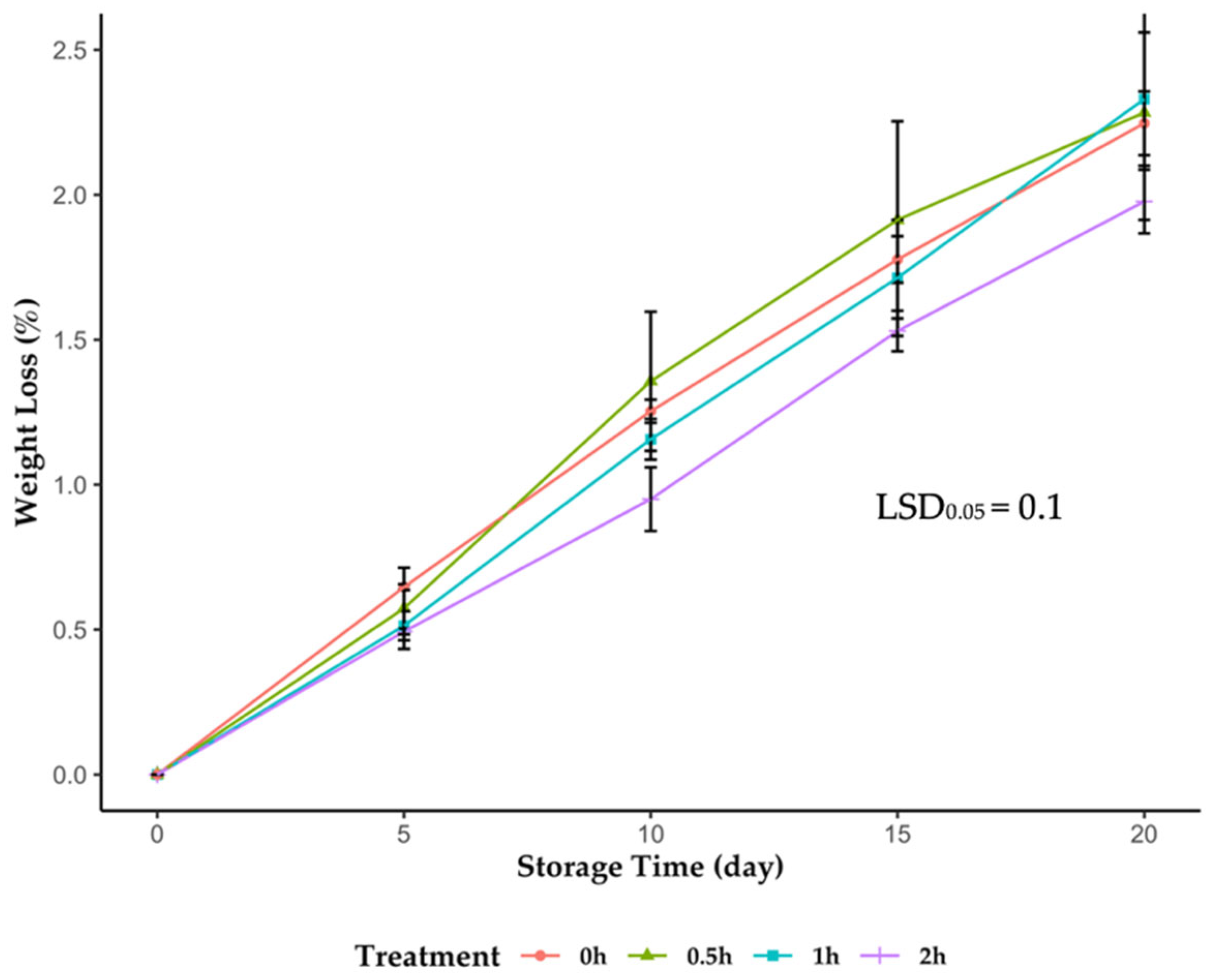
2.2. Weight Loss
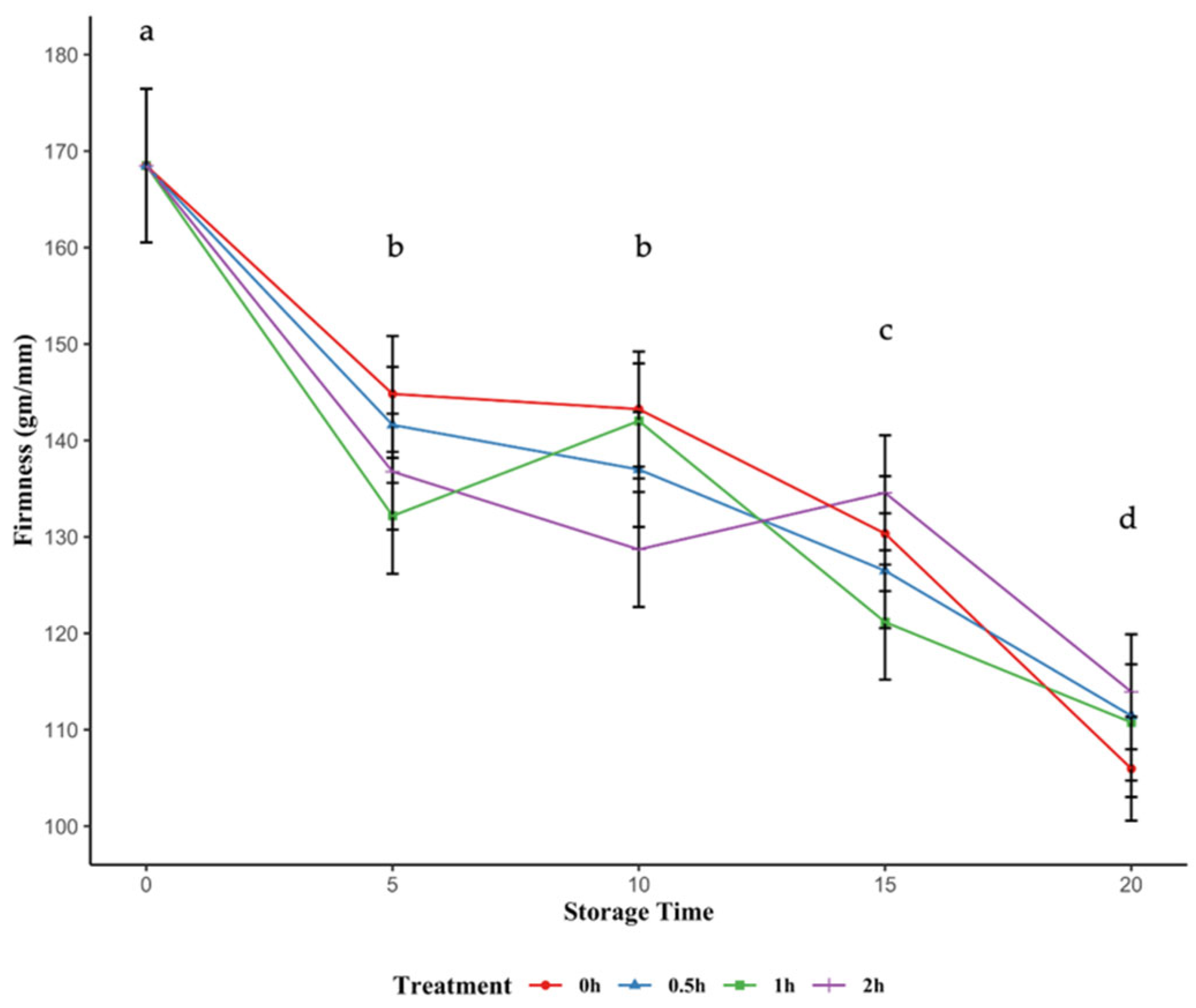
2.3. Firmness of Blackcurrants
2.4. Phytochemical Properties of Blackcurrants
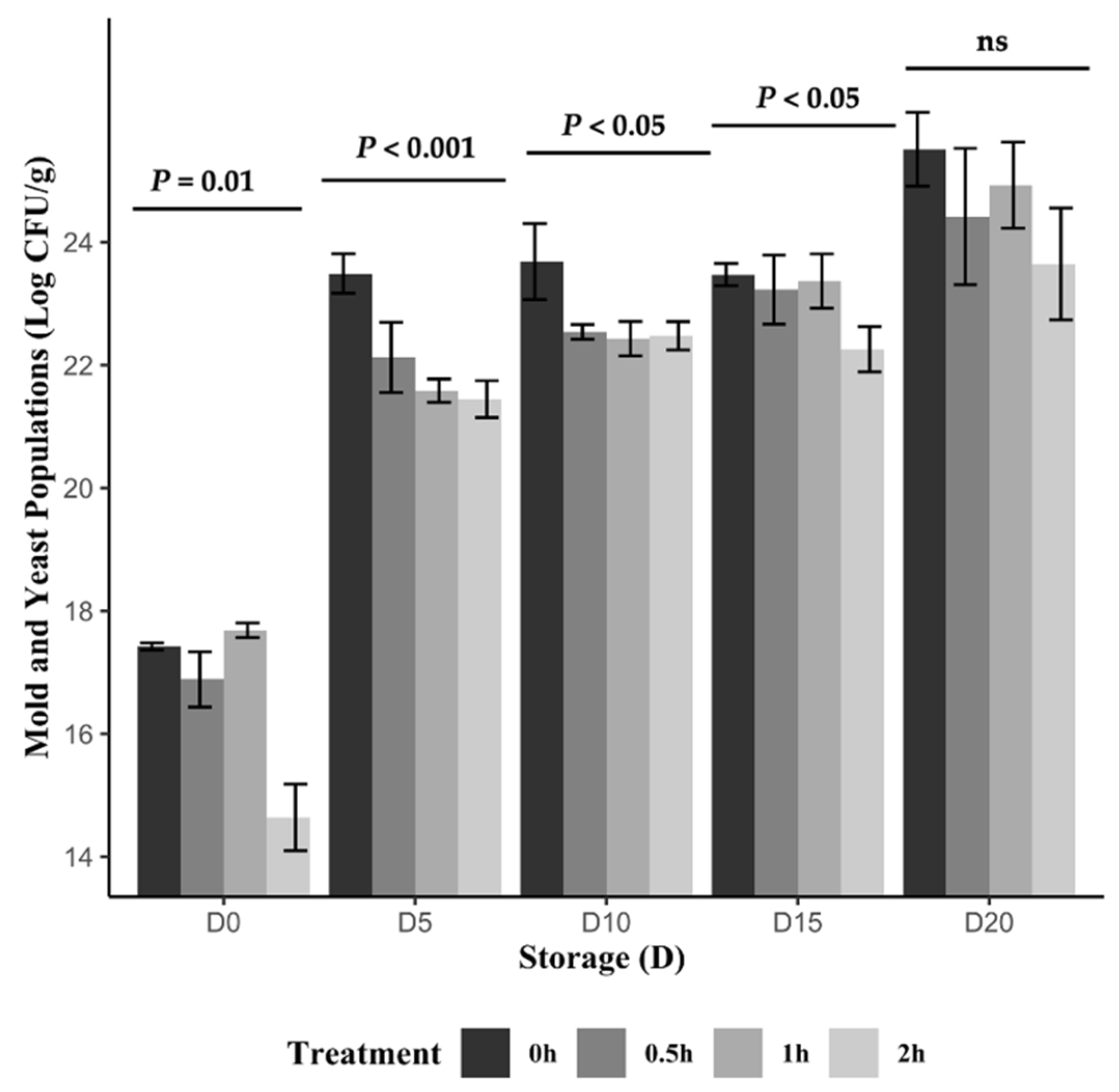
2.5. Microbial Population During Storage
2.6. Sample Extraction
2.7. Total Phenolics
2.8. Antioxidant Activity Assay
2.9. Ascorbic Acid Assay
2.10. Monophenolic Compound Measurements by LC-MS
2.11. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Variance of the Treatment
3.2. UVC Pretreatment Effects on Blackcurrant Postharvest Storage
3.3. Blackcurrant Physicochemical Changes During Storage
3.4. Blackcurrant Firmness Reduction During Storage
3.5. Yeast and Mold Population Changes During Blackcurrant Postharvest Storage
3.6. Blackcurrant Total Phenolic, Antioxidant Capacity, and Ascorbic Acid Changes During Storage
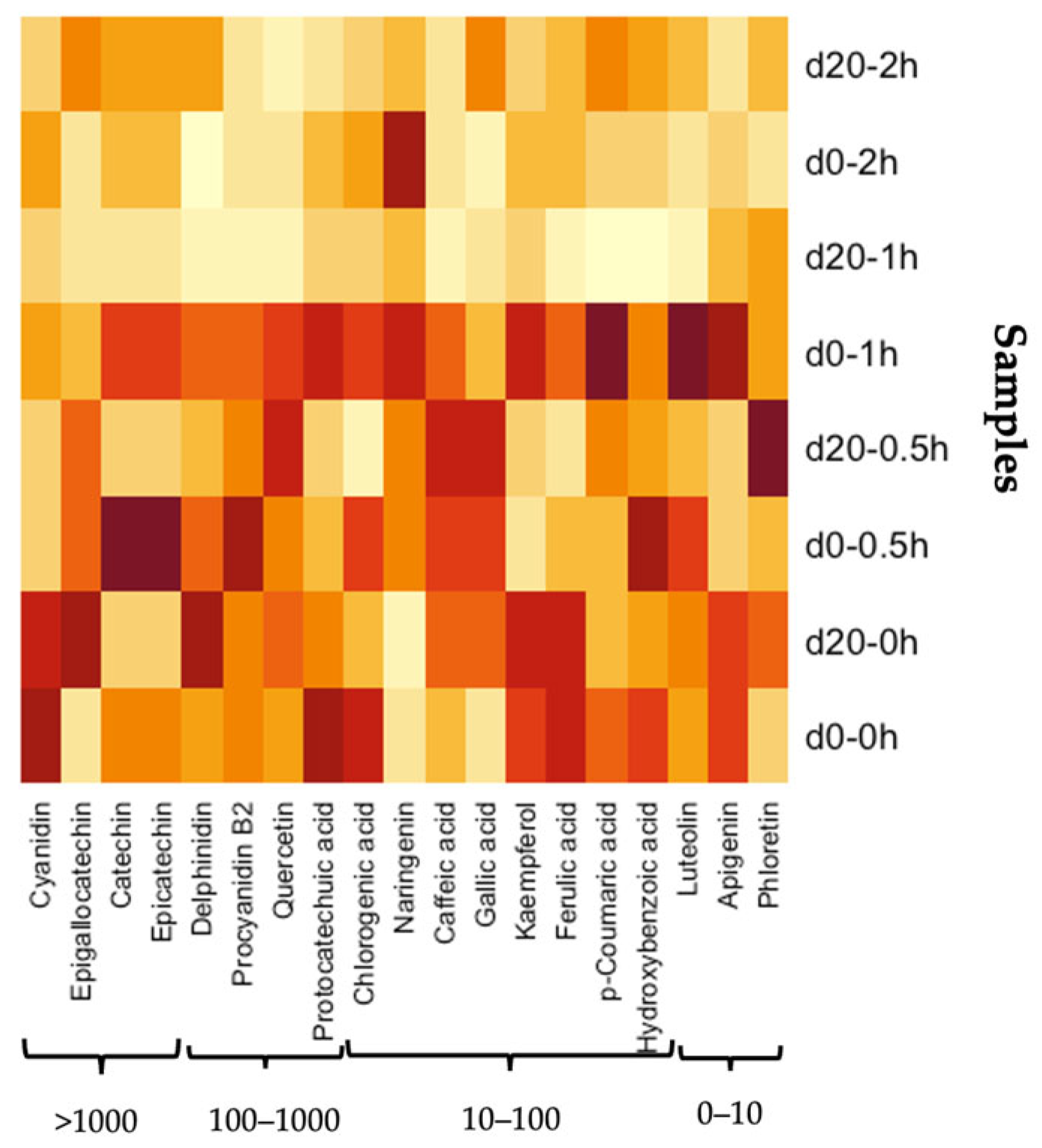
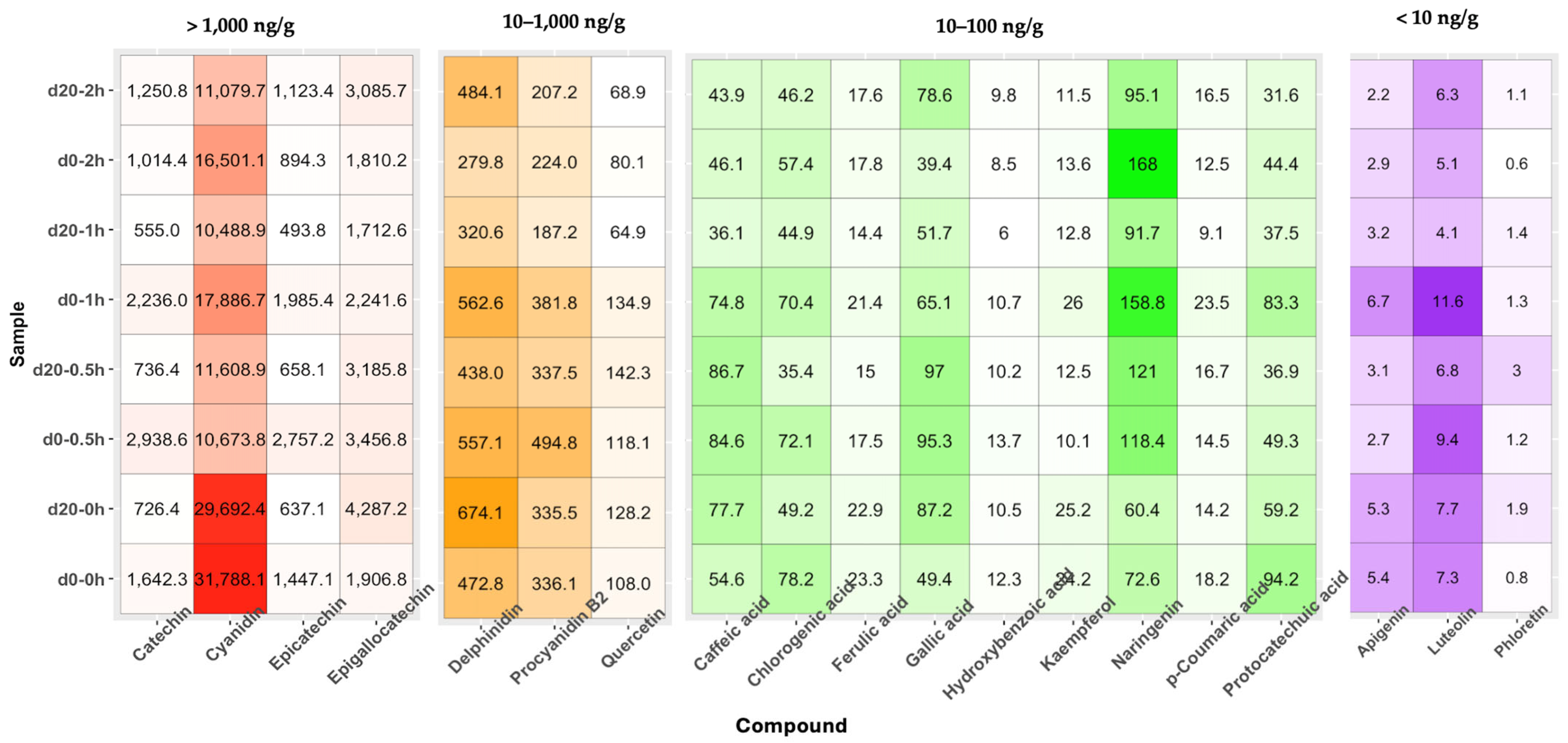
3.7. Antioxidant Phenolic Compounds in Blackcurrants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Black Currant Production. Available online: https://blackcurrant-wholesale.com/global-black-currant-production/ (accessed on 28 May 2025).
- Marsol-Vall, A.; Laaksonen, O.; Yang, B. Effects of Processing and Storage Conditions on Volatile Composition and Odor Characteristics of Blackcurrant (Ribes nigrum) Juices. Food Chem. 2019, 293, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kampuse, S.; Kampuss, K.; Pizika, L. Stability of anthocyanins and ascorbic acid in raspberry and blackcurrant cultivars during frozen storage. Acta Hortic. 2002, 585, 507–510. [Google Scholar] [CrossRef]
- Giné Bordonaba, J.; Chope, G.A.; Terry, L.A. Maximising Blackcurrant Anthocyanins: Temporal Changes during Ripening and Storage in Different Genotypes. J. Berry Res. 2010, 1, 73–80. [Google Scholar] [CrossRef]
- Buchweitz, M.; Speth, M.; Kammerer, D.R.; Carle, R. Impact of Pectin Type on the Storage Stability of Black Currant (Ribes nigrum L.) Anthocyanins in Pectic Model Solutions. Food Chem. 2013, 139, 1168–1178. [Google Scholar] [CrossRef]
- Dobson, G.; McDougall, G.J.; Stewart, D.; Cubero, M.Á.; Karjalainen, R.O. Effects of Juice Matrix and Pasteurization on Stability of Black Currant Anthocyanins during Storage. J. Food Sci. 2017, 82, 44–52. [Google Scholar] [CrossRef]
- Gudkovskii, V.A.; Kozhina, L.V.; Akimov, M.Y.; Zhidekhina, T.V. Innovative Storage Technology of Modern Commercial Black Currant Cultivars. Acta Hortic. 2020, 1277, 487–494. [Google Scholar] [CrossRef]
- Kouniaki, S.; Kajda, P.; Zabetakis, I. The Effect of High Hydrostatic Pressure on Anthocyanins and Ascorbic Acid in Blackcurrants (Ribes nigrum). Flavour Fragr. J. 2004, 19, 281–286. [Google Scholar] [CrossRef]
- Affonso, A.D. The Effects of Cofermentation of Cider and Apple Pomace on Cider Attributes. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2022. [Google Scholar]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest Treatments of Fresh Produce. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry Fruit Response to Postharvest Application of Ultraviolet Radiation. Postharvest Biol. Technol. 2008, 47, 280–285. [Google Scholar] [CrossRef]
- Jaramillo Sánchez, G.; Contigiani, E.V.; Coronel, M.B.; Alzamora, S.M.; García-Loredo, A.; Nieto, A.B. Study of UV-C Treatments on Postharvest Life of Blueberries ‘O’Neal’ and Correlation between Structure and Quality Parameters. Heliyon 2021, 7, e07190. [Google Scholar] [CrossRef]
- Eichholz, I.; Huyskens-Keil, S.; Keller, A.; Ulrich, D.; Kroh, L.W.; Rohn, S. UV-B-Induced Changes of Volatile Metabolites and Phenolic Compounds in Blueberries (Vaccinium corymbosum L.). Food Chem. 2011, 126, 60–64. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed Light Treatments for Food Preservation. A Review. Food Bioprocess Technol. 2010, 3, 13–23. [Google Scholar] [CrossRef]
- Joshi, K.; Mahendran, R.; Alagusundaram, K.; Norton, T.; Tiwari, B.K. Novel Disinfectants for Fresh Produce. Trends Food Sci. Technol. 2013, 34, 54–61. [Google Scholar] [CrossRef]
- Xing, Y.; Yue, T.; Wu, Y.; Xu, Q.; Guo, X.; Wang, X.; Yang, S.; Xu, L.; Yang, P. Effect of Chitosan Composite Coatings with Salicylic Acid and Titanium Dioxide Nanoparticles on the Storage Quality of Blackcurrant Berries. Coatings 2021, 11, 738. [Google Scholar] [CrossRef]
- Darré, M.; Vicente, A.R.; Cisneros-Zevallos, L.; Artés-Hernández, F. Postharvest Ultraviolet Radiation in Fruit and Vegetables: Applications and Factors Modulating Its Efficacy on Bioactive Compounds and Microbial Growth. Foods 2022, 11, 653. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of Postharvest Water Loss in Fruits: Mechanisms, Influencing Factors, and Effective Control Strategies—A Comprehensive Review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Kim, J.; Yoo, K.S.; Lim, S.; Lee, E.J. Effect of Prestorage UV-A, -B, and -C Radiation on Fruit Quality and Anthocyanin of ‘Duke’ Blueberries during Cold Storage. J. Agric. Food Chem. 2014, 62, 12144–12151. [Google Scholar] [CrossRef]
- Valerga, L.; González, R.E.; Mauricci, M.; Concellón, A.; Cavagnaro, P.F. Use of UVC Radiation as a Postharvest Stressor to Increase Phenolic Compounds Concentration and Antioxidant Status in Purple, Orange, and White Carrots. Postharvest Biol. Technol. 2024, 211, 112817. [Google Scholar] [CrossRef]
- Sonntag, F.; Liu, H.; Neugart, S. Nutritional and Physiological Effects of Postharvest UV Radiation on Vegetables: A Review. J. Agric. Food Chem. 2023, 71, 9951–9972. [Google Scholar] [CrossRef]
- Andrade Cuvi, M.J.; Vicente, A.R.; Concellón, A.; Chaves, A.R. Changes in Red Pepper Antioxidants as Affected by UV-C Treatments and Storage at Chilling Temperatures. LWT-Food Sci. Technol. 2011, 44, 1666–1671. [Google Scholar] [CrossRef]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet Irradiation and the Mechanisms Underlying Its Inactivation of Infectious Agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Gąstoł, M.; Błaszczyk, U. Effect of Magnetic Field and UV-C Radiation on Postharvest Fruit Properties. Agriculture 2024, 14, 1167. [Google Scholar] [CrossRef]
- Civello, P.; Vicente, A.; Martínez, G. UV-C Technology to Control Postharvest Diseases of Fruits and Vegetables. Recent Advances in Alternative Postharvest Technologies to Control Fungal Diseases in Fruits and Vegetables; 37/661 (2); Transworld Research Network: Trivandrum, India, 2006; pp. 71–102. [Google Scholar]
- Wang, Z.; Svyantek, A.; Miller, Z.; Watrelot, A.A.; Kadium, V.R. Postharvest Evaluations of Blackcurrant Fruits with Chitosan and Ultraviolet A Treatments. Appl. Sci. 2024, 14, 12052. [Google Scholar] [CrossRef]
- Luby, C.H.; Doane, S.; Mackey, T.; Yang, W.Q. A Comparison of Two Firmness-Testing Machines for Measuring Blueberry Firmness and Size. HortTechnology 2022, 33, 98–102. [Google Scholar] [CrossRef]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef] [PubMed]
- Carlos Sánchez-Rangel, J.; Benavides, J.; Basilio Heredia, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Peng, J.; Ling, J.; Zhang, X.-Q.; Zhang, L.-Y.; Cao, Q.-E.; Ding, Z.-T. A Rapid, Sensitive and Selective Colorimetric Method for Detection of Ascorbic Acid. Sens. Actuators B Chem. 2015, 221, 708–716. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef]
- Popat, R.; Banakara, K. Doebioresearch: Analysis of Design of Experiments for Biological Research. R Package Version 0.1.0. 2020. Available online: https://CRAN.R-project.org/package=doebioresearch (accessed on 25 July 2025).
- Fujisawa, H.; Kawai, Y.; Yoshida, M. Influence of Light-Reflecting Mulching Sheet and Shading Net on Ascorbic Acid Content in Blackcurrant Fruits (Ribes nigrum). Acta Hortic. 2023, 1381, 245–250. [Google Scholar] [CrossRef]
- Djordjevic, B.; Šavikin, K.; Djurovic, D.; Veberic, R.; Mikulič Petkovšek, M.; Zdunić, G.; Vulic, T. Biological and Nutritional Properties of Blackcurrant Berries (Ribes nigrum L.) under Conditions of Shading Nets. J. Sci. Food Agric. 2015, 95, 2416–2423. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Naseri, N. Combination Method of UV-B and UV-C Prevents Post-Harvest Decay and Improves Organoleptic Quality of Peach Fruit. Sci. Hortic. 2019, 256, 108564. [Google Scholar] [CrossRef]
- Pinheiro, J.; Alegria, C.; Abreu, M.; Gonçalves, E.M.; Silva, C.L.M. Use of UV-C Postharvest Treatment for Extending Fresh Whole Tomato (Solanum lycopersicum, cv. Zinac) Shelf-Life. J. Food Sci. Technol. 2015, 52, 5066–5074. [Google Scholar] [CrossRef] [PubMed]
- Jaruekdee, H.; Promyou, S. Effect of Pre-Treatment by Ultraviolet-C (UV-C) Irradiation Incorporated with Hydro-Cooling on Postharvest Quality of Red Hot Chili (Capsicum annuum L.). J. Food Sci. Agric. Technol. (JFAT) 2019, 5, 158–162. [Google Scholar]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. The Effect of the Application of Edible Coatings on or Before Ultraviolet Treatment on Postharvested Longan Fruits. J. Food Qual. 2017, 2017, 5454263. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV Treatment Improved the Quality of Postharvest Fruits and Vegetables by Inducing Resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Pataro, G.; Donsi, G.; Ferrari, G. Post-Harvest UV-C and PL Irradiation of Fruits and Vegetables. Chem. Eng. Trans. 2015, 44, 31–36. [Google Scholar] [CrossRef]
- Dassamiour, S.; Boujouraf, O.; Sraoui, L.; Bensaad, M.S.; Derardja, A.E.; Alsufyani, S.J.; Sami, R.; Algarni, E.; Aljumayi, H.; Aljahani, A.H. Effect of Postharvest UV-C Radiation on Nutritional Quality, Oxidation and Enzymatic Browning of Stored Mature Date. Appl. Sci. 2022, 12, 4947. [Google Scholar] [CrossRef]
- Obande, M.A.; Tucker, G.A.; Shama, G. Effect of Preharvest UV-C Treatment of Tomatoes (Solanum lycopersicon Mill.) on Ripening and Pathogen Resistance. Postharvest Biol. Technol. 2011, 62, 188–192. [Google Scholar] [CrossRef]
- Ortiz Araque, L.C.; Ortiz, C.M.; Darré, M.; Rodoni, L.M.; Civello, P.M.; Vicente, A.R. Role of UV-C Irradiation Scheme on Cell Wall Disassembly and Surface Mechanical Properties in Strawberry Fruit. Postharvest Biol. Technol. 2019, 150, 122–128. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Model of Fungal Development in Stored Barley Ecosystems as a Prognostic Auxiliary Tool for Postharvest Preservation Systems. Food Bioprocess Technol. 2021, 14, 298–309. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Uddin, M.S.; Hawlader, M.N.A.; Ding, L.; Mujumdar, A.S. Degradation of Ascorbic Acid in Dried Guava During Storage. J. Food Eng. 2002, 51, 21–26. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of Flavonoid and Phenolic Antioxidants in Black Currants, Blueberries, Raspberries, Red Currants, and Cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and Biological Properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Tennakone, K.; Kumara, G.R.R.A.; Kottegoda, I.R.M.; Wijayantha, K.G.U. The Photostability of Dye-Sensitized Solid State Photovoltaic Cells: Factors Determining the Stability of the Pigment in a Nanoporous n-/Cyanidin/p-CuI Cell. Semicond. Sci. Technol. 1997, 12, 128. [Google Scholar] [CrossRef]
- Prajapati, U.; Asrey, R.; Varghese, E.; Singh, A.K.; Pal Singh, M. Effects of Postharvest Ultraviolet-C Treatment on Shelf-Life and Quality of Bitter Gourd Fruit during Storage. Food Packag. Shelf Life 2021, 28, 100665. [Google Scholar] [CrossRef]
- Vergne, M.J.; Patras, A.; Bhullar, M.S.; Shade, L.M.; Sasges, M.; Rakariyatham, K.; Pan, C.; Xiao, H. UV-C Irradiation on the Quality of Green Tea: Effect on Catechins, Antioxidant Activity, and Cytotoxicity. J. Food Sci. 2018, 83, 1258–1264. [Google Scholar] [CrossRef]
- Wade, N.L.; Tan, S.C.; Kavanagh, E.E. White Light Prevents Increased Catechin Synthesis by Ultraviolet Irradiation in Banana Fruits. J. Hortic. Sci. 1993, 68, 637–644. [Google Scholar] [CrossRef]
- Cebulak, T.; Oszmiański, J.; Kapusta, I.; Lachowicz, S. Effect of UV-C Radiation, Ultra-Sonication Electromagnetic Field and Microwaves on Changes in Polyphenolic Compounds in Chokeberry (Aronia melanocarpa). Molecules 2017, 22, 1161. [Google Scholar] [CrossRef] [PubMed]
- Untea, A.E.; Oancea, A.-G.; Vlaicu, P.A.; Varzaru, I.; Saracila, M. Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization Before and After In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation. Foods 2024, 13, 1514. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-Gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant Activities of Peel, Pulp and Seed Fractions of Common Fruits as Determined by FRAP Assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Estévez, L.; Mosquera, R.A. Molecular Structure and Antioxidant Properties of Delphinidin. J. Phys. Chem. A 2008, 112, 10614–10623. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Jing, Y.; Liu, S.; Qin, S.; Peng, F.; Li, D.; Peng, C. Procyanidin B2: A Promising Multi-Functional Food-Derived Pigment for Human Diseases. Food Chem 2023, 420, 136101. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic Acid: A Review of Its Potential Use in Medications and Cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a Potential Immunomodulator in Therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Clé, C.; Hill, L.M.; Niggeweg, R.; Martin, C.R.; Guisez, Y.; Prinsen, E.; Jansen, M.A.K. Modulation of Chlorogenic Acid Biosynthesis in Solanum lycopersicum; Consequences for Phenolic Accumulation and UV-Tolerance. Phytochemistry 2008, 69, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L.; Nabavi, S.M. Health Effects of Phloretin: From Chemistry to Medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health Functionality of Apigenin: A Review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]

| Factor | Pretreatment | Storage Time | Pretreatment × Storage Time |
|---|---|---|---|
| df | 3 | 3 for weight loss percentage or 4 for others | 6 or 8 |
| Weight loss (%) | 1.053 × 10−5 *** | <2.2 × 10−16 *** | ns |
| SSC (°Brix) | 0.0001709 *** | ns | 6.934 × 10−5 *** |
| pH | ns | 6.863 × 10−7 *** | 5.569 × 10−5 *** |
| Acid (%) | ns | 9.039 × 10−8 *** | 0.001989 ** |
| Firmness | ns | <2.2 × 10−16 *** | ns |
| Polyphenol content | ns | 0.058 | ns |
| Ascorbic acid content | ns | 0.004 ** | ns |
| DPPH | ns | 0.00032 *** | 0.04463 * |
| FRAP | ns | 2.712 × 10−7 *** | 0.04384 * |
| Mold | 5.97 × 10−7 *** | <2.2 × 10−16 *** | ns |
| Pretreatment (UVC) | SSC (°Brix) | p-Value | F-Value |
|---|---|---|---|
| 0 h | 14.31 ± 0.31 b | 0.0001709 *** | 11.37 |
| 0.5 h | 14.42 ± 0.26 b | ||
| 1 h | 14.61 ± 0.13 a | ||
| 2 h | 14.58 ± 0.21 a | ||
| Storage Duration (days) | pH | p-Value | F-Value |
| 0 | 3.27 ± 0.01 c | 6.863 × 10−7 *** | 21.85 |
| 5 | 3.27 ± 0.02 c | ||
| 10 | 2.28 ± 0.03 c | ||
| 15 | 3.29 ± 0.01 b | ||
| 20 | 3.31 ± 0.02 a | ||
| Storage Duration (days) | Acidity (%) | p-Value | F-Value |
| 0 | 2.37 ± 0.06 a | 9.04 × 10−8 *** | 28.31 |
| 5 | 2.40 ± 0.09 a | ||
| 10 | 2.41 ± 0.05 a | ||
| 15 | 2.39 ± 0.06 a | ||
| 20 | 2.19 ± 0.08 b |
| Storage Duration (Days) | Total Phenolics (µg GAE/mL) | DPPH (TEAC mM/mg) | FRAP (Fe2+ Equivalent nmol/mg) | Ascorbic Acids (mg/100 g) |
|---|---|---|---|---|
| 0 | 520.33 ± 30.19 a | 4.14 ± 0.08 a | 321.87 ± 34.40 a | 90.87 ± 13.54 bc |
| 5 | 518.69 ± 52.33 a | 4.07 ± 0.15 a | 246.79 ± 47.55 b | 82.13 ± 3.99 c |
| 10 | 524.09 ± 87.43 a | 4.10 ± 0.14 a | 243.10 ± 39.70 bc | 92.39 ± 9.64 ab |
| 15 | 474.98 ± 37.81 ab | 3.91 ± 0.10 b | 187.40 ± 38.98 ab | 98.81 ± 4.35 a |
| 20 | 454.71 ± 61.64 b | 3.97 ± 0.07 b | 215.10 ± 25.78 c | 94.95 ± 6.52 ab |
| F-value | 2.74 | 8.88 | 24.64 | 5.46 |
| p-Value | 0.058 | 0.00032 *** | 2.712 × 10−7 *** | 0.004 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Svyantek, A.; Miller, Z.; Davis, H.; Kapus, A. The Influence of Ultraviolet-C Light Pretreatment on Blackcurrant (Ribes nigrum) Quality During Storage. Appl. Sci. 2025, 15, 8452. https://doi.org/10.3390/app15158452
Wang Z, Svyantek A, Miller Z, Davis H, Kapus A. The Influence of Ultraviolet-C Light Pretreatment on Blackcurrant (Ribes nigrum) Quality During Storage. Applied Sciences. 2025; 15(15):8452. https://doi.org/10.3390/app15158452
Chicago/Turabian StyleWang, Zhuoyu, Andrej Svyantek, Zachariah Miller, Haydon Davis, and Ashley Kapus. 2025. "The Influence of Ultraviolet-C Light Pretreatment on Blackcurrant (Ribes nigrum) Quality During Storage" Applied Sciences 15, no. 15: 8452. https://doi.org/10.3390/app15158452
APA StyleWang, Z., Svyantek, A., Miller, Z., Davis, H., & Kapus, A. (2025). The Influence of Ultraviolet-C Light Pretreatment on Blackcurrant (Ribes nigrum) Quality During Storage. Applied Sciences, 15(15), 8452. https://doi.org/10.3390/app15158452






